Neonatal Brains Exhibit Higher Neural Reparative Activities than Adult Brains in a Mouse Model of Ischemic Stroke
Abstract
1. Introduction
2. Materials and Methods
2.1. Induction of Ischemic Stroke in Mice
2.2. Ischemic Volume Evaluation
2.3. Preparation of Poststroke Brain Samples
2.4. Ischemic Area Evaluation
2.5. Immunohistochemistry
2.6. Cell Culture
2.7. Reverse Transcription Polymerase Chain Reaction (RT-PCR)
2.8. Microarray Analysis
2.9. Statistical Analysis
3. Results
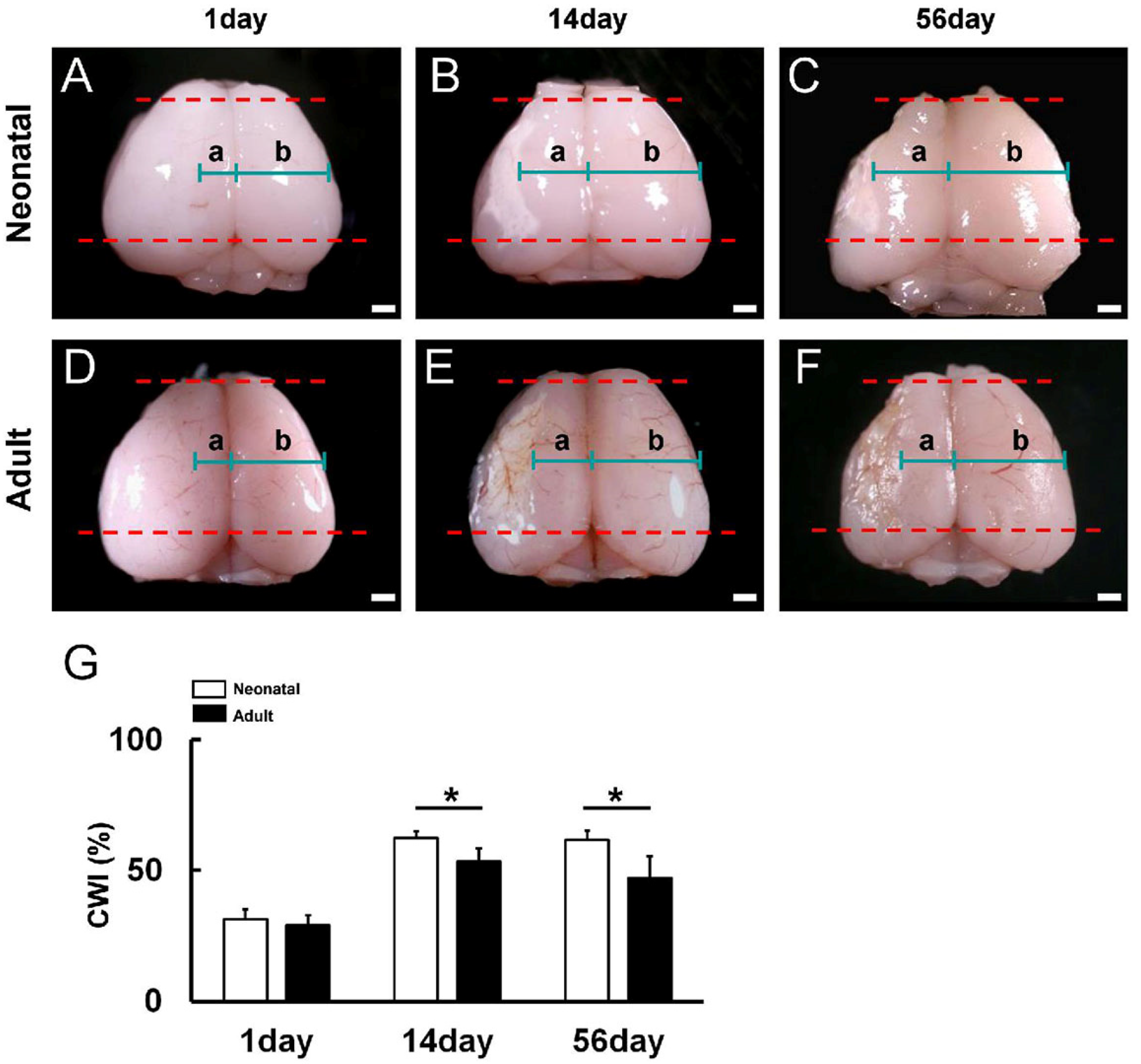
3.1. Reduced Poststroke Cortical Degeneration in Neonatal Brain Compared to Adult Brain following Ischemic Stroke
3.2. Reduced Ischemic Area Size in Neonatal Brain following Ischemic Stroke
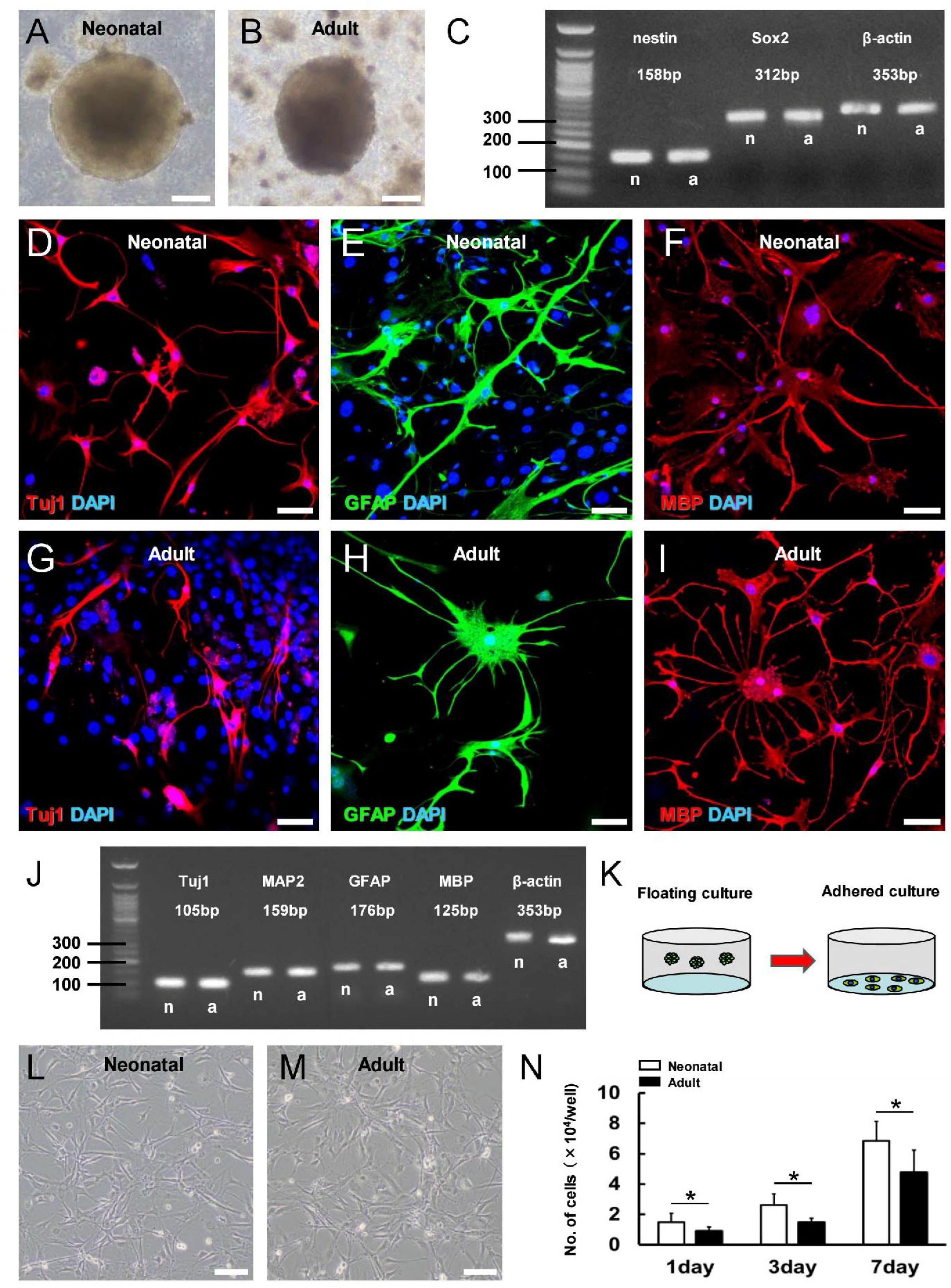
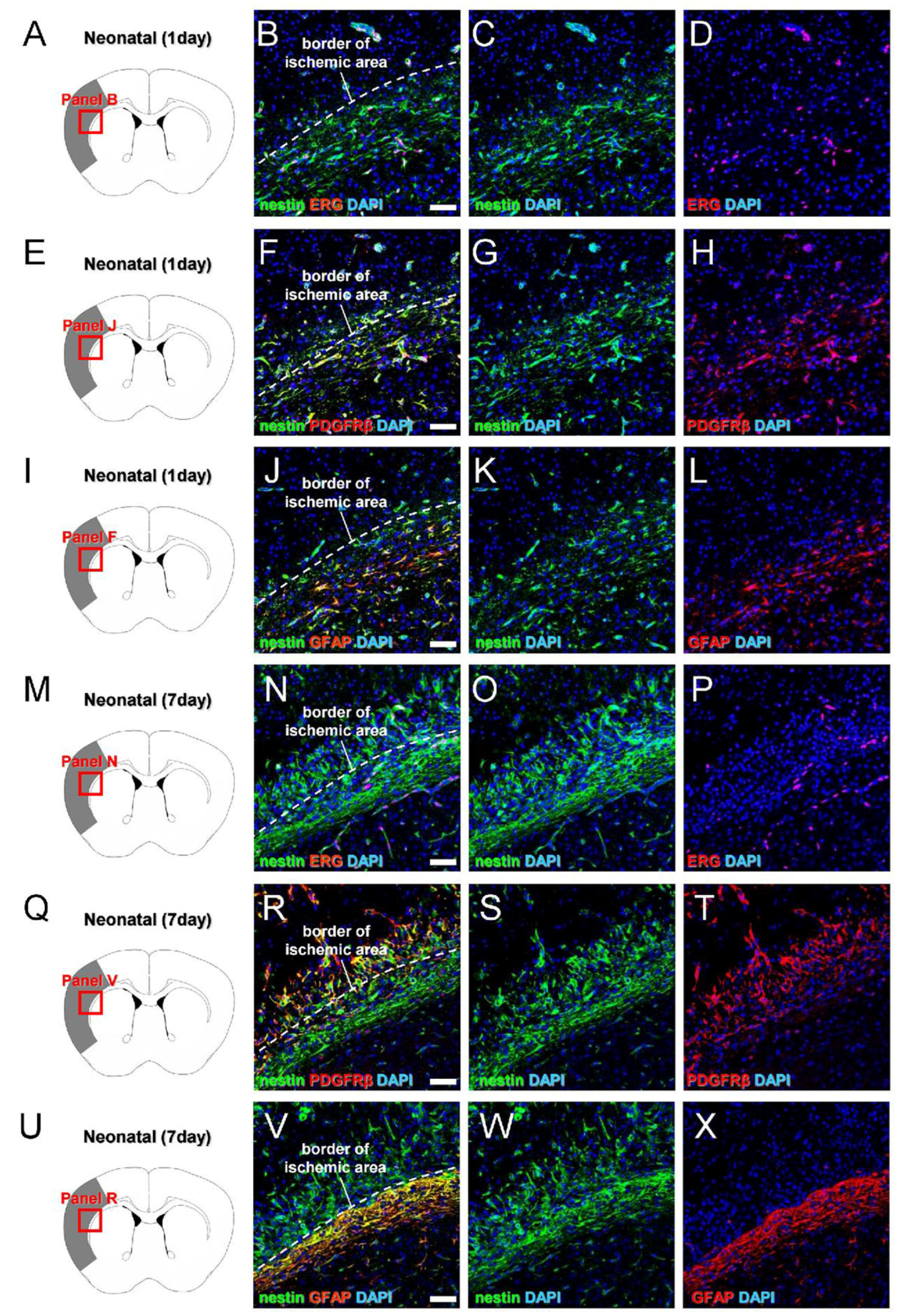
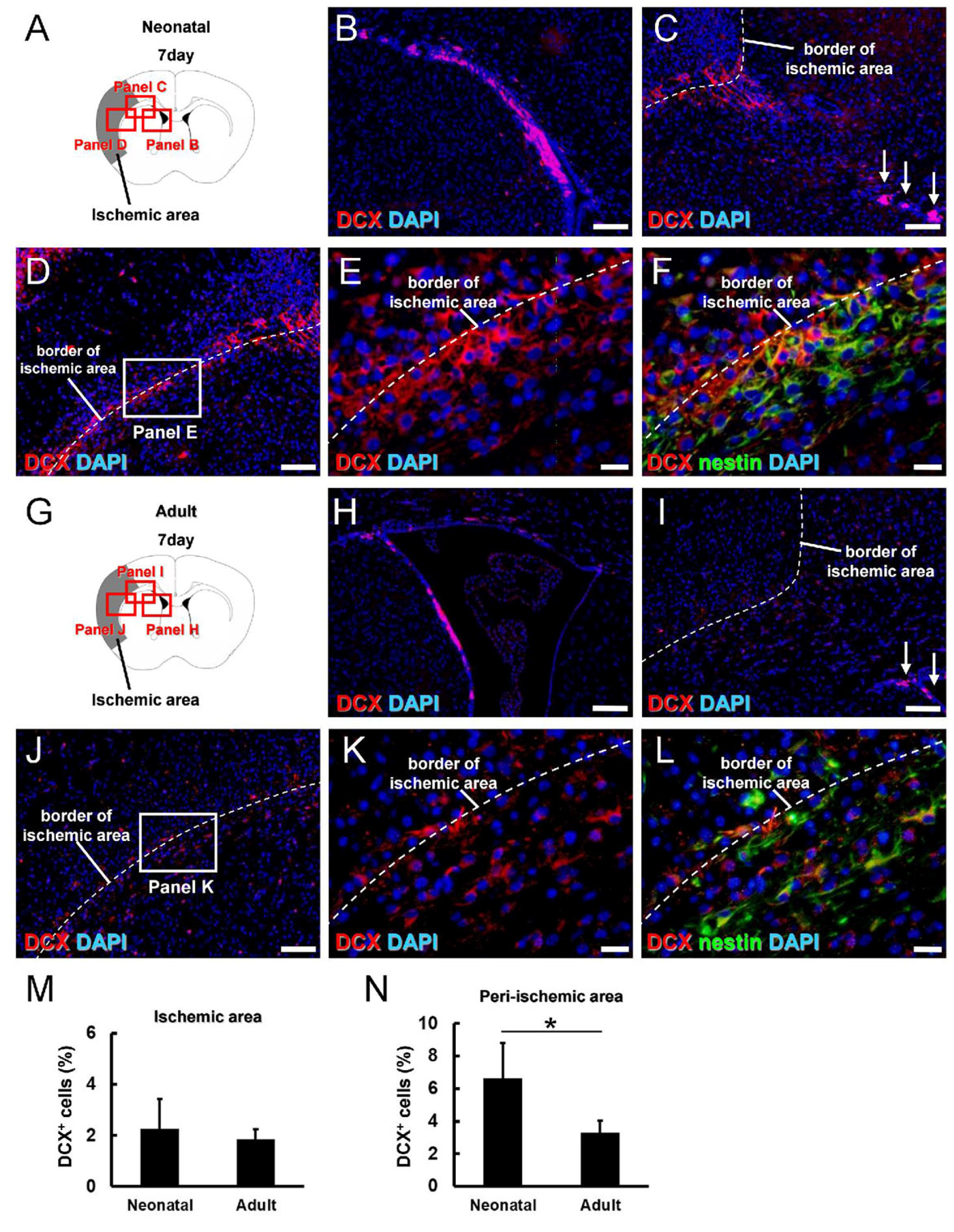
3.3. Greater Neural Stem/Progenitor Cell Generation in Neonatal Brain following Ischemic Stroke
3.4. Characterization of iNSPC Lineages and Phenotypes in Neonatal Brain after Ischemic Stroke
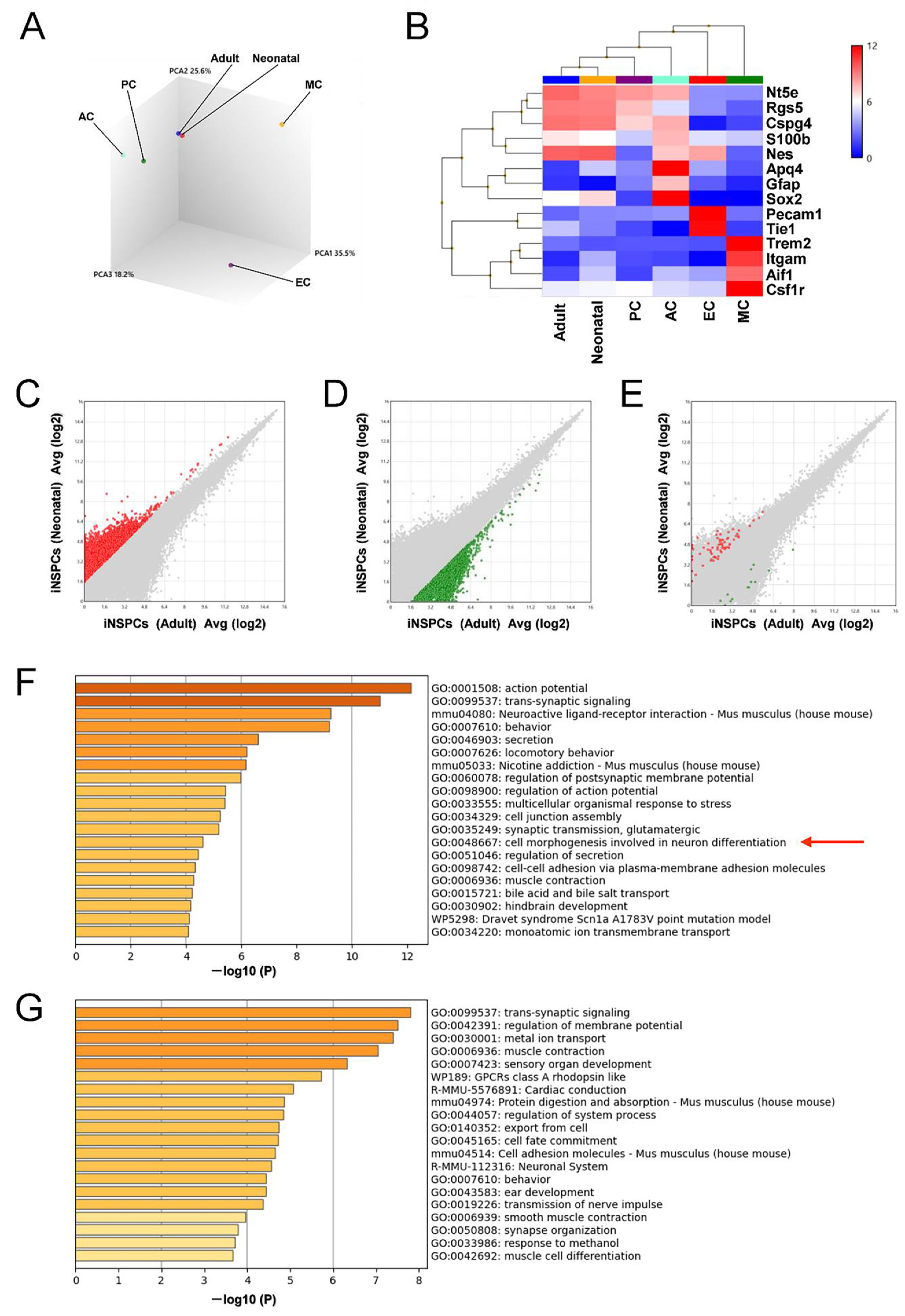
3.5. iNSPCs from Neonatal Brain Show a Greater Potential for Neurogenesis after Ischemic Stroke
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Merida-Rodrigo, L.; Poveda-Gomez, F.; Camafort-Babkowski, M.; Rivas-Ruiz, F.; Martin-Escalante, M.D.; Quiros-Lopez, R.; Garcia-Alegria, J. Long-term survival of ischemic stroke. Rev. Clin. Esp. 2012, 212, 223–228. [Google Scholar]
- Hankey, G.J.; Jamrozik, K.; Broadhurst, R.J.; Forbes, S.; Burvill, P.W.; Anderson, C.S.; Stewart-Wynne, E.G. Five-year survival after first-ever stroke and related prognostic factors in the Perth Community Stroke Study. Stroke 2000, 31, 2080–2086. [Google Scholar] [CrossRef]
- Varona, J.F.; Bermejo, F.; Guerra, J.M.; Molina, J.A. Long-term prognosis of ischemic stroke in young adults. Study of 272 cases. J. Neurol. 2004, 251, 1507–1514. [Google Scholar] [CrossRef]
- Clive, B.; Vincer, M.; Ahmad, T.; Khan, N.; Afifi, J.; El-Naggar, W. Epidemiology of neonatal stroke: A population-based study. Paediatr. Child. Health 2020, 25, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Perlman, J.M.; Rollins, N.K.; Evans, D. Neonatal stroke: Clinical characteristics and cerebral blood flow velocity measurements. Pediatr. Neurol. 1994, 11, 281–284. [Google Scholar] [CrossRef] [PubMed]
- Dunbar, M.; Mineyko, A.; Hill, M.; Hodge, J.; Floer, A.; Kirton, A. Population Based Birth Prevalence of Disease-Specific Perinatal Stroke. Pediatrics 2020, 146, e2020013201. [Google Scholar] [CrossRef] [PubMed]
- Elgendy, M.M.; Puthuraya, S.; LoPiccolo, C.; Liu, W.; Aly, H.; Karnati, S. Neonatal stroke: Clinical characteristics and neurodevelopmental outcomes. Pediatr. Neonatol. 2022, 63, 41–47. [Google Scholar] [CrossRef]
- Boardman, J.P.; Ganesan, V.; Rutherford, M.A.; Saunders, D.E.; Mercuri, E.; Cowan, F. Magnetic resonance image correlates of hemiparesis after neonatal and childhood middle cerebral artery stroke. Pediatrics 2005, 115, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Lynch, J.K.; Hirtz, D.G.; DeVeber, G.; Nelson, K.B. Report of the National Institute of Neurological Disorders and Stroke workshop on perinatal and childhood stroke. Pediatrics 2002, 109, 116–123. [Google Scholar] [CrossRef]
- Aden, U. Neonatal stroke is not a harmless condition. Stroke 2009, 40, 1948–1949. [Google Scholar] [CrossRef][Green Version]
- Doetsch, F.; Caille, I.; Lim, D.A.; Garcia-Verdugo, J.M.; Alvarez-Buylla, A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell 1999, 97, 703–716. [Google Scholar] [CrossRef]
- Mignone, J.L.; Kukekov, V.; Chiang, A.S.; Steindler, D.; Enikolopov, G. Neural stem and progenitor cells in nestin-GFP transgenic mice. J. Comp. Neurol. 2004, 469, 311–324. [Google Scholar] [CrossRef]
- Kuhn, H.G.; Dickinson-Anson, H.; Gage, F.H. Neurogenesis in the dentate gyrus of the adult rat: Age-related decrease of neuronal progenitor proliferation. J. Neurosci. 1996, 16, 2027–2033. [Google Scholar] [CrossRef]
- Nishie, H.; Nakano-Doi, A.; Sawano, T.; Nakagomi, T. Establishment of a Reproducible Ischemic Stroke Model in Nestin-GFP Mice with High Survival Rates. Int. J. Mol. Sci. 2021, 22, 12997. [Google Scholar] [CrossRef]
- Tanaka, Y.; Nakagomi, N.; Doe, N.; Nakano-Doi, A.; Sawano, T.; Takagi, T.; Matsuyama, T.; Yoshimura, S.; Nakagomi, T. Early Reperfusion Following Ischemic Stroke Provides Beneficial Effects, Even After Lethal Ischemia with Mature Neural Cell Death. Cells 2020, 9, 1374. [Google Scholar] [CrossRef]
- Tsuji, M.; Ohshima, M.; Taguchi, A.; Kasahara, Y.; Ikeda, T.; Matsuyama, T. A novel reproducible model of neonatal stroke in mice: Comparison with a hypoxia-ischemia model. Exp. Neurol. 2013, 247, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Hirano, Y.; Nakagomi, T.; Nakano-Doi, A.; Kubo, S.; Minato, Y.; Sawano, T.; Sakagami, M.; Tsuzuki, K. Microglia Negatively Regulate the Proliferation and Neuronal Differentiation of Neural Stem/Progenitor Cells Isolated from Poststroke Mouse Brains. Cells 2023, 12, 2040. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, A.; Soma, T.; Tanaka, H.; Kanda, T.; Nishimura, H.; Yoshikawa, H.; Tsukamoto, Y.; Iso, H.; Fujimori, Y.; Stern, D.M.; et al. Administration of CD34+ cells after stroke enhances neurogenesis via angiogenesis in a mouse model. J. Clin. Invest. 2004, 114, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Beppu, M.; Nakagomi, T.; Takagi, T.; Nakano-Doi, A.; Sakuma, R.; Kuramoto, Y.; Tatebayashi, K.; Matsuyama, T.; Yoshimura, S. Isolation and Characterization of Cerebellum-Derived Stem Cells in Poststroke Human Brain. Stem Cells Dev. 2019, 28, 528–542. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhou, B.; Pache, L.; Chang, M.; Khodabakhshi, A.H.; Tanaseichuk, O.; Benner, C.; Chanda, S.K. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat. Commun. 2019, 10, 1523. [Google Scholar] [CrossRef] [PubMed]
- Isayama, K.; Pitts, L.H.; Nishimura, M.C. Evaluation of 2,3,5-triphenyltetrazolium chloride staining to delineate rat brain infarcts. Stroke 1991, 22, 1394–1398. [Google Scholar] [CrossRef]
- Liszczak, T.M.; Hedley-Whyte, E.T.; Adams, J.F.; Han, D.H.; Kolluri, V.S.; Vacanti, F.X.; Heros, R.C.; Zervas, N.T. Limitations of tetrazolium salts in delineating infarcted brain. Acta Neuropathol. 1984, 65, 150–157. [Google Scholar]
- Nakagomi, T.; Kubo, S.; Nakano-Doi, A.; Sakuma, R.; Lu, S.; Narita, A.; Kawahara, M.; Taguchi, A.; Matsuyama, T. Brain vascular pericytes following ischemia have multipotential stem cell activity to differentiate into neural and vascular lineage cells. Stem Cells 2015, 33, 1962–1974. [Google Scholar]
- Shimada, I.S.; LeComte, M.D.; Granger, J.C.; Quinlan, N.J.; Spees, J.L. Self-renewal and differentiation of reactive astrocyte-derived neural stem/progenitor cells isolated from the cortical peri-infarct area after stroke. J. Neurosci. 2012, 32, 7926–7940. [Google Scholar] [CrossRef]
- Shimada, I.S.; Peterson, B.M.; Spees, J.L. Isolation of locally derived stem/progenitor cells from the peri-infarct area that do not migrate from the lateral ventricle after cortical stroke. Stroke 2010, 41, e552–e560. [Google Scholar] [CrossRef]
- Nakagomi, T.; Molnar, Z.; Taguchi, A.; Nakano-Doi, A.; Lu, S.; Kasahara, Y.; Nakagomi, N.; Matsuyama, T. Leptomeningeal-derived doublecortin-expressing cells in poststroke brain. Stem Cells Dev. 2012, 21, 2350–2354. [Google Scholar] [CrossRef]
- Decimo, I.; Bifari, F.; Rodriguez, F.J.; Malpeli, G.; Dolci, S.; Lavarini, V.; Pretto, S.; Vasquez, S.; Sciancalepore, M.; Montalbano, A.; et al. Nestin- and doublecortin-positive cells reside in adult spinal cord meninges and participate in injury-induced parenchymal reaction. Stem Cells 2011, 29, 2062–2076. [Google Scholar] [PubMed]
- Semple, B.D.; Blomgren, K.; Gimlin, K.; Ferriero, D.M.; Noble-Haeusslein, L.J. Brain development in rodents and humans: Identifying benchmarks of maturation and vulnerability to injury across species. Prog. Neurobiol. 2013, 106–107, 1–16. [Google Scholar]
- Zalewska, A. Developmental milestones in neonatal and juvenile C57Bl/6 mouse—Indications for the design of juvenile toxicity studies. Reprod. Toxicol. 2019, 88, 91–128. [Google Scholar] [CrossRef] [PubMed]
- Orr, M.E.; Garbarino, V.R.; Salinas, A.; Buffenstein, R. Extended Postnatal Brain Development in the Longest-Lived Rodent: Prolonged Maintenance of Neotenous Traits in the Naked Mole-Rat Brain. Front. Neurosci. 2016, 10, 504. [Google Scholar] [PubMed]
- Alvarez-Buylla, A.; Garcia-Verdugo, J.M. Neurogenesis in adult subventricular zone. J. Neurosci. 2002, 22, 629–634. [Google Scholar] [CrossRef]
- Magavi, S.S.; Leavitt, B.R.; Macklis, J.D. Induction of neurogenesis in the neocortex of adult mice. Nature 2000, 405, 951–955. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Covey, M.V.; Bitel, C.L.; Ni, L.; Jonakait, G.M.; Levison, S.W. Sustained neocortical neurogenesis after neonatal hypoxic/ischemic injury. Ann. Neurol. 2007, 61, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Gu, W.; Brannstrom, T.; Rosqvist, R.; Wester, P. Cortical neurogenesis in adult rats after transient middle cerebral artery occlusion. Stroke 2001, 32, 1201–1207. [Google Scholar] [CrossRef]
- Wilems, T.; Vardhan, S.; Wu, S.; Sakiyama-Elbert, S. The influence of microenvironment and extracellular matrix molecules in driving neural stem cell fate within biomaterials. Brain Res. Bull. 2019, 148, 25–33. [Google Scholar]
- Kazanis, I.; ffrench-Constant, C. Extracellular matrix and the neural stem cell niche. Dev. Neurobiol. 2011, 71, 1006–1017. [Google Scholar] [CrossRef] [PubMed]
- Porte, B.; Hardouin, J.; Zerdoumi, Y.; Derambure, C.; Hauchecorne, M.; Dupre, N.; Obry, A.; Lequerre, T.; Bekri, S.; Gonzalez, B.; et al. Major remodeling of brain microvessels during neonatal period in the mouse: A proteomic and transcriptomic study. J. Cereb. Blood Flow Metab. 2017, 37, 495–513. [Google Scholar] [CrossRef]
- Porte, B.; Chatelain, C.; Hardouin, J.; Derambure, C.; Zerdoumi, Y.; Hauchecorne, M.; Dupre, N.; Bekri, S.; Gonzalez, B.; Marret, S.; et al. Proteomic and transcriptomic study of brain microvessels in neonatal and adult mice. PLoS ONE 2017, 12, e0171048. [Google Scholar]
- Hamada, Y.; Ogata, S.; Masuda, T.; Ito, S.; Ohtsuki, S. Development of a method for isolating brain capillaries from a single neonatal mouse brain and comparison of proteomic profiles between neonatal and adult brain capillaries. Fluids Barriers CNS 2023, 20, 50. [Google Scholar] [CrossRef]
- Leroux, P.; Omouendze, P.L.; Roy, V.; Dourmap, N.; Gonzalez, B.J.; Brasse-Lagnel, C.; Carmeliet, P.; Leroux-Nicollet, I.; Marret, S. Age-dependent neonatal intracerebral hemorrhage in plasminogen activator inhibitor 1 knockout mice. J. Neuropathol. Exp. Neurol 2014, 73, 387–402. [Google Scholar] [CrossRef] [PubMed]
- Nakagomi, N.; Nakagomi, T.; Kubo, S.; Nakano-Doi, A.; Saino, O.; Takata, M.; Yoshikawa, H.; Stern, D.M.; Matsuyama, T.; Taguchi, A. Endothelial cells support survival, proliferation, and neuronal differentiation of transplanted adult ischemia-induced neural stem/progenitor cells after cerebral infarction. Stem Cells 2009, 27, 2185–2195. [Google Scholar] [PubMed]
- Takata, M.; Nakagomi, T.; Kashiwamura, S.; Nakano-Doi, A.; Saino, O.; Nakagomi, N.; Okamura, H.; Mimura, O.; Taguchi, A.; Matsuyama, T. Glucocorticoid-induced TNF receptor-triggered T cells are key modulators for survival/death of neural stem/progenitor cells induced by ischemic stroke. Cell Death Differ. 2012, 19, 756–767. [Google Scholar] [CrossRef]
- Sutherland, T.C.; Mathews, K.J.; Mao, Y.; Nguyen, T.; Gorrie, C.A. Differences in the Cellular Response to Acute Spinal Cord Injury between Developing and Mature Rats Highlights the Potential Significance of the Inflammatory Response. Front. Cell Neurosci. 2016, 10, 310. [Google Scholar] [CrossRef] [PubMed]
- Etchevers, H.C.; Vincent, C.; Le Douarin, N.M.; Couly, G.F. The cephalic neural crest provides pericytes and smooth muscle cells to all blood vessels of the face and forebrain. Development 2001, 128, 1059–1068. [Google Scholar] [CrossRef] [PubMed]
- Kondo, T.; Raff, M. Chromatin remodeling and histone modification in the conversion of oligodendrocyte precursors to neural stem cells. Genes. Dev. 2004, 18, 2963–2972. [Google Scholar] [CrossRef] [PubMed]
- Kondo, T.; Raff, M. Oligodendrocyte precursor cells reprogrammed to become multipotential CNS stem cells. Science 2000, 289, 1754–1757. [Google Scholar] [CrossRef] [PubMed]
- Kojima, T.; Hirota, Y.; Ema, M.; Takahashi, S.; Miyoshi, I.; Okano, H.; Sawamoto, K. Subventricular zone-derived neural progenitor cells migrate along a blood vessel scaffold toward the post-stroke striatum. Stem Cells 2010, 28, 545–554. [Google Scholar] [CrossRef]
- Ramaswamy, S.; Goings, G.E.; Soderstrom, K.E.; Szele, F.G.; Kozlowski, D.A. Cellular proliferation and migration following a controlled cortical impact in the mouse. Brain Res. 2005, 1053, 38–53. [Google Scholar] [CrossRef] [PubMed]
- Palma-Tortosa, S.; Garcia-Culebras, A.; Moraga, A.; Hurtado, O.; Perez-Ruiz, A.; Duran-Laforet, V.; Parra, J.; Cuartero, M.I.; Pradillo, J.M.; Moro, M.A.; et al. Specific Features of SVZ Neurogenesis After Cortical Ischemia: A Longitudinal Study. Sci. Rep. 2017, 7, 16343. [Google Scholar] [CrossRef] [PubMed]


| Primers | Sequence (5′→3′) (F: Forward; R: Reverse) | Size |
|---|---|---|
| β-actin | F: GCTCGTCGTCGACAAGGGCTC R: CAAACATGATCTGGGTCATCTTCTC | 353 bp |
| GFAP | F: TCGGCCAGTTACCAGGAGG R: ATGGTGATGCGGTTTTCTTCG | 176 bp |
| MAP2 | F: CTCATTCGCTGAGCCTTTAGAC R: ACTGGAGGCAACTTTTCTCCT | 159 bp |
| MBP | F: TCACAGCGATCCAAGTACCTG R: CCCCTGTCACCGCTAAAGAA | 125 bp |
| nestin | F: CGCTGGAACAGAGATTGGAAG R: CATCTTGAGGTGTGCCAGTT | 158 bp |
| Sox2 | F: TTGGGAGGGGTGCAAAAAGA R: CCTGCGAAGCGCCTAACGTA | 312 bp |
| Tuj1 | F: TGAGGCCTCCTCTCACAAGT R: GGCCTGAATAGGTGTCCAAA | 105 bp |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nishiyama, R.; Nakagomi, T.; Nakano-Doi, A.; Kuramoto, Y.; Tsuji, M.; Yoshimura, S. Neonatal Brains Exhibit Higher Neural Reparative Activities than Adult Brains in a Mouse Model of Ischemic Stroke. Cells 2024, 13, 519. https://doi.org/10.3390/cells13060519
Nishiyama R, Nakagomi T, Nakano-Doi A, Kuramoto Y, Tsuji M, Yoshimura S. Neonatal Brains Exhibit Higher Neural Reparative Activities than Adult Brains in a Mouse Model of Ischemic Stroke. Cells. 2024; 13(6):519. https://doi.org/10.3390/cells13060519
Chicago/Turabian StyleNishiyama, Ryo, Takayuki Nakagomi, Akiko Nakano-Doi, Yoji Kuramoto, Masahiro Tsuji, and Shinichi Yoshimura. 2024. "Neonatal Brains Exhibit Higher Neural Reparative Activities than Adult Brains in a Mouse Model of Ischemic Stroke" Cells 13, no. 6: 519. https://doi.org/10.3390/cells13060519
APA StyleNishiyama, R., Nakagomi, T., Nakano-Doi, A., Kuramoto, Y., Tsuji, M., & Yoshimura, S. (2024). Neonatal Brains Exhibit Higher Neural Reparative Activities than Adult Brains in a Mouse Model of Ischemic Stroke. Cells, 13(6), 519. https://doi.org/10.3390/cells13060519






