Immunophenotyping of Peripheral Blood Cells in Patients with Chronic Lymphocytic Leukemia Treated with Ibrutinib
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples for Spectral Flow Cytometry Analyses
2.2. Flow Cytometry
2.3. Statistical Analyses
3. Results
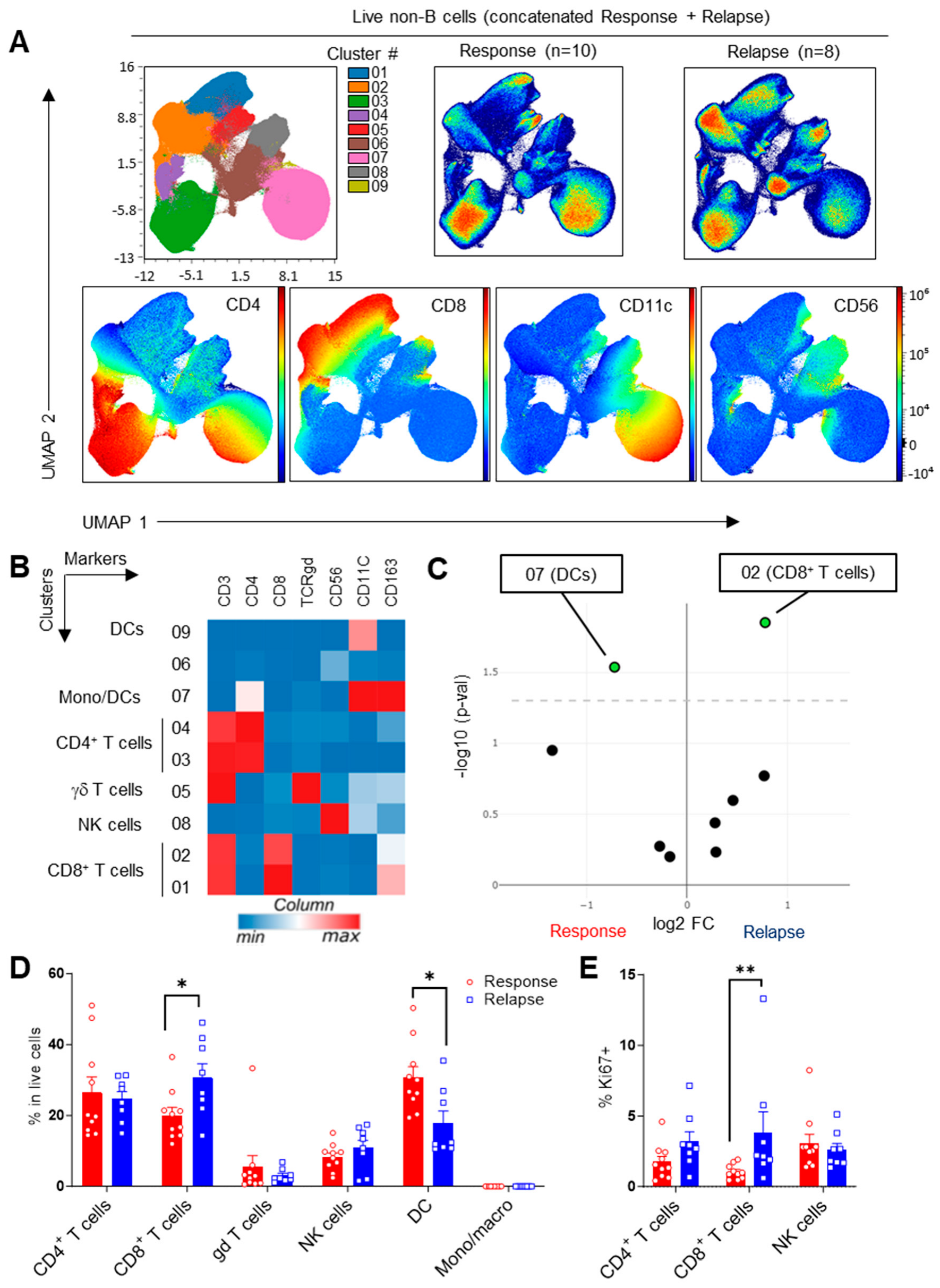
3.1. Increased CD8+ T-Cell Frequencies upon Ibrutinib Relapse
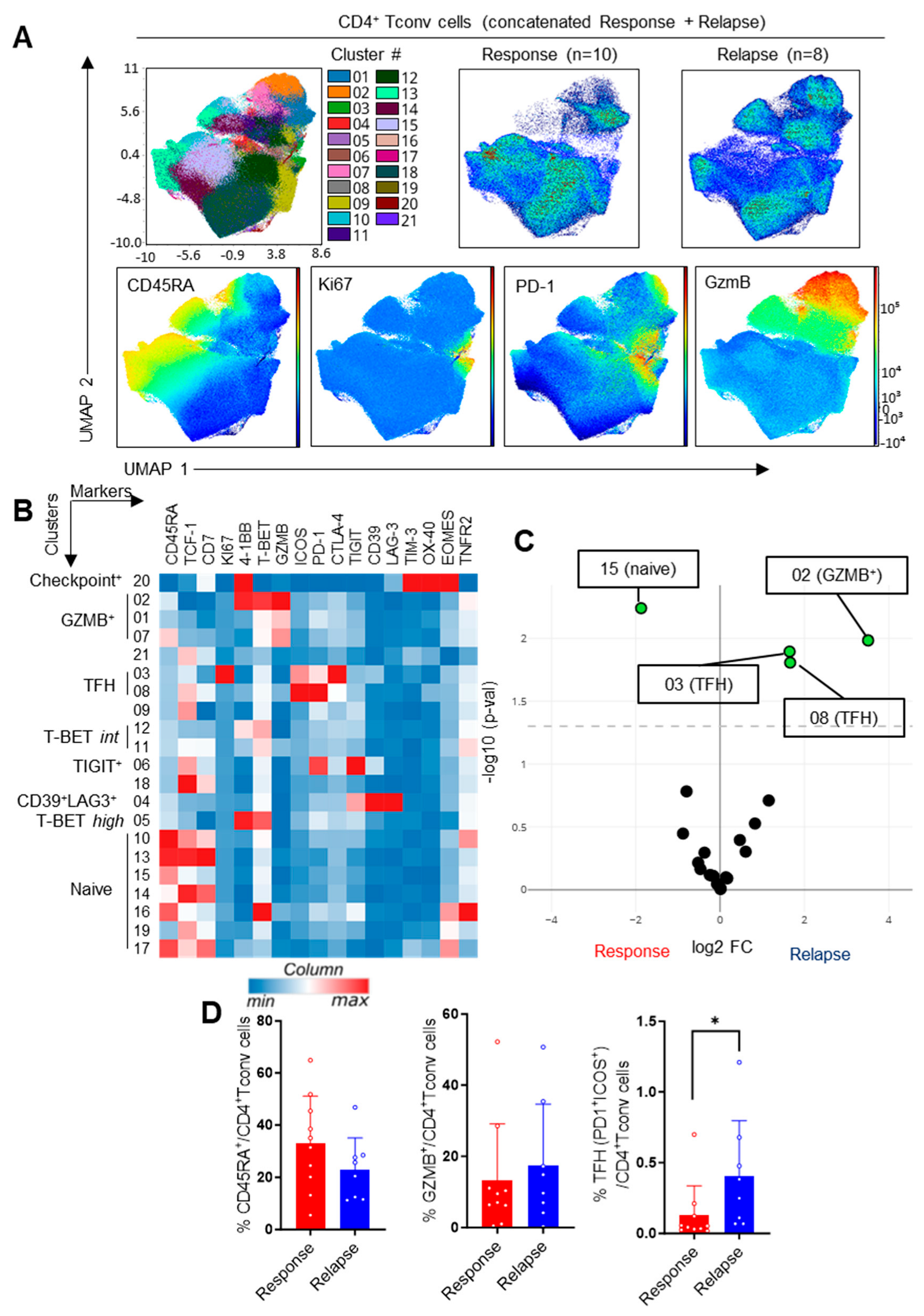
3.2. Impact of the Response Status on CD4+ T-Cell Subsets
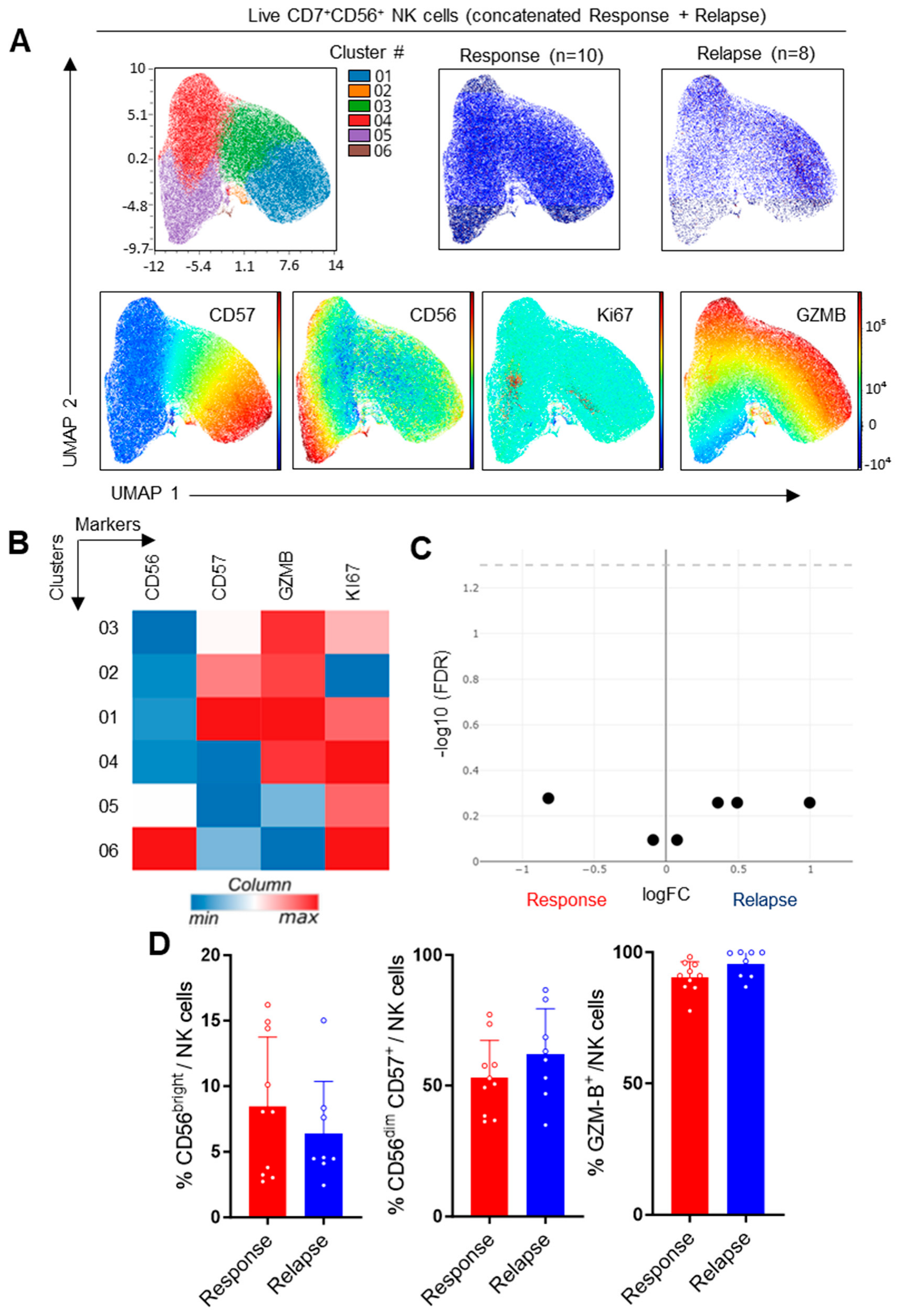
3.3. Study of NK Cells in CLL Patients
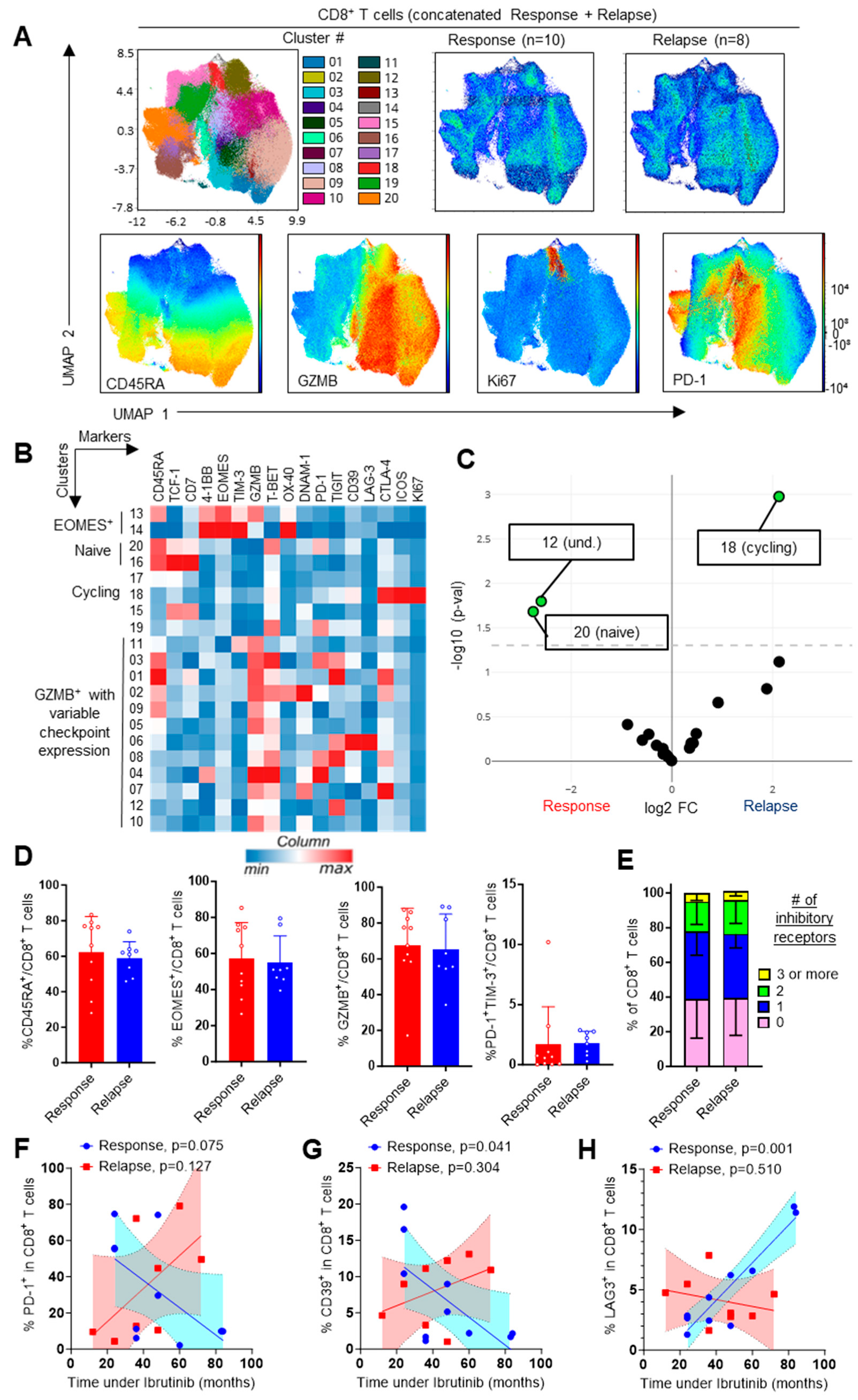
3.4. Evaluation of Checkpoint Receptors on T and NK Cells
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ribas, A.; Wolchok, J.D. Cancer immunotherapy using checkpoint blockade. Science 2018, 359, 1350–1355. [Google Scholar] [CrossRef] [PubMed]
- Chiorazzi, N.; Rai, K.R.; Ferrarini, M. Chronic lymphocytic leukemia. N. Engl. J. Med. 2005, 352, 804–815. [Google Scholar] [CrossRef] [PubMed]
- Arruga, F.; Gyau, B.B.; Iannello, A.; Vitale, N.; Vaisitti, T.; Deaglio, S. Immune Response Dysfunction in Chronic Lymphocytic Leukemia: Dissecting Molecular Mechanisms and Microenvironmental Conditions. Int. J. Mol. Sci. 2020, 21, 1825. [Google Scholar] [CrossRef] [PubMed]
- Roessner, P.M.; Seiffert, M. T-cells in chronic lymphocytic leukemia: Guardians or drivers of disease? Leukemia 2020, 34, 2012–2024. [Google Scholar] [CrossRef] [PubMed]
- D’Arena, G.; Simeon, V.; D’Auria, F.; Statuto, T.; Sanzo, P.D.; Martino, L.D.; Marandino, A.; Sangiorgio, M.; Musto, P.; Feo, V.D. Regulatory T-cells in chronic lymphocytic leukemia: Actor or innocent bystander? Am. J. Blood Res. 2013, 3, 52–57. [Google Scholar] [PubMed]
- Giannopoulos, K.; Schmitt, M.; Kowal, M.; Wlasiuk, P.; Bojarska-Junak, A.; Chen, J.; Rolinski, J.; Dmoszynska, A. Characterization of regulatory T cells in patients with B-cell chronic lymphocytic leukemia. Oncol. Rep. 2008, 20, 677–682. [Google Scholar] [CrossRef]
- Riches, J.C.; Davies, J.K.; McClanahan, F.; Fatah, R.; Iqbal, S.; Agrawal, S.; Ramsay, A.G.; Gribben, J.G. T cells from CLL patients exhibit features of T-cell exhaustion but retain capacity for cytokine production. Blood 2013, 121, 1612–1621. [Google Scholar] [CrossRef]
- Palma, M.; Gentilcore, G.; Heimersson, K.; Mozaffari, F.; Nasman-Glaser, B.; Young, E.; Rosenquist, R.; Hansson, L.; Osterborg, A.; Mellstedt, H. T cells in chronic lymphocytic leukemia display dysregulated expression of immune checkpoints and activation markers. Haematologica 2017, 102, 562–572. [Google Scholar] [CrossRef]
- Hanna, B.S.; Roessner, P.M.; Yazdanparast, H.; Colomer, D.; Campo, E.; Kugler, S.; Yosifov, D.; Stilgenbauer, S.; Schmidt, M.; Gabriel, R.; et al. Control of chronic lymphocytic leukemia development by clonally-expanded CD8+ T-cells that undergo functional exhaustion in secondary lymphoid tissues. Leukemia 2019, 33, 625–637. [Google Scholar] [CrossRef]
- Sportoletti, P.; de Falco, F.; del Papa, B.; Baldoni, S.; Guarente, V.; Marra, A.; Dorillo, E.; Rompietti, C.; Adamo, F.M.; Ruggeri, L.; et al. NK Cells in Chronic Lymphocytic Leukemia and Their Therapeutic Implications. Int. J. Mol. Sci. 2021, 22, 6665. [Google Scholar] [CrossRef]
- MacFarlane, A.W.t.; Jillab, M.; Smith, M.R.; Alpaugh, R.K.; Cole, M.E.; Litwin, S.; Millenson, M.M.; Al-Saleem, T.; Cohen, A.D.; Campbell, K.S. NK cell dysfunction in chronic lymphocytic leukemia is associated with loss of the mature cells expressing inhibitory killer cell Ig-like receptors. Oncoimmunology 2017, 6, e1330235. [Google Scholar] [CrossRef]
- Hofland, T.; Endstra, S.; Gomes, C.K.P.; de Boer, R.; de Weerdt, I.; Bobkov, V.; Riedl, J.A.; Heukers, R.; Smit, M.J.; Eldering, E.; et al. Natural Killer Cell Hypo-responsiveness in Chronic Lymphocytic Leukemia can be Circumvented In Vitro by Adequate Activating Signaling. Hemasphere 2019, 3, e308. [Google Scholar] [CrossRef] [PubMed]
- Burger, J.A.; Tedeschi, A.; Barr, P.M.; Robak, T.; Owen, C.; Ghia, P.; Bairey, O.; Hillmen, P.; Bartlett, N.L.; Li, J.; et al. Ibrutinib as Initial Therapy for Patients with Chronic Lymphocytic Leukemia. N. Engl. J. Med. 2015, 373, 2425–2437. [Google Scholar] [CrossRef]
- Skanland, S.S.; Mato, A.R. Overcoming resistance to targeted therapies in chronic lymphocytic leukemia. Blood Adv. 2021, 5, 334–343. [Google Scholar] [CrossRef]
- Solman, I.G.; Blum, L.K.; Burger, J.A.; Kipps, T.J.; Dean, J.P.; James, D.F.; Mongan, A. Impact of long-term ibrutinib treatment on circulating immune cells in previously untreated chronic lymphocytic leukemia. Leuk. Res. 2021, 102, 106520. [Google Scholar] [CrossRef] [PubMed]
- Mhibik, M.; Wiestner, A.; Sun, C. Harnessing the Effects of BTKi on T Cells for Effective Immunotherapy against CLL. Int. J. Mol. Sci. 2019, 21, 68. [Google Scholar] [CrossRef]
- Papazoglou, D.; Wang, X.V.; Shanafelt, T.D.; Lesnick, C.E.; Ioannou, N.; de Rossi, G.; Herter, S.; Bacac, M.; Klein, C.; Tallman, M.S.; et al. Ibrutinib-based therapy reinvigorates CD8+ T cells compared to chemoimmunotherapy: Immune monitoring from the E1912 trial. Blood 2024, 143, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Hallek, M.; Cheson, B.D.; Catovsky, D.; Caligaris-Cappio, F.; Dighiero, G.; Dohner, H.; Hillmen, P.; Keating, M.; Montserrat, E.; Chiorazzi, N.; et al. iwCLL guidelines for diagnosis, indications for treatment, response assessment, and supportive management of CLL. Blood 2018, 131, 2745–2760. [Google Scholar] [CrossRef]
- Stephan, P.; Perrot, J.; Voisin, A.; Barbery, M.; Andrieu, T.; Grimont, M.; Caramel, J.; Bardou, M.; Tondeur, G.; Missiaglia, E.; et al. Deep phenotyping of nodal T-cell lymphomas reveals immune alterations and therapeutic targets. Haematologica 2024. [Google Scholar] [CrossRef]
- Sakuishi, K.; Apetoh, L.; Sullivan, J.M.; Blazar, B.R.; Kuchroo, V.K.; Anderson, A.C. Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J. Exp. Med. 2010, 207, 2187–2194. [Google Scholar] [CrossRef]
- Cass, S.H.; Tobin, J.W.D.; Seo, Y.D.; Gener-Ricos, G.; Keung, E.Z.; Burton, E.M.; Davies, M.A.; McQuade, J.L.; Lazar, A.J.; Mason, R.; et al. Efficacy of immune checkpoint inhibitors for the treatment of advanced melanoma in patients with concomitant chronic lymphocytic leukemia. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2023, 34, 796–805. [Google Scholar] [CrossRef] [PubMed]
- Smithy, J.W.; Pianko, M.J.; Maher, C.; Postow, M.A.; Shoushtari, A.N.; Momtaz, P.; Chapman, P.B.; Wolchok, J.D.; Park, J.H.; Callahan, M.K. Checkpoint Blockade in Melanoma Patients with Underlying Chronic Lymphocytic Leukemia. J. Immunother. 2021, 44, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Perutelli, F.; Jones, R.; Griggio, V.; Vitale, C.; Coscia, M. Immunotherapeutic Strategies in Chronic Lymphocytic Leukemia: Advances and Challenges. Front. Oncol. 2022, 12, 837531. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.; LaPlant, B.R.; Call, T.G.; Parikh, S.A.; Leis, J.F.; He, R.; Shanafelt, T.D.; Sinha, S.; Le-Rademacher, J.; Feldman, A.L.; et al. Pembrolizumab in patients with CLL and Richter transformation or with relapsed CLL. Blood 2017, 129, 3419–3427. [Google Scholar] [CrossRef]
- Pula, B.; Golos, A.; Gorniak, P.; Jamroziak, K. Overcoming Ibrutinib Resistance in Chronic Lymphocytic Leukemia. Cancers 2019, 11, 1834. [Google Scholar] [CrossRef]
- Solman, I.G.; Blum, L.K.; Hoh, H.Y.; Kipps, T.J.; Burger, J.A.; Barrientos, J.C.; O’Brien, S.; Mulligan, S.P.; Kay, N.E.; Hillmen, P.; et al. Ibrutinib restores immune cell numbers and function in first-line and relapsed/refractory chronic lymphocytic leukemia. Leuk. Res. 2020, 97, 106432. [Google Scholar] [CrossRef]
- Cadot, S.; Valle, C.; Tosolini, M.; Pont, F.; Largeaud, L.; Laurent, C.; Fournie, J.J.; Ysebaert, L.; Quillet-Mary, A. Longitudinal CITE-Seq profiling of chronic lymphocytic leukemia during ibrutinib treatment: Evolution of leukemic and immune cells at relapse. Biomark. Res. 2020, 8, 72. [Google Scholar] [CrossRef]
- Le Saos-Patrinos, C.; Loizon, S.; Zouine, A.; Turpin, D.; Dilhuydy, M.S.; Blanco, P.; Sisirak, V.; Forcade, E.; Duluc, D. Elevated levels of circulatory follicular T helper cells in chronic lymphocytic leukemia contribute to B cell expansion. J. Leukoc. Biol. 2023, 113, 305–314. [Google Scholar] [CrossRef]
- Wu, X.; Fajardo-Despaigne, J.E.; Zhang, C.; Neppalli, V.; Banerji, V.; Johnston, J.B.; Gibson, S.B.; Marshall, A.J. Altered T Follicular Helper Cell Subsets and Function in Chronic Lymphocytic Leukemia. Front. Oncol. 2021, 11, 674492. [Google Scholar] [CrossRef]
- Chavez, J.C.; Foss, F.M.; William, B.M.; Brammer, J.E.; Smith, S.M.; Prica, A.; Zain, J.M.; Tuscano, J.M.; Shah, H.; Mehta-Shah, N.; et al. Targeting the Inducible T-cell Costimulator (ICOS) in Patients with Relapsed/Refractory T-follicular Helper Phenotype Peripheral T-cell and Angioimmunoblastic T-cell Lymphoma. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2023, 29, 1869–1878. [Google Scholar] [CrossRef]
- Kater, A.P.; Arslan, O.; Demirkan, F.; Herishanu, Y.; Ferhanoglu, B.; Diaz, M.G.; Leber, B.; Montillo, M.; Panayiotidis, P.; Rossi, D.; et al. Activity of venetoclax in patients with relapsed or refractory chronic lymphocytic leukaemia: Analysis of the VENICE-1 multicentre, open-label, single-arm, phase 3b trial. Lancet Oncol. 2024, 25, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Todorovic, Z.; Todorovic, D.; Markovic, V.; Ladjevac, N.; Zdravkovic, N.; Djurdjevic, P.; Arsenijevic, N.; Milovanovic, M.; Arsenijevic, A.; Milovanovic, J. CAR T Cell Therapy for Chronic Lymphocytic Leukemia: Successes and Shortcomings. Curr. Oncol. 2022, 29, 3647–3657. [Google Scholar] [CrossRef] [PubMed]


| Patient ID | Sex | Age (yrs) | TP53 Mutation (Yes/No) | Cytogenetic Status | IGHV Mutation Status | # of Previous Treatment Lines | Disease Status | Months under Ibrutinib Therapy |
|---|---|---|---|---|---|---|---|---|
| 1 | M | 52 | No | del11q | mutated | 2 | CR | 84 |
| 2 | M | 73 | Yes | del17p | unmutated | 1 | CR | 83 |
| 3 | M | 68 | No | None | unmutated | 1 | CR | 36 |
| 4 | F | 77 | No | None | unmutated | 0 | CR | 36 |
| 5 | F | 68 | No | None | unmutated | 0 | CR | 60 |
| 6 | M | 72 | No | None | unmutated | 1 | CR | 48 |
| 7 | F | 80 | No | None | unmutated | 0 | CR | 24 |
| 8 | F | 67 | No | None | unmutated | 0 | CR | 24 |
| 9 | M | 79 | No | None | unmutated | 1 | CR | 24 |
| 10 | F | 62 | No | None | unmutated | 1 | CR | 48 |
| 12 | F | 84 | Yes | del17p, complex karyotype | unmutated | 1 | Relapse | 12 |
| 13 | M | 61 | Yes | del17p, complex karyotype | unmutated | 2 | Relapse | 36 |
| 14 | F | 45 | No | del11q | unmutated | 1 | Relapse | 48 |
| 16 | M | 76 | No | None | unmutated | 1 | Relapse | 24 |
| 17 | F | 89 | No | None | unmutated | 1 | Relapse | 36 |
| 18 | M | 51 | No | None | unmutated | 1 | Relapse | 72 |
| 19 | M | 67 | Yes | del17p, complex karyotype | unmutated | 0 | Relapse | 60 |
| 20 | M | 67 | No | del11q | unmutated | 1 | Relapse | 48 |
| Patient ID | Disease Status | Months under Ibrutinib Therapy | BTK Mutation | Bulky Disease | Lymphocyte Count (G/L) | Months between Relapse Diagnosis and Sampling |
|---|---|---|---|---|---|---|
| 12 | Relapse | 12 | N/A | No | 23.8 | 1 |
| 13 | Relapse | 36 | C481S | No | 13.3 | 2 |
| 14 | Relapse | 48 | C481S | No | 7 | 2 |
| 16 | Relapse | 24 | N/A | No | 20.39 | 5 |
| 17 | Relapse | 36 | N/A | No | 28.6 | 24 |
| 18 | Relapse | 72 | C481S | No | 54 | 1 |
| 19 | Relapse | 60 | C481S | No | 11.7 | 1 |
| 20 | Relapse | 48 | None | No | 8.22 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stéphan, P.; Bouherrou, K.; Guillermin, Y.; Michallet, A.-S.; Grinberg-Bleyer, Y. Immunophenotyping of Peripheral Blood Cells in Patients with Chronic Lymphocytic Leukemia Treated with Ibrutinib. Cells 2024, 13, 1458. https://doi.org/10.3390/cells13171458
Stéphan P, Bouherrou K, Guillermin Y, Michallet A-S, Grinberg-Bleyer Y. Immunophenotyping of Peripheral Blood Cells in Patients with Chronic Lymphocytic Leukemia Treated with Ibrutinib. Cells. 2024; 13(17):1458. https://doi.org/10.3390/cells13171458
Chicago/Turabian StyleStéphan, Pierre, Khaled Bouherrou, Yann Guillermin, Anne-Sophie Michallet, and Yenkel Grinberg-Bleyer. 2024. "Immunophenotyping of Peripheral Blood Cells in Patients with Chronic Lymphocytic Leukemia Treated with Ibrutinib" Cells 13, no. 17: 1458. https://doi.org/10.3390/cells13171458






