Role and Function of Peroxisomes in Neuroinflammation
Abstract
1. Introduction
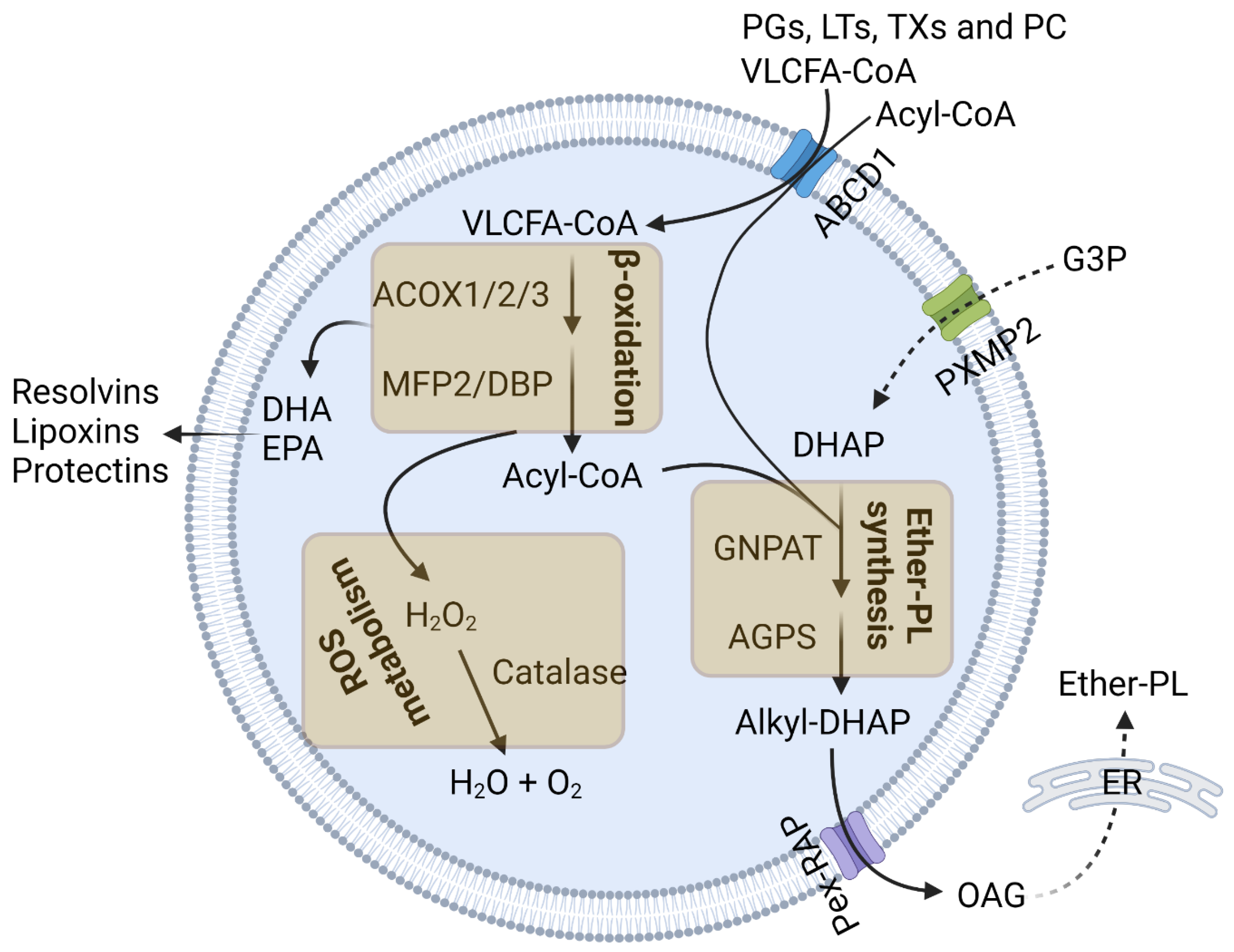
2. Peroxisomal Functions in Immune Response
2.1. Peroxisomal β-Oxidation
2.2. Ether-PL Synthesis
2.3. Peroxisomal Redox Metabolism
3. Peroxisomes in Innate Immune Signaling during Viral Infection
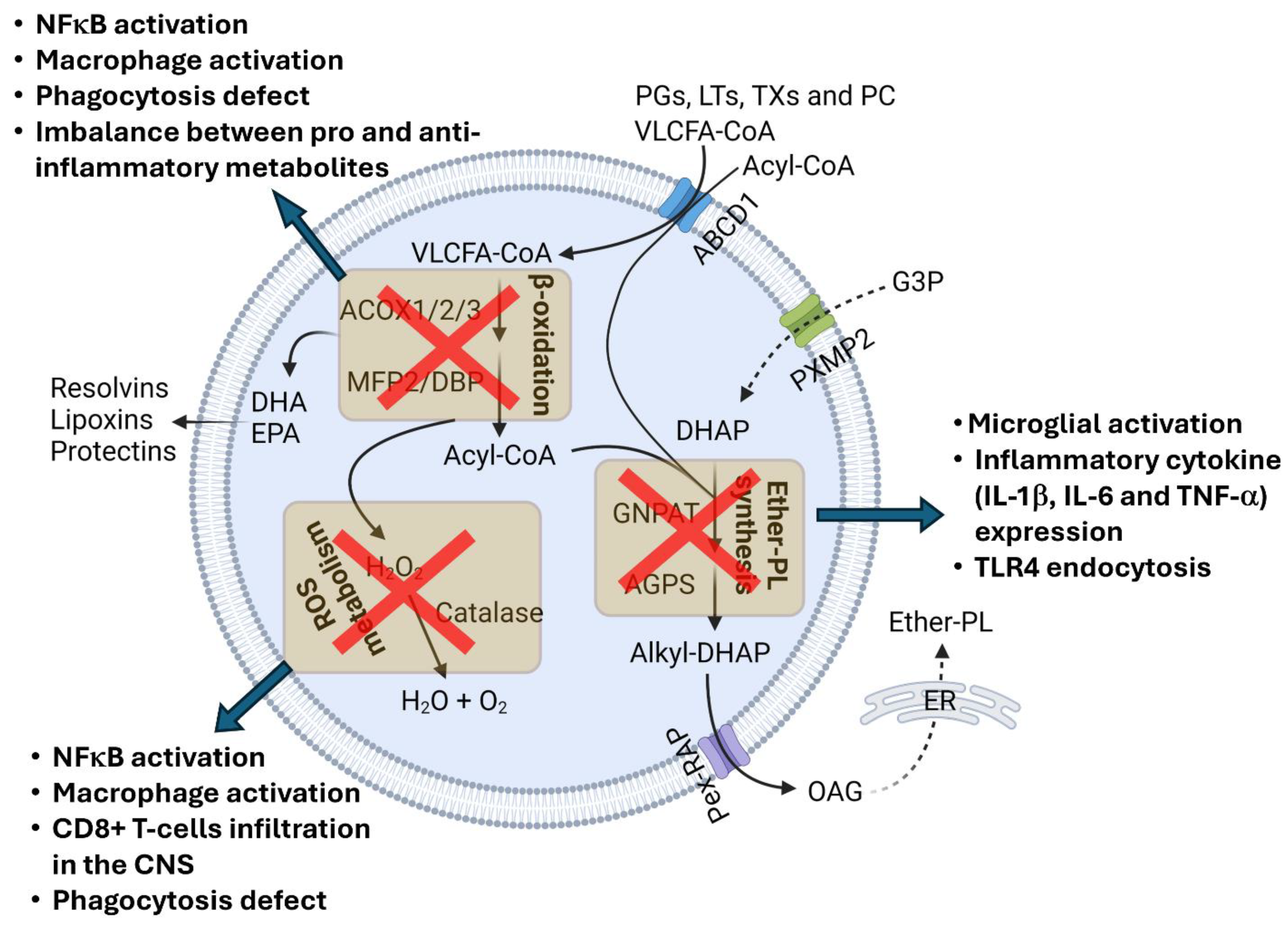
4. Neuroinflammation in Peroxisomal Disorders
4.1. Peroxisomal Biogenesis Disorders (PBDs)
4.2. Single-Peroxisomal-Protein Deficiencies
| Peroxisomal Disorder | Gene Affected | Inflammatory Response | Species | Ref |
|---|---|---|---|---|
| ZSD | PEX6 | Severe inflammatory response | Human | [52] |
| Pex5 | Increased expression of proinflammatory cytokines and chemokines Infiltration of B and CD8+ T cells in the brain | Mouse | [36] | |
| Pex7 | Defect in phagocytosis | Mouse | [35] | |
| Pex13 | Astrogliosis and microgliosis | Mouse | [53] | |
| X-ALD | ABCD1 | Progressive inflammatory demyelination Microglial activation Proinflammatory cytokine and chemokine expression CD8+ T-cell and B-cell infiltration in the brain | Human | [54,55,56,57,58] |
| Abcd1 | Microgliosis and increased levels of c1q and TREM2 in the spinal cord | Mouse | [61] | |
| RCDP | GNPAT | Gliosis | Human | [65] |
| Gnpat | Astrogliosis | Mouse | [29] |
5. Perspective
Author Contributions
Funding
Conflicts of Interest
References
- Wanders, R.J.A.; Baes, M.; Ribeiro, D.; Ferdinandusse, S.; Waterham, H.R. The physiological functions of human peroxisomes. Physiol. Rev. 2023, 103, 957–1024. [Google Scholar] [CrossRef] [PubMed]
- Fourcade, S.; Ferrer, I.; Pujol, A. Oxidative stress, mitochondrial and proteostasis malfunction in adrenoleukodystrophy: A paradigm for axonal degeneration. Free Radic. Biol. Med. 2015, 88, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Islinger, M.; Voelkl, A.; Fahimi, H.D.; Schrader, M. The peroxisome: An update on mysteries 2.0. Histochem. Cell Biol. 2018, 150, 443–471. [Google Scholar] [CrossRef]
- De Duve, C.; Baudhuin, P. Peroxisomes (microbodies and related particles). Physiol. Rev. 1966, 46, 323–357. [Google Scholar] [CrossRef] [PubMed]
- de Araújo Boleti, A.P.; de Oliveira Flores, T.M.; Moreno, S.E.; Dos Anjos, L.; Mortari, M.R.; Migliolo, L. Neuroinflammation: An overview of neurodegenerative and metabolic diseases and of biotechnological studies. Neurochem. Int. 2020, 136, 104714. [Google Scholar] [CrossRef] [PubMed]
- Simpson, D.S.A.; Oliver, P.L. ROS Generation in Microglia: Understanding Oxidative Stress and Inflammation in Neurodegenerative Disease. Antioxidants 2020, 9, 743. [Google Scholar] [CrossRef] [PubMed]
- Devanney, N.A.; Stewart, A.N.; Gensel, J.C. Microglia and macrophage metabolism in CNS injury and disease: The role of immunometabolism in neurodegeneration and neurotrauma. Exp. Neurol. 2020, 329, 113310. [Google Scholar] [CrossRef]
- Leng, F.; Edison, P. Neuroinflammation and microglial activation in Alzheimer disease: Where do we go from here? Nat. Rev. Neurol. 2021, 17, 157–172. [Google Scholar] [CrossRef]
- Trompier, D.; Vejux, A.; Zarrouk, A.; Gondcaille, C.; Geillon, F.; Nury, T.; Savary, S.; Lizard, G. Brain peroxisomes. Biochimie 2014, 98, 102–110. [Google Scholar] [CrossRef]
- Dean, J.M.; Lodhi, I.J. Structural and functional roles of ether lipids. Protein Cell 2018, 9, 196–206. [Google Scholar] [CrossRef]
- He, A.; Dean, J.M.; Lodhi, I.J. Peroxisomes as cellular adaptors to metabolic and environmental stress. Trends Cell Biol. 2021, 31, 656–670. [Google Scholar] [CrossRef]
- Griffin, E.N.; Ackerman, S.L. Lipid Metabolism and Axon Degeneration: An ACOX1 Balancing Act. Neuron 2020, 106, 551–553. [Google Scholar] [CrossRef] [PubMed]
- Di Cara, F.; Savary, S.; Kovacs, W.J.; Kim, P.; Rachubinski, R.A. The peroxisome: An up-and-coming organelle in immunometabolism. Trends Cell Biol. 2023, 33, 70–86. [Google Scholar] [CrossRef] [PubMed]
- Nath, A.S.; Parsons, B.D.; Makdissi, S.; Chilvers, R.L.; Mu, Y.; Weaver, C.M.; Euodia, I.; Fitze, K.A.; Long, J.; Scur, M.; et al. Modulation of the cell membrane lipid milieu by peroxisomal beta-oxidation induces Rho1 signaling to trigger inflammatory responses. Cell Rep. 2022, 38, 110433. [Google Scholar] [CrossRef] [PubMed]
- Zierfuss, B.; Buda, A.; Villoria-González, A.; Logist, M.; Fabjan, J.; Parzer, P.; Battin, C.; Vandersteene, S.; Dijkstra, I.M.E.; Waidhofer-Söllner, P.; et al. Saturated very long-chain fatty acids regulate macrophage plasticity and invasiveness. J. Neuroinflamm. 2022, 19, 305. [Google Scholar] [CrossRef] [PubMed]
- Vijayan, V.; Srinu, T.; Karnati, S.; Garikapati, V.; Linke, M.; Kamalyan, L.; Mali, S.R.; Sudan, K.; Kollas, A.; Schmid, T.; et al. A New Immunomodulatory Role for Peroxisomes in Macrophages Activated by the TLR4 Ligand Lipopolysaccharide. J. Immunol. 2017, 198, 2414–2425. [Google Scholar] [CrossRef]
- Chung, H.-L.; Ye, Q.; Park, Y.-J.; Zuo, Z.; Mok, J.-W.; Kanca, O.; Tattikota, S.G.; Lu, S.; Perrimon, N.; Lee, H.K.; et al. Very-long-chain fatty acids induce glial-derived sphingosine-1-phosphate synthesis, secretion, and neuroinflammation. Cell Metab. 2023, 35, 855–874.e5. [Google Scholar] [CrossRef]
- Radzikowska, U.; Rinaldi, A.O.; Çelebi, Z.C.; Karaguzel, D.; Wojcik, M.; Cypryk, K.; Akdis, M.; Akdis, C.A.; Sokolowska, M. The Influence of Dietary Fatty Acids on Immune Responses. Nutrients 2019, 11, 2990. [Google Scholar] [CrossRef]
- Dorninger, F.; Gundacker, A.; Zeitler, G.; Pollak, D.D.; Berger, J. Ether Lipid Deficiency in Mice Produces a Complex Behavioral Phenotype Mimicking Aspects of Human Psychiatric Disorders. Int. J. Mol. Sci. 2019, 20, 3929. [Google Scholar] [CrossRef]
- Hossain, M.S.; Abe, Y.; Ali, F.; Youssef, M.; Honsho, M.; Fujiki, Y.; Katafuchi, T. Reduction of Ether-Type Glycerophospholipids, Plasmalogens, by NF-kappaB Signal Leading to Microglial Activation. J. Neurosci. 2017, 37, 4074–4092. [Google Scholar] [CrossRef]
- Hossain, S.; Mawatari, S.; Fujino, T. Plasmalogens inhibit neuroinflammation and promote cognitive function. Brain Res. Bull. 2023, 192, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Ali, F.; Hossain, S.; Sejimo, S.; Akashi, K. Plasmalogens Inhibit Endocytosis of Toll-like Receptor 4 to Attenuate the Inflammatory Signal in Microglial Cells. Mol. Neurobiol. 2019, 56, 3404–3419. [Google Scholar] [CrossRef]
- Gu, J.; Chen, L.; Sun, R.; Wang, J.-L.; Wang, J.; Lin, Y.; Lei, S.; Zhang, Y.; Lv, D.; Jiang, F.; et al. Plasmalogens Eliminate Aging-Associated Synaptic Defects and Microglia-Mediated Neuroinflammation in Mice. Front. Mol. Biosci. 2022, 9, 815320. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.S.; Tajima, A.; Kotoura, S.; Katafuchi, T. Oral ingestion of plasmalogens can attenuate the LPS-induced memory loss and microglial activation. Biochem. Biophys. Res. Commun. 2018, 496, 1033–1039. [Google Scholar] [CrossRef] [PubMed]
- Sejimo, S.; Hossain, M.S.; Akashi, K. Scallop-derived plasmalogens attenuate the activation of PKCdelta associated with the brain inflammation. Biochem. Biophys. Res. Commun. 2018, 503, 837–842. [Google Scholar] [CrossRef]
- Youssef, M.; Ibrahim, A.; Akashi, K.; Hossain, M.S. PUFA-Plasmalogens Attenuate the LPS-Induced Nitric Oxide Production by Inhibiting the NF-kB, p38 MAPK and JNK Pathways in Microglial Cells. Neuroscience 2019, 397, 18–30. [Google Scholar] [CrossRef]
- Bozelli, J.C.; Azher, S., Jr.; Epand, R.M. Plasmalogens and Chronic Inflammatory Diseases. Front. Physiol. 2021, 12, 730829. [Google Scholar] [CrossRef]
- Facciotti, F.; Ramanjaneyulu, G.S.; Lepore, M.; Sansano, S.; Cavallari, M.; Kistowska, M.; Forss-Petter, S.; Ni, G.; Colone, A.; Singhal, A.; et al. Peroxisome-derived lipids are self antigens that stimulate invariant natural killer T cells in the thymus. Nat. Immunol. 2012, 13, 474–480. [Google Scholar] [CrossRef]
- Bottelbergs, A.; Verheijden, S.; Van Veldhoven, P.P.; Just, W.; Devos, R.; Baes, M. Peroxisome deficiency but not the defect in ether lipid synthesis causes activation of the innate immune system and axonal loss in the central nervous system. J. Neuroinflamm. 2012, 9, 61. [Google Scholar] [CrossRef]
- Andrés, C.M.C.; de la Lastra, J.M.P.; Juan, C.A.; Plou, F.J.; Pérez-Lebeña, E. The Role of Reactive Species on Innate Immunity. Vaccines 2022, 10, 1735. [Google Scholar] [CrossRef]
- Fan, H.; Bai, Q.; Yang, Y.; Shi, X.; Du, G.; Yan, J.; Shi, J.; Wang, D. The key roles of reactive oxygen species in microglial inflammatory activation: Regulation by endogenous antioxidant system and exogenous sulfur-containing compounds. Eur. J. Pharmacol. 2023, 956, 175966. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, H.-L.; Yang, C.-M. Role of redox signaling in neuroinflammation and neurodegenerative diseases. BioMed Res. Int. 2013, 2013, 484613. [Google Scholar] [CrossRef] [PubMed]
- Solleiro-Villavicencio, H.; Rivas-Arancibia, S. Effect of Chronic Oxidative Stress on Neuroinflammatory Response Mediated by CD4(+)T Cells in Neurodegenerative Diseases. Front. Cell. Neurosci. 2018, 12, 114. [Google Scholar] [CrossRef] [PubMed]
- Di Cara, F.; Andreoletti, P.; Trompier, D.; Vejux, A.; Bülow, M.H.; Sellin, J.; Lizard, G.; Cherkaoui-Malki, M.; Savary, S. Peroxisomes in Immune Response and Inflammation. Int. J. Mol. Sci. 2019, 20, 3877. [Google Scholar] [CrossRef]
- Di Cara, F.; Sheshachalam, A.; Braverman, N.E.; Rachubinski, R.A.; Simmonds, A.J. Peroxisome-Mediated Metabolism Is Required for Immune Response to Microbial Infection. Immunity 2017, 47, 93–106.e7. [Google Scholar] [CrossRef]
- Kassmann, C.M.; Lappe-Siefke, C.; Baes, M.; Brügger, B.; Mildner, A.; Werner, H.B.; Natt, O.; Michaelis, T.; Prinz, M.; Frahm, J.; et al. Axonal loss and neuroinflammation caused by peroxisome-deficient oligodendrocytes. Nat. Genet. 2007, 39, 969–976. [Google Scholar] [CrossRef]
- Cook, K.C.; Moreno, J.A.; Beltran, P.M.J.; Cristea, I.M. Peroxisome Plasticity at the Virus-Host Interface. Trends Microbiol. 2019, 27, 906–914. [Google Scholar] [CrossRef]
- Ferreira, A.R.; Marques, M.; Ramos, B.; Kagan, J.C.; Ribeiro, D. Emerging roles of peroxisomes in viral infections. Trends Cell Biol. 2022, 32, 124–139. [Google Scholar] [CrossRef]
- Ferreira, A.R.; Marques, M.; Ribeiro, D. Peroxisomes and Innate Immunity: Antiviral Response and Beyond. Int. J. Mol. Sci. 2019, 20, 3795. [Google Scholar] [CrossRef]
- Dixit, E.; Boulant, S.; Zhang, Y.; Lee, A.S.; Odendall, C.; Shum, B.; Hacohen, N.; Chen, Z.J.; Whelan, S.P.; Fransen, M.; et al. Peroxisomes are signaling platforms for antiviral innate immunity. Cell 2010, 141, 668–681. [Google Scholar] [CrossRef]
- Odendall, C.; Dixit, E.; Stavru, F.; Bierne, H.; Franz, K.M.; Durbin, A.F.; Boulant, S.; Gehrke, L.; Cossart, P.; Kagan, J.C. Diverse intracellular pathogens activate type III interferon expression from peroxisomes. Nat. Immunol. 2014, 15, 717–726. [Google Scholar] [CrossRef]
- Bender, S.; Reuter, A.; Eberle, F.; Einhorn, E.; Binder, M.; Bartenschlager, R. Activation of Type I and III Interferon Response by Mitochondrial and Peroxisomal MAVS and Inhibition by Hepatitis C Virus. PLoS Pathog. 2015, 11, e1005264. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.P.; Xu, Z.; Hou, S.; Limonta, D.; Kumar, A.; Power, C.; Hobman, T.C. Interplay between Zika Virus and Peroxisomes during Infection. Cells 2019, 8, 725. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Asahchop, E.L.; Branton, W.G.; Gelman, B.B.; Power, C.; Hobman, T.C. MicroRNAs upregulated during HIV infection target peroxisome biogenesis factors: Implications for virus biology, disease mechanisms and neuropathology. PLoS Pathog. 2017, 13, e1006360. [Google Scholar] [CrossRef] [PubMed]
- Knoblach, B.; Ishida, R.; Hobman, T.C.; Rachubinski, R.A. Peroxisomes exhibit compromised structure and matrix protein content in SARS-CoV-2-infected cells. Mol. Biol. Cell 2021, 32, 1273–1282. [Google Scholar] [CrossRef] [PubMed]
- Cohen, G.B.; Rangan, V.S.; Chen, B.K.; Smith, S.; Baltimore, D. The human thioesterase II protein binds to a site on HIV-1 Nef critical for CD4 down-regulation. J. Biol. Chem. 2000, 275, 23097–23105. [Google Scholar] [CrossRef]
- Mohan, K.V.; Som, I.; Atreya, C.D. Identification of a type 1 peroxisomal targeting signal in a viral protein and demonstration of its targeting to the organelle. J. Virol. 2002, 76, 2543–2547. [Google Scholar] [CrossRef][Green Version]
- Beltran, P.M.J.; Cook, K.C.; Hashimoto, Y.; Galitzine, C.; Murray, L.A.; Vitek, O.; Cristea, I.M. Infection-Induced Peroxisome Biogenesis Is a Metabolic Strategy for Herpesvirus Replication. Cell Host Microbe 2018, 24, 526–541.e7. [Google Scholar] [CrossRef]
- Queiroz, A.; Pinto, I.F.D.; Lima, M.; Giovanetti, M.; de Jesus, J.G.; Xavier, J.; Barreto, F.K.; Canuto, G.A.B.; Amaral, H.R.D.; de Filippis, A.M.B.; et al. Lipidomic Analysis Reveals Serum Alteration of Plasmalogens in Patients Infected With ZIKA Virus. Front. Microbiol. 2019, 10, 753. [Google Scholar] [CrossRef]
- De Munter, S.; Verheijden, S.; Régal, L.; Baes, M. Peroxisomal Disorders: A Review on Cerebellar Pathologies. Brain Pathol. 2015, 25, 663–678. [Google Scholar] [CrossRef]
- Wanders, R.; Waterham, H. Peroxisomal disorders I: Biochemistry and genetics of peroxisome biogenesis disorders. Clin. Genet. 2005, 67, 107–133. [Google Scholar] [CrossRef] [PubMed]
- Lucaccioni, L.; Righi, B.; Cingolani, G.M.; Lugli, L.; Della Casa, E.; Torcetta, F.; Iughetti, L.; Berardi, A. Overwhelming sepsis in a neonate affected by Zellweger syndrome due to a compound heterozygosis in PEX 6 gene: A case report. BMC Med. Genet. 2020, 21, 229. [Google Scholar] [CrossRef] [PubMed]
- Müller, C.C.; Nguyen, T.H.; Ahlemeyer, B.; Meshram, M.; Santrampurwala, N.; Cao, S.; Sharp, P.; Fietz, P.B.; Baumgart-Vogt, E.; Crane, D.I. PEX13 deficiency in mouse brain as a model of Zellweger syndrome: Abnormal cerebellum formation, reactive gliosis and oxidative stress. Dis. Models Mech. 2011, 4, 104–119. [Google Scholar] [CrossRef] [PubMed]
- Wanders, R.J.; Waterham, H.R. Peroxisomal disorders: The single peroxisomal enzyme deficiencies. Biochim. Biophys. Acta 2006, 1763, 1707–1720. [Google Scholar] [CrossRef]
- Turk, B.R.; Theda, C.; Fatemi, A.; Moser, A.B. X-linked adrenoleukodystrophy: Pathology, pathophysiology, diagnostic testing, newborn screening and therapies. Int. J. Dev. Neurosci. 2020, 80, 52–72. [Google Scholar] [CrossRef]
- Weinhofer, I.; Rommer, P.; Gleiss, A.; Ponleitner, M.; Zierfuss, B.; Waidhofer-Söllner, P.; Fourcade, S.; Grabmeier-Pfistershammer, K.; Reinert, M.-C.; Göpfert, J.; et al. Biomarker-based risk prediction for the onset of neuroinflammation in X-linked adrenoleukodystrophy. EBioMedicine 2023, 96, 104781. [Google Scholar] [CrossRef]
- Berger, J.; Forss-Petter, S.; Eichler, F. Pathophysiology of X-linked adrenoleukodystrophy. Biochimie 2014, 98, 135–142. [Google Scholar] [CrossRef]
- Yu, J.; Chen, T.; Guo, X.; Zafar, M.I.; Li, H.; Wang, Z.; Zheng, J. The Role of Oxidative Stress and Inflammation in X-Link Adrenoleukodystrophy. Front. Nutr. 2022, 9, 864358. [Google Scholar] [CrossRef]
- Jang, J.; Park, S.; Hur, H.J.; Cho, H.-J.; Hwang, I.; Kang, Y.P.; Im, I.; Lee, H.; Lee, E.; Yang, W.; et al. 25-hydroxycholesterol contributes to cerebral inflammation of X-linked adrenoleukodystrophy through activation of the NLRP3 inflammasome. Nat. Commun. 2016, 7, 13129. [Google Scholar] [CrossRef]
- Rutherford, H.A.; Hamilton, N. Animal models of leukodystrophy: A new perspective for the development of therapies. FEBS J. 2019, 286, 4176–4191. [Google Scholar] [CrossRef]
- Manor, J.; Chung, H.; Bhagwat, P.K.; Wangler, M.F. ABCD1 and X-linked adrenoleukodystrophy: A disease with a markedly variable phenotype showing conserved neurobiology in animal models. J. Neurosci. Res. 2021, 99, 3170–3181. [Google Scholar] [CrossRef] [PubMed]
- Verheijden, S.; Bottelbergs, A.; Krysko, O.; Krysko, D.V.; Beckers, L.; De Munter, S.; Van Veldhoven, P.P.; Wyns, S.; Kulik, W.; Nave, K.-A.; et al. Peroxisomal multifunctional protein-2 deficiency causes neuroinflammation and degeneration of Purkinje cells independent of very long chain fatty acid accumulation. Neurobiol. Dis. 2013, 58, 258–269. [Google Scholar] [CrossRef] [PubMed]
- El Hajj, H.I.; Vluggens, A.; Andreoletti, P.; Ragot, K.; Mandard, S.; Kersten, S.; Waterham, H.R.; Lizard, G.; Wanders, R.J.; Reddy, J.K.; et al. The inflammatory response in acyl-CoA oxidase 1 deficiency (pseudoneonatal adrenoleukodystrophy). Endocrinology 2012, 153, 2568–2575. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Viswakarma, N.; Yu, S.; Jia, Y.; Bai, L.; Vluggens, A.; Cherkaoui-Malki, M.; Khan, M.; Singh, I.; Yang, G.; et al. Progressive endoplasmic reticulum stress contributes to hepatocarcinogenesis in fatty acyl-CoA oxidase 1-deficient mice. Am. J. Pathol. 2011, 179, 703–713. [Google Scholar] [CrossRef]
- Viola, A.; Confort-Gouny, S.; Ranjeva, J.-P.; Chabrol, B.; Raybaud, C.; Vintila, F.; Cozzone, P.J. MR imaging and MR spectroscopy in rhizomelic chondrodysplasia punctata. AJNR Am. J. Neuroradiol. 2002, 23, 480–483. [Google Scholar]
- Cipolla, C.M.; Lodhi, I.J. Peroxisomal Dysfunction in Age-Related Diseases. Trends Endocrinol. Metab. 2017, 28, 297–308. [Google Scholar] [CrossRef]
- Andronie-Cioara, F.L.; Ardelean, A.I.; Nistor-Cseppento, C.D.; Jurcau, A.; Jurcau, M.C.; Pascalau, N.; Marcu, F. Molecular Mechanisms of Neuroinflammation in Aging and Alzheimer’s Disease Progression. Int. J. Mol. Sci. 2023, 24, 1869. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarkar, C.; Lipinski, M.M. Role and Function of Peroxisomes in Neuroinflammation. Cells 2024, 13, 1655. https://doi.org/10.3390/cells13191655
Sarkar C, Lipinski MM. Role and Function of Peroxisomes in Neuroinflammation. Cells. 2024; 13(19):1655. https://doi.org/10.3390/cells13191655
Chicago/Turabian StyleSarkar, Chinmoy, and Marta M. Lipinski. 2024. "Role and Function of Peroxisomes in Neuroinflammation" Cells 13, no. 19: 1655. https://doi.org/10.3390/cells13191655
APA StyleSarkar, C., & Lipinski, M. M. (2024). Role and Function of Peroxisomes in Neuroinflammation. Cells, 13(19), 1655. https://doi.org/10.3390/cells13191655






