In Vitro Induction of Hypertrophic Chondrocyte Differentiation of Naïve MSCs by Strain
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples Preparation
2.2. Bioreactor and Mechanical Deformation
2.3. Cell Viability
2.4. Gene Expression Analysis
2.5. Protein Expression
2.5.1. Histology and Immunohistochemistry Staining
2.5.2. Biochemistry Analysis (GAG)
2.6. Mechanical Testing
2.7. Statistical Analysis
3. Results
3.1. Construct Integrity and Cell Viability Is Not Impaired by Application of Strain
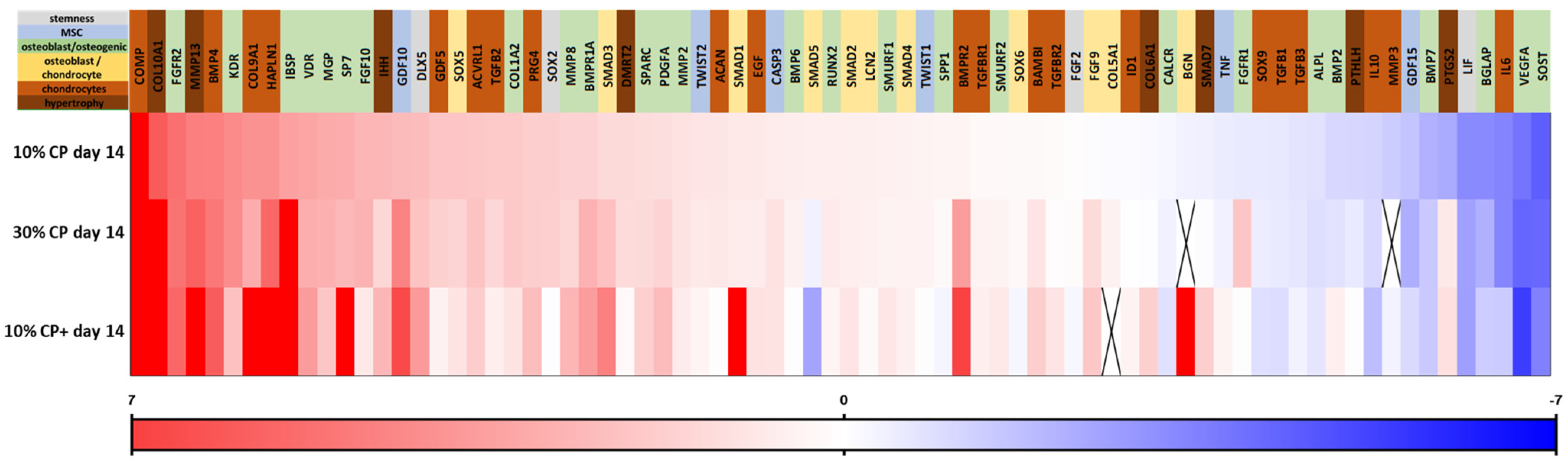
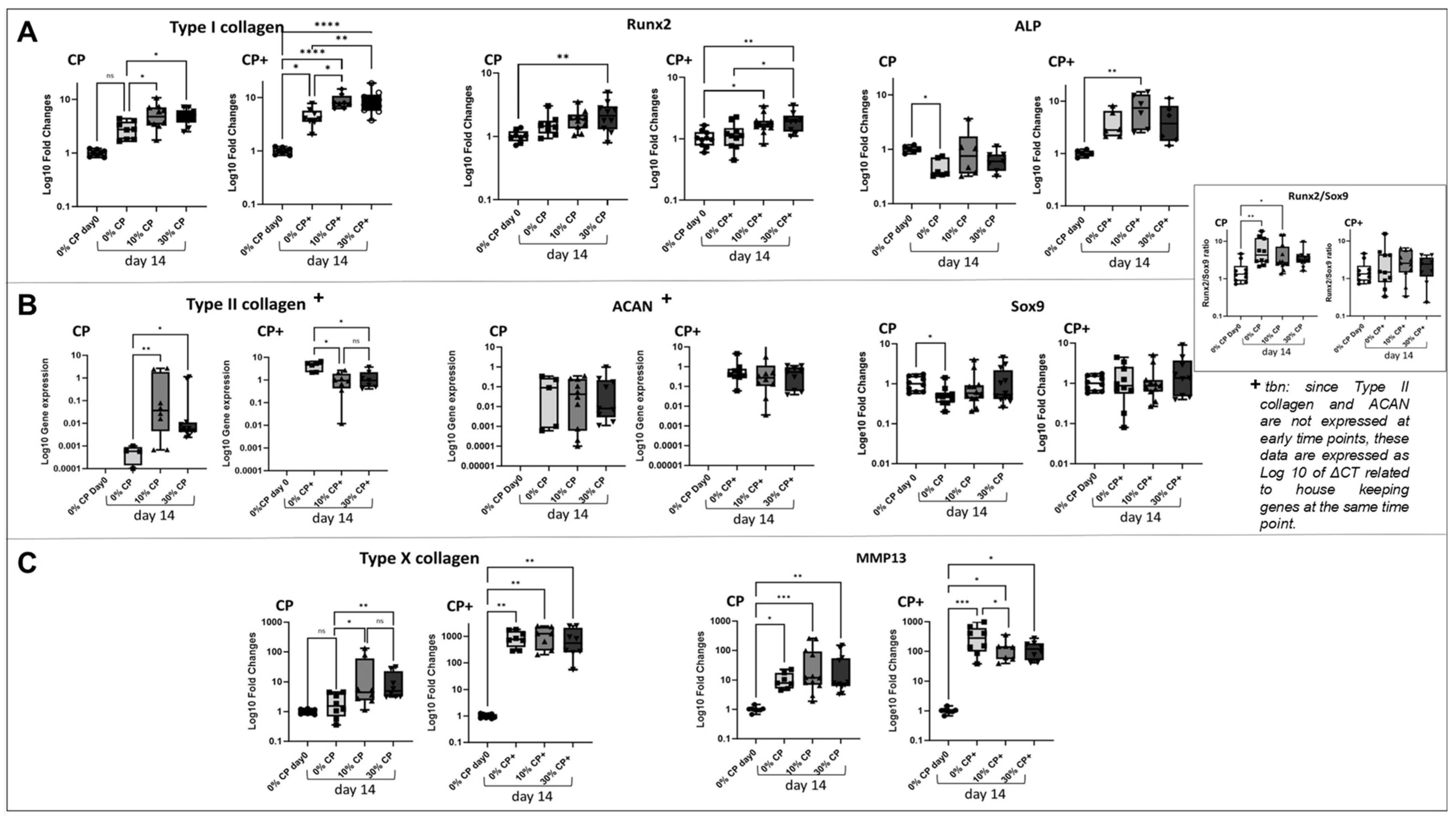
3.2. Effect of Strain on MSC Gene Expression
3.3. Extracellular Matrix Synthesis
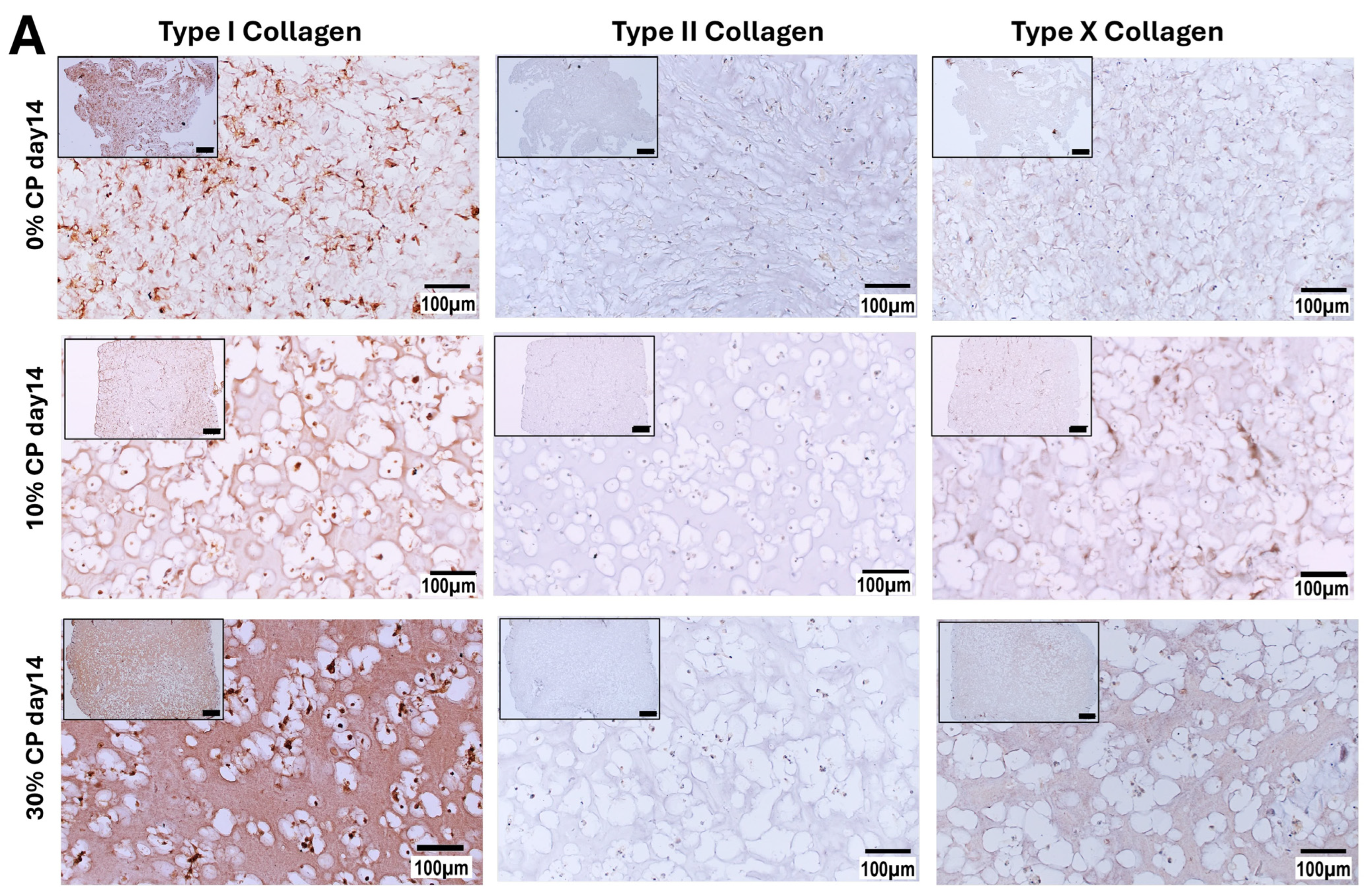
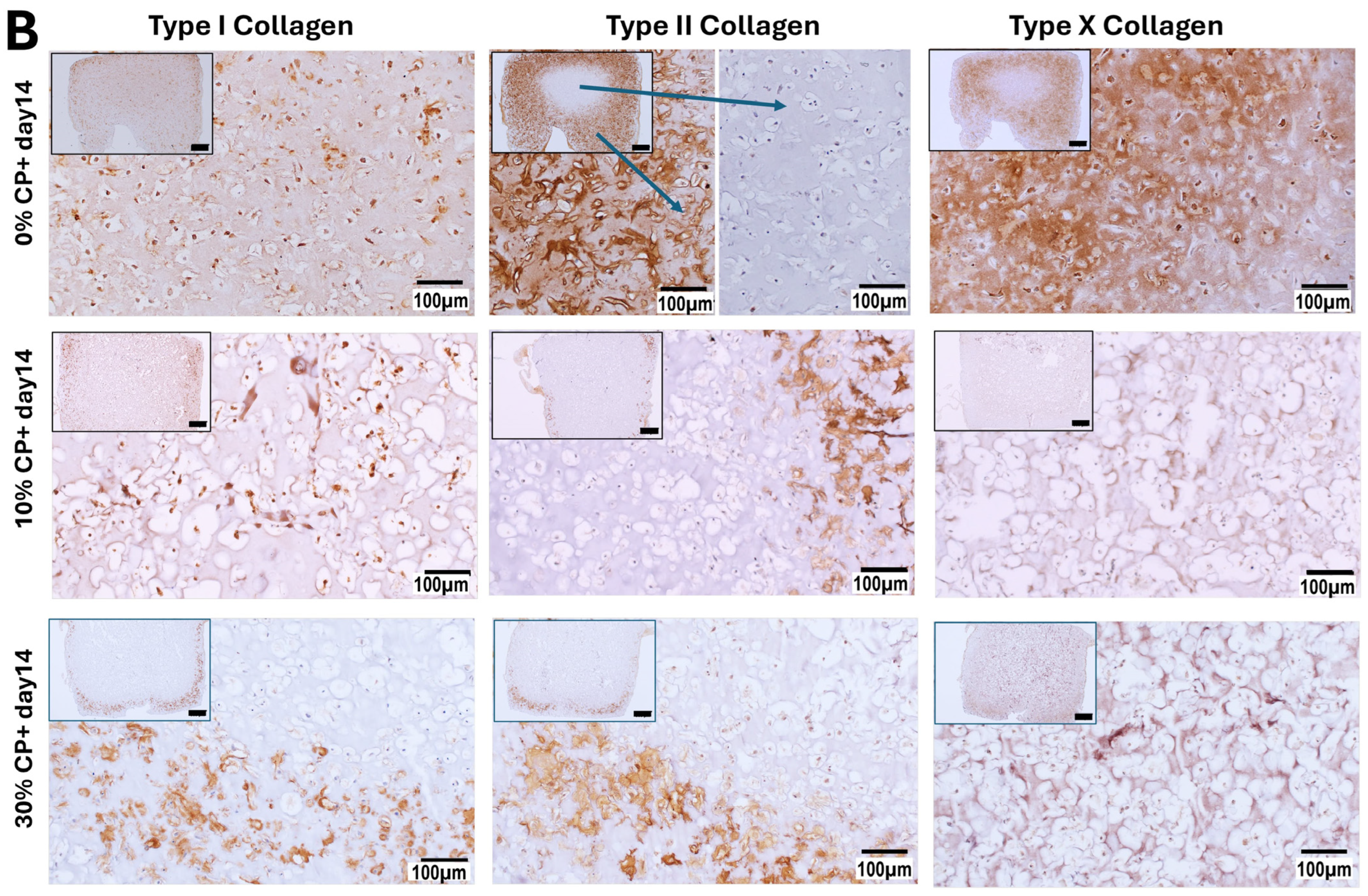
3.3.1. Immunohistochemistry Staining
3.3.2. Glycosaminoglycans Synthesis
4. Discussion
4.1. GelMa Enables Mechanical Deformation of Embedded Naïve MSCs
4.2. Uniaxial Cyclic Deformation Induces the Hypertrophic Differentiation of Naïve MSCs
4.3. Mechanical Strain and TGFβ1 Differentially Regulate Mesenchymal Stem Cell Fate
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Claes, L.E.; Heigele, C.A.; Neidlinger-Wilke, C.; Kaspar, D.; Seidl, W.; Margevicius, K.J.; Augat, P. Effects of mechanical factors on the fracture healing process. Clin. Orthop. Relat. Res. 1998, 355, S132–S147. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.L.; Goswami, T. A review of locking compression plate biomechanics and their advantages as internal fixators in fracture healing. Clin. Biomech. 2007, 22, 1049–1062. [Google Scholar] [CrossRef]
- Marsell, R.; Einhorn, T.A. The biology of fracture healing. Injury 2011, 42, 551–555. [Google Scholar] [CrossRef] [PubMed]
- Einhorn, T.A.; Gerstenfeld, L.C. Fracture healing: Mechanisms and interventions. Nat. Rev. Rheumatol. 2015, 11, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Perren, S.M.; Cordey, J. The concept of interfragmentary strain. In Current Concepts of Internal Fixation of Fractures; Uhthoff, H.K., Ed.; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 1980; pp. 63–77. [Google Scholar]
- Augat, P.; Hollensteiner, M.; von Rüden, C. The role of mechanical stimulation in the enhancement of bone healing. Injury 2021, 52 (Suppl. S2), S78–S83. [Google Scholar] [CrossRef] [PubMed]
- Goodship, A.E.; Cunningham, J.L.; Kenwright, J. Strain rate and timing of stimulation in mechanical modulation of fracture healing. Clin. Orthop. Relat. Res. 1998, 355, S105–S115. [Google Scholar] [CrossRef]
- Liu, C.; Carrera, R.; Flamini, V.; Kenny, L.; Cabahug-Zuckerman, P.; George, B.M.; Hunter, D.; Liu, B.; Singh, G.; Leucht, P.; et al. Effects of mechanical loading on cortical defect repair using a novel mechanobiological model of bone healing. Bone 2018, 108, 145–155. [Google Scholar] [CrossRef]
- Claes, L.; Blakytny, R.; Gockelmann, M.; Schoen, M.; Ignatius, A.; Willie, B. Early dynamization by reduced fixation stiffness does not improve fracture healing in a rat femoral osteotomy model. J. Orthop. Res. 2009, 27, 22–27. [Google Scholar] [CrossRef]
- Hente, R.; Perren, S.M. Mechanical Stimulation of Fracture Healing–Stimulation of Callus by Improved Recovery [Mechanická stimulace hojení zlomenin–stimulace svalku prodloužením fáze zotavení]. Acta Chir. Orthop. Traumatol. Cech 2018, 85, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Tufekci, P.; Tavakoli, A.; Dlaska, C.; Neumann, M.; Shanker, M.; Saifzadeh, S.; Steck, R.; Schuetz, M.; Epari, D. Early mechanical stimulation only permits timely bone healing in sheep. J. Orthop. Res. 2018, 36, 1790–1796. [Google Scholar] [CrossRef]
- Glatt, V.; Bartnikowski, N.; Quirk, N.; Schuetz, M.; Evans, C. Reverse Dynamization: Influence of Fixator Stiffness on the Mode and Efficiency of Large-Bone-Defect Healing at Different Doses of rhBMP-2. J. Bone Jt. Surg. 2016, 98, 677–687. [Google Scholar] [CrossRef] [PubMed]
- Glatt, V.; Evans, C.H.; Tetsworth, K. A Concert between Biology and Biomechanics: The Influence of the Mechanical Environment on Bone Healing. Front. Physiol. 2016, 7, 678. [Google Scholar] [CrossRef] [PubMed]
- Glatt, V.; Tepic, S.; Evans, C. Reverse Dynamization: A Novel Approach to Bone Healing. J. Am. Acad. Orthop. Surg. 2016, 24, e60–e61. [Google Scholar] [CrossRef] [PubMed]
- Haller, S.J.; Dudley, A.T. Extracellular mechanotransduction. J. Gen. Physiol. 2022, 154. [Google Scholar] [CrossRef]
- Burke, D.P.; Khayyeri, H.; Kelly, D.J. Substrate stiffness and oxygen availability as regulators of mesenchymal stem cell differentiation within a mechanically loaded bone chamber. Biomech. Model. Mechanobiol. 2015, 14, 93–105. [Google Scholar] [CrossRef] [PubMed]
- Steward, A.J.; Kelly, D.J. Mechanical regulation of mesenchymal stem cell differentiation. J. Anat. 2015, 227, 717–731. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Wan, B.; Wang, R.; Zhang, B.; Luo, P.; Wang, D.; Nie, J.J.; Chen, D.; Wu, X. Mechanical Stimulation on Mesenchymal Stem Cells and Surrounding Microenvironments in Bone Regeneration: Regulations and Applications. Front. Cell Dev. Biol. 2022, 10, 808303. [Google Scholar] [CrossRef]
- Bancroft, G.N.; Sikavitsas, V.I.; van den Dolder, J.; Sheffield, T.L.; Ambrose, C.G.; Jansen, J.A.; Mikos, A.G. Fluid flow increases mineralized matrix deposition in 3D perfusion culture of marrow stromal osteoblasts in a dose-dependent manner. Proc. Natl. Acad. Sci. USA 2002, 99, 12600–12605. [Google Scholar] [CrossRef] [PubMed]
- Braccini, A.; Wendt, D.; Jaquiery, C.; Jakob, M.; Heberer, M.; Kenins, L.; Wodnar-Filipowicz, A.; Quarto, R.; Martin, I. Three-dimensional perfusion culture of human bone marrow cells and generation of osteoinductive grafts. Stem Cells 2005, 23, 1066–1072. [Google Scholar] [CrossRef]
- Hoffmann, W.; Feliciano, S.; Martin, I.; de Wild, M.; Wendt, D. Novel Perfused Compression Bioreactor System as an in vitro Model to Investigate Fracture Healing. Front. Bioeng. Biotechnol. 2015, 3, 10. [Google Scholar] [CrossRef]
- Lee, D.A.; Martin, I. Bioreactor culture techniques for cartilage-tissue engineering. Methods Mol. Biol. 2004, 238, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Papadimitropoulos, A.; Piccinini, E.; Brachat, S.; Braccini, A.; Wendt, D.; Barbero, A.; Jacobi, C.; Martin, I. Expansion of human mesenchymal stromal cells from fresh bone marrow in a 3D scaffold-based system under direct perfusion. PLoS ONE 2014, 9, e102359. [Google Scholar] [CrossRef] [PubMed]
- Peroglio, M.; Gaspar, D.; Zeugolis, D.I.; Alini, M. Relevance of bioreactors and whole tissue cultures for the translation of new therapies to humans. J. Orthop. Res. 2018, 36, 10–21. [Google Scholar] [CrossRef]
- Fang, B.; Liu, Y.; Zheng, D.; Shan, S.; Wang, C.; Gao, Y.; Wang, J.; Xie, Y.; Zhang, Y.; Li, Q. The effects of mechanical stretch on the biological characteristics of human adipose-derived stem cells. J. Cell. Mol. Med. 2019, 23, 4244–4255. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Shan, S.; Wang, C.; Wang, J.; Li, J.; Hu, G.; Dai, K.; Li, Q.; Zhang, X. Mechanical stimulation promote the osteogenic differentiation of bone marrow stromal cells through epigenetic regulation of Sonic Hedgehog. Exp. Cell Res. 2017, 352, 346–356. [Google Scholar] [CrossRef]
- Yue, D.; Zhang, M.; Lu, J.; Zhou, J.; Bai, Y.; Pan, J. The rate of fluid shear stress is a potent regulator for the differentiation of mesenchymal stem cells. J. Cell. Physiol. 2019, 234, 16312–16319. [Google Scholar] [CrossRef]
- Ravichandran, A.; Lim, J.; Chong, M.S.K.; Wen, F.; Liu, Y.; Pillay, Y.T.; Chan, J.K.Y.; Teoh, S.H. In vitro cyclic compressive loads potentiate early osteogenic events in engineered bone tissue. J. Biomed. Mater. Res. B Appl. Biomater. 2017, 105, 2366–2375. [Google Scholar] [CrossRef] [PubMed]
- Baas, E.; Kuiper, J.H.; Yang, Y.; Wood, M.A.; El Haj, A.J. In vitro bone growth responds to local mechanical strain in three-dimensional polymer scaffolds. J. Biomech. 2010, 43, 733–739. [Google Scholar] [CrossRef]
- Schreivogel, S.; Kuchibhotla, V.; Knaus, P.; Duda, G.N.; Petersen, A. Load-induced osteogenic differentiation of mesenchymal stromal cells is caused by mechano-regulated autocrine signaling. J. Tissue Eng. Regen. Med. 2019, 13, 1992–2008. [Google Scholar] [CrossRef]
- Li, Z.; Kupcsik, L.; Yao, S.J.; Alini, M.; Stoddart, M.J. Mechanical load modulates chondrogenesis of human mesenchymal stem cells through the TGF-beta pathway. J. Cell. Mol. Med. 2010, 14, 1338–1346. [Google Scholar] [CrossRef]
- Zhao, X.; Lang, Q.; Yildirimer, L.; Lin, Z.Y.; Cui, W.; Annabi, N.; Ng, K.W.; Dokmeci, M.R.; Ghaemmaghami, A.M.; Khademhosseini, A. Photocrosslinkable Gelatin Hydrogel for Epidermal Tissue Engineering. Adv. Healthc. Mater. 2016, 5, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Farndale, R.W.; Buttle, D.J.; Barrett, A.J. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochim. Biophys. Acta 1986, 883, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Herlofsen, S.R.; Kuchler, A.M.; Melvik, J.E.; Brinchmann, J.E. Chondrogenic differentiation of human bone marrow-derived mesenchymal stem cells in self-gelling alginate discs reveals novel chondrogenic signature gene clusters. Tissue Eng. Part A 2011, 17, 1003–1013. [Google Scholar] [CrossRef]
- Li, X.; Zhang, J.; Kawazoe, N.; Chen, G. Fabrication of Highly Crosslinked Gelatin Hydrogel and Its Influence on Chondrocyte Proliferation and Phenotype. Polymers 2017, 9, 309. [Google Scholar] [CrossRef]
- Martyniak, K.; Lokshina, A.; Cruz, M.A.; Karimzadeh, M.; Kemp, R.; Kean, T.J. Biomaterial composition and stiffness as decisive properties of 3D bioprinted constructs for type II collagen stimulation. Acta Biomater. 2022, 152, 221–234. [Google Scholar] [CrossRef]
- Karim, A.; Hall, A.C. Chondrocyte Morphology in Stiff and Soft Agarose Gels and the Influence of Fetal Calf Serum. J. Cell. Physiol. 2017, 232, 1041–1052. [Google Scholar] [CrossRef] [PubMed]
- Mauck, R.L.; Yuan, X.; Tuan, R.S. Chondrogenic differentiation and functional maturation of bovine mesenchymal stem cells in long-term agarose culture. Osteoarthr. Cartil. 2006, 14, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Spyropoulou, A.; Karamesinis, K.; Basdra, E.K. Mechanotransduction pathways in bone pathobiology. Biochim. Biophys. Acta 2015, 1852, 1700–1708. [Google Scholar] [CrossRef]
- Lee, W.; Nims, R.J.; Savadipour, A.; Zhang, Q.; Leddy, H.A.; Liu, F.; McNulty, A.L.; Chen, Y.; Guilak, F.; Liedtke, W.B. Inflammatory signaling sensitizes Piezo1 mechanotransduction in articular chondrocytes as a pathogenic feed-forward mechanism in osteoarthritis. Proc. Natl. Acad. Sci. USA 2021, 118. [Google Scholar] [CrossRef]
- Lee, W.; Leddy, H.A.; Chen, Y.; Lee, S.H.; Zelenski, N.A.; McNulty, A.L.; Wu, J.; Beicker, K.N.; Coles, J.; Zauscher, S.; et al. Synergy between Piezo1 and Piezo2 channels confers high-strain mechanosensitivity to articular cartilage. Proc. Natl. Acad. Sci. USA 2014, 111, E5114–E5122. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Jeon, O.; Kong, M.; Abdeen, A.A.; Shin, J.Y.; Lee, H.N.; Lee, Y.B.; Sun, W.; Bandaru, P.; Alt, D.S.; et al. Combinatorial screening of biochemical and physical signals for phenotypic regulation of stem cell-based cartilage tissue engineering. Sci. Adv. 2020, 6, eaaz5913. [Google Scholar] [CrossRef]
- Chen, P.; Zhang, G.; Jiang, S.; Ning, Y.; Deng, B.; Pan, X.; Liu, S.; He, Y.; Zhang, L.; Wan, R.; et al. Mechanosensitive Piezo1 in endothelial cells promotes angiogenesis to support bone fracture repair. Cell Calcium 2021, 97, 102431. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Tian, H.; Hu, Y.; Cao, Y.; Song, H.; Lan, S.; Dai, Z.; Chen, W.; Zhang, Y.; Shao, Z.; et al. Mechanosensitive Piezo1 is crucial for periosteal stem cell-mediated fracture healing. Int. J. Biol. Sci. 2022, 18, 3961–3980. [Google Scholar] [CrossRef]
- Wang, L.; You, X.; Lotinun, S.; Zhang, L.; Wu, N.; Zou, W. Mechanical sensing protein PIEZO1 regulates bone homeostasis via osteoblast-osteoclast crosstalk. Nat. Commun. 2020, 11, 282. [Google Scholar] [CrossRef] [PubMed]
- Delco, M.L.; Bonassar, L.J. Targeting calcium-related mechanotransduction in early OA. Nat. Rev. Rheumatol. 2021, 17, 445–446. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Hasan, H.; Anderson, D.E.; Lee, W. The Role of Mechanically-Activated Ion Channels Piezo1, Piezo2, and TRPV4 in Chondrocyte Mechanotransduction and Mechano-Therapeutics for Osteoarthritis. Front. Cell Dev. Biol. 2022, 10, 885224. [Google Scholar] [CrossRef]
- Zhang, M.; Meng, N.; Wang, X.; Chen, W.; Zhang, Q. TRPV4 and PIEZO Channels Mediate the Mechanosensing of Chondrocytes to the Biomechanical Microenvironment. Membranes 2022, 12, 237. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Li, Y.; Wang, M.; Zhao, S.; Zhao, Z.; Fang, J. Mechanotransduction pathways in the regulation of cartilage chondrocyte homoeostasis. J. Cell. Mol. Med. 2020, 24, 5408–5419. [Google Scholar] [CrossRef] [PubMed]
- Khatib, N.S.; Monsen, J.; Ahmed, S.; Huang, Y.; Hoey, D.A.; Nowlan, N.C. Mechanoregulatory role of TRPV4 in prenatal skeletal development. Sci. Adv. 2023, 9, eade2155. [Google Scholar] [CrossRef]
- Masuyama, R.; Vriens, J.; Voets, T.; Karashima, Y.; Owsianik, G.; Vennekens, R.; Lieben, L.; Torrekens, S.; Moermans, K.; Vanden Bosch, A.; et al. TRPV4-mediated calcium influx regulates terminal differentiation of osteoclasts. Cell Metab. 2008, 8, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Shi, G.X.; Zheng, X.F.; Zhu, C.; Li, B.; Wang, Y.R.; Jiang, S.D.; Jiang, L.S. Evidence of the Role of R-Spondin 1 and Its Receptor Lgr4 in the Transmission of Mechanical Stimuli to Biological Signals for Bone Formation. Int. J. Mol. Sci. 2017, 18, 564. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Sharma, A.R.; Jagga, S.; Lee, S.S.; Nam, J.S. Differential Expression Patterns of Rspondin Family and Leucine-Rich Repeat-Containing G-Protein Coupled Receptors in Chondrocytes and Osteoblasts. Cell J. 2021, 22, 437–449. [Google Scholar] [CrossRef] [PubMed]
- Takegami, Y.; Ohkawara, B.; Ito, M.; Masuda, A.; Nakashima, H.; Ishiguro, N.; Ohno, K. R-spondin 2 facilitates differentiation of proliferating chondrocytes into hypertrophic chondrocytes by enhancing Wnt/beta-catenin signaling in endochondral ossification. Biochem. Biophys. Res. Commun. 2016, 473, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Okura, T.; Ohkawara, B.; Takegami, Y.; Ito, M.; Masuda, A.; Seki, T.; Ishiguro, N.; Ohno, K. Mianserin suppresses R-spondin 2-induced activation of Wnt/β-catenin signaling in chondrocytes and prevents cartilage degradation in a rat model of osteoarthritis. Sci. Rep. 2019, 9, 2808. [Google Scholar] [CrossRef] [PubMed]
- Bradley, E.W.; Drissi, M.H. WNT5A regulates chondrocyte differentiation through differential use of the CaN/NFAT and IKK/NF-kappaB pathways. Mol. Endocrinol. 2010, 24, 1581–1593. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Topol, L.; Lee, H.; Wu, J. Wnt5a and Wnt5b exhibit distinct activities in coordinating chondrocyte proliferation and differentiation. Development 2003, 130, 1003–1015. [Google Scholar] [CrossRef]
- Hallett, S.A.; Ono, W.; Ono, N. The hypertrophic chondrocyte: To be or not to be. Histol. Histopathol. 2021, 36, 1021–1036. [Google Scholar] [CrossRef]
- Larson, B.L.; Yu, S.N.; Park, H.; Estes, B.T.; Moutos, F.T.; Bloomquist, C.J.; Wu, P.B.; Welter, J.F.; Langer, R.; Guilak, F.; et al. Chondrogenic, hypertrophic, and osteochondral differentiation of human mesenchymal stem cells on three-dimensionally woven scaffolds. J. Tissue Eng. Regen. Med. 2019, 13, 1453–1465. [Google Scholar] [CrossRef]
- Gu, J.; Lu, Y.; Li, F.; Qiao, L.; Wang, Q.; Li, N.; Borgia, J.A.; Deng, Y.; Lei, G.; Zheng, Q. Identification and characterization of the novel Col10a1 regulatory mechanism during chondrocyte hypertrophic differentiation. Cell Death Dis. 2014, 5, e1469. [Google Scholar] [CrossRef] [PubMed]
- Giannoni, P.; Siegrist, M.; Hunziker, E.B.; Wong, M. The mechanosensitivity of cartilage oligomeric matrix protein (COMP). Biorheology 2003, 40, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Berven, S.; Simpson, H.; Triffitt, J.T. Expression of BMP-4 mRNA during distraction osteogenesis in rabbits. Acta Orthop. Scand. 1998, 69, 420–425. [Google Scholar] [CrossRef] [PubMed]
- Gerstenfeld, L.C.; Shapiro, F.D. Expression of bone-specific genes by hypertrophic chondrocytes: Implication of the complex functions of the hypertrophic chondrocyte during endochondral bone development. J. Cell. Biochem. 1996, 62, 1–9. [Google Scholar] [CrossRef]
- Chawla, S.; Mainardi, A.; Majumder, N.; Dönges, L.; Kumar, B.; Occhetta, P.; Martin, I.; Egloff, C.; Ghosh, S.; Bandyopadhyay, A.; et al. Chondrocyte Hypertrophy in Osteoarthritis: Mechanistic Studies and Models for the Identification of New Therapeutic Strategies. Cells 2022, 11, 4034. [Google Scholar] [CrossRef] [PubMed]
- Dreier, R. Hypertrophic differentiation of chondrocytes in osteoarthritis: The developmental aspect of degenerative joint disorders. Arthritis Res. Ther. 2010, 12, 216. [Google Scholar] [CrossRef] [PubMed]
- Lian, C.; Wang, X.; Qiu, X.; Wu, Z.; Gao, B.; Liu, L.; Liang, G.; Zhou, H.; Yang, X.; Peng, Y.; et al. Collagen type II suppresses articular chondrocyte hypertrophy and osteoarthritis progression by promoting integrin beta1-SMAD1 interaction. Bone Res. 2019, 7, 8. [Google Scholar] [CrossRef] [PubMed]
- Loebel, C.; Czekanska, E.M.; Bruderer, M.; Salzmann, G.; Alini, M.; Stoddart, M.J. In vitro osteogenic potential of human mesenchymal stem cells is predicted by Runx2/Sox9 ratio. Tissue Eng. Part A 2015, 21, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Johnstone, B.; Hering, T.M.; Caplan, A.I.; Goldberg, V.M.; Yoo, J.U. In vitro chondrogenesis of bone marrow-derived mesenchymal progenitor cells. Exp. Cell Res. 1998, 238, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.U.; Barthel, T.S.; Nishimura, K.; Solchaga, L.; Caplan, A.I.; Goldberg, V.M.; Johnstone, B. The chondrogenic potential of human bone-marrow-derived mesenchymal progenitor cells. J. Bone Jt. Surg. 1998, 80, 1745–1757. [Google Scholar] [CrossRef]
- Mueller, M.B.; Fischer, M.; Zellner, J.; Berner, A.; Dienstknecht, T.; Prantl, L.; Kujat, R.; Nerlich, M.; Tuan, R.S.; Angele, P. Hypertrophy in mesenchymal stem cell chondrogenesis: Effect of TGF-beta isoforms and chondrogenic conditioning. Cells Tissues Organs 2010, 192, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Mueller, M.B.; Tuan, R.S. Functional characterization of hypertrophy in chondrogenesis of human mesenchymal stem cells. Arthritis Rheum. 2008, 58, 1377–1388. [Google Scholar] [CrossRef] [PubMed]
- Scotti, C.; Tonnarelli, B.; Papadimitropoulos, A.; Scherberich, A.; Schaeren, S.; Schauerte, A.; Lopez-Rios, J.; Zeller, R.; Barbero, A.; Martin, I. Recapitulation of endochondral bone formation using human adult mesenchymal stem cells as a paradigm for developmental engineering. Proc. Natl. Acad. Sci. USA 2010, 107, 7251–7256. [Google Scholar] [CrossRef]
- Ballock, R.T.; Heydemann, A.; Wakefield, L.M.; Flanders, K.C.; Roberts, A.B.; Sporn, M.B. TGF-beta 1 prevents hypertrophy of epiphyseal chondrocytes: Regulation of gene expression for cartilage matrix proteins and metalloproteases. Dev. Biol. 1993, 158, 414–429. [Google Scholar] [CrossRef] [PubMed]
- Angele, P.; Yoo, J.U.; Smith, C.; Mansour, J.; Jepsen, K.J.; Nerlich, M.; Johnstone, B. Cyclic hydrostatic pressure enhances the chondrogenic phenotype of human mesenchymal progenitor cells differentiated in vitro. J. Orthop. Res. 2003, 21, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Mauck, R.L.; Soltz, M.A.; Wang, C.C.; Wong, D.D.; Chao, P.H.; Valhmu, W.B.; Hung, C.T.; Ateshian, G.A. Functional tissue engineering of articular cartilage through dynamic loading of chondrocyte-seeded agarose gels. J. Biomech. Eng. 2000, 122, 252–260. [Google Scholar] [CrossRef]
- Meyer, E.G.; Buckley, C.T.; Steward, A.J.; Kelly, D.J. The effect of cyclic hydrostatic pressure on the functional development of cartilaginous tissues engineered using bone marrow derived mesenchymal stem cells. J. Mech. Behav. Biomed. Mater. 2011, 4, 1257–1265. [Google Scholar] [CrossRef] [PubMed]
- Schatti, O.; Grad, S.; Goldhahn, J.; Salzmann, G.; Li, Z.; Alini, M.; Stoddart, M.J. A combination of shear and dynamic compression leads to mechanically induced chondrogenesis of human mesenchymal stem cells. Eur. Cell Mater. 2011, 22, 214–225. [Google Scholar] [CrossRef] [PubMed]
- Bian, L.; Zhai, D.Y.; Zhang, E.C.; Mauck, R.L.; Burdick, J.A. Dynamic compressive loading enhances cartilage matrix synthesis and distribution and suppresses hypertrophy in hMSC-laden hyaluronic acid hydrogels. Tissue Eng. Part A 2012, 18, 715–724. [Google Scholar] [CrossRef] [PubMed]
- Pfeifer, C.G.; Karl, A.; Kerschbaum, M.; Berner, A.; Lang, S.; Schupfner, R.; Koch, M.; Angele, P.; Nerlich, M.; Mueller, M.B. TGF-beta Signalling is Suppressed under Pro-Hypertrophic Conditions in MSC Chondrogenesis Due to TGF-beta Receptor Downregulation. Int. J. Stem Cells 2019, 12, 139–150. [Google Scholar] [CrossRef]
- Rutgers, M.; Bach, F.; Vonk, L.; van Rijen, M.; Akrum, V.; van Boxtel, A.; Dhert, W.; Creemers, L. PTH decreases in vitro human cartilage regeneration without affecting hypertrophic differentiation. PLoS ONE 2019, 14, e0213483. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Kupcsik, L.; Yao, S.J.; Alini, M.; Stoddart, M.J. Chondrogenesis of human bone marrow mesenchymal stem cells in fibrin-polyurethane composites. Tissue Eng. Part A 2009, 15, 1729–1737. [Google Scholar] [CrossRef]
- Bian, L.; Angione, S.L.; Ng, K.W.; Lima, E.G.; Williams, D.Y.; Mao, D.Q.; Ateshian, G.A.; Hung, C.T. Influence of decreasing nutrient path length on the development of engineered cartilage. Osteoarthr. Cartil. 2009, 17, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Foyt, D.A.; Taheem, D.K.; Ferreira, S.A.; Norman, M.D.A.; Petzold, J.; Jell, G.; Grigoriadis, A.E.; Gentleman, E. Hypoxia impacts human MSC response to substrate stiffness during chondrogenic differentiation. Acta Biomater. 2019, 89, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Studer, D.; Millan, C.; Ozturk, E.; Maniura-Weber, K.; Zenobi-Wong, M. Molecular and biophysical mechanisms regulating hypertrophic differentiation in chondrocytes and mesenchymal stem cells. Eur. Cells Mater. 2012, 24, 118–135; discussion 135. [Google Scholar] [CrossRef]
- Zhong, L.; Huang, X.; Karperien, M.; Post, J.N. The Regulatory Role of Signaling Crosstalk in Hypertrophy of MSCs and Human Articular Chondrocytes. Int. J. Mol. Sci. 2015, 16, 19225–19247. [Google Scholar] [CrossRef] [PubMed]
- Hall, A.C. The Role of Chondrocyte Morphology and Volume in Controlling Phenotype-Implications for Osteoarthritis, Cartilage Repair, and Cartilage Engineering. Curr. Rheumatol. Rep. 2019, 21, 38. [Google Scholar] [CrossRef] [PubMed]
- Lewis, R.; Feetham, C.H.; Barrett-Jolley, R. Cell volume regulation in chondrocytes. Cell. Physiol. Biochem. 2011, 28, 1111–1122. [Google Scholar] [CrossRef] [PubMed]
- Cooper, K.L.; Oh, S.; Sung, Y.; Dasari, R.R.; Kirschner, M.W.; Tabin, C.J. Multiple phases of chondrocyte enlargement underlie differences in skeletal proportions. Nature 2013, 495, 375–378. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jörimann, T.; Füllemann, P.; Jose, A.; Matthys, R.; Wehrle, E.; Stoddart, M.J.; Verrier, S. In Vitro Induction of Hypertrophic Chondrocyte Differentiation of Naïve MSCs by Strain. Cells 2025, 14, 25. https://doi.org/10.3390/cells14010025
Jörimann T, Füllemann P, Jose A, Matthys R, Wehrle E, Stoddart MJ, Verrier S. In Vitro Induction of Hypertrophic Chondrocyte Differentiation of Naïve MSCs by Strain. Cells. 2025; 14(1):25. https://doi.org/10.3390/cells14010025
Chicago/Turabian StyleJörimann, Thomas, Priscilla Füllemann, Anita Jose, Romano Matthys, Esther Wehrle, Martin J. Stoddart, and Sophie Verrier. 2025. "In Vitro Induction of Hypertrophic Chondrocyte Differentiation of Naïve MSCs by Strain" Cells 14, no. 1: 25. https://doi.org/10.3390/cells14010025
APA StyleJörimann, T., Füllemann, P., Jose, A., Matthys, R., Wehrle, E., Stoddart, M. J., & Verrier, S. (2025). In Vitro Induction of Hypertrophic Chondrocyte Differentiation of Naïve MSCs by Strain. Cells, 14(1), 25. https://doi.org/10.3390/cells14010025







