Circulating CD16-Positive Monocyte-like Myeloid-Derived Suppressor Cells and Intermediate Monocytes Associated with Clinical and Immunological Complications in Pars Planitis Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Cytometric Analysis
2.3. Statistical Analysis
3. Results
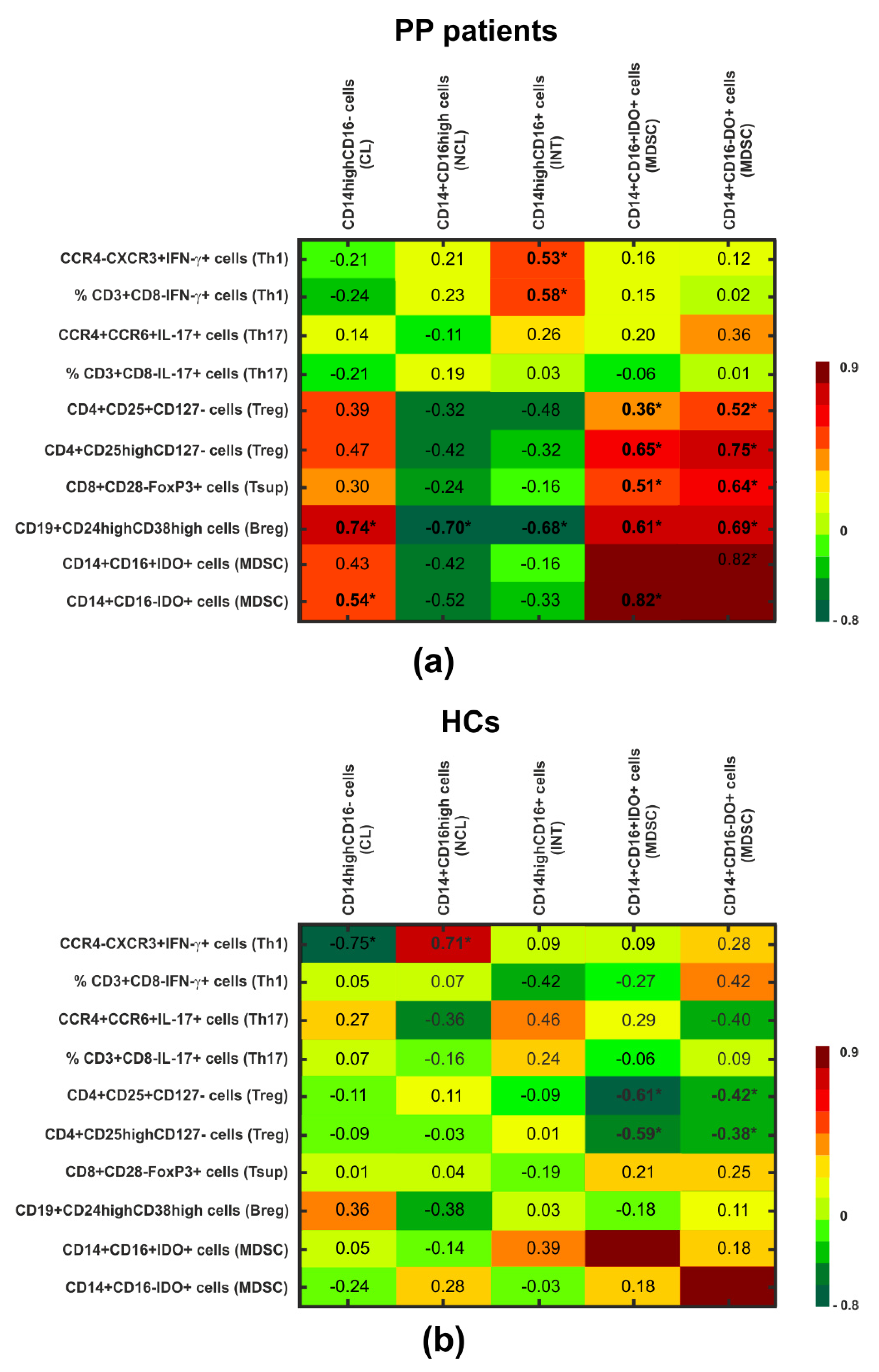
3.1. Adaptive Immune Cells (Pro- and Anti-Inflammatory Lymphocytes)
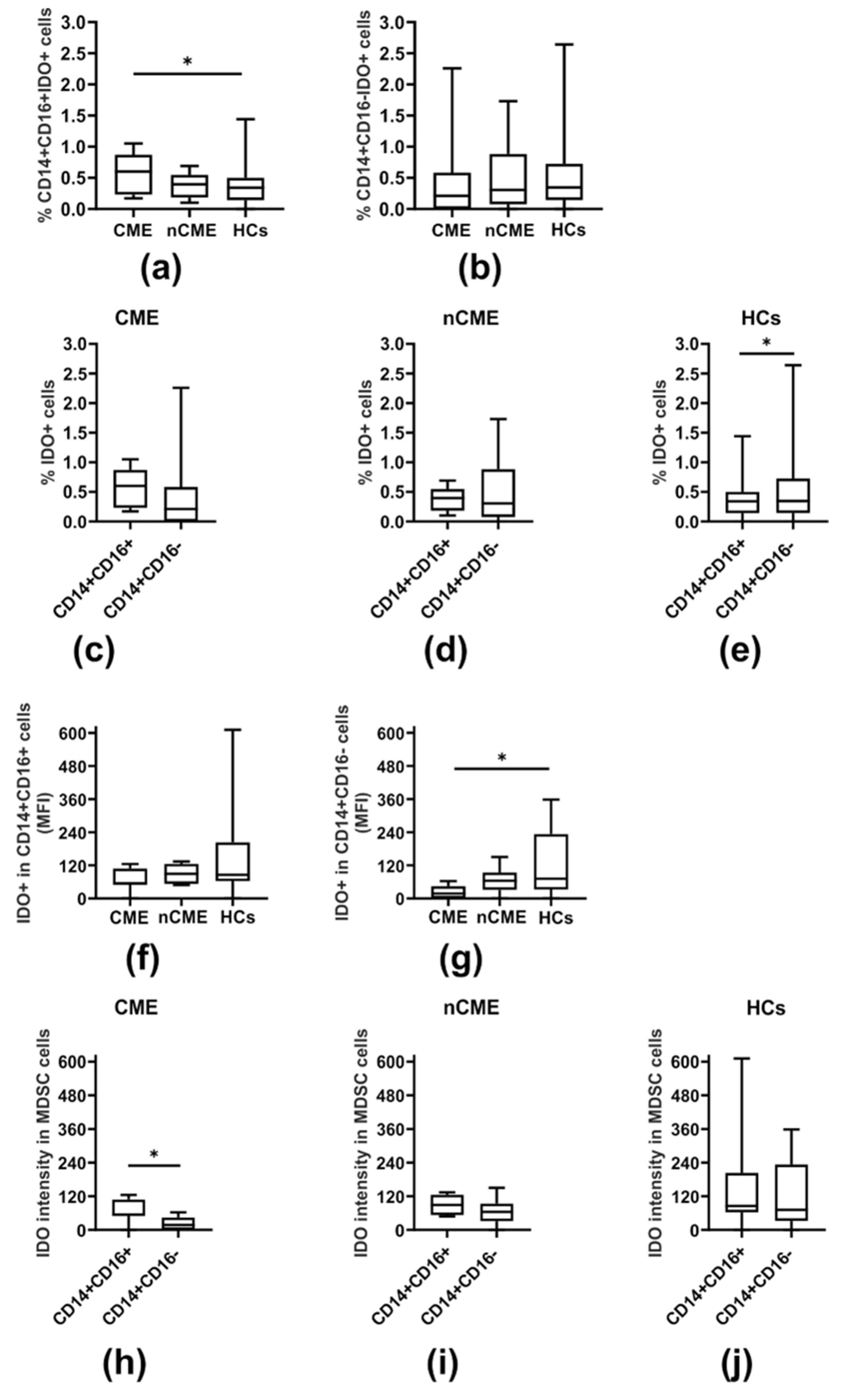
3.2. IDO-Expressing MDSCs
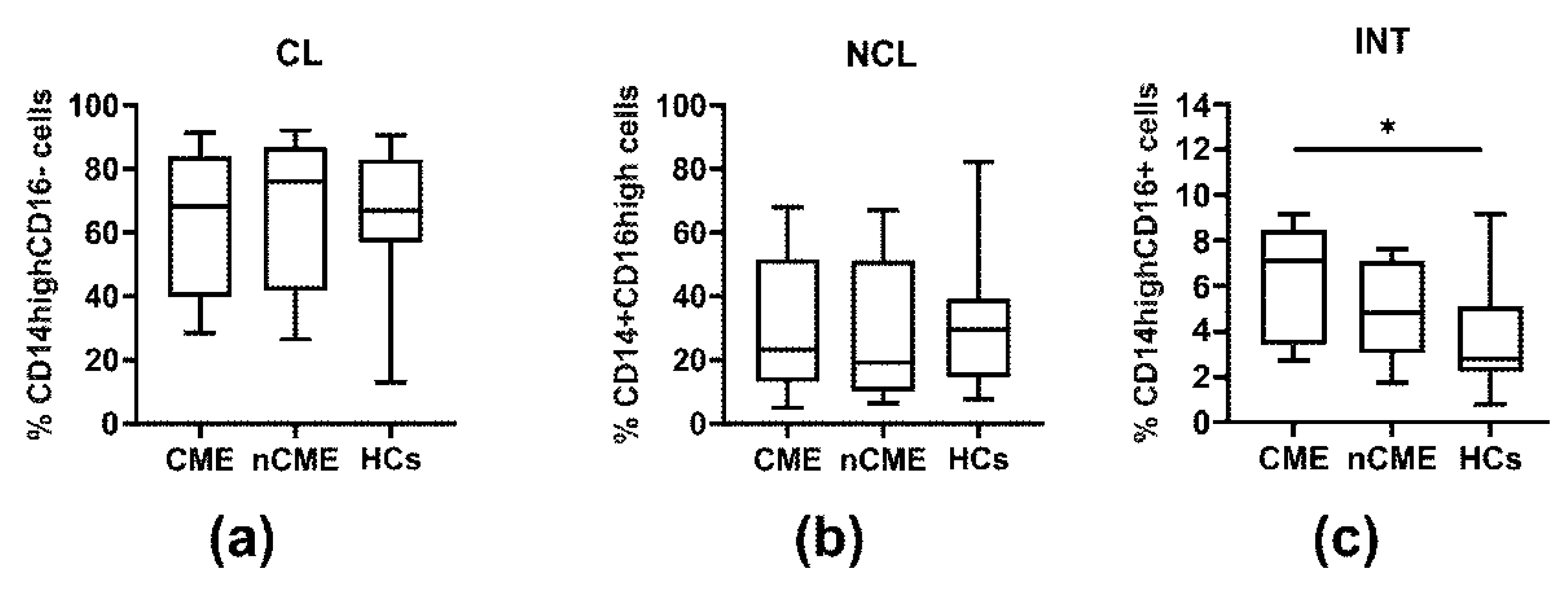
3.3. Monocyte Subpopulations
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PP | pars planitis |
| PB | peripheral blood |
| MDSC | myeloid-derived suppressor cell |
| CME | cystoid macular edema |
| nCME | no cystoid macular edema |
| HC | healthy control |
| Th | T helper |
| IDO | indoleamine 2,3-dioxygenase |
| Treg | T regulatory |
| EAU | experimental autoimmune uveitis |
| TNF | tumor necrosis factor |
| IFN | interferon |
| IL | interleukin |
| BRB | blood–retina barrier |
| Tsup | T suppressor |
| Breg | B regulatory |
| LPS | lipopolysaccharide |
| PBMC | peripheral blood mononuclear cell |
| mAbs | monoclonal antibodies |
| PMA | phorbol 12-myristate 23-acetate |
| Ion | ionomycin |
| BFA | brefeldin A |
| CCR | C-C chemokine receptor |
| CXCR | C-X-C chemokine receptor |
| PerCP | peridinin-chlorophyll-protein |
| PE | phycoerythrin |
| FITC | fluorescein |
| FOXP3 | Forkhead Box P3 |
| BD | Becton Dickinson |
| M-MDSC | monocytic-like myeloid-derived suppressor cell |
| IQ | interquartile |
| TGF | tumor growth factor |
| 1-MT | 1-methyltryptophan |
| DC | dendritic cell |
References
- Jabs, D.A.; Nussenblatt, R.B.; Rosenbaum, J.T. Standardization of uveitis nomenclature for reporting clinical data. Results of the First International Workshop. Am. J. Ophthalmol. 2005, 140, 509–516. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, Z.; Chong, W.P.; Wu, S.; Wang, W.; Zhou, H.; Chen, J. Comparative Analysis of the Interphotoreceptor Retinoid Binding Protein Induced Models of Experimental Autoimmune Uveitis in B10.RIII versus C57BL/6 Mice. Curr. Mol. Med. 2018, 18, 602–611. [Google Scholar] [CrossRef] [PubMed]
- Dagkalis, A.; Wallace, C.; Hing, B.; Liversidge, J.; Crane, I.J. CX3CR1-deficiency is associated with increased severity of disease in experimental autoimmune uveitis. Immunology 2009, 128, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Forrester, J.V.; Kuffova, L.; Dick, A.D. Autoimmunity, Autoinflammation, and Infection in Uveitis. Am. J. Ophthalmol. 2018, 189, 77–85. [Google Scholar] [CrossRef]
- Luger, D.; Silver, P.B.; Tang, J.; Cua, D.; Chen, Z.; Iwakura, Y.; Bowman, E.P.; Sgambellone, N.M.; Chan, C.-C.; Caspi, R.R. Either a Th17 or a Th1 effector response can drive autoimmunity: Conditions of disease induction affect dominant effector category. J. Exp. Med. 2008, 205, 799–810. [Google Scholar] [CrossRef]
- Merida, S.; Palacios, E.; Navea, A.; Bosch-Morell, F. Macrophages and Uveitis in Experimental Animal Models. Mediat. Inflamm. 2015, 2015, 671417. [Google Scholar] [CrossRef]
- Robertson, M.J.; Erwig, L.P.; Liversidge, J.; Forrester, J.V.; Rees, A.J.; Dick, A.D. Retinal microenvironment controls resident and infiltrating macrophage function during uveoretinitis. Investig. Ophthalmol. Vis. Sci. 2002, 43, 2250–2257. [Google Scholar] [PubMed]
- Saraswathy, S.; Nguyen, A.M.; Rao, N.A. The role of TLR4 in photoreceptor alphaa crystallin upregulation during early experimental autoimmune uveitis. Investig. Ophthalmol. Vis. Sci. 2010, 51, 3680–3686. [Google Scholar] [CrossRef]
- Auffray, C.; Sieweke, M.H.; Geissmann, F. Blood monocytes: Development, heterogeneity, and relationship with dendritic cells. Annu. Rev. Immunol. 2009, 27, 669–692. [Google Scholar] [CrossRef]
- Weaver, C.T.; Harrington, L.E.; Mangan, P.R.; Gavrieli, M.; Murphy, K.M. Th17: An effector CD4 T cell lineage with regulatory T cell ties. Immunity 2006, 24, 677–688. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Forrester, J.V.; Liversidge, J.; Crane, I.J. Leukocyte Trafficking in Experimental Autoimmune Uveitis: Breakdown of Blood–Retinal Barrier and Upregulation of Cellular Adhesion Molecules. Investig. Opthalmol. Vis. Sci. 2003, 44, 226–234. [Google Scholar] [CrossRef]
- Kosmaczewska, A.; Przeździecka-Dołyk, J.; Turno-Kręcicka, A.; Ciszak, L.; Szteblich, A.; Węgrzyn, A.; Frydecka, I.; Misiuk-Hojło, M. Imbalance in PB IL-17-Secreting and Regulatory Cells in Pars Planitis Is Associated with Dysregulation of IFN-gamma-Secreting Cells, Especially in Patients with Clinical Complications. Mediat. Inflamm. 2020, 2020, 9175083–9175092. [Google Scholar] [CrossRef]
- Przeździecka-Dołyk, J.; Wegrzyn, A.; Turno-Krecicka, A.; Misiuk-Hojło, M. Immunopathogenic Background of Pars Planitis. Arch. Immunol. Ther. Exp. 2016, 64, 127–137. [Google Scholar] [CrossRef]
- Lescoat, A.; Lecureur, V.; Roussel, M.; Sunnaram, B.L.; Ballerie, A.; Coiffier, G.; Jouneau, S.; Fardel, O.; Fest, T.; Jẻgo, P. CD16-positive circulating monocytes and fibrotic manifestations of systemic sclerosis. Clin. Rheumatol. 2017, 36, 1649–1654. [Google Scholar] [CrossRef] [PubMed]
- Wijbrandts, C.A.; Dijkgraaf, M.G.; Kraan, M.C.; Vinkenoog, M.; Smeets, T.J.; Dinant, H.; Vos, K.; Lems, W.F.; Wolbink, G.J.; Sijpkens, D.; et al. The clinical response to infliximab in rheumatoid arthritis is in part dependent on pretreatment tumour necrosis factor alpha expression in the synovium. Ann. Rheum. Dis. 2008, 67, 1139–1144. [Google Scholar] [CrossRef]
- Wildgruber, M.; Aschenbrenner, T.; Wendorff, H.; Czubba, M.; Glinzer, A.; Haller, B.; Schiemann, M.; Zimmermann, A.; Berger, H.; Eckstein, H.-H.; et al. The “Intermediate” CD14(++)CD16(+) monocyte subset increases in severe peripheral artery disease in humans. Sci. Rep. 2016, 6, 39483–39491. [Google Scholar] [CrossRef]
- Passlick, B.; Flieger, D.; Ziegler-Heitbrock, H.W. Identification and characterization of a novel monocyte subpopulation in human peripheral blood. Blood 1989, 74, 2527–2534. [Google Scholar] [CrossRef] [PubMed]
- Ingersoll, M.A.; Spanbroek, R.; Lottaz, C.; Gautier, E.L.; Frankenberger, M.; Hoffmann, R.; Lang, R.; Haniffa, M.; Collin, M.; Tacke, F.; et al. Comparison of gene expression profiles between human and mouse monocyte subsets. Blood 2010, 115, e10–e19. [Google Scholar] [CrossRef]
- Schlitt, A.; Heine, G.H.; Blankenberg, S.; Espinola-Klein, C.; Dopheide, J.F.; Bickel, C.; Lackner, K.J.; Iz, M.; Meyer, J.; Darius, H.; et al. CD14+CD16+ monocytes in coronary artery disease and their relationship to serum TNF-alpha levels. Thromb. Haemost. 2004, 92, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Crook, K.R.; Liu, P. Role of myeloid-derived suppressor cells in autoimmune disease. World J. Immunol. 2014, 4, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Cheng, D.; Jia, Q.; Xiong, H.; Zhang, J. Mechanisms Underlying the Role of Myeloid-Derived Suppressor Cells in Clinical Diseases: Good or Bad. Immune Netw. 2021, 21, e21–e43. [Google Scholar] [CrossRef]
- Jeong, H.J.; Lee, H.J.; Ko, J.H.; Cho, B.J.; Park, S.Y.; Park, J.W.; Choi, S.R.; Heo, J.W.; Yoon, S.-O.; Oh, J.Y. Myeloid-Derived Suppressor Cells Mediate Inflammation Resolution in Humans and Mice with Autoimmune Uveoretinitis. J. Immunol. 2018, 200, 1306–1315. [Google Scholar] [CrossRef]
- Luker, A.J.; Graham, L.J.; Smith, T.M., Jr.; Camarena, C.; Zellner, M.P.; Gilmer, J.S.; Damle, S.R.; Conrad, D.H.; Bear, H.D.; Martin, R.K. The DNA methyltransferase inhibitor, guadecitabine, targets tumor-induced myelopoiesis and recovers T cell activity to slow tumor growth in combination with adoptive immunotherapy in a mouse model of breast cancer. BMC Immunol. 2020, 21, 8. [Google Scholar] [CrossRef] [PubMed]
- Friberg, M.; Jennings, R.; Alsarraj, M.; Dessureault, S.; Cantor, A.; Extermann, M.; Mellor, A.L.; Munn, D.H.; Antonia, S.J. Indoleamine 2,3-dioxygenase contributes to tumor cell evasion of T cell-mediated rejection. Int. J. Cancer 2002, 101, 151–155. [Google Scholar] [CrossRef]
- Lee, G.K.; Park, H.J.; Macleod, M.; Chandler, P.; Munn, D.H.; Mellor, A.L. Tryptophan deprivation sensitizes activated T cells to apoptosis prior to cell division. Immunology 2002, 107, 452–460. [Google Scholar] [CrossRef]
- Mellor, A.L.; Munn, D.H. IDO expression by dendritic cells: Tolerance and tryptophan catabolism. Nat. Rev. Immunol. 2004, 4, 762–774. [Google Scholar] [CrossRef]
- Zoso, A.; Mazza, E.M.; Bicciato, S.; Mandruzzato, S.; Bronte, V.; Serafini, P.; Inverardi, L. Human fibrocytic myeloid-derived suppressor cells express IDO and promote tolerance via Treg-cell expansion. Eur. J. Immunol. 2014, 44, 3307–3319. [Google Scholar] [CrossRef]
- Fallarino, F.; Grohmann, U.; You, S.; McGrath, B.C.; Cavener, D.R.; Vacca, C.; Orabona, C.; Bianchi, R.; Belladonna, M.L.; Volpi, C.; et al. Tryptophan catabolism generates autoimmune-preventive regulatory T cells. Transpl. Immunol. 2006, 17, 58–60. [Google Scholar] [CrossRef] [PubMed]
- Munn, D.H.; Mellor, A.L. Indoleamine 2,3-dioxygenase and tumor-induced tolerance. J. Clin. Investig. 2007, 117, 1147–1154. [Google Scholar] [CrossRef] [PubMed]
- Curnow, S.J.; Falciani, F.; Durrani, O.M.; Cheung, C.M.; Ross, E.J.; Wloka, K.; Rauz, S.; Wallace, G.R.; Salmon, M.; Murray, P.I. Multiplex bead immunoassay analysis of aqueous humor reveals distinct cytokine profiles in uveitis. Investig. Ophthalmol. Vis. Sci. 2005, 46, 4251–4259. [Google Scholar] [CrossRef]
- Hennessy, B.; North, J.; Deru, A.; Llewellyn-Smith, N.; Lowdell, M.W. Use of Leu3a/3b for the accurate determination of CD4 subsets for measurement of intracellular cytokines. Cytometry 2001, 44, 148–152. [Google Scholar] [CrossRef]
- Kwak, Y.; Kim, H.E.; Park, S.G. Insights into Myeloid-Derived Suppressor Cells in Inflammatory Diseases. Arch. Immunol. Ther. Exp. 2015, 63, 269–285. [Google Scholar] [CrossRef]
- Nakamura, T.; Ushigome, H. Myeloid-Derived Suppressor Cells as a Regulator of Immunity in Organ Transplantation. Int. J. Mol. Sci. 2018, 19, 2357. [Google Scholar] [CrossRef] [PubMed]
- Melero-Jerez, C.; Ortega, M.C.; Moline-Velazquez, V.; Clemente, D. Myeloid derived suppressor cells in inflammatory conditions of the central nervous system. Biochim. Biophys. Acta 2016, 1862, 368–380. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Ji, F.; Wang, Y.; Zhang, Y.; Liu, Q.; Zhang, J.Z.; Matsushima, K.; Cao, Q.; Zhang, Y. Synovial autoreactive T cells in rheumatoid arthritis resist IDO-mediated inhibition. J. Immunol. 2006, 177, 8226–8233. [Google Scholar] [CrossRef]
- Evans, H.G.; Gullick, N.J.; Kelly, S.; Pitzalis, C.; Lord, G.M.; Kirkham, B.W.; Taams, L.S. In vivo activated monocytes from the site of inflammation in humans specifically promote Th17 responses. Proc. Natl. Acad. Sci. USA 2009, 106, 6232–6237. [Google Scholar] [CrossRef]
- Walter, G.J.; Evans, H.G.; Menon, B.; Gullick, N.J.; Kirkham, B.W.; Cope, A.P.; Geissmann, F.; Taams, L.S. Interaction with activated monocytes enhances cytokine expression and suppressive activity of human CD4+CD45ro+CD25+CD127(low) regulatory T cells. Arthritis Rheum. 2013, 65, 627–638. [Google Scholar] [CrossRef]
- Hepburn, A.L.; Mason, J.C.; Davies, K.A. Expression of Fcgamma and complement receptors on peripheral blood monocytes in systemic lupus erythematosus and rheumatoid arthritis. Rheumatology 2004, 43, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Katayama, K.; Matsubara, T.; Fujiwara, M.; Koga, M.; Furukawa, S. CD14+CD16+ monocyte subpopulation in Kawasaki disease. Clin. Exp. Immunol. 2000, 121, 566–570. [Google Scholar] [CrossRef]
- Liu, B.; Dhanda, A.; Hirani, S.; Williams, E.L.; Sen, H.N.; Martinez, E.F.; Ling, D.; Thompson, I.; Casady, M.; Li, Z.; et al. CD14++CD16+ Monocytes Are Enriched by Glucocorticoid Treatment and Are Functionally Attenuated in Driving Effector T Cell Responses. J. Immunol. 2015, 194, 5150–5160. [Google Scholar] [CrossRef]
- Okamoto, H.; Mizuno, K.; Horio, T. Circulating CD14+ CD16+ monocytes are expanded in sarcoidosis patients. J. Dermatol. 2003, 30, 503–509. [Google Scholar] [CrossRef]
- Rossol, M.; Kraus, S.; Pierer, M.; Baerwald, C.; Wagner, U. The CD14(bright) CD16+ monocyte subset is expanded in rheumatoid arthritis and promotes expansion of the Th17 cell population. Arthritis Rheum. 2012, 64, 671–677. [Google Scholar] [CrossRef]
- Schneider, L.; Marcondes, N.A.; Hax, V.; da Silva Moreira, I.F.; Ueda, C.Y.; Piovesan, R.R.; Xavier, R.; Chakr, R. Flow cytometry evaluation of CD14/CD16 monocyte subpopulations in systemic sclerosis patients: A cross sectional controlled study. Adv. Rheumatol. 2021, 61, 27. [Google Scholar] [CrossRef]
- Liyanage, S.E.; Gardner, P.J.; Ribeiro, J.; Cristante, E.; Sampson, R.D.; Luhmann, U.F.; Ali, R.R.; Bainbridge, J.W. Flow cytometric analysis of inflammatory and resident myeloid populations in mouse ocular inflammatory models. Exp. Eye Res. 2016, 151, 160–170. [Google Scholar] [CrossRef] [PubMed]
- Walscheid, K.; Weinhage, T.; Foell, D.; Heinz, C.; Kasper, M.; Heiligenhaus, A. Phenotypic changes of peripheral blood mononuclear cells upon corticosteroid treatment in idiopathic intermediate uveitis. Clin. Immunol. 2016, 173, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Amiano, N.O.; Pellegrini, J.M.; Morelli, M.P.; Martinena, C.; Rolandelli, A.; Castello, F.A.; Casco, N.; Ciallella, L.M.; de Casado, G.C.; Armitano, R.; et al. Circulating Monocyte-Like Myeloid Derived Suppressor Cells and CD16 Positive Monocytes Correlate With Immunological Responsiveness of Tuberculosis Patients. Front. Cell Infect. Microbiol. 2022, 12, 841741–841752. [Google Scholar] [CrossRef]
- Koch, S.; Kucharzik, T.; Heidemann, J.; Nusrat, A.; Luegering, A. Investigating the role of proinflammatory CD16+ monocytes in the pathogenesis of inflammatory bowel disease. Clin. Exp. Immunol. 2010, 161, 332–341. [Google Scholar] [CrossRef]
- Poitou, C.; Dalmas, E.; Renovato, M.; Benhamo, V.; Hajduch, F.; Abdennour, M.; Kahn, J.F.; Veyrie, N.; Rizkalla, S.; Fridman, W.H.; et al. CD14dimCD16+ and CD14+CD16+ monocytes in obesity and during weight loss: Relationships with fat mass and subclinical atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 2322–2330. [Google Scholar] [CrossRef] [PubMed]
- Tsukamoto, M.; Seta, N.; Yoshimoto, K.; Suzuki, K.; Yamaoka, K.; Takeuchi, T. CD14(bright)CD16+ intermediate monocytes are induced by interleukin-10 and positively correlate with disease activity in rheumatoid arthritis. Arthritis Res. Ther. 2017, 19, 28. [Google Scholar] [CrossRef]
- Zimmermann, H.W.; Seidler, S.; Nattermann, J.; Gassler, N.; Hellerbrand, C.; Zernecke, A.; Tischendorf, J.J.; Luedde, T.; Weiskirchen, R.; Trautwein, C.; et al. Functional contribution of elevated circulating and hepatic non-classical CD14CD16 monocytes to inflammation and human liver fibrosis. PLoS ONE 2010, 5, e11049–e11064. [Google Scholar] [CrossRef]
- Lee, S.J.; Yoon, B.R.; Kim, H.Y.; Yoo, S.J.; Kang, S.W.; Lee, W.W. Activated Platelets Convert CD14(+)CD16(-) Into CD14(+)CD16(+) Monocytes With Enhanced FcgammaR-Mediated Phagocytosis and Skewed M2 Polarization. Front. Immunol. 2021, 11, 611133–611147. [Google Scholar] [CrossRef]
- Phillips, J.H.; Chang, C.W.; Lanier, L.L. Platelet-induced expression of Fc gamma RIII (CD16) on human monocytes. Eur. J. Immunol. 1991, 21, 895–899. [Google Scholar] [CrossRef] [PubMed]
- Yoon, B.R.; Yoo, S.J.; Choi, Y.; Chung, Y.H.; Kim, J.; Yoo, I.S.; Kang, S.W.; Lee, W.W. Functional phenotype of synovial monocytes modulating inflammatory T-cell responses in rheumatoid arthritis (RA). PLoS ONE 2014, 9, e109775–e109788. [Google Scholar] [CrossRef] [PubMed]
- Hijdra, D.; Vorselaars, A.D.; Grutters, J.C.; Claessen, A.M.; Rijkers, G.T. Phenotypic characterization of human intermediate monocytes. Front. Immunol. 2013, 4, 339–342. [Google Scholar] [CrossRef]
- Valentincic, N.V.; de Groot-Mijnes, J.D.; Kraut, A.; Korosec, P.; Hawlina, M.; Rothova, A. Intraocular and serum cytokine profiles in patients with intermediate uveitis. Mol. Vis. 2011, 17, 2003–2010. [Google Scholar] [PubMed]
- Passos, S.; Carvalho, L.P.; Costa, R.S.; Campos, T.M.; Novais, F.O.; Magalhaes, A.; Machado, P.R.; Beiting, D.; Mosser, D.; Carvalho, E.M.; et al. Intermediate monocytes contribute to pathologic immune response in Leishmania braziliensis infections. J. Infect. Dis. 2015, 211, 274–282. [Google Scholar] [CrossRef]
- Shantsila, E.; Tapp, L.D.; Wrigley, B.J.; Pamukcu, B.; Apostolakis, S.; Montoro-Garcia, S.; Lip, G.Y. Monocyte subsets in coronary artery disease and their associations with markers of inflammation and fibrinolysis. Atherosclerosis 2014, 234, 4–10. [Google Scholar] [CrossRef]
- Cordero-Coma, M.; Sobrin, L. Anti-tumor necrosis factor-alpha therapy in uveitis. Surv. Ophthalmol. 2015, 60, 575–589. [Google Scholar] [CrossRef]
- Takeuchi, M. A systematic review of biologics for the treatment of noninfectious uveitis. Immunotherapy 2013, 5, 91–102. [Google Scholar] [CrossRef]
- Gonzalez, A.; Varo, N.; Alegre, E.; Diaz, A.; Melero, I. Immunosuppression routed via the kynurenine pathway: A biochemical and pathophysiologic approach. Adv. Clin. Chem. 2008, 45, 155–197. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.Y.; Wu, C.L.; Lai, M.D.; Lin, C.C.; Yo, Y.T.; Jou, I.M.; Lee, C.H.; Weng, C.T.; Shiau, A.L.; Wang, C.R. Amelioration of rat collagen-induced arthritis through CD4+ T cells apoptosis and synovial interleukin-17 reduction by indoleamine 2,3-dioxygenase gene therapy. Hum. Gene Ther. 2011, 22, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Criado, G.; Simelyte, E.; Inglis, J.J.; Essex, D.; Williams, R.O. Indoleamine 2,3 dioxygenase-mediated tryptophan catabolism regulates accumulation of Th1/Th17 cells in the joint in collagen-induced arthritis. Arthritis Rheum. 2009, 60, 1342–1351. [Google Scholar] [CrossRef]
- Kenney, L.L.; Chiu, R.S.; Dutra, M.N.; Wactor, A.; Honan, C.; Shelerud, L.; Corrigan, J.J.; Yu, K.; Ferrari, J.D.; Jeffrey, K.L.; et al. mRNA-delivery of IDO1 suppresses T cell-mediated autoimmunity. Cell Rep. Med. 2024, 5, 101717–101727. [Google Scholar] [CrossRef]
- Lemos, H.; Mohamed, E.; Ou, R.; McCardle, C.; Zheng, X.; McGuire, K.; Homer, N.Z.M.; Mole, D.J.; Huang, L.; Mellor, A.L. Co-treatments to Boost IDO Activity and Inhibit Production of Downstream Catabolites Induce Durable Suppression of Experimental Autoimmune Encephalomyelitis. Front. Immunol. 2020, 17, 1256–1266. [Google Scholar] [CrossRef]
- Wetzel, L.A.; Hurtado, M.; MacDowell Kaswan, Z.A.; McCusker, R.H.; Steelman, A.J. Deletion of indoleamine 2,3 dioxygenase (Ido)1 but not Ido2 exacerbates disease symptoms of MOG(35-55)-induced experimental autoimmune encephalomyelitis. Brain Behav. Immun. Health 2020, 25, 100116–100123. [Google Scholar] [CrossRef]
- Choi, B.K.; Asai, T.; Vinay, D.S.; Kim, Y.H.; Kwon, B.S. 4-1BB-mediated amelioration of experimental autoimmune uveoretinitis is caused by indoleamine 2,3-dioxygenase-dependent mechanisms. Cytokine 2006, 34, 233–242. [Google Scholar] [CrossRef]
- Palabiyik, S.S.; Keles, S.; Girgin, G.; Arpali-Tanas, E.; Topdagi, E.; Baydar, T. Neopterin Release and Tryptophan Degradation in Patients with Uveitis. Curr. Eye Res. 2016, 41, 1513–1515. [Google Scholar] [CrossRef]
- Williams, R.O. Exploitation of the IDO Pathway in the Therapy of Rheumatoid Arthritis. Int. J. Tryptophan Res. 2013, 6, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Furuzawa-Carballeda, J.; Lima, G.; Jakez-Ocampo, J.; Llorente, L. Indoleamine 2,3-dioxygenase-expressing peripheral cells in rheumatoid arthritis and systemic lupus erythematosus: A cross-sectional study. Eur. J. Clin. Investig. 2011, 41, 1037–1046. [Google Scholar] [CrossRef] [PubMed]
- Tu, Z.; Li, Y.; Smith, D.; Doller, C.; Sugita, S.; Chan, C.C.; Qian, S.; Fung, J.; Caspi, R.R.; Lu, L.; et al. Myeloid suppressor cells induced by retinal pigment epithelial cells inhibit autoreactive T-cell responses that lead to experimental autoimmune uveitis. Investig. Ophthalmol. Vis. Sci. 2012, 53, 959–966. [Google Scholar] [CrossRef] [PubMed]


| Cell Population (%) | Patients (overall) (O) n = 15 | Patients with Macular Edema (CME) n = 9 | Patients Without Macular Edema (nCME) n = 6 | Healthy Controls (HCs) n = 17 | p-Values |
|---|---|---|---|---|---|
| CD3+CD8−IFN-γ+ (Th1) | 4.89 (2.80–6.68) | 5.35 (2.88–6.68) | 4.44 (2.34–5.68) | 8.43 (7.58–10.00) | O vs. HCs: <0.001 CME vs. nCME: 0.999 CME vs. HCs: 0.020 nCME vs. HCs: 0.014 |
| CCR4−CXCR3+IFN-γ+ (Th1) | 4.10 (3.06–5.28) | 4.94 (3.23–5.28) | 3.74 (3.06–4.39) | 8.77 (5.13–9.71) | O vs. HCs: 0.006 CME vs. nCME: 0.999 CME vs. HCs: 0.058 nCME vs. HCs: 0.099 |
| CD3+CD8−IL-17+ (Th17) | 0.70 (0.60–0.94) | 0.73 (0.62–0.78) | 0.69 (0.60–0.94) | 0.32 (0.22–0.48) | O vs. HCs: <0.001 CME vs. nCME: 0.999 CME vs. HCs: <0.001 nCME vs. HCs: 0.009 |
| CCR4+CCR6+IL-17+ (Th17) | 1.44 (1.02–2.03) | 1.39 (1.02–1.71) | 1.80 (1.44–2.03) | 0.36 (0.26–0.45) | O vs. HCs: <0.001 CME vs. nCME: 0.999 CME vs. HCs: <0.001 nCME vs. HCs: 0.001 |
| CD4+CD25+CD127− (Treg) | 2.34 (1.33–4.31) | 2.31 (1.33–2.56) | 3.35 (1.95–4.34) | 3.90 (3.16–5.33) | O vs. HCs: 0.004 CME vs. nCME: 0.981 CME vs. HCs: 0.008 nCME vs. HCs: 0.421 |
| CD4+CD25highCD127− (Treg) | 0.30 (0.11–0.44) | 0.20 (0.11–0.44) | 0.35 (0.16–0.43) | 0.66 (0.51–1.10) | O vs. HCs: <0.001 CME vs. nCME: 0.981 CME vs. HCs: 0.004 nCME vs. HCs: 0.020 |
| CD8+CD28−FoxP3+ (Tsup) | 0.65 (0.24–2.64) | 0.54 (0.39–2.35) | 0.94 (0.22–2.69) | 3.17 (1.75–5.51) | O vs. HCs: 0.005 CME vs. nCME: 0.988 CME vs. HCs: 0.045 nCME vs. HCs: 0.109 |
| CD19+CD24highCD38high (Breg) | 0.98 (0.26–2.37) | 0.82 (0.52–1.86) | 1.25 (0.26–2.99) | 1.35 (0.60–2.98) | O vs. HCs: 0.363 CME vs. nCME: 0.999 CME vs. HCs: 0.989 nCME vs. HCs: 0.989 |
| IDO Expression in MDSC Subsets | Patients (overall) (O) n = 15 | Patients with Macular Edema (CME) n = 9 | Patients Without Macular Edema (nCME) n = 6 | Healthy Controls (HCs) n = 17 | p-Values |
|---|---|---|---|---|---|
| CD14+CD16+IDO+ (%) | 0.45 (0.21–0.64) | 0.58 (0.20–0.76) | 0.40 (0.21–0.50) | 0.11 (0.10–0.18) | O vs. HCs: 0.011 CME vs. nCME: 0.999 CME vs. HCs: 0.037 nCME vs. HCs: 0.204 |
| CD14+CD16−IDO+ (%) | 0.21 (0. 01–0.60) | 0.21 (0. 01–0.37) | 0.31 (0.10–0.60) | 0.35 (0.15–0.65) | O vs. HCs: 0.333 CME vs. nCME: 0.999 CME vs. HCs: 0.652 nCME vs. HCs: 0.997 |
| p-Values | 0.345 | 0.398 | 0.345 | 0.016 | |
| IDO in CD14+CD16+ cells (MFI) | 69.57 (54.38–108.43) | 54.46 (49.89–108.43) | 89.00 (54.75–122.61) | 104.06 (78.65–204.45) | O vs. HCs: 0.169 CME vs. nCME: 0.999 CME vs. HCs: 0.141 nCME vs. HCs: 0.999 |
| IDO in CD14+CD16−cells (MFI) | 40.65 (0.00–62.93) | 17.78 (0.00–42.58) | 64.35 (41.83–74.99) | 71.71 (32.45–233.38) | O vs. HCs: 0.071 CME vs. nCME: 0.202 CME vs. HCs: 0.041 nCME vs. HCs: 0.999 |
| p-Values | 0.071 | 0.028 | 0.363 | 0.388 |
| Monocyte Subpopulation (%) | Patients (overall) (O) n = 15 | Patients with Macular Edema (CME) n = 9 | Patients Without Macular Edema (nCME) n = 6 | Healthy Controls (HCs) n = 17 | p-Values |
|---|---|---|---|---|---|
| CD14highCD16− (CL) | 72.12 (45.14–83.88) | 68.21 (45.14–83.88) | 76.02 (57.06–81.54) | 66.92 (56.98–81.74) | O vs. HCs: 0.984 CME vs. nCME: 0.844 CME vs. HCs: 0.984 nCME vs. HCs: 0.817 |
| CD14+CD16high (NCL) | 21.25 (13.53–46.97) | 23.20 (13.53–46.97) | 19.29 (14.23–35.59) | 29.44 (15.92–37.72) | O vs. HCs: 0.796 CME vs. nCME: 0.981 CME vs. HCs: 0.999 nCME vs. HCs: 0.973 |
| CD14highCD16+ (INT) | 5.56 (3.57–7.63) | 6.90 (3.57–7.88) | 4.68 (4.22–6.44) | 2.70 (2.20–4.91) | O vs. HCs: 0.020 CME vs. nCME: 0.999 CME vs. HCs: 0.052 nCME vs. HCs: 0.711 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kosmaczewska, A.; Przeździecka-Dołyk, J.; Ciszak, L.; Rojek-Gajda, Z.; Frydecka, I.; Turno-Kręcicka, A.; Misiuk-Hojło, M.; Pawlak, E. Circulating CD16-Positive Monocyte-like Myeloid-Derived Suppressor Cells and Intermediate Monocytes Associated with Clinical and Immunological Complications in Pars Planitis Patients. Cells 2025, 14, 1636. https://doi.org/10.3390/cells14201636
Kosmaczewska A, Przeździecka-Dołyk J, Ciszak L, Rojek-Gajda Z, Frydecka I, Turno-Kręcicka A, Misiuk-Hojło M, Pawlak E. Circulating CD16-Positive Monocyte-like Myeloid-Derived Suppressor Cells and Intermediate Monocytes Associated with Clinical and Immunological Complications in Pars Planitis Patients. Cells. 2025; 14(20):1636. https://doi.org/10.3390/cells14201636
Chicago/Turabian StyleKosmaczewska, Agata, Joanna Przeździecka-Dołyk, Lidia Ciszak, Zofia Rojek-Gajda, Irena Frydecka, Anna Turno-Kręcicka, Marta Misiuk-Hojło, and Edyta Pawlak. 2025. "Circulating CD16-Positive Monocyte-like Myeloid-Derived Suppressor Cells and Intermediate Monocytes Associated with Clinical and Immunological Complications in Pars Planitis Patients" Cells 14, no. 20: 1636. https://doi.org/10.3390/cells14201636
APA StyleKosmaczewska, A., Przeździecka-Dołyk, J., Ciszak, L., Rojek-Gajda, Z., Frydecka, I., Turno-Kręcicka, A., Misiuk-Hojło, M., & Pawlak, E. (2025). Circulating CD16-Positive Monocyte-like Myeloid-Derived Suppressor Cells and Intermediate Monocytes Associated with Clinical and Immunological Complications in Pars Planitis Patients. Cells, 14(20), 1636. https://doi.org/10.3390/cells14201636







