Mitigating Effect of Trans-Zeatin on Cadmium Toxicity in Desmodesmus armatus
Abstract
1. Introduction
2. Materials and Methods
2.1. Algal Growth Conditions
2.2. Number of Cells Determination
2.3. Determination of Optimum Cytokinin Concentrations for Algal Growth and Metal Toxicity
2.4. Heavy Metal Determination
2.5. Photosynthetic Pigment Determination
2.6. Malondialdehyde and Hydrogen Peroxide Content Determination
2.7. Determination of Ascorbate and Proline
2.8. Determination of the Antioxidant Enzymes’ Activities
2.9. Determination of Cysteine, γ-Glutamylcysteine, Glutathione, and Phytochelatins
2.10. Phytochelatin Synthase Activity Assay
2.11. Determination of Plant Hormone Content
2.12. Statistical Analysis
3. Results and Discussion
3.1. Algal Growth
3.2. Cd Uptake
3.3. Photosynthetic Pigments
3.4. Oxidative Stress
3.5. Antioxidant Response
3.6. Phytochelatin Synthesis
3.7. Phytohormone Content
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huang, J.; Su, B.; Fei, X.; Che, J.; Yao, T.; Zhang, R.; Yi, S. Enhanced microalgal biomass and lipid production with simultaneous effective removal of Cd using algae-bacteria-activated carbon consortium added with organic carbon source. Chemosphere 2024, 350, 141088. [Google Scholar] [CrossRef]
- Bouida, L.; Rafatullah, M.; Kerrouche, A.; Qutob, M.; Alosaimi, A.M.; Alorfi, H.S.; Hussein, M.A. A review on cadmium and lead contamination: Sources, fate, mechanism, health effects and remediation methods. Water 2022, 14, 3432. [Google Scholar] [CrossRef]
- Cheng, J.; Qiu, H.; Chang, Z.; Jiang, Z.; Yin, W. The effect of cadmium on the growth and antioxidant response for freshwater algae Chlorella vulgaris. Springerplus 2016, 5, 1290. [Google Scholar] [CrossRef] [PubMed]
- Rawat, I.; Ranjith Kumar, R.; Mutanda, T.; Bux, F. Dual role of microalgae: Phycoremediation of domestic wastewater and biomass production for sustainable biofuels production. Appl. Energy 2011, 88, 3411–3424. [Google Scholar] [CrossRef]
- Tu, X.; Xu, P.; Zhu, Y.; Mi, W.; Bi, Y. Molecular complexation properties of Cd2+ by algal organic matter from Scenedesmus obliquus. Ecotox. Environ. Safe 2023, 263, 115378. [Google Scholar] [CrossRef]
- Al-Khiat, S.H.; Bukhari, N.A.; Ameen, F.; Abdel-Raouf, N. Comparison of the microalgae Phormidium tenue and Chlorella vulgaris as biosorbents of Cd and Zn from aqueous environments. Environ. Res. 2023, 235, 116675. [Google Scholar] [CrossRef] [PubMed]
- Baumann, H.A.; Morrison, L.; Stengel, D.B. Metal accumulation and toxicity measured by PAM-chlorophyll fluorescence in seven species of marine macroalgae. Ecotox. Environ. Safe 2009, 72, 1063–1075. [Google Scholar] [CrossRef]
- Wang, M.-J.; Wang, W.-X. Cadmium sensitivity, uptake, subcellular distribution and thiol induction in a marine diatom: Exposure to cadmium. Aquat. Toxicol. 2011, 101, 377–386. [Google Scholar] [CrossRef]
- El-Naggar, N.E.-A.; Hamouda, R.A.; Mousa, I.E.; Abdel-Hamid, M.S.; Rabei, N.H. Statistical optimization for cadmium removal using Ulva fasciata biomass: Characterization, immobilization and application for almost-complete cadmium removal from aqueous solutions. Sci. Rep. 2018, 8, 12456. [Google Scholar] [CrossRef]
- Ordóñez, J.I.; Cortés, S.; Maluenda, P.; Soto, I. Biosorption of heavy metals with algae: Critical review of its application in real effluents. Sustainability 2023, 15, 5521. [Google Scholar] [CrossRef]
- Chandrashekharaiah, P.S.; Sanyal, D.; Dasgupta, S.; Banik, A. Cadmium biosorption and biomass production by two freshwater microalgae Scenedesmus acutus and Chlorella pyrenoidosa: An integrated approach. Chemosphere 2021, 269, 128755. [Google Scholar] [CrossRef]
- Qi, F.; Gao, Y.; Liu, J.; Yao, X.; Han, K.; Wu, Z.; Wang, Y. Alleviation of cadmium-induced photoinhibition and oxidative stress by melatonin in Chlamydomonas reinhardtii. Environ. Sci. Pollut. Res. 2023, 30, 78423–78437. [Google Scholar] [CrossRef]
- Piotrowska-Niczyporuk, A.; Bajguz, A.; Kotowska, U.; Zambrzycka-Szelewa, E.; Sienkiewicz, A. Auxins and cytokinins regulate phytohormone homeostasis and thiol-mediated detoxification in the green alga Acutodesmus obliquus exposed to lead stress. Sci. Rep. 2020, 10, 10193. [Google Scholar] [CrossRef] [PubMed]
- Talarek-Karwel, M.; Bajguz, A.; Piotrowska-Niczyporuk, A. Hormonal response of Acutodesmus obliquus exposed to combined treatment with 24-epibrassinolide and lead. J. Appl. Phycol. 2020, 32, 2903–2914. [Google Scholar] [CrossRef]
- Pinto, E.; Sigaud-Kutner, T.C.S.; Leitão, M.A.S.; Okamoto, O.K.; Morse, D.; Colepicolo, P. Heavy metal–induced oxidative stress in algae. J. Phycol. 2003, 39, 1008–1018. [Google Scholar] [CrossRef]
- Yan, A.; Wang, Y.; Tan, S.N.; Mohd Yusof, M.L.; Ghosh, S.; Chen, Z. Phytoremediation: A promising approach for revegetation of heavy metal-polluted land. Front. Plant Sci. 2020, 11, 359. [Google Scholar] [CrossRef]
- Wątły, J.; Łuczkowski, M.; Padjasek, M.; Krężel, A. Phytochelatins as a dynamic system for Cd(II) buffering from the micro- to femtomolar range. Inorg. Chem. 2021, 60, 4657–4675. [Google Scholar] [CrossRef]
- Wu, X.; He, J.; Ding, H.; Zhu, Z.; Chen, J.; Xu, S.; Zha, D. Modulation of zinc-induced oxidative damage in Solanum melongena by 6-benzylaminopurine involves ascorbate–glutathione cycle metabolism. Environ. Exp. Bot. 2015, 116, 1–11. [Google Scholar] [CrossRef]
- Grill, E.; Löffler, S.; Winnacker, E.L.; Zenk, M.H. Phytochelatins, the heavy-metal-binding peptides of plants, are synthesized from glutathione by a specific g-glutamylcysteine dipeptidyl transpeptidase (phytochelatin synthase). Proc. Natl. Acad. Sci. USA 1989, 86, 6838–6842. [Google Scholar] [CrossRef]
- Kováčik, J.; Klejdus, B.; Babula, P.; Hedbavny, J. Age affects not only metabolome but also metal toxicity in Scenedesmus quadricauda cultures. J. Hazard. Mater. 2016, 306, 58–66. [Google Scholar] [CrossRef]
- Vingiani, G.M.; Gasulla, F.; Barón-Sola, Á.; Sobrino-Plata, J.; Henández, L.E.; Casano, L.M. Physiological and molecular alterations of phycobionts of genus Trebouxia and Coccomyxa exposed to cadmium. Microb. Ecol. 2021, 82, 334–343. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, W.-X. Differential acclimation of a marine diatom to inorganic mercury and methylmercury exposure. Aquat. Toxicol. 2013, 138–139, 52–59. [Google Scholar] [CrossRef]
- Balzano, S.; Sardo, A.; Blasio, M.; Chahine, T.B.; Dell’Anno, F.; Sansone, C.; Brunet, C. Microalgal metallothioneins and phytochelatins and their potential use in bioremediation. Front. Microbiol. 2020, 11, 517. [Google Scholar] [CrossRef] [PubMed]
- Bücker-Neto, L.; Paiva, A.L.S.; Machado, R.D.; Arenhart, R.A.; Margis-Pinheiro, M. Interactions between plant hormones and heavy metals responses. Genet. Mol. Biol. 2017, 40, 373–386. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.L.; Waqas, M.; Hussain, J.; Al-Harrasi, A.; Hamayun, M.; Lee, I.-J. Phytohormones enabled endophytic fungal symbiosis improve aluminum phytoextraction in tolerant Solanum lycopersicum: An examples of Penicillium janthinellum LK5 and comparison with exogenous GA3. J. Hazard. Mater. 2015, 295, 70–78. [Google Scholar] [CrossRef]
- Nguyen, N.H.; Nguyen, Q.T.; Dang, D.H.; Emery, R.J.N. Phytohormones enhance heavy metal responses in Euglena gracilis: Evidence from uptake of Ni, Pb and Cd and linkages to hormonomic and metabolomic dynamics. Environ. Pollut. 2023, 320, 121094. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.Q.; Sesin, V.; Kisiala, A.; Emery, R.J.N. The role of phytohormones in enhancing metal remediation capacity of algae. Bull. Environ. Contam. Toxicol. 2020, 105, 671–678. [Google Scholar] [CrossRef]
- Bajguz, A.; Hayat, S. Effects of brassinosteroids on the plant responses to environmental stresses. Plant Physiol. Biochem. 2009, 47, 1–8. [Google Scholar] [CrossRef]
- Ahanger, M.A.; Ashraf, M.; Bajguz, A.; Ahmad, P. Brassinosteroids regulate growth in plants under stressful environments and crosstalk with other potential phytohormones. J. Plant Growth Regul. 2018, 37, 1007–1024. [Google Scholar] [CrossRef]
- Zulfiqar, F.; Ashraf, M. Bioregulators: Unlocking their potential role in regulation of the plant oxidative defense system. Plant Mol. Biol. 2021, 105, 11–41. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, Q.; Zhang, S.; Hamid, Y.; Lian, J.; Huang, X.; Zou, T.; Lin, Q.; Feng, Y.; He, Z.; et al. Foliar application of plant growth regulators for enhancing heavy metal phytoextraction efficiency by Sedum alfredii Hance in contaminated soils: Lab to field experiments. Sci. Total Environ. 2024, 913, 169788. [Google Scholar] [CrossRef] [PubMed]
- Bilal, S.; Saad Jan, S.; Shahid, M.; Asaf, S.; Khan, A.L.; Lubna; Al-Rawahi, A.; Lee, I.-J.; Al-Harrasi, A. Novel insights into exogenous phytohormones: Central regulators in the modulation of physiological, biochemical, and molecular responses in rice under metal(loid) stress. Metabolites 2023, 13, 1036. [Google Scholar] [CrossRef] [PubMed]
- Veselova, S.V.; Farhutdinov, R.G.; Veselov, S.Y.; Kudoyarova, G.R.; Veselov, D.S.; Hartung, W. The effect of root cooling on hormone content, leaf conductance and root hydraulic conductivity of durum wheat seedlings (Triticum durum L.). J. Plant Physiol. 2005, 162, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Atanasova, L.Y.; Pissarska, M.G.; Popov, G.S.; Georgiev, G.I. Growth and endogenous cytokinins of juniper shoots as affected by high metal concentrations. Biol. Plant. 2004, 48, 157–159. [Google Scholar] [CrossRef]
- Hayward, A.R.; Coates, K.E.; Galer, A.L.; Hutchinson, T.C.; Emery, R.J.N. Chelator profiling in Deschampsia cespitosa (L.) Beauv. Reveals a Ni reaction, which is distinct from the ABA and cytokinin associated response to Cd. Plant Physiol. Biochem. 2013, 64, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Leonardo, B.; Emanuela, T.; Letizia, M.M.; Antonella, M.; Marco, M.; Fabrizio, A.; Beatrice, B.M.; Adriana, C. Cadmium affects cell niches maintenance in Arabidopsis thaliana post-embryonic shoot and root apical meristem by altering the expression of WUS/WOX homolog genes and cytokinin accumulation. Plant Physiol. Biochem. 2021, 167, 785–794. [Google Scholar] [CrossRef] [PubMed]
- Sameena, P.P.; Kalaji, H.M.; Żuk-Gołaszewska, K.; Horaczek, T.; Sierka, E.; Puthur, J.T. 6-Benzylaminopurine alleviates the impact of Cu2+ toxicity on photosynthetic performance of Ricinus communis L. seedlings. Int. J. Mol. Sci. 2021, 22, 13349. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Zehra, A.; Sahito, Z.A.; Wang, W.; Chen, S.; Feng, Y.; He, Z.; Yang, X. Cytokinin-mediated shoot proliferation and its correlation with phytoremediation effects in Cd-hyperaccumulator ecotype of Sedum alfredii. Sci. Total Environ. 2024, 912, 168993. [Google Scholar] [CrossRef] [PubMed]
- Piotrowska-Niczyporuk, A.; Bajguz, A.; Zambrzycka-Szelewa, E.; Bralska, M. Exogenously applied auxins and cytokinins ameliorate lead toxicity by inducing antioxidant defence system in green alga Acutodesmus obliquus. Plant Physiol. Biochem. 2018, 132, 535–546. [Google Scholar] [CrossRef]
- Piotrowska-Niczyporuk, A.; Bajguz, A.; Zambrzycka-Szelewa, E. Response and the detoxification strategies of green alga Acutodesmus obliquus (Chlorophyceae) under lead stress. Environ. Exp. Bot. 2017, 144, 25–36. [Google Scholar] [CrossRef]
- Stirk, W.A.; Ordog, V.; Novak, O.; Rolcik, J.; Strnad, M.; Balint, P.; van Staden, J. Auxin and cytokinin relationships in 24 microalgal strains. J. Phycol. 2013, 49, 459–467. [Google Scholar] [CrossRef]
- Žižková, E.; Dobrev, P.I.; Motyka, V.; Kubeš, M.; Šimura, J.; Přibyl, P.; Zahajská, L.; Záveská Drábková, L.; Novák, O. Control of cytokinin and auxin homeostasis in cyanobacteria and algae. Ann. Bot. 2017, 119, 151–166. [Google Scholar] [CrossRef]
- Andersen, R.A.; Berges, J.A.; Harrison, P.J.; Watanabe, M.M. Appendix A—Recipes for freshwater and seawater media. In Algal Culturing Techniques; Andersen, R.A., Ed.; Academic Press: Amsterdam, The Netherlands, 2005; pp. 429–538. [Google Scholar]
- Zapata, M.; Rodríguez, F.; Garrido, J.L. Separation of chlorophylls and carotenoids from marine phytoplankton: A new HPLC method using a reversed phase C8 column and pyridine-containing mobile phases. Mar. Ecol. Prog. Ser. 2000, 195, 29–45. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Alexieva, V.; Sergiev, I.; Mapelli, S.; Karanov, E. The effect of drought and ultraviolet radiation on growth and stress markers in pea and wheat. Plant Cell Environ. 2001, 24, 1337–1344. [Google Scholar] [CrossRef]
- Kampfenkel, K.; Vanmontagu, M.; Inze, D. Extraction and determination of ascorbate and dehydroascorbate from plant tissue. Anal. Biochem. 1995, 225, 165–167. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef] [PubMed]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar] [CrossRef]
- Schaedle, M.; Bassham, J.A. Chloroplast glutathione reductase. Plant Physiol. 1977, 59, 1011–1012. [Google Scholar] [CrossRef] [PubMed]
- Scheidegger, C.; Behra, R.; Sigg, L. Phytochelatin formation kinetics and toxic effects in the freshwater alga Chlamydomonas reinhardtii upon short- and long-term exposure to lead(II). Aquat. Toxicol. 2011, 101, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Finkemeier, I.; Kluge, C.; Metwally, A.; Georgi, M.; Grotjohann, N.; Dietz, K.J. Alterations in Cd-induced gene expression under nitrogen deficiency in Hordeum vulgare. Plant Cell Environ. 2003, 26, 821–833. [Google Scholar] [CrossRef] [PubMed]
- Šimura, J.; Antoniadi, I.; Široká, J.; Tarkowská, D.; Strnad, M.; Ljung, K.; Novák, O. Plant hormonomics: Multiple phytohormone profiling by targeted metabolomics. Plant Physiol. 2018, 177, 476–489. [Google Scholar] [CrossRef] [PubMed]
- Ran, Y.; Sun, D.; Liu, X.; Zhang, L.; Niu, Z.; Chai, T.; Hu, Z.; Qiao, K. Chlorella pyrenoidosa as a potential bioremediator: Its tolerance and molecular responses to cadmium and lead. Sci. Total Environ. 2024, 912, 168712. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Tang, Y.; Yu, F.; Peng, Z.; Yao, S.; Deng, X.; Long, H.; Wang, X.; Huang, K. Translatomics and physiological analyses of the detoxification mechanism of green alga Chlamydomonas reinhardtii to cadmium toxicity. J. Hazard. Mater. 2023, 448, 130990. [Google Scholar] [CrossRef] [PubMed]
- Mo, L.; Yang, Y.; Zhao, D.; Qin, L.; Yuan, B.; Liang, N. Time-dependent toxicity and health effects mechanism of cadmium to three green algae. Int. J. Environ. Res. Public Health 2022, 19, 10974. [Google Scholar] [CrossRef]
- Nayar, S. Exploring the role of a cytokinin-activating enzyme LONELY GUY in unicellular microalga Chlorella variabilis. Front. Plant Sci. 2021, 11, 611871. [Google Scholar] [CrossRef] [PubMed]
- Ali, E.F.; Aljarani, A.M.; Mohammed, F.A.; Desoky, E.-S.M.; Mohamed, I.A.A.; El-Sharnouby, M.; Tammam, S.A.; Hassan, F.A.S.; Rady, M.M.; Shaaban, A. Exploring the potential enhancing effects of trans-zeatin and silymarin on the productivity and antioxidant defense capacity of cadmium-stressed wheat. Biology 2022, 11, 1173. [Google Scholar] [CrossRef]
- Ahanger, M.A.; Aziz, U.; Sahli, A.A.; Alyemeni, M.N.; Ahmad, P. Combined kinetin and spermidine treatments ameliorate growth and photosynthetic inhibition in Vigna angularis by up-regulating antioxidant and nitrogen metabolism under cadmium stress. Biomolecules 2020, 10, 147. [Google Scholar] [CrossRef]
- Singh, S.; Singh, A.; Srivastava, P.K.; Prasad, S.M. Cadmium toxicity and its amelioration by kinetin in tomato seedlings vis-à-vis ascorbate-glutathione cycle. J. Photochem. Photobiol. B-Biol. 2018, 178, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Piotrowska-Niczyporuk, A.; Bajguz, A.; Zambrzycka, E.; Godlewska-Zylkiewicz, B. Phytohormones as regulators of heavy metal biosorption and toxicity in green alga Chlorella vulgaris (Chlorophyceae). Plant Physiol. Biochem. 2012, 52, 52–65. [Google Scholar] [CrossRef] [PubMed]
- Maoka, T. Carotenoids as natural functional pigments. J. Nat. Med. 2020, 74, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Torricelli, E.; Gorbi, G.; Pawlik-Skowronska, B.; di Toppi, L.S.; Corradi, M.G. Cadmium tolerance, cysteine and thiol peptide levels in wild type and chromium-tolerant strains of Scenedesmus acutus (Chlorophyceae). Aquat. Toxicol. 2004, 68, 315–323. [Google Scholar] [CrossRef]
- Wu, Y.; Guo, Z.; Zhang, W.; Tan, Q.; Zhang, L.; Ge, X.; Chen, M. Quantitative relationship between cadmium uptake and the kinetics of phytochelatin induction by cadmium in a marine diatom. Sci. Rep. 2016, 6, 35935. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Ghnaya, T.; Dailly, H.; Cui, G.; Vanpee, B.; Han, R.; Lutts, S. The cytokinin trans-zeatine riboside increased resistance to heavy metals in the halophyte plant species Kosteletzkya pentacarpos in the absence but not in the presence of NaCl. Chemosphere 2019, 233, 954–965. [Google Scholar] [CrossRef]
- Cassina, L.; Tassi, E.; Morelli, E.; Giorgetti, L.; Remorini, D.; Chaney, R.L.; Barbafieri, M. Exogenous cytokinin treatments of an ni hyper-accumulator, Alyssum murale, grown in a serpentine soil: Implications for phytoextraction. Int. J. Phytoremediat. 2011, 13, 90–101. [Google Scholar] [CrossRef]

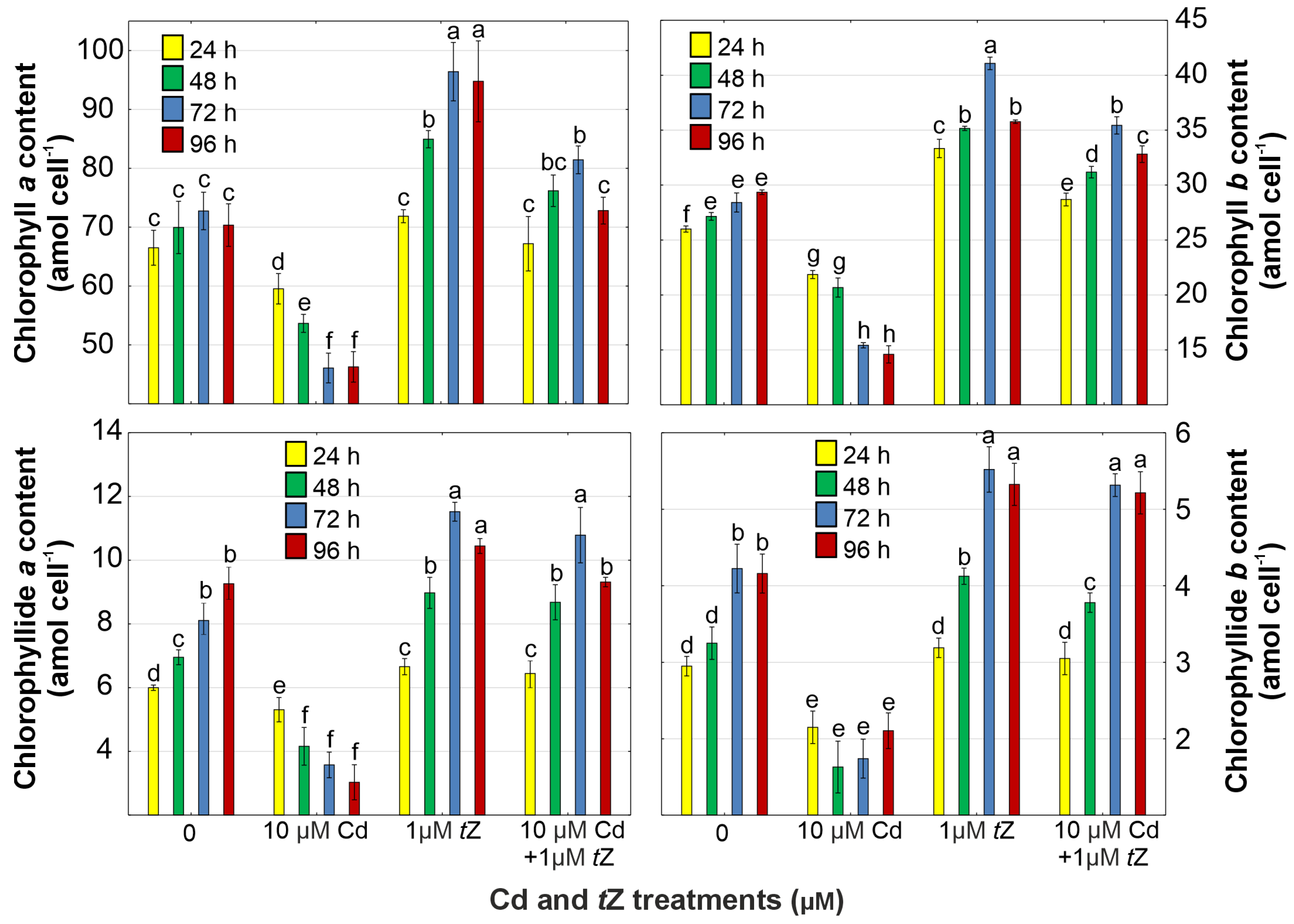

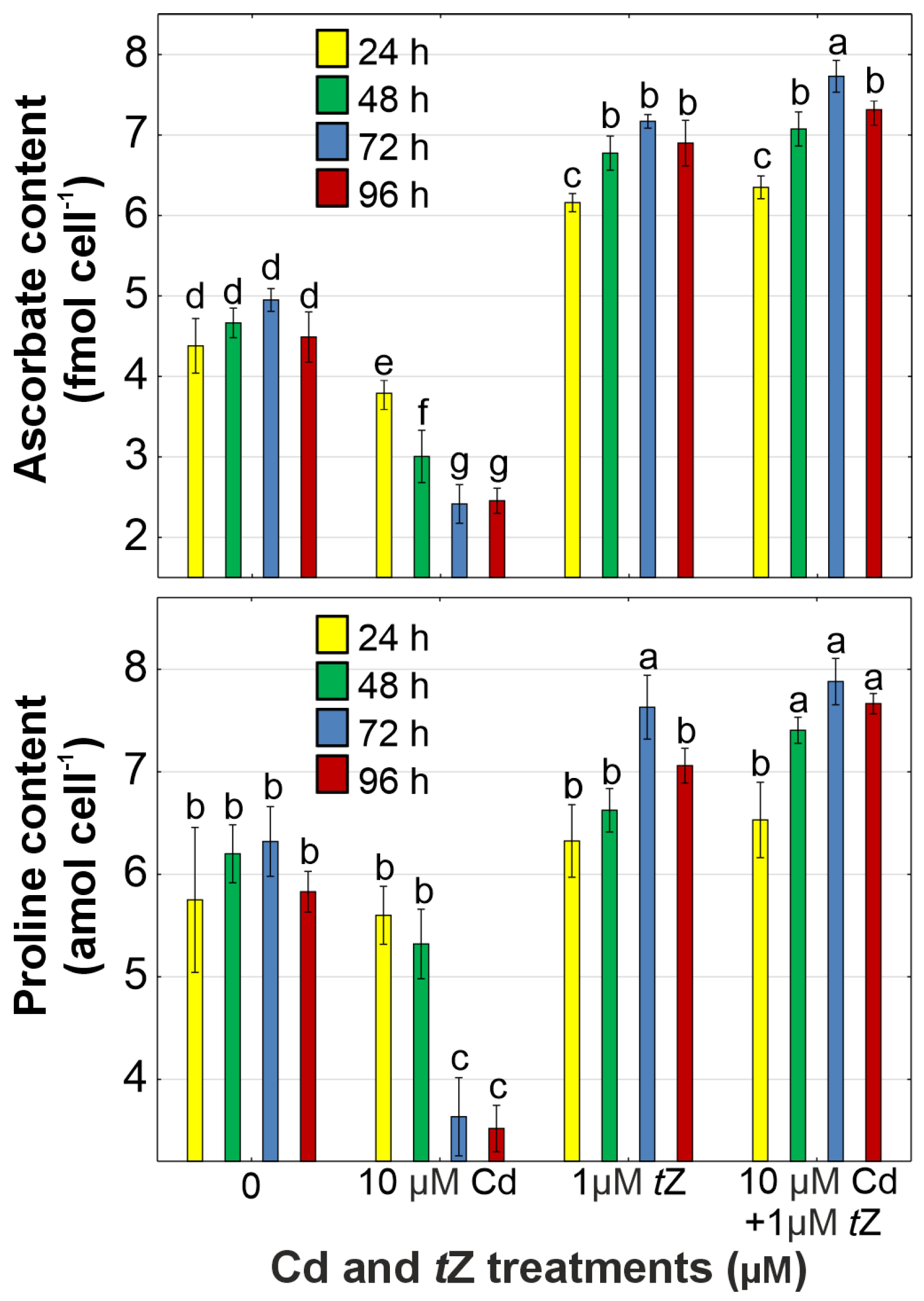

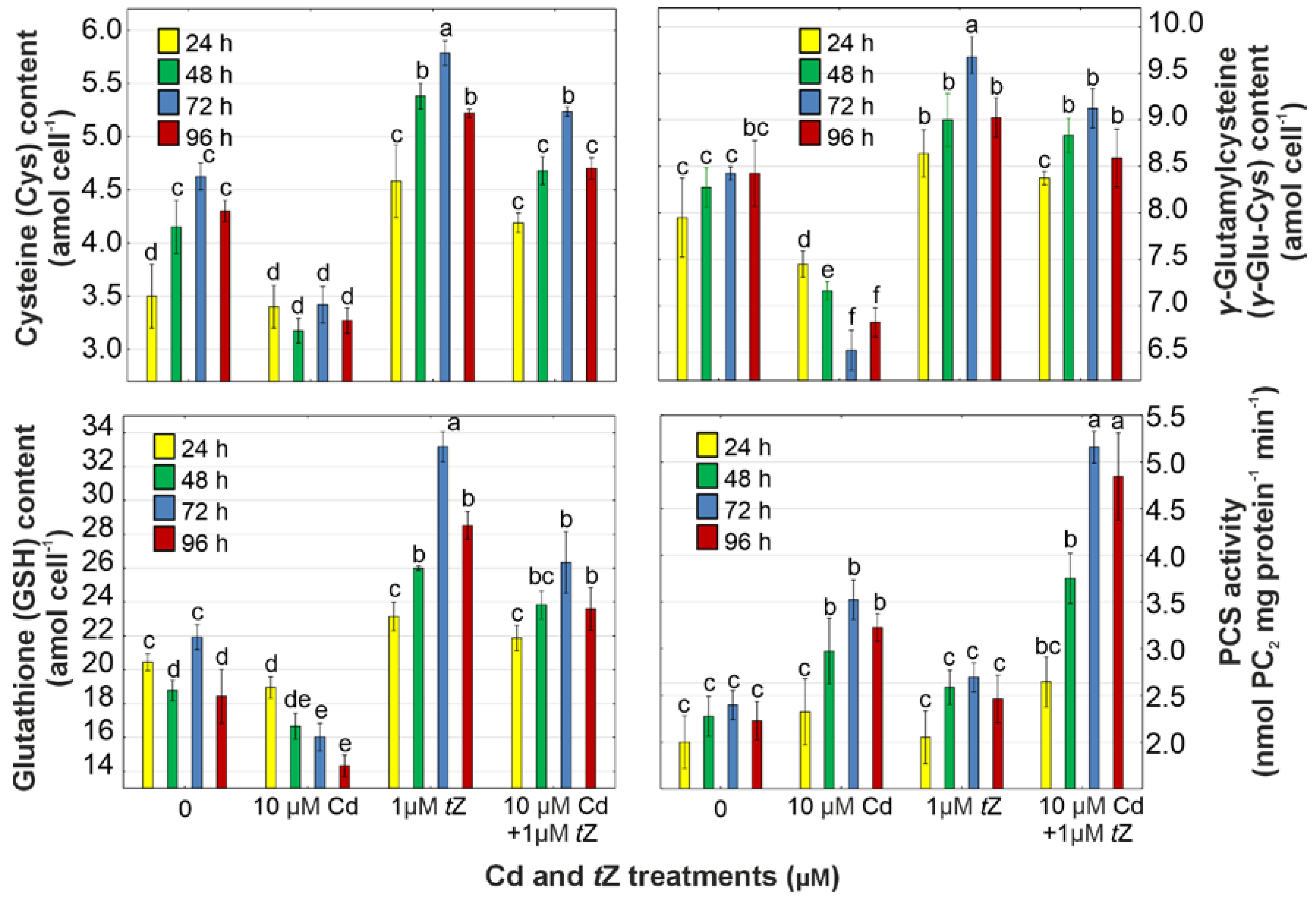
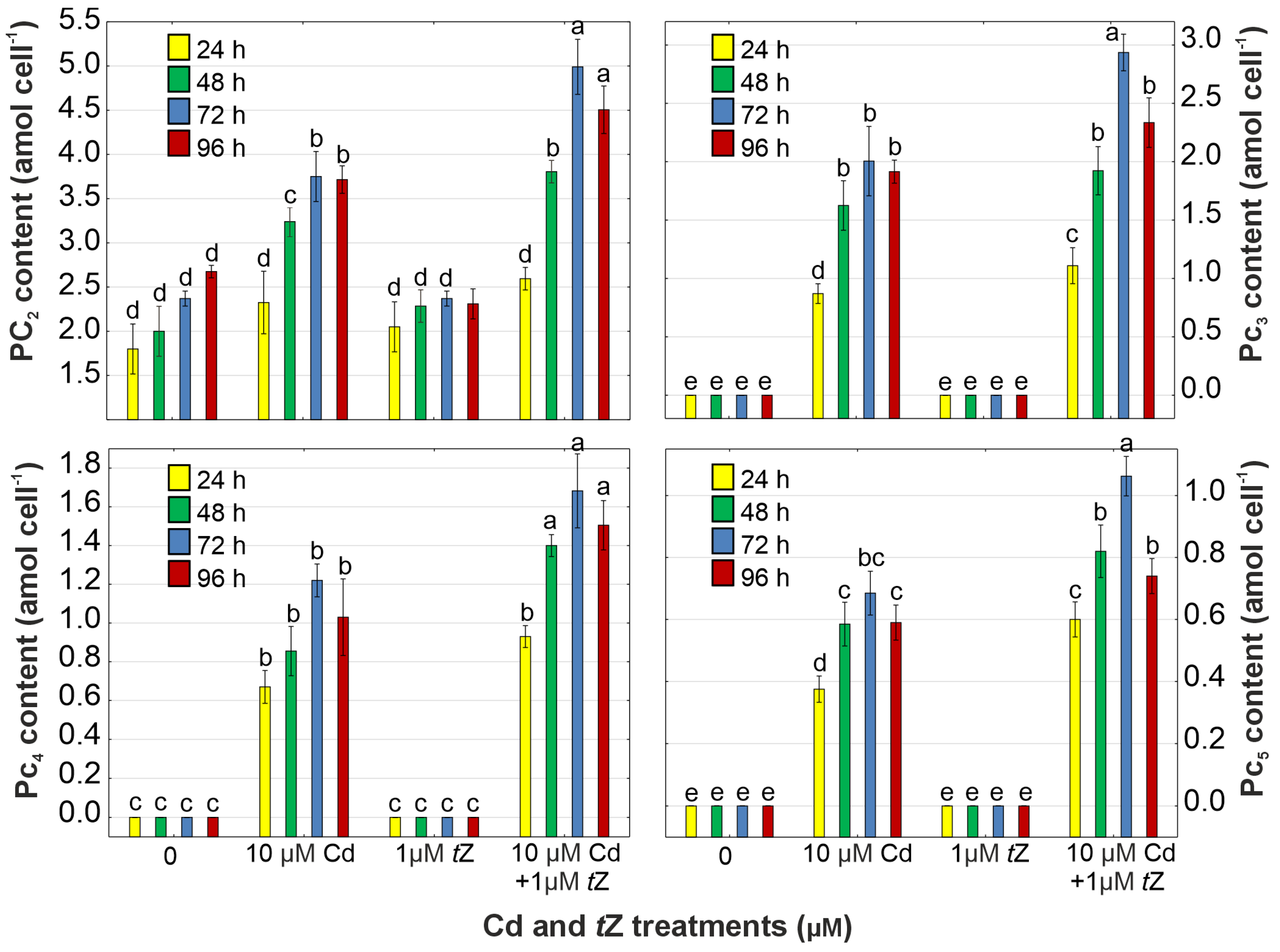

| Cd Content (amol cell−1) | ||||
|---|---|---|---|---|
| Treatments | Time of Culture | |||
| 24 h | 48 h | 72 h | 96 h | |
| Control | 0 | 0 | 0 | 0 |
| 10 µM Cd | 76.25 ± 2.002 h | 89.14 ± 1.237 f | 112.74 ± 1.307 d | 121.03 ± 1.884 c |
| 1 µM tZ | 0 | 0 | 0 | 0 |
| 10 µM Cd + 1 µM tZ | 83.61 ± 1.148 g | 94.77 ± 0.941 e | 138.32 ± 3.115 b | 145.16 ± 3.551 a |
| Carotene and Xanthophyll Contents (amol cell−1) | |||||
|---|---|---|---|---|---|
| Pigments | Treatments | Time of Culture | |||
| 24 h | 48 h | 72 h | 96 h | ||
| α-Carotene | Control | 2.09 ± 0.012 h | 2.11 ± 0.101 h | 2.56 ± 0.011 g | 2.67 ± 0.014 g |
| 10 µM Cd | 2.13 ± 0.023 h | 2.55 ± 0.055 g | 2.68 ± 0.004 g | 3.09 ± 0.021 e | |
| 1 µM tZ | 2.85 ± 0.041 f | 2.87 ± 0.016 f | 3.29 ± 0.033 d | 3.31 ± 0.036 d | |
| 10 µM Cd + 1 µM tZ | 2.97 ± 0.043 e | 3.48 ± 0.038 c | 4.44 ± 0.031 a | 4.26 ± 0.010 b | |
| β-Carotene | Control | 4.12 ± 0.064 f | 4.08 ± 0.033 f | 4.52 ± 0.032 e | 4.66 ± 0.088 e |
| 10 µM Cd | 4.51 ± 0.030 e | 4.92 ± 0.075 d | 5.17 ± 0.009 d | 5.22 ± 0.025 d | |
| 1 µM tZ | 4.99 ± 0.022 d | 4.95 ± 0.019 d | 5.78 ± 0.018 c | 5.31 ± 0.012 d | |
| 10 µM Cd + 1 µM tZ | 5.22 ± 0.009 d | 5.68 ± 0.099 c | 6.99 ± 0.033 b | 7.19 ± 0.093 a | |
| Antheraxanthin | Control | 4.37 ± 0.046 g | 4.52 ± 0.084 f | 4.58 ± 0.009 f | 4.71 ± 0.014 e |
| 10 µM Cd | 4.45 ± 0.006 f | 4.72 ± 0.005 e | 4.88 ± 0.016 e | 4.83 ± 0.066 e | |
| 1 µM tZ | 4.46 ± 0.022 f | 4.63 ± 0.077 e | 4.77 ± 0.044 e | 4.69 ± 0.021 e | |
| 10 µM Cd + 1 µM tZ | 4.99 ± 0.077 d | 5.54 ± 0.093 c | 7.25 ± 0.064 a | 6.63 ± 0.026 b | |
| Violaxanthin | Control | 3.78 ± 0.033 f | 4.05 ± 0.014 e | 4.23 ± 0.067 d | 4.19 ± 0.068 e |
| 10 µM Cd | 4.11 ± 0.092 e | 4.32 ± 0.021 d | 4.66 ± 0.011 d | 4.47 ± 0.019 d | |
| 1 µM tZ | 3.97 ± 0.004 f | 4.38 ± 0.015 d | 5.45 ± 0.020 c | 5.11 ± 0.066 c | |
| 10 µM Cd + 1 µM tZ | 4.39 ± 0.081 d | 5.64 ± 0.085 c | 6.93 ± 0.095 a | 6.75 ± 0.037 b | |
| Zeaxanthin | Control | 10.38 ± 0.921 e | 10.46 ± 0.083 e | 11.25 ± 0.712 d | 11.72 ± 0.997 d |
| 10 µM Cd | 11.16 ± 0.893 d | 12.67 ± 0.285 d | 12.59 ± 0.805 d | 12.31 ± 0.831 d | |
| 1 µM tZ | 10.48 ± 0.755 d | 11.09 ± 0.843 d | 12.12 ± 0.722 d | 10.75 ± 0.104 d | |
| 10 µM Cd + 1 µM tZ | 11.65 ± 0.011 d | 13.73 ± 0.306 c | 14.84 ± 0.101 a | 14.21 ± 0.399 b | |
| Phytohormone Content (fmol cell−1) | Treatments | |||
|---|---|---|---|---|
| Control | 10 µM Cd | 1 µM tZ | 10 µM Cd + 1 µM tZ | |
| Cytokinins | ||||
| trans-Zeatin (tZ) | 0.421 ± 0.011 c | 0.225 ± 0.015 d | 0.865 ± 0.055 a | 0.781 ± 0.033 b |
| trans-Zeatin-Riboside (tZR) | 0.112 ± 0.015 c | 0.082 ± 0.023 d | 0.203 ± 0.019 a | 0.196 ± 0.008 b |
| trans-Zeatin-9-Glucoside (tZ9G) | 0.174 ± 0.028 c | 0.301 ± 0.101 a | 0.292 ± 0.071 b | 0.198 ± 0.017 c |
| trans-Zeatin-7-Glucoside (tZ7G) | 0.071 ± 0.016 c | 0.125 ± 0.077 a | 0.113 ± 0.007 b | 0.072 ± 0.005 c |
| trans-Zeatin-O-Glucoside (tZOG) | 0.783 ± 0.205 c | 0.936 ± 0.069 a | 0.855 ± 0.059 b | 0.724 ± 0.062 c |
| trans-Zeatin-O-Glucoside Riboside (tZROG) | 0.097 ± 0.008 c | 0.135 ± 0.026 a | 0.109 ± 0.013 b | 0.088 ± 0.011 c |
| cis-Zeatin (cZ) | 7.012 ± 0.308 c | 4.891 ± 0.274 d | 8.804 ± 0.933 b | 9.552 ± 0.809 a |
| cis-Zeatin-Riboside (cZR) | 3.088 ± 0.264 b | 4.294 ± 0.286 a | 3.227 ± 0.213 b | 3.304 ± 0.244 b |
| cis-Zeatin-O-Glucoside (cZOG) | 0.585 ± 0.089 d | 0.926 ± 0.309 a | 0.744 ± 0.019 b | 0.633 ± 0.043 c |
| cis-Zeatin-O-Glucoside Riboside (cZROG) | 0.451 ± 0.042 c | 0.739 ± 0.034 a | 0.592 ± 0.044 b | 0.507 ± 0.013 b |
| Dihydrozeatin (DHZ) | 0.883 ± 0.055 b | 0.571 ± 0.028 c | 0.992 ± 0.026 a | 0.974 ± 0.066 a |
| Dihydrozeatin Riboside (DHZR) | 1.325 ± 0.808 c | 1.576 ± 0.605 a | 1.489 ± 0.621 b | 1.486 ± 0.508 b |
| Dihydrozeatin-9-Glucoside (DHZ9G) | 0.113 ± 0.007 c | 0.273 ± 0.080 a | 0.178 ± 0.010 b | 0.119 ± 0.031 c |
| Dihydrozeatin-7-Glucoside (DHZ7G) | 0.085 ± 0.009 b | 0.124 ± 0.018 a | 0.099 ± 0.006 b | 0.068 ± 0.008 c |
| Dihydrozeatin-O-Glucoside (DHZOG) | 0.381 ± 0.026 c | 0.519 ± 0.022 a | 0.394 ± 0.018 c | 0.401 ± 0.016 b |
| N6-Isopentenyladenine (iP) | 1.807 ± 0.103 b | 1.115 ± 0.129 c | 1.993 ± 0.237 b | 2.155 ± 0.115 a |
| N6-Isopentenyladenosine (iPR) | 1.749 ± 0.664 b | 1.127 ± 0.211 c | 1.953 ± 0.075 b | 2.038 ± 0.808 a |
| N6-Isopentenyladenine-7-Glucoside (iP7G) | 0.173 ± 0.059 b | 0.244 ± 0.007 a | 0.196 ± 0.013 b | 0.168 ± 0.037 b |
| Auxins | ||||
| Indole-3-acetic acid (IAA) | 18.045 ± 1.044 b | 11.663 ± 0.803 c | 23.251 ± 1.569 a | 24.023 ± 2.001 a |
| Indole-3-butyric acid (IBA) | 2.801 ± 0.341 b | 1.088 ± 0.017 c | 2.955 ± 0.903 b | 3.177 ± 0.455 a |
| Phenylacetic acid (PAA) | 5.902 ± 0.099 c | 3.605 ± 0.081 d | 6.736 ± 0.078 b | 7.302 ± 0.811 a |
| Abscisic acid (ABA) | 6.318 ± 0.312 c | 11.043 ± 0.668 a | 5.329 ± 0.408 d | 7.034 ± 0.552 b |
| Gibberellin (GA3) | 1.755 ± 0.308 b | 0.822 ± 0.055 c | 1.968 ± 0.117 b | 2.113 ± 0.081 a |
| Brassinosteroids | ||||
| Brassinolide (BL) | 2.535 ± 0.088 b | 1.604 ± 0.029 c | 2.877 ± 0.047 a | 2.803 ± 0.048 a |
| Epibrassinolide (epiBL) | 0.671 ± 0.016 a | 0.358 ± 0.014 b | 0.711 ± 0.085 a | 0.739 ± 0.111 a |
| Castasterone (CS) | 3.466 ± 0.052 b | 1.684 ± 0.266 c | 4.199 ± 0.609 b | 5.231 ± 0.446 a |
| 24-Epicastasterone (epiCS) | 0.165 ± 0.027 b | 0.122 ± 0.037 b | 0.227 ± 0.042 a | 0.253 ± 0.039 a |
| Cathasterone (CT) | 3.419 ± 0.087 b | 2.015 ± 0.807 c | 3.881 ± 0.114 a | 4.116 ± 0.258 a |
| 28-Homobrassinolide (HBL) | 0.137 ± 0.019 b | 0.078 ± 0.021 c | 0.188 ± 0.035 a | 0.175 ± 0.088 a |
| Typhasterol (TY) | 0.275 ± 0.008 b | 0.139 ± 0.018 c | 0.281 ± 0.067 b | 0.316 ± 0.057 a |
| 6-Deoxytyphasterol (6dTY) | 0.195 ± 0.033 b | 0.108 ± 0.055 c | 0.235 ± 0.031 a | 0.204 ± 0.041 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piotrowska-Niczyporuk, A.; Bonda-Ostaszewska, E.; Bajguz, A. Mitigating Effect of Trans-Zeatin on Cadmium Toxicity in Desmodesmus armatus. Cells 2024, 13, 686. https://doi.org/10.3390/cells13080686
Piotrowska-Niczyporuk A, Bonda-Ostaszewska E, Bajguz A. Mitigating Effect of Trans-Zeatin on Cadmium Toxicity in Desmodesmus armatus. Cells. 2024; 13(8):686. https://doi.org/10.3390/cells13080686
Chicago/Turabian StylePiotrowska-Niczyporuk, Alicja, Elżbieta Bonda-Ostaszewska, and Andrzej Bajguz. 2024. "Mitigating Effect of Trans-Zeatin on Cadmium Toxicity in Desmodesmus armatus" Cells 13, no. 8: 686. https://doi.org/10.3390/cells13080686
APA StylePiotrowska-Niczyporuk, A., Bonda-Ostaszewska, E., & Bajguz, A. (2024). Mitigating Effect of Trans-Zeatin on Cadmium Toxicity in Desmodesmus armatus. Cells, 13(8), 686. https://doi.org/10.3390/cells13080686







