Chloroquine Causes Aging-like Changes in Diaphragm Neuromuscular Junction Morphology in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Experimental Treatment
2.3. Neuromuscular Junction Morphology
2.4. Confocal Imaging and Analysis
2.5. Statistics
3. Results
3.1. Chloroquine Treatment
3.2. Chloroquine Effects on Diaphragm Neuromuscular Junction Volume
3.3. Morphological Assessment of Denervation of Diaphragm Neuromuscular Junctions
3.4. Relationship Between NMJ Innervation and Morphology
4. Discussion
4.1. Age-Related Effects on Neuromuscular Junction Morphology
4.2. Chloroquine Has Deleterious Effects on the Neuromuscular System
4.3. Autophagy-Related Mechanisms of Chloroquine
4.4. Autophagy Inhibition and Muscle Denervation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| NMJ | Neuromuscular junction |
References
- Shippey, E.A.; Wagler, V.D.; Collamer, A.N. Hydroxychloroquine: An old drug with new relevance. Cleve Clin. J. Med. 2018, 85, 459–467. [Google Scholar] [CrossRef] [PubMed]
- Edington, F.L.B.; Gadellha, S.R.; Santiago, M.B. Safety of treatment with chloroquine and hydroxychloroquine: A ten-year systematic review and meta-analysis. Eur. J. Intern. Med. 2021, 88, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, A.A.S. Side Effects of Chloroquine and Hydroxychloroquine on Skeletal Muscle: A Narrative Review. Curr. Pharmacol. Rep. 2020, 6, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Biguetti, C.C.; Junior, J.F.S.; Fiedler, M.W.; Marrelli, M.T.; Brotto, M. The toxic effects of chloroquine and hydroxychloroquine on skeletal muscle: A systematic review and meta-analysis. Sci. Rep. 2021, 11, 6589. [Google Scholar] [CrossRef]
- Sieb, J.P.; Milone, M.; Engel, A.G. Effects of the quinoline derivatives quinine, quinidine, and chloroquine on neuromuscular transmission. Brain Res. 1996, 712, 179–189. [Google Scholar] [CrossRef]
- Hong, S.J. Reduction of quantal size and inhibition of neuromuscular transmission by bafilomycin A. Neuropharmacology 2001, 41, 609–617. [Google Scholar] [CrossRef]
- Okwuasaba, F.K.; Otubu, J.A.; Udoh, F.V. Mode of inhibitory actions of acute and chronic chloroquine administration on the electrically stimulated mouse diaphragm in vitro. Br. J. Pharmacol. 1990, 101, 133–139. [Google Scholar] [CrossRef]
- Saldarriaga, C.A.; Alatout, M.H.; Khurram, O.U.; Gransee, H.M.; Sieck, G.C.; Mantilla, C.B. Chloroquine impairs maximal transdiaphragmatic pressure generation in old mice. J. Appl. Physiol. 2023, 135, 1126–1134. [Google Scholar] [CrossRef]
- Mauthe, M.; Orhon, I.; Rocchi, C.; Zhou, X.; Luhr, M.; Hijlkema, K.-J.; Coppes, R.P.; Engedal, N.; Mari, M.; Reggiori, F. Chloroquine inhibits autophagic flux by decreasing autophagosome-lysosome fusion. Autophagy 2018, 14, 1435–1455. [Google Scholar] [CrossRef]
- Liu, S.; Yao, S.; Yang, H.; Liu, S.; Wang, Y. Autophagy: Regulator of cell death. Cell Death Dis. 2023, 14, 648. [Google Scholar] [CrossRef]
- Zhao, Y.G.; Codogno, P.; Zhang, H. Machinery, regulation and pathophysiological implications of autophagosome maturation. Nat. Rev. Mol. Cell Biol. 2021, 22, 733–750. [Google Scholar] [CrossRef] [PubMed]
- Pareja-Cajiao, M.; Gransee, H.M.; Stowe, J.M.; Rana, S.; Sieck, G.C.; Mantilla, C.B. Age-related impairment of autophagy in cervical motor neurons. Exp. Gerontol. 2021, 144, 111193. [Google Scholar] [CrossRef]
- Jahanian, S.; Pareja-Cajiao, M.; Gransee, H.M.; Sieck, G.C.; Mantilla, C.B. Autophagy markers LC3 and p62 in aging lumbar motor neurons. Exp. Gerontol. 2024, 194, 112483. [Google Scholar] [CrossRef]
- Manic, G.; Obrist, F.; Kroemer, G.; Vitale, I.; Galluzzi, L. Chloroquine and hydroxychloroquine for cancer therapy. Mol. Cell Oncol. 2014, 1, e29911. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Chen, K.; Lu, X.; Gao, H.J.; Qin, Z.H.; Lin, F. Exercise ameliorates the detrimental effect of chloroquine on skeletal muscles in mice via restoring autophagy flux. Acta Pharmacol. Sin. 2014, 35, 135–142. [Google Scholar] [CrossRef]
- Iwai-Kanai, E.; Yuan, H.; Huang, C.; Sayen, M.R.; Perry-Garza, C.N.; Kim, L.; Gottlieb, R.A. A method to measure cardiac autophagic flux in vivo. Autophagy 2008, 4, 322–329. [Google Scholar] [CrossRef]
- Greising, S.M.; Stowe, J.M.; Sieck, G.C.; Mantilla, C.B. Role of TrkB kinase activity in aging diaphragm neuromuscular junctions. Exp. Gerontol. 2015, 72, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Mantilla, C.B.; Stowe, J.M.; Sieck, D.C.; Ermilov, L.G.; Greising, S.M.; Zhang, C.; Shokat, K.M.; Sieck, G.C. TrkB Kinase Activity Maintains Synaptic Function and Structural Integrity at Adult Neuromuscular Junctions. J. Appl. Physiol. 2014, 117, 910–920. [Google Scholar] [CrossRef]
- Willadt, S.; Nash, M.; Slater, C.R. Age-related fragmentation of the motor endplate is not associated with impaired neuromuscular transmission in the mouse diaphragm. Sci. Rep. 2016, 6, 24849. [Google Scholar] [CrossRef]
- Prakash, Y.S.; Sieck, G.C. Age-related remodeling of neuromuscular junctions on type-identified diaphragm fibers. Muscle Nerve 1998, 21, 887–895. [Google Scholar] [CrossRef]
- Mantilla, C.B.; Rowley, K.L.; Fahim, M.A.; Zhan, W.Z.; Sieck, G.C. Synaptic vesicle cycling at type-identified diaphragm neuromuscular junctions. Muscle Nerve 2004, 30, 774–783. [Google Scholar] [CrossRef]
- Mantilla, C.B.; Rowley, K.L.; Zhan, W.Z.; Fahim, M.A.; Sieck, G.C. Synaptic vesicle pools at diaphragm neuromuscular junctions vary with motoneuron soma, not axon terminal, inactivity. Neuroscience 2007, 146, 178–189. [Google Scholar] [CrossRef] [PubMed]
- Greising, S.M.; Ermilov, L.G.; Sieck, G.C.; Mantilla, C.B. Ageing and neurotrophic signalling effects on diaphragm neuromuscular function. J. Physiol. 2015, 593, 431–440. [Google Scholar] [CrossRef] [PubMed]
- Greising, S.M.; Mantilla, C.B.; Medina-Martinez, J.S.; Stowe, J.M.; Sieck, G.C. Functional Impact of Diaphragm Muscle Sarcopenia in both Male and Female Mice. Am. J. Physiol. Lung Cell Mol. Physiol. 2015, 309, L46–L52. [Google Scholar] [CrossRef] [PubMed]
- Khurram, O.U.; Fogarty, M.J.; Sarrafian, T.L.; Bhatt, A.; Mantilla, C.B.; Sieck, G.C. Impact of aging on diaphragm muscle function in male and female Fischer 344 rats. Physiol. Rep. 2018, 6, e13786. [Google Scholar] [CrossRef]
- Gonzalez-Freire, M.; de Cabo, R.; Studenski, S.A.; Ferrucci, L. The Neuromuscular Junction: Aging at the Crossroad between Nerves and Muscle. Front. Aging Neurosci. 2014, 6, 208. [Google Scholar] [CrossRef]
- Iyer, S.R.; Shah, S.B.; Lovering, R.M. The Neuromuscular Junction: Roles in Aging and Neuromuscular Disease. Int. J. Mol. Sci. 2021, 22, 8058. [Google Scholar] [CrossRef]
- Tsentsevitsky, A.N.; Sibgatullina, G.V.; Odoshivkina, Y.G.; Khuzakhmetova, V.F.; Tokmakova, A.R.; Ponomareva, A.A.; Salnikov, V.V.; Zakirjanova, G.F.; Petrov, A.M.; Bukharaeva, E.A. Functional and Structural Changes in Diaphragm Neuromuscular Junctions in Early Aging. Int. J. Mol. Sci. 2024, 25, 8959. [Google Scholar] [CrossRef]
- Valdez, G.; Tapia, J.C.; Lichtman, J.W.; Fox, M.A.; Sanes, J.R. Shared resistance to aging and ALS in neuromuscular junctions of specific muscles. PLoS ONE 2012, 7, e34640. [Google Scholar] [CrossRef]
- Simpson, L.L. The interaction between aminoquinolines and presynaptically acting neurotoxins. J. Pharmacol. Exp. Ther. 1982, 222, 43–48. [Google Scholar] [CrossRef]
- Chinyanga, H.M.; Vartanian, G.A.; Okai, E.A.; Greenberger, D.V. Chloroquine-induced depression of neuromuscular transmission. Eur. J. Pharmacol. 1972, 18, 256–260. [Google Scholar] [CrossRef] [PubMed]
- O’Shaughnessy, T.J.; Zim, B.; Ma, W.; Shaffer, K.M.; Stenger, D.A.; Zamani, K.; Gross, G.W.; Pancrazio, J.J. Acute neuropharmacologic action of chloroquine on cortical neurons in vitro. Brain Res. 2003, 959, 280–286. [Google Scholar] [CrossRef]
- Ayitey-Smith, E.; Vartanian, G.A. Dual action of chloroquine on frog’s skeletal muscle contraction. Eur. J. Pharmacol. 1975, 30, 29–35. [Google Scholar] [CrossRef]
- Naddaf, E.; Paul, P.; AbouEzzeddine, O.F. Chloroquine and Hydroxychloroquine Myopathy: Clinical Spectrum and Treatment Outcomes. Front. Neurol. 2020, 11, 616075. [Google Scholar] [CrossRef] [PubMed]
- Smit, C.; Peeters, M.Y.M.; van den Anker, J.N.; Knibbe, C.A.J. Chloroquine for SARS-CoV-2: Implications of Its Unique Pharmacokinetic and Safety Properties. Clin. Pharmacokinet. 2020, 59, 659–669. [Google Scholar] [CrossRef]
- Coban, C. The host targeting effect of chloroquine in malaria. Curr. Opin. Immunol. 2020, 66, 98–107. [Google Scholar] [CrossRef]
- Cho, H.I.; Choi, J.W.; Lee, S.M. Impairment of autophagosome-lysosome fusion contributes to chronic ethanol-induced liver injury. J. Alcohol. 2014, 48, 717–725. [Google Scholar] [CrossRef] [PubMed]
- Budi, Y.P.; Li, Y.H.; Huang, C.; Wang, M.E.; Lin, Y.C.; Jong, D.S.; Chiu, C.H.; Jiang, Y.F. The role of autophagy in high-fat diet-induced insulin resistance of adipose tissues in mice. PeerJ 2022, 10, e13867. [Google Scholar] [CrossRef]
- Chen, J.L.; Luo, C.; Pu, D.; Zhang, G.Q.; Zhao, Y.X.; Sun, Y.; Zhao, K.X.; Liao, Z.Y.; Lv, A.K.; Zhu, S.Y.; et al. Metformin attenuates diabetes-induced tau hyperphosphorylation in vitro and in vivo by enhancing autophagic clearance. Exp. Neurol. 2019, 311, 44–56. [Google Scholar] [CrossRef]
- Leidal, A.M.; Levine, B.; Debnath, J. Autophagy and the cell biology of age-related disease. Nat. Cell Biol. 2018, 20, 1338–1348. [Google Scholar] [CrossRef]
- Nieto-Torres, J.L.; Hansen, M. Macroautophagy and aging: The impact of cellular recycling on health and longevity. Mol. Aspects Med. 2021, 82, 101020. [Google Scholar] [CrossRef]
- Gonzalez Porras, M.A.; Sieck, G.C.; Mantilla, C.B. Impaired Autophagy in Motor Neurons: A Final Common Mechanism of Injury and Death. Physiology 2018, 33, 211–224. [Google Scholar] [CrossRef] [PubMed]
- Greising, S.M.; Mantilla, C.B.; Gorman, B.A.; Ermilov, L.G.; Sieck, G.C. Diaphragm muscle sarcopenia in aging mice. Exp. Gerontol. 2013, 48, 881–887. [Google Scholar] [CrossRef] [PubMed]
- Vang, P.; Vasdev, A.; Zhan, W.Z.; Gransee, H.M.; Sieck, G.C.; Mantilla, C.B. Diaphragm muscle sarcopenia into very old age in mice. Physiol. Rep. 2020, 8, e14305. [Google Scholar] [CrossRef]
- Fogarty, M.J.; Omar, T.S.; Zhan, W.Z.; Mantilla, C.B.; Sieck, G.C. Phrenic Motor Neuron Loss in Aged Rats. J. Neurophysiol. 2018, 119, 1852–1862. [Google Scholar] [CrossRef]
- Schrezenmeier, E.; Dorner, T. Mechanisms of action of hydroxychloroquine and chloroquine: Implications for rheumatology. Nat. Rev. Rheumatol. 2020, 16, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Kwon, D.; Choi, C.; Oh, J.W.; Benveniste, E.N. Chloroquine induces activation of nuclear factor-kappaB and subsequent expression of pro-inflammatory cytokines by human astroglial cells. J. Neurochem. 2003, 84, 1266–1274. [Google Scholar] [CrossRef]
- Gao, L.; Zhu, H.; Fan, H.; Liu, Z. Chloroquine exacerbates serum withdrawal-induced G1 phase arrest via an autophagy-independent mechanism. RSC Adv. 2017, 7, 46082–46091. [Google Scholar] [CrossRef]
- Kazama, I.; Maruyama, Y.; Murata, Y.; Sano, M. Voltage-dependent biphasic effects of chloroquine on delayed rectifier K+-channel currents in murine thymocytes. J. Physiol. Sci. 2012, 62, 267–274. [Google Scholar] [CrossRef]
- Maes, H.; Kuchnio, A.; Peric, A.; Moens, S.; Nys, K.; De Bock, K.; Quaegebeur, A.; Schoors, S.; Georgiadou, M.; Wouters, J.; et al. Tumor vessel normalization by chloroquine independent of autophagy. Cancer Cell 2014, 26, 190–206. [Google Scholar] [CrossRef]
- Ashrafi, G.; Schlehe, J.S.; LaVoie, M.J.; Schwarz, T.L. Mitophagy of damaged mitochondria occurs locally in distal neuronal axons and requires PINK1 and Parkin. J. Cell Biol. 2014, 206, 655–670. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Martin, S.; Papadopulos, A.; Harper, C.B.; Mavlyutov, T.A.; Niranjan, D.; Glass, N.R.; Cooper-White, J.J.; Sibarita, J.B.; Choquet, D.; et al. Control of autophagosome axonal retrograde flux by presynaptic activity unveiled using botulinum neurotoxin type a. J. Neurosci. 2015, 35, 6179–6194. [Google Scholar] [CrossRef] [PubMed]
- Tammineni, P.; Ye, X.; Feng, T.; Aikal, D.; Cai, Q. Impaired retrograde transport of axonal autophagosomes contributes to autophagic stress in Alzheimer’s disease neurons. eLife 2017, 6, e21776. [Google Scholar] [CrossRef] [PubMed]
- Javaid, H.M.A.; Lim, H.; Shin, S.; Huh, J.Y. Inhibition of autophagy with chloroquine dysregulates mitochondrial quality control and energetics in adipocytes. Arch. Pharm. Res. 2022, 45, 731–742. [Google Scholar] [CrossRef]
- Filippone, A.; Esposito, E.; Mannino, D.; Lyssenko, N.; Pratico, D. The contribution of altered neuronal autophagy to neurodegeneration. Pharmacol. Ther. 2022, 238, 108178. [Google Scholar] [CrossRef]
- Salmon, A.B.; Richardson, A.; Perez, V.I. Update on the oxidative stress theory of aging: Does oxidative stress play a role in aging or healthy aging? Free Radic. Biol. Med. 2010, 48, 642–655. [Google Scholar] [CrossRef]
- Muller, F.L.; Song, W.; Liu, Y.; Chaudhuri, A.; Pieke-Dahl, S.; Strong, R.; Huang, T.T.; Epstein, C.J.; Roberts, L.J., 2nd; Csete, M.; et al. Absence of CuZn superoxide dismutase leads to elevated oxidative stress and acceleration of age-dependent skeletal muscle atrophy. Free Radic. Biol. Med. 2006, 40, 1993–2004. [Google Scholar] [CrossRef]
- Zhou, J.; Li, X.Y.; Liu, Y.J.; Feng, J.; Wu, Y.; Shen, H.M.; Lu, G.D. Full-coverage regulations of autophagy by ROS: From induction to maturation. Autophagy 2022, 18, 1240–1255. [Google Scholar] [CrossRef]
- Lee, J.; Giordano, S.; Zhang, J. Autophagy, mitochondria and oxidative stress: Cross-talk and redox signalling. Biochem. J. 2012, 441, 523–540. [Google Scholar] [CrossRef]
- Uzar, E.; Ozay, R.; Evliyaoglu, O.; Aktas, A.; Ulkay, M.B.; Uyar, M.E.; Ersoy, A.; Burakgazi, A.Z.; Turkay, C.; Ilhan, A. Hydroxycloroquine-induced oxidative stress on sciatic nerve and muscle tissue of rats: A stereological and biochemical study. Hum. Exp. Toxicol. 2012, 31, 1066–1073. [Google Scholar] [CrossRef]
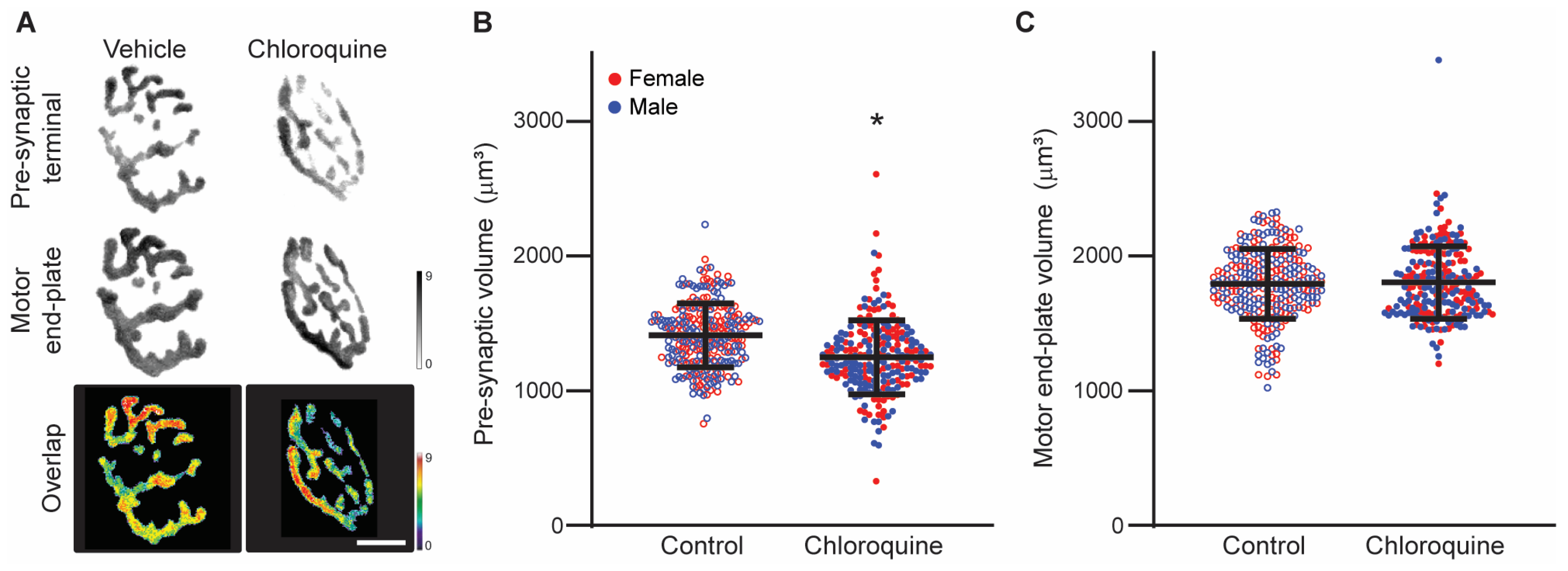

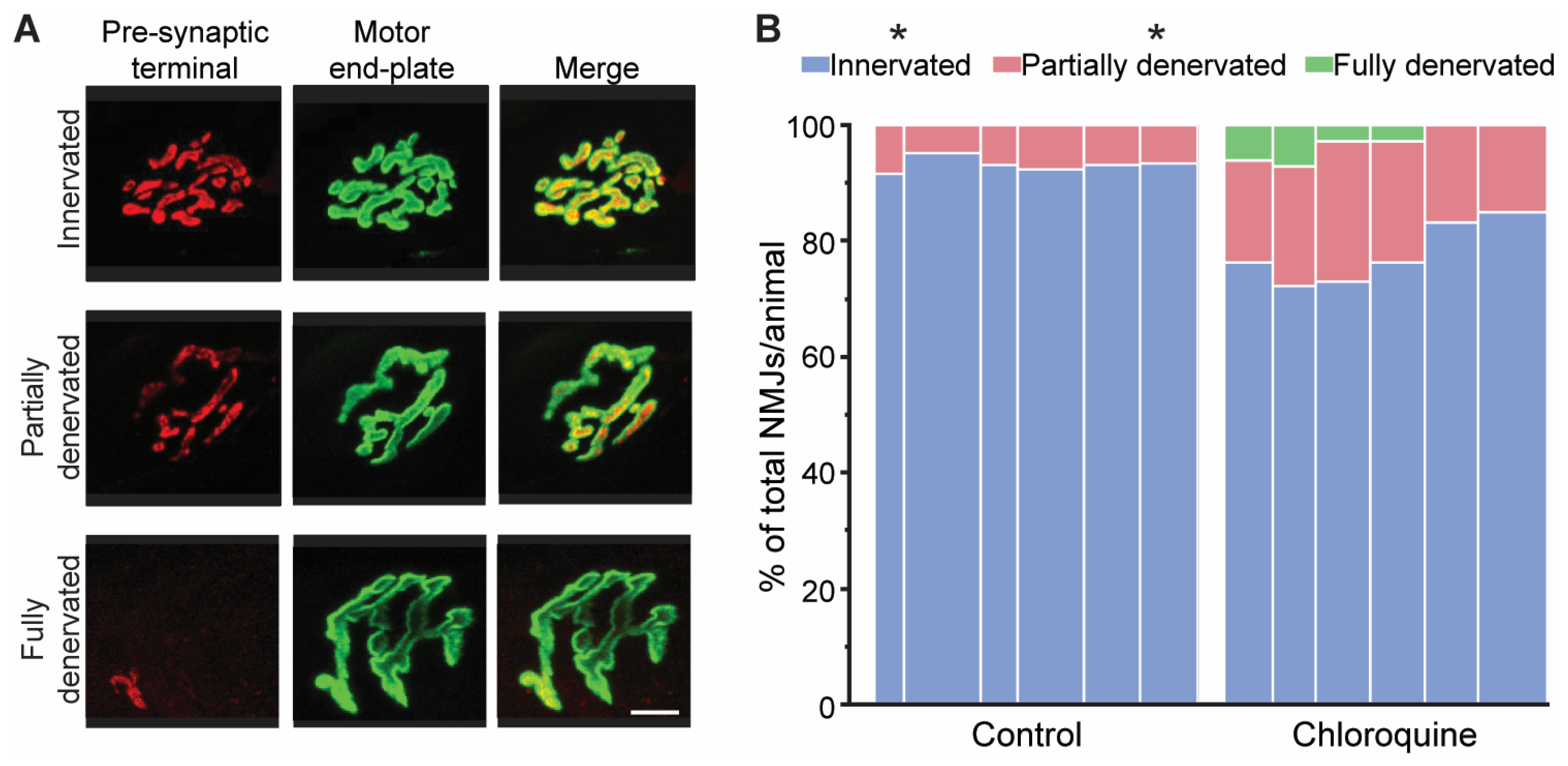
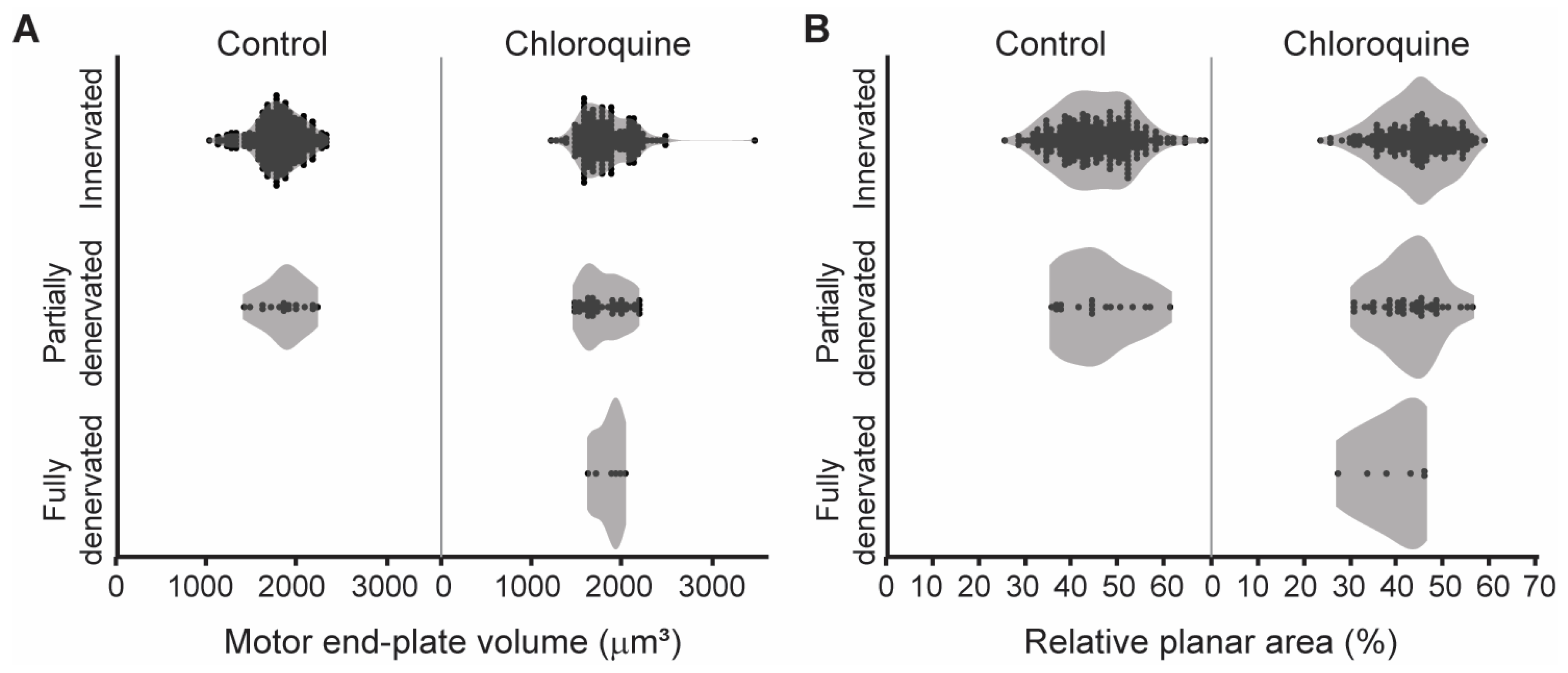
| Vehicle | Chloroquine | |||
|---|---|---|---|---|
| Male | Female | Male | Female | |
| NMJs analyzed | 136 | 134 | 106 | 109 |
| Age (mo) | 7.7 ± 0.6 | 6.7 ± 1.5 | 6.7 ± 0.6 | 7.3 ± 0.6 |
| Pre-treatment body mass (g) | 33.3 ± 2.9 | 22.6 ± 1.2 | 30.8 ± 3.4 | 22.8 ± 2.1 |
| Post-treatment body mass (g) | 32.3 ± 3.6 | 22.6 ± 0.9 | 30.6 ± 3.0 | 23.8 ± 2.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gulbronson, C.I.; Jahanian, S.; Gransee, H.M.; Sieck, G.C.; Mantilla, C.B. Chloroquine Causes Aging-like Changes in Diaphragm Neuromuscular Junction Morphology in Mice. Cells 2025, 14, 390. https://doi.org/10.3390/cells14060390
Gulbronson CI, Jahanian S, Gransee HM, Sieck GC, Mantilla CB. Chloroquine Causes Aging-like Changes in Diaphragm Neuromuscular Junction Morphology in Mice. Cells. 2025; 14(6):390. https://doi.org/10.3390/cells14060390
Chicago/Turabian StyleGulbronson, Chloe I., Sepideh Jahanian, Heather M. Gransee, Gary C. Sieck, and Carlos B. Mantilla. 2025. "Chloroquine Causes Aging-like Changes in Diaphragm Neuromuscular Junction Morphology in Mice" Cells 14, no. 6: 390. https://doi.org/10.3390/cells14060390
APA StyleGulbronson, C. I., Jahanian, S., Gransee, H. M., Sieck, G. C., & Mantilla, C. B. (2025). Chloroquine Causes Aging-like Changes in Diaphragm Neuromuscular Junction Morphology in Mice. Cells, 14(6), 390. https://doi.org/10.3390/cells14060390







