Natural Autophagy Activators to Fight Age-Related Diseases
Abstract
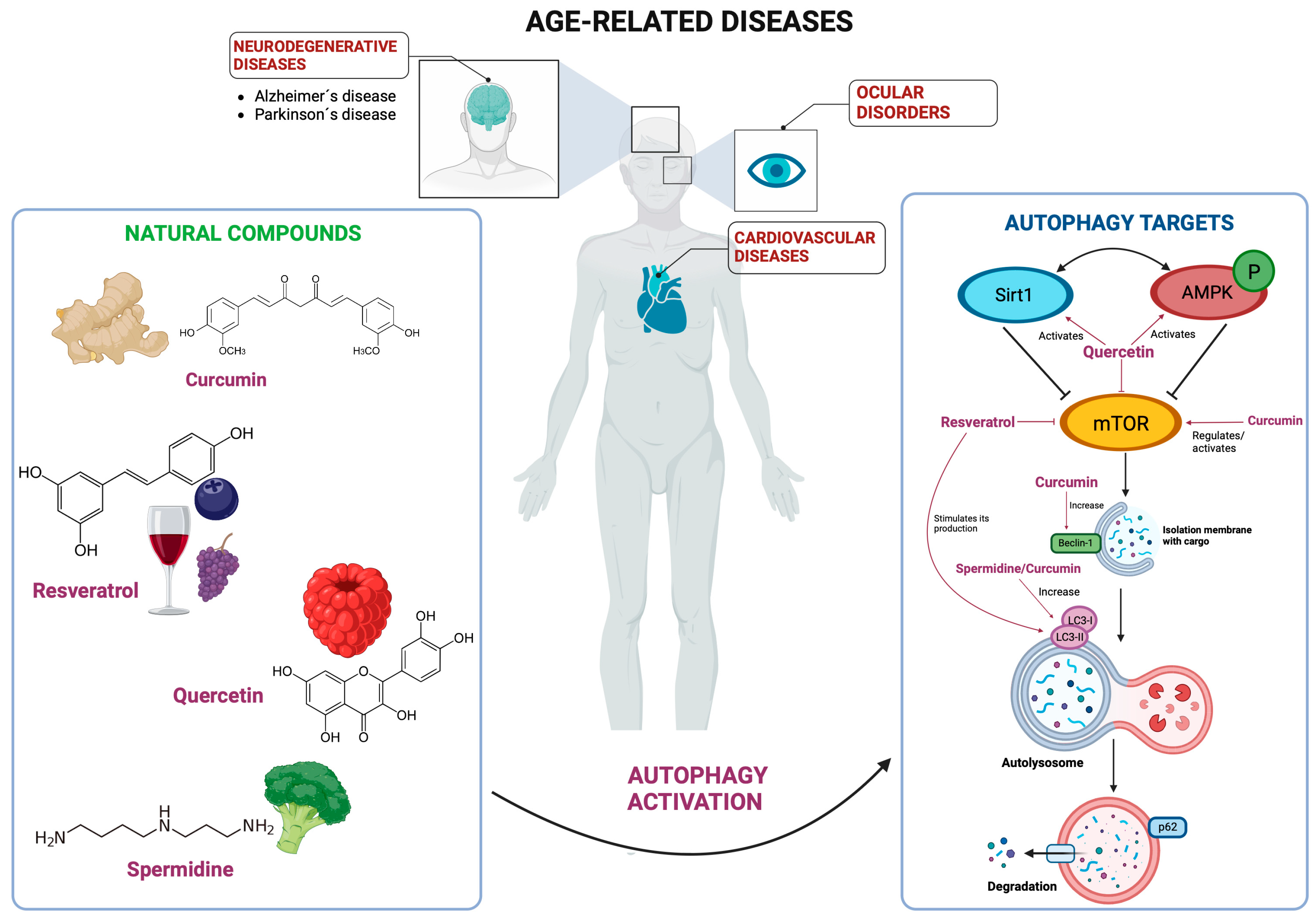
:1. Introduction
2. Cardiopathies
2.1. Curcumin
2.2. Resveratrol
2.3. Quercetin
2.4. Polyamines
3. Age-Related Neurodegenerative Diseases: Alzheimer’s and Parkinson’s
3.1. Curcumin
3.2. Resveratrol
3.3. Quercetin
3.4. Polyamines
4. Age-Related Ocular Diseases: Cataracts
4.1. Curcumin
4.2. Resveratrol
4.3. Quercetin
4.4. Polyamines
5. Natural Autophagy Activators in Other ARDs
6. Conclusions
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
Glossary
| Abbreviation | Meaning |
| Abl | Abelson kinase |
| AD | Alzheimer’s disease |
| AKT | Alpha serine-threonine protein kinase |
| AMBRA1 | Activating molecule in Beclin1-regulated autophagy protein 1 |
| AMD | Age-related macular degeneration |
| AMPK | 5′-adenosine monophosphate (AMP)-activated protein kinase |
| APP | Amyloid precursor protein |
| ARDs | Age-related diseases |
| Atg | Agy-related proteins |
| Aβ | Amyloid-β |
| Bcl-2 | B-cell lymphoma 2 protein |
| BECN1 | Beclin 1 gene |
| C1 | curcumin analog |
| CAT | Catalase |
| CNS | Central nervous system |
| CXCL16 | CXC motif chemokine ligand 16 |
| EGFR | Epidermal growth factor |
| eIF5A | Initial Translate factor 5A Eukaryotic |
| EP300 | Histone acetyltransferase P300 |
| ERK | Extracellular signal-regulated kinase |
| FDA | Food and Drug Administration |
| FoxO | Forkhead box O proteins |
| HIIT | High intensity interval training |
| HLECs | Cultured lens epithelial cells |
| HSP70 | 70-kDa heat shock proteins |
| IGF | Insulin-like growth factor |
| JAK/STAT | Janus kinase-signal transducers and activators of transcription |
| JNK | c-jun-N-terminal kinase |
| LC3 | Microtubule-associated protein 1A/1B-light chain 3 (LC3) |
| LV | Left ventricular |
| MAPK | Mitogen-activated protein kinase |
| MPP | 1-methyl-4-phenylpyridium |
| MPTP | 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine |
| MSVI | Severe vision impairment |
| mTOR | Mammalian target of rapamycin |
| NF-kB | Nuclear factor κB |
| NFTs | Neurofibrillary tangles |
| NMDA | N-methyl-D-aspartate |
| NRF2 | Nuclear factor erythroid 2-related factor 2 |
| Ox-LDL | Oxidized Low-Density lipoprotein |
| P62/SQSTM1 | Sequestosome 1 (SQSTM1)/p62 |
| PAs | Polyamines |
| PD | Parkinson´s disease |
| PdNPs | Palladium nanomaterial |
| PI3K | Phosphoinositide 3-kinase |
| PINK1 | PTEN-induced putative kinase 1 |
| PKA | Protein kinase A |
| p-TBK1 | phospho-TANK-binding kinase 1 |
| RPE | Retinal pigment epithelium |
| Sirt1 | Silent information regulator 1 |
| SNAP29 | synaptosome associated protein 29 |
| SNARE | Soluble N-ethylmaleimide-sensitive factor attachment protein receptor |
| SNCA | Synuclein alpha gene |
| SNHG1 | small nucleolar RNA host gene 1 |
| SOD | Superoxide dismutase |
| STX | Syntaxin 17 |
| TBC1D20 | TBC1 domain family, member 20 |
| TFEB | Transcriptional Factor EB |
| THC | Tetrahydrocurcumin |
| TRAP | Translocon-associated protein |
| ULK | Ubiquitin-like-kinase |
| UPS | Ubiquitin-proteasome system |
| UVRAG | UV Radiation Resistance Associated |
| VAMP8 | Vesicle associated membrane protein 8 |
| VPS | Vacuolar protein sorting |
References
- United Nations Department of Economic; Social Affairs. World Social Report 2023; United Nations: New York, NY, USA, 2023. [Google Scholar]
- Chang, A.Y.; Skirbekk, V.F.; Tyrovolas, S.; Kassebaum, N.J.; Dieleman, J.L. Measuring population ageing: An analysis of the Global Burden of Disease Study 2017. Lancet Public Health 2019, 4, e159–e167. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, T.; Zulfiqar, A.; Ishaq, S. Age-Related Diseases. In Nutrients and Nutraceuticals for Active & Healthy Ageing; Nabavi, S.M., D’Onofrio, G., Nabavi, S.F., Eds.; Springer: Singapore, 2020; pp. 27–51. [Google Scholar]
- Li, Z.; Zhang, Z.; Ren, Y.; Wang, Y.; Fang, J.; Yue, H.; Ma, S.; Guan, F. Aging and age-related diseases: From mechanisms to therapeutic strategies. Biogerontology 2021, 22, 165–187. [Google Scholar] [CrossRef] [PubMed]
- Di Ciaula, A.; Portincasa, P. The environment as a determinant of successful aging or frailty. Mech. Ageing Dev. 2020, 188, 111244. [Google Scholar] [CrossRef] [PubMed]
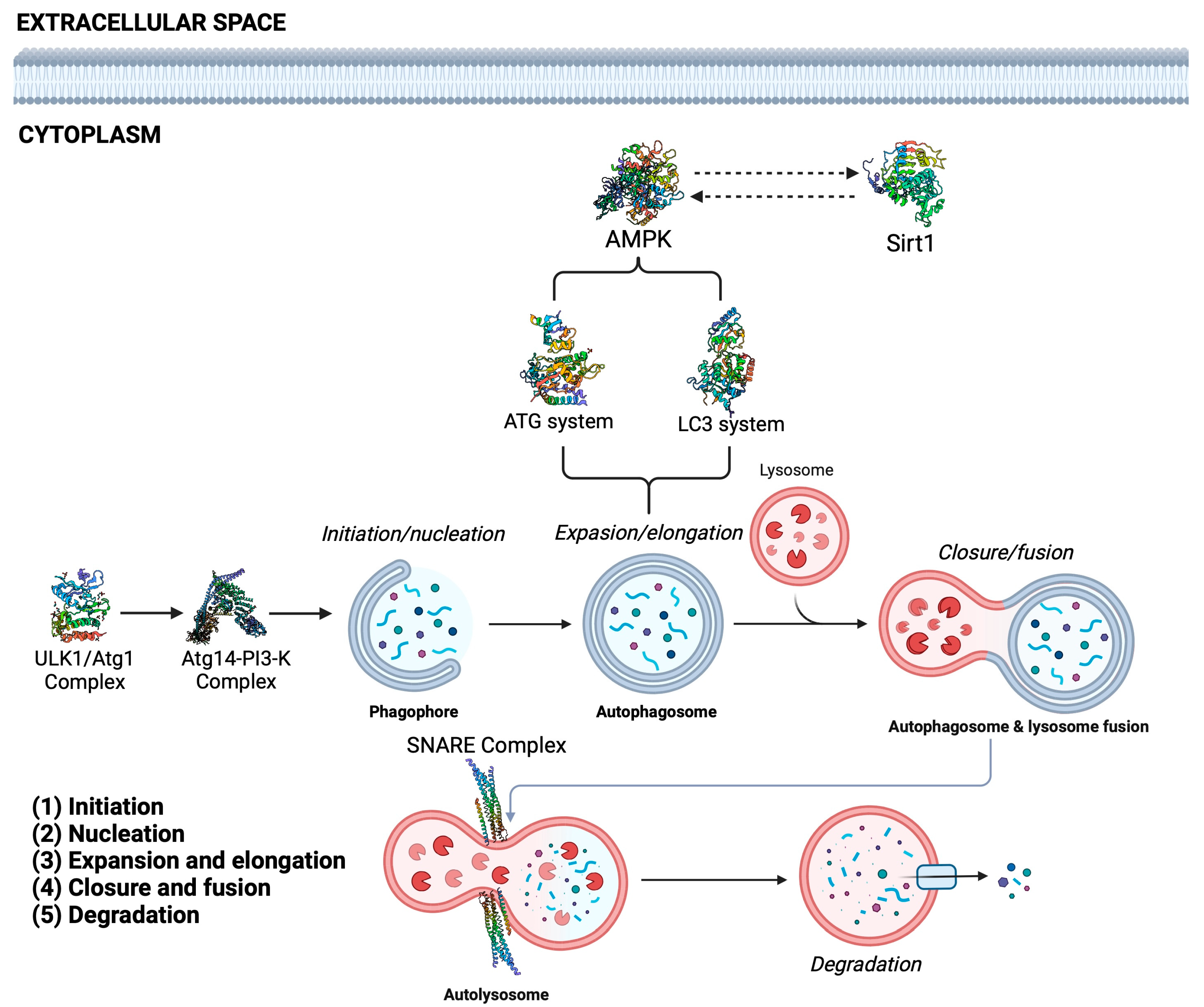
- Hurley, J.H.; Young, L.N. Mechanisms of Autophagy Initiation. Annu. Rev. Biochem. 2017, 86, 225–244. [Google Scholar] [CrossRef]
- Baba, M.; Takeshige, K.; Baba, N.; Ohsumi, Y. Ultrastructural analysis of the autophagic process in yeast: Detection of autophagosomes and their characterization. J. Cell Biol. 1994, 124, 903–913. [Google Scholar] [CrossRef]
- Chen, T.; Tu, S.; Ding, L.; Jin, M.; Chen, H.; Zhou, H. The role of autophagy in viral infections. J. Biomed. Sci. 2023, 30, 5. [Google Scholar] [CrossRef]
- Mizushima, N.; Komatsu, M. Autophagy: Renovation of cells and tissues. Cell 2011, 147, 728–741. [Google Scholar] [CrossRef]
- Yang, Y.; Pan, Z.; Sun, J.; Welch, J.; Klionsky, D.J. Autophagy and machine learning: Unanswered questions. Biochim. Biophys. Acta Mol. Basis Dis. 2024, 1870, 167263. [Google Scholar] [CrossRef]
- Wang, L.; Klionsky, D.J.; Shen, H.M. The emerging mechanisms and functions of microautophagy. Nat. Rev. Mol. Cell Biol. 2023, 24, 186–203. [Google Scholar] [CrossRef]
- Dice, J.F. Altered degradation of proteins microinjected into senescent human fibroblasts. J. Biol. Chem. 1982, 257, 14624–14627. [Google Scholar] [CrossRef]
- Valdor, R.; Martinez-Vicente, M. The Role of Chaperone-Mediated Autophagy in Tissue Homeostasis and Disease Pathogenesis. Biomedicines 2024, 12, 257. [Google Scholar] [CrossRef] [PubMed]
- Mulcahy Levy, J.M.; Thorburn, A. Autophagy in cancer: Moving from understanding mechanism to improving therapy responses in patients. Cell Death Differ. 2020, 27, 843–857. [Google Scholar] [CrossRef] [PubMed]
- Axe, E.L.; Walker, S.A.; Manifava, M.; Chandra, P.; Roderick, H.L.; Habermann, A.; Griffiths, G.; Ktistakis, N.T. Autophagosome formation from membrane compartments enriched in phosphatidylinositol 3-phosphate and dynamically connected to the endoplasmic reticulum. J. Cell Biol. 2008, 182, 685–701. [Google Scholar] [CrossRef] [PubMed]
- Parzych, K.R.; Klionsky, D.J. An overview of autophagy: Morphology, mechanism, and regulation. Antioxid Redox Signal. 2014, 20, 460–473. [Google Scholar] [CrossRef]
- Pyo, J.O.; Nah, J.; Jung, Y.K. Molecules and their functions in autophagy. Exp. Mol. Med. 2012, 44, 73–80. [Google Scholar] [CrossRef]
- Pankiv, S.; Clausen, T.H.; Lamark, T.; Brech, A.; Bruun, J.A.; Outzen, H.; Øvervatn, A.; Bjørkøy, G.; Johansen, T. p62/SQSTM1 binds directly to Atg8/LC3 to facilitate degradation of ubiquitinated protein aggregates by autophagy. J. Biol. Chem. 2007, 282, 24131–24145. [Google Scholar] [CrossRef]
- Fujita, N.; Itoh, T.; Omori, H.; Fukuda, M.; Noda, T.; Yoshimori, T. The Atg16L complex specifies the site of LC3 lipidation for membrane biogenesis in autophagy. Mol. Biol. Cell 2008, 19, 2092–2100. [Google Scholar] [CrossRef]
- Fahmy, A.M.; Labonté, P. The autophagy elongation complex (ATG5-12/16L1) positively regulates HCV replication and is required for wild-type membranous web formation. Sci. Rep. 2017, 7, 40351. [Google Scholar] [CrossRef]
- Ke, P.Y. Molecular Mechanism of Autophagosome-Lysosome Fusion in Mammalian Cells. Cells 2024, 13, 500. [Google Scholar] [CrossRef]
- Zhen, Y.; Stenmark, H. A dual-purpose fusion complex in autophagy. Cell Res. 2024, 34, 183–184. [Google Scholar] [CrossRef]
- Mizushima, N. Autophagy: Process and function. Genes Dev. 2007, 21, 2861–2873. [Google Scholar] [CrossRef] [PubMed]
- Ortega, M.A.; Fraile-Martinez, O.; de Leon-Oliva, D.; Boaru, D.L.; Lopez-Gonzalez, L.; García-Montero, C.; Alvarez-Mon, M.A.; Guijarro, L.G.; Torres-Carranza, D.; Saez, M.A.; et al. Autophagy in Its (Proper) Context: Molecular Basis, Biological Relevance, Pharmacological Modulation, and Lifestyle Medicine. Int. J. Biol. Sci. 2024, 20, 2532–2554. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Klionsky, D.J. Regulation mechanisms and signaling pathways of autophagy. Annu. Rev. Genet. 2009, 43, 67–93. [Google Scholar] [CrossRef] [PubMed]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. Hallmarks of aging: An expanding universe. Cell 2023, 186, 243–278. [Google Scholar] [CrossRef]
- Hipp, M.S.; Kasturi, P.; Hartl, F.U. The proteostasis network and its decline in ageing. Nat. Rev. Mol. Cell Biol. 2019, 20, 421–435. [Google Scholar] [CrossRef]
- Kumar, P.; Choudhary, A.; Kinger, S.; Jagtap, Y.A.; Dubey, A.R.; Gutti, R.K.; Chitkara, D.; Suresh, A.K.; Mishra, A. Proteostasis defects: Medicinal challenges of imperfect aging & neurodegeneration. Transl. Med. Aging 2023, 7, 87–97. [Google Scholar]
- Wu, X.; Liu, Z.; Yu, X.Y.; Xu, S.; Luo, J. Autophagy and cardiac diseases: Therapeutic potential of natural products. Med. Res. Rev. 2021, 41, 314–341. [Google Scholar] [CrossRef]
- Klionsky, D.J.; Petroni, G.; Amaravadi, R.K.; Baehrecke, E.H.; Ballabio, A.; Boya, P.; Bravo-San Pedro, J.M.; Cadwell, K.; Cecconi, F.; Choi, A.M.K.; et al. Autophagy in major human diseases. Embo J. 2021, 40, e108863. [Google Scholar] [CrossRef]
- Eisenberg, T.; Abdellatif, M.; Schroeder, S.; Primessnig, U.; Stekovic, S.; Pendl, T.; Harger, A.; Schipke, J.; Zimmermann, A.; Schmidt, A.; et al. Cardioprotection and lifespan extension by the natural polyamine spermidine. Nat. Med. 2016, 22, 1428–1438. [Google Scholar] [CrossRef]
- Jiang, B.; Zhou, X.; Yang, T.; Wang, L.; Feng, L.; Wang, Z.; Xu, J.; Jing, W.; Wang, T.; Su, H.; et al. The role of autophagy in cardiovascular disease: Cross-interference of signaling pathways and underlying therapeutic targets. Front. Cardiovasc. Med. 2023, 10, 1088575. [Google Scholar] [CrossRef]
- Luo, G.; Jian, Z.; Zhu, Y.; Zhu, Y.; Chen, B.; Ma, R.; Tang, F.; Xiao, Y. Sirt1 promotes autophagy and inhibits apoptosis to protect cardiomyocytes from hypoxic stress. Int. J. Mol. Med. 2019, 43, 2033–2043. [Google Scholar] [CrossRef] [PubMed]
- Farhan, M.; Silva, M.; Xingan, X.; Huang, Y.; Zheng, W. Role of FOXO Transcription Factors in Cancer Metabolism and Angiogenesis. Cells 2020, 9, 1586. [Google Scholar] [CrossRef] [PubMed]
- Hariharan, N.; Maejima, Y.; Nakae, J.; Paik, J.; Depinho, R.A.; Sadoshima, J. Deacetylation of FoxO by Sirt1 Plays an Essential Role in Mediating Starvation-Induced Autophagy in Cardiac Myocytes. Circ. Res. 2010, 107, 1470–1482. [Google Scholar] [CrossRef] [PubMed]
- Maithili Karpaga Selvi, N.; Sridhar, M.G.; Swaminathan, R.P.; Sripradha, R. Curcumin Attenuates Oxidative Stress and Activation of Redox-Sensitive Kinases in High Fructose- and High-Fat-Fed Male Wistar Rats. Sci. Pharm. 2015, 83, 159–175. [Google Scholar] [CrossRef]
- Kong, F.; Ye, B.; Cao, J.; Cai, X.; Lin, L.; Huang, S.; Huang, W.; Huang, Z. Curcumin Represses NLRP3 Inflammasome Activation via TLR4/MyD88/NF-κB and P2X7R Signaling in PMA-Induced Macrophages. Front. Pharmacol. 2016, 7, 369. [Google Scholar] [CrossRef]
- Jang, S.Y.; Kim, J.; Hong, E.; Lee, K.; Na, Y.; Yeom, C.H.; Park, S. Curcumin inhibits human cancer cell growth and migration through downregulation of SVCT2. Cell Biochem. Funct. 2023, 41, 696–703. [Google Scholar] [CrossRef]
- Akaberi, M.; Sahebkar, A.; Emami, S.A. Turmeric and Curcumin: From Traditional to Modern Medicine. Adv. Exp. Med. Biol. 2021, 1291, 15–39. [Google Scholar]
- Moustapha, A.; Pérétout, P.A.; Rainey, N.E.; Sureau, F.; Geze, M.; Petit, J.M.; Dewailly, E.; Slomianny, C.; Petit, P.X. Curcumin induces crosstalk between autophagy and apoptosis mediated by calcium release from the endoplasmic reticulum, lysosomal destabilization and mitochondrial events. Cell Death Discov. 2015, 1, 15017. [Google Scholar] [CrossRef]
- Zhu, Y.; Bu, S. Curcumin Induces Autophagy, Apoptosis, and Cell Cycle Arrest in Human Pancreatic Cancer Cells. Evid. Based Complement. Alternat. Med. 2017, 2017, 5787218. [Google Scholar] [CrossRef]
- Kim, J.Y.; Cho, T.J.; Woo, B.H.; Choi, K.U.; Lee, C.H.; Ryu, M.H.; Park, H.R. Curcumin-induced autophagy contributes to the decreased survival of oral cancer cells. Arch. Oral Biol. 2012, 57, 1018–1025. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, J.; Xu, J.; Lu, Y.; Jiang, J.; Wang, L.; Shen, H.M.; Xia, D. Curcumin targets the TFEB-lysosome pathway for induction of autophagy. Oncotarget 2016, 7, 75659–75671. [Google Scholar] [CrossRef] [PubMed]
- Alers, S.; Löffler, A.S.; Wesselborg, S.; Stork, B. Role of AMPK-mTOR-Ulk1/2 in the regulation of autophagy: Cross talk, shortcuts, and feedbacks. Mol Cell Biol 2012, 32, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Du, P.; Liu, X.; Xu, X.; Ge, Y.; Zhang, C. Curcumin supplementation increases longevity and antioxidant capacity in Caenorhabditis elegans. Front. Pharmacol. 2023, 14, 1195490. [Google Scholar] [CrossRef] [PubMed]
- Suckow, B.K.; Suckow, M.A. Lifespan extension by the antioxidant curcumin in Drosophila melanogaster. Int. J. Biomed. Sci. 2006, 2, 402–405. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.S.; Lee, B.S.; Semnani, S.; Avanesian, A.; Um, C.Y.; Jeon, H.J.; Seong, K.M.; Yu, K.; Min, K.J.; Jafari, M. Curcumin extends life span, improves health span, and modulates the expression of age-associated aging genes in Drosophila melanogaster. Rejuvenation Res. 2010, 13, 561–570. [Google Scholar] [CrossRef]
- Stępień, K.; Wojdyła, D.; Nowak, K.; Mołoń, M. Impact of curcumin on replicative and chronological aging in the Saccharomyces cerevisiae yeast. Biogerontology 2020, 21, 109–123. [Google Scholar] [CrossRef]
- Kuo, J.J.; Chang, H.H.; Tsai, T.H.; Lee, T.Y. Curcumin ameliorates mitochondrial dysfunction associated with inhibition of gluconeogenesis in free fatty acid-mediated hepatic lipoapoptosis. Int. J. Mol. Med. 2012, 30, 643–649. [Google Scholar] [CrossRef]
- Wang, D.; Yang, Y.; Zou, X.; Zheng, Z.; Zhang, J. Curcumin ameliorates CKD-induced mitochondrial dysfunction and oxidative stress through inhibiting GSK-3β activity. J. Nutr. Biochem. 2020, 83, 108404. [Google Scholar] [CrossRef]
- Sathyabhama, M.; Priya Dharshini, L.C.; Karthikeyan, A.; Kalaiselvi, S.; Min, T. The Credible Role of Curcumin in Oxidative Stress-Mediated Mitochondrial Dysfunction in Mammals. Biomolecules 2022, 12, 1405. [Google Scholar] [CrossRef]
- Sadeghi, S.; Delphan, M.; Shams, M.; Esmaeili, F.; Shanaki-Bavarsad, M.; Shanaki, M. The high-intensity interval training (HIIT) and curcumin supplementation can positively regulate the autophagy pathway in myocardial cells of STZ-induced diabetic rats. BMC Res. Notes 2023, 16, 21. [Google Scholar] [CrossRef]
- Yu, W.; Qin, X.; Zhang, Y.; Qiu, P.; Wang, L.; Zha, W.; Ren, J. Curcumin suppresses doxorubicin-induced cardiomyocyte pyroptosis via a PI3K/Akt/mTOR-dependent manner. Cardiovasc. Diagn. Ther. 2020, 10, 752–769. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Shi, J.; Wang, X.; Zhang, R. Curcumin Alleviates D-Galactose-Induced Cardiomyocyte Senescence by Promoting Autophagy via the SIRT1/AMPK/mTOR Pathway. Evid.-Based Complement. Altern. Med. 2022, 2022, 2990843. [Google Scholar] [CrossRef] [PubMed]
- (US), N.C.I. Curcumin (Curcuma, Turmeric) and Cancer (PDQ®): Patient Version. Available online: https://www.ncbi.nlm.nih.gov/books/NBK578436/ (accessed on 28 May 2024).
- Thapa, S.B.; Pandey, R.P.; Park, Y.I.; Kyung Sohng, J. Biotechnological Advances in Resveratrol Production and its Chemical Diversity. Molecules 2019, 24, 2571. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Vinayak, M. Resveratrol alleviates inflammatory hyperalgesia by modulation of reactive oxygen species (ROS), antioxidant enzymes and ERK activation. Inflamm. Res. 2017, 66, 911–921. [Google Scholar] [CrossRef]
- Inglés, M.; Gambini, J.; Miguel, M.G.; Bonet-Costa, V.; Abdelaziz, K.M.; El Alami, M.; Viña, J.; Borrás, C. PTEN mediates the antioxidant effect of resveratrol at nutritionally relevant concentrations. Biomed. Res. Int. 2014, 2014, 580852. [Google Scholar] [CrossRef]
- Meng, T.; Xiao, D.; Muhammed, A.; Deng, J.; Chen, L.; He, J. Anti-Inflammatory Action and Mechanisms of Resveratrol. Molecules 2021, 26, 229. [Google Scholar] [CrossRef]
- Song, B.; Wang, W.; Tang, X.; Goh, R.M.W.; Thuya, W.L.; Ho, P.C.L.; Chen, L.; Wang, L. Inhibitory Potential of Resveratrol in Cancer Metastasis: From Biology to Therapy. Cancers 2023, 15, 2758. [Google Scholar] [CrossRef]
- Park, D.; Jeong, H.; Lee, M.N.; Koh, A.; Kwon, O.; Yang, Y.R.; Noh, J.; Suh, P.-G.; Park, H.; Ryu, S.H. Resveratrol induces autophagy by directly inhibiting mTOR through ATP competition. Sci. Rep. 2016, 6, 21772. [Google Scholar] [CrossRef]
- Mauthe, M.; Jacob, A.; Freiberger, S.; Hentschel, K.; Stierhof, Y.D.; Codogno, P.; Proikas-Cezanne, T. Resveratrol-mediated autophagy requires WIPI-1-regulated LC3 lipidation in the absence of induced phagophore formation. Autophagy 2011, 7, 1448–1461. [Google Scholar] [CrossRef]
- Ferretta, A.; Gaballo, A.; Tanzarella, P.; Piccoli, C.; Capitanio, N.; Nico, B.; Annese, T.; Di Paola, M.; Dell’Aquila, C.; De Mari, M.; et al. Effect of resveratrol on mitochondrial function: Implications in parkin-associated familiar Parkinson’s disease. Biochim. Biophys. Acta (BBA)—Mol. Basis Dis. 2014, 1842, 902–915. [Google Scholar] [CrossRef]
- Tomou, E.M.; Papakyriakopoulou, P.; Skaltsa, H.; Valsami, G.; Kadoglou, N.P.E. Bio-Actives from Natural Products with Potential Cardioprotective Properties: Isolation, Identification, and Pharmacological Actions of Apigenin, Quercetin, and Silibinin. Molecules 2023, 28, 2387. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.M.A.; Akram, M.; Riaz, M.; Munir, N.; Rasool, G. Cardioprotective Potential of Plant-Derived Molecules: A Scientific and Medicinal Approach. Dose Response 2019, 17, 1559325819852243. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Huang, A.; Yan, J.; Liu, B.; Liu, Q.; Zhang, J.; Zhang, X.; Ou, C.; Chen, M. Resveratrol Ameliorates Cardiac Dysfunction by Inhibiting Apoptosis via the PI3K/Akt/FoxO3a Pathway in a Rat Model of Diabetic Cardiomyopathy. J. Cardiovasc. Pharmacol. 2017, 70, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Yang, J.; Zhou, M.; Zhang, Y.; Liu, Y.; Hou, P.; Zeng, X.; Yi, L.; Mi, M. Resveratrol attenuates endothelial oxidative injury by inducing autophagy via the activation of transcription factor EB. Nutr. Metab. 2019, 16, 42. [Google Scholar] [CrossRef]
- Wang, B.; Yang, Q.; Sun, Y.Y.; Xing, Y.F.; Wang, Y.B.; Lu, X.T.; Bai, W.W.; Liu, X.Q.; Zhao, Y.X. Resveratrol-enhanced autophagic flux ameliorates myocardial oxidative stress injury in diabetic mice. J. Cell. Mol. Med. 2014, 18, 1599–1611. [Google Scholar] [CrossRef]
- Michala, A.S.; Pritsa, A. Quercetin: A Molecule of Great Biochemical and Clinical Value and Its Beneficial Effect on Diabetes and Cancer. Diseases 2022, 10, 37. [Google Scholar] [CrossRef]
- Shabir, I.; Kumar Pandey, V.; Shams, R.; Dar, A.H.; Dash, K.K.; Khan, S.A.; Bashir, I.; Jeevarathinam, G.; Rusu, A.V.; Esatbeyoglu, T.; et al. Promising bioactive properties of quercetin for potential food applications and health benefits: A review. Front. Nutr. 2022, 9, 999752. [Google Scholar] [CrossRef]
- Deepika; Maurya, P.K. Health Benefits of Quercetin in Age-Related Diseases. Molecules 2022, 27, 2498. [Google Scholar] [CrossRef]
- Rahmani, A.H.; Alsahli, M.A.; Khan, A.A.; Almatroodi, S.A. Quercetin, a Plant Flavonol Attenuates Diabetic Complications, Renal Tissue Damage, Renal Oxidative Stress and Inflammation in Streptozotocin-Induced Diabetic Rats. Metabolites 2023, 13, 130. [Google Scholar] [CrossRef]
- Dhanya, R. Quercetin for managing type 2 diabetes and its complications, an insight into multitarget therapy. Biomed. Pharmacother. 2022, 146, 112560. [Google Scholar] [CrossRef]
- Shahbaz, M.; Naeem, H.; Momal, U.; Imran, M.; Alsagaby, S.A.; Al Abdulmonem, W.; Waqar, A.B.; El-Ghorab, A.H.; Ghoneim, M.M.; Abdelgawad, M.A.; et al. Anticancer and apoptosis inducing potential of quercetin against a wide range of human malignancies. Int. J. Food Prop. 2023, 26, 2590–2626. [Google Scholar] [CrossRef]
- Asgharian, P.; Tazekand, A.P.; Hosseini, K.; Forouhandeh, H.; Ghasemnejad, T.; Ranjbar, M.; Hasan, M.; Kumar, M.; Beirami, S.M.; Tarhriz, V.; et al. Potential mechanisms of quercetin in cancer prevention: Focus on cellular and molecular targets. Cancer Cell Int. 2022, 22, 257. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Zhang, B.; Yin, S.; Xie, S.; Huang, K.; Wang, J.; Yang, W.; Liu, H.; Zhang, G.; Liu, X.; et al. Quercetin induces autophagy-associated death in HL-60 cells through CaMKKβ/AMPK/mTOR signal pathway. Acta Biochim. Biophys. Sin. 2022, 54, 1244–1256. [Google Scholar] [PubMed]
- Guo, H.; Ding, H.; Tang, X.; Liang, M.; Li, S.; Zhang, J.; Cao, J. Quercetin induces pro-apoptotic autophagy via SIRT1/AMPK signaling pathway in human lung cancer cell lines A549 and H1299 in vitro. Thorac. Cancer 2021, 12, 1415–1422. [Google Scholar] [CrossRef]
- Peng, J.; Yang, Z.; Li, H.; Hao, B.; Cui, D.; Shang, R.; Lv, Y.; Liu, Y.; Pu, W.; Zhang, H.; et al. Quercetin Reprograms Immunometabolism of Macrophages via the SIRT1/PGC-1α Signaling Pathway to Ameliorate Lipopolysaccharide-Induced Oxidative Damage. Int. J. Mol. Sci. 2023, 24, 5542. [Google Scholar] [CrossRef]
- Chen, Y.F.; Qiu, Q.; Wang, L.; Li, X.R.; Zhou, S.; Wang, H.; Jiang, W.D.; Geng, J.Y.; Qin, G.; Tang, B.; et al. Quercetin Ameliorates Myocardial Injury in Diabetic Rats by Regulating Autophagy and Apoptosis through AMPK/mTOR Signaling Pathway. Am. J. Chin. Med. 2024, 52, 841–864. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Z.Z.; Wu, Y.; Ke, J.J.; He, X.H.; Wang, Y.L. Quercetin postconditioning attenuates myocardial ischemia/reperfusion injury in rats through the PI3K/Akt pathway. Braz. J. Med. Biol. Res. 2013, 46, 861–867. [Google Scholar] [CrossRef]
- Lin, X.; Han, T.; Fan, Y.; Wu, S.; Wang, F.; Wang, C. Quercetin improves vascular endothelial function through promotion of autophagy in hypertensive rats. Life Sci. 2020, 258, 118106. [Google Scholar] [CrossRef]
- Hu, J.; Wang, X.; Cui, X.; Kuang, W.; Li, D.; Wang, J. Quercetin prevents isoprenaline-induced myocardial fibrosis by promoting autophagy via regulating miR-223-3p/FOXO3. Cell Cycle 2021, 20, 1253–1269. [Google Scholar] [CrossRef]
- Muñoz-Esparza, N.C.; Costa-Catala, J.; Comas-Basté, O.; Toro-Funes, N.; Latorre-Moratalla, M.L.; Veciana-Nogués, M.T.; Vidal-Carou, M.C. Occurrence of Polyamines in Foods and the Influence of Cooking Processes. Foods 2021, 10, 1752. [Google Scholar] [CrossRef]
- Bouchereau, A.; Guénot, P.; Larher, F. Analysis of amines in plant materials. Chromatogr. B Biomed. Sci. Appl. 2000, 747, 49–67. [Google Scholar] [CrossRef] [PubMed]
- Herbst, E.J.; Tanguay, R.B. The Interaction of Polyamines with Nucleic Acids. In Proceedings of the Research Symposium on Complexes of Biologically Active Substances with Nucleic Acids and Their Modes of Action: Held at the Walter Reed Army Institute of Research Washington, Washington, DC, USA, 16–19 March 1970; Hahn, F.E., Ed.; Springer: Berlin/Heidelberg, Germany, 1971; pp. 166–180. [Google Scholar]
- Schuster, I.; Bernhardt, R. Interactions of natural polyamines with mammalian proteins. Biomol. Concepts 2011, 2, 79–94. [Google Scholar] [CrossRef] [PubMed]
- Michael, A.J. Biosynthesis of polyamines and polyamine-containing molecules. Biochem. J. 2016, 473, 2315–2329. [Google Scholar] [CrossRef] [PubMed]
- Hofer, S.J.; Simon, A.K.; Bergmann, M.; Eisenberg, T.; Kroemer, G.; Madeo, F. Mechanisms of spermidine-induced autophagy and geroprotection. Nat. Aging 2022, 2, 1112–1129. [Google Scholar] [CrossRef]
- Wang, J.; Li, S.; Wang, J.; Wu, F.; Chen, Y.; Zhang, H.; Guo, Y.; Lin, Y.; Li, L.; Yu, X.; et al. Spermidine alleviates cardiac aging by improving mitochondrial biogenesis and function. Aging 2020, 12, 650–671. [Google Scholar] [CrossRef]
- Fairley, L.H.; Lejri, I.; Grimm, A.; Eckert, A. Spermidine Rescues Bioenergetic and Mitophagy Deficits Induced by Disease-Associated Tau Protein. Int. J. Mol. Sci. 2023, 24, 5297. [Google Scholar] [CrossRef]
- Zhang, H.; Alsaleh, G.; Feltham, J.; Sun, Y.; Napolitano, G.; Riffelmacher, T.; Charles, P.; Frau, L.; Hublitz, P.; Yu, Z.; et al. Polyamines Control eIF5A Hypusination, TFEB Translation, and Autophagy to Reverse B Cell Senescence. Mol. Cell 2019, 76, 110–125.e9. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, S.; Zhang, Y.; Lin, X.; Song, Y.; Xue, Z.; Qian, H.; Wang, S.; Wan, G.; Zheng, X.; et al. Induction of autophagy by spermidine is neuroprotective via inhibition of caspase 3-mediated Beclin 1 cleavage. Cell Death Dis. 2017, 8, e2738. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, J.; Li, L.; Chai, N.; Chen, Y.; Wu, F.; Zhang, W.; Wang, L.; Shi, S.; Zhang, L.; et al. Spermine and spermidine reversed age-related cardiac deterioration in rats. Oncotarget 2017, 8, 64793–64808. [Google Scholar] [CrossRef]
- Michiels, C.F.; Kurdi, A.; Timmermans, J.P.; De Meyer, G.R.Y.; Martinet, W. Spermidine reduces lipid accumulation and necrotic core formation in atherosclerotic plaques via induction of autophagy. Atherosclerosis 2016, 251, 319–327. [Google Scholar] [CrossRef]
- Yan, J.; Yan, J.Y.; Wang, Y.X.; Ling, Y.N.; Song, X.D.; Wang, S.Y.; Liu, H.Q.; Liu, Q.C.; Zhang, Y.; Yang, P.Z.; et al. Spermidine-enhanced autophagic flux improves cardiac dysfunction following myocardial infarction by targeting the AMPK/mTOR signalling pathway. Br. J. Pharmacol. 2019, 176, 3126–3142. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Dan, X.; Babbar, M.; Wei, Y.; Hasselbalch, S.G.; Croteau, D.L.; Bohr, V.A. Ageing as a risk factor for neurodegenerative disease. Nat. Rev. Neurol. 2019, 15, 565–581. [Google Scholar] [CrossRef] [PubMed]
- Leidal, A.M.; Levine, B.; Debnath, J. Autophagy and the cell biology of age-related disease. Nat. Cell Biol. 2018, 20, 1338–1348. [Google Scholar] [CrossRef] [PubMed]
- Lynch-Day, M.A.; Mao, K.; Wang, K.; Zhao, M.; Klionsky, D.J. The role of autophagy in Parkinson’s disease. Cold Spring Harb. Perspect. Med. 2012, 2, a009357. [Google Scholar] [CrossRef]
- Deng, Z.; Zhou, X.; Lu, J.H.; Yue, Z. Autophagy deficiency in neurodevelopmental disorders. Cell Biosci. 2021, 11, 214. [Google Scholar] [CrossRef]
- Uddin, M.S.; Stachowiak, A.; Mamun, A.A.; Tzvetkov, N.T.; Takeda, S.; Atanasov, A.G.; Bergantin, L.B.; Abdel-Daim, M.M.; Stankiewicz, A.M. Autophagy and Alzheimer’s Disease: From Molecular Mechanisms to Therapeutic Implications. Front. Aging Neurosci. 2018, 10, 4. [Google Scholar] [CrossRef]
- François, A.; Rioux Bilan, A.; Quellard, N.; Fernandez, B.; Janet, T.; Chassaing, D.; Paccalin, M.; Terro, F.; Page, G. Longitudinal follow-up of autophagy and inflammation in brain of APPswePS1dE9 transgenic mice. J. Neuroinflamm. 2014, 11, 139. [Google Scholar] [CrossRef]
- Tan, C.; Ai, J.; Zhu, Y. mTORC1-Dependent Protein and Parkinson’s Disease: A Mendelian Randomization Study. Brain Sci. 2023, 13, 536. [Google Scholar] [CrossRef]
- Batiha, G.E.; Al-Kuraishy, H.M.; Al-Gareeb, A.I.; Elekhnawy, E. SIRT1 pathway in Parkinson’s disease: A faraway snapshot but so close. Inflammopharmacology 2023, 31, 37–56. [Google Scholar] [CrossRef]
- Mehramiz, M.; Porter, T.; O’Brien, E.K.; Rainey-Smith, S.R.; Laws, S.M. A Potential Role for Sirtuin-1 in Alzheimer’s Disease: Reviewing the Biological and Environmental Evidence. J. Alzheimers Dis. Rep. 2023, 7, 823–843. [Google Scholar] [CrossRef]
- Maruyama, H.; Ooizumi, T.; Kawakami, F.; Lwin, T.-T.; Akita, H.; Kunii, T.; Shirai, R.; Takeda, T. Long-term oral administration of curcumin is effective in preventing short-term memory deterioration and prolonging lifespan in a mouse model of Alzheimer’s disease. Adv. Tradit. Med. 2024, 24, 373–385. [Google Scholar] [CrossRef]
- Yi, L.-T.; Dong, S.-Q.; Wang, S.-S.; Chen, M.; Li, C.-F.; Geng, D.; Zhu, J.-X.; Liu, Q.; Cheng, J. Curcumin attenuates cognitive impairment by enhancing autophagy in chemotherapy. Neurobiol. Dis. 2020, 136, 104715. [Google Scholar] [CrossRef] [PubMed]
- Rathore, A.S.; Singh, S.S.; Birla, H.; Zahra, W.; Keshri, P.K.; Dilnashin, H.; Singh, R.; Singh, S.; Singh, S.P. Curcumin Modulates p62–Keap1–Nrf2-Mediated Autophagy in Rotenone-Induced Parkinson’s Disease Mouse Models. ACS Chem. Neurosci. 2023, 14, 1412–1423. [Google Scholar] [CrossRef] [PubMed]
- Kou, X.; Chen, N. Resveratrol as a Natural Autophagy Regulator for Prevention and Treatment of Alzheimer’s Disease. Nutrients 2017, 9, 927. [Google Scholar] [CrossRef]
- Wang, N.; He, J.; Pan, C.; Wang, J.; Ma, M.; Shi, X.; Xu, Z. Resveratrol Activates Autophagy via the AKT/mTOR Signaling Pathway to Improve Cognitive Dysfunction in Rats with Chronic Cerebral Hypoperfusion. Front. Neurosci. 2019, 13, 859. [Google Scholar] [CrossRef]
- Xu, J.; Jackson, C.W.; Khoury, N.; Escobar, I.; Perez-Pinzon, M.A. Brain SIRT1 Mediates Metabolic Homeostasis and Neuroprotection. Front. Endocrinol. 2018, 9, 702. [Google Scholar] [CrossRef]
- Baeken, M.W. Sirtuins and their influence on autophagy. J. Cell Biochem. 2023. [Google Scholar] [CrossRef]
- Hao, Y.; Shao, L.; Hou, J.; Zhang, Y.; Ma, Y.; Liu, J.; Xu, C.; Chen, F.; Cao, L.H.; Ping, Y. Resveratrol and Sir2 Reverse Sleep and Memory Defects Induced by Amyloid Precursor Protein. Neurosci. Bull. 2023, 39, 1117–1130. [Google Scholar] [CrossRef]
- Turner, R.S.; Thomas, R.G.; Craft, S.; van Dyck, C.H.; Mintzer, J.; Reynolds, B.A.; Brewer, J.B.; Rissman, R.A.; Raman, R.; Aisen, P.S. A randomized, double-blind, placebo-controlled trial of resveratrol for Alzheimer disease. Neurology 2015, 85, 1383–1391. [Google Scholar] [CrossRef]
- Zhang, L.F.; Yu, X.L.; Ji, M.; Liu, S.Y.; Wu, X.L.; Wang, Y.J.; Liu, R.T. Resveratrol alleviates motor and cognitive deficits and neuropathology in the A53T α-synuclein mouse model of Parkinson’s disease. Food Funct. 2018, 9, 6414–6426. [Google Scholar] [CrossRef]
- Illes-Toth, E.; Rempel, D.L.; Gross, M.L. Exploration of Resveratrol as a Potent Modulator of α-Synuclein Fibril Formation. ACS Chem. Neurosci. 2024, 15, 503–516. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.J.; Dong, S.Y.; Cui, X.X.; Feng, Y.; Liu, T.; Yin, M.; Kuo, S.H.; Tan, E.K.; Zhao, W.J.; Wu, Y.C. Resveratrol alleviates MPTP-induced motor impairments and pathological changes by autophagic degradation of α-synuclein via SIRT1-deacetylated LC3. Mol. Nutr. Food Res. 2016, 60, 2161–2175. [Google Scholar] [CrossRef]
- Shen, D.F.; Qi, H.P.; Zhang, W.N.; Sang, W.X. Resveratrol Promotes Autophagy to Improve neuronal Injury in Parkinson’s Disease by Regulating SNHG1/miR-128-3p/SNCA Axis. Brain Sci. 2023, 13, 1124. [Google Scholar] [CrossRef] [PubMed]
- Ning, B.; Hang, S.; Zhang, W.; Mao, C.; Li, D. An update on the bridging factors connecting autophagy and Nrf2 antioxidant pathway. Front. Cell Dev. Biol. 2023, 11, 1232241. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z. The FoxO-Autophagy Axis in Health and Disease. Trends Endocrinol. Metab. 2019, 30, 658–671. [Google Scholar] [CrossRef]
- Collier, J.J.; Suomi, F.; Oláhová, M.; McWilliams, T.G.; Taylor, R.W. Emerging roles of ATG7 in human health and disease. EMBO Mol. Med. 2021, 13, e14824. [Google Scholar] [CrossRef]
- Sabarathinam, S. Unraveling the therapeutic potential of quercetin and quercetin-3-O-glucuronide in Alzheimer’s disease through network pharmacology, molecular docking, and dynamic simulations. Sci. Rep. 2024, 14, 14852. [Google Scholar] [CrossRef]
- León, R.; Gutiérrez, D.A.; Pinto, C.; Morales, C.; de la Fuente, C.; Riquelme, C.; Cortés, B.I.; González-Martin, A.; Chamorro, D.; Espinosa, N.; et al. c-Abl tyrosine kinase down-regulation as target for memory improvement in Alzheimer’s disease. Front. Aging Neurosci. 2023, 15, 1180987. [Google Scholar] [CrossRef]
- Schiavi, A.; Cirotti, C.; Gerber, L.-S.; Di Lauro, G.; Maglioni, S.; Shibao, P.Y.T.; Montresor, S.; Kirstein, J.; Petzsch, P.; Köhrer, K.; et al. Abl depletion via autophagy mediates the beneficial effects of quercetin against Alzheimer pathology across species. Cell Death Discov. 2023, 9, 376. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, H.; Yin, T.; Gong, Y.; Yuan, G.; Chen, L.; Liu, J. Quercetin-modified gold-palladium nanoparticles as a potential autophagy inducer for the treatment of Alzheimer’s disease. J. Colloid Interface Sci. 2019, 552, 388–400. [Google Scholar] [CrossRef]
- Kong, Y.; Li, K.; Fu, T.; Wan, C.; Zhang, D.; Song, H.; Zhang, Y.; Liu, N.; Gan, Z.; Yuan, L. Quercetin ameliorates Aβ toxicity in Drosophila AD model by modulating cell cycle-related protein expression. Oncotarget 2016, 7, 67716–67731. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Li, Y.; Mu, X. Effect of Quercetin on PC12 Alzheimer’s Disease Cell Model Induced by Aβ25-35 and Its Mechanism Based on Sirtuin1/Nrf2/HO-1 Pathway. BioMed Res. Int. 2020, 2020, 8210578. [Google Scholar] [CrossRef] [PubMed]
- Pakrashi, S.; Chakraborty, J.; Bandyopadhyay, J. Neuroprotective Role of Quercetin on Rotenone-Induced Toxicity in SH-SY5Y Cell Line Through Modulation of Apoptotic and Autophagic Pathways. Neurochem. Res. 2020, 45, 1962–1973. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.W.; Han, R.; He, H.J.; Li, J.; Chen, S.Y.; Gu, Y.; Xie, C. Administration of quercetin improves mitochondria quality control and protects the neurons in 6-OHDA-lesioned Parkinson’s disease models. Aging (Albany NY) 2021, 13, 11738–11751. [Google Scholar] [CrossRef]
- El-Horany, H.E.; El-Latif, R.N.; ElBatsh, M.M.; Emam, M.N. Ameliorative Effect of Quercetin on Neurochemical and Behavioral Deficits in Rotenone Rat Model of Parkinson’s Disease: Modulating Autophagy (Quercetin on Experimental Parkinson’s Disease). J. Biochem. Mol. Toxicol. 2016, 30, 360–369. [Google Scholar] [CrossRef]
- Jimenez Gutierrez, G.E.; Borbolla Jiménez, F.V.; Muñoz, L.G.; Tapia Guerrero, Y.S.; Murillo Melo, N.M.; Cristóbal-Luna, J.M.; Leyva Garcia, N.; Cordero-Martínez, J.; Magaña, J.J. The Molecular Role of Polyamines in Age-Related Diseases: An Update. Int. J. Mol. Sci. 2023, 24, 16469. [Google Scholar] [CrossRef]
- Williams, K.; Romano, C.; Dichter, M.A.; Molinoff, P.B. Modulation of the NMDA receptor by polyamines. Life Sci. 1991, 48, 469–498. [Google Scholar] [CrossRef]
- Morrison, L.D.; Kish, S.J. Brain polyamine levels are altered in Alzheimer’s disease. Neurosci. Lett. 1995, 197, 5–8. [Google Scholar] [CrossRef]
- Sandusky-Beltran, L.A.; Kovalenko, A.; Ma, C.; Calahatian, J.I.T.; Placides, D.S.; Watler, M.D.; Hunt, J.B.; Darling, A.L.; Baker, J.D.; Blair, L.J.; et al. Spermidine/spermine-N(1)-acetyltransferase ablation impacts tauopathy-induced polyamine stress response. Alzheimers Res. Ther. 2019, 11, 58. [Google Scholar] [CrossRef]
- Xu, T.T.; Li, H.; Dai, Z.; Lau, G.K.; Li, B.Y.; Zhu, W.L.; Liu, X.Q.; Liu, H.F.; Cai, W.W.; Huang, S.Q.; et al. Spermidine and spermine delay brain aging by inducing autophagy in SAMP8 mice. Aging 2020, 12, 6401–6414. [Google Scholar] [CrossRef]
- Lumkwana, D.; Peddie, C.; Kriel, J.; Michie, L.L.; Heathcote, N.; Collinson, L.; Kinnear, C.; Loos, B. Investigating the Role of Spermidine in a Model System of Alzheimer’s Disease Using Correlative Microscopy and Super-resolution Techniques. Front. Cell Dev. Biol. 2022, 10, 819571. [Google Scholar] [CrossRef] [PubMed]
- Mackenbrock, L.H.B.; Labuz, G.; Baur, I.D.; Yildirim, T.M.; Auffarth, G.U.; Khoramnia, R. Cataract Classification Systems: A Review. Klin. Monbl. Augenheilkd. 2024, 241, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Pesudovs, K.; Lansingh, V.C.; Kempen, J.H.; Tapply, I.; Fernandes, A.G.; Cicinelli, M.V.; Arrigo, A.; Leveziel, N.; Briant, P.S.; Vos, T.; et al. Global estimates on the number of people blind or visually impaired by cataract: A meta-analysis from 2000 to 2020. Eye 2024, 38, 2156–2172. [Google Scholar]
- Fernández-Albarral, J.A.; de Julián-López, E.; Soler-Domínguez, C.; de Hoz, R.; López-Cuenca, I.; Salobrar-García, E.; Ramírez, J.M.; Pinazo-Durán, M.D.; Salazar, J.J.; Ramírez, A.I. The Role of Autophagy in Eye Diseases. Life 2021, 11, 189. [Google Scholar] [CrossRef]
- Costello, M.J.; Brennan, L.A.; Basu, S.; Chauss, D.; Mohamed, A.; Gilliland, K.O.; Johnsen, S.; Menko, S.; Kantorow, M. Autophagy and mitophagy participate in ocular lens organelle degradation. Exp. Eye Res. 2013, 116, 141–150. [Google Scholar] [CrossRef]
- Morishita, H. Role of autophagy in the eye: From physiology to disease. Curr. Opin. Physiol. 2022, 30, 100592. [Google Scholar] [CrossRef]
- Chai, P.; Ni, H.; Zhang, H.; Fan, X. The Evolving Functions of Autophagy in Ocular Health: A Double-edged Sword. Int. J. Biol. Sci. 2016, 12, 1332–1340. [Google Scholar] [CrossRef]
- Sidjanin, D.J.; Park, A.K.; Ronchetti, A.; Martins, J.; Jackson, W.T. TBC1D20 mediates autophagy as a key regulator of autophagosome maturation. Autophagy 2016, 12, 1759–1775. [Google Scholar] [CrossRef]
- Cai, Y.; Liu, K.; Wu, P.; Yuan, R.; He, F.; Zou, J. Association of mTORC1-dependent circulating protein levels with cataract formation: A mendelian randomization study. BMC Genom. 2022, 23, 719. [Google Scholar] [CrossRef]
- Shukal, D.K.; Malaviya, P.B.; Sharma, T. Role of the AMPK signalling pathway in the aetiopathogenesis of ocular diseases. Hum. Exp. Toxicol. 2022, 41, 9603271211063165. [Google Scholar] [CrossRef]
- Zhou, M.; Luo, J.; Zhang, H. Role of Sirtuin 1 in the pathogenesis of ocular disease (Review). Int. J. Mol. Med. 2018, 42, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Radomska-Leśniewska, D.M.; Osiecka-Iwan, A.; Hyc, A.; Góźdź, A.; Dąbrowska, A.M.; Skopiński, P. Therapeutic potential of curcumin in eye diseases. Cent. Eur. J. Immunol. 2019, 44, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.F.; Hao, J.L.; Xie, T.; Mukhtar, N.J.; Zhang, W.; Malik, T.H.; Lu, C.W.; Zhou, D.D. Curcumin, A Potential Therapeutic Candidate for Anterior Segment Eye Diseases: A Review. Front. Pharmacol. 2017, 8, 66. [Google Scholar] [CrossRef] [PubMed]
- Pinelli, R.; Ferrucci, M.; Biagioni, F.; Bumah, V.; Scaffidi, E.; Puglisi-Allegra, S.; Fornai, F. Curcumin as a Perspective Protection for Retinal Pigment Epithelium during Autophagy Inhibition in the Course of Retinal Degeneration. Curr. Neuropharmacol. 2023, 21, 2227–2232. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, X.; Chen, B.; Huang, M.; Ma, P.; Xiong, L.; Huang, J.; Chen, J.; Huang, S.; Liu, Y. TFEB-Mediated Lysosomal Restoration Alleviates High Glucose-Induced Cataracts via Attenuating Oxidative Stress. Investig. Ophthalmol. Vis. Sci. 2022, 63, 26. [Google Scholar] [CrossRef]
- Josifovska, N.; Albert, R.; Nagymihály, R.; Lytvynchuk, L.; Moe, M.C.; Kaarniranta, K.; Veréb, Z.J.; Petrovski, G. Resveratrol as Inducer of Autophagy, Pro-Survival, and Anti-Inflammatory Stimuli in Cultured Human RPE Cells. Int. J. Mol. Sci. 2020, 21, 813. [Google Scholar] [CrossRef]
- Subirada, P.V.; Vaglienti, M.V.; Joray, M.B.; Paz, M.C.; Barcelona, P.F.; Sánchez, M.C. Rapamycin and Resveratrol Modulate the Gliotic and Pro-Angiogenic Response in Müller Glial Cells Under Hypoxia. Front. Cell Dev. Biol. 2022, 10, 855178. [Google Scholar] [CrossRef]
- Ye, M.J.; Meng, N. Resveratrol acts via the mitogen-activated protein kinase (MAPK) pathway to protect retinal ganglion cells from apoptosis induced by hydrogen peroxide. Bioengineered 2021, 12, 4878–4886. [Google Scholar] [CrossRef]
- Chen, P.; Yao, Z.; He, Z. Resveratrol protects against high glucose-induced oxidative damage in human lens epithelial cells by activating autophagy. Exp. Ther. Med. 2021, 21, 440. [Google Scholar] [CrossRef]
- Petrovski, G.; Berenyi, E.; Albert, R.; Moe, M.; Fesus, L.; Berta, A. Resveratrol, rapamycin and MG-132 as inducers of autophagy in ARPE-19 cells. Acta Ophthalmol. 2011, 89. [Google Scholar] [CrossRef]
- Wang, S.; Wang, Z.; Yang, S.; Yin, T.; Zhang, Y.; Qin, Y.; Weinreb, R.N.; Sun, X. Tissue Distribution of trans-Resveratrol and Its Metabolites after Oral Administration in Human Eyes. J. Ophthalmol. 2017, 2017, 4052094. [Google Scholar] [CrossRef] [PubMed]
- Hsu, M.Y.; Hsiao, Y.P.; Lin, Y.T.; Chen, C.; Lee, C.M.; Liao, W.C.; Tsou, S.C.; Lin, H.W.; Chang, Y.Y. Quercetin Alleviates the Accumulation of Superoxide in Sodium Iodate-Induced Retinal Autophagy by Regulating Mitochondrial Reactive Oxygen Species Homeostasis through Enhanced Deacetyl-SOD2 via the Nrf2-PGC-1α-Sirt1 Pathway. Antioxidants 2021, 10, 1125. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Wang, H.; Du, X. The therapeutic use of quercetin in ophthalmology: Recent applications. Biomed. Pharmacother. 2021, 137, 111371. [Google Scholar] [CrossRef] [PubMed]
- Sanderson, J.; McLauchlan, W.R.; Williamson, G. Quercetin inhibits hydrogen peroxide-induced oxidation of the rat lens. Free. Radic. Biol. Med. 1999, 26, 639–645. [Google Scholar] [CrossRef]
- Cornish, K.M.; Williamson, G.; Sanderson, J. Quercetin metabolism in the lens: Role in inhibition of hydrogen peroxide induced cataract. Free. Radic. Biol. Med. 2002, 33, 63–70. [Google Scholar] [CrossRef]
- Ferlemi, A.V.; Makri, O.E.; Mermigki, P.G.; Lamari, F.N.; Georgakopoulos, C.D. Quercetin glycosides and chlorogenic acid in highbush blueberry leaf decoction prevent cataractogenesis in vivo and in vitro: Investigation of the effect on calpains, antioxidant and metal chelating properties. Exp. Eye Res. 2016, 145, 258–268. [Google Scholar] [CrossRef]
- Bungau, S.; Abdel-Daim, M.M.; Tit, D.M.; Ghanem, E.; Sato, S.; Maruyama-Inoue, M.; Yamane, S.; Kadonosono, K. Health Benefits of Polyphenols and Carotenoids in Age-Related Eye Diseases. Oxid. Med. Cell Longev. 2019, 2019, 9783429. [Google Scholar] [CrossRef]
- Liu, X.; Chen, A.; Liang, Q.; Yang, X.; Dong, Q.; Fu, M.; Wang, S.; Li, Y.; Ye, Y.; Lan, Z.; et al. Spermidine inhibits vascular calcification in chronic kidney disease through modulation of SIRT1 signaling pathway. Aging Cell 2021, 20, e13377. [Google Scholar] [CrossRef]
- Ağaoğlu, N.B.; Varol, N.; Yıldız, S.H.; Karaosmanoğlu, C.; Duman, R.; Özdemir Erdoğan, M.; Solak, M. Relationship between SIRT1 gene expression level and disease in age-related cataract cases. Turk. J. Med. Sci. 2019, 49, 1068–1072. [Google Scholar] [CrossRef]
- Wu, F.; Xia, X.; Lei, T.; Du, H.; Hua, H.; Liu, W.; Xu, B.; Yang, T. Inhibition of SIRT1 promotes ultraviolet B induced cataract via downregulation of the KEAP1/NFE2L2 signaling pathway. J. Photochem. Photobiol. B Biol. 2023, 245, 112753. [Google Scholar] [CrossRef]
- Han, W.; Li, H.; Chen, B. Research Progress and Potential Applications of Spermidine in Ocular Diseases. Pharmaceutics 2022, 14, 1500. [Google Scholar] [CrossRef] [PubMed]
- Maekawa, S.; Hibasami, H.; Uji, Y.; Nakashima, K. Active transport and metabolic characteristics of polyamines in the rat lens. Biochim. Biophys. Acta (BBA)—Gen. Subj. 1989, 993, 199–203. [Google Scholar] [CrossRef]
- Kremzner, L.T.; Roy, D.; Spector, A. Polyamines in normal and cataractous human lenses: Evidence for post-translational modification. Exp. Eye Res. 1983, 37, 649–659. [Google Scholar] [CrossRef] [PubMed]
- Maekawa, S.; Hibasami, H.; Tsukada, T.; Furusako, S.; Nakashima, K.; Yokoyama, M. Induction of spermidine/spermine N1-acetyltransferase in needle-punctured rat lens as a model of traumatic cataract. Biochim. Biophys. Acta 1986, 883, 501–505. [Google Scholar] [CrossRef]
- Mischiati, C.; Feriotto, G.; Tabolacci, C.; Domenici, F.; Melino, S.; Borromeo, I.; Forni, C.; De Martino, A.; Beninati, S. Polyamine Oxidase Is Involved in Spermidine Reduction of Transglutaminase Type 2-Catalyzed βH-Crystallins Polymerization in Calcium-Induced Experimental Cataract. Int. J. Mol. Sci. 2020, 21, 5427. [Google Scholar] [CrossRef]
- Lentini, A.; Tabolacci, C.; Mattioli, P.; Provenzano, B.; Beninati, S. Spermidine delays eye lens opacification in vitro by suppressing transglutaminase-catalyzed crystallin cross-linking. Protein J. 2011, 30, 109–114. [Google Scholar] [CrossRef]
- Rao, Y.L.; Ganaraja, B.; Suresh, P.K.; Joy, T.; Ullal, S.D.; Manjrekar, P.A.; Murlimanju, B.V.; Sharma, B.G. Effect of resveratrol and combination of resveratrol and donepezil on the expression of microglial cells and astrocytes in Wistar albino rats of colchicine-induced Alzheimer’s disease. 3 Biotech 2023, 13, 319. [Google Scholar] [CrossRef]
- Yao, J.; Liu, X.; Sun, Y.; Dong, X.; Liu, L.; Gu, H. Curcumin-Alleviated Osteoarthritic Progression in Rats Fed a High-Fat Diet by Inhibiting Apoptosis and Activating Autophagy via Modulation of MicroRNA-34a. J. Inflamm. Res. 2021, 14, 2317–2331. [Google Scholar] [CrossRef]
- Jin, Z.; Chang, B.; Wei, Y.; Yang, Y.; Zhang, H.; Liu, J.; Piao, L.; Bai, L. Curcumin exerts chondroprotective effects against osteoarthritis by promoting AMPK/PINK1/Parkin-mediated mitophagy. Biomed. Pharmacother. 2022, 151, 113092. [Google Scholar] [CrossRef]
- Li, X.; Feng, K.; Li, J.; Yu, D.; Fan, Q.; Tang, T.; Yao, X.; Wang, X. Curcumin Inhibits Apoptosis of Chondrocytes through Activation ERK1/2 Signaling Pathways Induced Autophagy. Nutrients 2017, 9, 414. [Google Scholar] [CrossRef]
- Qin, N.; Wei, L.; Li, W.; Yang, W.; Cai, L.; Qian, Z.; Wu, S. Local intra-articular injection of resveratrol delays cartilage degeneration in C57BL/6 mice by inducing autophagy via AMPK/mTOR pathway. J. Pharmacol. Sci. 2017, 134, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Darwish, M.A.; Abdel-Bakky, M.S.; Messiha, B.A.S.; Abo-Saif, A.A.; Abo-Youssef, A.M. Resveratrol mitigates pancreatic TF activation and autophagy-mediated beta cell death via inhibition of CXCL16/ox-LDL pathway: A novel protective mechanism against type 1 diabetes mellitus in mice. Eur. J. Pharmacol. 2021, 901, 174059. [Google Scholar] [CrossRef] [PubMed]
- Sacitharan, P.K.; Lwin, S.; Gharios, G.B.; Edwards, J.R. Spermidine restores dysregulated autophagy and polyamine synthesis in aged and osteoarthritic chondrocytes via EP300. Exp. Mol. Med. 2018, 50, 1–10. [Google Scholar] [CrossRef] [PubMed]
- D’Adamo, S.; Cetrullo, S.; Guidotti, S.; Silvestri, Y.; Minguzzi, M.; Santi, S.; Cattini, L.; Filardo, G.; Flamigni, F.; Borzì, R.M. Spermidine rescues the deregulated autophagic response to oxidative stress of osteoarthritic chondrocytes. Free Radic. Biol. Med. 2020, 153, 159–172. [Google Scholar] [CrossRef]
- Hosoda, R.; Nakashima, R.; Yano, M.; Iwahara, N.; Asakura, S.; Nojima, I.; Saga, Y.; Kunimoto, R.; Horio, Y.; Kuno, A. Resveratrol, a SIRT1 activator, attenuates aging-associated alterations in skeletal muscle and heart in mice. J. Pharmacol. Sci. 2023, 152, 112–122. [Google Scholar] [CrossRef]
- Fan, J.; Yang, X.; Li, J.; Shu, Z.; Dai, J.; Liu, X.; Li, B.; Jia, S.; Kou, X.; Yang, Y.; et al. Spermidine coupled with exercise rescues skeletal muscle atrophy from D-gal-induced aging rats through enhanced autophagy and reduced apoptosis via AMPK-FOXO3a signal pathway. Oncotarget 2017, 8, 17475–17490. [Google Scholar] [CrossRef]
- Khater, S.I.; Dowidar, M.F.; Abdel-Aziz, A.E.; Khamis, T.; Dahran, N.; Alqahtani, L.S.; Metwally, M.M.M.; Al-Hady Abd-Elrahamn, A.S.; Alsieni, M.; Alosaimi, M.E.; et al. β-Cell Autophagy Pathway and Endoplasmic Reticulum Stress Regulating-Role of Liposomal Curcumin in Experimental Diabetes Mellitus: A Molecular and Morphometric Study. Antioxidants 2022, 11, 2400. [Google Scholar] [CrossRef]
- Zhang, P.; Fang, J.; Zhang, J.; Ding, S.; Gan, D. Curcumin Inhibited Podocyte Cell Apoptosis and Accelerated Cell Autophagy in Diabetic Nephropathy via Regulating Beclin1/UVRAG/Bcl2. Diabetes Metab. Syndr. Obes. 2020, 13, 641–652. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, L.; Chen, Z.; Li, S.; Che, B.; Wang, N.; Chen, J.; Xu, C.; Wei, C. Exogenous spermine attenuates diabetic kidney injury in rats by inhibiting AMPK/mTOR signaling pathway. Int. J. Mol. Med. 2021, 47, 27. [Google Scholar] [CrossRef]
- Aman, Y.; Schmauck-Medina, T.; Hansen, M.; Morimoto, R.I.; Simon, A.K.; Bjedov, I.; Palikaras, K.; Simonsen, A.; Johansen, T.; Tavernarakis, N.; et al. Autophagy in healthy aging and disease. Nat. Aging 2021, 1, 634–650. [Google Scholar] [CrossRef]
- Pavlova, J.A.; Guseva, E.A.; Dontsova, O.A.; Sergiev, P.V. Natural Activators of Autophagy. Biochemistry 2024, 89, 1–26. [Google Scholar] [PubMed]
- Tabibzadeh, S. Role of autophagy in aging: The good, the bad, and the ugly. Aging Cell 2023, 22, e13753. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.; Tang, H.; Li, W.; Gong, Y.; Li, S.; Huang, J.; Fang, Y.; Yuan, W.; Liu, Y.; Wang, S.; et al. AMPK and its Activator Berberine in the Treatment of Neurodegenerative Diseases. Curr. Pharm Des. 2020, 26, 5054–5066. [Google Scholar] [CrossRef] [PubMed]
- DiNicolantonio, J.J.; McCarty, M.F.; Assanga, S.I.; Lujan, L.L.; O’Keefe, J.H. Ferulic acid and berberine, via Sirt1 and AMPK, may act as cell cleansing promoters of healthy longevity. Open Heart 2022, 9, e001801. [Google Scholar] [CrossRef]
- Ren, G.; Guo, J.H.; Qian, Y.Z.; Kong, W.J.; Jiang, J.D. Berberine Improves Glucose and Lipid Metabolism in HepG2 Cells Through AMPKα1 Activation. Front. Pharmacol. 2020, 11, 647. [Google Scholar] [CrossRef]
- Su, X.; Yang, D.; Hu, Y.; Yuan, Y.; Song, L. Berberine suppressed sarcopenia insulin resistance through SIRT1-mediated mitophagy. Open Life Sci. 2023, 18, 20220648. [Google Scholar] [CrossRef]
- Dang, Y.; An, Y.; He, J.; Huang, B.; Zhu, J.; Gao, M.; Zhang, S.; Wang, X.; Yang, B.; Xie, Z. Berberine ameliorates cellular senescence and extends the lifespan of mice via regulating p16 and cyclin protein expression. Aging Cell 2020, 19, e13060. [Google Scholar] [CrossRef]
- Cai, Y.; Xin, Q.; Lu, J.; Miao, Y.; Lin, Q.; Cong, W.; Chen, K. A New Therapeutic Candidate for Cardiovascular Diseases: Berberine. Front. Pharmacol. 2021, 12, 631100. [Google Scholar] [CrossRef]
- FDA Curcumin GRN No. 822. Available online: https://www.cfsanappsexternal.fda.gov/scripts/fdcc/index.cfm?set=GRASNotices&id=822&sort=GRN_No&order=DESC&startrow=1&type=basic&search=822 (accessed on 20 September 2024).
- FDA Quercetin GRN No. 341. Available online: https://www.cfsanappsexternal.fda.gov/scripts/fdcc/?set=GRASNotices&id=341&sort=GRN_No&order=DESC&startrow=1&type=basic&search=quercetin (accessed on 20 September 2024).
- Barber-Chamoux, N.; Milenkovic, D.; Verny, M.A.; Habauzit, V.; Pereira, B.; Lambert, C.; Richard, D.; Boby, C.; Mazur, A.; Lusson, J.R.; et al. Substantial Variability Across Individuals in the Vascular and Nutrigenomic Response to an Acute Intake of Curcumin: A Randomized Controlled Trial. Mol. Nutr. Food Res. 2018, 62, 1700418. [Google Scholar] [CrossRef]
- Mirza, M.A.; Mahmood, S.; Hilles, A.R.; Ali, A.; Khan, M.Z.; Zaidi, S.A.A.; Iqbal, Z.; Ge, Y. Quercetin as a Therapeutic Product: Evaluation of Its Pharmacological Action and Clinical Applications-A Review. Pharmaceuticals 2023, 16, 1631. [Google Scholar] [CrossRef]
- Tomé-Carneiro, J.; Gonzálvez, M.; Larrosa, M.; Yáñez-Gascón, M.J.; García-Almagro, F.J.; Ruiz-Ros, J.A.; Tomás-Barberán, F.A.; García-Conesa, M.T.; Espín, J.C. Grape resveratrol increases serum adiponectin and downregulates inflammatory genes in peripheral blood mononuclear cells: A triple-blind, placebo-controlled, one-year clinical trial in patients with stable coronary artery disease. Cardiovasc. Drugs Ther. 2013, 27, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, C.; Stekovic, S.; Wirth, M.; Benson, G.; Royer, P.; Sigrist, S.J.; Pieber, T.; Dammbrueck, C.; Magnes, C.; Eisenberg, T.; et al. Safety and tolerability of spermidine supplementation in mice and older adults with subjective cognitive decline. Aging (Albany NY) 2018, 10, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Quagliariello, V.; Iaffaioli, R.V.; Armenia, E.; Clemente, O.; Barbarisi, M.; Nasti, G.; Berretta, M.; Ottaiano, A.; Barbarisi, A. Hyaluronic Acid Nanohydrogel Loaded With Quercetin Alone or in Combination to a Macrolide Derivative of Rapamycin RAD001 (Everolimus) as a New Treatment for Hormone-Responsive Human Breast Cancer. J. Cell Physiol. 2017, 232, 2063–2074. [Google Scholar] [CrossRef] [PubMed]
- Alayev, A.; Berger, S.M.; Kramer, M.Y.; Schwartz, N.S.; Holz, M.K. The combination of rapamycin and resveratrol blocks autophagy and induces apoptosis in breast cancer cells. J. Cell Biochem. 2015, 116, 450–457. [Google Scholar] [CrossRef]
- Jin, H.G.; Wu, G.Z.; Wu, G.H.; Bao, Y.G. Combining the mammalian target of rapamycin inhibitor, rapamycin, with resveratrol has a synergistic effect in multiple myeloma. Oncol. Lett. 2018, 15, 6257–6264. [Google Scholar] [CrossRef]
- Shui, L.; Wang, W.; Xie, M.; Ye, B.; Li, X.; Liu, Y.; Zheng, M. Isoquercitrin induces apoptosis and autophagy in hepatocellular carcinoma cells via AMPK/mTOR/p70S6K signaling pathway. Aging 2020, 12, 24318–24332. [Google Scholar] [CrossRef]
- Dehzad, M.J.; Ghalandari, H.; Nouri, M.; Askarpour, M. Antioxidant and anti-inflammatory effects of curcumin/turmeric supplementation in adults: A GRADE-assessed systematic review and dose-response meta-analysis of randomized controlled trials. Cytokine 2023, 164, 156144. [Google Scholar] [CrossRef]
- Li, F.; Liu, J.; Tang, S.; Yan, J.; Chen, H.; Li, D.; Yan, X. Quercetin regulates inflammation, oxidative stress, apoptosis, and mitochondrial structure and function in H9C2 cells by promoting PVT1 expression. Acta Histochem. 2021, 123, 151819. [Google Scholar] [CrossRef]
- Hussain, T.; Tan, B.; Ren, W.; Rahu, N.; Dad, R.; Kalhoro, D.H.; Yin, Y. Polyamines: Therapeutic perspectives in oxidative stress and inflammatory diseases. Amino Acids 2017, 49, 1457–1468. [Google Scholar] [CrossRef]
| Age-Related Diseases | Natural Compound | Autophagic Effect | Ref. |
|---|---|---|---|
| Cardiopathies | Curcumin | Attenuation of oxidative stress and activation of autophagy via the AKT/mTOR regulation in diabetic rats with myocardial damage. | [52] |
| Restoration of Beclin-1 and LC3 I/II ratio in mice with doxorubicin-induced cardiomyopathy. | [54] | ||
| Resveratrol | Amelioration of cardiac dysfunction of neonatal rat ventricular myocytes via the negative regulation of the PI3K/Akt pathway and decreasing of apoptosis. | [66] | |
| Activation of TFEB for autophagy activation. | [67] | ||
| Increased the expression of LC3 and Beclin-1 via Sirt1on endothelial oxidative damage of cardiovascular models. | [68] | ||
| Quercetin | Activation of the AMPK pathway that leads to increased LC3 and Beclin-1 protein levels to ameliorate myocardial injury. | [79] | |
| Suppression of the PI3K/Akt pathway to attenuate myocardial ischemia. | [80] | ||
| Prevention of myocardial fibrosis via the regulation of the miR-223/FOXO3 that leads to increasing LC3 II/I ratio. | [81,82] | ||
| Polyamines | Prevention of lipidic plaque formation via increased expression of LC3 I/II ratio. | [94] | |
| Amelioration of myocardial dysfunction by promoting AMPK/mTOR pathway. | [95] | ||
| Alzheimer’s disease | Curcumin | Reverse cognitive disorders in an AD mice model induced by cisplatin by the activation of the AMPK-JNK signaling pathway to inhibit mTOR and Bcl-2, which modulates autophagy by the overexpression of LC3 and downregulation of p62. | [106] |
| Resveratrol | Enhance cognitive function in rats by stimulating the AKT/mTOR pathway. | [109] | |
| Activation of the Sirt1 pathway, leading to increased mitophagy and subsequent neuroprotection. | [171] | ||
| Improvements in sleep and memory dependent on the ortholog Sir2, a sirtuin ortholog that stimulates autophagy by activating HSP70 and ATG4 while it also interacts with LC3 and p62. | [111,112] | ||
| Quercetin | Activates Sirt1 pathway, influencing the expression of FOXO1 and NRF2. | [118,119] | |
| Reduction of the Ab1 kinase expression, preventing tau phosphorylation and neuronal apoptosis triggered by Aβ. | [122,123] | ||
| Research on a PC12 cell model showed quercetin improves cell survival and proliferation, mitigates Aβ toxicity, and enhances the expression of Sirt1. | [125,126] | ||
| Polyamines | Elevates LC3 and Beclin-1 levels compared to control in SY5Y cells stably expressing a mutant form of human tau protein. | [90] | |
| Spermidines induce autophagy and clear amyloid precursor protein clusters. | [135] | ||
| Parkison’s disease | Curcumin | Increases the expression of LC3-II, facilitating enhanced clearance of α-synuclein proteins in mice models. | [107] |
| Resveratrol | In mice model, ameliorates the deficit in cognitive and motor function in a dose-dependent manner along with a decrease in α-synuclein aggregation. | [114] | |
| In mice model, autophagic degradation of α-synuclein was promoted via the activation of the Sirt1 pathway. | [116] | ||
| In two cell models, resveratrol promoted autophagy by increasing beclin-1 and the LC3-II/LC3-I ratio, and the reduction in p62. | [117] | ||
| Quercetin | Ameliorates neuronal death, mitochondrial dysfunction, and α-synuclein accumulation in animal and cell models by activating autophagy. | [127] | |
| Increase of ULK1(Ser555)/ULK1, p-TBK1(Ser172)/TBK1, and LC3 levels. | [128] | ||
| Increase autophagy and attenuate behavioral impairments. | [129] | ||
| Polyamines | Autophagy stimulation | [90] | |
| Ocular disorders | Curcumin | * Activation of TFEB to promote lysosomal biogenesis on rats with glucose-induced cataracts. | [149] |
| Resveratrol | Increased expression of LC3 and Beclin-1 in high glucose treated HLEC cells. | [153] | |
| Polyamines | Downregulation of Sirt1 in age-related cataracts may be indirectly linked to altered polyamine (PA) levels in the human lens. Spermidine, known to positively regulate Sirt1, could play a role in this process. | [163,164] |
| Age-Related Disease | Natural Compound | Autophagic Effect | Ref. |
|---|---|---|---|
| Osteoarthritis | Curcumin Resveratrol Polyamines (Spermidine) | Reduction of miR-34a expression and probably through the Akt/mTOR pathway | [172] |
| mTOR via the PI3K-Akt pathway | [172] | ||
| Mitophagy AMPK/PINK1/Parkin pathway | [173] | ||
| Activation of MAPK/ERK1/2 pathway | [174] | ||
| AMPK/mTOR pathway | [175] | ||
| Inhibition of CXCL16/ox-LDL pathway | [176] | ||
| Overexpression of EP300 acetyltransferase | [177] | ||
| Overexpression of BECN-1, LC3-II and p62 | [178] | ||
| Sarcopenia | Resveratrol Polyamines (Spermidine) | SIRT1 | [179] |
| Probably AMPK-FOXO3a | [180] | ||
| Diabetes | Curcumin Polyamines (Spermidine) | In β cells miR-29b and miR-137 overexpression | [181] |
| In podocyte via regulating Beclin1/UVRAG/Bcl2 | [182] | ||
| In podocyte regulate the AMPK/mTOR pathway | [183] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mundo Rivera, V.M.; Tlacuahuac Juárez, J.R.; Murillo Melo, N.M.; Leyva Garcia, N.; Magaña, J.J.; Cordero Martínez, J.; Jiménez Gutierrez, G.E. Natural Autophagy Activators to Fight Age-Related Diseases. Cells 2024, 13, 1611. https://doi.org/10.3390/cells13191611
Mundo Rivera VM, Tlacuahuac Juárez JR, Murillo Melo NM, Leyva Garcia N, Magaña JJ, Cordero Martínez J, Jiménez Gutierrez GE. Natural Autophagy Activators to Fight Age-Related Diseases. Cells. 2024; 13(19):1611. https://doi.org/10.3390/cells13191611
Chicago/Turabian StyleMundo Rivera, Vianey M., José Roberto Tlacuahuac Juárez, Nadia Mireya Murillo Melo, Norberto Leyva Garcia, Jonathan J. Magaña, Joaquín Cordero Martínez, and Guadalupe Elizabeth Jiménez Gutierrez. 2024. "Natural Autophagy Activators to Fight Age-Related Diseases" Cells 13, no. 19: 1611. https://doi.org/10.3390/cells13191611
APA StyleMundo Rivera, V. M., Tlacuahuac Juárez, J. R., Murillo Melo, N. M., Leyva Garcia, N., Magaña, J. J., Cordero Martínez, J., & Jiménez Gutierrez, G. E. (2024). Natural Autophagy Activators to Fight Age-Related Diseases. Cells, 13(19), 1611. https://doi.org/10.3390/cells13191611







