Correlation between Periostin Expression and Pro-Angiogenic Factors in Non-Small-Cell Lung Carcinoma
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Group
2.2. Formation of Tissue Microarrays (TMAs)
2.3. Immunohistochemistry
2.4. Assessment of IHC Reactions
2.5. Statistical Analyses
3. Results
3.1. Expression of POSTN and Pro-Angiogenic Factors (CD31, CD34, CD 105, VEGF-A) in NSCLC Cells and Non-Malignant Lung Tissue (NMLT)
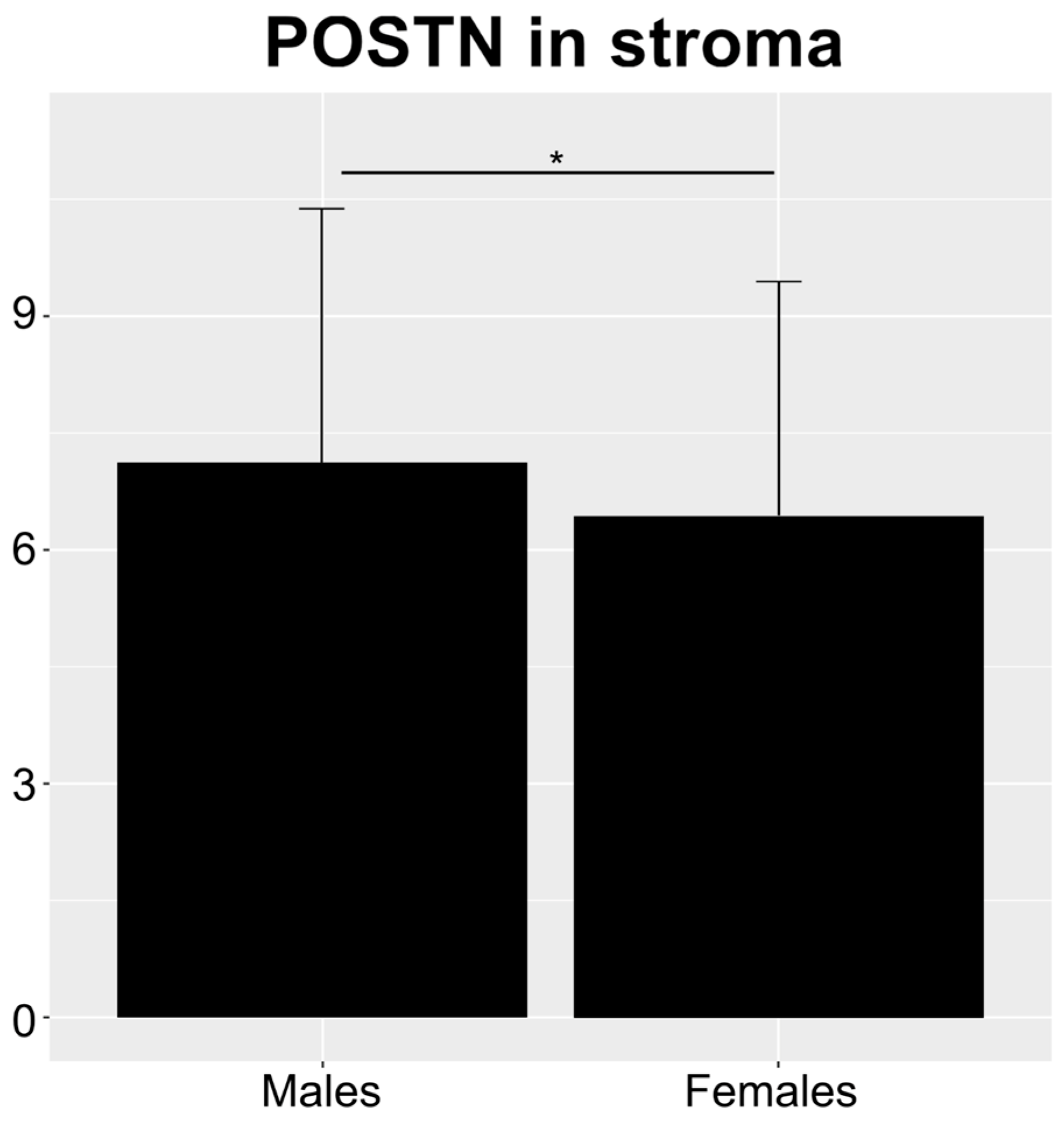
3.2. Relationship between Immunohistochemical (IHC) Expression of POSTN in NSCLC and Clinicopathological Data of Patients
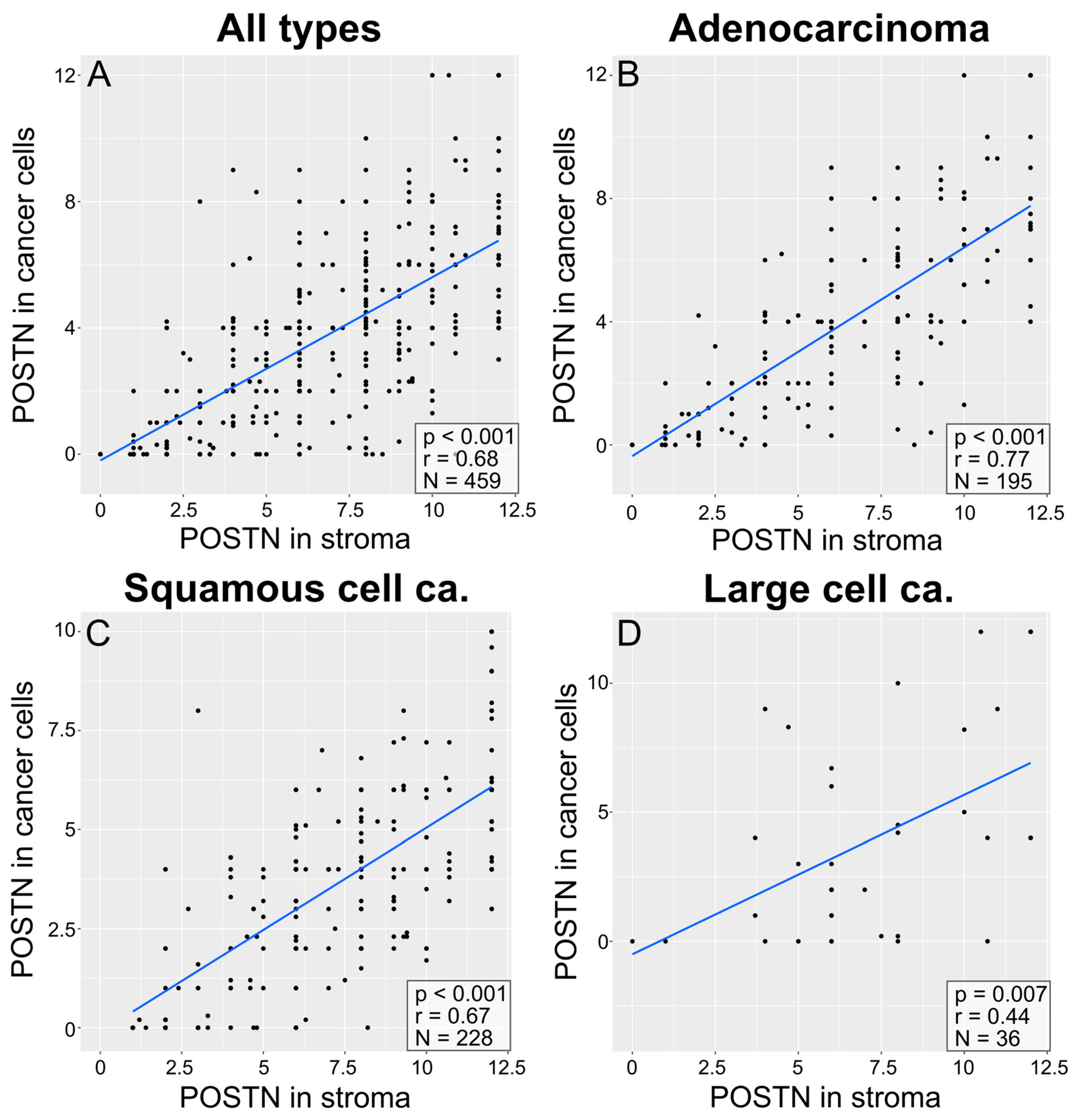
3.3. Correlations between POSTN Expression in Cancer Cells and POSTN Expression in NSCLC Stroma
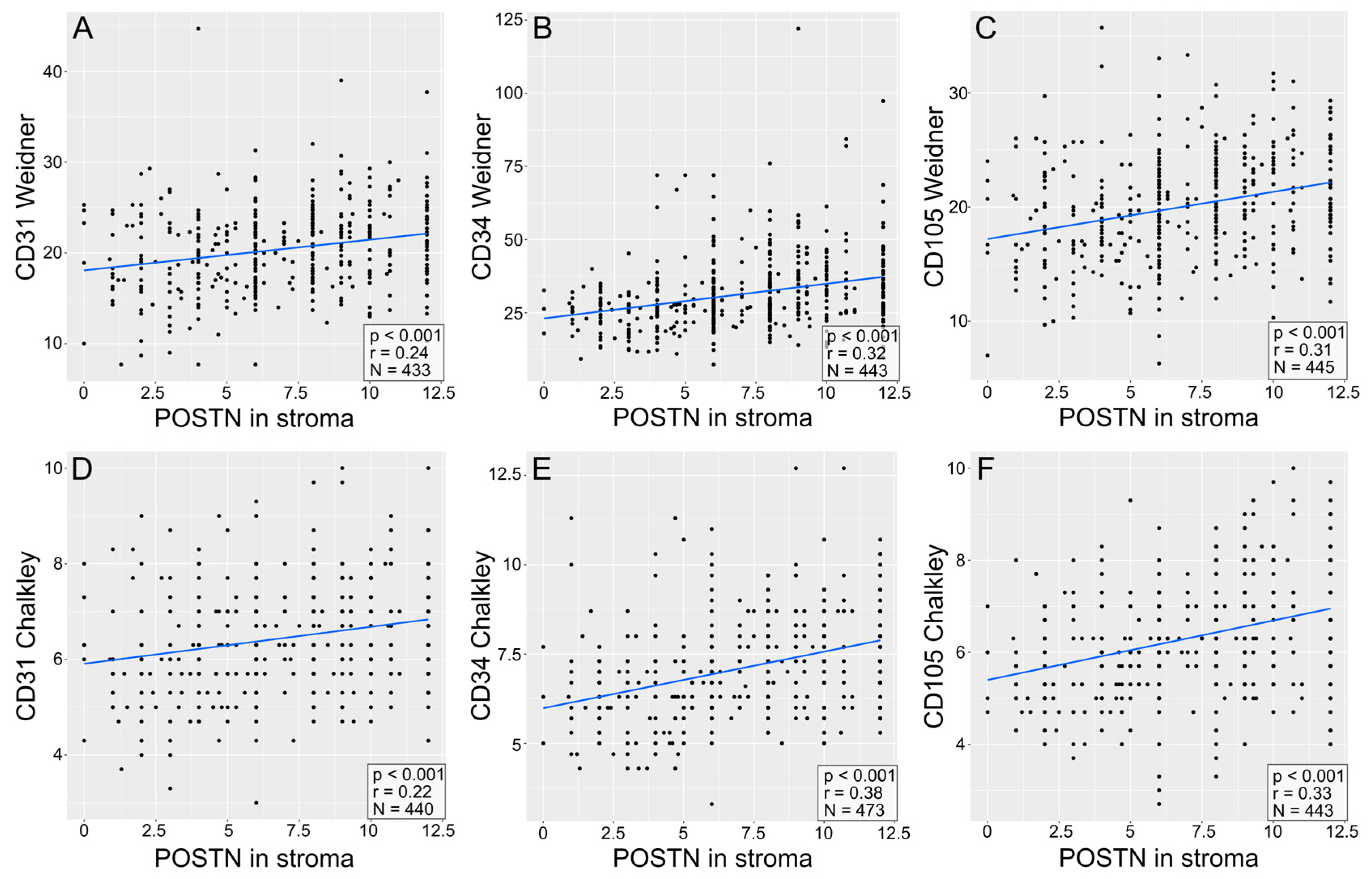
3.4. Correlations of the Expression of Pro-Angiogenic Factors (CD31, CD34, CD105) in NSCLC as Examined with the Methods of Weidner and Chalkley
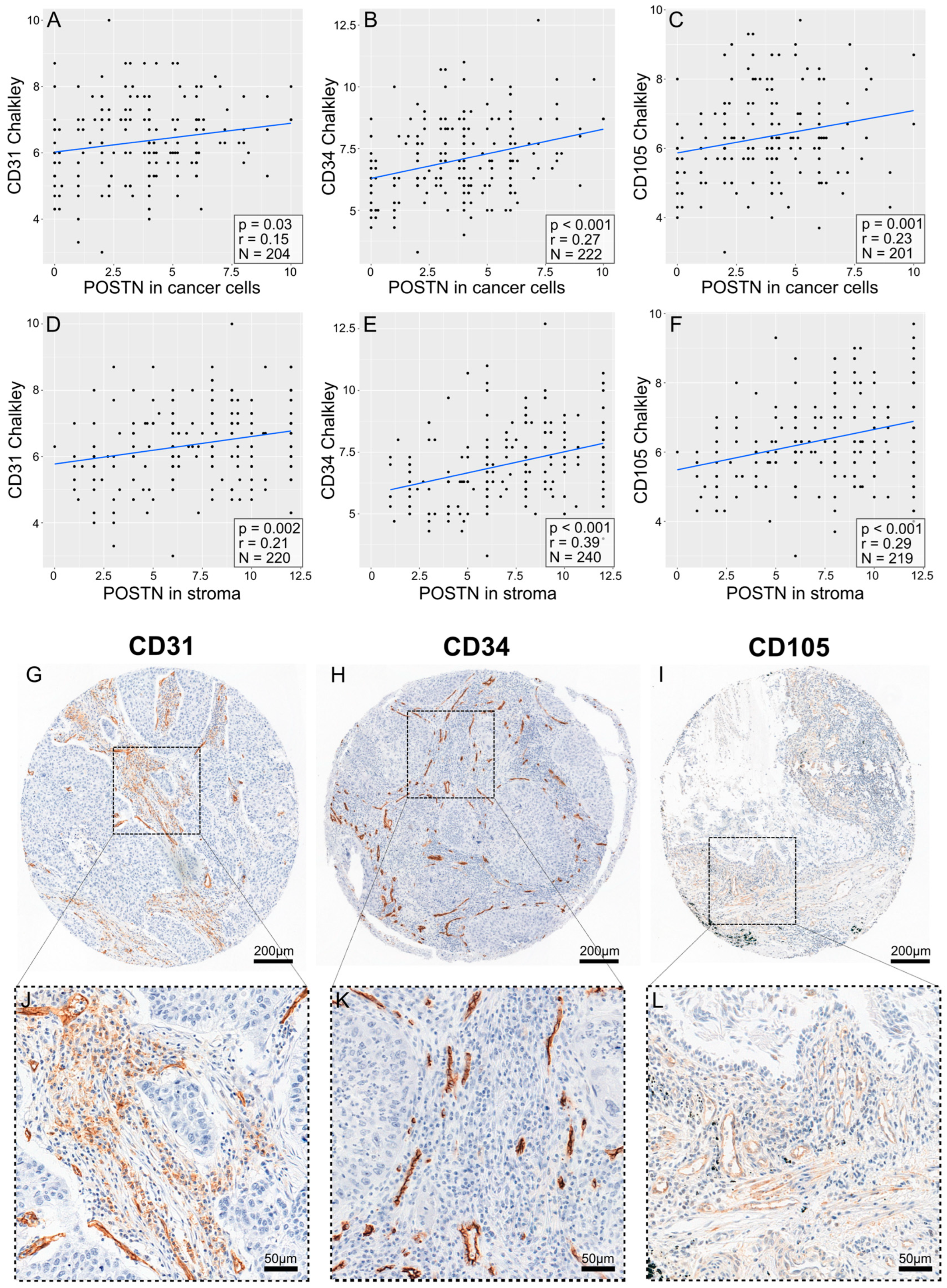
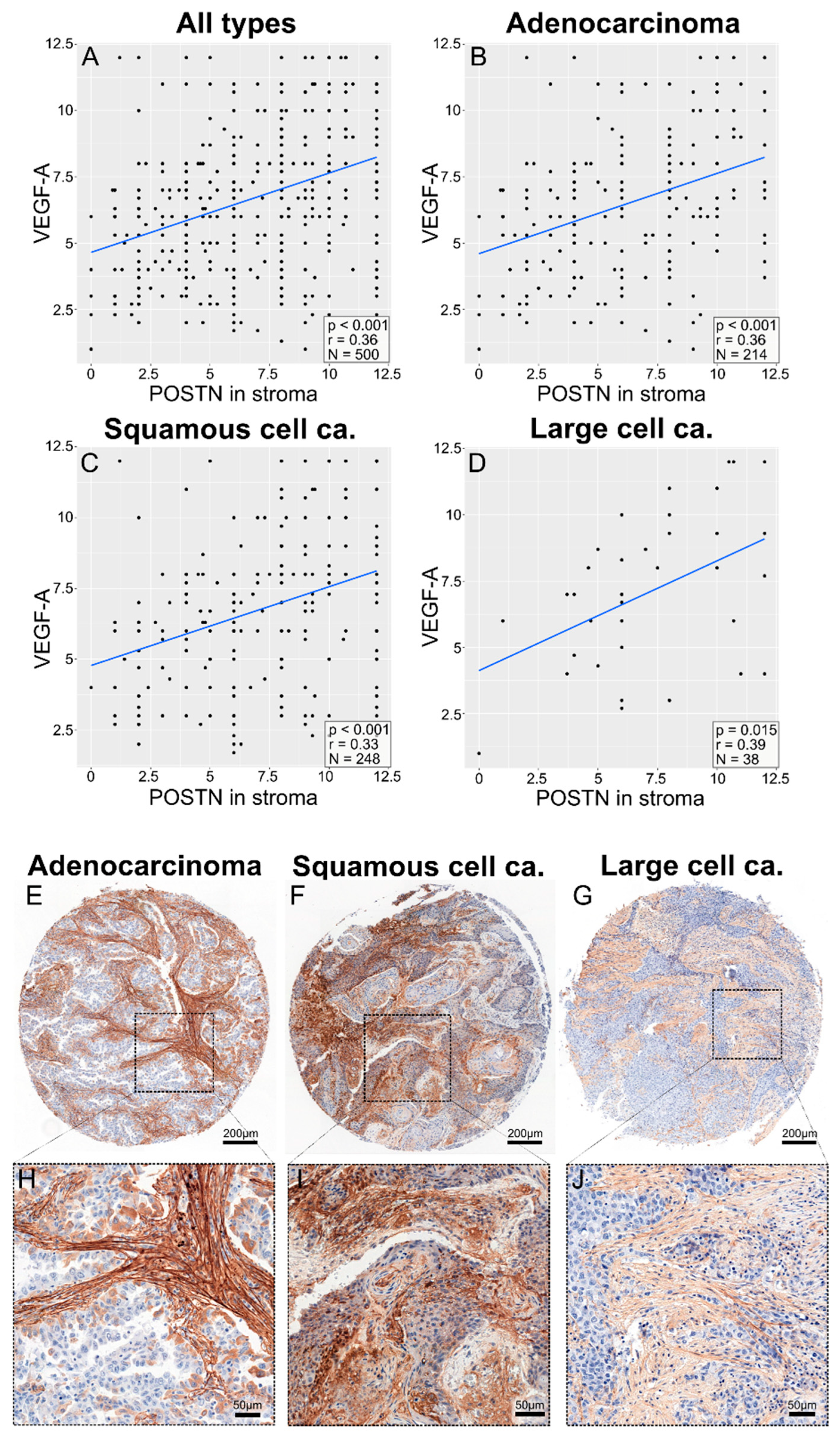
3.5. Correlations between POSTN Expression and Pro-Angiogenic Factor Expression in NSCLC
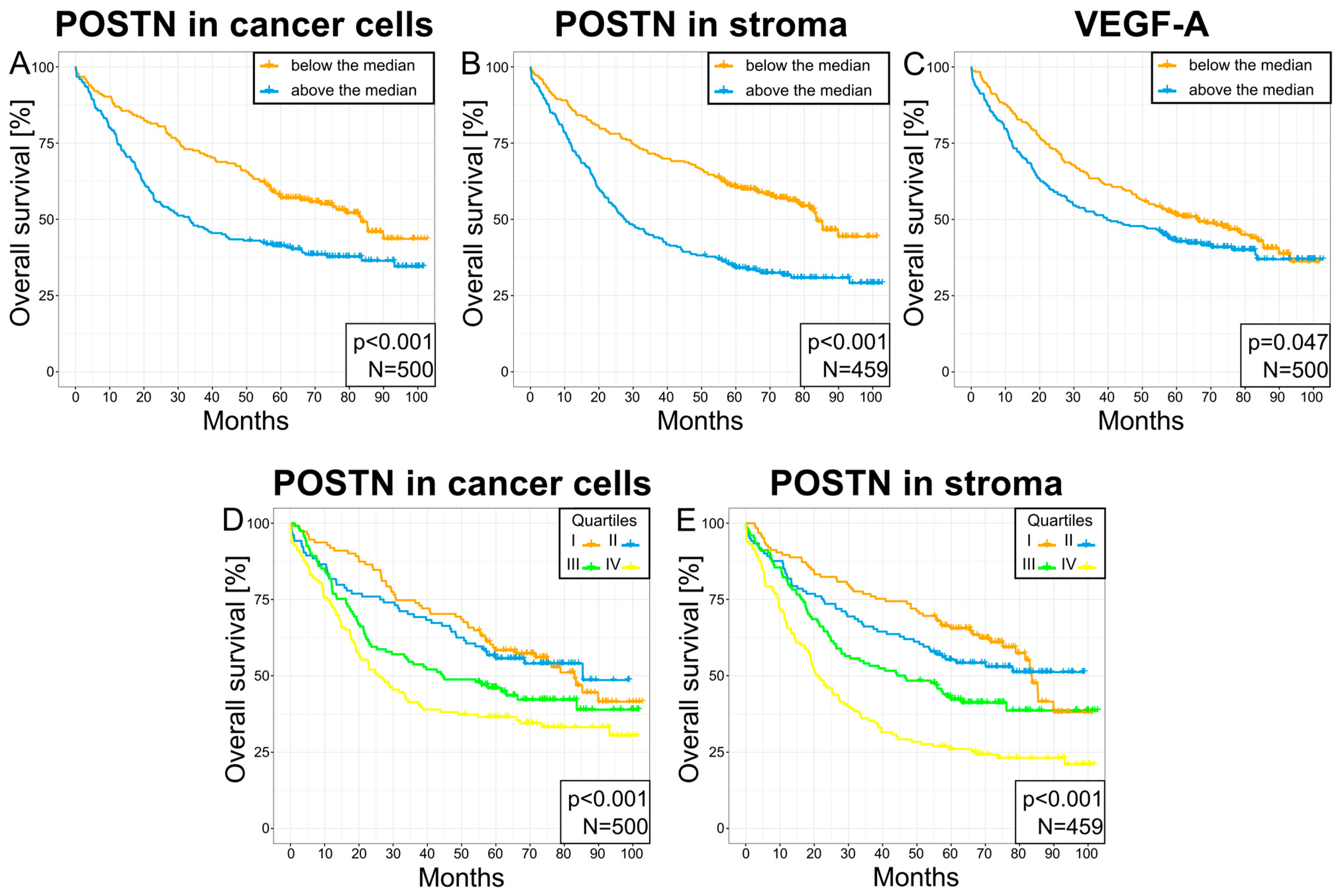
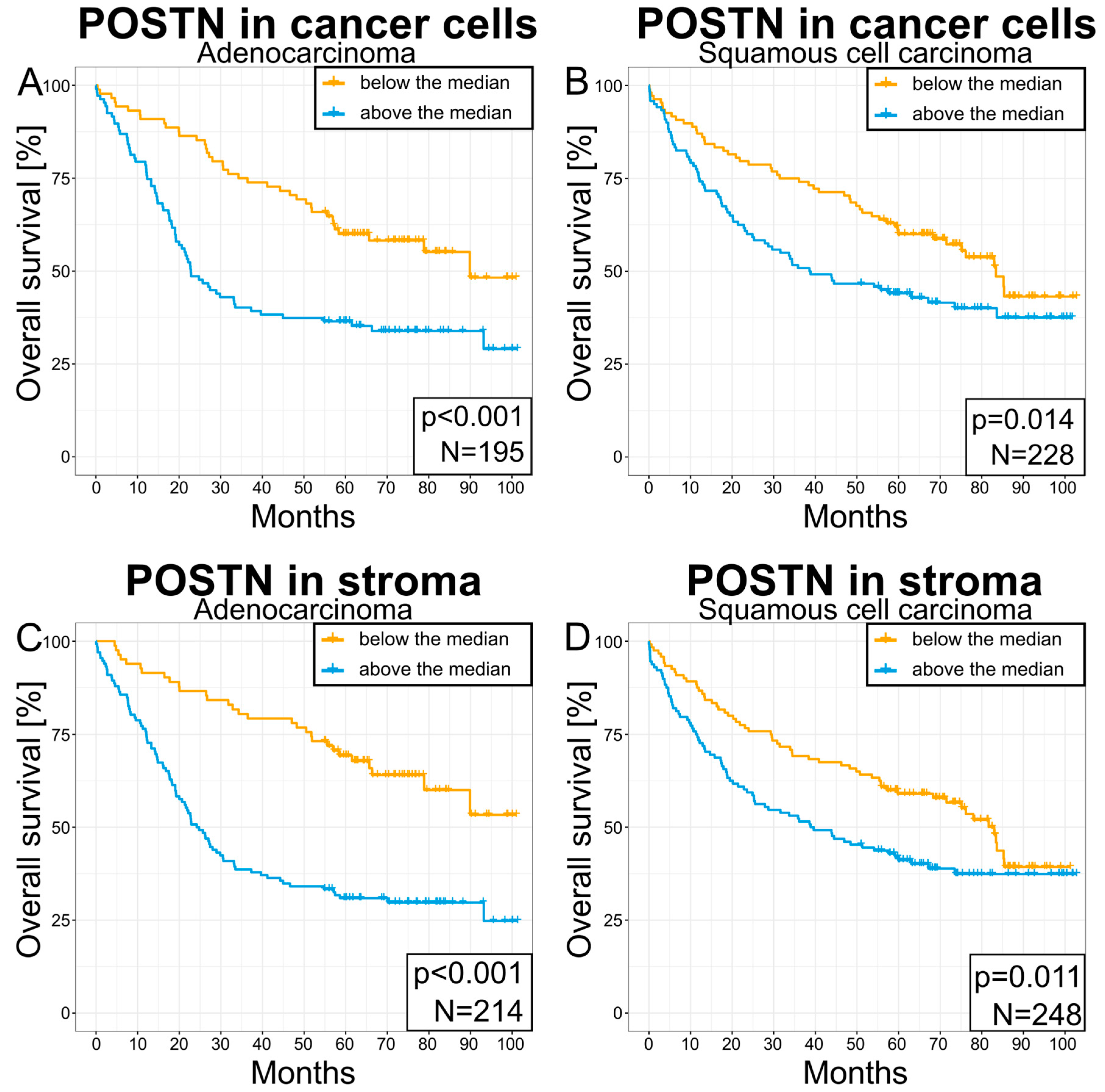
3.6. Survival Analysis
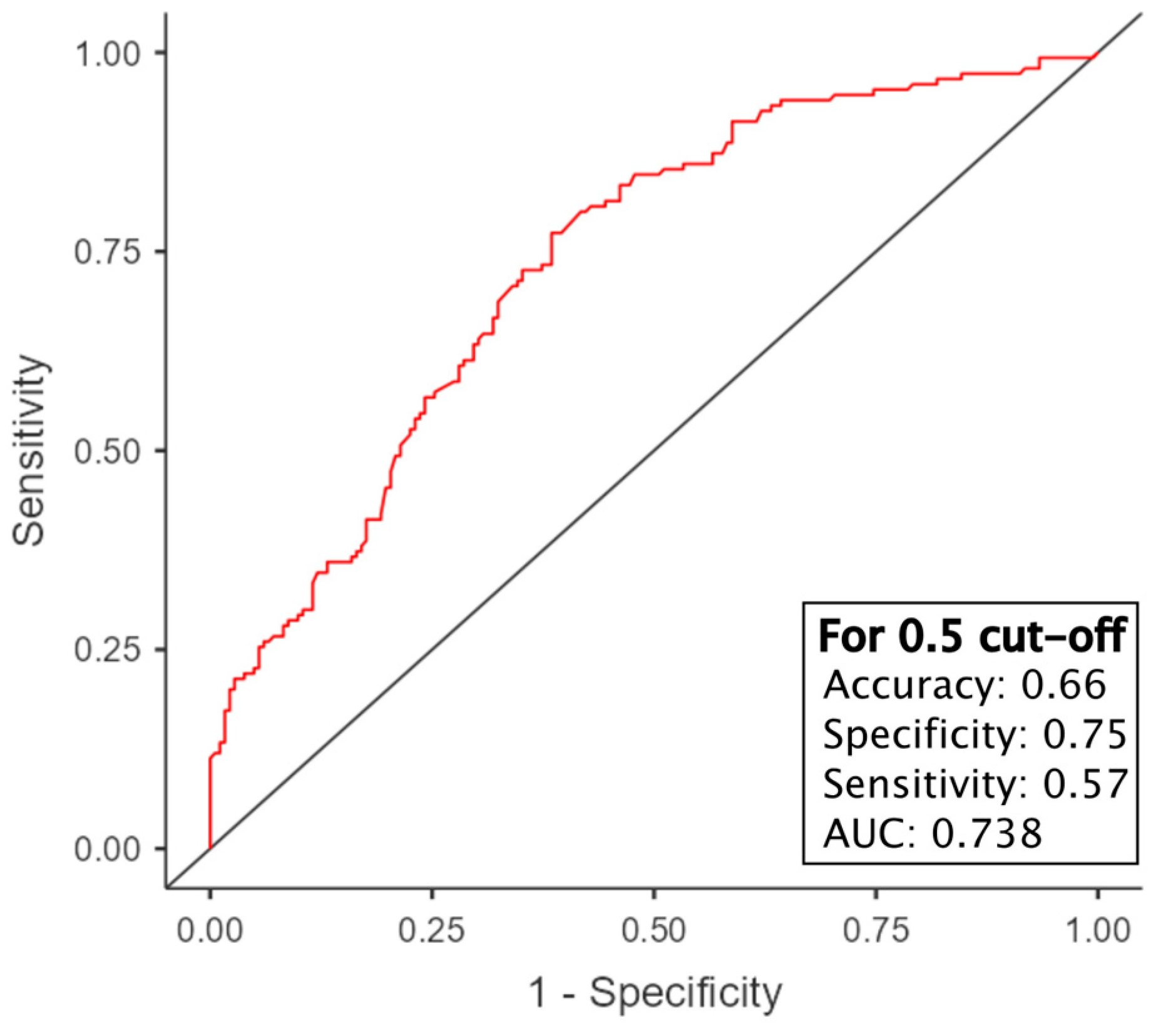
3.7. Survival Prediction Models
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wasik, A.; Ratajczak-Wielgomas, K.; Badzinski, A.; Dziegiel, P.; Podhorska-Okolow, M. The Role of Periostin in Angiogenesis and Lymphangiogenesis in Tumors. Cancers 2022, 14, 4225. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Khajuria, O.; Sharma, N. Epigenetic targeting for lung cancer treatment via CRISPR/Cas9 technology. Adv. Cancer Biol.-Metastasis 2021, 3, 100012. [Google Scholar] [CrossRef]
- American Cancer Society. What Is Non-Small Cell Lung Cancer? [Internet]. Available online: https://web.archive.org/web/20170117074735/http://www.cancer.org/cancer/lungcancer-non-smallcell/detailedguide/non-small-cell-lung-cancer-what-is-non-small-cell-lung-cancer (accessed on 20 August 2024).
- Molina, J.R.; Yang, P.; Cassivi, S.D.; Schild, S.E.; Adjei, A.A. Non-small cell lung cancer: Epidemiology, risk factors, treatment, and survivorship. Mayo Clin. Proc. 2008, 83, 584–594. [Google Scholar] [CrossRef] [PubMed]
- Bajbouj, K.; Al-ali, A.; Ramakrishnan, R.K.; Saber-ayad, M.; Hamid, Q. Histone modification in nsclc: Molecular mechanisms and therapeutic targets. Int. J. Mol. Sci. 2021, 22, 1701. [Google Scholar] [CrossRef]
- American Cancer Society. Lung Cancer Early Detection, Diagnosis, and Staging [Internet]. Available online: https://www.cancer.org/cancer/types/lung-cancer/detection-diagnosis-staging.html (accessed on 20 August 2024).
- Manzo, A.; Montanino, A.; Carillio, G.; Costanzo, R.; Sandomenico, C.; Normanno, N.; Piccirillo, M.C.; Daniele, G.; Perrone, F.; Rocco, G.; et al. Angiogenesis Inhibitors in NSCLC. Int. J. Mol. Sci. 2017, 18, 2021. [Google Scholar] [CrossRef]
- Sacewicz, I.; Wiktorska, M.; Wysocki, T. Mechanisms of cancer angiogenesis Mechanizmy angiogenezy nowotworowej * Mechanisms of cancer angiogenesis. Postep. Hig. Med. Dosw. 2009, 63, 159–168. [Google Scholar]
- Birbrair, A.; Zhang, T.; Wang, Z.M.; Messi, M.L.; Olson, J.D.; Mintz, A.; Delbono, O. Type-2 pericytes participate in normal and tumoral angiogenesis. Am. J. Physiol.-Cell Physiol. 2014, 307, C25–C38. [Google Scholar] [CrossRef]
- Birbrair, A.; Zhang, T.; Wang, Z.-M.; Messi, M.L.; Mintz, A.; Delbono, O. Pericytes at the intersection between tissue regeneration and pathology. Clin. Sci. 2015, 128, 81–93. [Google Scholar] [CrossRef]
- Younes, A. Angiogenesis in Lymphoma: A Short Review. Curr. Mol. Med. 2005, 5, 609–613. [Google Scholar] [CrossRef]
- Carmeliet, P.; Jain, R.K. Molecular mechanisms and clinical applications of angiogenesis. Nature 2011, 473, 298–307. [Google Scholar] [CrossRef]
- Folkman, J.; Shing, Y. Angiogenesis. J. Biol. Chem. 1992, 267, 10931–10934. [Google Scholar] [CrossRef] [PubMed]
- Potente, M.; Carmeliet, P. The Link between Angiogenesis and Endothelial Metabolism. Annu. Rev. Physiol. 2017, 79, 43–66. [Google Scholar] [CrossRef]
- Dusseau, J.W.; Hutchins, P.M. Hypoxia-induced angiogenesis in chick chorioallantoic membranes: A role for adenosine. Respir. Physiol. 1988, 71, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Hirota, K.; Semenza, G.L. Regulation of angiogenesis by hypoxia-inducible factor 1. Crit. Rev. Oncol. Hematol. 2006, 59, 15–26. [Google Scholar] [CrossRef]
- Hlatky, L.; Tsionou, C.; Hahnfeldt, P.; Coleman, C.N. Mammary fibroblasts may influence breast tumor angiogenesis via hypoxia-induced vascular endothelial growth factor up-regulation and protein expression. Cancer Res. 1994, 54, 6083–6086. [Google Scholar]
- Prior, B.M.; Yang, H.T.; Terjung, R.L. What makes vessels grow with exercise training? J. Appl. Physiol. 2004, 97, 1119–1128. [Google Scholar] [CrossRef]
- Rust, R.; Grönnert, L.; Gantner, C.; Enzler, A.; Mulders, G.; Weber, R.Z.; Siewert, A.; Limasale, Y.D.P.; Meinhardt, A.; Maurer, M.A.; et al. Nogo-A targeted therapy promotes vascular repair and functional recovery following stroke. Proc. Natl. Acad. Sci. USA 2019, 116, 14270–14279. [Google Scholar] [CrossRef] [PubMed]
- Rust, R.; Weber, R.Z.; Grönnert, L.; Mulders, G.; Maurer, M.A.; Hofer, A.S.; Sartori, A.M.; Schwab, M.E. Anti-Nogo-A antibodies prevent vascular leakage and act as pro-angiogenic factors following stroke. Sci. Rep. 2019, 9, 20040. [Google Scholar] [CrossRef]
- Pavlakovic, H.; Havers, W.; Schweigerer, L. Multiple angiogenesis stimulators in a single malignancy: Implications for anti-angiogenic tumour therapy. Angiogenesis 2001, 4, 259–262. [Google Scholar] [CrossRef]
- Kerbel, R.S. Tumor Angiogenesis. N. Engl. J. Med. 2008, 358, 2039–2049. [Google Scholar] [CrossRef] [PubMed]
- Liotta, L.A.; Steeg, P.S.; Stetler-Stevenson, W.G. Cancer metastasis and angiogenesis: An imbalance of positive and negative regulation. Cell 1991, 64, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Mariscalco, G.; Lorusso, R.; Sessa, F.; Bruno, V.D.; Piffaretti, G.; Banach, M.; Cattaneo, P.; Cozzi, G.P.; Sala, A. Imbalance between pro-angiogenic and anti-angiogenic factors in rheumatic and mixomatous mitral valves. Int. J. Cardiol. 2011, 152, 337–344. [Google Scholar] [CrossRef]
- Ramanujan, S.; Koenig, G.C.; Padera, T.P.; Stoll, B.R.; Jain, R.K. Local imbalance of proangiogenic and antiangiogenic factors: A potential mechanism of focal necrosis and dormancy in tumors. Cancer Res. 2000, 60, 1442–1448. [Google Scholar] [PubMed]
- Sehra, S.; Yao, W.; Nguyen, E.T.; Ahyi, A.-N.N.; Barbé Tuana, F.M.; Ahlfeld, S.K.; Snider, P.; Tepper, R.S.; Petrache, I.; Conway, S.J.; et al. Periostin Regulates Goblet Cell Metaplasia in a Model of Allergic Airway Inflammation. J. Immunol. 2011, 186, 4959–4966. [Google Scholar] [CrossRef]
- Kudo, Y.; Siriwardena, B.S.M.S.; Hatano, H.; Ogawa, I.; Takata, T. Periostin: Novel diagnostic and therapeutic target for cancer. Histol. Histopathol. 2007, 22, 1167–1174. [Google Scholar] [CrossRef]
- Takeshita, S.; Kikuno, R.; Tezuka, K.; Amann, E. Osteoblast-specific factor 2: Cloning of a putative bone adhesion protein with homology with the insect protein fasciclin I. Biochem. J. 1993, 294 Pt 1, 271–278. [Google Scholar] [CrossRef]
- Bao, S.; Ouyang, G.; Bai, X.; Huang, Z.; Ma, C.; Liu, M.; Shao, R.; Anderson, R.M.; Rich, J.N.; Wang, X.F. Periostin potently promotes metastatic growth of colon cancer by augmenting cell survival via the Akt/PKB pathway. Cancer Cell 2004, 5, 329–339. [Google Scholar] [CrossRef]
- Ratajczak-Wielgomas, K.; Dziegiel, P. The role of periostin in neoplastic processes. Folia Histochem. Cytobiol. 2015, 53, 120–132. [Google Scholar] [CrossRef]
- Morra, L.; Rechsteiner, M.; Casagrande, S.; von Teichman, A.; Schraml, P.; Moch, H.; Soltermann, A. Characterization of periostin isoform pattern in non-small cell lung cancer. Lung Cancer 2012, 76, 183–190. [Google Scholar] [CrossRef]
- Kikuchi, Y.; Kunita, A.; Iwata, C.; Komura, D.; Nishiyama, T.; Shimazu, K.; Takeshita, K.; Shibahara, J.; Kii, I.; Morishita, Y.; et al. The niche component periostin is produced by cancer-associated fibroblasts, supporting growth of gastric cancer through ERK activation. Am. J. Pathol. 2014, 184, 859–870. [Google Scholar] [CrossRef]
- Molkentin, S.J.C. and J.D. Periostin as a Heterofunctional Regulator of Cardiac Development and Disease. Curr. Genom. 2008, 9, 548–555. [Google Scholar]
- Hakuno, D.; Kimura, N.; Yoshioka, M.; Mukai, M.; Kimura, T.; Okada, Y.; Yozu, R.; Shukunami, C.; Hiraki, Y.; Kudo, A.; et al. Periostin advances atherosclerotic and rheumatic cardiac valve degeneration by inducing angiogenesis and MMP production in humans and rodents. J. Clin. Investig. 2011, 121, 454. [Google Scholar] [CrossRef]
- Kühn, B.; del Monte, F.; Hajjar, R.J.; Chang, Y.-S.; Lebeche, D.; Arab, S.; Keating, M.T. Periostin induces proliferation of differentiated cardiomyocytes and promotes cardiac repair. Nat. Med. 2007, 13, 962–969. [Google Scholar] [CrossRef]
- Shimazaki, M.; Nakamura, K.; Kii, I.; Kashima, T.; Amizuka, N.; Li, M.; Saito, M.; Fukuda, K.; Nishiyama, T.; Kitajima, S.; et al. Periostin is essential for cardiac healing after acute myocardial infarction. J. Exp. Med. 2008, 205, 295–303. [Google Scholar] [CrossRef]
- Horiuchi, K.; Amizuka, N.; Takeshita, S.; Takamatsu, H.; Katsuura, M.; Ozawa, H.; Toyama, Y.; Bonewald, L.F.; Kudo, A. Identification and Characterization of a Novel Protein, Periostin, with Restricted Expression to Periosteum and Periodontal Ligament and Increased Expression by Transforming Growth Factor β. J. Bone Miner. Res. 1999, 14, 1239–1249. [Google Scholar] [CrossRef]
- Hamilton, D.W. Functional role of periostin in development and wound repair: Implications for connective tissue disease. J. Cell Commun. Signal. 2008, 2, 9. [Google Scholar] [CrossRef] [PubMed]
- Conway, S.J.; Izuhara, K.; Kudo, Y.; Litvin, J.; Markwald, R.; Ouyang, G.; Arron, J.R.; Holweg, C.T.J.; Kudo, A. The role of periostin in tissue remodeling across health and disease. Cell. Mol. Life Sci. 2014, 71, 1279–1288. [Google Scholar] [CrossRef]
- Dorafshan, S.; Razmi, M.; Safaei, S.; Gentilin, E.; Madjd, Z.; Ghods, R. Periostin: Biology and function in cancer. Cancer Cell Int. 2022, 22, 315. [Google Scholar] [CrossRef]
- Wang, Z.; An, J.; Zhu, D.; Chen, H.; Lin, A.; Kang, J.; Liu, W.; Kang, X. Periostin: An emerging activator of multiple signaling pathways. J. Cell Commun. Signal. 2022, 16, 515–530. [Google Scholar] [CrossRef]
- Yue, H.; Li, W.; Chen, R.; Wang, J.; Lu, X.; Li, J. Stromal POSTN induced by TGF-β1 facilitates the migration and invasion of ovarian cancer. Gynecol. Oncol. 2021, 160, 530–538. [Google Scholar] [CrossRef]
- Kudo, Y.; Iizuka, S.; Yoshida, M.; Nguyen, P.T.; Siriwardena, S.B.S.M.; Tsunematsu, T.; Ohbayashi, M.; Ando, T.; Hatakeyama, D.; Shibata, T.; et al. Periostin Directly and Indirectly Promotes Tumor Lymphangiogenesis of Head and Neck Cancer. PLoS ONE 2012, 7. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Wang, J.; Zhao, X.; Wu, T.; Huang, Z.; Chen, D.; Liu, Y.; Ouyang, G. Periostin Promotes Colorectal Tumorigenesis through Integrin-FAK-Src Pathway-Mediated YAP/TAZ Activation. Cell Rep. 2020, 30, 793–806.e6. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Oparil, S.; Feng, W.; Chen, Y.F. Hypoxia-responsive growth factors upregulate periostin and osteopontin expression via distinct signaling pathways in rat pulmonary arterial smooth muscle cells. J. Appl. Physiol. 2004, 97, 1550–1558. [Google Scholar] [CrossRef]
- Lindsley, A.; Snider, P.; Zhou, H.; Rogers, R.; Wang, J.; Olaopa, M.; Kruzynska-Frejtag, A.; Koushik, S.V.; Lilly, B.; Burch, J.B.E.; et al. Identification and characterization of a novel Schwann and outflow tract endocardial cushion lineage-restricted periostin enhancer. Dev. Biol. 2007, 307, 340–355. [Google Scholar] [CrossRef]
- Gillan, L.; Matei, D.; Fishman, D.A.; Gerbin, C.S.; Karlan, B.Y.; Chang, D.D. Periostin secreted by epithelial ovarian carcinoma is a ligand for αVβ3 and αVβ5 integrins and promotes cell motility. Cancer Res. 2002, 62, 5358–5364. [Google Scholar]
- Yan, W.; Shao, R. Transduction of a mesenchyme-specific gene periostin into 293T cells induces cell invasive activity through epithelial-mesenchymal transformation. J. Biol. Chem. 2006, 281, 19700–19709. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Sun, Q.K.; He, Y.F.; Ma, D.C.; Xie, M.R.; Ji, C.S.; Hu, B. Overexpression of periostin is significantly correlated to the tumor angiogenesis and poor prognosis in patients with esophageal squamous cell carcinoma. Int. J. Clin. Exp. Pathol. 2014, 7, 593–601. [Google Scholar]
- Nguyen, T.H. Mechanisms of metastasis. Clin. Dermatol. 2004, 22, 209–216. [Google Scholar] [CrossRef]
- Ribatti, D.; Vacca, A.; Dammacco, F. New non-angiogenesis dependent pathways for tumour growth. Eur. J. Cancer 2003, 39, 1835–1841. [Google Scholar] [CrossRef]
- Shao, R.; Bao, S.; Bai, X.; Blanchette, C.; Anderson, R.M.; Dang, T.; Gishizky, M.L.; Marks, J.R.; Wang, X.-F. Acquired Expression of Periostin by Human Breast Cancers Promotes Tumor Angiogenesis through Up-Regulation of Vascular Endothelial Growth Factor Receptor 2 Expression. Mol. Cell. Biol. 2004, 24, 3992–4003. [Google Scholar] [CrossRef]
- Erber, R.; Thurnher, A.; Katsen, A.D.; Groth, G.; Kerger, H.; Hammes, H.P.; Menger, M.D.; Ullrich, A.; Vajkoczy, P. Combined inhibition of VEGF and PDGF signaling enforces tumor vessel regression by interfering with pericyte-mediated endothelial cell survival mechanisms. FASEB J. 2004, 18, 338–340. [Google Scholar] [CrossRef]
- Takanami, I.; Abiko, T.; Koizumi, S. Expression of periostin in patients with non-small cell lung cancer: Correlation with angiogenesis and lymphangiogenesis. Int. J. Biol. Markers 2008, 23, 182–186. [Google Scholar] [CrossRef]
- Nicholson, A.G.; Tsao, M.S.; Beasley, M.B.; Borczuk, A.C.; Brambilla, E.; Cooper, W.A.; Dacic, S.; Jain, D.; Kerr, K.M.; Lantuejoul, S.; et al. The 2021 WHO Classification of Lung Tumors: Impact of Advances Since 2015. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2022, 17, 362–387. [Google Scholar] [CrossRef] [PubMed]
- Asamura, H.; Nishimura, K.K.; Giroux, D.J.; Chansky, K.; Hoering, A.; Rusch, V.; Rami-Porta, R. IASLC Lung Cancer Staging Project: The New Database to Inform Revisions in the Ninth Edition of the TNM Classification of Lung Cancer. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2023, 18, 564–575. [Google Scholar] [CrossRef] [PubMed]
- Jawhar, N.M.T. Tissue Microarray: A rapidly evolving diagnostic and research tool. Ann. Saudi Med. 2009, 29, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Vogel, U. Overview on Techniques to Construct Tissue Arrays with Special Emphasis on Tissue Microarrays. Microarrays 2014, 3, 103–136. [Google Scholar] [CrossRef] [PubMed]
- Remmele, W.; Stegner, H.E. Recommendation for uniform definition of an immunoreactive score (IRS) for immunohistochemical estrogen receptor detection (ER-ICA) in breast cancer tissue. Pathologe 1987, 8, 138–140. [Google Scholar]
- Zhang, Q.; Wu, J.; Bai, X.; Liang, T. Evaluation of Intra-Tumoral Vascularization in Hepatocellular Carcinomas. Front. Med. 2020, 7, 584250. [Google Scholar] [CrossRef]
- Vermeulen, P.B.; Gasparini, G.; Fox, S.B.; Colpaert, C.; Marson, L.P.; Gion, M.; Beliën, J.A.M.; de Waal, R.M.W.; Van Marck, E.; Magnani, E.; et al. Second international consensus on the methodology and criteria of evaluation of angiogenesis quantification in solid human tumours. Eur. J. Cancer 2002, 38, 1564–1579. [Google Scholar] [CrossRef]
- Nick, T.G.; Campbell, K.M. Logistic regression. Methods Mol. Biol. 2007, 404, 273–301. [Google Scholar] [CrossRef] [PubMed]
- Carter, J.V.; Pan, J.; Rai, S.N.; Galandiuk, S. ROC-ing along: Evaluation and interpretation of receiver operating characteristic curves. Surgery 2016, 159, 1638–1645. [Google Scholar] [CrossRef] [PubMed]
- Ratajczak-Wielgomas, K.; Kmiecik, A.; Dziegiel, P. Role of Periostin Expression in Non-Small Cell Lung Cancer: Periostin Silencing Inhibits the Migration and Invasion of Lung Cancer Cells via Regulation of MMP-2 Expression. Int. J. Mol. Sci. 2022, 23, 1240. [Google Scholar] [CrossRef]
- Ratajczak-Wielgomas, K.; Kmiecik, A.; Grzegrzołka, J.; Piotrowska, A.; Gomulkiewicz, A.; Partynska, A.; Pawelczyk, K.; Nowinska, K.; Podhorska-Okolow, M.; Dziegiel, P. Prognostic Significance of Stromal Periostin Expression in Non-Small Cell Lung Cancer. Int. J. Mol. Sci. 2020, 21, 7025. [Google Scholar] [CrossRef]
- Balcerak, A.; Wakuła, M.; Trȩbińska, A.; Grzybowska, E.A. Migracja i inwazyjność komórek nowotworowych; rola plastyczności komórek i udział macierzy zewnątrzkomórkowej w tworzeniu przerzutów. Nowotwory 2016, 66, 45–52. [Google Scholar] [CrossRef]
- Folkman, J. What is the evidence that tumors are angiogenesis dependent? J. Natl. Cancer Inst. 1990, 82, 4–7. [Google Scholar] [CrossRef] [PubMed]
- Hillen, F.; Griffioen, A.W. Tumour vascularization: Sprouting angiogenesis and beyond. Cancer Metastasis Rev. 2007, 26, 489–502. [Google Scholar] [CrossRef]
- Liu, Y.; Li, F.; Gao, F.; Xing, L.; Qin, P.; Liang, X.; Zhang, J.; Qiao, X.; Lin, L.; Zhao, Q.; et al. Periostin promotes tumor angiogenesis in pancreatic cancer via Erk/VEGF signaling. Oncotarget 2016, 7, 40148. [Google Scholar] [CrossRef]
- Morra, L.; Moch, H. Periostin expression and epithelial-mesenchymal transition in cancer: A review and an update. Virchows Arch. 2011, 459, 465–475. [Google Scholar] [CrossRef]
- Baril, P.; Gangeswaran, R.; Mahon, P.C.; Caulee, K.; Kocher, H.M.; Harada, T.; Zhu, M.; Kalthoff, H.; Crnogorac-Jurcevic, T.; Lemoine, N.R. Periostin promotes invasiveness and resistance of pancreatic cancer cells to hypoxia-induced cell death: Role of the β4 integrin and the PI3k pathway. Oncogene 2007, 26, 2082–2094. [Google Scholar] [CrossRef]
- Fearnley, G.W.; Smith, G.A.; Abdul-Zani, I.; Yuldasheva, N.; Mughal, N.A.; Homer-Vanniasinkam, S.; Kearney, M.T.; Zachary, I.C.; Tomlinson, D.C.; Harrison, M.A.; et al. VEGF-A isoforms program differential VEGFR2 signal transduction, trafficking and proteolysis. Biol. Open 2016, 5, 571–583. [Google Scholar] [CrossRef] [PubMed]
- Olsson, A.K.; Dimberg, A.; Kreuger, J.; Claesson-Welsh, L. VEGF receptor signalling—In control of vascular function. Nat. Rev. Mol. Cell Biol. 2006, 7, 359–371. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.Q.; Lv, Y.E.; Lin, B.H.; Luo, L.M.; Lv, S.L.; Bi, A.H.; Jia, Y.S. Perisilencing of periostin inhibits nicotine-mediated tumor cell growth and epithelial-mesenchymal transition in lung cancer cells. Mol. Med. Rep. 2013, 7, 875–880. [Google Scholar] [CrossRef] [PubMed]
- González-González, L.; Alonso, J. Periostin: A Matricellular Protein with Multiple Functions in Cancer Development and Progression. Front. Oncol. 2018, 8, 225. [Google Scholar] [CrossRef]
- Liu, A.Y.; Zheng, H.; Ouyang, G. Periostin, a multifunctional matricellular protein in inflammatory and tumor microenvironments. Matrix Biol. 2014, 37, 150–156. [Google Scholar] [CrossRef]
- Yao, Y.; Kubota, T.; Takeuchi, H.; Sato, K. Prognostic significance of microvessel density determined by an anti-CD105/endoglin monoclonal antibody in astrocytic tumors: Comparison with an anti-CD31 monoclonal antibody. Neuropathology 2005, 25, 201–206. [Google Scholar] [CrossRef]
- Yao, Y.; Pan, Y.; Chen, J.; Sun, X.; Qiu, Y.; Ding, Y. Endoglin (CD105) expression in angiogenesis of primary hepatocellular carcinomas: Analysis using tissue microarrays and comparisons with CD34 and VEGF. Ann. Clin. Lab. Sci. 2007, 37, 39–48. [Google Scholar]
- da Silva, B.B.; Lopes-Costa, P.V.; dos Santos, A.R.; de Sousa-Júnior, E.C.; Alencar, A.P.; Pires, C.G.; Rosal, M.A. Comparison of three vascular endothelial markers in the evaluation of microvessel density in breast cancer. Eur. J. Gynaecol. Oncol. 2009, 30, 285–288. [Google Scholar]
- Randall, L.M.; Monk, B.J.; Darcy, K.M.; Tian, C.; Burger, R.A.; Liao, S.-Y.; Peters, W.A.; Stock, R.J.; Fruehauf, J.P. Markers of angiogenesis in high-risk, early-stage cervical cancer: A Gynecologic Oncology Group study. Gynecol. Oncol. 2009, 112, 583–589. [Google Scholar] [CrossRef]
- Rubatt, J.M.; Darcy, K.M.; Hutson, A.; Bean, S.M.; Havrilesky, L.J.; Grace, L.A.; Berchuck, A.; Secord, A.A. Independent prognostic relevance of microvessel density in advanced epithelial ovarian cancer and associations between CD31, CD105, p53 status, and angiogenic marker expression: A Gynecologic Oncology Group study. Gynecol. Oncol. 2009, 112, 469–474. [Google Scholar] [CrossRef]
- Kumar, S.; Ghellal, A.; Li, C.; Byrne, G.; Haboubi, N.; Wang, J.M.; Bundred, N. Breast carcinoma: Vascular density determined using CD105 antibody correlates with tumor prognosis. Cancer Res. 1999, 59, 856–861. [Google Scholar] [PubMed]
- Tanaka, F.; Otake, Y.; Yanagihara, K.; Kawano, Y.; Miyahara, R.; Li, M.; Yamada, T.; Hanaoka, N.; Inui, K.; Wada, H. Evaluation of angiogenesis in non-small cell lung cancer: Comparison between anti-CD34 antibody and anti-CD105 antibody. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2001, 7, 3410–3415. [Google Scholar]
- Senger, D.R.; Galli, S.J.; Dvorak, A.M.; Perruzzi, C.A.; Susan Harvey, V.; Dvorak, H.F. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science 1983, 219, 983–985. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, N. Role of vascular endothelial growth factor in the regulation of angiogenesis. Kidney Int. 1999, 56, 794–814. [Google Scholar] [CrossRef] [PubMed]
- Su, J.L.; Yen, C.J.; Chen, P.S.; Chuang, S.E.; Hong, C.C.; Kuo, I.H.; Chen, H.Y.; Hung, M.C.; Kuo, M.L. The role of the VEGF-C/VEGFR-3 axis in cancer progression. Br. J. Cancer 2007, 96, 541–545. [Google Scholar] [CrossRef]
- Ziehe, M.; Morbidelli, L.; Choudhuri, R.; Zhang, H.T.; Donnini, S.; Granger, H.J.; Bicknell, R. Nitric oxide synthase lies downstream from vascular endothelial growth factor-induced but not basic fibroblast growth factor-induced angiogenesis. J. Clin. Investig. 1997, 99, 2625–2634. [Google Scholar] [CrossRef]
- Soltermann, A.; Tischler, V.; Arbogast, S.; Braun, J.; Probst-Hensch, N.; Weder, W.; Moch, H.; Kristiansen, G. Prognostic significance of epithelial-mesenchymal and mesenchymal- epithelial transition protein expression in non-small cell lung cancer. Clin. Cancer Res. 2008, 14, 7430–7437. [Google Scholar] [CrossRef]
- Zhu, M.; Fejzo, M.S.; Anderson, L.; Dering, J.; Ginther, C.; Ramos, L.; Gasson, J.C.; Karlan, B.Y.; Slamon, D.J. Periostin promotes ovarian cancer angiogenesis and metastasis. Gynecol. Oncol. 2010, 119, 337–344. [Google Scholar] [CrossRef]
- Jang, S.Y.; Park, S.Y.; Lee, H.W.; Choi, Y.K.; Park, K.G.; Yoon, G.S.; Tak, W.Y.; Kweon, Y.O.; Hur, K.; Lee, W.K. The combination of periostin overexpression and microvascular invasion is related to a poor prognosis for hepatocellular carcinoma. Gut Liver 2016, 10, 948–954. [Google Scholar] [CrossRef]
- Lv, Y.; Wang, W.; Jia, W.-D.; Sun, Q.-K.; Li, J.-S.; Ma, J.-L.; Liu, W.-B.; Zhou, H.-C.; Ge, Y.-S.; Yu, J.-H.; et al. High-level expression of periostin is closely related to metastatic potential and poor prognosis of hepatocellular carcinoma. Med. Oncol. 2012, 30, 385. [Google Scholar] [CrossRef]
- Morra, L.; Rechsteiner, M.; Casagrande, S.; Duc Luu, V.; Santimaria, R.; Diener, P.A.; Sulser, T.; Kristiansen, G.; Schraml, P.; Moch, H.; et al. Relevance of periostin splice variants in renal cell carcinoma. Am. J. Pathol. 2011, 179, 1513–1521. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Ke, S.Q.; Huang, Z.; Flavahan, W.; Fang, X.; Paul, J.; Wu, L.; Sloan, A.E.; McLendon, R.E.; Li, X.; et al. Periostin secreted by glioblastoma stem cells recruits M2 tumour-associated macrophages and promotes malignant growth. Nat. Cell Biol. 2015, 17, 170–182. [Google Scholar] [CrossRef] [PubMed]
- Hong, L.Z.; Wei, X.W.; Chen, J.F.; Shi, Y. Overexpression of periostin predicts poor prognosis in non-small cell lung cancer. Oncol. Lett. 2013, 6, 1595–1603. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.-H.; Wang, W.; Lin, Y.; Qian, L.-H.; Zhang, X.-W.; Wang, Q.-B.; Yu, L.-K. Diagnostic and prognostic value of serum periostin in patients with non-small cell lung cancer. Oncotarget 2017, 8, 18746–18753. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yuan, D.; Yao, Y.; Sun, W.; Shi, Y.; Su, X. Predictive and prognostic value of serum periostin in advanced non-small cell lung cancer patients receiving chemotherapy. Tumour Biol. J. Int. Soc. Oncodevelopmental Biol. Med. 2017, 39, 1010428317698367. [Google Scholar] [CrossRef]





| Variables | NSCLC N = 500 (100%) | SCC N = 248 (49.6%) | AC N = 214 (42.8%) | LCC N = 38 (7.6%) |
|---|---|---|---|---|
| Age (median 62): <62, ≥62 | <62—N = 224 (44.8%) ≥62—N = 276 (55.2%) | <62—N = 97 (39.11%) ≥62—N = 151 (60.89%) | <62—N = 106 (49.53%) ≥62—N = 108 (50.47%) | <62—N = 21 (55.26%) ≥62—N = 17 (44.74%) |
| Sex: males/females | males—N = 365 (73%) females—N = 135 (27%) | males—N = 196 (79.03%) females—N = 52 (20.97%) | males—N = 139 (64.95%) females—N = 75 (35.05%) | males—N = 30 (78.95%) females—N = 8 (21.05%) |
| Size of the primary tumor (pT): pT1, pT2, pT3, pT4 | pT1—N = 131 (26.2%) pT2—N = 223 (44.6%) pT3—N = 97 (19.4%) pT4—N = 49 (9.8%) | pT1—N = 67 (27.01%) pT2—N = 101 (40.73%) pT3—N = 54 (21.78%) pT4—N = 26 (10.48%) | pT1—N = 53 (24.76%) pT2—N = 105 (49.07%) pT3—N = 38 (17.76%) pT4—N = 18 (8.41%) | pT1—N = 11 (28.94%) pT2—N = 17 (44.74%) pT3—N = 5 (13.16%) pT4—N = 5 (13.16%) |
| Involvement of regional lymph nodes in NSCLC cells (pN): pN0, pN1, pN2 | pN0—N = 321 (64.2%) pN1—N = 96 (19.2%) pN2—N = 83 (16.6%) | pN0—N = 160 (64.52%) pN1—N = 62 (25%) pN2—N = 26 (10.48%) | pN0—N = 135 (63.08%) pN1—N = 29 (13.56%) pN2—N = 50 (23.36%) | pN0—N = 26 (68.42%) pN1—N = 5 (13.16%) pN2—N = 7 (18.42%) |
| Presence (or absence) of distant metastases (M): pM0, pM1 | pM0—N = 497 (99.4%) pM1—N = 3 (0.6%) | pM0—N = 248 (100%) | pM0—N = 211 (98.60%) pM1—N = 3 (1.40%) | pM0—N = 38 (100%) |
| Histological grade (G): G1, G2, G3 | G1—N = 24 (4.8%) G2—N = 383 (76.6%) G3—N = 93 (18.6%) | G1—N = 6 (2.42%) G2—N = 206 (83.06%) G3—N = 36 (14.52%) | G1—N = 16 (7.48%) G2—N = 145 (67.76%) G3—N = 53 (24.76%) | G1—N = 2 (5.26%) G2—N = 32 (84.21%) G3—N = 4 (10.53%) |
| Cancer stage: I, II, III, IV | I—N = 186 (37.2%) II—N = 164 (32.8%) III—N = 147 (29.4%) IV—N = 3 (0.6%) | I—N = 93 (37.5%) II—N = 90 (36.29%) III—N = 65 (26.21%) | I—N = 79 (36.92%) II—N = 62 (28.97%) III—N = 70 (32.71%) IV—N = 3 (1.4%) | I—N = 14 (36.84%) II—N = 12 (31.58%) III—N = 12 (31.58%) |
| A—The Percentage of Stained Cells | B—The Intensity of the Color Reaction |
|---|---|
| 0 pts—no stained cells | 0 pts—no reaction |
| 1 pt < 10% of stained cells | 1 pt—weak reaction |
| 2 pts—10–50% of stained cells | 2 pts—moderate reaction |
| 3 pts—51–80% of stained cells | 3 pts—strong reaction |
| 4 pts > 80% of stained cells | |
| IRS (A × B): 0–12 | |
| Variable | p-Value |
|---|---|
| CD 105 Chalkley | 0.840 |
| CD 105 Weidner | 0.656 |
| CD 34 Chalkley | 0.817 |
| CD 34 Weidner | 0.976 |
| CD 31 Chalkley | 0.816 |
| CD 31 Weidner | 0.195 |
| VEGF-A | 0.611 |
| POSTN in cells | 0.663 |
| POSTN in stroma | 0.032 |
| Predictor | Estimate | SE | t | p |
|---|---|---|---|---|
| Intercept | 2851.3 | 249.8 | 11.42 | <0.001 |
| POSTN in stroma | −77.7 | 15.0 | −5.19 | <0.001 |
| CD 34 Chalkley | −70.9 | 34.7 | −2.04 | 0.042 |
| T-stage: | ||||
| 2–1 | −224.8 | 110.3 | −2.04 | 0.042 |
| 3–1 | −545.2 | 134.5 | −4.05 | <0.001 |
| 4–1 | −593.8 | 159.1 | −3.73 | <0.001 |
| N-stage: | ||||
| 1–0 | −189.3 | 114.8 | −1.65 | 0.100 |
| 2–0 | −521.3 | 119.2 | −4.37 | <0.001 |
| Predictor | Estimate | SE | Z | p |
|---|---|---|---|---|
| Intercept | 3.402 | 0.8020 | 4.24 | <0.001 |
| POSTN in stroma | −0.21 | 0.0404 | −5.20 | <0.001 |
| CD 31 Chalkley | −0.227 | 0.1128 | −2.01 | 0.044 |
| T-stage: | ||||
| 2–1 | −0.558 | 0.3019 | −1.85 | 0.065 |
| 3–1 | −1.201 | 0.3670 | −3.27 | 0.001 |
| 4–1 | −1.635 | 0.4897 | −3.34 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wasik, A.; Podhorska-Okolow, M.; Dziegiel, P.; Piotrowska, A.; Kulus, M.J.; Kmiecik, A.; Ratajczak-Wielgomas, K. Correlation between Periostin Expression and Pro-Angiogenic Factors in Non-Small-Cell Lung Carcinoma. Cells 2024, 13, 1406. https://doi.org/10.3390/cells13171406
Wasik A, Podhorska-Okolow M, Dziegiel P, Piotrowska A, Kulus MJ, Kmiecik A, Ratajczak-Wielgomas K. Correlation between Periostin Expression and Pro-Angiogenic Factors in Non-Small-Cell Lung Carcinoma. Cells. 2024; 13(17):1406. https://doi.org/10.3390/cells13171406
Chicago/Turabian StyleWasik, Adrian, Marzenna Podhorska-Okolow, Piotr Dziegiel, Aleksandra Piotrowska, Michal Jerzy Kulus, Alicja Kmiecik, and Katarzyna Ratajczak-Wielgomas. 2024. "Correlation between Periostin Expression and Pro-Angiogenic Factors in Non-Small-Cell Lung Carcinoma" Cells 13, no. 17: 1406. https://doi.org/10.3390/cells13171406







