Spectral Algal Fingerprinting and Long Sequencing in Synthetic Algal–Microbial Communities
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation and Instrument Setup
2.2. Autofluorescence Finder Tool and Virtual Filters Setup
2.3. Data Analysis
2.4. Sequencing Library Preparation and Bioinformatic Analysis
3. Results
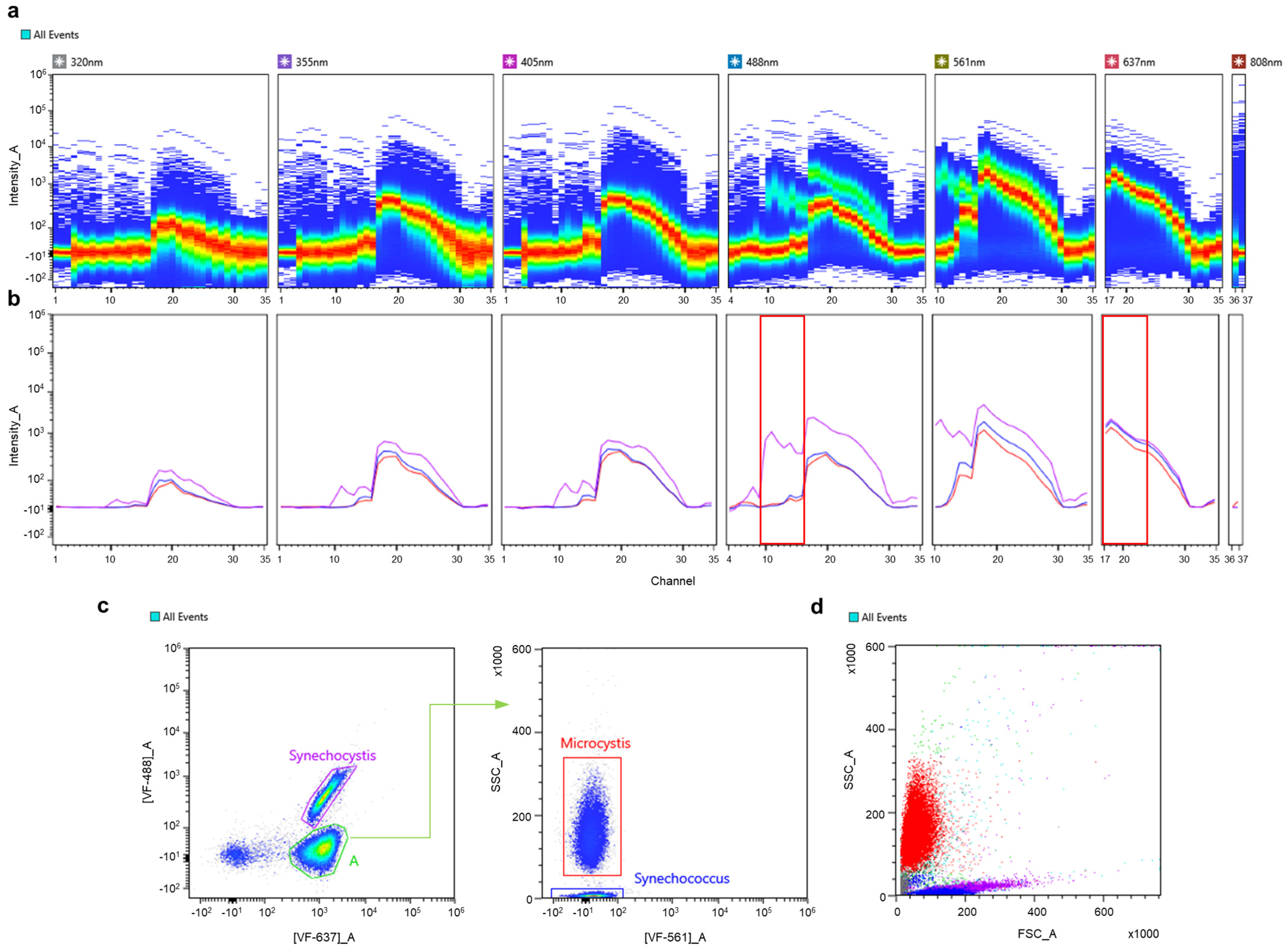
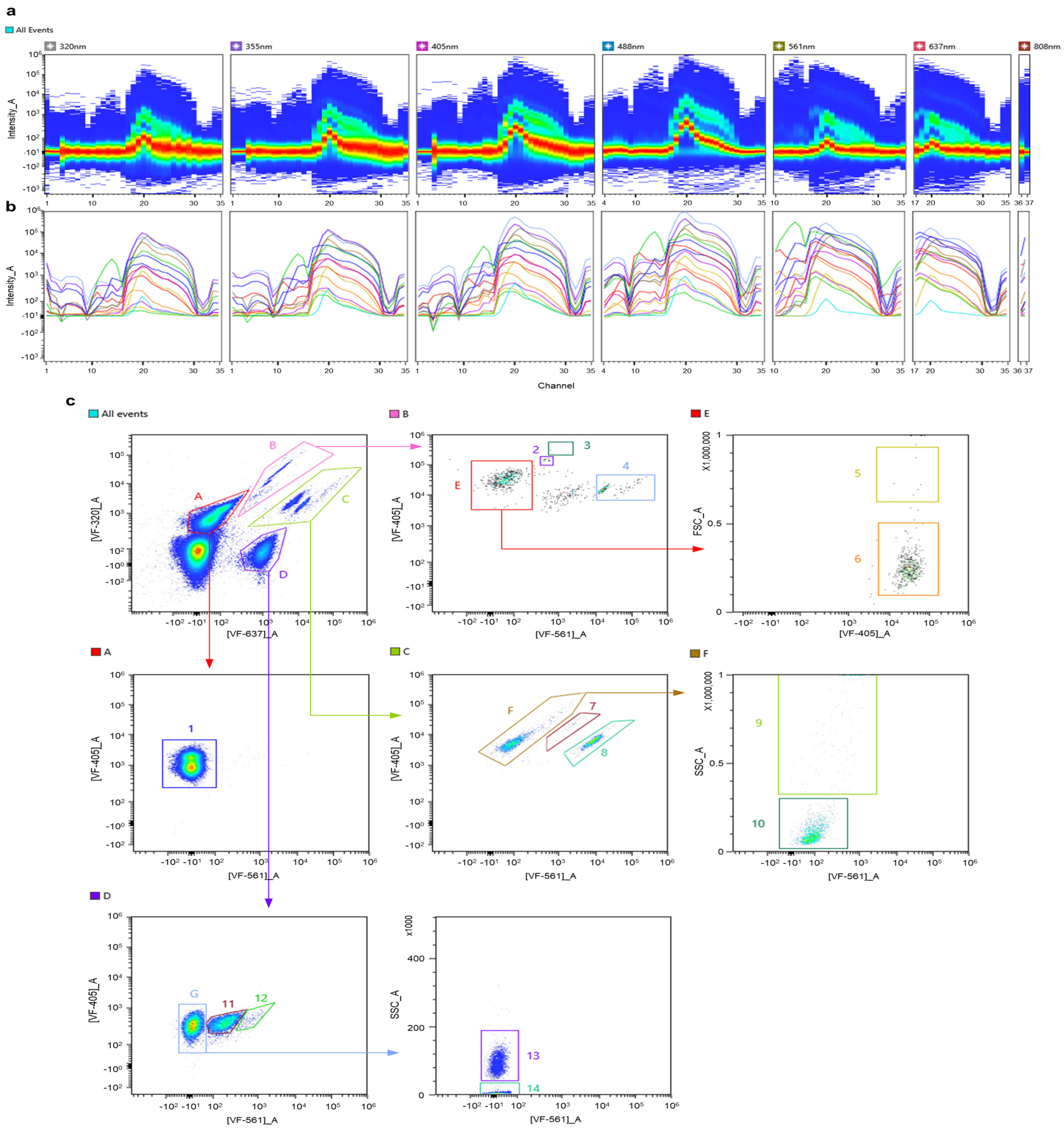
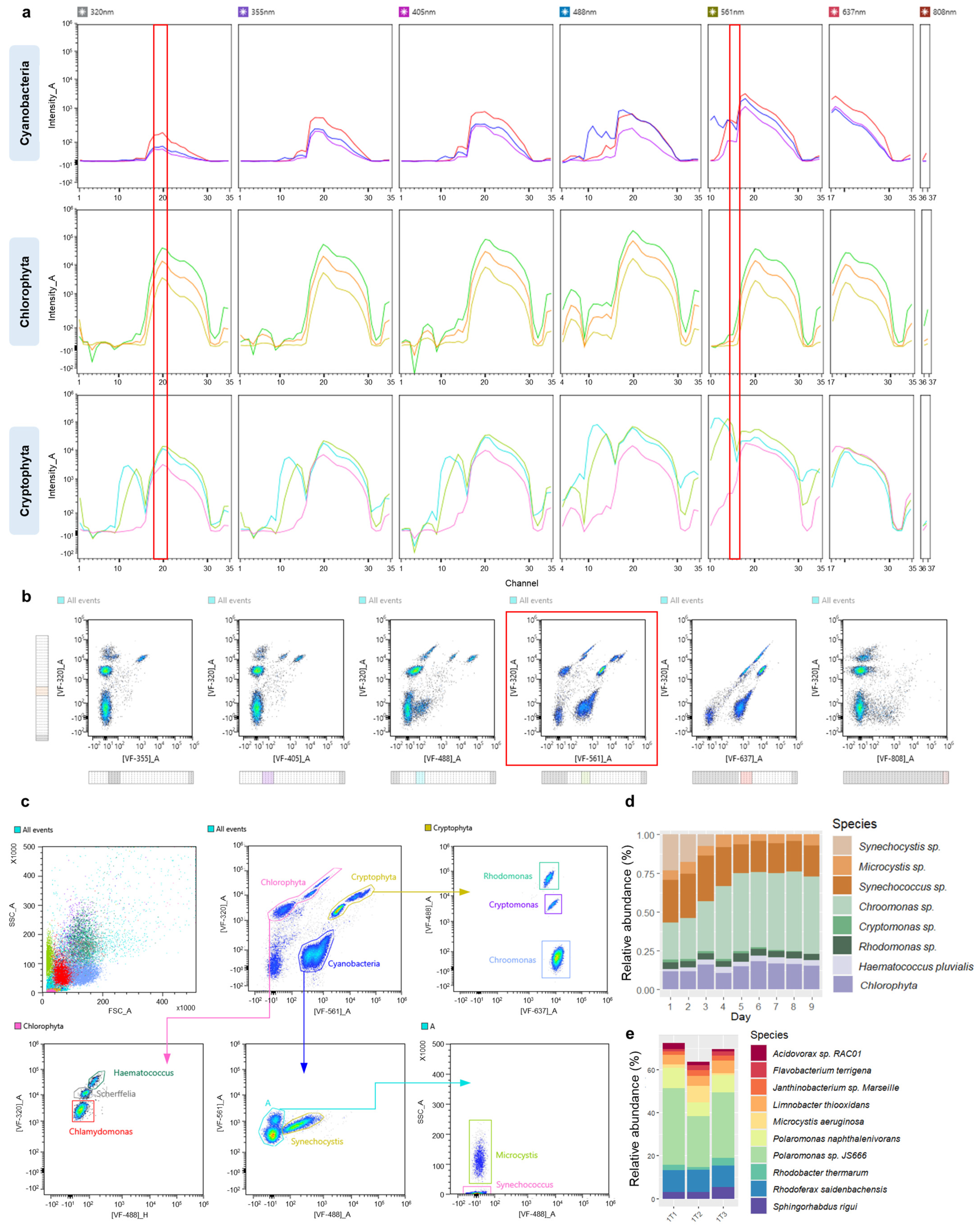
3.1. Construction of a Spectral Signature Library for Phytoplankton Groups
3.2. Autofluorescence-Based Discrimination of Multi-Component Suspensions
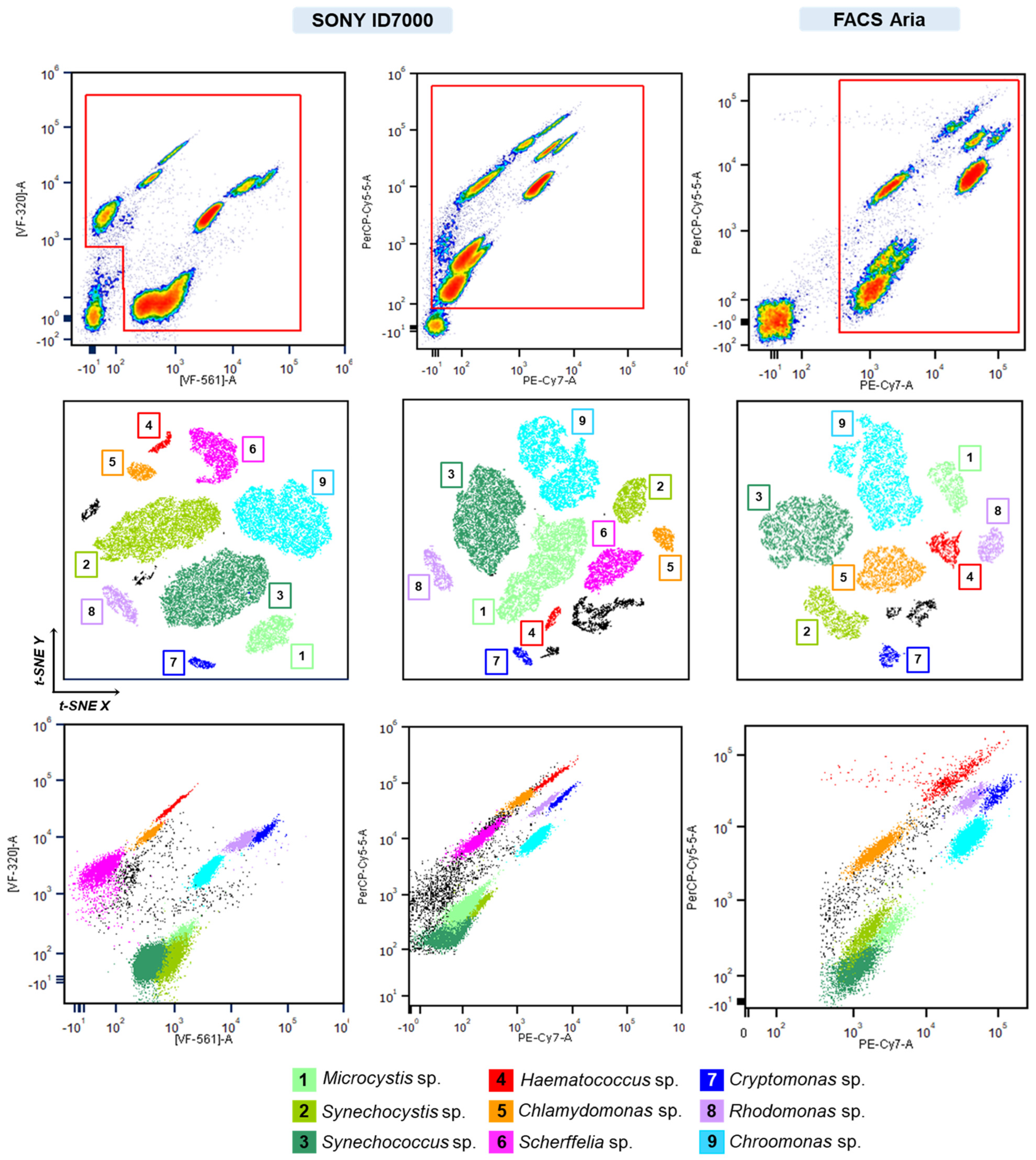
3.3. Parallel Monitoring of Synthetic Phytoplankton Communities Using Full-Spectrum Cytometry and Long Sequencing
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Deng, Y.; Mauri, M.; Vallet, M.; Staudinger, M.; Allen, R.J.; Pohnert, G. Dynamic Diatom-Bacteria Consortia in Synthetic Plankton Communities. Appl. Environ. Microbiol. 2022, 88, e01619–e01622. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.; Uchimiya, M.; Gore, J.; Moran, M.A. Ecological Drivers of Bacterial Community Assembly in Synthetic Phycospheres. Proc. Natl. Acad. Sci. USA 2020, 117, 3656–3662. [Google Scholar] [CrossRef]
- Hartwell, L.H.; Hopfield, J.J.; Leibler, S.; Murray, A.W. From Molecular to Modular Cell Biology. Nature 1999, 402, C47–C52. [Google Scholar] [CrossRef] [PubMed]
- Andersen, R.A. Algal Culturing Techniques; Elsevier: Amsterdam, The Netherlands, 2005. [Google Scholar]
- De-Bashan, L.E.; Bashan, Y.; Moreno, M.; Lebsky, V.K.; Bustillos, J.J. Increased Pigment and Lipid Content, Lipid Variety, and Cell and Population Size of the Microalgae Chlorella Spp. When Co-Immobilized in Alginate Beads with the Microalgae-Growth-Promoting Bacterium Azospirillum Brasilense. Can. J. Microbiol. 2002, 48, 514–521. [Google Scholar] [CrossRef] [PubMed]
- Cho, D.-H.; Ramanan, R.; Heo, J.; Lee, J.; Kim, B.-H.; Oh, H.-M.; Kim, H.-S. Enhancing Microalgal Biomass Productivity by Engineering a Microalgal–Bacterial Community. Bioresour. Technol. 2015, 175, 578–585. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, Y.; Yang, Y.; Zhou, D. The Role of Starvation in Biomass Harvesting and Lipid Accumulation: Co-culture of Microalgae–Bacteria in Synthetic Wastewater. Environ. Prog. Sustain. Energy 2016, 35, 103–109. [Google Scholar] [CrossRef]
- Segev, E.; Wyche, T.P.; Kim, K.H.; Petersen, J.; Ellebrandt, C.; Vlamakis, H.; Barteneva, N.; Paulson, J.N.; Chai, L.; Clardy, J.; et al. Dynamic Metabolic Exchange Governs a Marine Algal-Bacterial Interaction. eLife 2016, 5, e17473. [Google Scholar] [CrossRef]
- Kolter, R. Asking a Question. J. Bacteriol. 2024, 206, e00050-24. [Google Scholar] [CrossRef]
- Castle, S.D.; Grierson, C.S.; Gorochowski, T.E. Towards an Engineering Theory of Evolution. Nat. Commun. 2021, 12, 3326. [Google Scholar] [CrossRef]
- Brenner, K.; You, L.; Arnold, F.H. Engineering Microbial Consortia: A New Frontier in Synthetic Biology. Trends Biotechnol. 2008, 26, 483–489. [Google Scholar] [CrossRef]
- Perera, I.A.; Abinandan, S.; Subashchandrabose, S.R.; Venkateswarlu, K.; Naidu, R.; Megharaj, M. Advances in the Technologies for Studying Consortia of Bacteria and Cyanobacteria/Microalgae in Wastewaters. Crit. Rev. Biotechnol. 2019, 39, 709–731. [Google Scholar] [CrossRef] [PubMed]
- Curry, K.D.; Wang, Q.; Nute, M.G.; Tyshaieva, A.; Reeves, E.; Soriano, S.; Wu, Q.; Graeber, E.; Finzer, P.; Mendling, W.; et al. Emu: Species-Level Microbial Community Profiling of Full-Length 16S rRNA Oxford Nanopore Sequencing Data. Nat. Methods 2022, 19, 845–853. [Google Scholar] [CrossRef] [PubMed]
- Meirkhanova, A.; Zhumakhanova, A.; Len, P.; Schoenbach, C.; Levi, E.E.; Jeppesen, E.; Davidson, T.A.; Barteneva, N.S. Dynamics of Associated Microbiomes during Algal Bloom Development: To See and to Be Seeing. bioRxiv 2023, 2023-09. [Google Scholar] [CrossRef]
- Poryvkina, L.; Babichenko, S.; Kaitala, S.; Kuosa, H.; Shalapjonok, A. Spectral Fluorescence Signatures in the Characterization of Phytoplankton Community Composition. J. Plankton Res. 1994, 16, 1315–1327. [Google Scholar] [CrossRef]
- Falkowski, P.G.; Oliver, M.J. Mix and Match: How Climate Selects Phytoplankton. Nat. Rev. Microbiol. 2007, 5, 813–819. [Google Scholar] [CrossRef]
- Roy, S.; Llewellyn, C.A.; Egeland, E.S.; Johnsen, G. (Eds.) Phytoplankton Pigments: Characterization, Chemotaxonomy and Applications in Oceanography; Cambridge University Press: Cambridge, UK, 2011. [Google Scholar]
- Swan, C.M.; Vogt, M.; Gruber, N.; Laufkoetter, C. A Global Seasonal Surface Ocean Climatology of Phytoplankton Types Based on CHEMTAX Analysis of HPLC Pigments. Deep Sea Res. Part I Oceanogr. Res. Pap. 2016, 109, 137–156. [Google Scholar] [CrossRef]
- Liu, J.-Y.; Zeng, L.-H.; Ren, Z.-H. The Application of Spectroscopy Technology in the Monitoring of Microalgae Cells Concentration. Appl. Spectrosc. Rev. 2021, 56, 171–192. [Google Scholar] [CrossRef]
- Catlett, D.; Siegel, D. Phytoplankton Pigment Communities Can Be Modeled Using Unique Relationships with Spectral Absorption Signatures in a Dynamic Coastal Environment. J. Geophys. Res. Oceans 2018, 123, 246–264. [Google Scholar] [CrossRef]
- Kramer, S.J.; Siegel, D.A. How Can Phytoplankton Pigments Be Best Used to Characterize Surface Ocean Phytoplankton Groups for Ocean Color Remote Sensing Algorithms? J. Geophys. Res. Oceans 2019, 124, 7557–7574. [Google Scholar] [CrossRef]
- Hochberg, E.J.; Atkinson, M.J. Capabilities of Remote Sensors to Classify Coral, Algae, and Sand as Pure and Mixed Spectra. Remote Sens. Environ. 2003, 85, 174–189. [Google Scholar] [CrossRef]
- Rodríguez, Y.C.; Gómez, J.D.; Sánchez-Carnero, N.; Rodríguez-Pérez, D. A Comparison of Spectral Macroalgae Taxa Separability Methods Using an Extensive Spectral Library. Algal Res. 2017, 26, 463–473. [Google Scholar] [CrossRef]
- Cawse-Nicholson, K.; Townsend, P.A.; Schimel, D.; Assiri, A.M.; Blake, P.L.; Buongiorno, M.F.; Campbell, P.; Carmon, N.; Casey, K.A.; Correa-Pabón, R.E.; et al. NASA’s Surface Biology and Geology Designated Observable: A Perspective on Surface Imaging Algorithms. Remote Sens. Environ. 2021, 257, 112349. [Google Scholar] [CrossRef]
- Futamura, K.; Sekino, M.; Hata, A.; Ikebuchi, R.; Nakanishi, Y.; Egawa, G.; Kabashima, K.; Watanabe, T.; Furuki, M.; Tomura, M. Novel Full-spectral Flow Cytometry with Multiple Spectrally-adjacent Fluorescent Proteins and Fluorochromes and Visualization of in Vivo Cellular Movement. Cytom. Part A 2015, 87, 830–842. [Google Scholar] [CrossRef] [PubMed]
- Robinson, J.P. Flow Cytometry: Past and Future. Biotechniques 2022, 72, 159–169. [Google Scholar] [CrossRef]
- Dott, T.; Culina, S.; Chemali, R.; Mansour, C.A.; Dubois, F.; Jagla, B.; Doisne, J.M.; Rogge, L.; Huetz, F.; Jönsson, F.; et al. Standardized High-dimensional Spectral Cytometry Protocol and Panels for Whole Blood Immune Phenotyping in Clinical and Translational Studies. Cytom. Part A 2024, 105, 124–138. [Google Scholar] [CrossRef]
- Barteneva, N.S.; Dashkova, V.; Vorobjev, I. Probing Complexity of Microalgae Mixtures with Novel Spectral Flow Cytometry Approach and “Virtual Filtering”. bioRxiv 2019, 516146. [Google Scholar] [CrossRef]
- Barteneva, N.S.; Kussanova, A.; Dashkova, V.; Meirkhanova, A.; Vorobjev, I.A. Using Virtual Filtering Approach to Discriminate Microalgae by Spectral Flow Cytometer. In Spectral and Imaging Cytometry: Methods and Protocols; Springer: New York, NY, USA, 2023; pp. 23–40. [Google Scholar]
- Jeppesen, E.; Søndergaard, M.; Jensen, J.P.; Havens, K.E.; Anneville, O.; Carvalho, L.; Coveney, M.F.; Deneke, R.; Dokulil, M.T.; Foy, B.; et al. Lake Responses to Reduced Nutrient Loading—An Analysis of Contemporary Long-term Data from 35 Case Studies. Freshwater Biol. 2005, 50, 1747–1771. [Google Scholar] [CrossRef]
- Wanner, N.; Barnhart, J.; Apostolakis, N.; Zlojutro, V.; Asosingh, K. Using the Autofluorescence Finder on the Sony ID7000TM Spectral Cell Analyzer to Identify and Unmix Multiple Highly Autofluorescent Murine Lung Populations. Front. Bioeng. Biotechnol. 2022, 10, 827987. [Google Scholar] [CrossRef]
- Geng, H.; Sale, K.L.; Tran-Gyamfi, M.B.; Lane, T.W.; Yu, E.T. Longitudinal Analysis of Microbiota in Microalga Nannochloropsis Salina Cultures. Microb. Ecol. 2016, 72, 14–24. [Google Scholar] [CrossRef]
- Barteneva, N.S.; Vorobjev, I.A. (Eds.) Spectral and Imaging Cytometry: Methods and Protocols; Springer: New York, NY, USA, 2023. [Google Scholar]
- Frank, J.A.; Reich, C.I.; Sharma, S.; Weisbaum, J.S.; Wilson, B.A.; Olsen, G.J. Critical Evaluation of Two Primers Commonly Used for Amplification of Bacterial 16S rRNA Genes. Appl. Environ. Microbiol. 2008, 74, 2461–2470. [Google Scholar] [CrossRef]
- Mao, D.P.; Zhou, Q.; Chen, C.Y.; Quan, Z.X. Coverage evaluation of Universal Bacterial Primers Using the metagenomic datasets. BMC Microbiol. 2012, 12, 66. [Google Scholar] [CrossRef] [PubMed]
- Len, P.; Meirkhanova, A.; Nugumanova, G.; Cestaro, A.; Jeppesen, E.; Vorobjev, I.A.; Donati, C.; Barteneva, N.S. Species-Level Classification Provides New Insights into the Biogeographical Patterns of Microbial Communities in Shallow Saline Lakes. bioRxiv 2023, 2023-12. [Google Scholar] [CrossRef]
- Trask, B.J.; Van den Engh, G.J.; Elgershuizen, J.H.B.W. Analysis of Phytoplankton by Flow Cytometry. Cytometry 1982, 2, 258–264. [Google Scholar] [CrossRef]
- Yentsch, C.M.; Horan, P.K.; Muirhead, K.; Dortch, Q.; Haugen, E.; Legendre, L.; Murphy, L.S.; Perry, M.J.; Phinney, D.A.; Pomponi, S.A.; et al. Flow Cytometry and Cell Sorting: A Technique for Analysis and Sorting of Aquatic Particles. Limnol. Oceanogr. 1983, 28, 1275–1280. [Google Scholar] [CrossRef]
- Sosik, H.M.; Olson, R.J.; Armbrust, E.V. Flow Cytometry in Phytoplankton Research. In Chlorophyll a Fluorescence in Aquatic Sciences: Methods and Applications; Springer: Dordrecht, The Netherlands, 2010; pp. 171–185. [Google Scholar]
- Paau, A.S.; Oro, J.; Cowles, J.R. Application of Flow Microflorometry to the Study of Algal Cells and Isolated Chloroplasts. J. Exp. Bot. 1978, 29, 1011–1020. [Google Scholar] [CrossRef]
- Hutter, K.J.; Eipel, H.E. Flow Cytometric Determinations of Cellular Substances in Algae, Bacteria, Moulds and Yeasts. Antonie Leeuwenhoek 1978, 44, 269–282. [Google Scholar] [CrossRef]
- Phinney, D.A.; Cucci, T.L. Flow Cytometry and Phytoplankton. Cytometry 1989, 10, 511–521. [Google Scholar] [CrossRef]
- Li, W.K.W.; Dickie, P.M. Monitoring Phytoplankton, Bacterioplankton, and Virioplankton in a Coastal Inlet (Bedford Basin) by Flow Cytometry. Cytometry 2001, 44, 236–246. [Google Scholar] [CrossRef]
- Ning, M.; Li, H.; Xu, Z.; Chen, L.; He, Y. Picophytoplankton Identification by Flow Cytometry and High-Throughput Sequencing in a Clean Reservoir. Ecotoxicol. Environ. Saf. 2021, 216, 112216. [Google Scholar] [CrossRef]
- Robicheau, B.M.; Tolman, J.; Bertrand, E.M.; LaRoche, J. Highly-resolved Interannual Phytoplankton Community Dynamics of the Coastal Northwest Atlantic. ISME Commun. 2022, 2, 38. [Google Scholar] [CrossRef]
- Vorobjev, I.A.; Kussanova, A.; Barteneva, N.S. Development of Spectral Imaging Cytometry. In Spectral and Imaging Cytometry: Methods and Protocols; Springer: New York, NY, USA, 2023; pp. 3–22. [Google Scholar]
- Culverhouse, P.F. Human and Machine Factors in Algae Monitoring Performance. Ecol. Inform. 2007, 2, 361–366. [Google Scholar] [CrossRef]
- Willén, E. Phytoplankton in Water Quality Assessment–an Indicator Concept. In Hydrological and Limnological Aspects of Lake Monitring; John Wiley & Sons: Chichester, UK, 2000; pp. 58–80. [Google Scholar]
- Catherine, A.; Escoffier, N.; Belhocine, A.; Nasri, A.B.; Hamlaoui, S.; Yéprémian, C.; Bernard, C.; Troussellier, M. On the Use of the FluoroProbe®, a Phytoplankton Quantification Method Based on Fluorescence Excitation Spectra for Large-Scale Surveys of Lakes and Reservoirs. Water Res. 2012, 46, 1771–1784. [Google Scholar] [CrossRef] [PubMed]
- Tamm, M.; Freiberg, R.; Tõnno, I.; Nõges, P.; Nõges, T. Pigment-based Chemotaxonomy-a Quick Alternative to Determine Algal Assemblages in Large Shallow Eutrophic Lake? PLoS ONE 2015, 10, e0122526. [Google Scholar] [CrossRef] [PubMed]
- Simmons, L.J.; Sandgren, C.D.; Berges, J.A. Problems and Pitfalls in Using HPLC Pigment Analysis to Distinguish Lake Michigan Phytoplankton Taxa. J. Great Lakes Res. 2016, 42, 397–404. [Google Scholar] [CrossRef]
- Havskum, H.; Schlüter, L.; Scharek, R.; Berdalet, E.; Jacquet, S. Routine quantification of phytoplankton groups microscopy or pigment analyses? Mar. Ecol. Prog. Ser. 2004, 273, 31–42. [Google Scholar] [CrossRef]
- Jameson, V.J.; Luke, T.; Yan, Y.; Hind, A.; Evrard, M.; Man, K.; Mackay, L.K.; Kallies, A.; Villadangos, J.A.; McWilliam, H.E.G.; et al. Unlocking Autofluorescence in the Era of Full Spectrum Analysis: Implications for Immunophenotype Discovery Projects. Cytom. Part A 2022, 101, 922–941. [Google Scholar] [CrossRef]
- Sommeria-Klein, G.; Watteaux, R.; Ibarbalz, F.M.; Pierella Karlusich, J.J.; Iudicone, D.; Bowler, C.; Morlon, H. Global Drivers of Eukaryotic Plankton Biogeography in the Sunlit Ocean. Science 2021, 374, 594–599. [Google Scholar] [CrossRef]
- Camp, B.; Jorde, I.; Sittel, F.; Pausder, A.; Jeron, A.; Bruder, D.; Schreiber, J.; Stegemann-Koniszewski, S. Comprehensive Analysis of Lung Macrophages and Dendritic Cells in Two Murine Models of Allergic Airway Inflammation Reveals Model- and Subset-Specific Accumulation and Phenotypic Alterations. Front. Immunol. 2024, 15, 1374670. [Google Scholar] [CrossRef]
- Bourdely, P.; Petti, L.; Khou, S.; Meghraoui-Kheddar, A.; Elaldi, R.; Cazareth, J.; Mossadegh-Keller, N.; Boyer, J.; Sieweke, M.H.; Poissonnet, G.; et al. Autofluorescence Identifies Highly Phagocytic Tissue-resident Macrophages in Mouse and Human Skin and Cutaneous Squamous Cell Carcinoma. Front. Immunol. 2022, 13, 903069. [Google Scholar] [CrossRef]
- Schmutz, S.; Commere, P.H.; Montcuquet, N.; Cumano, A.; Ait-Mansour, C.; Novault, S.; Hasan, M. Beyond 40 fluorescent probes for deep phenotyping of blood mononuclear cells, using spectral technology. Front. Immunol. 2024, 15, 1285215. [Google Scholar] [CrossRef]
- Peixoto, M.M.; Soares-da-Silva, F.; Schmutz, S.; Mailhe, M.; Novault, S.; Cumano, A.; Ait-Mansour, C. Identification of Fetal Liver Stroma in Spectral Cytometry Using the Parameter Autofluorescence. Cytom. Part A 2022, 101, 960–969. [Google Scholar] [CrossRef]
- Dubelaar, G.B.; Groenewegen, A.C.; Stokdijk, W.; Van Den Engh, G.; Visser, J.W. Optical Plankton Analyser: A Flow Cytometer for Plankton Analysis, II: Specifications. Cytometry 1989, 10, 529–539. [Google Scholar] [CrossRef] [PubMed]
- Sieracki, C.K.; Sieracki, M.E.; Yentsch, C.S. An Imaging-in-Flow System for Automated Analysis of Marine Microplankton. Mar. Ecol. Prog. Ser. 1998, 168, 285–296. [Google Scholar] [CrossRef]
- Dubelaar, G.; Gerritzen, P. CytoBuoy: A Step Forward towards Using Flow Cytometry in Operational Oceanography. Sci. Mar. 2000, 64, 255–265. [Google Scholar] [CrossRef]
- Matsuo, Y.; Komiya, S.; Yasumizu, Y.; Yasuoka, Y.; Mizushima, K.; Takagi, T.; Kryukov, K.; Fukuda, A.; Morimoto, Y.; Naito, Y.; et al. Full-length 16S rRNA Gene Amplicon Analysis of Human Gut Microbiota using MinION™ Nanopore Sequencing Confers Species-level Resolution. BMC Microbiol. 2021, 21, 35. [Google Scholar] [CrossRef] [PubMed]
- Szoboszlay, M.; Schramm, L.; Pinzauti, D.; Scerri, J.; Sandionigi, A.; Biazzo, M. Nanopore is Preferable over Illumina for 16S Amplicon Sequencing of the Gut Microbiota When Species-level Taxonomic Classification, Accurate Estimation of Richness, or Focus on Rare Taxa is Required. Microorganisms 2023, 11, 804. [Google Scholar] [CrossRef]
- Li, X.; Zhou, Z.; Li, W.; Yan, Y.; Shen, X.; Wang, J.; Sun, X.; Yuan, Q. Design of Stable and Self-Regulated Microbial Consortia for Chemical Synthesis. Nat. Commun. 2022, 13, 1554. [Google Scholar] [CrossRef]
- De Roy, K.; Marzorati, M.; Van den Abbeele, P.; Van de Wiele, T.; Boon, N. Synthetic Microbial Ecosystems: An Exciting Tool to Understand and Apply Microbial Communities. Environ. Microbiol. 2014, 16, 1472–1481. [Google Scholar] [CrossRef]


| Phylum | Order | Species | Habitat | Strain Number |
|---|---|---|---|---|
| Cyanobacteria | Nostocales | Dolichospermum urugayensis | Freshwater | 2498 |
| Nodularia sphaerocarpa | Freshwater | 50.79 | ||
| Gloeobacterales | Gloeobacter violaceus | Freshwater | 35.87 | |
| Chroococcales | Microcystis sp. | Freshwater | CCAC 7146B | |
| Synechococcales | Synechococcus sp. | Freshwater | CCAC 2944B | |
| Synechocystis sp. | Freshwater | CCAC 4059B | ||
| Chlorophyta | Chlorodendrales | Scherffelia dubia | Freshwater | CCAC 1398 B |
| Chlamydomonadales | Chlamydomonas sp. | Freshwater | CCAC 4527 B | |
| Haematococcus pluvialis | Freshwater | CCAC 2072B | ||
| Chlorellales | Chlorella vulgaris | Freshwater | 211-12 | |
| Sphaeropleales | Acutodesmus obliquus | Freshwater | 22.81 | |
| Cryptophyta | Pyrenomonadales | Chroomonas sp. | Freshwater | CCAC 2614B |
| Rhodomonas sp. | Freshwater | CCAC 1479B | ||
| Cryptomonadales | Cryptomonas sp. | Freshwater | CCAC 2345B | |
| Rhodophyta | Stylonematales | Chroothece richteriana | Marine | 104.79 |
| Porphyridiales | Porphyridium cruentum cf. | Marine | CCAC 3771 B | |
| Dinophyta | Peridiniales | Peridinium sp. | Freshwater | CCAC 1612 B |
| Euglenophyta | Euglenida | Euglena sanguinea | Freshwater | CCAC 3518 B |
| Bacillariophyta | Fragilariales | Fragilaria sp. | Freshwater | CCAC 5509 B |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meirkhanova, A.; Marks, S.; Feja, N.; Vorobjev, I.A.; Barteneva, N.S. Spectral Algal Fingerprinting and Long Sequencing in Synthetic Algal–Microbial Communities. Cells 2024, 13, 1552. https://doi.org/10.3390/cells13181552
Meirkhanova A, Marks S, Feja N, Vorobjev IA, Barteneva NS. Spectral Algal Fingerprinting and Long Sequencing in Synthetic Algal–Microbial Communities. Cells. 2024; 13(18):1552. https://doi.org/10.3390/cells13181552
Chicago/Turabian StyleMeirkhanova, Ayagoz, Sabina Marks, Nicole Feja, Ivan A. Vorobjev, and Natasha S. Barteneva. 2024. "Spectral Algal Fingerprinting and Long Sequencing in Synthetic Algal–Microbial Communities" Cells 13, no. 18: 1552. https://doi.org/10.3390/cells13181552
APA StyleMeirkhanova, A., Marks, S., Feja, N., Vorobjev, I. A., & Barteneva, N. S. (2024). Spectral Algal Fingerprinting and Long Sequencing in Synthetic Algal–Microbial Communities. Cells, 13(18), 1552. https://doi.org/10.3390/cells13181552






