Targeting Cleavage of C-Terminal Fragment of Cytoskeletal Filamin A in Cancers
Abstract
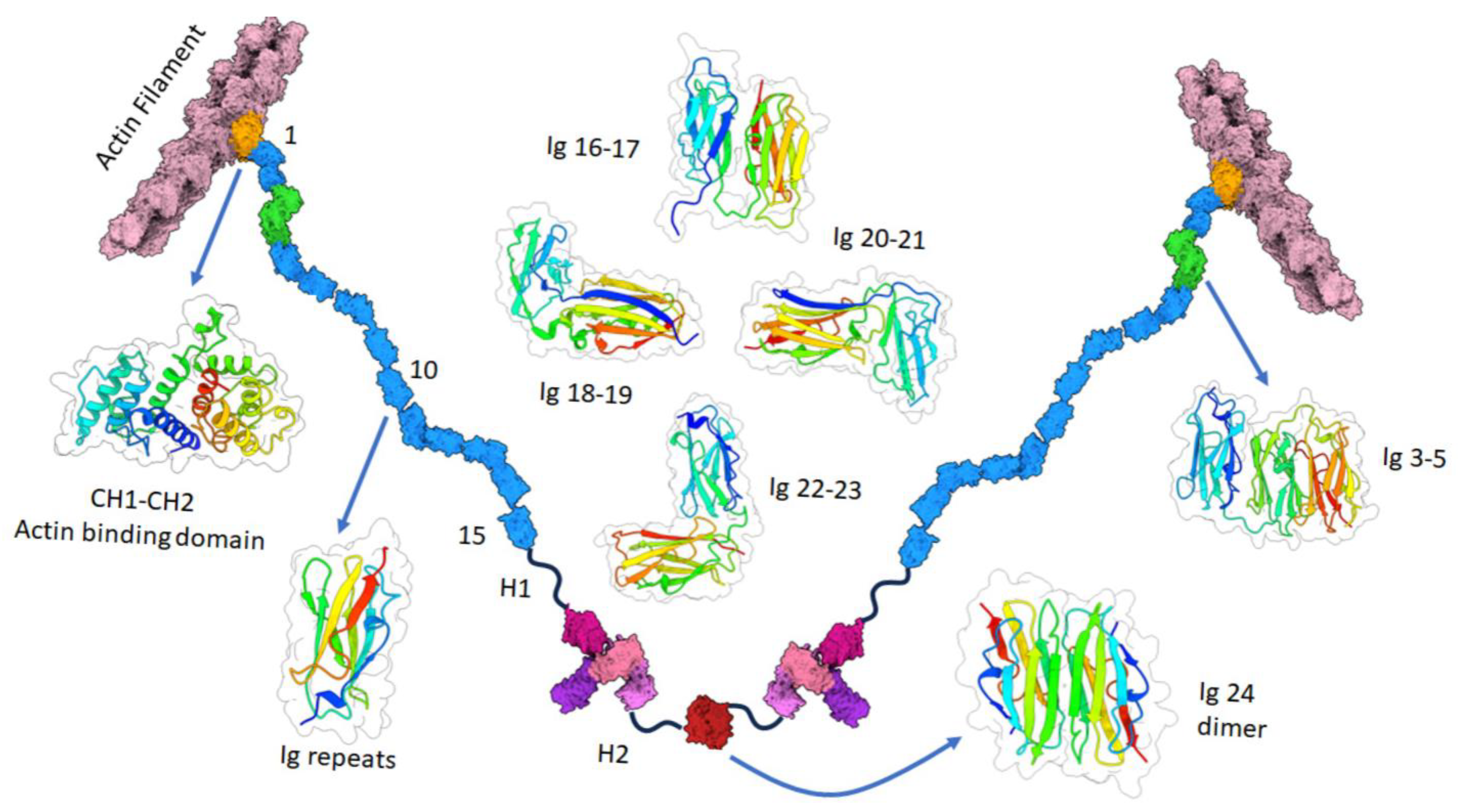
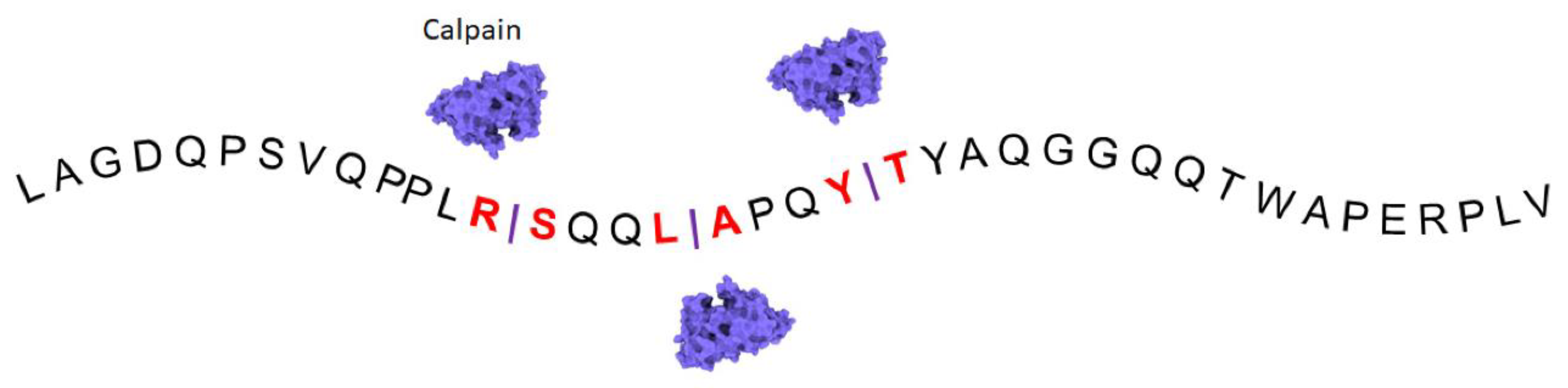
:1. Structure and Function of Filamin A
2. Interaction Partners of Filamin A Involved in Cancers
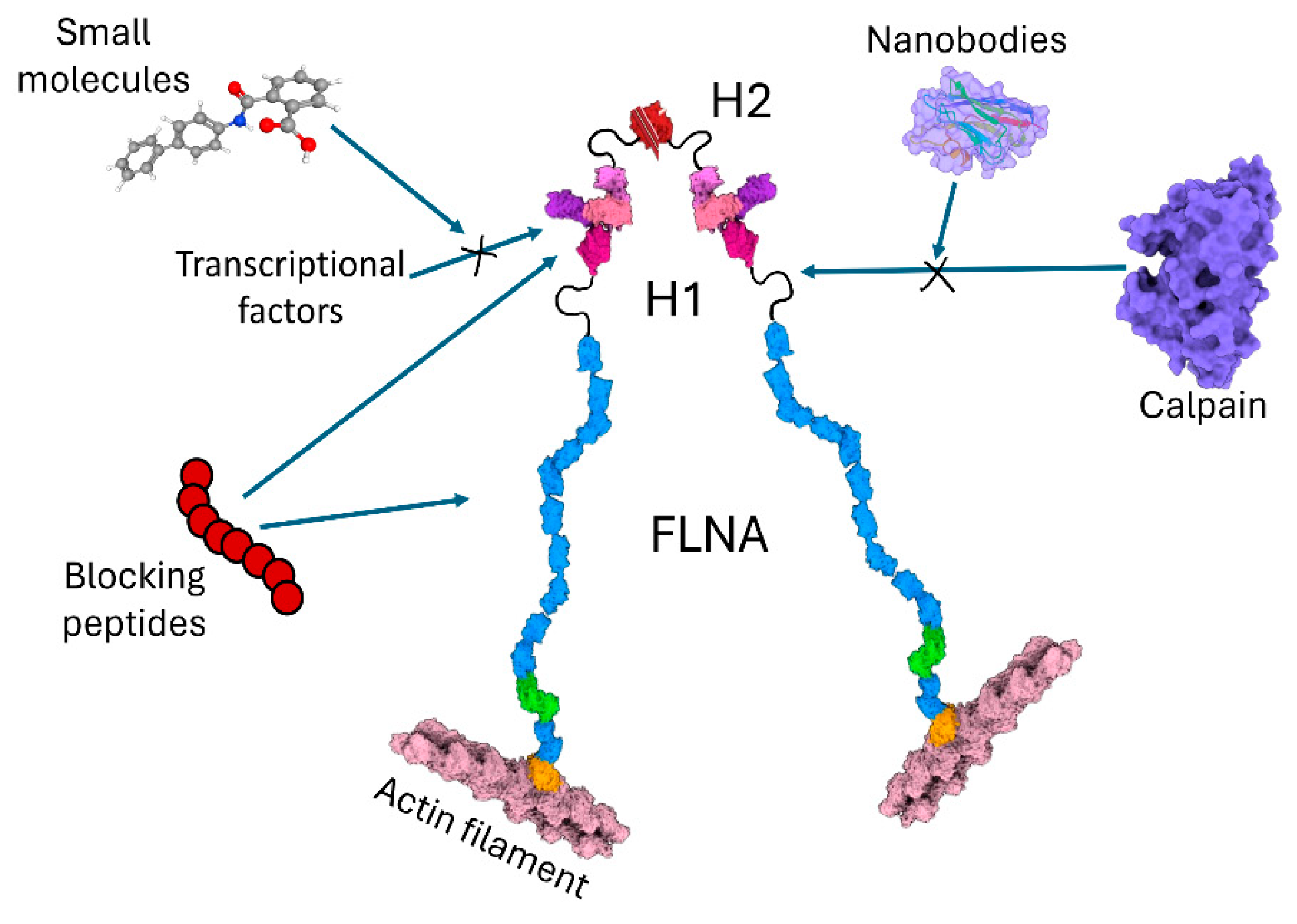
3. Approaches Targeting Cleavage of Filamin A in Cancers
| Cancer Type | Cell Lines | Inhibition of FLNA Cleavage | Outcome of FLNACT Inhibition | Reference |
|---|---|---|---|---|
| Glioblastoma multiforme | U87 A172 | Calpeptin | Increased cell growth and invasion | [27] |
| Melanoma, Prostate Fibrosarcoma |
A7 PC3 T241 | Calpeptin | Reduced cell proliferation, migration, and colony formation | [26] |
| Melanoma | A7 M2 | Calpeptin Mutant protein resistant to calpain cleavage | Reduced tumor angiogenesis by HIF-1α/VEGF signaling | [12] |
| Breast | MCF-7 MDA-231 BT-20 | Mutant protein resistant to calpain cleavage | Increased cell migration and invasion by focal adhesion disassembly | [40] |
| Prostate | COS1 LNCaP DU145 22Rv1 | Calpeptin | Decreased FHL2 accumulation and decreased AR co-activation | [41] |
| Prostate | DU145 PC-3 | Leupeptin and ALLM | Reduced cell migration | [37] |
| Melanoma | UACC903 M93-047 | Klotho protein | Inhibition of cell motility by Wnt5A signaling | [35] |
| Melanoma | UACC90 M93-047 UACC647 Franklin Square G-361 | BAPTA-AM chelation of intracellular calcium | Inhibition of cell motility by Wnt5A signaling | [36] |
| Melanoma | M2 A7 | Granzyme B | Inhibition of caspase-independent cell death | [38] |
| Leukemia and lymphoma | U937 Jurkat | Caspase 3 inhibitor Ac-DEVD-cho | - | [39] |
4. Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Stossel, T.P.; Condeelis, J.; Cooley, L.; Hartwig, J.H.; Noegel, A.; Schleicher, M.; Shapiro, S.S. Filamins as integrators of cell mechanics and signalling. Nat. Rev. Mol. Cell Biol. 2001, 2, 138–145. [Google Scholar] [CrossRef]
- Nakamura, F.; Osborn, T.M.; Hartemink, C.A.; Hartwig, J.H.; Stossel, T.P. Structural basis of filamin A functions. J. Cell Biol. 2007, 179, 1011–1025. [Google Scholar] [CrossRef] [PubMed]
- Zhou, A.X.; Hartwig, J.H.; Akyürek, L.M. Filamins in cell signaling, transcription and organ development. Trends Cell Biol. 2010, 20, 113–123. [Google Scholar] [CrossRef]
- Bandaru, S.; Ala, C.; Zhou, A.X.; Akyürek, L.M. Filamin A regulates cardiovascular remodeling. Int. J. Mol. Sci. 2021, 22, 6555. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, C.C.; Gorlin, J.B.; Kwiatkowski, D.J.; Hartwig, J.H.; Janmey, P.A.; Byers, H.R.; Stossel, T.P. Actin-binding protein requirement for cortical stability and efficient locomotion. Science 1992, 255, 325–327. [Google Scholar] [CrossRef]
- Ohta, Y.; Suzuki, N.; Nakamura, S.; Hartwig, J.H.; Stossel, T.P. The small GTPase RalA targets filamin to induce filopodia. Proc. Natl. Acad. Sci. USA 1999, 96, 2122–2128. [Google Scholar] [CrossRef]
- Hart, A.W.; Morgan, J.E.; Schneider, J.; West, K.; McKie, L.; Bhattacharya, S.; Jackson, I.J.; Cross, S.H. Cardiac malformations and midline skeletal defects in mice lacking filamin A. Hum. Mol. Genet. 2006, 15, 2457–2467. [Google Scholar] [CrossRef] [PubMed]
- Robertson, S.P.; Twigg, S.R.; Sutherland-Smith, A.J.; Biancalana, V.; Gorlin, R.J.; Horn, D.; Kenwrick, S.J.; Kim, C.A.; Morava, E.; Newbury-Ecob, R.; et al. Localized mutations in the gene encoding the cytoskeletal protein filamin A cause diverse malformations in humans. Nat. Genet. 2003, 33, 487–491. [Google Scholar] [CrossRef]
- Percipalle, P.; Vartiainen, M. Cytoskeletal proteins in the cell nucleus: A special nuclear actin perspective. Mol. Biol. Cell 2019, 30, 1781–1785. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Lopez-Camacho, C.; Tang, J.Y.; Mendoza-Villanueva, D.; Maya-Mendoza, A.; Jackson, D.A.; Shore, P. Cytoskeletal protein filamin A is a nucleolar protein that suppresses ribosomal RNA gene transcription. Proc. Natl. Acad. Sci. USA 2012, 109, 1524–1529. [Google Scholar] [CrossRef]
- Izdebska, M.; Zielinska, W.; Halas-Wisniewska, M.; Grzanka, A. Involvement of actin and actin-binding proteins in carcinogenesis. Cells 2020, 9, 2245. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Zhou, A.X.; Rouhi, P.; Uramoto, H.; Borén, J.; Cao, Y.; Pereira, T.; Akyürek, L.M.; Poellinger, L. Hypoxia-induced and calpain-dependent cleavage of filamin A regulates the hypoxic response. Proc. Natl. Acad. Sci. USA 2014, 111, 2560–2565. [Google Scholar] [CrossRef]
- Bandaru, S.; Prajapati, B.; Juvvuna, P.K.; Dosa, S.; Kogner, P.; Johnsen, J.I.; Kanduri, C.; Akyürek, L.M. Filamin A increases aggressiveness of human neuroblastoma. Neurooncol. Adv. 2022, 4, vdac028. [Google Scholar] [CrossRef] [PubMed]
- Zhou, A.X.; Toylu, A.; Nallapalli, R.K.; Nilsson, G.; Atabey, N.; Heldin, C.H.; Borén, J.; Bergo, M.O.; Akyürek, L.M. Filamin A mediates HGF/c-MET signaling in tumor cell migration. Int. J. Cancer 2011, 128, 839–846. [Google Scholar] [CrossRef] [PubMed]
- Di Donato, M.; Zamagni, A.; Galasso, G.; Di Zazzo, E.; Giovannelli, P.; Barone, M.V.; Zanoni, M.; Gunelli, R.; Costantini, M.; Auricchio, F.; et al. The androgen receptor/filamin A complex as a target in prostate cancer microenvironment. Cell Death Dis. 2021, 12, 127. [Google Scholar] [CrossRef]
- Velkova, A.; Carvalho, M.A.; Johnson, J.O.; Tavtigian, S.V.; Monteiro, A.N. Identification of filamin A as a BRCA1-interacting protein required for efficient DNA repair. Cell Cycle 2010, 9, 1421–1433. [Google Scholar] [CrossRef]
- Yuan, Y.; Shen, Z. Interaction with BRCA2 suggests a role for filamin-1 (hsFLNa) in DNA damage response. J. Biol. Chem. 2001, 276, 48318–48324. [Google Scholar] [CrossRef]
- Meng, X.; Yuan, Y.; Maestas, A.; Shen, Z. Recovery from DNA damage-induced G2 arrest requires actin-binding protein filamin-A/actin-binding protein 280. J. Biol. Chem. 2004, 279, 6098–6105. [Google Scholar] [CrossRef]
- Yue, J.; Lu, H.; Liu, J.; Berwick, M.; Shen, Z. Filamin-A as a marker and target for DNA damage based cancer therapy. DNA Repair 2012, 11, 192–200. [Google Scholar] [CrossRef]
- Najib, S.; Saint-Laurent, N.; Esteve, J.P.; Schulz, S.; Boutet-Robinet, E.; Fourmy, D.; Lattig, J.; Mollereau, C.; Pyronnet, S.; Susini, C.; et al. A switch of G protein-coupled receptor binding preference from phosphoinositide 3-kinase (PI3K)-p85 to filamin A negatively controls the PI3K pathway. Mol. Cell Biol. 2012, 32, 1004–1016. [Google Scholar] [CrossRef]
- Momeni, H.R. Role of calpain in apoptosis. Cell J. 2011, 13, 65–72. [Google Scholar] [PubMed]
- Perez-Siles, G.; Ellis, M.; Ashe, A.; Grosz, B.; Vucic, S.; Kiernan, M.C.; Morris, K.A.; Reddel, S.W.; Kennerson, M.L. A Compound Heterozygous Mutation in calpain 1 identifies a new genetic cause for spinal muscular atrophy type 4 (SMA4). Front. Genet. 2021, 12, 801253. [Google Scholar] [CrossRef]
- Tang, S.; Yin, Q.; Liu, F.; Zhang, Y. Calpain small subunit 1 protein in the prognosis of cancer survivors and its clinicopathological correlation. Biomed. Res. Int. 2019, 2019, 8053706. [Google Scholar] [CrossRef]
- Zheng, P.C.; Chen, X.; Zhu, H.W.; Zheng, W.; Mao, L.H.; Lin, C.; Liu, J.N.; Zheng, M. Capn4 is a marker of poor clinical outcomes and promotes nasopharyngeal carcinoma metastasis via nuclear factor-kappaB-induced matrix metalloproteinase 2 expression. Cancer Sci. 2014, 105, 630–638. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Yuan, G.; Jiang, Y.; Xu, J.; Ye, L.; Zhan, W.; Wang, J. Capn4 contributes to tumor invasion and metastasis in gastric cancer via activation of the Wnt/beta-catenin/MMP9 signalling pathways. Exp. Cell Res. 2020, 395, 112220. [Google Scholar] [CrossRef] [PubMed]
- Salimi, R.; Bandaru, S.; Devarakonda, S.; Gökalp, S.; Ala, C.; Alvandian, A.; Yener, N.; Akyürek, L.M. Blocking the cleavage of filamin A by calpain inhibitor decreases tumor cell growth. Anticancer. Res. 2018, 38, 2079–2085. [Google Scholar] [CrossRef]
- Cai, L.; Li, Q.; Li, W.; Wang, C.; Tu, M.; Zhu, Z.; Su, Z.; Lu, X. Calpain suppresses cell growth and invasion of glioblastoma multiforme by producing the cleavage of filamin A. Int. J. Clin. Oncol. 2020, 25, 1055–1066. [Google Scholar] [CrossRef]
- Shapovalov, I.; Harper, D.; Greer, P.A. Calpain as a therapeutic target in cancer. Expert. Opin. Ther. Targets 2022, 26, 217–231. [Google Scholar] [CrossRef]
- Vitali, E.; Franceschini, B.; Milana, F.; Soldani, C.; Polidoro, M.A.; Carriero, R.; Kunderfranco, P.; Trivellin, G.; Costa, G.; Milardi, G.; et al. Filamin A is involved in human intrahepatic cholangiocarcinoma aggressiveness and progression. Liver Int. 2024, 44, 518–531. [Google Scholar] [CrossRef]
- Lyu, L.; Lin, T.C.; McCarty, N. TRIM44 mediated p62 deubiquitination enhances DNA damage repair by increasing nuclear FLNA and 53BP1 expression. Oncogene 2021, 40, 5116–5130. [Google Scholar] [CrossRef]
- Zhang, S.; Cao, M.; Yan, S.; Liu, Y.; Fan, W.; Cui, Y.; Tian, F.; Gu, R.; Cui, Y.; Zhan, Y.; et al. TRIM44 promotes BRCA1 functions in HR repair to induce cisplatin chemoresistance in lung adenocarcinoma by deubiquitinating FLNA. Int. J. Biol. Sci. 2022, 18, 2962–2979. [Google Scholar] [CrossRef]
- Razinia, Z.; Baldassarre, M.; Cantelli, G.; Calderwood, D.A. ASB2α, an E3 ubiquitin ligase specificity subunit, regulates cell spreading and triggers proteasomal degradation of filamins by targeting the filamin calponin homology 1 domain. J. Biol. Chem. 2013, 288, 32093–32105. [Google Scholar] [CrossRef]
- Kircher, P.; Hermanns, C.; Nossek, M.; Drexler, M.K.; Grosse, R.; Fischer, M.; Sarikas, A.; Penkava, J.; Lewis, T.; Prywes, R.; et al. Filamin A interacts with the coactivator MKL1 to promote the activity of the transcription factor SRF and cell migration. Sci. Signal 2015, 8, ra112. [Google Scholar] [CrossRef]
- Shao, Q.Q.; Zhang, T.P.; Zhao, W.J.; Liu, Z.W.; You, L.; Zhou, L.; Guo, J.C.; Zhao, Y.P. Filamin A: Insights into its exact role in cancers. Pathol. Oncol. Res. 2016, 22, 245–252. [Google Scholar] [CrossRef]
- Camilli, T.C.; Xu, M.; O’Connell, M.P.; Chien, B.; Frank, B.P.; Subaran, S.; Indig, F.E.; Morin, P.J.; Hewitt, S.M.; Weeraratna, A.T. Loss of Klotho during melanoma progression leads to increased filamin cleavage, increased Wnt5A expression, and enhanced melanoma cell motility. Pigment. Cell Melanoma Res. 2011, 24, 175–186. [Google Scholar] [CrossRef]
- O’Connell, M.P.; Fiori, J.L.; Baugher, K.M.; Indig, F.E.; French, A.D.; Camilli, T.C.; Frank, B.P.; Earley, R.; Hoek, K.S.; Hasskamp, J.H.; et al. Wnt5A activates the calpain-mediated cleavage of filamin A. J. Investig. Dermatol. 2009, 129, 1782–1789. [Google Scholar] [CrossRef]
- Huang, C.; Miller, R.T.; Freter, C.E. Signaling regulation and role of filamin A cleavage in Ca2+-stimulated migration of androgen receptor-deficient prostate cancer cells. Oncotarget 2017, 8, 3840–3853. [Google Scholar] [CrossRef] [PubMed]
- Browne, K.A.; Johnstone, R.W.; Jans, D.A.; Trapani, J.A. Filamin (280-kDa actin-binding protein) is a caspase substrate and is also cleaved directly by the cytotoxic T lymphocyte protease granzyme B during apoptosis. J. Biol. Chem. 2000, 275, 39262–39266. [Google Scholar] [CrossRef] [PubMed]
- Umeda, T.; Kouchi, Z.; Kawahara, H.; Tomioka, S.; Sasagawa, N.; Maeda, T.; Sorimachi, H.; Ishiura, S.; Suzuki, K. Limited proteolysis of filamin is catalyzed by caspase-3 in U937 and Jurkat cells. J. Biochem. 2001, 130, 535–542. [Google Scholar] [CrossRef]
- Xu, Y.; Bismar, T.A.; Su, J.; Xu, B.; Kristiansen, G.; Varga, Z.; Teng, L.; Ingber, D.E.; Mammoto, A.; Kumar, R.; et al. Filamin A regulates focal adhesion disassembly and suppresses breast cancer cell migration and invasion. J. Exp. Med. 2010, 207, 2421–2437. [Google Scholar] [CrossRef]
- McGrath, M.J.; Binge, L.C.; Sriratana, A.; Wang, H.; Robinson, P.A.; Pook, D.; Fedele, C.G.; Brown, S.; Dyson, J.M.; Cottle, D.L.; et al. Regulation of the transcriptional coactivator FHL2 licenses activation of the androgen receptor in castrate-resistant prostate cancer. Cancer Res. 2013, 73, 5066–5079. [Google Scholar] [CrossRef]
- Donkor, I.O. An update on the therapeutic potential of calpain inhibitors: A patent review. Expert. Opin. Ther. Pat. 2020, 30, 659–675. [Google Scholar] [CrossRef] [PubMed]
- Havasi, A.; Lu, W.; Cohen, H.T.; Beck, L.; Wang, Z.; Igwebuike, C.; Borkan, S.C. Blocking peptides and molecular mimicry as treatment for kidney disease. Am. J. Physiol. Renal Physiol. 2017, 312, F1016–F1025. [Google Scholar] [CrossRef]
- Johnson, K.; Zhu, S.; Tremblay, M.S.; Payette, J.N.; Wang, J.; Bouchez, L.C.; Meeusen, S.; Althage, A.; Cho, C.Y.; Wu, X.; et al. A stem cell-based approach to cartilage repair. Science 2012, 336, 717–721. [Google Scholar] [CrossRef]
- Jin, B.K.; Odongo, S.; Radwanska, M.; Magez, S. Nanobodies: A review of generation, diagnostics and therapeutics. Int. J. Mol. Sci. 2023, 24, 5994. [Google Scholar] [CrossRef]
- Mullard, A. FDA approves second BCMA-targeted CAR-T cell therapy. Nat. Rev. Drug Discov. 2022, 21, 249. [Google Scholar] [CrossRef] [PubMed]
- Silva-Pilipich, N.; Martisova, E.; Ballesteros-Briones, M.C.; Hervas-Stubbs, S.; Casares, N.; Gonzalez-Sapienza, G.; Smerdou, C.; Vanrell, L. Long-term systemic expression of a novel PD-1 blocking nanobody from an AAV vector provides antitumor activity without toxicity. Biomedicines 2020, 8, 562. [Google Scholar] [CrossRef]

| Interaction Partner | Cellular Outcome | Cancer Types | Cell Lines | Reference |
|---|---|---|---|---|
| HIF-1α | Nuclear localization, promotes tumor growth and angiogenesis | Melanoma | M2 A7 | [12] |
| STAT3 | Increases MYCN expression and aggressiveness of tumors | Neuroblastoma | SKNBE2 SHSY5 Kelly IMR-32 | [13] |
| SMAD2 | Increases C-MET expression and tumor cell migration | Liver Pancreas Prostate Lung | HepG2 H69 BON PC-3 M2 A7 | [14] |
| Androgen receptor | Nuclear localization, promotes tumor growth | Prostate | LNCaP PC3 DU145 MCF-7 T47D | [15] |
| BRCA1 | Stabilizes DNA repair, acts as checkpoint in tumor progression | Breast | HCT116 293FT M2 A7 | [16] |
| BRCA2 | DNA damage response pathways in tumors | Melanoma Breast Pancreas | M2 A7 MCF-7 Capan-1 | [17] |
| GPCR | Down regulates PI3K pathway in tumor signaling | Melanoma Neuroblastoma Pancreas | BON CHO M2 A7 HaCaT SH-SY5Y BxPC3 | [20] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cakici, O.; Bandaru, S.; Lee, G.Y.; Mustafa, D.; Akyürek, L.M. Targeting Cleavage of C-Terminal Fragment of Cytoskeletal Filamin A in Cancers. Cells 2024, 13, 1394. https://doi.org/10.3390/cells13161394
Cakici O, Bandaru S, Lee GY, Mustafa D, Akyürek LM. Targeting Cleavage of C-Terminal Fragment of Cytoskeletal Filamin A in Cancers. Cells. 2024; 13(16):1394. https://doi.org/10.3390/cells13161394
Chicago/Turabian StyleCakici, Ozgur, Sashidar Bandaru, Grace Yankun Lee, Dyar Mustafa, and Levent M. Akyürek. 2024. "Targeting Cleavage of C-Terminal Fragment of Cytoskeletal Filamin A in Cancers" Cells 13, no. 16: 1394. https://doi.org/10.3390/cells13161394






