Metabolic Alteration Bridging the Prediabetic State and Colorectal Cancer
Abstract
1. Introduction
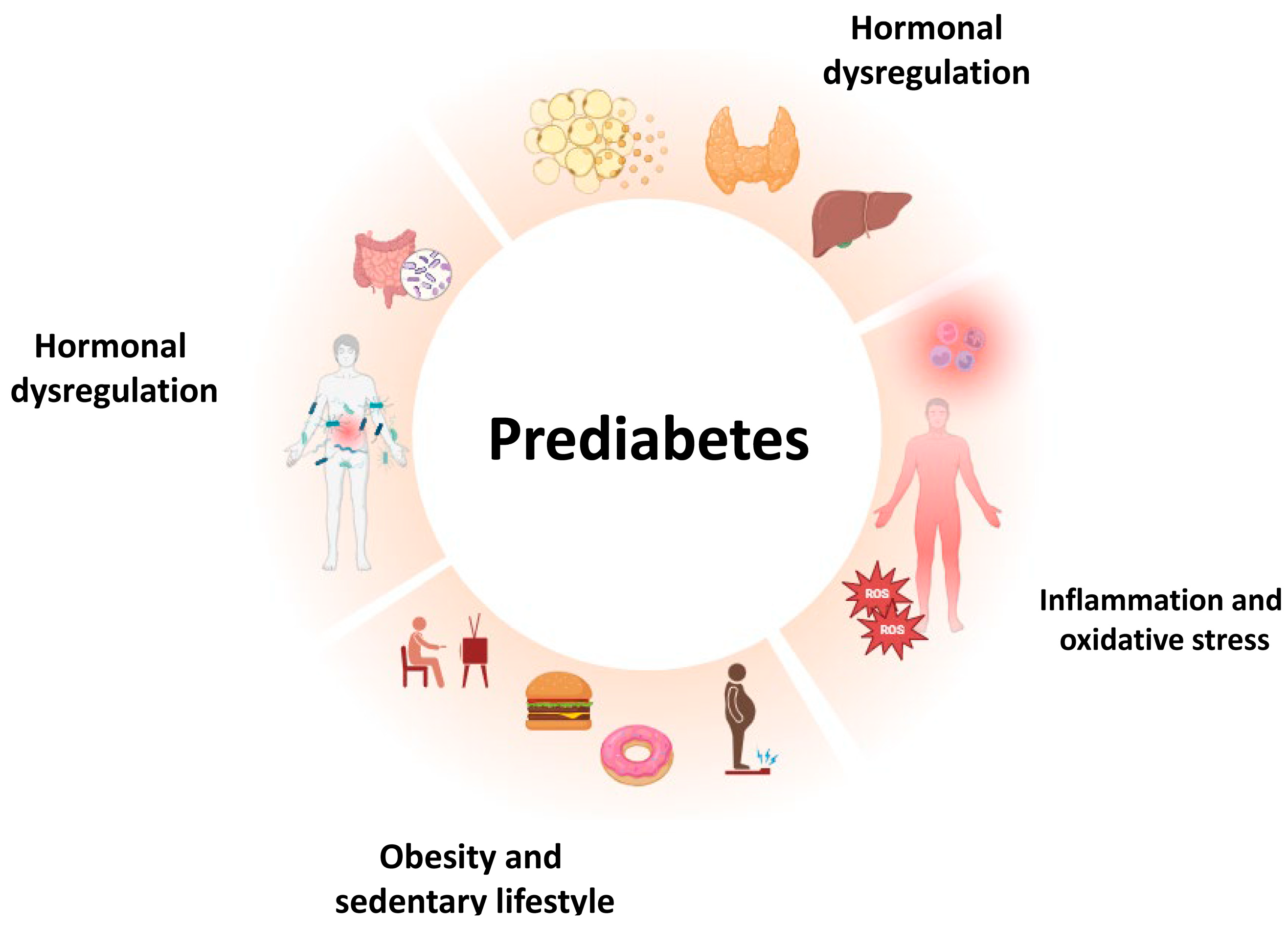
2. Prediabetes: Risk Factors and Determinants
2.1. Chronic Inflammation
2.2. Obesity and Sarcopenia
2.3. Hormonal Dysregulation
2.4. Microbiota
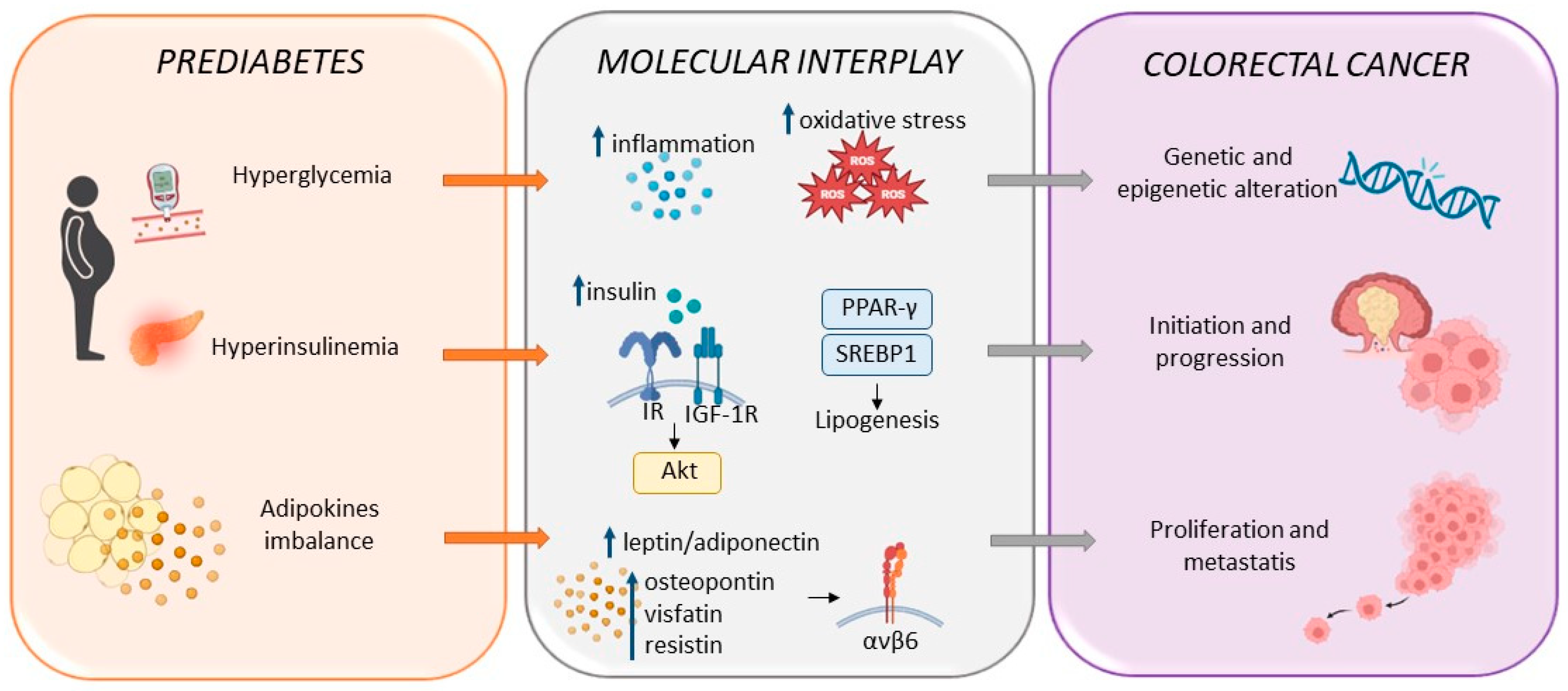
3. Prediabetes as an Independent Risk Factor for Colorectal Cancer
4. Common Therapeutic Approaches in Prediabetes and Colorectal Cancer
4.1. Nutrition
4.2. Physical Activity
4.3. Pharmacotherapy
| Drug | Effects on Prediabetes | Effects on Colorectal Cancer |
|---|---|---|
| Metformin | Glucose homeostasis enhancement [152,153,155] Inhibition of prediabetes to DM progression [152,153] Increase in GLUT4 expression levels [157] Promotion of GLUT4 membrane translocation [157] Reduction in systemic inflammation and oxidative stress and miR-195 and miR-27 [158] SIRT6 upregulation [159] SGLT2 downregulation [159] Leptin/adiponectin ratio reduction [159] | Overall and disease-free CRC survival increase Reduced liver metastasis [174,175,179] Inhibition of mTOR and PI3K/Akt signaling [176,183] AMPK activation [97,176,183] TGF-β/INHBA signaling suppression [177] CyclinD downregulation [177] Urea cycle suppression [178] Reduced putrescin levels [178] Caspase 3-mediated apoptosis [180] Disruption of tumor-mediated immunosuppression [181,182,183] Chemosensitivity increase [97,184] |
| Gliflozins | Reduced cardiovascular-related death [161] Reduced heart failure [161] Inhibition of prediabetes to DM progression [162] | Suppression of hyperinsulinemia pro-tumoral effect [185] Reduced cell adhesion [185,186] Synergic cytotoxic effect with cetuximab [185] β-catenin suppression [187] Farnesylated Ras levels downregulation [188] Insulin levels downregulation [188] |
| GLP-1RA | Glucose homeostasis enhancement [164,168] Weight loss promotion [164,171] Amelioration of insulin sensitivity [166] Insulin secretion enhancement via HCNP, M3R [167] Choline acetyltransferase upregulation [167] Reduced systemic inflammation [168,169] Cardiovascular function enhancement [170] | Suppression of cell survival [189] Suppression of cell migration ability [189] Inhibition of PI3K/Akt/mTOR pathway [189] Reduced cancer relative risk [190] |
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zand, A.; Ibrahim, K.; Patham, B. Prediabetes, Why Should We Care? Methodist. DeBakey Cardiovasc. J. 2018, 14, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Rooney, M.R.; Fang, M.; Ogurtsova, K.; Ozkan, B.; Echouffo-Tcheugui, J.B.; Boyko, E.J.; Magliano, D.J.; Selvin, E. Global Prevalence of Prediabetes. Diabetes Care 2023, 46, 1388–1394. [Google Scholar] [CrossRef] [PubMed]
- Rett, K.; Gottwald-Hostalek, U. Understanding prediabetes, definition; prevalence; burden and treatment options for an emerging disease. Curr. Med. Res. Opin. 2019, 35, 1529–1534. [Google Scholar] [CrossRef] [PubMed]
- ElSayed, N.A.; Aleppo, G.; Aroda, V.R.; Bannuru, R.R.; Brown, F.M.; Bruemmer, D.; Collins, B.S.; Hilliard, M.E.; Isaacs, D.; Johnson, E.L.; et al. 2. Classification and Diagnosis of Diabetes, Standards of Care in Diabetes—2023. Diabetes Care 2023, 46 (Suppl. S1), S19–S40. [Google Scholar]
- Schleicher, E.; Gerdes, C.; Petersmann, A.; Müller-Wieland, D.; Müller, U.A.; Freckmann, G.; Heinemann, L.; Nauck, M.; Landgraf, R. Definition, Classification and Diagnosis of Diabetes Mellitus. Exp. Clin. Endocrinol. Diabetes 2022, 130, S1–S8. [Google Scholar] [CrossRef] [PubMed]
- Petersmann, A.; Müller-Wieland, D.; Müller, U.A.; Landgraf, R.; Nauck, M.; Freckmann, G.; Heinemann, L.; Schleicher, E. Definition, Classification and Diagnosis of Diabetes Mellitus. Exp. Clin. Endocrinol. Diabetes 2019, 127, S1–S7. [Google Scholar] [CrossRef] [PubMed]
- Tinajero, M.G.; Malik, V.S. An Update on the Epidemiology of Type 2 Diabetes, A Global Perspective. Endocrinol. Metab. Clin. N. Am. 2021, 50, 337–355. [Google Scholar] [CrossRef]
- Park, J.J. Epidemiology; Pathophysiology; Diagnosis and Treatment of Heart Failure in Diabetes. Diabetes Metab. J. 2021, 45, 146–157. [Google Scholar] [CrossRef] [PubMed]
- Joharatnam-Hogan, N.; Morganstein, D.L. Diabetes and cancer: Optimising glycaemic control. J. Hum. Nutr. Diet. 2023, 36, 504–513. [Google Scholar] [CrossRef] [PubMed]
- Volpe, M.; Ferrera, A.; Piccinocchi, R.; Morisco, C. The Emerging Role of Prediabetes and Its Management, Focus on L-Arginine and a Survey in Clinical Practice. High. Blood Press. Cardiovasc. Prev. 2023, 30, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Cai, X.; Qiu, M.; Chen, P.; Tang, H.; Hu, Y.; Huang, Y. Prediabetes and the risk of cancer, a meta-analysis. Diabetologia. 2014, 57, 2261–2269. [Google Scholar] [CrossRef] [PubMed]
- Cha, J.M.; Lee, J.I.; Joo, K.R.; Shin, H.P.; Jeun, J.W.; Lim, J.U. Prediabetes is associated with a high-risk colorectal adenoma. Dig. Dis. Sci. 2013, 58, 2061–2067. [Google Scholar] [CrossRef] [PubMed]
- Morgan, E.; Arnold, M.; Gini, A.; Lorenzoni, V.; Cabasag, C.J.; Laversanne, M.; Vignat, J.; Ferlay, J.; Murphy, N.; Bray, F. Global burden of colorectal cancer in 2020 and 2040, incidence and mortality estimates from GLOBOCAN. Gut. 2023, 72, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Sninsky, J.A.; Shore, B.M.; Lupu, G.V.; Crockett, S.D. Risk Factors for Colorectal Polyps and Cancer. Gastrointest. Endosc. Clin. N. 2022, 32, 195–213. [Google Scholar] [CrossRef] [PubMed]
- Imkeller, K.; Ambrosi, G.; Klemm, N.; Claveras Cabezudo, A.; Henkel, L.; Huber, W.; Boutros, M. Metabolic balance in colorectal cancer is maintained by optimal Wnt signaling levels. Mol. Sys. Biol. 2022, 18, e10874. [Google Scholar] [CrossRef] [PubMed]
- Saeed, H.; Leibowitz, B.J.; Zhang, L.; Yu, J. Targeting Myc-driven stress addiction in colorectal cancer. Drug Resist. Updat. 2023, 69, 100963. [Google Scholar] [CrossRef] [PubMed]
- Vande Voorde, J.; Steven, R.T.; Najumudeen, A.K.; Ford, C.A.; Dexter, A.; Gonzalez-Fernandez, A.; Nikula, C.J.; Xiang, Y.; Ford, L.; Maneta Stavrakaki, S.; et al. Metabolic profiling stratifies colorectal cancer and reveals adenosylhomocysteinase as a therapeutic target. Nat. Met. 2023, 5, 1303–1318. [Google Scholar] [CrossRef] [PubMed]
- Schlesinger, S.; Neuenschwander, M.; Barbaresko, J.; Lang, A.; Maalmi, H.; Rathmann, W.; Roden, M.; Herder, C. Prediabetes and risk of mortality; diabetes-related complications and comorbidities, umbrella review of meta-analyses of prospective studies. Diabetologia 2022, 65, 275–285. [Google Scholar] [CrossRef]
- Saltiel, A.R. Insulin signaling in health and disease. J. Clin. Investig. 2021, 131, e142241. [Google Scholar] [CrossRef] [PubMed]
- Khan, R.M.M.; Chua, Z.J.Y.; Tan, J.C.; Yang, Y.; Liao, Z.; Zhao, Y. From Pre-Diabetes to Diabetes, Diagnosis; Treatments and Translational Research. Medicina 2019, 55, 546. [Google Scholar] [CrossRef] [PubMed]
- Onyango, A.N. Cellular Stresses and Stress Responses in the Pathogenesis of Insulin Resistance. Oxid. Med. Cell. Longev. 2018, 2018, 4321714. [Google Scholar] [CrossRef] [PubMed]
- Shu, Y.; Wu, X.; Wang, J.; Ma, X.; Li, H.; Xiang, Y. Associations of Dietary Inflammatory Index with Prediabetes and Insulin Resistance. Front. Endocrinol. 2022, 13, 820932. [Google Scholar] [CrossRef] [PubMed]
- Brannick, B.; Wynn, A.; Dagogo-Jack, S. Prediabetes as a toxic environment for the initiation of microvascular and macrovascular complications. Exp. Biol. Med. 2016, 241, 1323–1331. [Google Scholar] [CrossRef] [PubMed]
- Integrative HMP (iHMP) Research Network Consortium. The Integrative Human Microbiome Project. Nature 2019, 569, 641–648. [Google Scholar] [CrossRef] [PubMed]
- Luc, K.; Schramm-Luc, A.; Guzik, T.J.; Mikolajczyk, T.P. Oxidative stress and inflammatory markers in prediabetes and diabetes. J. Physiol. Pharmacol. 2019, 70, 70. [Google Scholar] [CrossRef]
- Malenica, M.; Klisić, A.; Meseldžić, N.; Dujić, T.; Bego, T.; Kotur-Stevuljević, J. Principal component analysis of the oxidative stress; inflammation; and dyslipidemia influence in patients with different levels of glucoregulation. J. Med. Biochem. 2023, 42, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Weaver, J.R.; Odanga, J.J.; Breathwaite, E.K.; Treadwell, M.L.; Murchinson, A.C.; Walters, G.; Fuentes, D.P.; Lee, J.B. An increase in inflammation and islet dysfunction is a feature of prediabetes. Diabetes/Metab. Res. Rev. 2021, 37, e3405. [Google Scholar] [CrossRef] [PubMed]
- Demirkol, M.E.; Alisik, M.; Yis, O.M. C-Reactive Protein to Albumin Ratio in Patients with Prediabetes and Diabetes Mellitus, HbA1c and Inflammation. Clin. Lab. 2021, 68, 1688–1694. [Google Scholar] [CrossRef] [PubMed]
- González Delgado, A.; Hernández, A.F.; Marrero, D.; Maside, A.F.; Barroso, G.H.; Carreño, E.P.; Acosta Sørensen, C.; Rodríguez-Rodríguez, A.E.; Collantes, T.; Anabel, R.; et al. Inflammation on the Waiting List Is a Risk Factor for New-Onset Prediabetes and Post-Transplant Diabetes Mellitus, A Prospective Study. Nephron 2023, 147, 560–571. [Google Scholar] [CrossRef] [PubMed]
- Diamanti, K.; Cavalli, M.; Pereira, M.J.; Pan, G.; Castillejo-López, C.; Kumar, C.; Mundt, F.; Komorowski, J.; Deshmukh, A.S.; Mann, M.; et al. Organ-specific metabolic pathways distinguish prediabetes; type 2 diabetes; and normal tissues. Cell Rep. Med. 2022, 3, 100763. [Google Scholar] [CrossRef]
- Liu, R.; Pugh, G.H.; Tevonian, E.; Thompson, K.; Lauffenburger, D.A.; Kern, P.A.; Nikolajczyk, B.S. Regulatory T Cells Control Effector T Cell Inflammation in Human Prediabetes. Diabetes 2022, 71, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Mei, Y.; Li, A.; Zhao, J.; Zhou, Q.; Zhao, M.; Xu, J.; Li, R.; Li, Y.; Li, K.; Ge, X.; et al. Association of long-term air pollution exposure with the risk of prediabetes and diabetes, Systematic perspective from inflammatory mechanisms; glucose homeostasis pathway to preventive strategies. Environ. Res. 2023, 216 Pt 1, 114472. [Google Scholar] [CrossRef] [PubMed]
- Hong, H.; Lin, X.; Xu, Y.; Tong, T.; Zhang, J.; He, H.; Yang, L.; Lu, Y.; Zhou, Z. Cadmium induces ferroptosis mediated inflammation by activating Gpx4/Ager/p65 axis in pancreatic β-cells. Sci. Total Env. 2022, 849, 157819. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Deng, P.; Lin, M.; Yang, L.; Li, L.; Guo, L.; Zhang, L.; He, M.; Lu, Y.; Pi, H.; et al. Long-term bisphenol A exposure exacerbates diet-induced prediabetes via TLR4-dependent hypothalamic inflammation. J. Hazard. Mater. 2021, 402, 123926. [Google Scholar] [CrossRef] [PubMed]
- Sardu, C.; Trotta, M.C.; Pieretti, G.; Gatta, G.; Ferraro, G.; Nicoletti, G.F.; D’ Onofrio, N.; Balestrieri, M.L.; D’ Amico, M.; Abbatecola, A.; et al. MicroRNAs modulation and clinical outcomes at 1 year of follow-up in obese patients with pre-diabetes treated with metformin vs. placebo. Acta Diabetol. 2021, 58, 1381–1393. [Google Scholar] [CrossRef] [PubMed]
- Greco, M.; Mirabelli, M.; Salatino, A.; Accattato, F.; Aiello, V.; Brunetti, F.S.; Chiefari, E.; Pullano, S.A.; Fiorillo, A.S.; Foti, D.P.; et al. From Euglycemia to Recent Onset of Type 2 Diabetes Mellitus, A Proof-of-Concept Study on Circulating microRNA Profiling Reveals Distinct; and Early microRNA Signatures. Diagnostics 2023, 13, 2443. [Google Scholar] [CrossRef] [PubMed]
- Bou Malhab, L.J.; Abdel-Rahman, W.M. Obesity and Inflammation, Colorectal Cancer Engines. Curr. Mol. Pharmacol. 2022, 15, 620–646. [Google Scholar] [CrossRef]
- Purnell, J.Q. Definitions, Classification, and Epidemiology of Obesity. In Endotext; MDText.com, Inc.: South Dartmouth, MA, USA, 2023. [Google Scholar]
- Tutor, A.W.; Lavie, C.J.; Kachur, S.; Milani, R.V.; Ventura, H.O. Updates on obesity and the obesity paradox in cardiovascular diseases. Prog. Cardiovasc. Dis. 2023, 78, 2–10. [Google Scholar] [CrossRef]
- Osei, K.; Gaillard, T. Pathogenic Mechanisms of Prediabetes in Obese vs. Very Obese African American Women, Implications for Diabetes Prevention. J. Natl. Med. Assoc. 2019, 111, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Aliluev, A.; Tritschler, S.; Sterr, M.; Oppenländer, L.; Hinterdobler, J.; Greisle, T.; Irmler, M.; Beckers, J.; Sun, N.; Walch, A.; et al. Diet-induced alteration of intestinal stem cell function underlies obesity and prediabetes in mice. Nat. Metab. 2021, 3, 1202–1216. [Google Scholar] [CrossRef] [PubMed]
- Kuppuswami, J.; Senthilkumar, G.P. Nutri-stress, mitochondrial dysfunction; and insulin resistance-role of heat shock proteins. Cell Stress Chaperon. 2023, 28, 35–48. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, I.; Machado de Oliveira, R.; Carvalho, A.S.; Teshima, A.; Beck, H.C.; Matthiesen, R.; Costa-Silva, B.; Macedo, M.P. Messages from the Small Intestine Carried by Extracellular Vesicles in Prediabetes, A Proteomic Portrait. J. Prot. Res. 2021, 21, 910–920. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, B.; Sultana, R.; Greene, M.W. Adipose tissue and insulin resistance in obese. Biomed. Pharmacother. 2021, 137, 111315. [Google Scholar] [CrossRef] [PubMed]
- Kostara, C.E.; Karakitsou, K.S.; Florentin, M.; Bairaktari, E.T.; Tsimihodimos, V. Progressive, Qualitative, and Quantitative Alterations in HDL Lipidome from Healthy Subjects to Patients with Prediabetes and Type 2 Diabetes. Metabolites 2022, 12, 683. [Google Scholar] [CrossRef] [PubMed]
- Lepretti, M.; Martucciello, S.; Burgos Aceves, M.A.; Putti, R.; Lionetti, L. Omega-3 Fatty Acids and Insulin Resistance, Focus on the Regulation of Mitochondria and Endoplasmic Reticulum Stress. Nutrients 2018, 10, 350. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Ha, T.Y.; Jung, C.H.; Nirmala, F.S.; Park, S.Y.; Hu, Y.H.; Ahn, J. Mitochondrial dysfunction in skeletal muscle contributes to the development of acute insulin resistance in mice. J. Cachexia Sarcopenia Muscle 2021, 12, 1925–1939. [Google Scholar] [CrossRef] [PubMed]
- Sangwung, P.; Petersen, K.F.; Shulman, G.I.; Knowles, J.W. Mitochondrial Dysfunction, Insulin Resistance, and Potential Genetic Implications. Endocrinology 2020, 161, bqaa017. [Google Scholar] [CrossRef] [PubMed]
- Roszczyc-Owsiejczuk, K.; Zabielski, P. Sphingolipids as a Culprit of Mitochondrial Dysfunction in Insulin Resistance and Type 2 Diabetes. Front. Endocrinol. 2021, 12, 635175. [Google Scholar] [CrossRef] [PubMed]
- Martino, E.; Balestrieri, A.; Anastasio, C.; Maione, M.; Mele, L.; Cautela, D.; Campanile, G.; Balestrieri, M.L.; D’Onofrio, N. SIRT3 Modulates Endothelial Mitochondrial Redox State during Insulin Resistance. Antioxidants 2022, 11, 1611. [Google Scholar] [CrossRef] [PubMed]
- D’Onofrio, N.; Pieretti, G.; Ciccarelli, F.; Gambardella, A.; Passariello, N.; Rizzo, M.R.; Barbieri, M.; Marfella, R.; Nicoletti, G.; Balestrieri, M.L.; et al. Abdominal Fat SIRT6 Expression and Its Relationship with Inflammatory and Metabolic Pathways in Pre-Diabetic Overweight Patients. Int. J. Mol. Sci. 2019, 20, 1153. [Google Scholar] [CrossRef] [PubMed]
- Sardu, C.; Pieretti, G.; D’Onofrio, N.; Ciccarelli, F.; Paolisso, P.; Passavanti, M.B.; Marfella, R.; Cioffi, M.; Mone, P.; Dalise, A.M.; et al. Inflammatory Cytokines and SIRT1 Levels in Subcutaneous Abdominal Fat, Relationship with Cardiac Performance in Overweight Pre-diabetics Patients. Front. Physiol. 2018, 9, 1030. [Google Scholar] [CrossRef] [PubMed]
- Bensellam, M.; Shi, Y.C.; Chan, J.Y.; Laybutt, D.R.; Chae, H.; Abou-Samra, M.; Pappas, E.G.; Thomas, H.E.; Gilon, P.; Jonas, J.C. Metallothionein 1 negatively regulates glucose-stimulated insulin secretion and is differentially expressed in conditions of beta cell compensation and failure in mice and humans. Diabetologia 2019, 62, 2273–2286. [Google Scholar] [CrossRef] [PubMed]
- Corezola do Amaral, M.E.; Kravets, V.; Dwulet, J.M.; Farnsworth, N.L.; Piscopio, R.; Schleicher, W.E.; Miranda, J.G.; Benninger, R.K.P. Caloric restriction recovers impaired β-cell-β-cell gap junction coupling; calcium oscillation coordination; and insulin secretion in prediabetic mice. Am. J. Physiol. Endocrinol. Metab. 2020, 319, E709–E720. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Du, H.; Wei, S.; Feng, L.; Li, J.; Yao, F.; Zhang, M.; Hatch, G.M.; Chen, L. Adipocyte-Derived Exosomal MiR-27a Induces Insulin Resistance in Skeletal Muscle Through Repression of PPARγ. Theranostics 2018, 8, 2171–2188. [Google Scholar] [CrossRef] [PubMed]
- Donini, L.M.; Busetto, L.; Bischoff, S.C.; Cederholm, T.; Ballesteros-Pomar, M.D.; Batsis, J.A.; Bauer, J.M.; Boirie, Y.; Cruz-Jentoft, A.J.; Dicker, D.; et al. Definition and Diagnostic Criteria for Sarcopenic Obesity, ESPEN and EASO Consensus Statement. Obes. Facts 2022, 15, 321–335. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Xiong, B.; Liao, Z.; Xiao, M.; Chen, W. Association between dietary inflammatory index and low muscle mass in diabetes/prediabetes patients. Exp. Gerontol. 2023, 179, 112258. [Google Scholar] [CrossRef] [PubMed]
- Merz, K.E.; Thurmond, D.C. Role of Skeletal Muscle in Insulin Resistance and Glucose Uptake. Compr. Physiol. 2020, 10, 785–809. [Google Scholar] [PubMed]
- Qiu, S.; Cai, X.; Yuan, Y.; Xie, B.; Sun, Z.; Wang, D.; Wu, T. Muscle strength and prediabetes progression and regression in middle-aged and older adults, a prospective cohort study. J. Cachexia Sarcopenia Muscle 2022, 13, 909–918. [Google Scholar] [CrossRef] [PubMed]
- Patarrão, R.S.; Duarte, N.; Coelho, I.; Ward, J.; Ribeiro, R.T.; Meneses, M.J.; Andrade, R.; Costa, J.; Correia, I.; Boavida, J.M.; et al. Prediabetes blunts DPP4 genetic control of postprandial glycaemia and insulin secretion. Diabetologia 2022, 65, 861–871. [Google Scholar] [CrossRef] [PubMed]
- Chueire, V.B.; Muscelli, E. Effect of free fatty acids on insulin secretion; insulin sensitivity and incretin effect—A narrative review. Arch. Endocrinol. Metab. 2021, 65, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Pestel, J.; Blangero, F.; Watson, J.; Pirola, L.; Eljaafari, A. Adipokines in obesity and metabolic-related-diseases. Biochimie 2023, 212, 48–59. [Google Scholar] [CrossRef] [PubMed]
- Bungau, S.; Behl, T.; Tit, D.M.; Banica, F.; Bratu, O.G.; Diaconu, C.C.; Nistor-Cseppento, C.D.; Bustea, C.; Aron, R.A.C.; Vesa, C.M. Interactions between leptin and insulin resistance in patients with prediabetes; with and without NAFLD. Exp. Ther. Med. 2020, 20, 197. [Google Scholar] [CrossRef] [PubMed]
- Wolf, R.M.; Jaffe, A.E.; Rodriguez, S.; Lei, X.; Sarver, D.C.; Straub, A.T.; Wong, G.W.; Magge, S.N. Altered adipokines in obese adolescents, a cross-sectional and longitudinal analysis across the spectrum of glycemia. Am. J. Physiol. Endocrinol. Metab. 2021, 320, E1044–E1052. [Google Scholar] [CrossRef] [PubMed]
- Szczepańska, E.; Gietka-Czernel, M. FGF21, A Novel Regulator of Glucose and Lipid Metabolism and Whole-Body Energy Balance. Horm. Metab. Res. 2022, 54, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Wu, L.; Gao, Q.; Long, X.; Hou, X.; Qian, L.; Ni, J.; Fang, Q.; Li, H.; Jia, W. FGF21/adiponectin ratio predicts deterioration in glycemia, a 4.6-year prospective study in China. Cardiovasc. Diabetol. 2021, 20, 157. [Google Scholar] [CrossRef] [PubMed]
- Brismar, K.; Hilding, A.; Ansurudeen, I.; Flyvbjerg, A.; Frystyk, J.; Östenson, C.G. Adiponectin, IGFBP-1 and -2 are independent predictors in forecasting prediabetes and type 2 diabetes. Front. Endocrinol. 2023, 13, 1092307. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Liang, Y.; Ma, Y.; Wu, J.; Luo, H.; Yi, B. The Variation and Correlation of Serum Adiponectin; Nesfatin-1; IL-6; and TNF-α Levels in Prediabetes. Front. Endocrinol. 2022, 13, 774272. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Ryu, J.; Liu, J.; Luo, H.; Lv, Y.; Langlais, P.R.; Wen, J.; Dong, F.; Sun, Z.; Xia, W.; et al. LRG1 is an adipokine that mediates obesity-induced hepatosteatosis and insulin resistance. J. Clin. Investig. 2021, 131, e148545. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Wang, Z.; Fu, J.; Guan, H.; Lyu, Z.; Wang, W. Sensitivity to Thyroid Hormones and Risk of Prediabetes, A Cross-Sectional Study. Front. Endocrinol. 2021, 12, 657114. [Google Scholar] [CrossRef] [PubMed]
- El-Zawawy, H.T. Serum leptin in male hypothyroid prediabetic patients, Association with cardiovascular risk. Cardiovasc. Endocrinol. Metab. 2018, 7, 84–87. [Google Scholar] [CrossRef] [PubMed]
- Lundqvist, M.H.; Pereira, M.J.; Almby, K.; Hetty, S.; Eriksson, J.W. Regulation of the cortisol axis; glucagon and growth hormone by glucose is altered in prediabetes and type 2 diabetes. J. Clin. Endocrinol. Metab. 2023, 109, e675–e688. [Google Scholar] [CrossRef] [PubMed]
- Mosialou, I.; Shikhel, S.; Luo, N.; Petropoulou, P.I.; Panitsas, K.; Bisikirska, B.; Rothman, N.J.; Tenta, R.; Cariou, B.; Wargny, M.; et al. Lipocalin-2 counteracts metabolic dysregulation in obesity and diabetes. J. Exp. Med. 2020, 217, e20191261. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Chen, J.; Wang, P.; Li, L.; Hu, S.; Liu, H.; Huang, Y.; Mo, X.; Yan, H.; Shan, Z.; et al. The role of peripheral β-amyloid in insulin resistance; insulin secretion; and prediabetes, in vitro and population-based studies. Front. Endocrinol. 2023, 14, 1195658. [Google Scholar] [CrossRef] [PubMed]
- Allin, K.H.; Tremaroli, V.; Caesar, R.; Jensen, B.A.H.; Damgaard, M.T.F.; Bahl, M.I.; Licht, T.R.; Hansen, T.H.; Nielsen, T.; Dantoft, T.M.; et al. Aberrant intestinal microbiota in individuals with prediabetes. Diabetologia 2018, 61, 810–820. [Google Scholar] [CrossRef] [PubMed]
- Di Tommaso, N.; Gasbarrini, A.; Ponziani, F.R. Intestinal Barrier in Human Health and Disease. Int. J. Environ. Res. Public. Health 2021, 18, 12836. [Google Scholar] [CrossRef] [PubMed]
- Palmnäs-Bédard, M.S.A.; Costabile, G.; Vetrani, C.; Åberg, S.; Hjalmarsson, Y.; Dicksved, J.; Riccardi, G.; Landberg, R. The human gut microbiota and glucose metabolism, a scoping review of key bacteria and the potential role of SCFAs. Am. J. Clin. Nutr. 2022, 116, 862–874. [Google Scholar] [CrossRef] [PubMed]
- Letchumanan, G.; Abdullah, N.; Marlini, M.; Baharom, N.; Lawley, B.; Omar, M.R.; Mohideen, F.B.S.; Addnan, F.H.; Nur Fariha, M.M.; Ismail, Z.; et al. Gut Microbiota Composition in Prediabetes and Newly Diagnosed Type 2 Diabetes, A Systematic Review of Observational Studies. Front. Cell Infect. Microbiol. 2022, 12, 943427. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Bao, J.; Chang, Y.; Wang, M.; Chen, B.; Yan, F. Gut Microbiota May Mediate the Influence of Periodontitis on Prediabetes. J. Dent. Res. 2021, 100, 1387–1396. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Tremaroli, V.; Schmidt, C.; Lundqvist, A.; Olsson, L.M.; Krämer, M.; Gummesson, A.; Perkins, R.; Bergström, G.; Bäckhed, F. The Gut Microbiota in Prediabetes and Diabetes, A Population-Based Cross-Sectional Study. Cell Metab. 2020, 32, 379–390.e3. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Zhou, L.; Song, X.; Yin, M.; Liang, G.; Xu, H.; Zhang, L.; Jiang, G.; Huang, F. Alteration of Intestinal Microbiota in 3-Deoxyglucosone-Induced Prediabetic Rats. Biomed. Res. Int. 2020, 2020, 8406846. [Google Scholar] [CrossRef] [PubMed]
- Kawano, Y.; Edwards, M.; Huang, Y.; Bilate, A.M.; Araujo, L.P.; Tanoue, T.; Atarashi, K.; Ladinsky, M.S.; Reiner, S.L.; Wang, H.H.; et al. Microbiota imbalance induced by dietary sugar disrupts immune-mediated protection from metabolic syndrome. Cell. 2022, 185, 3501–3519.e20. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.C.; Yu, J. Gut microbiota in colorectal cancer development and therapy. Nat. Rev. Clin. Oncol. 2023, 20, 429–452. [Google Scholar] [CrossRef] [PubMed]
- An, X.; Zhang, Y.; Sun, W.; Kang, X.; Ji, H.; Sun, Y.; Jiang, L.; Zhao, X.; Gao, Q.; Lian, F.; et al. Early effective intervention can significantly reduce all-cause mortality in prediabetic patients: A systematic review and meta-analysis based on high-quality clinical studies. Front. Endocrinol. 2024, 15, 1294819. [Google Scholar] [CrossRef]
- Ke, J.; Lin, T.; Liu, X.; Wu, K.; Ruan, X.; Ding, Y.; Liu, W.; Qiu, H.; Tan, X.; Wang, X.; et al. Glucose Intolerance and Cancer Risk, A Community-Based Prospective Cohort Study in Shanghai; China. Front. Oncol. 2021, 11, 726672. [Google Scholar] [CrossRef] [PubMed]
- Scappaticcio, L.; Maiorino, M.I.; Bellastella, G.; Giugliano, D.; Esposito, K. Insights into the relationships between diabetes, prediabetes, and cancer. Endocrine 2017, 56, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Ionescu, V.A.; Gheorghe, G.; Bacalbasa, N.; Chiotoroiu, A.L.; Diaconu, C. Colorectal Cancer, from Risk Factors to Oncogenesis. Medicina 2023, 59, 1646. [Google Scholar] [CrossRef] [PubMed]
- Puzzono, M.; Mannucci, A.; Grannò, S.; Zuppardo, R.A.; Galli, A.; Danese, S.; Cavestro, G.M. The Role of Diet and Lifestyle in Early-Onset Colorectal Cancer, A Systematic Review. Cancers 2021, 13, 5933. [Google Scholar] [CrossRef] [PubMed]
- Diakité, M.T.; Diakité, B.; Koné, A.; Balam, S.; Fofana, D.; Diallo, D.; Kassogué, Y.; Traoré, C.B.; Kamaté, B.; Ba, D.; et al. Relationships between gut microbiota, red meat consumption and colorectal cancer. J. Carcinog. Mutagen. 2022, 13, 1000385. [Google Scholar] [PubMed]
- Bolla, A.M.; Caretto, A.; Laurenzi, A.; Scavini, M.; Piemonti, L. Low-Carb and Ketogenic Diets in Type 1 and Type 2 Diabetes. Nutrients 2019, 11, 962. [Google Scholar] [CrossRef] [PubMed]
- McGaugh, E.; Barthel, B. A Review of Ketogenic Diet and Lifestyle. Mo. Med. 2022, 119, 84–88. [Google Scholar] [PubMed]
- Zhang, J.; Zou, S.; Fang, L. Metabolic reprogramming in colorectal cancer, regulatory networks and therapy. Cell Biosci. 2023, 13, 25. [Google Scholar] [CrossRef] [PubMed]
- Onitilo, A.A.; Berg, R.L.; Engel, J.M.; Glurich, I.; Stankowski, R.V.; Williams, G.; Doi, S.A. Increased risk of colon cancer in men in the pre-diabetes phase. PLoS ONE 2013, 8, e70426. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yu, G.H.; Li, S.F.; Wei, R.; Jiang, Z. Diabetes and Colorectal Cancer Risk, Clinical and Therapeutic Implications. J. Diabetes Res. 2022, 2022, 1747326. [Google Scholar] [CrossRef] [PubMed]
- Pourvali, K.; Monji, H. Obesity and intestinal stem cell susceptibility to carcinogenesis. Nutr. Metab. 2021, 18, 37. [Google Scholar] [CrossRef] [PubMed]
- Murphy, N.; Song, M.; Papadimitriou, N.; Carreras-Torres, R.; Langenberg, C.; Martin, R.M.; Tsilidis, K.K.; Barroso, I.; Chen, J.; Frayling, T.M.; et al. Associations Between Glycemic Traits and Colorectal Cancer, A Mendelian Randomization Analysis. J. Natl. Cancer Inst. 2022, 114, 740–752. [Google Scholar] [CrossRef]
- Liu, C.; Liu, Q.; Yan, A.; Chang, H.; Ding, Y.; Tao, J.; Qiao, C. Metformin revert insulin-induced oxaliplatin resistance by activating mitochondrial apoptosis pathway in human colon cancer HCT116 cells. Cancer Med. 2020, 9, 3875–3884. [Google Scholar] [CrossRef]
- Yang, M.; Zhang, Q.; Ge, Y.Z.; Tang, M.; Zhang, X.; Song, M.M.; Ruan, G.T.; Zhang, X.W.; Zhang, K.P.; Shi, H.P. Association between insulin resistance related indicators with the prognosis of patients with colorectal cancer. Cancer Epidemiol. 2023, 87, 102478. [Google Scholar] [CrossRef] [PubMed]
- Cirillo, F.; Catellani, C.; Sartori, C.; Lazzeroni, P.; Amarri, S.; Street, M.E. Obesity; Insulin Resistance; and Colorectal Cancer, could miRNA Dysregulation Play A Role? Int. J. Mol. Sci. 2019, 20, 2922. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Xiong, L.; Li, J.; Cao, H.; Jiang, W.; Liu, B.; Chen, X.; Liu, C.; Liu, K.; Wang, G.; et al. A Linear Dose-Response Relationship between Fasting Plasma Glucose and Colorectal Cancer Risk, Systematic Review and Meta-analysis. Sci. Rep. 2015, 5, 17591. [Google Scholar] [CrossRef] [PubMed]
- Yoo, T.K.; Lee, M.Y.; Lee, S.A.; Cheong, E.S.; Seo, M.H.; Sung, K.C. Association of Glycosylated Hemoglobin Level and Cancer-Related Mortality in Patients without Diabetes. J. Clin. Med. 2022, 11, 5933. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Salmerón, M.; Lucena, S.R.; Chocarro-Calvo, A.; García-Martínez, J.M.; Martín Orozco, R.M.; García-Jiménez, C. Metabolic and hormonal remodeling of colorectal cancer cell signalling by diabetes. Endocr. Relat. Cancer 2021, 28, R191–R206. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Wang, S.; Wang, W.; Liu, E.; Guo, S.; Zhao, C.; Niu, J.; Zhang, Z. Hyperglycemia Promotes Liver Metastasis of Colorectal Cancer via Upregulation of Integrin αvβ6. Med. Sci. Monit. 2021, 27, e930921. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.S.; Scherer, P.E. Obesity, Diabetes, and Increased Cancer Progression. Diabetes Metab. J. 2021, 45, 799–812. [Google Scholar] [CrossRef] [PubMed]
- Farahani, H.; Mahmoudi, T.; Asadi, A.; Nobakht, H.; Dabiri, R.; Hamta, A. Insulin Resistance and Colorectal Cancer Risk, the Role of Elevated Plasma Resistin Levels. J. Gastrointest. Cancer 2020, 51, 478–483. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhou, L.; Zhu, J. Correlations of high molecular weight adiponectin; tumor necrosis factor-alpha and vascular endothelial growth factors with occurrence of colonic polyps in the prediabetic population. Nagoya J. Med. Sci. 2023, 85, 465–475. [Google Scholar] [PubMed]
- Deng, L.; Zhao, X.; Chen, M.; Ji, H.; Zhang, Q.; Chen, R.; Wang, Y. Plasma adiponectin, visfatin, leptin, and resistin levels and the onset of colonic polyps in patients with prediabetes. BMC Endocr. Disord. 2020, 20, 63. [Google Scholar] [CrossRef] [PubMed]
- Ghule, A.; Kamble, T.K.; Talwar, D.; Kumar, S.; Acharya, S.; Wanjari, A.; Gaidhane, S.A.; Agrawal, S. Association of Serum High Sensitivity C-Reactive Protein with Pre-diabetes in Rural Population: A Two-Year Cross-Sectional Study. Cureus 2021, 13, e19088. [Google Scholar] [CrossRef] [PubMed]
- Echouffo-Tcheugui, J.B.; Perreault, L.; Ji, L.; Dagogo-Jack, S. Diagnosis and Management of Prediabetes, A Review. JAMA 2023, 329, 1206–1216. [Google Scholar] [CrossRef] [PubMed]
- Shah, V.; Geller, G.; Xu, D.; Taylor, L.; Griffin, S.; Usher-Smith, J.A. Evaluating the potential impact of lifestyle-based behavior change interventions delivered at the time of colorectal cancer screening. Cancer Causes Control. 2023, 35, 561–574. [Google Scholar] [CrossRef] [PubMed]
- Gales, L.; Forsea, L.; Mitrea, D.; Stefanica, I.; Stanculescu, I.; Mitrica, R.; Georgescu, M.; Trifanescu, O.; Anghel, R.; Serbanescu, L. Antidiabetics; Anthelmintics; Statins; and Beta-Blockers as Co-Adjuvant Drugs in Cancer Therapy. Medicina 2022, 58, 1239. [Google Scholar] [CrossRef] [PubMed]
- He, D.; Qiao, Y.; Xiong, S.; Liu, S.; Ke, C.; Shen, Y. Association between Dietary Quality and Prediabetes based on the Diet Balance Index. Sci. Rep. 2020, 10, 3190. [Google Scholar] [CrossRef]
- Kim, S.H.; Park, D.H.; Lim, Y.J. Impact of Diet on Colorectal Cancer Progression and Prevention, from Nutrients to Neoplasms. Korean J. Gastroenterol. 2023, 82, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Syed Soffian, S.S.; Mohammed Nawi, A.; Hod, R.; Ja’afar, M.H.; Isa, Z.M.; Chan, H.K.; Hassan, M.R.A. Meta-Analysis of the Association between Dietary Inflammatory Index (DII) and Colorectal Cancer. Nutrients 2022, 14, 1555. [Google Scholar] [CrossRef] [PubMed]
- Thanikachalam, K.; Khan, G. Colorectal Cancer and Nutrition. Nutrients 2019, 11, 164. [Google Scholar] [CrossRef] [PubMed]
- Briggs Early, K.; Stanley, K. Position of the Academy of Nutrition and Dietetics, The Role of Medical Nutrition Therapy and Registered Dietitian Nutritionists in the Prevention and Treatment of Prediabetes and Type 2 Diabetes. J. Acad. Nutr. Diet. 2018, 118, 343–353. [Google Scholar] [CrossRef]
- Ivan, C.R.; Messina, A.; Cibelli, G.; Messina, G.; Polito, R.; Losavio, F.; Torre, E.; Monda, V.; Monda, M.; Quiete, S.; et al. Italian Ketogenic Mediterranean Diet in Overweight and Obese Patients with Prediabetes or Type 2 Diabetes. Nutrients 2022, 14, 4361. [Google Scholar] [CrossRef]
- Dmitrieva-Posocco, O.; Wong, A.C.; Lundgren, P.; Golos, A.M.; Descamps, H.C.; Dohnalová, L.; Cramer, Z.; Tian, Y.; Yueh, B.; Eskiocak, O.; et al. β-Hydroxybutyrate suppresses colorectal cancer. Nature 2022, 605, 160–165. [Google Scholar] [CrossRef]
- Dai, X.C.; Zhang, Y.H.; Huang, Y.L.; Wu, X.T.; Fang, Y.J.; Gao, Y.J.; Wang, F. Calorie restriction remodels gut microbiota and suppresses tumorigenesis of colorectal cancer in mice. Exp. Ther. Med. 2022, 25, 59. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.R.; Akash, S.; Rahman, M.M.; Nowrin, F.T.; Akter, T.; Shohag, S.; Rauf, A.; Aljohani, A.S.M.; Simal-Gandara, J. Colon cancer and colorectal cancer, Prevention and treatment by potential natural products. Chem. Biol. Interact. 2022, 368, 110170. [Google Scholar] [CrossRef]
- Hull, M.A. Nutritional prevention of colorectal cancer. Proc. Nutr. Soc. 2021, 80, 59–64. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Chen, J.; Yi, K.; Peng, L.; Xie, J.; Gou, X.; Peng, T.; Tang, L. Phlorizin ameliorates obesity-associated endotoxemia and insulin resistance in high-fat diet-fed mice by targeting the gut microbiota and intestinal barrier integrity. Gut Microbes 2020, 12, 1842990. [Google Scholar] [CrossRef]
- Gómez-Martínez, S.; Díaz-Prieto, L.E.; Vicente Castro, I.; Jurado, C.; Iturmendi, N.; Martín-Ridaura, M.C.; Calle, N.; Dueñas, M.; Picón, M.J.; Marcos, A.; et al. Moringa oleifera Leaf Supplementation as a Glycemic Control Strategy in Subjects with Prediabetes. Nutrients 2021, 14, 57. [Google Scholar] [CrossRef] [PubMed]
- Naseri, K.; Saadati, S.; Sadeghi, A.; Asbaghi, O.; Ghaemi, F.; Zafarani, F.; Li, H.B.; Gan, R.Y. The Efficacy of Ginseng (Panax) on Human Prediabetes and Type 2 Diabetes Mellitus, A Systematic Review and Meta-Analysis. Nutrients 2022, 14, 2401. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhao, A.; Sandhu, A.K.; Edirisinghe, I.; Burton-Freeman, B.M. Red Raspberry and Fructo-Oligosaccharide Supplementation, Metabolic Biomarkers, and the Gut Microbiota in Adults with Prediabetes, A Randomized Crossover Clinical Trial. J. Nutr. 2022, 152, 1438–1449. [Google Scholar] [CrossRef] [PubMed]
- Martino, E.; Luce, A.; Balestrieri, A.; Mele, L.; Anastasio, C.; D’Onofrio, N.; Balestrieri, M.L.; Campanile, G. Whey Improves In Vitro Endothelial Mitochondrial Function and Metabolic Redox Status in Diabetic State. Antioxidants 2023, 12, 1311. [Google Scholar] [CrossRef] [PubMed]
- Alzate-Yepes, T.; Pérez-Palacio, L.; Martínez, E.; Osorio, M. Mechanisms of Action of Fruit and Vegetable Phytochemicals in Colorectal Cancer Prevention. Molecules 2023, 28, 4322. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.T.; Cui, W.Q.; Pan, D.; Jiang, M.; Chang, B.; Sang, L.X. Tea polyphenols and their chemopreventive and therapeutic effects on colorectal cancer. World J. Gastroenterol. 2020, 26, 562–597. [Google Scholar] [CrossRef] [PubMed]
- Cacciola, N.A.; Venneri, T.; Salzano, A.; D’Onofrio, N.; Martano, M.; Saggese, A.; Vinale, F.; Neglia, G.; Campanile, C.; Baccigalupi, L.; et al. Chemopreventive effect of a milk whey by-product derived from Buffalo (Bubalus bubalis) in protecting from colorectal carcinogenesis. Cell Commun. Signal. 2023, 21, 245. [Google Scholar] [CrossRef] [PubMed]
- D’Onofrio, N.; Martino, E.; Mele, L.; Colloca, A.; Maione, M.; Cautela, D.; Cataldo, D.; Balestrieri, M.L. Colorectal Cancer Apoptosis Induced by Dietary δ-Valerobetaine Involves PINK1/Parkin Dependent-Mitophagy and SIRT3. Int. J. Mol. Sci. 2021, 22, 8117. [Google Scholar] [CrossRef]
- D’Onofrio, N.; Cacciola, N.A.; Martino, E.; Borrelli, F.; Fiorino, F.; Lombardi, A.; Neglia, G.; Balestrieri, M.L.; Campanile, G. ROS-Mediated Apoptotic Cell Death of Human Colon Cancer LoVo Cells by Milk δ-Valerobetaine. Sci. Rep. 2020, 10, 8978. [Google Scholar] [CrossRef]
- Martino, E.; Balestrieri, A.; Mele, L.; Sardu, C.; Marfella, R.; D’Onofrio, N.; Campanile, G.; Balestrieri, M.L. Milk Exosomal miR-27b Worsen Endoplasmic Reticulum Stress Mediated Colorectal Cancer Cell Death. Nutrients 2022, 14, 5081. [Google Scholar] [CrossRef] [PubMed]
- D’Onofrio, N.; Martino, E.; Balestrieri, A.; Mele, L.; Cautela, D.; Castaldo, D.; Balestrieri, M.L. Diet-derived ergothioneine induces necroptosis in colorectal cancer cells by activating the SIRT3/MLKL pathway. FEBS Lett. 2022, 596, 1313–1329. [Google Scholar] [CrossRef] [PubMed]
- Katzmarzyk, P.T.; Friedenreich, C.; Shiroma, E.J.; Lee, I.M. Physical inactivity and non-communicable disease burden in low-income; middle-income and high-income countries. Br. J. Sports Med. 2022, 56, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Friedenreich, C.M.; Ryder-Burbidge, C.; McNeil, J. Physical activity; obesity and sedentary behavior in cancer etiology, epidemiologic evidence and biologic mechanisms. Mol. Oncol. 2021, 15, 790–800. [Google Scholar] [CrossRef] [PubMed]
- Gillen, J.B.; Estafanos, S.; Govette, A. Exercise-nutrient interactions for improved postprandial glycemic control and insulin sensitivity. Appl. Physiol. Nutr. Metab. 2021, 46, 856–865. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, Y.; Ni, Y.; Cheung, C.K.Y.; Lam, K.S.L.; Wang, Y.; Xia, Z.; Ye, D.; Guo, J.; Tse, M.A.; et al. Gut Microbiome Fermentation Determines the Efficacy of Exercise for Diabetes Prevention. Cell Metab. 2020, 31, 77–91.e5. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, R.A.; Maiya, G.A.; Hombali, A.; Umakanth, S.; Shivashankar, K.N. Effect of physical activity promotion on adiponectin; leptin and other inflammatory markers in prediabetes, a systematic review and meta-analysis of randomized controlled trials. Acta Diabetol. 2021, 58, 419–429. [Google Scholar] [CrossRef] [PubMed]
- Motiani, K.K.; Collado, M.C.; Eskelinen, J.J.; Virtanen, K.A.; Löyttyniemi, E.; Salminen, S.; Nuutila, P.; Kalliokoski, K.K.; Hannukainen, J.C. Exercise Training Modulates Gut Microbiota Profile and Improves Endotoxemia. Med. Sci. Sports Exerc. 2020, 52, 94–104. [Google Scholar] [CrossRef] [PubMed]
- Beals, J.W.; Kayser, B.D.; Smith, G.I.; Schweitzer, G.G.; Kirbach, K.; Kearney, M.L.; Yoshino, J.; Rahman, G.; Knight, R.; Patterson, B.W.; et al. Dietary weight loss-induced improvements in metabolic function are enhanced by exercise in people with obesity and prediabetes. Nat. Metab. 2023, 5, 1221–1235. [Google Scholar] [CrossRef]
- Xie, F.; You, Y.; Huang, J.; Guan, C.; Chen, Z.; Fang, M.; Yao, F.; Han, J. Association between physical activity and digestive-system cancer, An updated systematic review and meta-analysis. J. Sport Health Sci. 2021, 10, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Botteri, E.; Peveri, G.; Berstad, P.; Bagnardi, V.; Chen, S.L.F.; Sandanger, T.M.; Hoff, G.; Dahm, C.C.; Antoniussen, C.S.; Tjønneland, A.; et al. Changes in Lifestyle and Risk of Colorectal Cancer in the European Prospective Investigation into Cancer and Nutrition. Am. J. Gastroenterol. 2023, 118, 702–711. [Google Scholar] [CrossRef]
- Orange, S.T. What is the optimal type and dose of physical activity for colorectal cancer prevention? Best. Pract. Res. Clin. Gastroenterol. 2023, 66, 101841. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Guo, F.; Chang-Claude, J.; Hoffmeister, M.; Brenner, H. Physical activity; polygenic risk score; and colorectal cancer risk. Cancer Med. 2023, 12, 4655–4666. [Google Scholar] [CrossRef] [PubMed]
- Choy, K.T.; Lam, K.; Kong, J.C. Exercise and colorectal cancer survival, an updated systematic review and meta-analysis. Int. J. Color. Dis. 2022, 37, 1751–1758. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.Y.; Chiu, H.M. Beyond colonoscopy, Physical activity as a viable adjunct to prevent colorectal cancer. Dig. Endosc. 2023, 35, 33–46. [Google Scholar] [CrossRef] [PubMed]
- Himbert, C.; Stephens, W.Z.; Gigic, B.; Hardikar, S.; Holowatyj, A.N.; Lin, T.; Ose, J.; Swanson, E.; Ashworth, A.; Warby, C.A.; et al. Differences in the gut microbiome by physical activity and BMI among colorectal cancer patients. Am. J. Cancer Res. 2022, 12, 4789–4801. [Google Scholar] [PubMed]
- Wang, T.; Zhang, Y.; Taaffe, D.R.; Kim, J.S.; Luo, H.; Yang, L.; Fairman, C.M.; Qiao, Y.; Newton, R.U.; Galvão, D.A. Protective effects of physical activity in colon cancer and underlying mechanisms, A review of epidemiological and biological evidence. Crit. Rev. Oncol. Hematol. 2022, 170, 103578. [Google Scholar] [CrossRef] [PubMed]
- Warrilow, A.; Somerset, S.; Pumpa, K.; Fleet, R. Metformin use in prediabetes, is earlier intervention better? Acta Diabetol. 2020, 57, 1359–1366. [Google Scholar] [CrossRef] [PubMed]
- Rendell, M. Pharmacotherapeutic options for prediabetes. Expert Opin. Pharmacother. 2021, 22, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Davidson, M.B. Metformin Should Not Be Used to Treat Prediabetes. Diabetes Care 2020, 43, 1983–1987. [Google Scholar] [CrossRef]
- Patel, D.; Ayesha, I.E.; Monson, N.R.; Klair, N.; Patel, U.; Saxena, A.; Hamid, P. The Effectiveness of Metformin in Diabetes Prevention, A Systematic Review and Meta-Analysis. Cureus 2023, 15, e46108. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, Y.; Shen, S.; Wang, X.; Dong, L.; Li, Q.; Ren, W.; Li, Y.; Bai, J.; Gong, Q.; et al. Safety and effectiveness of metformin plus lifestyle intervention compared with lifestyle intervention alone in preventing progression to diabetes in a Chinese population with impaired glucose regulation, a multicentre; open-label; randomised controlled trial. Lancet Diabetes Endocrinol. 2023, 11, 567–577. [Google Scholar]
- Zheng, M.; Soumya; Begum, M.; Bernardo, C.O.; Stocks, N.; Jahan, H.; Gonzalez-Chica, D. Do patients with prediabetes managed with metformin achieve better glycaemic control? A national study using primary care medical records. Diabet. Med. 2023, 40, e15170. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Cabañas, A.; Morales-Palomo, F.; Alvarez-Jimenez, L.; Mora-Gonzalez, D.; Ortega, J.F.; Mora-Rodriguez, R. Metformin and exercise effects on postprandial insulin sensitivity and glucose kinetics in pre-diabetic and diabetic adults. Am. J. Physiol. Endocrinol. Metab. 2023, 325, E310–E324. [Google Scholar] [CrossRef] [PubMed]
- Şahin, K.; Şahintürk, Y.; Köker, G.; Özçelik Köker, G.; Bostan, F.; Kök, M.; Uyar, S.; Çekin, A.H. Metformin with Versus without Concomitant Probiotic Therapy in Newly Diagnosed Patients with Type 2 Diabetes or Prediabetes, A Comparative Analysis in Relation to Glycemic Control; Gastrointestinal Side Effects; and Treatment Compliance. Turk. J. Gastroenterol. 2022, 33, 925–933. [Google Scholar] [CrossRef] [PubMed]
- Herman, R.; Kravos, N.A.; Jensterle, M.; Janež, A.; Dolžan, V. Metformin and Insulin Resistance, A Review of the Underlying Mechanisms behind Changes in GLUT4-Mediated Glucose Transport. Int. J. Mol. Sci. 2022, 23, 1264. [Google Scholar] [CrossRef] [PubMed]
- Sardu, C.; Paolisso, P.; Sacra, C.; Mauro, C.; Minicucci, F.; Portoghese, M.; Rizzo, M.R.; Barbieri, M.; Sasso, F.C.; D’Onofrio, N.; et al. Effects of Metformin Therapy on Coronary Endothelial Dysfunction in Patients with Prediabetes with Stable Angina and Nonobstructive Coronary Artery Stenosis, The CODYCE Multicenter Prospective Study. Diabetes Care 2019, 42, 1946–1955. [Google Scholar] [CrossRef] [PubMed]
- Sardu, C.; D’Onofrio, N.; Torella, M.; Portoghese, M.; Mureddu, S.; Loreni, F.; Ferraraccio, F.; Panarese, I.; Trotta, M.C.; Gatta, G.; et al. Metformin Therapy Effects on the Expression of Sodium-Glucose Cotransporter 2, Leptin, and SIRT6 Levels in Pericoronary Fat Excised from Pre-Diabetic Patients with Acute Myocardial Infarction. Biomedicines 2021, 9, 904. [Google Scholar] [CrossRef] [PubMed]
- Sardu, C.; D’Onofrio, N.; Torella, M.; Portoghese, M.; Loreni, F.; Mureddu, S.; Signoriello, G.; Scisciola, L.; Barbieri, M.; Rizzo, M.R.; et al. Pericoronary fat inflammation and Major Adverse Cardiac Events (MACE) in prediabetic patients with acute myocardial infarction, effects of metformin. Cardiovasc. Diabetol. 2019, 18, 126. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.M.Y.; Brooksbank, K.J.M.; Wetherall, K.; Mangion, K.; Roditi, G.; Campbell, R.T.; Berry, C.; Chong, V.; Coyle, L.; Docherty, K.F.; et al. Effect of Empagliflozin on Left Ventricular Volumes in Patients with Type 2 Diabetes; or Prediabetes; and Heart Failure with Reduced Ejection Fraction (SUGAR-DM-HF). Circulation 2021, 143, 516–525. [Google Scholar] [CrossRef]
- Mori, Y.; Duru, O.K.; Tuttle, K.R.; Fukuma, S.; Taura, D.; Harada, N.; Inagaki, N.; Inoue, K. Sodium-Glucose Cotransporter 2 Inhibitors and New-onset Type 2 Diabetes in Adults with Prediabetes, Systematic Review and Meta-analysis of Randomized Controlled Trials. J. Clin. Endocrinol. Metab. 2022, 108, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Mistry, S.; Eschler, D.C. Euglycemic Diabetic Ketoacidosis Caused by SGLT2 Inhibitors and a Ketogenic Diet: A Case Series and Review of Literature. AACE Clin. Case Rep. 2020, 7, 17–19. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wei, R.; Huang, Z.; Luo, J.; Pan, Q.; Guo, L. Effects of treatment with Glucagon-like peptide-1 receptor agonist on prediabetes with overweight/obesity, A systematic review and meta-analysis. Diabetes Metab. Res. Rev. 2023, 39, e3680. [Google Scholar] [CrossRef] [PubMed]
- Davidson, M.B. Should Prediabetes be Treated Pharmacologically? Diabetes Ther. 2023, 14, 1585–1593. [Google Scholar] [CrossRef]
- Mashayekhi, M.; Nian, H.; Mayfield, D.; Devin, J.K.; Gamboa, J.L.; Yu, C.; Silver, H.J.; Niswender, K.; Luther, J.M.; Brown, N.J. Weight Loss-Independent Effect of Liraglutide on Insulin Sensitivity in Individuals with Obesity and Prediabetes. Diabetes 2024, 73, 38–50. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Wu, D.; Guo, L.; Wang, L.; Hao, M.; Li, L.; Ni, D.; Hao, H. Liraglutide inhibits the progression of prediabetes in rats by reducing Raf-1 kinase inhibitor protein. Ann. Transl. Med. 2021, 9, 1157. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, A.D.; Gao, Z.; Hamidi, V.; Zhu, L.; Saint Andre, K.B.; Riggs, K.; Ruscheinsky, M.; Wang, H.; Yu, Y.; Miller, C.; et al. Anti-diabetic effects of GLP1 analogs are mediated by thermogenic interleukin-6 signaling in adipocytes. Cell Rep. Med. 2022, 3, 100813. [Google Scholar] [CrossRef] [PubMed]
- Mashayekhi, M.; Beckman, J.A.; Nian, H.; Garner, E.M.; Mayfield, D.; Devin, J.K.; Koethe, J.R.; Brown, J.D.; Cahill, K.N.; Yu, C.; et al. Comparative effects of weight loss and incretin-based therapies on vascular endothelial function; fibrinolysis and inflammation in individuals with obesity and prediabetes, A randomized controlled trial. Diabetes Obes. Metab. 2023, 25, 570–580. [Google Scholar] [CrossRef] [PubMed]
- Simeone, P.; Tripaldi, R.; Michelsen, A.; Ueland, T.; Liani, R.; Ciotti, S.; Birkeland, K.I.; Gulseth, H.L.; Di Castelnuovo, A.; Cipollone, F.; et al. Effects of liraglutide vs. lifestyle changes on soluble suppression of tumorigenesis-2 (sST2) and galectin-3 in obese subjects with prediabetes or type 2 diabetes after comparable weight loss. Cardiovasc. Diabetol. 2022, 21, 36. [Google Scholar] [CrossRef] [PubMed]
- Silver, H.J.; Olson, D.; Mayfield, D.; Wright, P.; Nian, H.; Mashayekhi, M.; Koethe, J.R.; Niswender, K.D.; Luther, J.M.; Brown, N.J. Effect of the glucagon-like peptide-1 receptor agonist liraglutide; compared to caloric restriction; on appetite, dietary intake, body fat distribution and cardiometabolic biomarkers, A randomized trial in adults with obesity and prediabetes. Diabetes Obes. Metab. 2023, 25, 2340–2350. [Google Scholar] [CrossRef] [PubMed]
- Kant, R.; Chandra, L.; Verma, V.; Nain, P.; Bello, D.; Patel, S.; Ala, S.; Chandra, R.; Antony, M.A. Gut microbiota interactions with anti-diabetic medications and pathogenesis of type 2 diabetes mellitus. World J. Methodol. 2022, 12, 246–257. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Luo, Z.; Zhou, J.; Sun, B. Gut Microbiota and Antidiabetic Drugs: Perspectives of Personalized Treatment in Type 2 Diabetes Mellitus. Front. Cell Infect. Microbiol. 2022, 12, 853771. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Shi, M. Effect of metformin use on the risk and prognosis of colorectal cancer in diabetes mellitus, a meta-analysis. Anticancer Drugs 2022, 33, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Tarhini, Z.; Manceur, K.; Magne, J.; Mathonnet, M.; Jost, J.; Christou, N. The effect of metformin on the survival of colorectal cancer patients with type 2 diabetes mellitus. Sci. Rep. 2022, 12, 12374. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, K.; Okabayashi, K.; Seishima, R.; Ishida, T.; Shigeta, K.; Tsuruta, M.; Hasegawa, H.; Kitagawa, Y. Metformin inhibits the development and metastasis of colorectal cancer. Med. Oncol. 2022, 39, 136. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Q.; Xiao, J.; Liu, J.; Liu, J.; Shu, G.; Yin, G. Metformin suppresses the growth of colorectal cancer by targeting INHBA to inhibit TGF-β/PI3K/AKT signaling transduction. Cell Death Dis. 2022, 13, 202. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Hu, L.; Tang, J.F.; Xu, H.; Tian, K.; Wu, M.N.; Huang, S.Y.; Du, Y.M.; Zhou, P.; Lu, R.J.; et al. Metformin Inhibits the Urea Cycle and Reduces Putrescine Generation in Colorectal Cancer Cell Lines. Molecules 2021, 26, 1990. [Google Scholar] [CrossRef] [PubMed]
- Chu, P.T.; Chuang, T.J.; Huang, S.H.; Wu, T.H.; Huang, W.C.; Wang, J.H. The impact of metformin on survival in diabetes patients with operable colorectal cancer, A nationwide retrospective cohort study. J. Int. Med. Res. 2023, 51, 3000605231168033. [Google Scholar] [CrossRef] [PubMed]
- Rosidi, B.; Priyatno, D.; Putra, T.P.; Yusuf, I. Metformin Induces a Caspase 3-Unrelated Apoptosis in Human Colorectal Cancer Cell Lines HCT116 and SW620. Cancer Manag. Res. 2023, 15, 475–485. [Google Scholar] [CrossRef] [PubMed]
- Ni, W.; Wu, J.; Feng, Y.; Hu, Y.; Liu, H.; Chen, J.; Chen, F.; Tian, H. Metformin reprograms tumor microenvironment and boosts chemoimmunotherapy in colorectal cancer. Biomater. Sci. 2022, 10, 5596–5607. [Google Scholar] [CrossRef]
- Huang, X.; Sun, T.; Wang, J.; Hong, X.; Chen, H.; Yan, T.; Zhou, C.; Sun, D.; Yang, C.; Yu, T.; et al. Metformin Reprograms Tryptophan Metabolism to Stimulate CD8+ T-cell Function in Colorectal Cancer. Cancer Res. 2023, 83, 2358–2371. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Lee, D.; Lee, K.J.; Yoon, J.E.; Kwon, J.H.; Seo, Y.; Kim, J.; Chang, S.Y.; Park, J.; Kang, E.A.; et al. Tumor-suppressive effect of metformin via the regulation of M2 macrophages and myeloid-derived suppressor cells in the tumor microenvironment of colorectal cancer. Cancers 2022, 14, 2881. [Google Scholar] [CrossRef] [PubMed]
- Nasiri, A.R.; Rodrigues, M.R.; Li, Z.; Leitner, B.P.; Perry, R.J. SGLT2 inhibition slows tumor growth in mice by reversing hyperinsulinemia. Cancer Metab. 2019, 7, 10. [Google Scholar] [CrossRef] [PubMed]
- Okada, J.; Yamada, E.; Saito, T.; Yokoo, H.; Osaki, A.; Shimoda, Y.; Ozawa, A.; Nakajima, Y.; Pessin, J.E.; Okada, S.; et al. Dapagliflozin Inhibits Cell Adhesion to Collagen I and IV and Increases Ectodomain Proteolytic Cleavage of DDR1 by Increasing ADAM10 Activity. Molecules. 2020, 25, 495. [Google Scholar] [CrossRef] [PubMed]
- Okada, J.; Matsumoto, S.; Kaira, K.; Saito, T.; Yamada, E.; Yokoo, H.; Katoh, R.; Kusano, M.; Okada, S.; Yamada, M. Sodium Glucose Cotransporter 2 Inhibition Combined with Cetuximab Significantly Reduced Tumor Size and Carcinoembryonic Antigen Level in Colon Cancer Metastatic to Liver. Clin. Color. Cancer 2018, 17, e45–e48. [Google Scholar] [CrossRef] [PubMed]
- Kato, J.; Shirakami, Y.; Ohnishi, M.; Mizutani, T.; Kubota, M.; Sakai, H.; Ibuka, T.; Tanaka, T.; Shimizu, M. Suppressive effects of the sodium-glucose cotransporter 2 inhibitor tofogliflozin on colorectal tumorigenesis in diabetic and obese mice. Oncol. Rep. 2019, 42, 2797–2805. [Google Scholar] [CrossRef]
- Chan, R.N.C.; Chan, R.N.F.; Chou, O.H.I.; Tse, G.; Lee, S. Lower risks of incident colorectal cancer in SGLT2i users compared to DPP4i users, A propensity score-matched study with competing risk analysis. Eur. J. Intern. Med. 2023, 110, 125–127. [Google Scholar] [CrossRef] [PubMed]
- Tong, G.; Peng, T.; Chen, Y.; Sha, L.; Dai, H.; Xiang, Y.; Zou, Z.; He, H.; Wang, S. Effects of GLP-1 Receptor Agonists on Biological Behavior of Colorectal Cancer Cells by Regulating PI3K/AKT/mTOR Signaling Pathway. Front. Pharmacol. 2022, 13, 901559. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, W.; Kaelber, D.C.; Xu, R.; Berger, N.A. GLP-1 Receptor Agonists and Colorectal Cancer Risk in Drug-Naive Patients with Type 2 Diabetes, with and Without Overweight/Obesity. JAMA Oncol. 2024, 10, 256. [Google Scholar] [CrossRef] [PubMed]
- Teodoro, J.S.; Nunes, S.; Rolo, A.P.; Reis, F.; Palmeira, C.M. Therapeutic Options Targeting Oxidative Stress, Mitochondrial Dysfunction and Inflammation to Hinder the Progression of Vascular Complications of Diabetes. Front. Physiol. 2019, 9, 1857. [Google Scholar] [CrossRef] [PubMed]
- Bardelčíková, A.; Šoltys, J.; Mojžiš, J. Oxidative Stress, Inflammation and Colorectal Cancer: An Overview. Antioxidants 2023, 12, 901. [Google Scholar] [CrossRef] [PubMed]
- Vekic, J.; Zeljkovic, A.; Stefanovic, A.; Giglio, R.V.; Ciaccio, M.; Rizzo, M. Diabetes and Colorectal Cancer Risk, A New Look at Molecular Mechanisms and Potential Role of Novel Antidiabetic Agents. Int. J. Mol. Sci. 2021, 22, 12409. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Colloca, A.; Donisi, I.; Anastasio, C.; Balestrieri, M.L.; D’Onofrio, N. Metabolic Alteration Bridging the Prediabetic State and Colorectal Cancer. Cells 2024, 13, 663. https://doi.org/10.3390/cells13080663
Colloca A, Donisi I, Anastasio C, Balestrieri ML, D’Onofrio N. Metabolic Alteration Bridging the Prediabetic State and Colorectal Cancer. Cells. 2024; 13(8):663. https://doi.org/10.3390/cells13080663
Chicago/Turabian StyleColloca, Antonino, Isabella Donisi, Camilla Anastasio, Maria Luisa Balestrieri, and Nunzia D’Onofrio. 2024. "Metabolic Alteration Bridging the Prediabetic State and Colorectal Cancer" Cells 13, no. 8: 663. https://doi.org/10.3390/cells13080663
APA StyleColloca, A., Donisi, I., Anastasio, C., Balestrieri, M. L., & D’Onofrio, N. (2024). Metabolic Alteration Bridging the Prediabetic State and Colorectal Cancer. Cells, 13(8), 663. https://doi.org/10.3390/cells13080663








