Lysine Distinctively Manipulates Myogenic Regulatory Factors and Wnt/Ca2+ Pathway in Slow and Fast Muscles, and Their Satellite Cells of Postnatal Piglets
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Animal Management and Study Design
2.3. Muscle Sample Collections and Processing
2.4. Protein Extraction and Assay
2.5. Western Blotting
2.6. Immunofluorescence Staining
2.7. Isolation and Culture of SCs
2.8. SCs Response to Lys Deficiency
2.9. Intracellular Ca2+ Assay
2.10. Statistical Analysis
3. Results
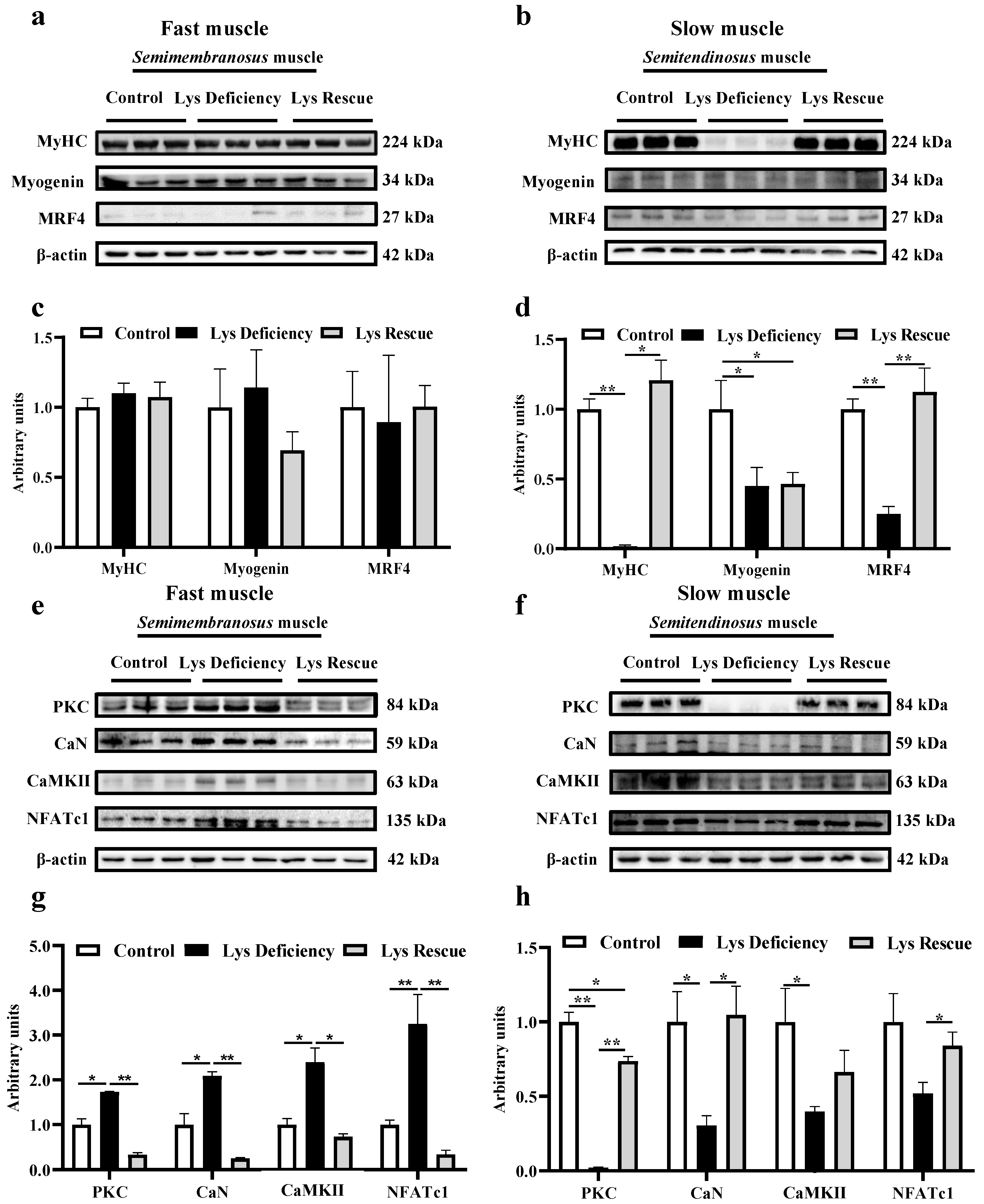
3.1. Dietary Lys Affect the Development of Both Fast (Semimembranosus) and Slow (Semitendinosus) Muscles
3.2. Lys Deficiency and Rescue Initiates Opposite Mechanism in Fast and Slow Muscles
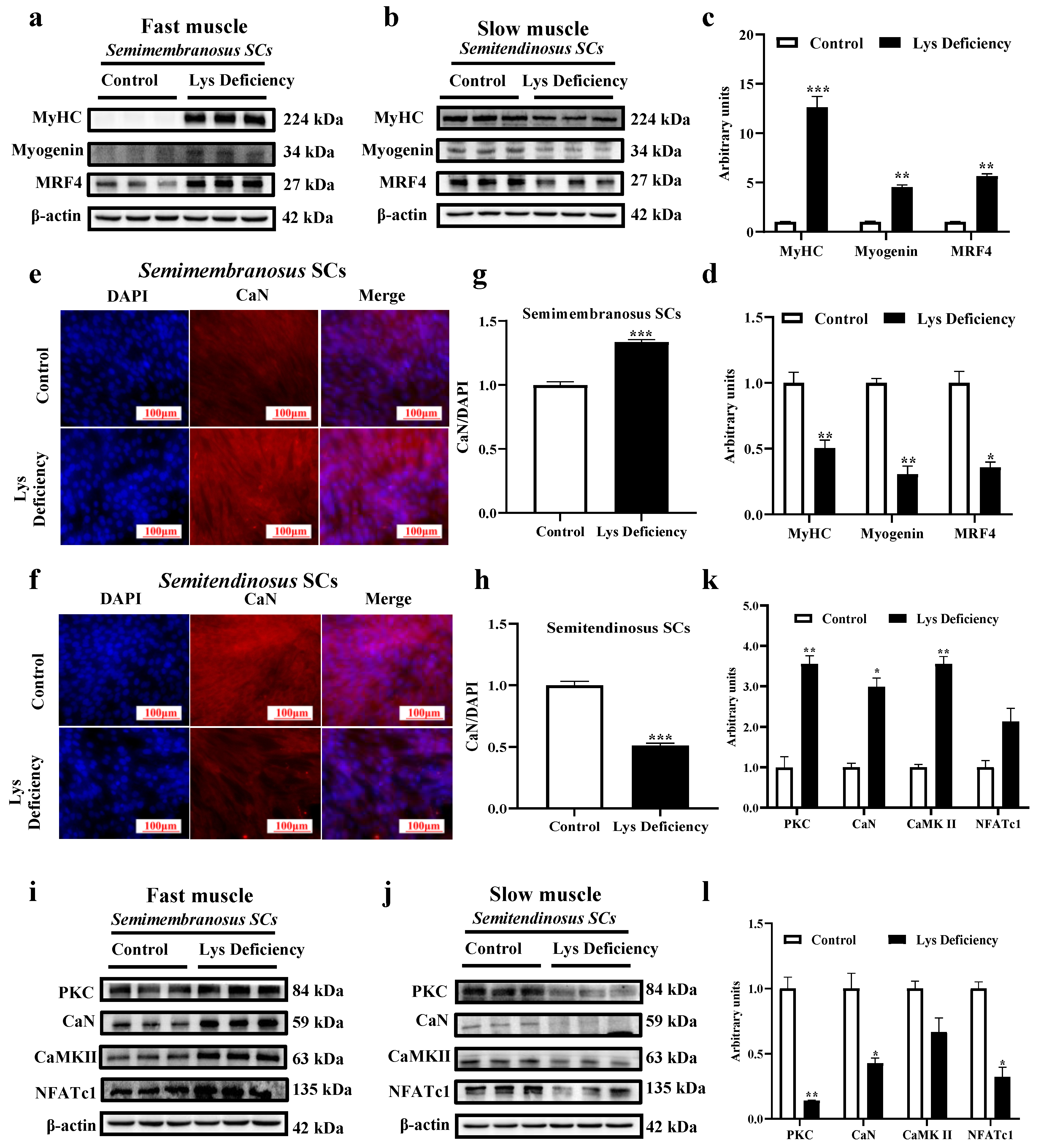
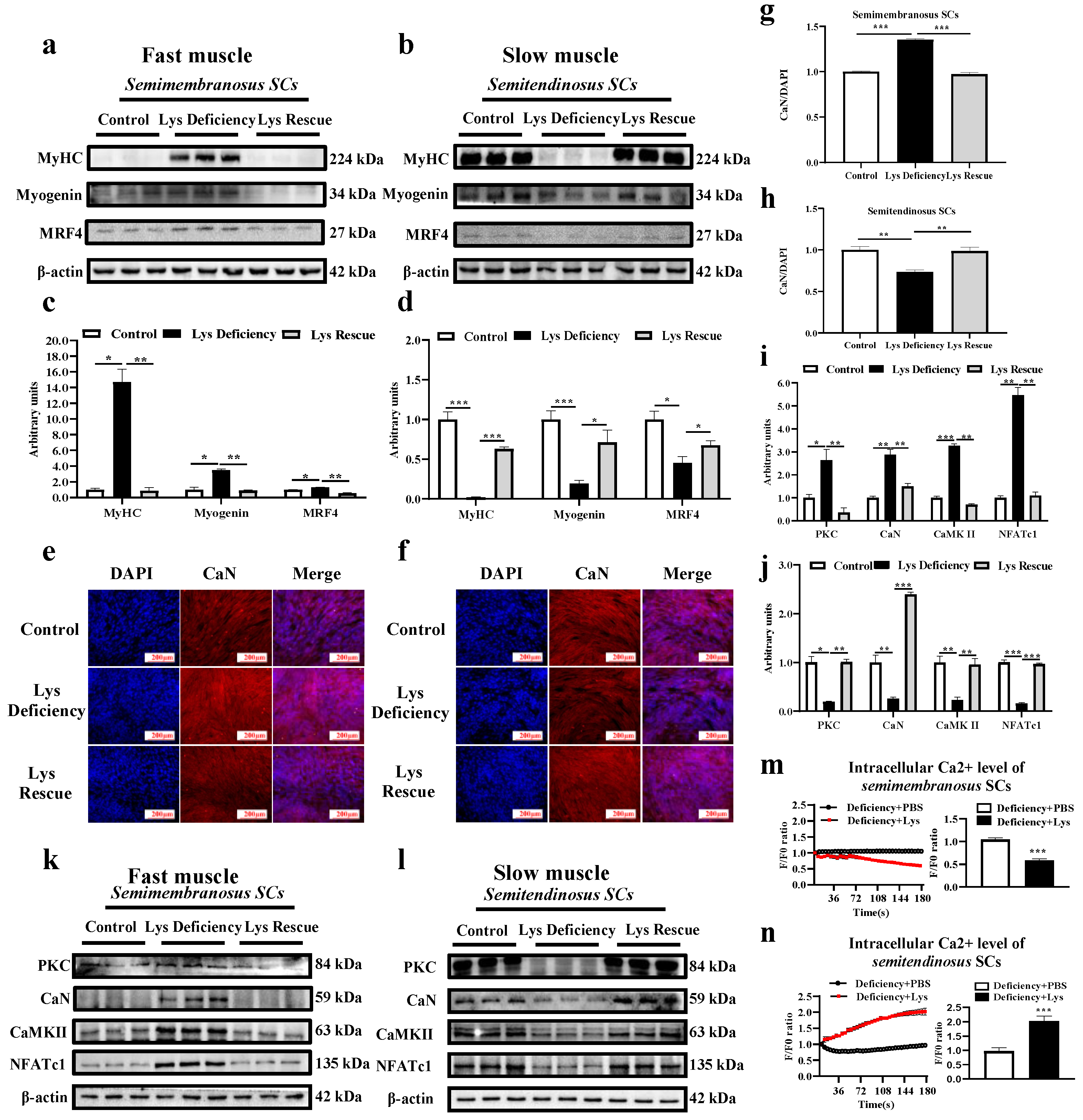
3.3. SC Differentiation in Response to Lys Deficiency
4. Discussion
4.1. Lys Affects Fast and Slow Muscle Development
4.2. Expressions of Myogenic Modulating Factors and MyHC in Fast and Slow Muscle SCs
4.3. Wnt/Ca2+ Pathway and Myogenic Differentiation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Mukund, K.; Subramaniam, S. Skeletal muscle: A review of molecular structure and function, in health and disease. Wiley Interdiscip. Rev. Syst. Biol. Med. 2020, 12, e1462. [Google Scholar] [CrossRef] [PubMed]
- Morgan, J.; Partridge, T. Skeletal muscle in health and disease. Dis. Models Mech. 2020, 13, dmm042192. [Google Scholar] [CrossRef] [PubMed]
- Bruusgaard, J.C.; Liestøl, K.; Gundersen, K. Distribution of myonuclei and microtubules in live muscle fibers of young, middle-aged, and old mice. J. Appl. Physiol. 2006, 100, 2024–2030. [Google Scholar] [CrossRef] [PubMed]
- Pala, F.; Di Girolamo, D.; Mella, S.; Yennek, S.; Chatre, L.; Ricchetti, M.; Tajbakhsh, S. Distinct metabolic states govern skeletal muscle stem cell fates during prenatal and postnatal myogenesis. J. Cell Sci. 2018, 131, jcs212977. [Google Scholar] [CrossRef] [PubMed]
- Wank, V.; Fischer, M.S.; Walter, B.; Bauer, R. Muscle growth and fiber type composition in hind limb muscles during postnatal development in pigs. Cells Tissues Organs 2006, 182, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Khodabukus, A.; Baar, K. Contractile and metabolic properties of engineered skeletal muscle derived from slow and fast phenotype mouse muscle. J. Cell. Physiol. 2015, 230, 1750–1757. [Google Scholar] [CrossRef] [PubMed]
- Collins, C.A.; Olsen, I.; Zammit, P.S.; Heslop, L.; Petrie, A.; Partridge, T.A.; Morgan, J.E. Stem cell function, self-renewal, and behavioral heterogeneity of cells from the adult muscle satellite cell niche. Cell 2005, 122, 289–301. [Google Scholar] [CrossRef] [PubMed]
- Zammit, P.S. Function of the myogenic regulatory factors Myf5, MyoD, Myogenin and MRF4 in skeletal muscle, satellite cells and regenerative myogenesis. Semin. Cell Dev. Biol. 2017, 72, 19–32. [Google Scholar] [CrossRef] [PubMed]
- Walters, E.H.; Stickland, N.C.; Loughna, P.T. MRF-4 exhibits fiber type- and muscle-specific pattern of expression in postnatal rat muscle. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2000, 278, R1381–R1384. [Google Scholar] [CrossRef]
- Voytik, S.L.; Przyborski, M.; Badylak, S.F.; Konieczny, S.F. Differential expression of muscle regulatory factor genes in normal and denervated adult rat hindlimb muscles. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 1993, 198, 214–224. [Google Scholar] [CrossRef]
- Hughes, S.M.; Taylor, J.M.; Tapscott, S.J.; Gurley, C.M.; Carter, W.J.; Peterson, C.A. Selective accumulation of MyoD and myogenin mRNAs in fast and slow adult skeletal muscle is controlled by innervation and hormones. Development 1993, 118, 1137–1147. [Google Scholar] [CrossRef]
- Otto, A.; Schmidt, C.; Luke, G.; Allen, S.; Valasek, P.; Muntoni, F.; Lawrence-Watt, D.; Patel, K. Canonical Wnt signalling induces satellite-cell proliferation during adult skeletal muscle regeneration. J. Cell Sci. 2008, 121, 2939–2950. [Google Scholar] [CrossRef] [PubMed]
- Le Grand, F.; Jones, A.E.; Seale, V.; Scimè, A.; Rudnicki, M.A. Wnt7a activates the planar cell polarity pathway to drive the symmetric expansion of satellite stem cells. Cell Stem Cell 2009, 4, 535–547. [Google Scholar] [CrossRef]
- Zhang, Z.K.; Li, J.; Guan, D.; Liang, C.; Zhuo, Z.; Liu, J.; Lu, A.; Zhang, G.; Zhang, B.T. A newly identified lncRNA MAR1 acts as a miR-487b sponge to promote skeletal muscle differentiation and regeneration. J. Cachexia Sarcopenia Muscle 2018, 9, 613–626. [Google Scholar] [CrossRef]
- Liao, S.F.; Wang, T.; Regmi, N. Lysine nutrition in swine and the related monogastric animals: Muscle protein biosynthesis and beyond. SpringerPlus 2015, 4, 147. [Google Scholar] [CrossRef]
- Liu, M.; Yue, Z.; Zhang, B.; Li, F.; Liu, L.; Li, F. mTORC1 Mediates the Processes of Lysine Regulating Satellite Cells Proliferation, Apoptosis, and Autophagy. Metabolites 2022, 12, 788. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.L.; Ye, M.; Song, Z.W.; Zhang, Z.M.; Gao, C.Q.; Yan, H.C.; Wang, X.Q. Lysine Interacts with Frizzled7 to Activate β-Catenin in Satellite Cell-Participated Skeletal Muscle Growth. J. Agric. Food Chem. 2022, 70, 3745–3756. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.L.; Zhang, Z.M.; Ye, J.L.; Gao, C.Q.; Yan, H.C.; Li, H.C.; Yang, J.Z.; Wang, X.Q. Lysine-induced swine satellite cell migration is mediated by the FAK pathway. Food Funct. 2019, 10, 583–591. [Google Scholar] [CrossRef]
- Jin, C.-L.; Ye, J.-L.; Yang, J.; Gao, C.-Q.; Yan, H.-C.; Li, H.-C.; Wang, X.-Q. mTORC1 mediates lysine-induced satellite cell activation to promote skeletal muscle growth. Cells 2019, 8, 1549. [Google Scholar] [CrossRef]
- Wang, X.Q.; Yang, W.J.; Yang, Z.; Shu, G.; Wang, S.B.; Jiang, Q.Y.; Yuan, L.; Wu, T.S. The differential proliferative ability of satellite cells in Lantang and Landrace pigs. PLoS ONE 2012, 7, e32537. [Google Scholar] [CrossRef]
- Jin, C.-L.; Zhang, Z.-M.; Song, Z.-W.; Gao, C.-Q.; Yan, H.-C.; Wang, X.-Q. mTORC1-mediated satellite cell differentiation is required for lysine-induced skeletal muscle growth. J. Agric. Food Chem. 2020, 68, 4884–4892. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Ito, Y.; Nagasawa, T. Dietary L-lysine suppresses autophagic proteolysis and stimulates Akt/mTOR signaling in the skeletal muscle of rats fed a low-protein diet. J. Agric. Food Chem. 2015, 63, 8192–8198. [Google Scholar] [CrossRef]
- Zeng, P.L.; Yan, H.C.; Wang, X.Q.; Zhang, C.M.; Zhu, C.; Shu, G.; Jiang, Q.Y. Effects of dietary lysine levels on apparent nutrient digestibility and serum amino Acid absorption mode in growing pigs. Asian-Australas J. Anim. Sci. 2013, 26, 1003–1011. [Google Scholar] [CrossRef] [PubMed]
- Tous, N.; Lizardo, R.; Vilà, B.; Gispert, M.; Font-i-Furnols, M.; Esteve-Garcia, E. Effect of reducing dietary protein and lysine on growth performance, carcass characteristics, intramuscular fat, and fatty acid profile of finishing barrows. J. Anim. Sci. 2014, 92, 129–140. [Google Scholar] [CrossRef]
- Verdijk, L.B.; Snijders, T.; Drost, M.; Delhaas, T.; Kadi, F.; Van Loon, L.J. Satellite cells in human skeletal muscle; from birth to old age. Age 2014, 36, 545–557. [Google Scholar] [CrossRef]
- Kuang, S.; Gillespie, M.A.; Rudnicki, M.A. Niche regulation of muscle satellite cell self-renewal and differentiation. Cell Stem Cell 2008, 2, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Hernández, J.M.; García-González, E.G.; Brun, C.E.; Rudnicki, M.A. The myogenic regulatory factors, determinants of muscle development, cell identity and regeneration. Semin. Cell Dev. Biol. 2017, 72, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Ekmark, M.; Rana, Z.A.; Stewart, G.; Hardie, D.G.; Gundersen, K. De-phosphorylation of MyoD is linking nerve-evoked activity to fast myosin heavy chain expression in rodent adult skeletal muscle. J. Physiol. 2007, 584, 637–650. [Google Scholar] [CrossRef]
- Agüera, E.; Castilla, S.; Luque, E.; Jimena, I.; Leiva Cepas, F.; Ruz Caracuel, I.; Peña, J. Muscular hypertrophy and atrophy in normal rats provoked by the administration of normal and denervated muscle extracts. Histol. Histopathol. 2016, 31, 1367–1379. [Google Scholar] [PubMed]
- Moreira-Pais, A.; Amado, F.; Vitorino, R.; Coriolano, H.-J.A.; Duarte, J.A.; Ferreira, R. The Signaling Pathways Involved in the Regulation of Skeletal Muscle Plasticity. In Tissue-Specific Cell Signaling; Silva, J.V., Freitas, M.J., Fardilha, M., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 383–408. [Google Scholar] [CrossRef]
- Motohashi, N.; Asakura, A. Muscle satellite cell heterogeneity and self-renewal. Front. Cell Dev. Biol. 2014, 2, 1. [Google Scholar] [CrossRef]
- Müller, C.; Leutz, A. Chromatin remodeling in development and differentiation. Curr. Opin. Genet. Dev. 2001, 11, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.H.; Chen, W.P.; Yan, W.L.; Huang, Y.C.; Chang, S.W.; Fu, W.M.; Su, M.J.; Yu, I.S.; Tsai, T.C.; Yan, Y.T.; et al. NRIP is newly identified as a Z-disc protein, activating calmodulin signaling for skeletal muscle contraction and regeneration. J. Cell Sci. 2015, 128, 4196–4209. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Song, C.; Li, H.; Cao, X.; Ma, Y.; Wang, X.; Huang, Y.; Lan, X.; Lei, C.; Chaogetu, B. Circular RNA SNX29 sponges miR-744 to regulate proliferation and differentiation of myoblasts by activating the Wnt5a/Ca2+ signaling pathway. Mol. Ther. -Nucleic Acids 2019, 16, 481–493. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Shi, S.; Deng, M.; Tang, L.; Zhang, G.; Liu, N.; Ding, B.; Liu, W.; Liu, Y.; Shi, H. High levels of β-catenin signaling reduce osteogenic differentiation of stem cells in inflammatory microenvironments through inhibition of the noncanonical Wnt pathway. J. Bone Miner. Res. 2011, 26, 2082–2095. [Google Scholar] [CrossRef] [PubMed]
- Rudolf, A.; Schirwis, E.; Giordani, L.; Parisi, A.; Lepper, C.; Taketo, M.M.; Le Grand, F. β-Catenin activation in muscle progenitor cells regulates tissue repair. Cell Rep. 2016, 15, 1277–1290. [Google Scholar] [CrossRef] [PubMed]
- Cong, W.; Liu, B.; Liu, S.; Sun, M.; Liu, H.; Yang, Y.; Wang, R.; Xiao, J. Implications of the Wnt5a/CaMKII pathway in retinoic acid-induced myogenic tongue abnormalities of developing mice. Sci. Rep. 2014, 4, 6082. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Kanatous, S.B.; Thurmond, F.A.; Gallardo, T.; Isotani, E.; Bassel-Duby, R.; Williams, R.S. Regulation of mitochondrial biogenesis in skeletal muscle by CaMK. Science 2002, 296, 349–352. [Google Scholar] [CrossRef] [PubMed]
- Chin, E.R. Role of Ca2+/calmodulin-dependent kinases in skeletal muscle plasticity. J. Appl. Physiol. 2005, 99, 414–423. [Google Scholar] [CrossRef] [PubMed]
- Anakwe, K.; Robson, L.; Hadley, J.; Buxton, P.; Church, V.; Allen, S.; Hartmann, C.; Harfe, B.; Nohno, T.; Brown, A.M. Wnt signalling regulates myogenic differentiation in the developing avian wing. Development 2003, 130, 3503–3514. [Google Scholar] [CrossRef]
- Gao, C.Q.; Zhao, Y.L.; Li, H.C.; Sui, W.G.; Yan, H.C.; Wang, X.Q. Heat stress inhibits proliferation, promotes growth, and induces apoptosis in cultured Lantang swine skeletal muscle satellite cells. J. Zhejiang Univ. Sci. B 2015, 16, 549–559. [Google Scholar] [CrossRef]
- Bassel-Duby, R.; Olson, E.N. Signaling pathways in skeletal muscle remodeling. Annu. Rev. Biochem. 2006, 75, 19–37. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Zong, X.; Ye, M.; Jin, C.; Xu, T.; Yang, J.; Gao, C.; Wang, X.; Yan, H. Lysine Distinctively Manipulates Myogenic Regulatory Factors and Wnt/Ca2+ Pathway in Slow and Fast Muscles, and Their Satellite Cells of Postnatal Piglets. Cells 2024, 13, 650. https://doi.org/10.3390/cells13070650
Wang X, Zong X, Ye M, Jin C, Xu T, Yang J, Gao C, Wang X, Yan H. Lysine Distinctively Manipulates Myogenic Regulatory Factors and Wnt/Ca2+ Pathway in Slow and Fast Muscles, and Their Satellite Cells of Postnatal Piglets. Cells. 2024; 13(7):650. https://doi.org/10.3390/cells13070650
Chicago/Turabian StyleWang, Xiaofan, Xiaoyin Zong, Mao Ye, Chenglong Jin, Tao Xu, Jinzeng Yang, Chunqi Gao, Xiuqi Wang, and Huichao Yan. 2024. "Lysine Distinctively Manipulates Myogenic Regulatory Factors and Wnt/Ca2+ Pathway in Slow and Fast Muscles, and Their Satellite Cells of Postnatal Piglets" Cells 13, no. 7: 650. https://doi.org/10.3390/cells13070650
APA StyleWang, X., Zong, X., Ye, M., Jin, C., Xu, T., Yang, J., Gao, C., Wang, X., & Yan, H. (2024). Lysine Distinctively Manipulates Myogenic Regulatory Factors and Wnt/Ca2+ Pathway in Slow and Fast Muscles, and Their Satellite Cells of Postnatal Piglets. Cells, 13(7), 650. https://doi.org/10.3390/cells13070650






