Immunophenotyping of Peripheral Blood Cells in Patients with Chronic Lymphocytic Leukemia Treated with Ibrutinib
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples for Spectral Flow Cytometry Analyses
2.2. Flow Cytometry
2.3. Statistical Analyses
3. Results
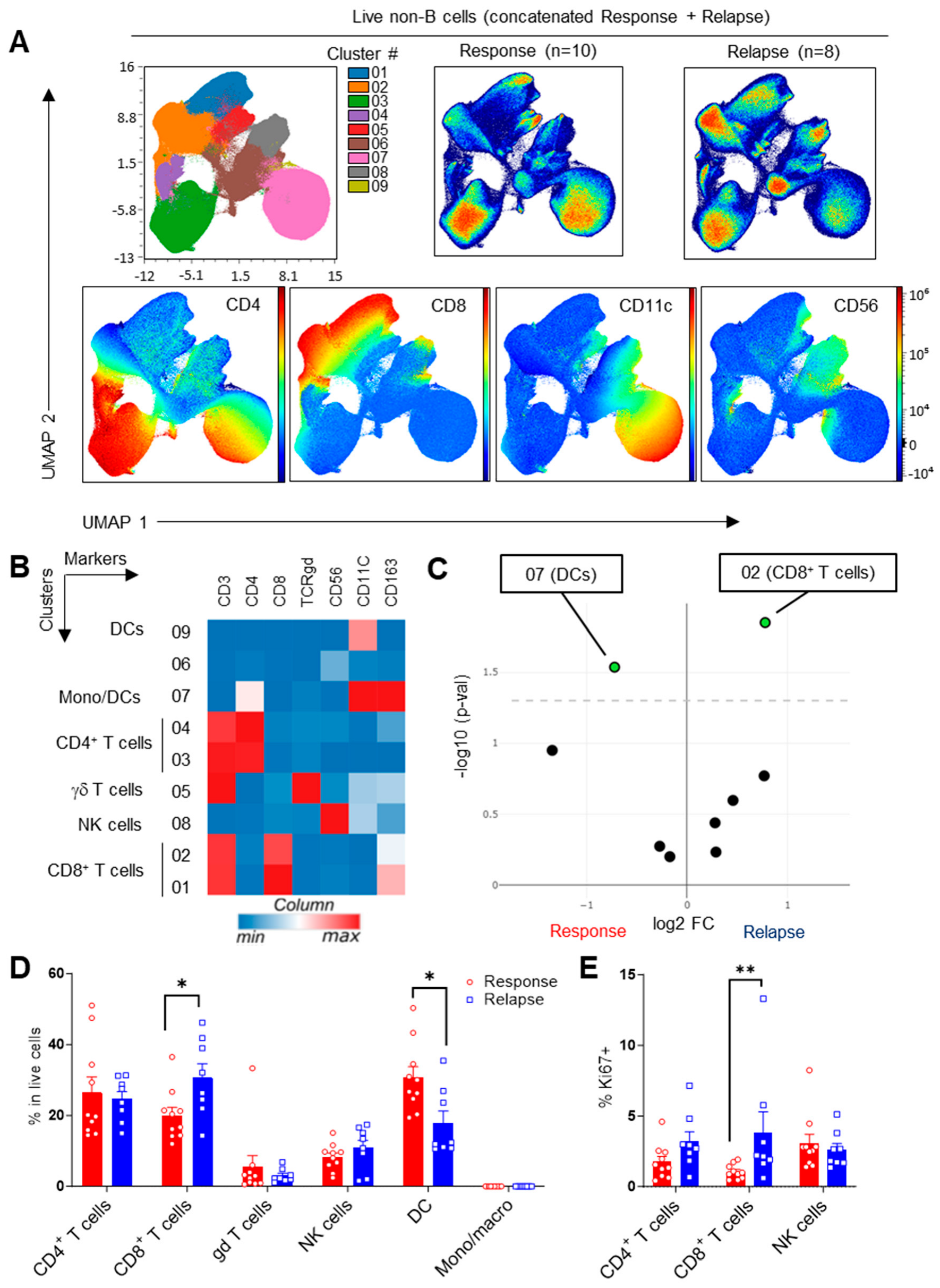
3.1. Increased CD8+ T-Cell Frequencies upon Ibrutinib Relapse
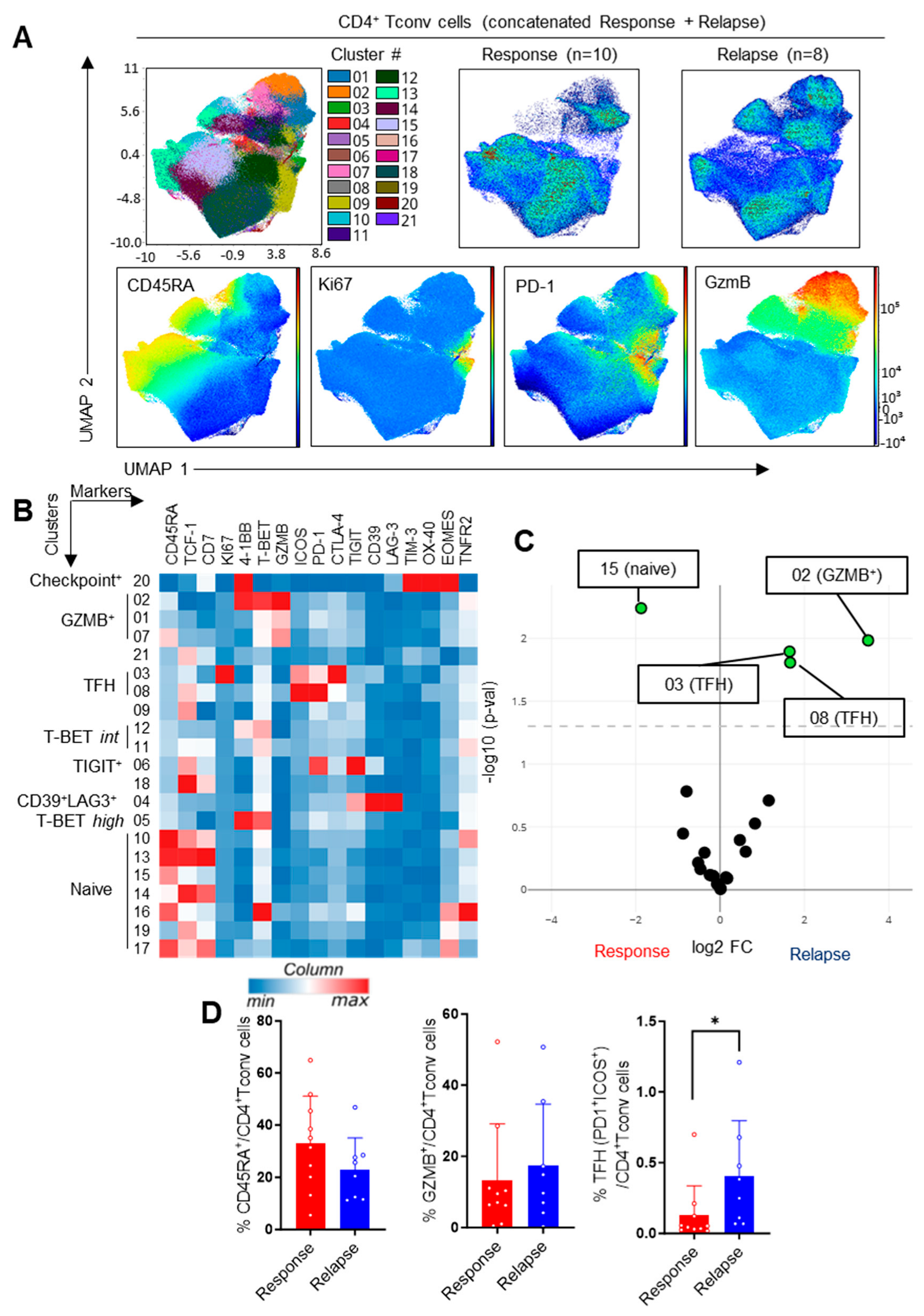
3.2. Impact of the Response Status on CD4+ T-Cell Subsets
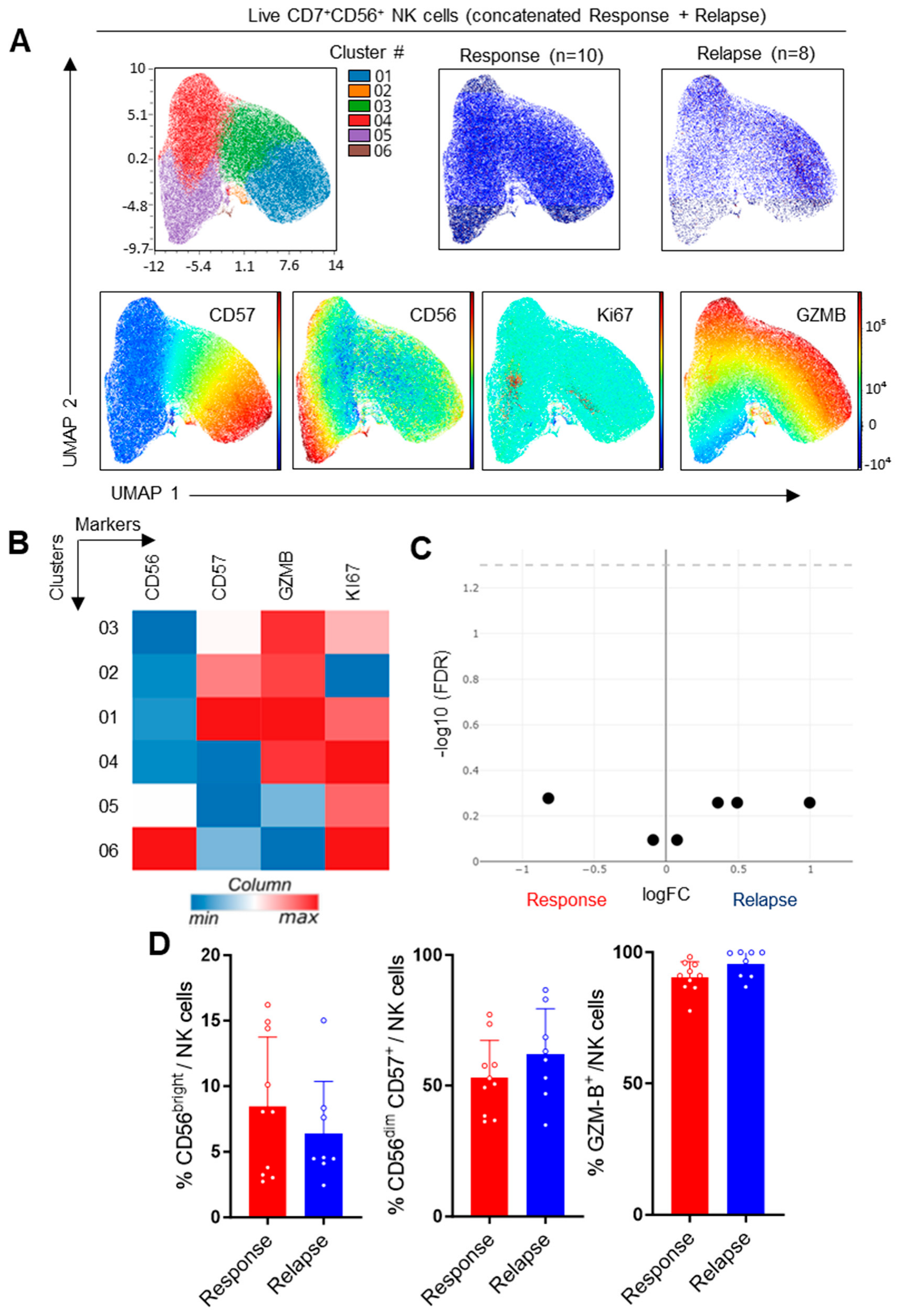
3.3. Study of NK Cells in CLL Patients
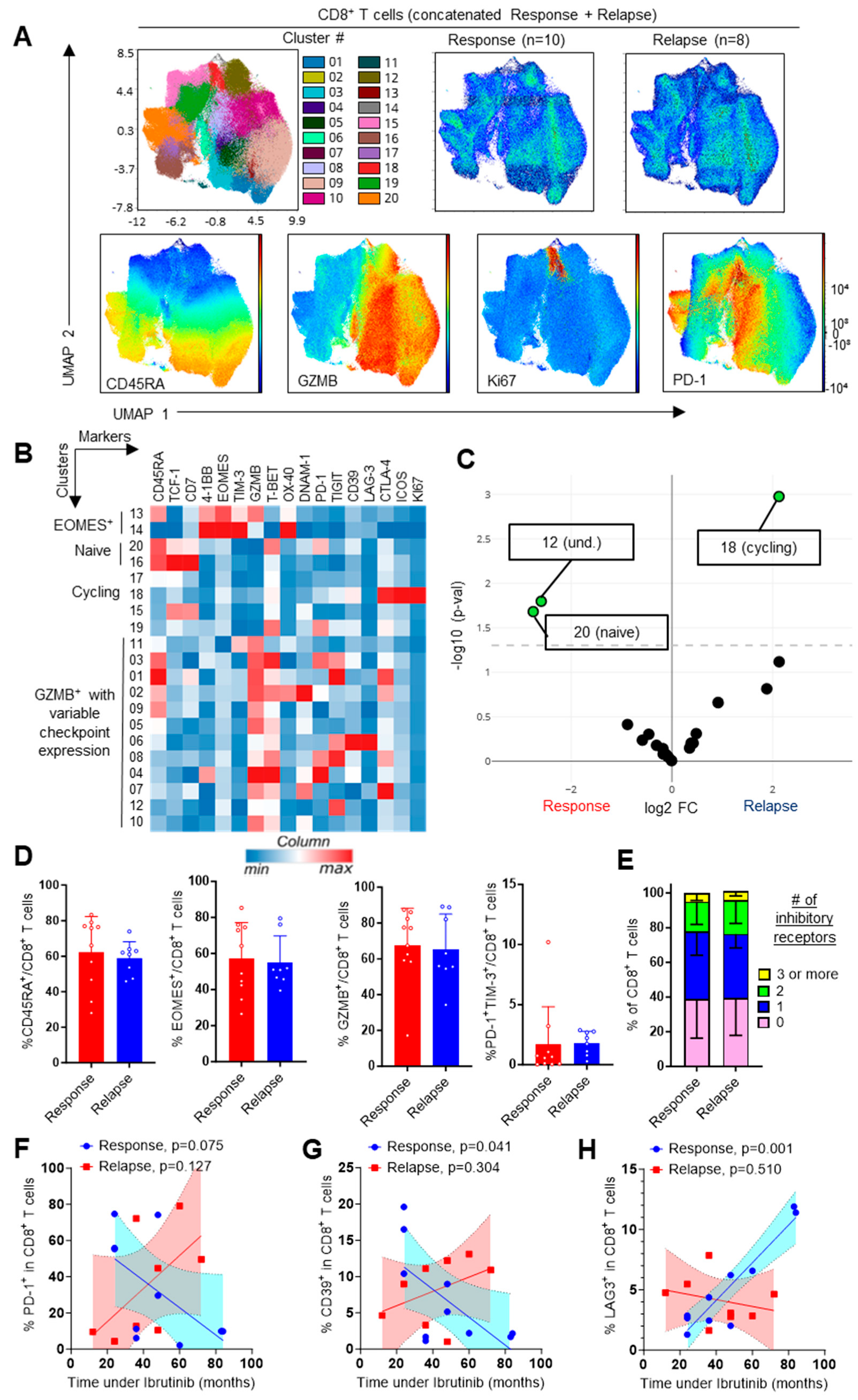
3.4. Evaluation of Checkpoint Receptors on T and NK Cells
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ribas, A.; Wolchok, J.D. Cancer immunotherapy using checkpoint blockade. Science 2018, 359, 1350–1355. [Google Scholar] [CrossRef] [PubMed]
- Chiorazzi, N.; Rai, K.R.; Ferrarini, M. Chronic lymphocytic leukemia. N. Engl. J. Med. 2005, 352, 804–815. [Google Scholar] [CrossRef] [PubMed]
- Arruga, F.; Gyau, B.B.; Iannello, A.; Vitale, N.; Vaisitti, T.; Deaglio, S. Immune Response Dysfunction in Chronic Lymphocytic Leukemia: Dissecting Molecular Mechanisms and Microenvironmental Conditions. Int. J. Mol. Sci. 2020, 21, 1825. [Google Scholar] [CrossRef] [PubMed]
- Roessner, P.M.; Seiffert, M. T-cells in chronic lymphocytic leukemia: Guardians or drivers of disease? Leukemia 2020, 34, 2012–2024. [Google Scholar] [CrossRef] [PubMed]
- D’Arena, G.; Simeon, V.; D’Auria, F.; Statuto, T.; Sanzo, P.D.; Martino, L.D.; Marandino, A.; Sangiorgio, M.; Musto, P.; Feo, V.D. Regulatory T-cells in chronic lymphocytic leukemia: Actor or innocent bystander? Am. J. Blood Res. 2013, 3, 52–57. [Google Scholar] [PubMed]
- Giannopoulos, K.; Schmitt, M.; Kowal, M.; Wlasiuk, P.; Bojarska-Junak, A.; Chen, J.; Rolinski, J.; Dmoszynska, A. Characterization of regulatory T cells in patients with B-cell chronic lymphocytic leukemia. Oncol. Rep. 2008, 20, 677–682. [Google Scholar] [CrossRef]
- Riches, J.C.; Davies, J.K.; McClanahan, F.; Fatah, R.; Iqbal, S.; Agrawal, S.; Ramsay, A.G.; Gribben, J.G. T cells from CLL patients exhibit features of T-cell exhaustion but retain capacity for cytokine production. Blood 2013, 121, 1612–1621. [Google Scholar] [CrossRef]
- Palma, M.; Gentilcore, G.; Heimersson, K.; Mozaffari, F.; Nasman-Glaser, B.; Young, E.; Rosenquist, R.; Hansson, L.; Osterborg, A.; Mellstedt, H. T cells in chronic lymphocytic leukemia display dysregulated expression of immune checkpoints and activation markers. Haematologica 2017, 102, 562–572. [Google Scholar] [CrossRef]
- Hanna, B.S.; Roessner, P.M.; Yazdanparast, H.; Colomer, D.; Campo, E.; Kugler, S.; Yosifov, D.; Stilgenbauer, S.; Schmidt, M.; Gabriel, R.; et al. Control of chronic lymphocytic leukemia development by clonally-expanded CD8+ T-cells that undergo functional exhaustion in secondary lymphoid tissues. Leukemia 2019, 33, 625–637. [Google Scholar] [CrossRef]
- Sportoletti, P.; de Falco, F.; del Papa, B.; Baldoni, S.; Guarente, V.; Marra, A.; Dorillo, E.; Rompietti, C.; Adamo, F.M.; Ruggeri, L.; et al. NK Cells in Chronic Lymphocytic Leukemia and Their Therapeutic Implications. Int. J. Mol. Sci. 2021, 22, 6665. [Google Scholar] [CrossRef]
- MacFarlane, A.W.t.; Jillab, M.; Smith, M.R.; Alpaugh, R.K.; Cole, M.E.; Litwin, S.; Millenson, M.M.; Al-Saleem, T.; Cohen, A.D.; Campbell, K.S. NK cell dysfunction in chronic lymphocytic leukemia is associated with loss of the mature cells expressing inhibitory killer cell Ig-like receptors. Oncoimmunology 2017, 6, e1330235. [Google Scholar] [CrossRef]
- Hofland, T.; Endstra, S.; Gomes, C.K.P.; de Boer, R.; de Weerdt, I.; Bobkov, V.; Riedl, J.A.; Heukers, R.; Smit, M.J.; Eldering, E.; et al. Natural Killer Cell Hypo-responsiveness in Chronic Lymphocytic Leukemia can be Circumvented In Vitro by Adequate Activating Signaling. Hemasphere 2019, 3, e308. [Google Scholar] [CrossRef] [PubMed]
- Burger, J.A.; Tedeschi, A.; Barr, P.M.; Robak, T.; Owen, C.; Ghia, P.; Bairey, O.; Hillmen, P.; Bartlett, N.L.; Li, J.; et al. Ibrutinib as Initial Therapy for Patients with Chronic Lymphocytic Leukemia. N. Engl. J. Med. 2015, 373, 2425–2437. [Google Scholar] [CrossRef]
- Skanland, S.S.; Mato, A.R. Overcoming resistance to targeted therapies in chronic lymphocytic leukemia. Blood Adv. 2021, 5, 334–343. [Google Scholar] [CrossRef]
- Solman, I.G.; Blum, L.K.; Burger, J.A.; Kipps, T.J.; Dean, J.P.; James, D.F.; Mongan, A. Impact of long-term ibrutinib treatment on circulating immune cells in previously untreated chronic lymphocytic leukemia. Leuk. Res. 2021, 102, 106520. [Google Scholar] [CrossRef] [PubMed]
- Mhibik, M.; Wiestner, A.; Sun, C. Harnessing the Effects of BTKi on T Cells for Effective Immunotherapy against CLL. Int. J. Mol. Sci. 2019, 21, 68. [Google Scholar] [CrossRef]
- Papazoglou, D.; Wang, X.V.; Shanafelt, T.D.; Lesnick, C.E.; Ioannou, N.; de Rossi, G.; Herter, S.; Bacac, M.; Klein, C.; Tallman, M.S.; et al. Ibrutinib-based therapy reinvigorates CD8+ T cells compared to chemoimmunotherapy: Immune monitoring from the E1912 trial. Blood 2024, 143, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Hallek, M.; Cheson, B.D.; Catovsky, D.; Caligaris-Cappio, F.; Dighiero, G.; Dohner, H.; Hillmen, P.; Keating, M.; Montserrat, E.; Chiorazzi, N.; et al. iwCLL guidelines for diagnosis, indications for treatment, response assessment, and supportive management of CLL. Blood 2018, 131, 2745–2760. [Google Scholar] [CrossRef]
- Stephan, P.; Perrot, J.; Voisin, A.; Barbery, M.; Andrieu, T.; Grimont, M.; Caramel, J.; Bardou, M.; Tondeur, G.; Missiaglia, E.; et al. Deep phenotyping of nodal T-cell lymphomas reveals immune alterations and therapeutic targets. Haematologica 2024. [Google Scholar] [CrossRef]
- Sakuishi, K.; Apetoh, L.; Sullivan, J.M.; Blazar, B.R.; Kuchroo, V.K.; Anderson, A.C. Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J. Exp. Med. 2010, 207, 2187–2194. [Google Scholar] [CrossRef]
- Cass, S.H.; Tobin, J.W.D.; Seo, Y.D.; Gener-Ricos, G.; Keung, E.Z.; Burton, E.M.; Davies, M.A.; McQuade, J.L.; Lazar, A.J.; Mason, R.; et al. Efficacy of immune checkpoint inhibitors for the treatment of advanced melanoma in patients with concomitant chronic lymphocytic leukemia. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2023, 34, 796–805. [Google Scholar] [CrossRef] [PubMed]
- Smithy, J.W.; Pianko, M.J.; Maher, C.; Postow, M.A.; Shoushtari, A.N.; Momtaz, P.; Chapman, P.B.; Wolchok, J.D.; Park, J.H.; Callahan, M.K. Checkpoint Blockade in Melanoma Patients with Underlying Chronic Lymphocytic Leukemia. J. Immunother. 2021, 44, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Perutelli, F.; Jones, R.; Griggio, V.; Vitale, C.; Coscia, M. Immunotherapeutic Strategies in Chronic Lymphocytic Leukemia: Advances and Challenges. Front. Oncol. 2022, 12, 837531. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.; LaPlant, B.R.; Call, T.G.; Parikh, S.A.; Leis, J.F.; He, R.; Shanafelt, T.D.; Sinha, S.; Le-Rademacher, J.; Feldman, A.L.; et al. Pembrolizumab in patients with CLL and Richter transformation or with relapsed CLL. Blood 2017, 129, 3419–3427. [Google Scholar] [CrossRef]
- Pula, B.; Golos, A.; Gorniak, P.; Jamroziak, K. Overcoming Ibrutinib Resistance in Chronic Lymphocytic Leukemia. Cancers 2019, 11, 1834. [Google Scholar] [CrossRef]
- Solman, I.G.; Blum, L.K.; Hoh, H.Y.; Kipps, T.J.; Burger, J.A.; Barrientos, J.C.; O’Brien, S.; Mulligan, S.P.; Kay, N.E.; Hillmen, P.; et al. Ibrutinib restores immune cell numbers and function in first-line and relapsed/refractory chronic lymphocytic leukemia. Leuk. Res. 2020, 97, 106432. [Google Scholar] [CrossRef]
- Cadot, S.; Valle, C.; Tosolini, M.; Pont, F.; Largeaud, L.; Laurent, C.; Fournie, J.J.; Ysebaert, L.; Quillet-Mary, A. Longitudinal CITE-Seq profiling of chronic lymphocytic leukemia during ibrutinib treatment: Evolution of leukemic and immune cells at relapse. Biomark. Res. 2020, 8, 72. [Google Scholar] [CrossRef]
- Le Saos-Patrinos, C.; Loizon, S.; Zouine, A.; Turpin, D.; Dilhuydy, M.S.; Blanco, P.; Sisirak, V.; Forcade, E.; Duluc, D. Elevated levels of circulatory follicular T helper cells in chronic lymphocytic leukemia contribute to B cell expansion. J. Leukoc. Biol. 2023, 113, 305–314. [Google Scholar] [CrossRef]
- Wu, X.; Fajardo-Despaigne, J.E.; Zhang, C.; Neppalli, V.; Banerji, V.; Johnston, J.B.; Gibson, S.B.; Marshall, A.J. Altered T Follicular Helper Cell Subsets and Function in Chronic Lymphocytic Leukemia. Front. Oncol. 2021, 11, 674492. [Google Scholar] [CrossRef]
- Chavez, J.C.; Foss, F.M.; William, B.M.; Brammer, J.E.; Smith, S.M.; Prica, A.; Zain, J.M.; Tuscano, J.M.; Shah, H.; Mehta-Shah, N.; et al. Targeting the Inducible T-cell Costimulator (ICOS) in Patients with Relapsed/Refractory T-follicular Helper Phenotype Peripheral T-cell and Angioimmunoblastic T-cell Lymphoma. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2023, 29, 1869–1878. [Google Scholar] [CrossRef]
- Kater, A.P.; Arslan, O.; Demirkan, F.; Herishanu, Y.; Ferhanoglu, B.; Diaz, M.G.; Leber, B.; Montillo, M.; Panayiotidis, P.; Rossi, D.; et al. Activity of venetoclax in patients with relapsed or refractory chronic lymphocytic leukaemia: Analysis of the VENICE-1 multicentre, open-label, single-arm, phase 3b trial. Lancet Oncol. 2024, 25, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Todorovic, Z.; Todorovic, D.; Markovic, V.; Ladjevac, N.; Zdravkovic, N.; Djurdjevic, P.; Arsenijevic, N.; Milovanovic, M.; Arsenijevic, A.; Milovanovic, J. CAR T Cell Therapy for Chronic Lymphocytic Leukemia: Successes and Shortcomings. Curr. Oncol. 2022, 29, 3647–3657. [Google Scholar] [CrossRef] [PubMed]


| Patient ID | Sex | Age (yrs) | TP53 Mutation (Yes/No) | Cytogenetic Status | IGHV Mutation Status | # of Previous Treatment Lines | Disease Status | Months under Ibrutinib Therapy |
|---|---|---|---|---|---|---|---|---|
| 1 | M | 52 | No | del11q | mutated | 2 | CR | 84 |
| 2 | M | 73 | Yes | del17p | unmutated | 1 | CR | 83 |
| 3 | M | 68 | No | None | unmutated | 1 | CR | 36 |
| 4 | F | 77 | No | None | unmutated | 0 | CR | 36 |
| 5 | F | 68 | No | None | unmutated | 0 | CR | 60 |
| 6 | M | 72 | No | None | unmutated | 1 | CR | 48 |
| 7 | F | 80 | No | None | unmutated | 0 | CR | 24 |
| 8 | F | 67 | No | None | unmutated | 0 | CR | 24 |
| 9 | M | 79 | No | None | unmutated | 1 | CR | 24 |
| 10 | F | 62 | No | None | unmutated | 1 | CR | 48 |
| 12 | F | 84 | Yes | del17p, complex karyotype | unmutated | 1 | Relapse | 12 |
| 13 | M | 61 | Yes | del17p, complex karyotype | unmutated | 2 | Relapse | 36 |
| 14 | F | 45 | No | del11q | unmutated | 1 | Relapse | 48 |
| 16 | M | 76 | No | None | unmutated | 1 | Relapse | 24 |
| 17 | F | 89 | No | None | unmutated | 1 | Relapse | 36 |
| 18 | M | 51 | No | None | unmutated | 1 | Relapse | 72 |
| 19 | M | 67 | Yes | del17p, complex karyotype | unmutated | 0 | Relapse | 60 |
| 20 | M | 67 | No | del11q | unmutated | 1 | Relapse | 48 |
| Patient ID | Disease Status | Months under Ibrutinib Therapy | BTK Mutation | Bulky Disease | Lymphocyte Count (G/L) | Months between Relapse Diagnosis and Sampling |
|---|---|---|---|---|---|---|
| 12 | Relapse | 12 | N/A | No | 23.8 | 1 |
| 13 | Relapse | 36 | C481S | No | 13.3 | 2 |
| 14 | Relapse | 48 | C481S | No | 7 | 2 |
| 16 | Relapse | 24 | N/A | No | 20.39 | 5 |
| 17 | Relapse | 36 | N/A | No | 28.6 | 24 |
| 18 | Relapse | 72 | C481S | No | 54 | 1 |
| 19 | Relapse | 60 | C481S | No | 11.7 | 1 |
| 20 | Relapse | 48 | None | No | 8.22 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stéphan, P.; Bouherrou, K.; Guillermin, Y.; Michallet, A.-S.; Grinberg-Bleyer, Y. Immunophenotyping of Peripheral Blood Cells in Patients with Chronic Lymphocytic Leukemia Treated with Ibrutinib. Cells 2024, 13, 1458. https://doi.org/10.3390/cells13171458
Stéphan P, Bouherrou K, Guillermin Y, Michallet A-S, Grinberg-Bleyer Y. Immunophenotyping of Peripheral Blood Cells in Patients with Chronic Lymphocytic Leukemia Treated with Ibrutinib. Cells. 2024; 13(17):1458. https://doi.org/10.3390/cells13171458
Chicago/Turabian StyleStéphan, Pierre, Khaled Bouherrou, Yann Guillermin, Anne-Sophie Michallet, and Yenkel Grinberg-Bleyer. 2024. "Immunophenotyping of Peripheral Blood Cells in Patients with Chronic Lymphocytic Leukemia Treated with Ibrutinib" Cells 13, no. 17: 1458. https://doi.org/10.3390/cells13171458
APA StyleStéphan, P., Bouherrou, K., Guillermin, Y., Michallet, A.-S., & Grinberg-Bleyer, Y. (2024). Immunophenotyping of Peripheral Blood Cells in Patients with Chronic Lymphocytic Leukemia Treated with Ibrutinib. Cells, 13(17), 1458. https://doi.org/10.3390/cells13171458







