Tomato Spotted Wilt Virus Suppresses the Antiviral Response of the Insect Vector, Frankliniella occidentalis, by Elevating an Immunosuppressive C18 Oxylipin Level Using Its Virulent Factor, NSs
Abstract
:1. Introduction
2. Materials and Methods
2.1. Insect Rearing
2.2. Chemicals
2.3. TSWV Infection to Thrips
2.4. Insect Sample Preparation to Quantify EpOMEs
2.5. LC-MS/MS Analyses
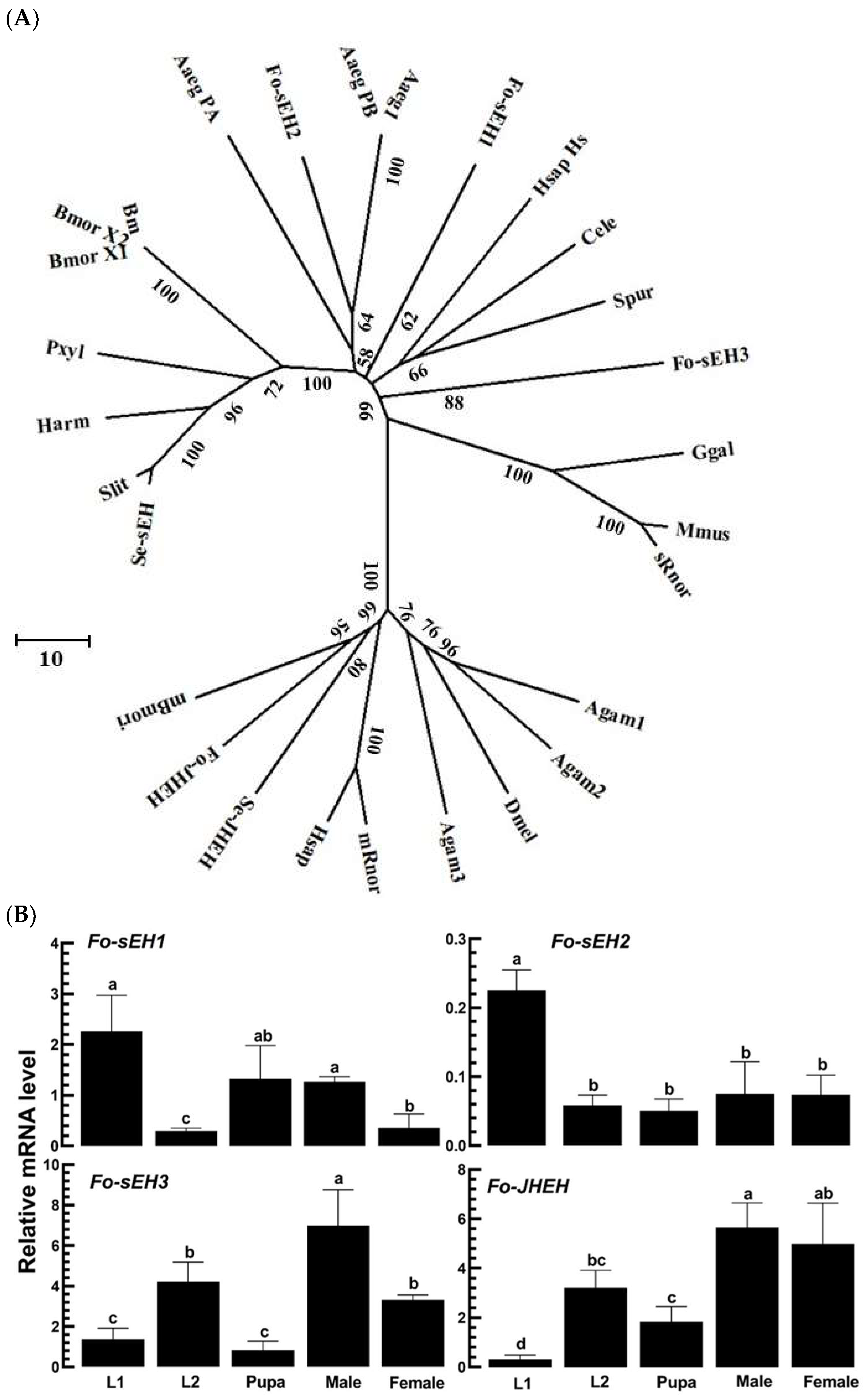
2.6. Bioinformatics to Predict EpOME Synthase (Fo-CYP) and Its Degradative Enzyme (Fo-sEH) Genes in F. occidentalis
2.7. RNA Extraction, cDNA Synthesis, RT-PCR, and RT-qPCR
2.8. dsRNA Preparation and RNA Interference (RNAi)
2.9. Fluorescence In Situ Hybridization (FISH) Assay
2.10. Terminal Deoxynucleotidyl Transferase dUTP Nick End Labeling (TUNEL) Assay
2.11. Preparation of Recombinant pIB-NSs Vector
2.12. IVTE of NSs in a Nonhost, Spodoptera Exigua
2.13. Quantification of cAMP and Ca2+ Signals
2.14. Hemocyte-Spreading Behavior
2.15. Nodulation Assay
2.16. Statistical Analysis
3. Results
3.1. Identification of EpOMEs in F. occidentalis
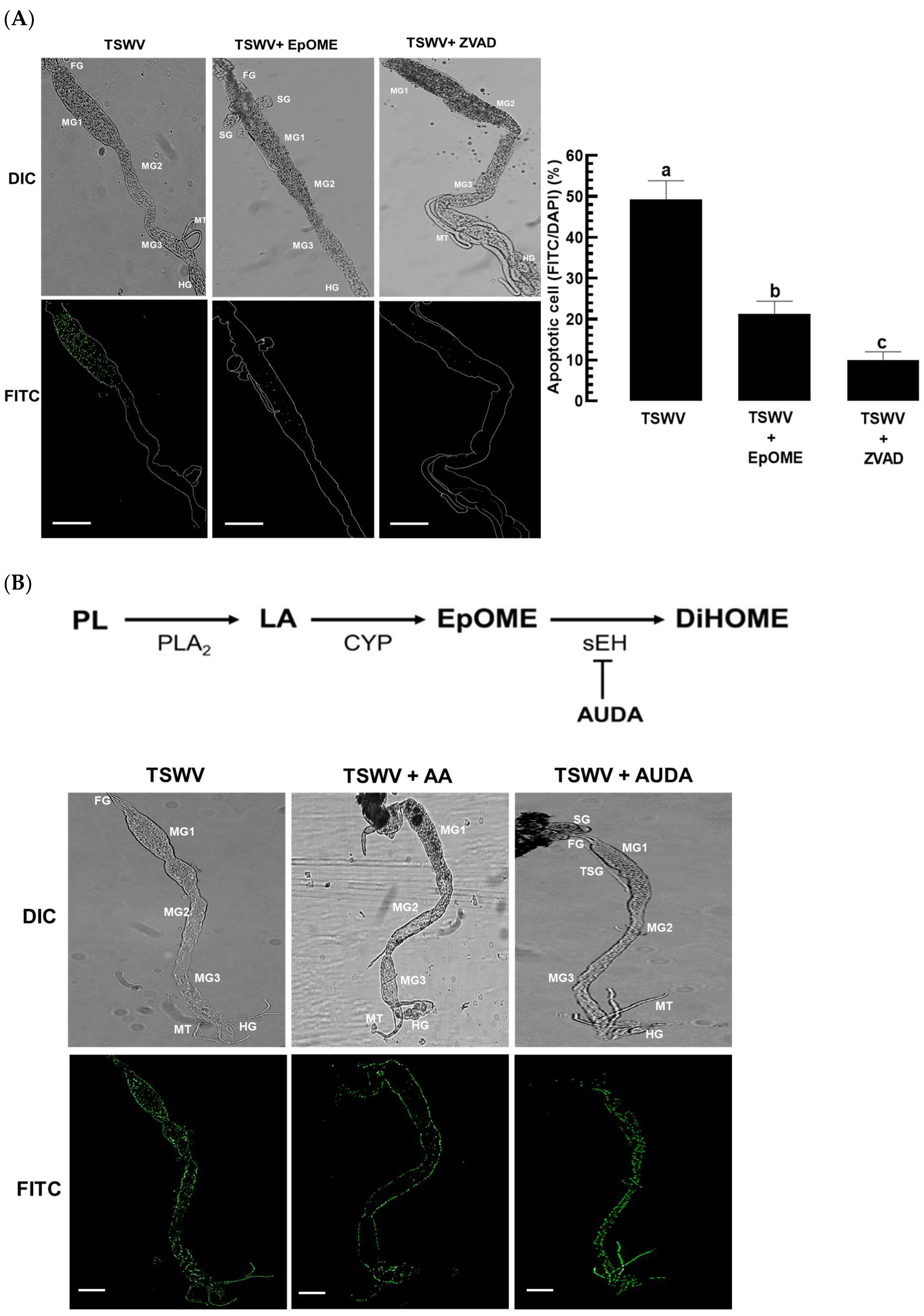
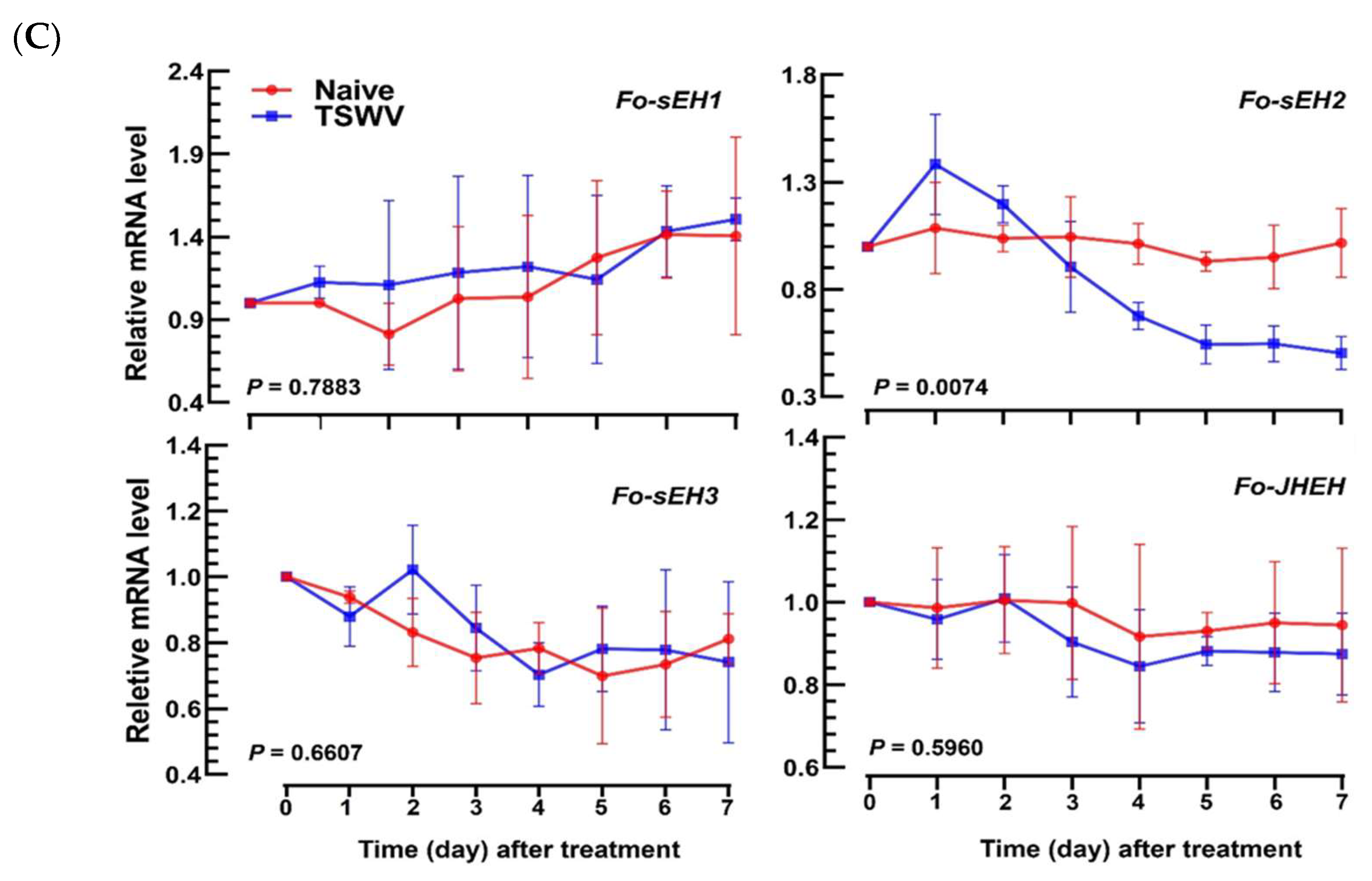
3.2. EpOME Suppresses the Antiviral Response in F. occidentalis Infected with TSWV
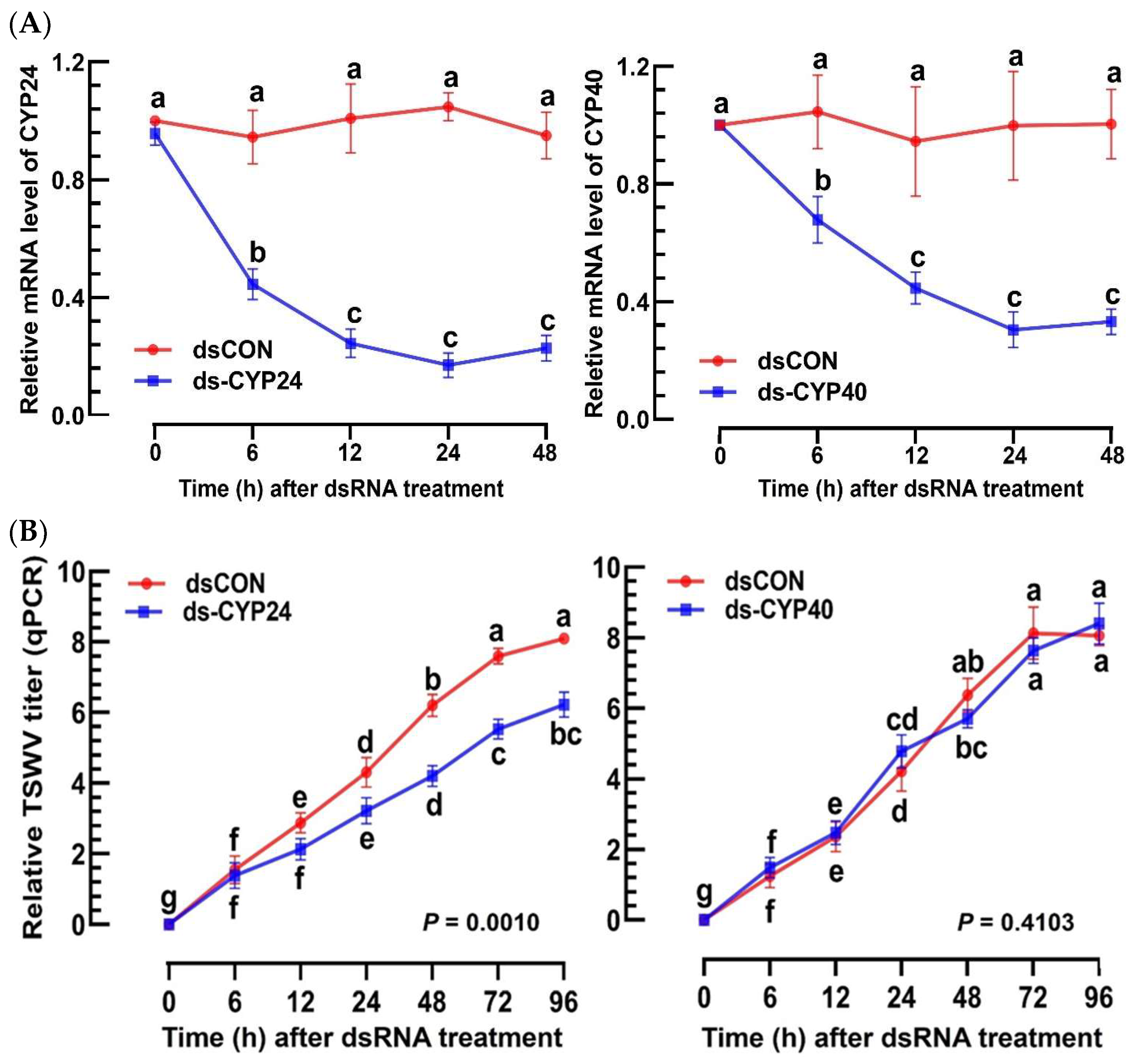
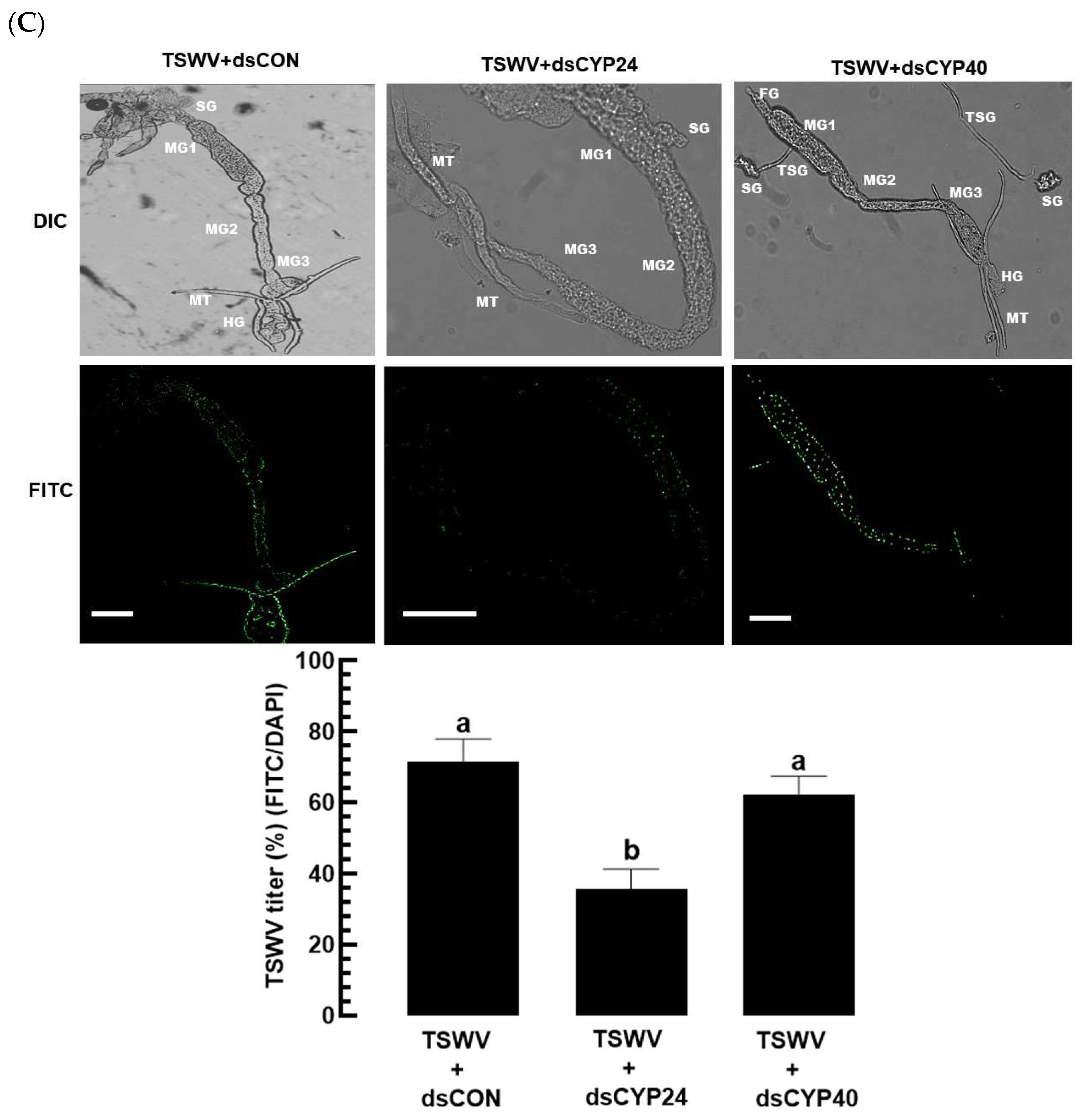
3.3. Prediction of EpOME Synthase in F. occidentalis and Its Functional Role in TSWV Infection
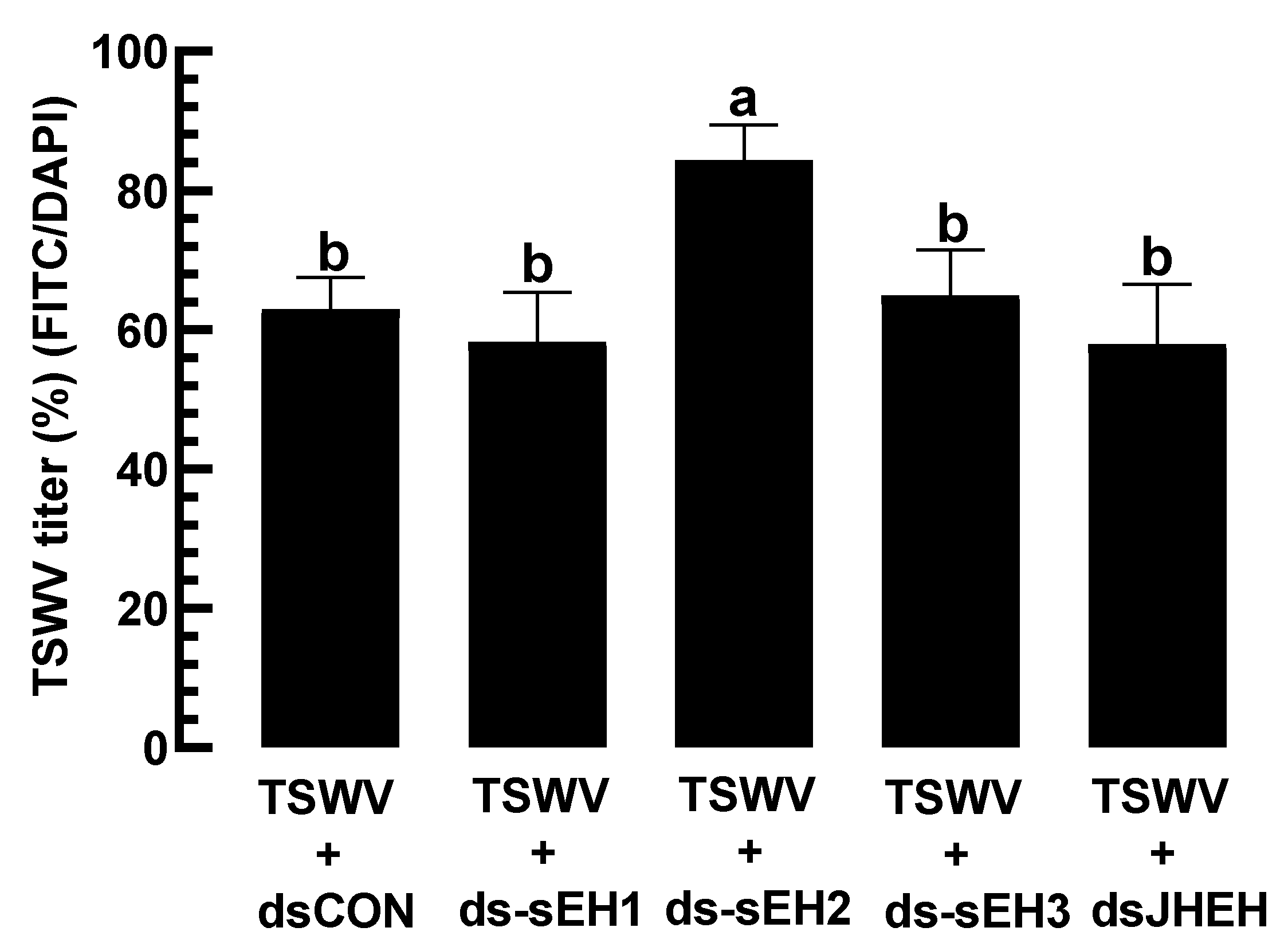
3.4. Prediction of sEH of F. occidentalis and Their Functional Association with TSWV Infection
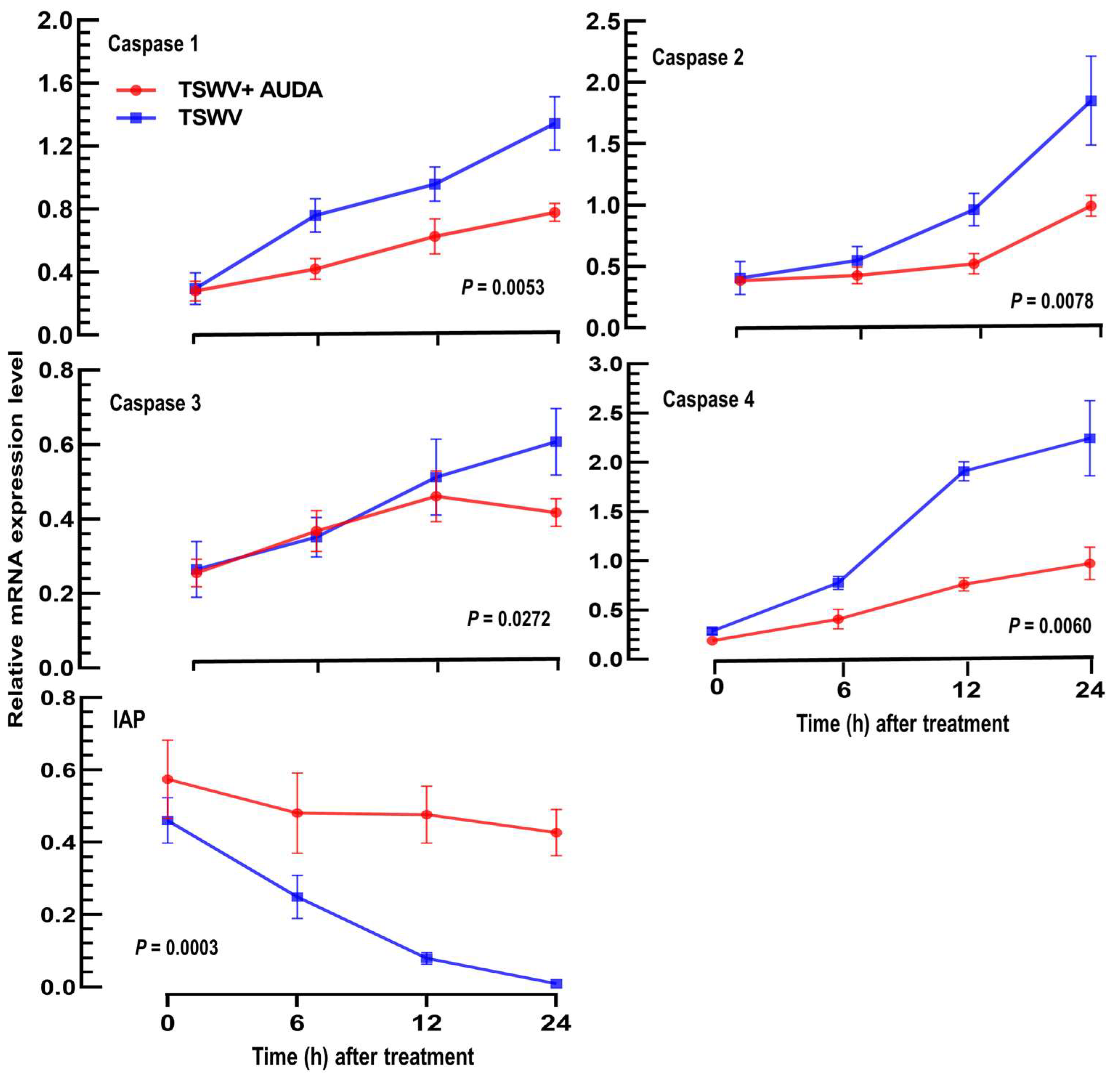
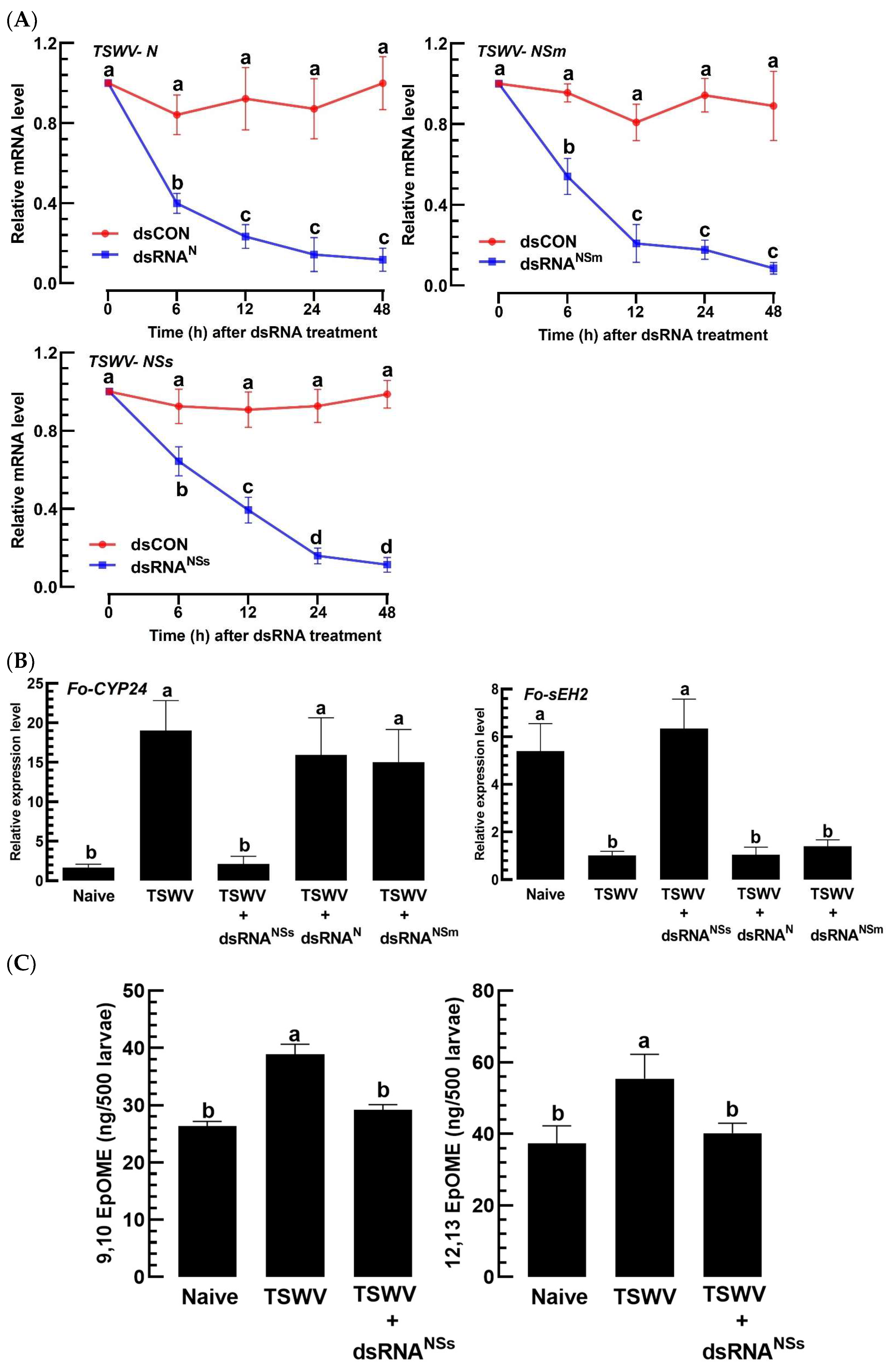
3.5. A Virulence Factor of TSWV in F. occidentalis Involves an Increase in EpOME Levels
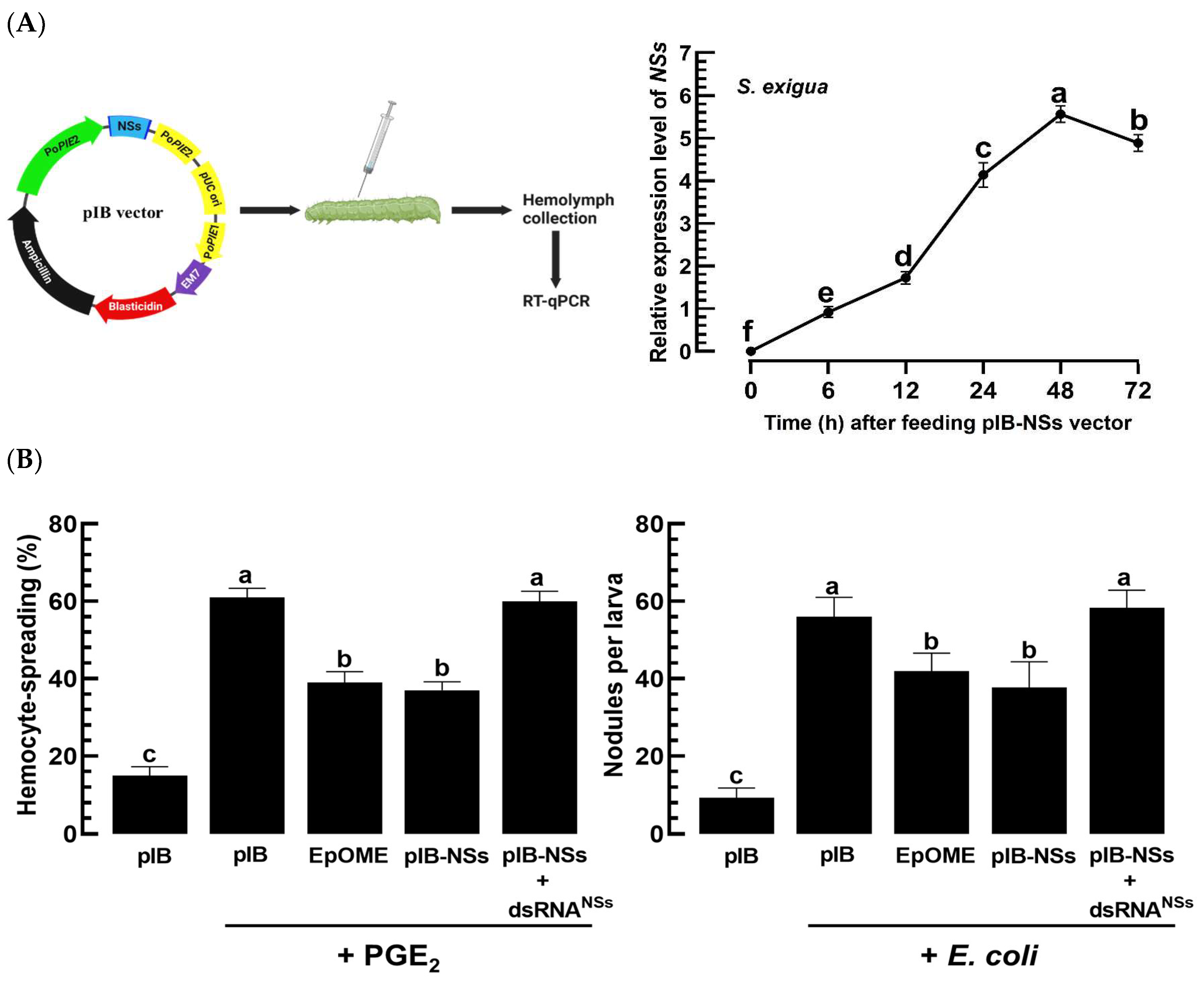
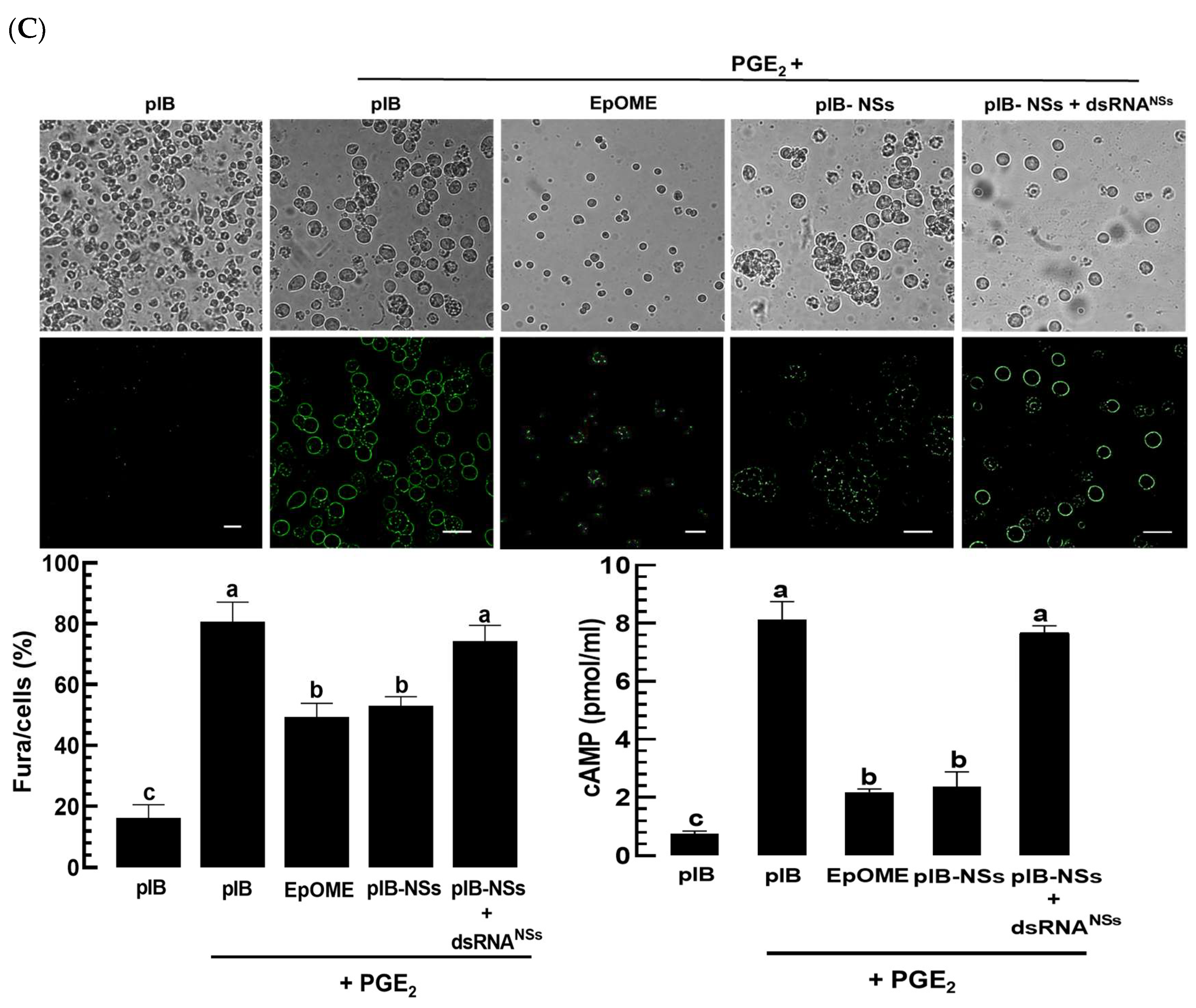
3.6. TSWV-NSs Mimics the EpOME Action to the Immune Response of a Non-Host Insect, S. exigua
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kuhn, J.H.; Abe, J.; Adkins, S.; Alkhovsky, S.V.; Avsic-Zupanc, T.; Ayllón, M.A.; Bahl, J.; Balkema-Buschmann, A.; Ballinger, M.J.; Kumar Baranwal, V.; et al. Annual (2023) taxonomic update of RNA- directed RNA polymerase- encoding negative- sense RNA viruses (realm Riboviria: Kingdom Orthornavirae: Phylum Negarnaviricota). J. Gen. Virol. 2023, 104, 001864. [Google Scholar] [CrossRef] [PubMed]
- Pappu, H.R.; Jones, R.A.C.; Jain, R.K. Global status of tospovirus epidemics in diverse cropping systems: Successes achieved and challenges ahead. Virus Res. 2009, 141, 219–236. [Google Scholar] [CrossRef] [PubMed]
- Oliver, J.E.; Whitfield, A.E. The genus Tospovirus: Emerging bunyaviruses that threaten food security. Annu. Rev. Virol. 2016, 3, 101–124. [Google Scholar] [CrossRef] [PubMed]
- Lewandowski, D.J.; Adkins, S. The tubule-forming NSm protein from tomato spotted wilt virus complements cell-to-cell and long-distance movement of tobacco mosaic virus hybrids. Virology 2005, 342, 26–37. [Google Scholar] [CrossRef] [PubMed]
- Ruark-Seward, C.L.; Bonville, B.; Kennedy, G.; Rasmussen, D.A. Evolutionary dynamics of tomato spotted wilt virus within and between alternate plant hosts and thrips. Sci. Rep. 2020, 10, 15797. [Google Scholar] [CrossRef] [PubMed]
- Rotenberg, D.; Jacobson, A.L.; Schneweis, D.J.; Whitfield, A.E. Thrips transmission of tospoviruses. Curr. Opin. Virol. 2015, 15, 80–89. [Google Scholar] [CrossRef]
- Nachappa, P.; Challacombe, J.; Margolies, D.C.; Nechols, J.R.; Whitfield, A.E.; Rotenberg, D. Tomato spotted wilt virus benefits its thrips vector by modulating metabolic and plant defense pathways in tomato. Front. Plant Sci. 2020, 11, 575564. [Google Scholar] [CrossRef]
- Ziegler-Graff, V. Molecular insights into host and vector manipulation by plant viruses. Viruses 2020, 12, 263. [Google Scholar] [CrossRef] [PubMed]
- Eigenbrode, S.D.; Bosque-Pérez, N.A.; Davis, T.S. Insect-borne plant pathogens and their vectors: Ecology, evolution, and complex interactions. Annu. Rev. Entomol. 2018, 63, 169–191. [Google Scholar] [CrossRef] [PubMed]
- Van De Wetering, F.; Goldbach, R.; Peters, D. Tomato spotted wilt tospovirus ingestion by first instar larvae of Frankliniella occidentalis is a prerequisite for transmission. Phytopathology 1996, 86, 900–905. [Google Scholar] [CrossRef]
- Nagata, T.; Inoue-Nagata, A.K.; Smid, H.M.; Goldbach, R.; Peters, D. Tissue tropism related to vector competence of Frankliniella occidentalis for tomato spotted wilt tospovirus. J. Gen. Virol. 1999, 80, 507–515. [Google Scholar] [CrossRef]
- Montero-Astúa, M.; Ullman, D.E.; Whitfield, A.E. Salivary gland morphology, tissue tropism and the progression of tospovirus infection in Frankliniella occidentalis. Virology 2016, 493, 39–51. [Google Scholar] [CrossRef]
- Rotenberg, D.; Whitfield, A.E. Molecular interactions between tospoviruses and thrips vectors. Curr. Opin. Virol. 2018, 33, 191–197. [Google Scholar] [CrossRef]
- Maris, P.C.; Joosten, N.N.; Goldbach, R.W.; Peters, D. Tomato spotted wilt virus infection improves host suitability for its vector Frankliniella occidentalis. Phytopathology 2004, 94, 706–711. [Google Scholar] [CrossRef]
- Belliure, B.; Janssen, A.; Maris, P.C.; Peters, D.; Sabelis, M.W. Herbivore arthropods benefit from vectoring plant viruses. Ecol. Lett. 2005, 8, 70–79. [Google Scholar] [CrossRef]
- Shrestha, A.; Srinivasan, R.; Riley, D.G.; Culbreath, A.K. Direct and indirect effects of a thrips-transmitted Tospovirus on the preference and fitness of its vector, Frankliniella fusca. Entomol. Exp. Appl. 2012, 145, 260–271. [Google Scholar] [CrossRef]
- Ogada, P.A.; Maiss, E.; Poehling, H.M. Influence of tomato spotted wilt virus on performance and behavior of western flower thrips (Frankliniella occidentalis). J. Appl. Entomol. 2013, 137, 488–498. [Google Scholar] [CrossRef]
- Wu, X.; Xu, S.; Zhao, P.; Zhang, X.; Yao, X.; Sun, Y.; Fang, R.; Ye, J. The Orthotospovirus nonstructural protein NSs suppresses plant MYC-regulated jasmonate signaling leading to enhanced vector attraction and performance. PLoS Pathog. 2019, 15, e1007897. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, J.; Li, X.; Zhang, J.; Wang, Y.; Lu, Y. The plant virus tomato spotted wilt orthotospovirus benefits its vector Frankliniella occidentalis by decreasing plant toxic alkaloids in host plant Datura stramonium. Int. J. Mol. Sci. 2023, 24, 14493. [Google Scholar] [CrossRef]
- Kim, C.Y.; Ahmed, S.; Stanley, D.; Kim, Y. HMG-like DSP1 is a damage signal to mediate the western flower thrips, Frankliniella occidentalis, immune responses to tomato spotted wilt virus infection. Dev. Comp. Immunol. 2023, 144, 104706. [Google Scholar] [CrossRef]
- Dunn, P.E.; Drake, D.R. Fate of bacteria injected into naïve and immunized larvae of the tobacco hornworm Manduca sexta. J. Invertebr. Pathol. 1983, 41, 77–85. [Google Scholar] [CrossRef]
- Hillyer, J.F. Insect immunology and hematopoiesis. Dev. Comp. Immunol. 2016, 58, 102–118. [Google Scholar] [CrossRef]
- Kim, Y.; Ahmed, S.; Stanley, D.; An, C. Eicosanoid-mediated immunity in insects. Dev. Comp. Immunol. 2018, 83, 130–143. [Google Scholar] [CrossRef]
- Shrestha, S.; Park, Y.; Stanley, D.; Kim, Y. Genes encoding phospholipases A2 mediate insect nodulation reactions to bacterial challenge. J. Insect Physiol. 2010, 56, 324–332. [Google Scholar] [CrossRef]
- Ahmed, S.; Kim, Y. Differential immunosuppression by inhibiting PLA2 affects virulence of Xenorhabdus hominickii and Photorhabdus temperata temperata. J. Invertebr. Pathol. 2018, 157, 136–146. [Google Scholar] [CrossRef]
- Stanley, D.W. Eicosanoids in Invertebrate Signal Transduction Systems; Princeton University Press: Princeton, NJ, USA, 1999; p. 292. [Google Scholar]
- Vatanparast, M.; Ahmed, S.; Lee, D.H.; Hwang, S.H.; Hammock, B.; Kim, Y. EpOMEs act as immune suppressors in a lepidopteran insect, Spodoptera exigua. Sci. Rep. 2020, 10, 20183. [Google Scholar] [CrossRef]
- Shahmohammadi, N.; Esmaeily, M.; Abdisa, E.; Mandal, E.; Kim, Y. Enhanced viral virulence by inhibition of EpOME biosynthesis in lepidopteran insects. J. Invertebr. Pathol. 2024, 204, 108095. [Google Scholar] [CrossRef] [PubMed]
- Vatanparast, M.; Lee, D.H.; Kim, Y. Biosynthesis and immunity of epoxyeicosatrienoic acids in a lepidopteran insect, Spodoptera exigua. Dev. Comp. Immunol. 2020, 107, 103643. [Google Scholar] [CrossRef]
- Jeong, S.Y.; Seol, D.W. The role of mitochondria in apoptosis. BMB Rep. 2008, 41, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Kvansakul, M. Viral infection and apoptosis. Viruses 2017, 9, 356. [Google Scholar] [CrossRef]
- Medeiros, R.B.; Resende Rde, O.; de Avila, A.C. The plant virus tomato spotted wilt tospovirus activates the immune system of its main insect vector, Frankliniella occidentalis. J. Virol. 2004, 78, 4976–4982. [Google Scholar] [CrossRef] [PubMed]
- Ogada, P.A.; Kiirika, L.M.; Lorenz, C.; Senkler, J.; Braun, H.P.; Poehling, H.M. Differential proteomics analysis of Frankliniella occidentalis immune response after infection with tomato spotted wilt virus (Tospovirus). Dev. Comp. Immunol. 2017, 67, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Rotenberg, D. Integration of transcriptomics and network analysis reveals co-expressed genes in Frankliniella occidentalis larval guts that respond to tomato spotted wilt virus infection. BMC Genome 2021, 22, 810. [Google Scholar] [CrossRef] [PubMed]
- Moran, J.H.; Mitchell, L.A.; Bradbury, J.A.; Qu, W.; Zeldin, D.C.; Schnellmann, R.G.; Grant, D.F. Analysis of the cytotoxic properties of linoleic acid metabolites produced by renal and hepatic P450s. Toxicol. Appl. Pharmacol. 2000, 168, 268–279. [Google Scholar] [CrossRef] [PubMed]
- Hanif, A.; Edin, M.L.; Zeldin, D.C.; Morisseau, C.; Nayeem, M.A. Effect of soluble epoxide hydrolase on the modulation of coronary reactive hyperemia: Role of oxylipins and PPARγ. PLoS ONE 2016, 11, e0162147. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular evolutionary genetics analysis version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Park, B.; Kim, Y. Transient transcription of a putative RNase containing BEN domain encoded in Cotesia plutellae bracovirus induces an immunosuppression of the diamondback moth, Plutella xylostella. J. Invertebr. Pathol. 2010, 105, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.Y.; Kim, Y. In vivo transient expression of a viral silencing suppressor, NSs, derived from tomato spotted wilt virus decreases insect RNAi efficiencies. Arch. Insect Biochem. Physiol. 2023, e21982. [Google Scholar] [CrossRef]
- Goh, H.G.; Lee, S.G.; Lee, B.P.; Choi, K.M.; Kim, J.H. Simple mass rearing of beet armyworm Spodoptera exigua (Hübner) (Lepidoptera: Noctuidae), on an artificial diet. Korean J. Appl. Entomol. 1990, 29, 180–183. [Google Scholar]
- Hepat, R.; Kim, Y. In vivo transient expression for the functional analysis of polydnaviral genes. J. Invertebr. Pathol. 2012, 111, 152–159. [Google Scholar] [CrossRef]
- SAS Institute, Inc. SAS/STAT User’s Guide, 3rd ed.; SAS Institute: Cary, NC, USA, 1989. [Google Scholar]
- Hossain Hrithik, M.T.; Shahmohammadi, N.; Jin, G.; Lee, D.H.; Singh, N.; Vik, A.; Hammock, B.D.; Kim, Y. Insect immune resolution with EpOME/DiHOME and its dysregulation by their analogs leading to pathogen hypersensitivity. Insect Biochem. Mol. Biol. 2024, 168, 104104. [Google Scholar] [CrossRef]
- Mandal, E.; Khan, F.; Kil, E.J.; Kim, Y. Virus-vectoring thrips regulate the excessive multiplication of tomato spotted wilt virus using their antiviral immune responses. J. Gen. Virol. 2024, 105. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Morisseau, C.; Yang, J.; Mamatha, D.M.; Hammock, B.D. Epoxide hydrolase activities and epoxy fatty acids in the mosquito Culex quinquefasciatus. Insect Biochem. Mol. Biol. 2015, 59, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.A.; Ahmed, S.; Mollah, M.M.I.; Lee, D.; Kim, Y. Variation in pathogenicity of different strains of Xenorhabdus nematophila; differential immunosuppressive activities and secondary metabolite production. J. Invertebr. Pathol. 2019, 166, 107221. [Google Scholar] [CrossRef]
- Xu, J.; Morisseau, C.; Yang, J.; Lee, K.S.S.; Kamita, S.G.; Hammock, B.D. Ingestion of the epoxide hydrolase inhibitor AUDA modulates immune responses of the mosquito, Culex quinquefasciatus during blood feeding. Insect Biochem. Mol. Biol. 2016, 76, 62–69. [Google Scholar] [CrossRef]
- Ribeiro, D.; Borst, J.W.; Goldbach, R.; Kormelink, R. Tomato spotted wilt virus nucleocapsid protein interacts with both viral glycoproteins Gn and Gc in planta. Virology 2009, 383, 121–130. [Google Scholar] [CrossRef]
- Margaria, P.; Bosco, L.; Vallino, M.; Ciuffo, M.; Mautino, G.C.; Tavella, L.; Turina, M. The NSs protein of tomato spotted wilt virus is required for persistent infection and transmission by Frankliniella occidentalis. J. Virol. 2014, 88, 5788–5802. [Google Scholar] [CrossRef]
- de Ronde, D.; Butterbach, P.; Lohuis, D.; Hedil, M.; van Lent, J.W.; Kormelink, R. TSWV gene-based resistance is triggered by a functional RNA silencing suppressor protein of the Tomato spotted wilt virus. Mol. Plant Pathol. 2013, 14, 405–415. [Google Scholar] [CrossRef]
- Hedil, M.; Kormelink, R. Viral RNA silencing suppression: The enigma of bunyavirus NSs proteins. Viruses 2016, 8, 208. [Google Scholar] [CrossRef]
- Takeda, A.; Sugiyama, K.; Nagano, H.; Mori, M.; Kaido, M.; Mise, K.; Tsuda, S.; Okuno, T. Identification of a novel RNA silencing suppressor, NSs protein of tomato spotted wilt virus. FEBS Lett. 2002, 532, 75–79. [Google Scholar] [CrossRef] [PubMed]
- de Ronde, D.; Pasquier, A.; Ying, S.; Butterbach, P.; Lohuis, D.; Kormelink, R. Analysis of tomato spotted wilt virus NSs protein indicates the importance of the N-terminal domain for avirulence and RNA silencing suppression. Mol. Plant Pathol. 2014, 15, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Y.; Bag, S.; Mitter, N.; Turina, M.; Pappu, H.R. Mutational analysis of two highly conserved motifs in the silencing suppressor encoded by tomato spotted wilt virus (genus Tospovirus, family Bunyaviridae). Arch. Virol. 2014, 159, 1499–1504. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shahmohammadi, N.; Khan, F.; Jin, G.; Kwon, M.; Lee, D.; Kim, Y. Tomato Spotted Wilt Virus Suppresses the Antiviral Response of the Insect Vector, Frankliniella occidentalis, by Elevating an Immunosuppressive C18 Oxylipin Level Using Its Virulent Factor, NSs. Cells 2024, 13, 1377. https://doi.org/10.3390/cells13161377
Shahmohammadi N, Khan F, Jin G, Kwon M, Lee D, Kim Y. Tomato Spotted Wilt Virus Suppresses the Antiviral Response of the Insect Vector, Frankliniella occidentalis, by Elevating an Immunosuppressive C18 Oxylipin Level Using Its Virulent Factor, NSs. Cells. 2024; 13(16):1377. https://doi.org/10.3390/cells13161377
Chicago/Turabian StyleShahmohammadi, Niayesh, Falguni Khan, Gahyeon Jin, Minji Kwon, Donghee Lee, and Yonggyun Kim. 2024. "Tomato Spotted Wilt Virus Suppresses the Antiviral Response of the Insect Vector, Frankliniella occidentalis, by Elevating an Immunosuppressive C18 Oxylipin Level Using Its Virulent Factor, NSs" Cells 13, no. 16: 1377. https://doi.org/10.3390/cells13161377
APA StyleShahmohammadi, N., Khan, F., Jin, G., Kwon, M., Lee, D., & Kim, Y. (2024). Tomato Spotted Wilt Virus Suppresses the Antiviral Response of the Insect Vector, Frankliniella occidentalis, by Elevating an Immunosuppressive C18 Oxylipin Level Using Its Virulent Factor, NSs. Cells, 13(16), 1377. https://doi.org/10.3390/cells13161377






