Effect of Paternal Body Mass Index on Cumulative Live Birth Rates: Retrospective Analysis of 3048 Embryo Transfers in Couples Using Autologous Gametes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. IVF Laboratory Procedures
2.3. Clinical Data Collection
2.4. Statistical Analysis
3. Results
3.1. Baseline Patients and ART Characteristics
3.2. Paternal BMI and Clinical Outcomes
3.3. CLBR According to ET
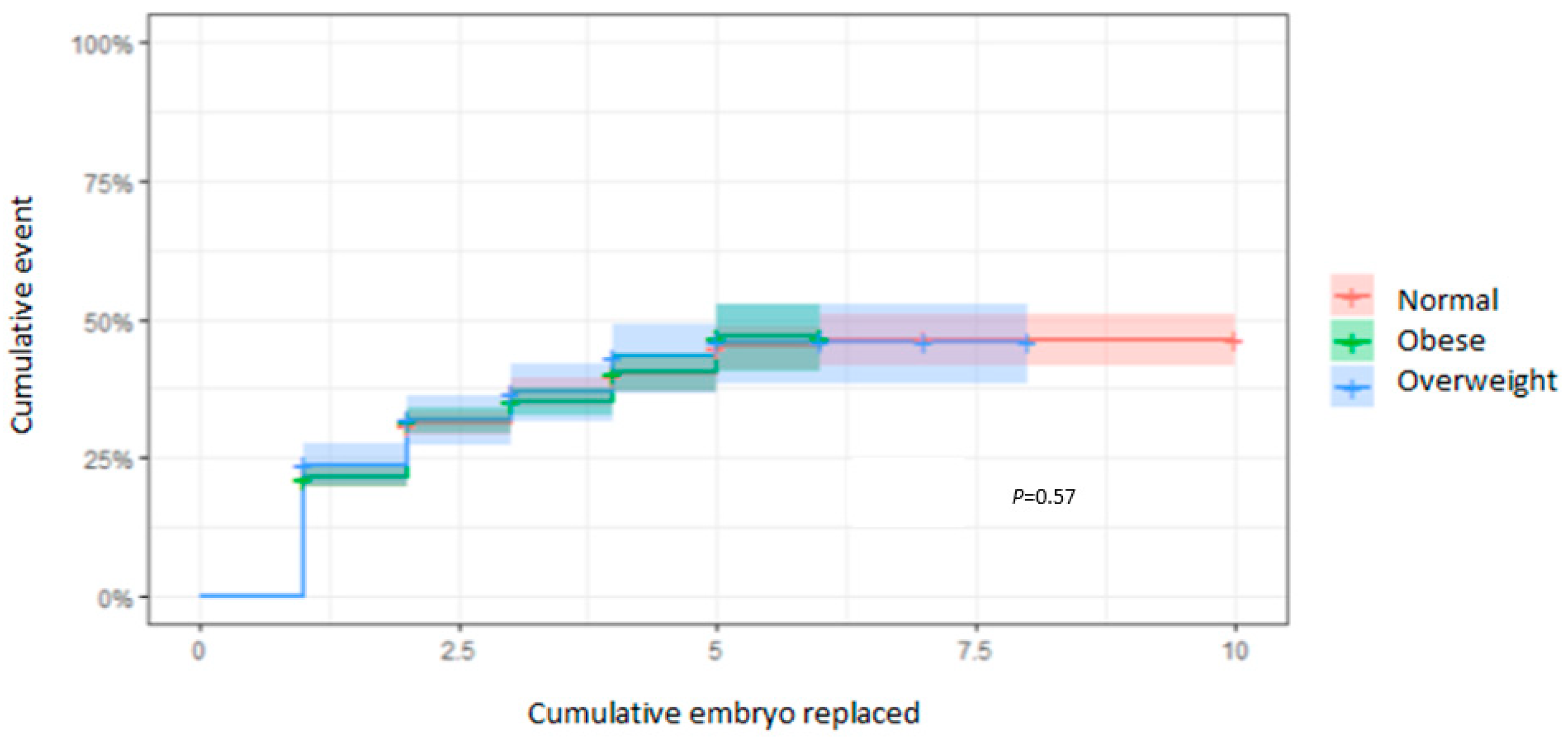
3.4. CLBR According to the EmbR
3.5. CLBR According to the Number of MII Oocytes Used
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Meldrum, D.; Morris, M.; Gambone, D. Obesity pandemic: Causes, consequences, and solutions-but do we have the will? Feril. Steril. 2017, 107, 833–839. [Google Scholar] [CrossRef] [PubMed]
- Visscher, T.; Seidell, J. The public health impact of obesity. Annu. Rev. Public Health 2001, 22, 355–375. [Google Scholar] [CrossRef] [PubMed]
- Jayawardena, R.; Ranasinghe, P.; Ranathunga, T.; Mathangasinghe, Y.; Wasalathanththri, S.; Hills, A.P. Novel anthropometric parameters to define obesity and obesity-related disease in adults: A systematic review. Nutr. Rev. 2020, 78, 498–513. [Google Scholar] [CrossRef] [PubMed]
- Chooi, Y.C.; Ding, C.; Magkos, F. The epidemiology of obesity. Metabolism 2019, 92, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Jing, X.; Wu, X.; Yan, M. Protective effect of resveratrol on spermatozoa function in male infertility induced by excess weight and obesity. Mol. Med. Rep. 2016, 14, 4659–4665. [Google Scholar] [CrossRef]
- Chavarro, J.; Toth, T.; Wright, D.; Meeker, J.; Hauser, R. Body mass index in relation to semen quality, sperm DNA integrity, and serum reproductive hormone levels among men attending an infertility clinic. Fertil. Steril. 2010, 93, 2222–2231. [Google Scholar] [CrossRef]
- Eisenberg, M.L.; Kim, S.; Chen, Z.; Sundaram, R.; Schisterman, E.F.; Buck Louis, G.M. The relationship between male BMI and waist circumference on semen quality: Data from the LIFE study. Hum. Reprod. 2014, 29, 193–200. [Google Scholar] [CrossRef]
- Arabipoor, A.; Ashrafi, M.; Hemat, M.; Zolfaghari, Z. The Effects of Maternal and Paternal Body Mass Index in Live Birth Rate after Intracytoplasmic Sperm Injection Cycles. Int. J. Fertil. Steril. 2019, 13, 24–31. [Google Scholar]
- Anifandis, G.; Dafopoulus, K.; Messini, C.I.; Polyzos, N.; Messinis, I.E. The BMI of men and not sperm parameters impact on embryo quality and the IVF outcome. Andrology 2013, 1, 85–89. [Google Scholar] [CrossRef]
- Provost, M.P.; Acharya, K.S.; Acharya, C.R.; Yeh, J.S.; Steward, R.G.; Eaton, J.L.; Goldfarb, J.M.; Muasher, S.J. Pregnancy outcomes decline with increasing body mass index: Analysis of 239,127 fresh autologous in vitro fertilization cycles from the 2008–2010 Society for Assisted Reproductive Technology registry. Fert. Steril. 2016, 105, 663–669. [Google Scholar] [CrossRef]
- Cancino-Villarreal, P.; González-Ortega, C.; Calull-Bagó, A.; Zúñiga-Sánchez, P.; Gutiérrez-Gutiérrez, A.M. Impact of male body mass index on ICSI. Ginecol. Obstet. Mex. 2017, 85, 531–540. [Google Scholar]
- Liu, Z.; Shi, X.; Wang, L.; Yang, Y.; Fu, Q.; Tao, M. Associations between male reproductive characteristics and the outcome of assisted reproductive technology (ART). Biosci. Rep. 2017, 37, BSR20170095. [Google Scholar] [CrossRef] [PubMed]
- Hervas, I.; Pacheco, A.; Gil, M.; Rivera, R.; Navarro, A.; Garrido, N. Sperm deoxyribonucleic acid fragmentation (by terminal deoxynucleotidyl transferase biotin dUTP nick end labeling assay) does not impair reproductive success measured as cumulative live birth rates per donor metaphase II oocyte used. Fertil. Steril. 2022, 118, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Esbert, M.; Pacheco, A.; Soares, S.; Amorós, D.; Florensa, M.; Ballesteros, A.; Meseguer, M. High Sperm DNA Fragmentation Delays Human Embryo Kinetics When Oocytes from Young and Healthy Donors Are Microinjected. Andrology 2018, 6, 697–706. [Google Scholar] [CrossRef]
- Cobo, A.; Garcia-Velasco, J.A.; Remohi, J.; Pellicer, A. Ooocyte vitrification for fertility preservation for both medical and non-medical reasons. Fertil. Steril. 2021, 115, 1091–1101. [Google Scholar] [CrossRef]
- Zheng, D.; Zeng, L.; Yang, R.; Lian, Y.; Zhu, Y.; Liang, X.; Tang, L.; Wang, H.; Cao, Y.; Hao, G.; et al. Intracytoplasmic sperm injection (ICSI) versus conventional in vitro fertilization (IVF) in couples with non-severe male infertility (NSMI-ICSI): Protocol for a multicenter randomized controlled trial. BMJ Open 2019, 9, e030366. [Google Scholar] [CrossRef]
- Bellver, J.; Melo, M.; Bosch, E.; Serra, V.; Remohi, J.; Pellicer, A. Obesity and poor reproductive outcome: The potential role of the endometrium. Fertil. Steril. 2007, 88, 446–451. [Google Scholar] [CrossRef]
- Jensen, T.K.; Andersson, A.M.; Jorgensen, N.; Andersen, A.G.; Carlsen, E.; Petersen, J.H. Body mass index in relation to semen quality and reproductive hormones among 1558 Danish men. Fertil. Steril. 2004, 82, 863–870. [Google Scholar] [CrossRef]
- Koloszar, S.; Fejes, I.; Zavaczki, Z.; Daru, J.; Szollosi, J.; Pal, A. Effect of body weight on sperm concentration in normozoospermic males. Arch. Androl. 2005, 51, 299–304. [Google Scholar] [CrossRef]
- Kort, H.I.; Massey, J.B.; Elsner, C.W.; Mitchell-Leef, D.; Shapiro, D.B.; Witt, M.A. Impact of body mass index values on sperm quantity and quality. J. Androl. 2006, 27, 450–452. [Google Scholar] [CrossRef]
- Fejes, I.; Koloszar, S.; Zavaczki, Z.; Daru, J.; Szollosi, J.; Pal, A. Effect of body weight on testosterone/estradiol ratio in oligozoospermic patients. Arch. Androl. 2006, 52, 97–102. [Google Scholar] [CrossRef]
- Hakonsen, L.B.; Thulstrup, A.M.; Aggerholm, A.S.; Olsen, J.; Bonde, J.P.; Andersen, C.Y. Does weight loss improve semen quality and reproductive hormones? Results from a cohort of severely obese men. Reprod. Healt. 2011, 8, 24. [Google Scholar] [CrossRef] [PubMed]
- Keltz, J.; Zapantis, A.; Jindal, S.K.; Lieman, H.J.; Santoro, N.; Polotsky, A.J. Overweight men: Clinical pregnancy after ART is decreased in IVF but not in ICSI cycles. J. Assist. Reprod. Genet. 2010, 27, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Merhi, Z.; Keltz, J.; Zapantis, A.; Younger, J.; Berger, D.; Lieman, H.; Jindal, S.; Polotsky, A. Male adiposity impairs clinical pregnancy rate by in vitro fertilization without a ecting day 3 embryo quality. Obesity 2013, 21, 1608–1612. [Google Scholar] [CrossRef] [PubMed]
- Colaci, D.; Afeiche, M.; Gaskins, A.; Wright, D.; Toth, T.; Tanrikut, C.; Hauser, R.; Chavarro, J. Men’s body mass index in relation to embryo quality and clinical outcomes in couples undergoing in vitro fertilization. Fertil. Steril. 2012, 98, 1193–1199. [Google Scholar] [CrossRef]
- Bakos, H.W.; Henshaw, R.C.; Mitchell, M.; Lane, M. Paternal body mass index is associated with decreased blastocyst development and reduced live birth rates following assisted reproductive technology. Fertil. Steril. 2011, 95, 1700–1704. [Google Scholar] [CrossRef]


| N (n = 1829) | OV (n = 1655) | OB (n = 451) | p Value | ||||
|---|---|---|---|---|---|---|---|
| Mean or Proportion | 95% CI | Mean or Proportion | 95% CI | Mean or Proportion | 95% CI | ||
| Paternal age (years) | 40.0 | 39.8–40.2 | 40.6 | 40.4–40.9 | 40.4 | 39.9–40.8 | <0.001 |
| Paternal BMI (kg/m2) | 23.2 | 23.1–23.3 | 27.0 | 26.9–27.1 | 32.6 | 32.4–32.8 | <0.001 |
| Maternal age (years) | 38.2 | 38.1–38.4 | 38.3 | 38.1–38.5 | 38.0 | 37.6–38.4 | 0.31 |
| Maternal BMI (kg/m2) | 22.2 | 22.0–22.3 | 23.4 | 23.3–23.6 | 25.5 | 25.1–25.9 | <0.001 |
| Female indications | 0.19 | ||||||
| Altered karyotype | 0.4% (9/2112) | 0.2–0.8 | 0.4% (8/1984) | 0.2–0.8 | 0.8% (4/515) | 0.2–2.1 | |
| Low ovarian reserve | 10.9% (230/2112) | 9.6–12.3 | 10.1% (200/1984) | 8.8–11.5 | 7.8% (40/515) | 5.7–10.5 | |
| Endometriosis | 1.9% (40/2112) | 1.4–2.6 | 1.2% (24/1984) | 0.8–1.8 | 2.5% (13/515) | 1.4–4.4 | |
| Premature ovarian failure | 0.1% (3/2112) | 0.04–0.4 | 0.05% (1/1984) | 0–0.3 | 0% (0/515) | 0–0.9 | |
| Polycystic ovary syndrome | 2.7% (58/2112) | 2.1–3.6 | 2.4% (47/1984) | 1.8–3.2 | 2.9% (15/515) | 1.7–4.9 | |
| Other factors | 83.9% (1772/2112) | 82.2–85.4 | 85.9% (1704/1984) | 84.3–87.4 | 86.0% (443/515) | 82.6–88.8 | |
| Number of MII oocytes | 8.3 | 8.1–8.4 | 8.3 | 8.0–8.5 | 8.3 | 8.0–8.5 | 0.89 |
| Oocyte state | 0.06 | ||||||
| Fresh | 80.5% (1758/2185) | 80.1–80.7 | 80% (1624/2030) | 78.5–82.5 | 85.6% (458/535) | 85.1–86.1 | |
| Vitrified | 2.2% (48/2185) | 2.1–2.3 | 2.2% (44/2030) | 2.1–2.3 | 1.7% (9/535) | 1.5–1.9 | |
| Mixed | 17.3% (379/2185) | 16.9–17.6 | 17.8% (362/2030) | 17.6–17.9 | 12.7% (68/535) | 12.1–13.2 | |
| Cycle type | 0.02 | ||||||
| Stimulated | 94.5% (2064/2185) | 94.1–94.8 | 92.1% (1870/2030) | 91.5–92.6 | 94.8% (507/535) | 94.4–95.2 | |
| Natural | 1.1% (24/2185) | 1.0–1.2 | 1.3% (27/2030) | 1.0–1.5 | 1.2% (4/535) | 1.0–1.4 | |
| Substituted | 4.4% (97/2185) | 4.1–4.6 | 6.5% (133/2030) | 6.2–6.9 | 4.5% (24/535) | 4.1–4.9 | |
| Capacitation method | 0.02 | ||||||
| Density gradient | 68.7% | 68.1–68.9 | 66.2% | 66.1–66.3 | 62.1% | 61.8–62.4 | |
| Swim-up | 26.5% | 26.1–26.8 | 28.7% | 28.2–29.1 | 33.6% | 33.4–33.9 | |
| Only washed | 4.7% | 4.1–4.9 | 5.1% | 4.7–5.3 | 4.2% | 3.9–4.4 | |
| ET with PGT-A | 0.08 | ||||||
| Yes | 14.6% (139/952) | 14.3–14.9 | 18.4% (175/953) | 18.1–18.6 | 17.2% (39/227) | 17.1–17.3 | |
| No | 85.4% (813/952) | 85.1–85.6 | 81.6% (778/953) | 81.3–81.9 | 82.8% (188/227) | 82.4–83.2 | |
| N (n = 1829) | OV (n = 1655) | OB (n = 451) | |||||
|---|---|---|---|---|---|---|---|
| Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | p Value | |
| Seminal volume (mL) | 2.8 | 2.7–2.8 | 2.8 | 2.7–2.8 | 2.7 | 2.6–2.8 | 0.81 |
| Sperm concentration (×106/mL) | 43.8 | 42.3–45.2 | 41.8 | 40.3–43.3 | 41.5 | 38.5–44.5 | 0.14 |
| Progressive sperm (%) | 39.2 | 38.5–39.9 | 39.3 | 38.5–40.1 | 40.4 | 38.8–41.9 | 0.42 |
| Non-progressive sperm (%) | 9.5 | 9.2–9.8 | 9.4 | 9.0–9.7 | 8.6 | 7.9–9.2 | 0.04 |
| Total motile sperm count | 118.0 | 108.0–128.1 | 112.8 | 101.6–124.1 | 115.1 | 92.3–137.9 | 0.79 |
| N | OV | OB | p Value | ||||
|---|---|---|---|---|---|---|---|
| Proportion (n = 1829) | 95% CI | Proportion (n = 1655) | 95% CI | Proportion (n = 451) | 95% CI | ||
| Implantation rate per EmbR | 57.6% (723/1255) | 54.8–60.4 | 54.6% (687/1258) | 51.8–57.4 | 58.5% (172/294) | 52.6–64.2 | 0.23 |
| Biochemical pregnancy rate per ET | 61.6% (842/1367) | 58.9–64.2 | 58.6% (801/1367) | 55.9–61.2 | 62.1% (195/314) | 56.5–67.4 | 0.22 |
| Clinical pregnancy rate per ET | 52.3% (715/1367) | 49.6–54.9 | 49.8% (681/1367) | 47.1–52.5 | 53.8% (169/314) | 48.1–59.4 | 0.28 |
| Ongoing pregnancy rate per ET | 42.6% (583/1367) | 40.0–45.3 | 39.5% (540/1367) | 36.9–42.2 | 43.3% (136/314) | 37.8–49.0 | 0.18 |
| Biochemical miscarriage rate per ET | 8.7% (119/1367) | 7.3–10.4 | 8.3% (113/1367) | 6.9–9.9 | 7.6% (24/314) | 5.1–11.3 | 0.81 |
| Clinical miscarriage rate per ET | 7.9% (109/1367) | 6.6–9.6 | 8.3% (114/1367) | 6.9–9.9 | 7.9% (25/314) | 5.3–11.7 | 0.93 |
| OR | 95% CI | p Value | |
|---|---|---|---|
| Implantation rate per EmbR | 1.0 | 0.9–1.1 | 0.79 |
| Biochemical pregnancy rate per ET | 0.9 | 0.9–1.0 | 0.82 |
| Clinical pregnancy rate per ET | 1.0 | 0.9–1.1 | 0.79 |
| Ongoing pregnancy rate per ET | 1.0 | 0.9–1.0 | 0.1 |
| Biochemical miscarriage rate per ET | 0.9 | 0.9–1.0 | 0.3 |
| Clinical miscarriage rate per ET | 1.0 | 0.9–1.0 | 0.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mossetti, L.; Hervás-Herrero, I.; Gil-Juliá, M.; Navarro Gomez-Lechon, A.; Pacheco-Rendón, R.M.; Rivera-Egea, R.; Garrido-Puchalt, N. Effect of Paternal Body Mass Index on Cumulative Live Birth Rates: Retrospective Analysis of 3048 Embryo Transfers in Couples Using Autologous Gametes. Cells 2024, 13, 1836. https://doi.org/10.3390/cells13221836
Mossetti L, Hervás-Herrero I, Gil-Juliá M, Navarro Gomez-Lechon A, Pacheco-Rendón RM, Rivera-Egea R, Garrido-Puchalt N. Effect of Paternal Body Mass Index on Cumulative Live Birth Rates: Retrospective Analysis of 3048 Embryo Transfers in Couples Using Autologous Gametes. Cells. 2024; 13(22):1836. https://doi.org/10.3390/cells13221836
Chicago/Turabian StyleMossetti, Laura, Irene Hervás-Herrero, María Gil-Juliá, Ana Navarro Gomez-Lechon, Rosa María Pacheco-Rendón, Rocío Rivera-Egea, and Nicolás Garrido-Puchalt. 2024. "Effect of Paternal Body Mass Index on Cumulative Live Birth Rates: Retrospective Analysis of 3048 Embryo Transfers in Couples Using Autologous Gametes" Cells 13, no. 22: 1836. https://doi.org/10.3390/cells13221836
APA StyleMossetti, L., Hervás-Herrero, I., Gil-Juliá, M., Navarro Gomez-Lechon, A., Pacheco-Rendón, R. M., Rivera-Egea, R., & Garrido-Puchalt, N. (2024). Effect of Paternal Body Mass Index on Cumulative Live Birth Rates: Retrospective Analysis of 3048 Embryo Transfers in Couples Using Autologous Gametes. Cells, 13(22), 1836. https://doi.org/10.3390/cells13221836






