Phospho-Tau Signature During Mitosis: AT8, p-T217 and p-S422 as Key Phospho-Epitopes
Abstract
1. Introduction
2. Material and Methods
2.1. Cell Culture
2.2. Fly Stocks
2.3. Immunocytochemistry
2.4. Antibodies
| Name of Antibody or Phospho-Epitope | Reference | Provider | Dilution |
| AT8 | MN1020 | Invitrogen (ThermoFisher Scientific) Waltham, MA, USA) | 1:200 |
| S202 | [EPR2402] ab108387 | Abcam, Cambridge, U.K. | 1:200 |
| T205 | 44-738 G | Invitrogen | 1:200 |
| PHF1 | Gift from P. Davies | 1:500 | |
| S396 | [EPR2731] ab109390 | Abcam | 1:200 |
| S404 | [EPR2605] ab92676 | Abcam | 1:200 |
| AT100 | MN1060 | Invitrogen | 1:200 |
| T212 | 44-740 G | Invitrogen | 1:200 |
| S214 | 44-742 G | Invitrogen | 1:200 |
| T217 | 44-744 | Invitrogen | 1:200 |
| S416 | Cell Signaling Technologies, Danvers, MA, USA | 1:200 | |
| D7U2P (#15013) | |||
| S422 | [EPR2866] ab79415 | Abcam | 1:200 |
2.5. Confocal Microscopy
2.6. Quantification and Statistics
3. Results
3.1. Tau Phosphorylation Pattern During Mitosis in Tau-Inducible SH-SY5Y Cell Line
3.2. AT8, p-T217 and p-S422 Phospho-Epitopes Are Specifically Associated with Mitotic Cells Under Conditions of Tau Overexpression In Vivo
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Weingarten, M.D.; Lockwood, A.H.; Hwo, S.Y.; Kirschner, M.W. A Protein Factor Essential for Microtubule Assembly. Proc. Natl. Acad. Sci. USA 1975, 72, 1858–1862. [Google Scholar] [CrossRef]
- Witman, G.B.; Cleveland, D.W.; Weingarten, M.D.; Kirschner, M.W. Tubulin Requires Tau for Growth onto Microtubule Initiating Sites. Proc. Natl. Acad. Sci. USA 1976, 73, 4070–4074. [Google Scholar] [CrossRef]
- Barbier, P.; Zejneli, O.; Martinho, M.; Lasorsa, A.; Belle, V.; Smet-Nocca, C.; Tsvetkov, P.O.; Devred, F.; Landrieu, I. Role of Tau as a Microtubule-Associated Protein: Structural and Functional Aspects. Front. Aging Neurosci. 2019, 11, 204. [Google Scholar] [CrossRef]
- Biernat, J.; Gustke, N.; Drewes, G.; Mandelkow, E.M.; Mandelkow, E. Phosphorylation of Ser262 Strongly Reduces Binding of Tau to Microtubules: Distinction between PHF-like Immunoreactivity and Microtubule Binding. Neuron 1993, 11, 153–163. [Google Scholar] [CrossRef]
- Scott, C.W.; Spreen, R.C.; Herman, J.L.; Chow, F.P.; Davison, M.D.; Young, J.; Caputo, C.B. Phosphorylation of Recombinant Tau by cAMP-Dependent Protein Kinase. Identification of Phosphorylation Sites and Effect on Microtubule Assembly. J. Biol. Chem. 1993, 268, 1166–1173. [Google Scholar] [CrossRef]
- Sengupta, A.; Kabat, J.; Novak, M.; Wu, Q.; Grundke-Iqbal, I.; Iqbal, K. Phosphorylation of Tau at Both Thr 231 and Ser 262 Is Required for Maximal Inhibition of Its Binding to Microtubules. Arch. Biochem. Biophys. 1998, 357, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Illenberger, S.; Zheng-Fischhöfer, Q.; Preuss, U.; Stamer, K.; Baumann, K.; Trinczek, B.; Biernat, J.; Godemann, R.; Mandelkow, E.-M.; Mandelkow, E. The Endogenous and Cell Cycle-Dependent Phosphorylation of Tau Protein in Living Cells: Implications for Alzheimer’s Disease. Mol. Biol. Cell 1998, 9, 1495–1512. [Google Scholar] [CrossRef]
- Schneider, A.; Biernat, J.; Von Bergen, M.; Mandelkow, E.; Mandelkow, E.-M. Phosphorylation That Detaches Tau Protein from Microtubules (Ser262, Ser214) Also Protects It against Aggregation into Alzheimer Paired Helical Filaments. Biochemistry 1999, 38, 3549–3558. [Google Scholar] [CrossRef] [PubMed]
- Evans, D.B.; Rank, K.B.; Bhattacharya, K.; Thomsen, D.R.; Gurney, M.E.; Sharma, S.K. Tau Phosphorylation at Serine 396 and Serine 404 by Human Recombinant Tau Protein Kinase II Inhibits Tau’s Ability to Promote Microtubule Assembly. J. Biol. Chem. 2000, 275, 24977–24983. [Google Scholar] [CrossRef] [PubMed]
- Otvos, L.; Feiner, L.; Lang, E.; Szendrei, G.I.; Goedert, M.; Lee, V.M. Monoclonal Antibody PHF-1 Recognizes Tau Protein Phosphorylated at Serine Residues 396 and 404. J. Neurosci. Res. 1994, 39, 669–673. [Google Scholar] [CrossRef]
- Wegmann, S.; Biernat, J.; Mandelkow, E. A Current View on Tau Protein Phosphorylation in Alzheimer’s Disease. Curr. Opin. Neurobiol. 2021, 69, 131–138. [Google Scholar] [CrossRef]
- Alonso, A.D.C.; Zaidi, T.; Novak, M.; Grundke-Iqbal, I.; Iqbal, K. Hyperphosphorylation Induces Self-Assembly of τ into Tangles of Paired Helical Filaments/Straight Filaments. Proc. Natl. Acad. Sci. USA 2001, 98, 6923–6928. [Google Scholar] [CrossRef]
- Buée, L.; Bussière, T.; Buée-Scherrer, V.; Delacourte, A.; Hof, P.R. Tau Protein Isoforms, Phosphorylation and Role in Neurodegenerative disorders11These Authors Contributed Equally to This Work. Brain Res. Rev. 2000, 33, 95–130. [Google Scholar] [CrossRef] [PubMed]
- Despres, C.; Byrne, C.; Qi, H.; Cantrelle, F.-X.; Huvent, I.; Chambraud, B.; Baulieu, E.-E.; Jacquot, Y.; Landrieu, I.; Lippens, G.; et al. Identification of the Tau Phosphorylation Pattern That Drives Its Aggregation. Proc. Natl. Acad. Sci. USA 2017, 114, 9080–9085. [Google Scholar] [CrossRef]
- Luna-Muñoz, J.; Chávez-Macías, L.; García-Sierra, F.; Mena, R. Earliest Stages of Tau Conformational Changes Are Related to the Appearance of a Sequence of Specific Phospho-Dependent Tau Epitopes in Alzheimer’s Disease1. J. Alzheimer’s Dis. 2007, 12, 365–375. [Google Scholar] [CrossRef] [PubMed]
- Augustinack, J.C.; Sanders, J.L.; Tsai, L.-H.; Hyman, B.T. Colocalization and Fluorescence Resonance Energy Transfer between Cdk5 and AT8 Suggests a Close Association in Pre-Neurofibrillary Tangles and Neurofibrillary Tangles. J. Neuropathol. Exp. Neurol. 2002, 61, 557–564. [Google Scholar] [CrossRef]
- Goedert, M.; Jakes, R.; Vanmechelen, E. Monoclonal Antibody AT8 Recognises Tau Protein Phosphorylated at Both Serine 202 and Threonine 205. Neurosci. Lett. 1995, 189, 167–170. [Google Scholar] [CrossRef]
- Barthélemy, N.R.; Li, Y.; Janelidze, S.; He, Y.; Xiong, C.; Stomrud, E.; Fagan, A.M.; Karch, C.M.; Benzinger, T.L.S.; McDade, E.; et al. Plasma Tau Phosphorylation at T217 Predicts Amyloid Deposition in Dominantly Inherited and Late Onset Alzheimer Disease Participants without Clinical Symptoms. Alzheimer’s Dement. 2023, 19, e079220. [Google Scholar] [CrossRef]
- Barthélemy, N.R.; Bateman, R.J.; Hirtz, C.; Marin, P.; Becher, F.; Sato, C.; Gabelle, A.; Lehmann, S. Cerebrospinal Fluid Phospho-Tau T217 Outperforms T181 as a Biomarker for the Differential Diagnosis of Alzheimer’s Disease and PET Amyloid-Positive Patient Identification. Alzheimer’s Res. Ther. 2020, 12, 26. [Google Scholar] [CrossRef] [PubMed]
- Grande, G.; Valletta, M.; Rizzuto, D.; Xia, X.; Qiu, C.; Orsini, N.; Dale, M.; Andersson, S.; Fredolini, C.; Winblad, B.; et al. Blood-Based Biomarkers of Alzheimer’s Disease and Incident Dementia in the Community. Nat. Med. 2025, 31, 2027–2035. [Google Scholar] [CrossRef]
- Janelidze, S.; Stomrud, E.; Smith, R.; Palmqvist, S.; Mattsson, N.; Airey, D.C.; Proctor, N.K.; Chai, X.; Shcherbinin, S.; Sims, J.R.; et al. Cerebrospinal Fluid P-Tau217 Performs Better than p-Tau181 as a Biomarker of Alzheimer’s Disease. Nat. Commun. 2020, 11, 1683. [Google Scholar] [CrossRef]
- Kanaan, N.M.; Cox, K.; Alvarez, V.E.; Stein, T.D.; Poncil, S.; McKee, A.C. Characterization of Early Pathological Tau Conformations and Phosphorylation in Chronic Traumatic Encephalopathy. J. Neuropathol. Exp. Neurol. 2016, 75, 19–34. [Google Scholar] [CrossRef]
- Voss, K.; Koren, J.; Dickey, C.A. The Earliest Tau Dysfunction in Alzheimer’s Disease? Am. J. Pathol. 2011, 179, 2148–2151. [Google Scholar] [CrossRef]
- Balczon, R.; Lin, M.T.; Lee, J.Y.; Abbasi, A.; Renema, P.; Voth, S.B.; Zhou, C.; Koloteva, A.; Michael Francis, C.; Sodha, N.R.; et al. Pneumonia Initiates a Tauopathy. FASEB J. 2021, 35, e21807. [Google Scholar] [CrossRef]
- Barker, R.M.; Chambers, A.; Kehoe, P.G.; Rowe, E.; Perks, C.M. Untangling the Role of Tau in Sex Hormone Responsive Cancers: Lessons Learnt from Alzheimer’s Disease. Clin. Sci. 2024, 138, 1357–1369. [Google Scholar] [CrossRef]
- Chapelet, G.; Béguin, N.; Castellano, B.; Grit, I.; De Coppet, P.; Oullier, T.; Neunlist, M.; Blottière, H.; Rolli-Derkinderen, M.; Le Dréan, G.; et al. Tau Expression and Phosphorylation in Enteroendocrine Cells. Front. Neurosci. 2023, 17, 1166848. [Google Scholar] [CrossRef]
- Gargini, R.; Segura-Collar, B.; Sánchez-Gómez, P. Novel Functions of the Neurodegenerative-Related Gene Tau in Cancer. Front. Aging Neurosci. 2019, 11, 231. [Google Scholar] [CrossRef] [PubMed]
- Sigala, J.; Jumeau, F.; Buée, L.; Sergeant, N.; Mitchell, V. La protéine microtubulaire Tau testiculaire: Une place dans la spermatogenèse? Morphologie 2015, 99, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Vanier, M.-T.; Neuville, P.; Michalik, L.; Launay, J.-F. Expression of Specific Tau Exons in Normal and Tumoral Pancreatic Acinar Cells. J. Cell Sci. 1998, 111, 1419–1432. [Google Scholar] [CrossRef] [PubMed]
- Clementi, L.; Sabetta, S.; Zelli, V.; Compagnoni, C.; Tessitore, A.; Mattei, V.; Angelucci, A. Mitotic Phosphorylation of Tau/MAPT Modulates Cell Cycle Progression in Prostate Cancer Cells. J. Cancer Res. Clin. Oncol. 2023, 149, 7689–7701. [Google Scholar] [CrossRef]
- Delobel, P.; Flament, S.; Hamdane, M.; Mailliot, C.; Sambo, A.; Bégard, S.; Sergeant, N.; Delacourte, A.; Vilain, J.; Buée, L. Abnormal Tau Phosphorylation of the Alzheimer-type Also Occurs during Mitosis. J. Neurochem. 2002, 83, 412–420. [Google Scholar] [CrossRef] [PubMed]
- Hamdane, M.; Sambo, A.-V.; Delobel, P.; Bégard, S.; Violleau, A.; Delacourte, A.; Bertrand, P.; Benavides, J.; Buée, L. Mitotic-like Tau Phosphorylation by P25-Cdk5 Kinase Complex. J. Biol. Chem. 2003, 278, 34026–34034. [Google Scholar] [CrossRef]
- Pope, W.B.; Lambert, M.P.; Leypold, B.; Seupaul, R.; Sletten, L.; Krafft, G.; Klein, W.L. Microtubule-Associated Protein Tau Is Hyperphosphorylated during Mitosis in the Human Neuroblastoma Cell Line SH-SY5Y. Exp. Neurol. 1994, 126, 185–194. [Google Scholar] [CrossRef]
- Preuss, U.; Döring, F.; Illenberger, S.; Mandelkow, E.M. Cell Cycle-Dependent Phosphorylation and Microtubule Binding of Tau Protein Stably Transfected into Chinese Hamster Ovary Cells. Mol. Biol. Cell 1995, 6, 1397–1410. [Google Scholar] [CrossRef]
- Preuss, U.; Mandelkow, E.-M. Mitotic Phosphorylation of Tau Protein in Neuronal Cell Lines Resembles Phosphorylation in Alzheimer’s Disease. Eur. J. Cell Biol. 1998, 76, 176–184. [Google Scholar] [CrossRef]
- Bretteville, A.; Ando, K.; Ghestem, A.; Loyens, A.; Bégard, S.; Beauvillain, J.-C.; Sergeant, N.; Hamdane, M.; Buée, L. Two-Dimensional Electrophoresis of Tau Mutants Reveals Specific Phosphorylation Pattern Likely Linked to Early Tau Conformational Changes. PLoS ONE 2009, 4, e4843. [Google Scholar] [CrossRef]
- Bougé, A.-L.; Parmentier, M.-L. Tau Excess Impairs Mitosis and Kinesin-5 Function, Leading to Aneuploidy and Cell Death. Dis. Models Mech. 2016, 9, 307–319. [Google Scholar] [CrossRef]
- Fulga, T.A.; Elson-Schwab, I.; Khurana, V.; Steinhilb, M.L.; Spires, T.L.; Hyman, B.T.; Feany, M.B. Abnormal Bundling and Accumulation of F-Actin Mediates Tau-Induced Neuronal Degeneration in Vivo. Nat. Cell Biol. 2007, 9, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An Open-Source Platform for Biological-Image Analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Yoshida, H.; Goedert, M. Sequential Phosphorylation of Tau Protein by cAMP-dependent Protein Kinase and SAPK4/P38δ or JNK2 in the Presence of Heparin Generates the AT100 Epitope. J. Neurochem. 2006, 99, 154–164. [Google Scholar] [CrossRef] [PubMed]
- Nadel, C.M.; Pokhrel, S.; Wucherer, K.; Oehler, A.; Thwin, A.C.; Basu, K.; Callahan, M.D.; Southworth, D.R.; Mordes, D.A.; Craik, C.S.; et al. Phosphorylation of Tau at a Single Residue Inhibits Binding to the E3 Ubiquitin Ligase, CHIP. Nat. Commun. 2024, 15, 7972. [Google Scholar] [CrossRef] [PubMed]
- Denus, M.; Filaquier, A.; Fargues, W.; Néel, E.; Stewart, S.E.; Colladant, M.; Curel, T.; Mezghrani, A.; Marin, P.; Claeysen, S.; et al. A Sensitive and Versatile Cell-Based Assay Combines Luminescence and Trapping Approaches to Monitor Unconventional Protein Secretion. Traffic 2025, 26, e70009. [Google Scholar] [CrossRef] [PubMed]
- Curel, T.; Denus, M.; Villeneuve, J.; Parmentier, M.-L. A New in Vivo Model of Human Tau Excess with Sustained Activation of Caspases. 2025; in preparation. [Google Scholar]
- Jackson, G.R.; Wiedau-Pazos, M.; Sang, T.-K.; Wagle, N.; Brown, C.A.; Massachi, S.; Geschwind, D.H. Human Wild-Type Tau Interacts with Wingless Pathway Components and Produces Neurofibrillary Pathology in Drosophila. Neuron 2002, 34, 509–519. [Google Scholar] [CrossRef] [PubMed]
- Steinhilb, M.L.; Dias-Santagata, D.; Fulga, T.A.; Felch, D.L.; Feany, M.B. Tau Phosphorylation Sites Work in Concert to Promote Neurotoxicity In Vivo. Mol. Biol. Cell 2007, 18, 5060–5068. [Google Scholar] [CrossRef]
- Cross, F.R.; Buchler, N.E.; Skotheim, J.M. Evolution of Networks and Sequences in Eukaryotic Cell Cycle Control. Phil. Trans. R. Soc. B 2011, 366, 3532–3544. [Google Scholar] [CrossRef]
- Glover, D.M. Mitosis in Drosophila. J. Cell Sci. 1989, 92, 137–146. [Google Scholar] [CrossRef]
- Canet, G.; Rocaboy, E.; Laliberté, F.; Boscher, E.; Guisle, I.; Diego-Diaz, S.; Fereydouni-Forouzandeh, P.; Whittington, R.A.; Hébert, S.S.; Pernet, V.; et al. Temperature-Induced Artifacts in Tau Phosphorylation: Implications for Reliable Alzheimer’s Disease Research. Exp. Neurobiol. 2023, 32, 423–440. [Google Scholar] [CrossRef]
- Duquette, A.; Pernègre, C.; Veilleux Carpentier, A.; Leclerc, N. Similarities and Differences in the Pattern of Tau Hyperphosphorylation in Physiological and Pathological Conditions: Impacts on the Elaboration of Therapies to Prevent Tau Pathology. Front. Neurol. 2021, 11, 607680. [Google Scholar] [CrossRef]
- León-Espinosa, G.; Murillo, A.M.M.; Turegano-Lopez, M.; DeFelipe, J.; Holgado, M. Phosphorylated Tau at T181 Accumulates in the Serum of Hibernating Syrian Hamsters and Rapidly Disappears after Arousal. Sci. Rep. 2024, 14, 20562. [Google Scholar] [CrossRef]
- Barrio-Alonso, E.; Hernández-Vivanco, A.; Walton, C.C.; Perea, G.; Frade, J.M. Cell Cycle Reentry Triggers Hyperploidization and Synaptic Dysfunction Followed by Delayed Cell Death in Differentiated Cortical Neurons. Sci. Rep. 2018, 8, 14316. [Google Scholar] [CrossRef]
- Wesseling, H.; Mair, W.; Kumar, M.; Schlaffner, C.N.; Tang, S.; Beerepoot, P.; Fatou, B.; Guise, A.J.; Cheng, L.; Takeda, S.; et al. Tau PTM Profiles Identify Patient Heterogeneity and Stages of Alzheimer’s Disease. Cell 2020, 183, 1699–1713.e13. [Google Scholar] [CrossRef]
- Warmenhoven, N.; Salvadó, G.; Janelidze, S.; Mattsson-Carlgren, N.; Bali, D.; Dolado, A.O.; Kolb, H.; Triana-Baltzer, G.; Barthélemy, N.R.; Schindler, S.E.; et al. A Comprehensive Head-to-Head Comparison of Key Plasma Phosphorylated Tau 217 Biomarker Tests. medRxiv 2024. [Google Scholar] [CrossRef]
- Lai, R.; Li, B.; Bishnoi, R. P-Tau217 as a Reliable Blood-Based Marker of Alzheimer’s Disease. Biomedicines 2024, 12, 1836. [Google Scholar] [CrossRef]
- Montalto, G.; Ricciarelli, R. Tau, Tau Kinases, and Tauopathies: An Updated Overview. BioFactors 2023, 49, 502–511. [Google Scholar] [CrossRef]
- Martin, L.; Latypova, X.; Wilson, C.M.; Magnaudeix, A.; Perrin, M.-L.; Yardin, C.; Terro, F. Tau Protein Kinases: Involvement in Alzheimer’s Disease. Ageing Res. Rev. 2013, 12, 289–309. [Google Scholar] [CrossRef] [PubMed]
- Shaul, Y.D.; Seger, R. ERK1c Regulates Golgi Fragmentation during Mitosis. J. Cell Biol. 2006, 172, 885–897. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, P.S.; Vaisberg, E.; Hunt, A.J.; Tolwinski, N.S.; Whalen, A.M.; McIntosh, J.R.; Ahn, N.G. Activation of the MKK/ERK Pathway during Somatic Cell Mitosis: Direct Interactions of Active ERK with Kinetochores and Regulation of the Mitotic 3F3/2 Phosphoantigen. J. Cell Biol. 1998, 142, 1533–1545. [Google Scholar] [CrossRef]
- Johnson, A.E.; Chen, J.-S.; Gould, K.L. CK1 Is Required for a Mitotic Checkpoint That Delays Cytokinesis. Curr. Biol. 2013, 23, 1920–1926. [Google Scholar] [CrossRef] [PubMed]
- Rashid, M.S.; Mazur, T.; Ji, W.; Liu, S.T.; Taylor, W.R. Analysis of the Role of GSK3 in the Mitotic Checkpoint. Sci. Rep. 2018, 8, 14259. [Google Scholar] [CrossRef]
- Salaun, P.; Rannou, Y.; Claude, P. Cdk1, Plks, Auroras, and Neks: The Mitotic Bodyguards. In Hormonal Carcinogenesis V.; Li, J.J., Li, S.A., Mohla, S., Rochefort, H., Maudelonde, T., Eds.; Advances in Experimental Medicine and Biology; Springer: New York, NY, USA, 2008; Volume 617, pp. 41–56. ISBN 978-0-387-69078-0. [Google Scholar]
- Chen, M.; Zhang, W.; Gou, Y.; Xu, D.; Wei, Y.; Liu, D.; Han, C.; Huang, X.; Li, C.; Ning, W.; et al. GPS 6.0: An Updated Server for Prediction of Kinase-Specific Phosphorylation Sites in Proteins. Nucleic Acids Res. 2023, 51, W243–W250. [Google Scholar] [CrossRef]
- Zhong, Q.; Congdon, E.E.; Nagaraja, H.N.; Kuret, J. Tau Isoform Composition Influences Rate and Extent of Filament Formation. J. Biol. Chem. 2012, 287, 20711–20719. [Google Scholar] [CrossRef] [PubMed]
- Prezel, E.; Elie, A.; Delaroche, J.; Stoppin-Mellet, V.; Bosc, C.; Serre, L.; Fourest-Lieuvin, A.; Andrieux, A.; Vantard, M.; Arnal, I. Tau Can Switch Microtubule Network Organizations: From Random Networks to Dynamic and Stable Bundles. Mol. Biol. Cell 2018, 29, 154–165. [Google Scholar] [CrossRef] [PubMed]
- Noble, W.; Hanger, D.P.; Miller, C.C.J.; Lovestone, S. The Importance of Tau Phosphorylation for Neurodegenerative Diseases. Front. Neurol. 2013, 4, 83. [Google Scholar] [CrossRef] [PubMed]
- Talmat-Amar, Y.; Arribat, Y.; Parmentier, M.-L. Vesicular Axonal Transport Is Modified In Vivo by Tau Deletion or Overexpression in Drosophila. Int. J. Mol. Sci. 2018, 19, 744. [Google Scholar] [CrossRef]
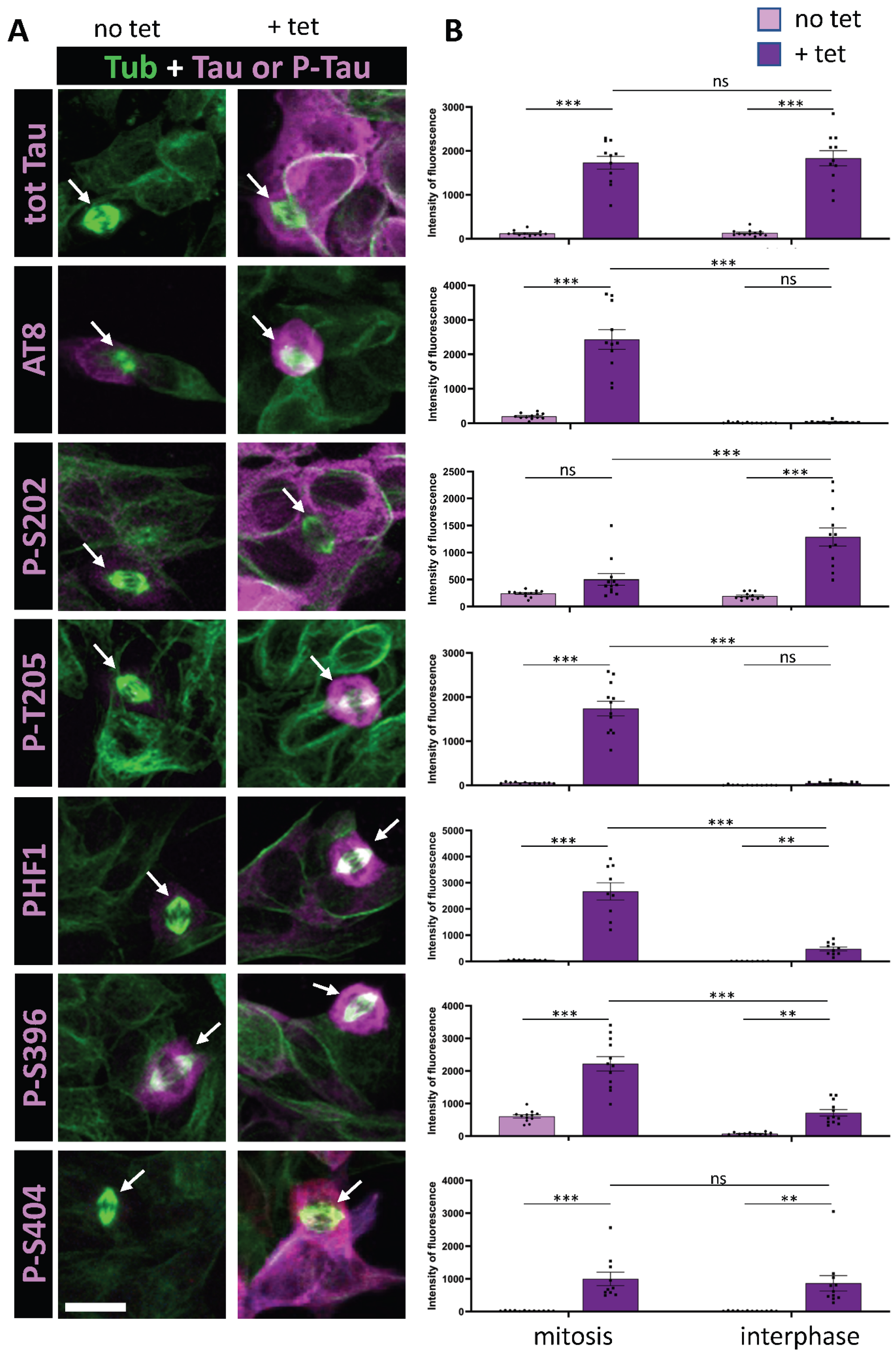
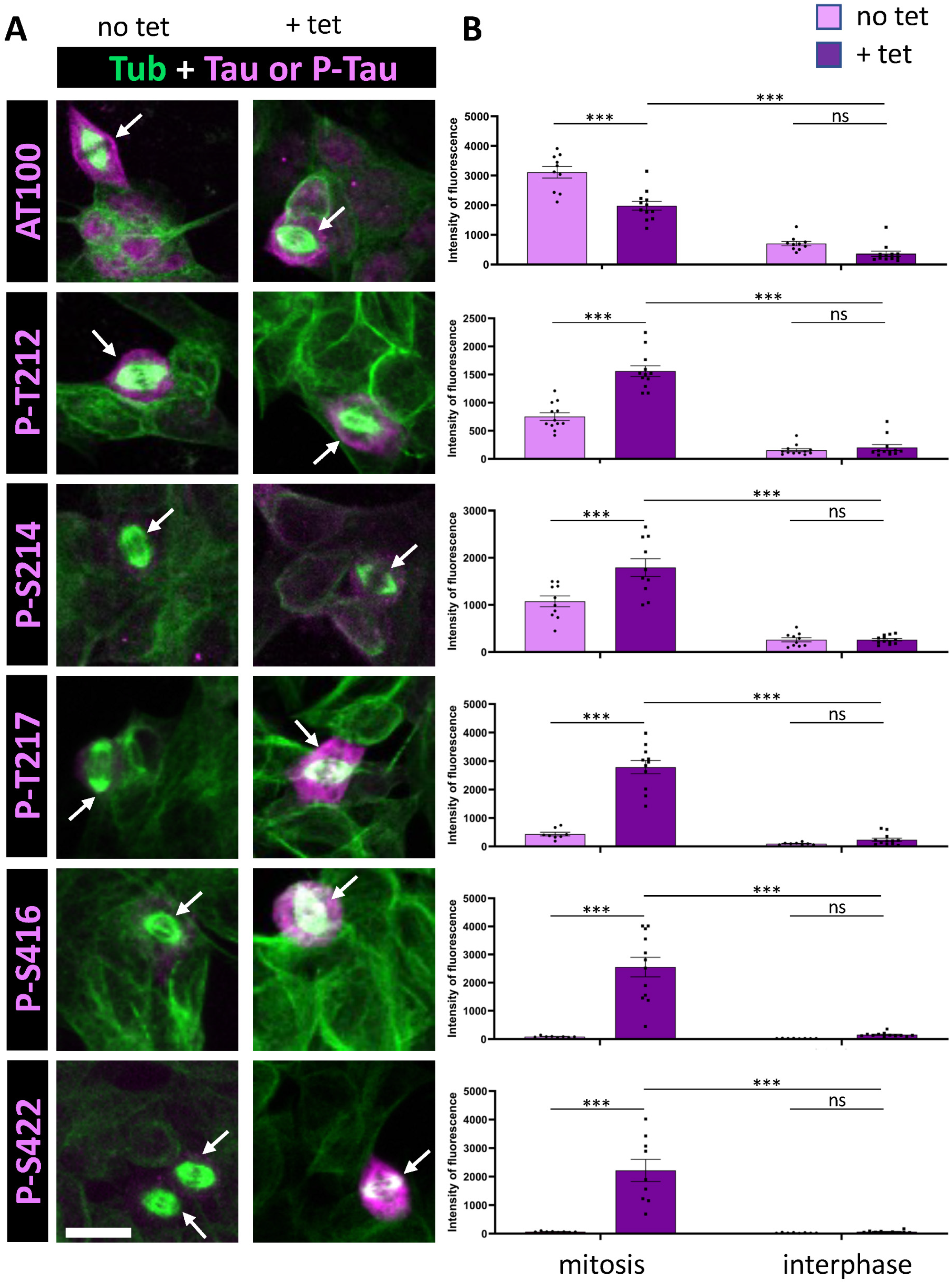


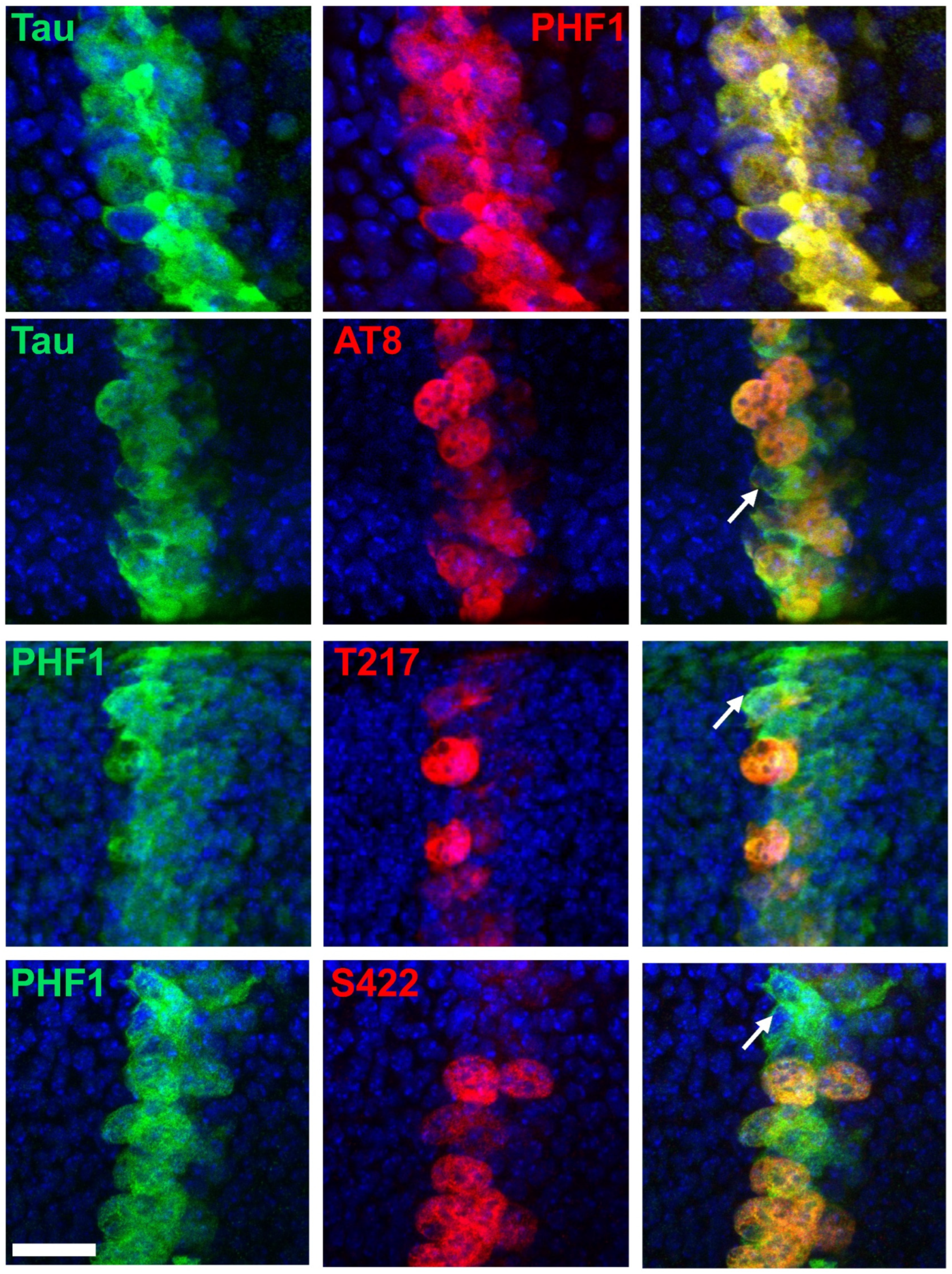
| Epitope | IR Increase (Mitosis/Interphase) in Tau Overexpressing Cells | IR Increase (Overexpression of Tau/Basal Conditions) During Mitosis | IR Increase (Overexpression of Tau/Basal Conditions) During Interphase |
|---|---|---|---|
| AT8 | 60.52 ± 7.34 * | 15.76 ± 2.80 | 1.42 ± 0.23 * |
| p-S202 | 0.43 ± 0.03 * | 4.41 ± 1.66 * | 19.11 ± 8.91 |
| p-T205 | 27.32 ± 5.70 * | 26.48 ± 3.04 | 5.34 ± 0.42 * |
| PHF1 | 3.95 ± 0.46 * | 26.02 ± 6.61 | 20.37 ± 3.36 |
| p-S396 | 3.10 ± 0.01 * | 3.38 ± 0.20 * | 8.99 ± 0.57 |
| p-S404 | 1.21 ± 0.05 * | 35.14 ± 1.75 | 30.41 ± 3.92 |
| AT100 | 3.97 ± 1.05 * | 0.75 ± 0.09 * | 0.70 ± 0.13 * |
| p-T212 | 6.61 ± 0.76 * | 2.41 ± 0.25 * | 1.75 ± 0.32 * |
| p-S214 | 6.69 ± 0.12 * | 1.42 ± 0.17 * | 0.9 ± 0.09 * |
| p-T217 | 30.52 ± 13.25 * | 8.0 ± 1.13 | 1.48 ± 0.65 * |
| p-S416 | 13.46 ± 1.93 * | 17.62 ± 7.90 | 3.39 ± 1.67 * |
| p-S422 | 27.96 ± 2.82 * | 28.17 ± 2.59 | 1.65 ± 0.41 * |
| Tot Tau | 0.95 ± 0.03 | 17.41 ± 4.93 | 19.51 ± 4.76 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goussard, M.; Zarka, K.; Denus, M.; Curel, T.; Claeysen, S.; Lefebvre, B.; Hamdane, M.; Marin, P.; Villeneuve, J.; Parmentier, M.-L. Phospho-Tau Signature During Mitosis: AT8, p-T217 and p-S422 as Key Phospho-Epitopes. Cells 2025, 14, 1638. https://doi.org/10.3390/cells14201638
Goussard M, Zarka K, Denus M, Curel T, Claeysen S, Lefebvre B, Hamdane M, Marin P, Villeneuve J, Parmentier M-L. Phospho-Tau Signature During Mitosis: AT8, p-T217 and p-S422 as Key Phospho-Epitopes. Cells. 2025; 14(20):1638. https://doi.org/10.3390/cells14201638
Chicago/Turabian StyleGoussard, Marion, Kelly Zarka, Morgane Denus, Thomas Curel, Sylvie Claeysen, Bruno Lefebvre, Malika Hamdane, Philippe Marin, Julien Villeneuve, and Marie-Laure Parmentier. 2025. "Phospho-Tau Signature During Mitosis: AT8, p-T217 and p-S422 as Key Phospho-Epitopes" Cells 14, no. 20: 1638. https://doi.org/10.3390/cells14201638
APA StyleGoussard, M., Zarka, K., Denus, M., Curel, T., Claeysen, S., Lefebvre, B., Hamdane, M., Marin, P., Villeneuve, J., & Parmentier, M.-L. (2025). Phospho-Tau Signature During Mitosis: AT8, p-T217 and p-S422 as Key Phospho-Epitopes. Cells, 14(20), 1638. https://doi.org/10.3390/cells14201638








