Abstract
Metabolic dysfunction leading to non-alcoholic fatty liver disease (NAFLD) exhibits distinct molecular and immune signatures that are influenced by factors like gut microbiota. The gut microbiome interacts with the liver via a bidirectional relationship with the gut–liver axis. Microbial metabolites, sirtuins, and immune responses are pivotal in different metabolic diseases. This extensive review explores the complex and multifaceted interrelationship between sirtuins and gut microbiota, highlighting their importance in health and disease, particularly metabolic dysfunction and hepatocellular carcinoma (HCC). Sirtuins (SIRTs), classified as a group of NAD+-dependent deacetylases, serve as crucial modulators of a wide spectrum of cellular functions, including metabolic pathways, the inflammatory response, and the process of senescence. Their subcellular localization and diverse functions link them to various health conditions, including NAFLD and cancer. Concurrently, the gut microbiota, comprising diverse microorganisms, significantly influences host metabolism and immune responses. Recent findings indicate that sirtuins modulate gut microbiota composition and function, while the microbiota can affect sirtuin activity. This bidirectional relationship is particularly relevant in metabolic disorders, where dysbiosis contributes to disease progression. The review highlights recent findings on the roles of specific sirtuins in maintaining gut health and their implications in metabolic dysfunction and HCC development. Understanding these interactions offers potential therapeutic avenues for managing diseases linked to metabolic dysregulation and liver pathology.
1. Background
Sirtuins (SIRTs), classified as class III histone deacetylases (HDACs), are crucial regulators of various cellular mechanisms, particularly those related to ageing, metabolism, and disease [1,2]. These NAD+-dependent enzymes play a key role in maintaining cellular homeostasis by deacetylating histones, transcription factors, and other proteins, thereby influencing gene expression and metabolic pathways. Research increasingly highlights the significance of sirtuins in health conditions ranging from metabolic dysfunction to cancer, particularly hepatocellular carcinoma (HCC) [3].
The gut microbiota, a complex community of microorganisms residing in the intestinal tract, significantly impacts host health [4]. Emerging evidence indicates that sirtuins and gut microbiota significantly influence metabolic health and disease outcomes [4]. Sirtuins enhance gut barrier integrity and modulate immune responses, while gut microbiota-derived metabolites can affect sirtuin activity, creating a dynamic feedback loop that influences overall health [5]. Understanding the interplay between sirtuins and gut microbiota is essential for studying non-alcoholic fatty liver disease (NAFLD) and its progression to HCC [3]. As NAFLD becomes more prevalent, it is vital to uncover its underlying biological mechanisms to enable the development of effective treatments [6].
Previous studies have underscored the vital role of gut microbiota in the onset of metabolic diseases, particularly non-alcoholic fatty liver disease (NAFLD) and hepatocellular carcinoma (HCC). Dysbiosis in gut microbiota is linked to these metabolic conditions. Additionally, different studies also highlight the association of sirtuins with different metabolic diseases.
This review addresses how sirtuins influence gut microbial diversity and composition, as well as their relationship with metabolic diseases. We also explore how microbial metabolites affect sirtuin activity. Furthermore, we propose future research directions to investigate sirtuin-microbiota interactions, paving the way for new strategies to prevent and treat metabolic disorders and HCC. Integrating sirtuin and gut microbiota research represents a novel approach in this field, offering potential pathways for targeted therapeutic interventions.
2. Overview of Sirtuins and Their Biological Functions
2.1. Sirtuins Classification and Subcellular Localization
Sirtuins (SIRTs), a family of class III histone deacetylases (HDACs), play crucial roles in various cellular processes and have significant implications for ageing and related diseases [1,2]. They facilitate NAD+-dependent deacetylation processes that connect them with metabolic control. In terms of structure, sirtuins possess an N-terminal, a C-terminal, and a Zinc-binding domain. The seven types of mammalian sirtuins (SIRT1-7) feature a conserved catalytic core made up of 275 amino acids, consisting of two bi-lobed globular domains with NAD+ acting as a cofactor. The unique lengths, compositions, and post-translational modifications (PTM) of the N- and C-terminal domains differ, influencing their localization and functionality [7].
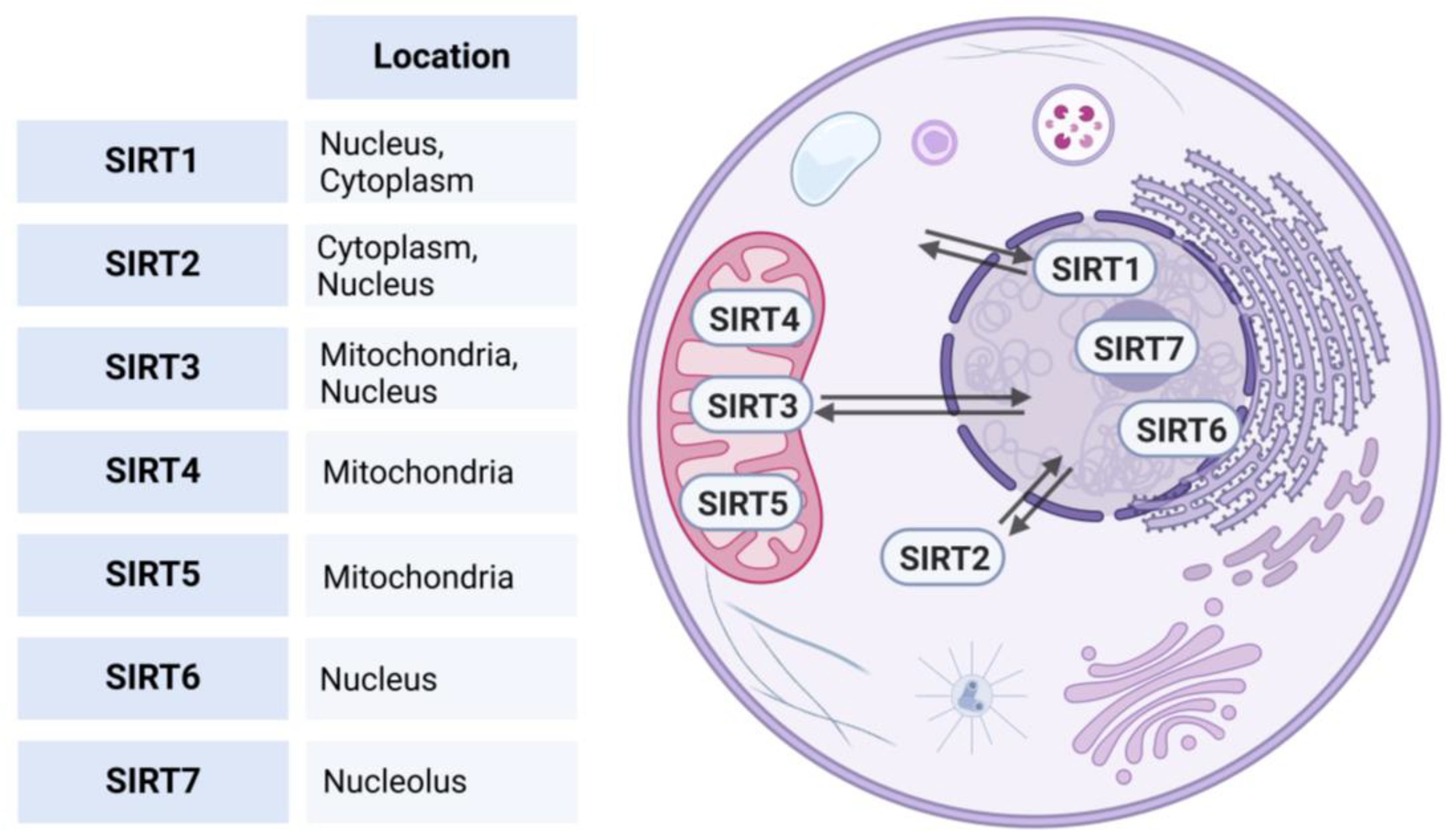
Phylogenetic analysis based on the amino acid sequence revealed four different classes of sirtuins. SIRT1-3 belong to Class I, SIRT-4 to Class II, SIRT 5 to Class III, SIRT6-7 to Class IV [7]. Each protein exhibits distinct subcellular compartmentalization [8]. SIRT1, 6, and 7 predominantly reside within the nucleus. However, SIRT1 can translocate to the cytoplasm under physiological or pathological stimuli [9]. SIRT2, on the other hand, is primarily situated within the cytoplasm but is capable of translocating to the nucleus under certain conditions to influence cellular processes [10]. Additionally, SIRT3 can also undergo relocation between the mitochondria and the nucleus, enabling it, to regulate a wide range of cellular processes [11], while SIRT 4, and 5 are typically located in the mitochondrial compartment (see Figure 1) [7,12].
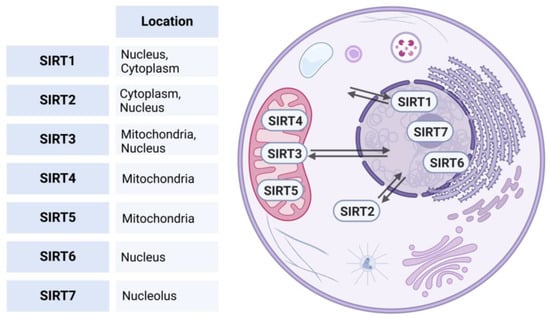
Figure 1.
Subcellular localizations of human sirtuins. This figure illustrates the subcellular localization of human sirtuins (SIRT1-7). SIRT1, 6, and 7 predominantly reside in the nucleus; SIRT1 can translocate to the cytoplasm under physiological or pathological stimuli. SIRT2 is primarily located in the cytoplasm but can translocate to the nucleus. SIRT3 is located in the mitochondria and is able to relocate between the mitochondria and nucleus, while SIRT4 and SIRT5 are typically found in the mitochondrial compartment. Created in BioRender. https://BioRender.com/r59u349 (accessed on 12 March 2025).
These dynamic subcellular distributions and trafficking abilities allow the sirtuins to coordinate their regulatory functions across different compartments of the cell [13]. SIRTs play a key regulatory role in metabolism, health-span, and longevity by deacetylating histones, transcriptional regulators, and other proteins, in addition to their ADP-ribosyltransferase and deacetylase activities [2,14].
2.2. Role of Sirtuins in Cellular Homeostasis and Metabolism
SIRTs are involved in post-translational modifications, particularly protein deacetylation, and contribute to gene expression regulation, DNA repair, and cellular senescence [15,16]. These enzymes enhance genomic stability through chromatin structural modulation and involvement in various DNA repair mechanisms, including base excision repair, nucleotide excision repair, and double-strand break repair [17,18]. SIRTs also serve a crucial role in the modification of histones and proteins, influencing cellular metabolism, mitochondrial function, and stem cell maintenance [19,20]. SIRT1, the most widely studied sirtuin, is recognised for its role in transcriptional regulation, genomic silencing, and epigenetic elements within the nucleus, while also contributing to metabolism and nutrient perception in the cytosol [21]. The involvement of SIRTs in ageing and cancer has been extensively studied, with different sirtuin family members showing diverse effects on stem cells and cancer cells [22,23,24].
SIRTs play vital roles in various physiological and pathological conditions, such as neurodegenerative diseases [25], kidney disorders [26], and cardiovascular diseases [8,27]. They are important for maintaining cellular integrity [28] and regulating metabolic balance, including glucose and lipid metabolism [29]. Additionally, they modulate mitochondrial activity and have been associated with various metabolic disorders [8]. SIRTs also contribute to the regulation of female reproductive processes [30] and influence women’s health, particularly in ovarian function and cancer development [31]. In the immune system, SIRTs modulate T cell metabolism and function, making them promising therapeutic targets for immune-related diseases [32]. Furthermore, SIRTs have been implicated in counteracting several hallmarks of ageing, potentially contributing to healthy longevity [33].
Different investigations underscore the crucial and multifaceted functions of sirtuins in neoplastic advancement, acting as tumour suppressors as well as activators of tumourigenesis, depending on the specific cellular environment [34,35]. SIRTs regulate various cancer-related processes, including cell viability, apoptosis, metastasis, and metabolism [34,36]. Among all SIRTs, SIRT1 has been extensively researched for its dual role in cancer, contributing to tumour suppression and promotion [21,37]. SIRT2′s role remains controversial, with evidence suggesting it can act as either an oncogene or a tumour suppressor across multiple malignancies [38]. The roles of other sirtuins (SIRT3-7) vary across different cancer types, influencing processes such as proliferation, invasion, and chemoresistance [39,40,41]. Furthermore, SIRTs play crucial roles in hepatocellular carcinoma (HCC) development and progression [3,42]. SIRT1 is overexpressed in HCC, promoting oncogenesis and multidrug resistance [43]. Collectively, these findings emphasise sirtuins’ vital role in cellular homeostasis and disease management, particularly in cancer, necessitating further investigation into their specific functions and therapeutic implications. (see Table 1).

Table 1.
Classification and functional roles of sirtuins in metabolism and cellular processes.
3. The Role of Gut Microbiota in Host Health and Disease
The gut microbiota includes a variety of microorganisms, such as bacteria, archaea, fungi, viruses, and parasites [69,70,71]. These microorganisms, inhabit within the gastrointestinal tract—particularly in the intestinal—and are commonly designated as gut flora or gut microbiota [72]. While bacteria dominate the gut microbiome, the significance of other microbes—often referred to as the “dark matter” of microbiomes—has become increasingly recognised [70]. The predominant bacterial phyla present within the human gut microbiome include Firmicutes, Bacteroidetes, Proteobacteria, Actinobacteria, and Fusobacteria [73]. Furthermore, this complex community plays essential roles in host physiology, metabolism, and immune function [74,75]. The gut microbiota helps maintain intestinal integrity through the production of microbial metabolites and anti-microbial peptides modulating the immunity [76]. Additionally, it serves as a potential source of novel antimicrobials, which could help address antimicrobial resistance [77]. Moreover, the gut microbiota contributes significantly to digestion by breaking down indigestible dietary components and producing essential nutrients like vitamins and enzymes [78]. Furthermore, the microbiota plays a critical role in modulating inflammation, cancer-related processes, and oxidative stress through the production of metabolites, especially short-chain fatty acids (SCFAs) and tryptophan catabolites [79,80,81,82].
Gut microbiota are essential for the fermentation of non-digestible carbohydrates, yielding SCFAs such as acetate, propionate, and butyrate [83,84]. These SCFAs serve as energy sources for colonocytes and maintain gut barrier integrity [85,86]. Moreover, SCFAs influence host metabolic processes and immune responses via diverse mechanisms, such as the stimulation of G-protein-coupled receptors and the suppression of histone deacetylases [87]. The gut microbiota is also participates in bile acid metabolism, which is essential for lipid digestion and absorption [88]. Consequently, microbial SCFA production and bile acid metabolism significantly impact host health via intricate interactions with the gut epithelium, immune response, and metabolic pathways [78,89]. Grasping these mechanisms is essential for developing approaches to enhance gut health and avoid metabolic diseases.
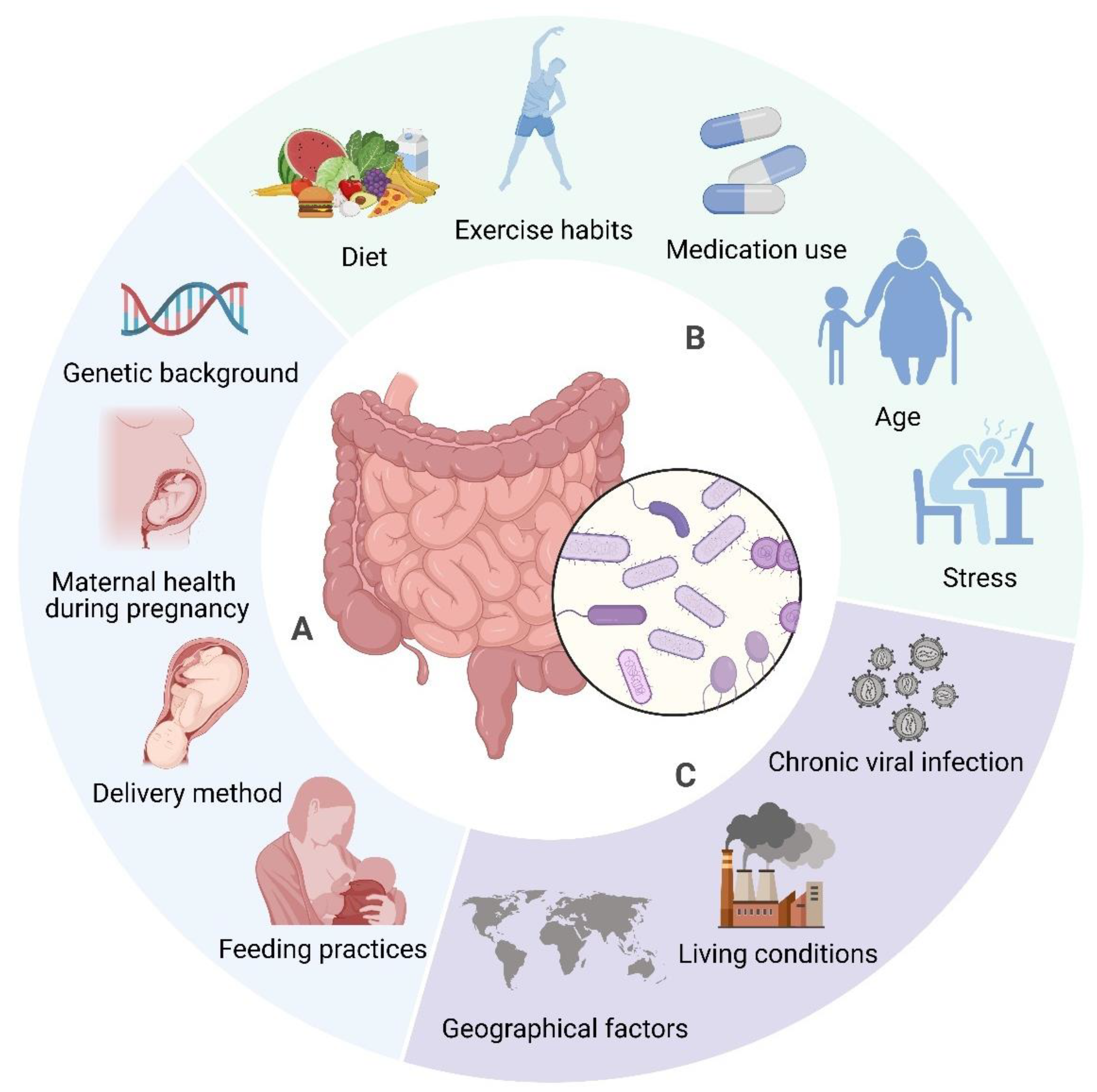
The composition of gut microbiota is modulated by factors including diet, genetics, and environmental conditions [90,91,92]. Diet significantly influences the microbiome, alongside seasonal and geographical factors impacting dietary habits and microbial composition [93]. Environmental determinants, such as communal residences, exert a more significant influence on microbiome composition than host genetics [94]. Nevertheless, host genetics continue to exert an influence in ascertaining microbiome composition, especially via immune system-related genes [95]. Early life factors, including birth mode, feeding methods, and antibiotic use, also contribute to microbiota development [96]. The gut microbiome is dynamic, changing throughout an individual’s lifetime due to factors like age, BMI, exercise, and lifestyle (see Figure 2) [92]. Recognising these influences is important for maintaining a healthy microbiome and preventing dysbiosis, which has been linked to several diseases [91,97].
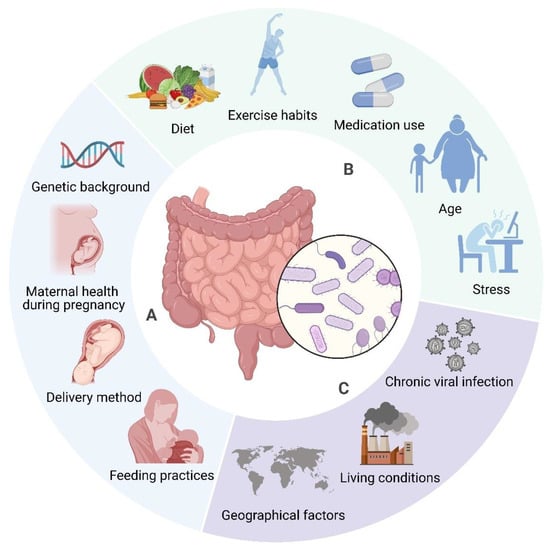
Figure 2.
Determinants of Gut Microbiome Composition. The composition of gut microbiota is affected by a wide range of factors. Some elements, such as genetic background (e.g., specific gene variants), delivery method (vaginal vs. caesarean), early infant feeding practises (breastfeeding vs. formula feeding), and maternal health during pregnancy (e.g., gestational diabetes, infections) are established early in life and tend to remain stable (A). In contrast, factors like diet (e.g., high fibre vs. high fat), medication use (antibiotics), exercise habits, age, and stress levels are more variable and can be modified throughout life (B). Furthermore, environmental influences, including chronic viral infections (e.g., Epstein–Barr virus), living conditions (urban vs. rural), and geographical factors (climate, culture), also contribute to microbiome composition, although these may be more challenging to alter (C). Created in BioRender. https://BioRender.com/r59u349 (accessed on 12 March 2025).
Dysbiosis, characterised by a perturbation in the assemblage of gastrointestinal microbiota, has been associated with numerous pathological states, encompassing inflammatory bowel disease (IBD), cardiovascular diseases, diabetes mellitus, obesity, and various neurological disorders [98,99,100,101,102]. This dysbiosis frequently presents as a diminution in microbial variety alongside an elevation in particular bacterial taxa [101]. Factors contributing to dysbiosis include antibiotic use, diet, and environmental stressors [100,101]. Dysbiosis can lead to altered microbial metabolite production, immune dysregulation, and chronic inflammation [103]. Therapeutic modalities aimed at addressing dysbiosis encompass faecal microbiota transplantation, the administration of probiotics, the utilisation of prebiotics, as well as various dietary interventions [104,105].
4. Interrelationship Between Sirtuins and Gut Microbiota: A Bidirectional Perspective
4.1. Influence of Sirtuins on Gut Microbiota Composition
Sirtuins are essential in gut function regulation, particularly in preserving the intestinal barrier and mucosal immune mechanism, which are vital for regulating intestinal microbiota composition [106].
4.1.1. SIRT1
Intestinal epithelial SIRT1 regulates gut microbial composition consequently preventing age-related intestinal inflammation [107]. The gut microbiome remotely regulates the expression of miR-204 subsequently impairing the endothelial function by targeting Sirt1 [108]. SIRT1 deficiency in the intestinal epithelium leads to increased faecal bile acid levels, reduced Lactobacillus abundance, and heightened susceptibility to intestinal inflammation and colitis [109]. Moreover, the gut microbiome modulates systemic hydrogen sulphide levels impacting SIRT1 activation followed by the regulation of neuroinflammation [110].
4.1.2. SIRT2
Similarly, SIRT2 deficiency enhances the progression of NAFLD by altering gut microbiota composition and inducing metabolic disorders [6], whereas inhibiting SIRT2 may improve gut barrier integrity and protect against colitis [111]. SIRT2 knockout mouse displayed increased susceptibility to obesity, liver injury, and metabolic dysfunction when placed on high fat/high sucrose/high cholesterol diet [6].
4.1.3. SIRT3
SIRT3 and the gut microbiota interaction leads to modulation of key cellular processes like mitochondrial function, inflammation, and energy metabolism [112]. SIRT3 deficiency promotes NAFLD progression through gut microbial dysbiosis and impaired intestinal permeability [113]. Moreover, the gut microbiota modulates hydrogen sulphide levels consequently affecting SIRT3 activation [110].
4.1.4. SIRT4
Mitochondrial SIRT4 regulates intestinal metabolism and homeostasis. It regulates lysozyme expression in the gut, influencing microbiota composition [114]. Loss of SIRT4 can lead to dysregulated glutamine and nucleotide metabolism in intestinal adenomas [115].
4.1.5. SIRT5-7
While the roles of SIRT5, SIRT6, and SIRT7 in gut microbiota regulation are not directly established, it is notable that downregulation of SIRT5 has been linked to increased bile acid production, which may contribute to an immunosuppressive tumour microenvironment and facilitate hepatocellular carcinoma (HCC) development [116]. Moreover, SIRT6 knockout mice exhibit premature ageing associated with gut dysbiosis, a condition reversible through faecal microbiota transplantation or a high-fat diet [117]. It is noteworthy that the presence of an inflammatory response of the colorectal mucosa is associated with higher concentrations of SIRT7 and lower concentrations of SIRT1 [118].
Together, these roles underscore the importance of sirtuins in gut health and their potential influence on microbiota dynamics, highlighting areas for future research (see Table 2).

Table 2.
Roles of sirtuins in gut health: impact on barrier integrity, inflammation, and microbial diversity.
4.2. Impact of Gut Microbiota on Sirtuin Activity
Conversely, gut microbiota also influences sirtuin activity and expression. Studies indicate that gut microbiota can regulate the expression of sirtuins, along with senescence-regulating miRNAs and mitochondrial DNA, all associated with overall well-being [131]. Moreover, gut microbiota interacts with sirtuin-activating compounds to influence molecular pathways that counteract ageing and inflammation, while enhancing specific gut microbiota groups to improve immune function [132].
Studies have elucidated that gastrointestinal microbiota may influence critical transcriptional co-activators, transcription factors, and enzymatic pathways pertinent to mitochondrial biogenesis, encompassing genes such as PGC-1α, SIRT1, and AMPK [133]. Additionally, the gut microbiome regulates vascular microRNA-204, which targets SIRT1 and affects endothelial function [108]. Furthermore, metabolites produced by gut microbiota from fermenting indigestible food components can impact sirtuin function [134]. For instance, gut microbiota-derived metabolites have anti-inflammatory and antioxidative properties that influence sirtuin activity [135]. Particularly, Urolithin A (UroA), a microbial metabolite derived from polyphenolics, exemplifies this by exhibiting anti-inflammatory and antioxidative effects [135]. These properties of microbial metabolites can directly affect sirtuin function, as sirtuins are involved in regulating oxidative stress and inflammation [136]. By modulating these pathways, gut microbiota-derived metabolites can indirectly affect sirtuin activity and contribute to overall host health.
Additionally, the gut microbiome possesses the capacity to affect sirtuin-associated pathways; for example, Saccharomyces boulardii has been demonstrated to alter necrotizing enterocolitis through the modulation of the SIRT1/NF-κB signalling pathway and the intestinal microbiota [137]. The overall outcomes shed light on the intricate relationship between sirtuins and gut microbiota, stressing the requirement for more extensive investigation to clarify current shortcomings in understanding their roles and interconnections.
5. Role of Sirtuins and Gut Microbiota in Non-Alcoholic Fatty Liver Disease (NAFLD)
NAFLD represents the most widespread form of chronic hepatic disorder globally and is projected to emerge as the primary etiology for liver transplantation by the year 2030 [138]. Furthermore, NAFLD is distinguished by an atypical aggregation of hepatic lipids, concomitant with insulin resistance, and demonstrable steatosis, while systematically ruling out secondary etiologies of hepatic steatosis, such as alcohol consumption [139]. Additionally, NAFLD comprises a spectrum of disorders extending from hepatic steatosis to steatohepatitis, leading to inflammation, hepatocirrhosis, hepatocellular carcinoma, and mortality [140]. Also, it is closely associated with insulin resistance and may function as both a cause and a result of metabolic syndrome [141].
Formerly, the “two-hit” hypothesis previously explained NAFLD pathogenesis, identifying lipid accumulation as the “first hit”. This initial accumulation of lipid predisposes the liver to further injury, termed the “second hit”, resulting in inflammation and fibrosis [142]. Recent research proposes the multiple-hit hypothesis, which more comprehensively describes the molecular and metabolic alterations in NAFLD. This updated framework includes interconnected mechanisms, such as insulin resistance, lipotoxicity, innate immune activation, and gut microbiome effects, influenced by genetic (PNPLA3) and dietary elements (saturated fat and fructose) [142].
The interplay between sirtuins and intestinal microbiota is a complex relationship. The gut microbiota plays a crucial role in host metabolism by breaking down nutrients and producing metabolites that influence metabolic processes and modulate immunity [143]. Sirtuins are recognised for their ability to govern the intestinal microbiome, thereby suggesting their participation in the pathophysiological mechanisms underlying a variety of diseases. They are integral to numerous physiological processes, encompassing glucose and lipid metabolism, insulin resistance, and mitochondrial function, thereby rendering them pivotal factors in the etiology of conditions such as type 2 diabetes, obesity, and NAFLD [107,144,145].
Furthermore, sirtuins are influenced essential metabolic hormones such as leptin, ghrelin, melatonin, insulin, and serotonin, which perform a crucial function in the regulation of gastrointestinal homeostasis [146]. Ghrelin, leptin, and insulin also play a critical role in neural plasticity and cognition [147]. SIRT1 has been documented to be associated with metabolic homeostasis melatonin activates SIRT1 in order to combat mitochondrial dysfunction, inflammation, and oxidative stress [148,149]. Ghrelin and ghrelin potentiator treatment can not only increase SIRT1 expression at the protein level but also its activity [150]. Leptin promotes the expression of SIRT1 by activating Nrf2, which may play a role in colon cancer development [151]. Additionally, leptin reduces the expression of BACE1 and the production of amyloid-β by triggering the SIRT1 signalling pathway, thereby diminishing NF-κB-driven transcription of BACE1 [152]. There is a connection between age-related weight gain and leptin resistance due to lower levels of SIRT1 in the hypothalamus. Preserving SIRT1 activity in the hypothalamus could enhance leptin sensitivity and help counteract weight gain associated with ageing [153]. Moreover, the gut bacteria also tend to influence the level of peptides like leptin, ghrelin, and melatonin [154].
Recent studies indicate a significant reduction in sirtuin levels in NAFLD patients. Wu et al. found decreased expression of SIRT1, SIRT3, SIRT5, and SIRT6 in NAFLD patients, alongside increased lipogenic gene expression and SIRT4 [155]. Furthermore, Bruce et al. demonstrated that excess dietary fat exposure during early and postnatal periods elevates NASH risk in adulthood, particularly affecting sirtuin levels. Offspring on a high-fat diet exhibited NAFLD, with those from high-fat diet mothers developing NASH, characterised by decreased NAD+/NADH and lower SIRT1 and SIRT3 levels, coupled with increased lipid metabolism gene expression [156].
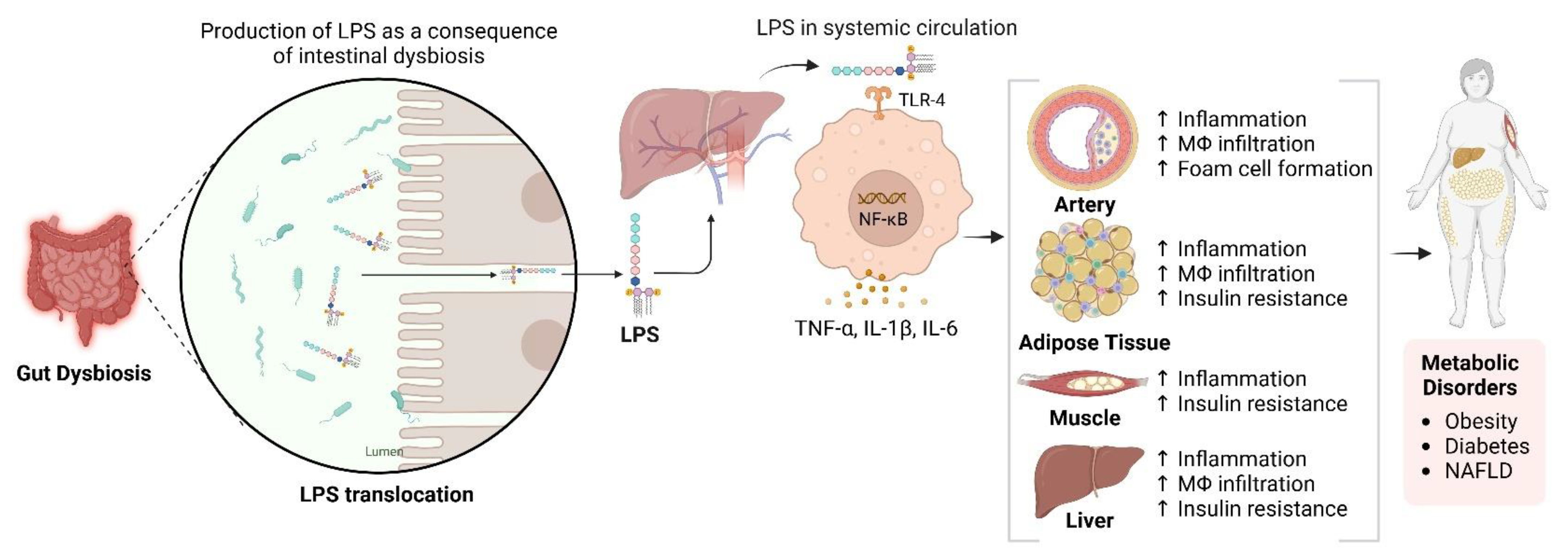
The gut microbiota is essential for NAFLD development through dietary metabolism, yielding vital nutrients and energy [157,158,159]. Its composition is diet-dependent, with high-fat/high-cholesterol (HFC) and high-fat/high-sucrose (HFS) diets causing dysbiosis linked to NAFLD [160,161,162]. This dysbiosis increases intestinal permeability, exposing the liver to bacterial products through the portal vein and inducing metabolic endotoxemia, thereby disrupting the gut-liver axis [163,164]. NAFLD microbiota differs from that of healthy individuals and is influenced by genetic factors related to metabolic syndrome 170]. Additionally, gastrointestinal microbiomes can synthesise lipopolysaccharides (LPS), which may infiltrate the circulatory system and impair the hepatic tissue when the intestinal barrier is compromised (see Figure 3) 171]. In contrast, SCFAs such as butyrate are crucial for maintaining gut barrier integrity. Zhou et al. demonstrated that sodium butyrate enhances gut microbiota and fortifies the intestinal barrier, mitigating LPS translocation and reducing steatohepatitis in mice [165]. This underscores the significance of a robust gut barrier.
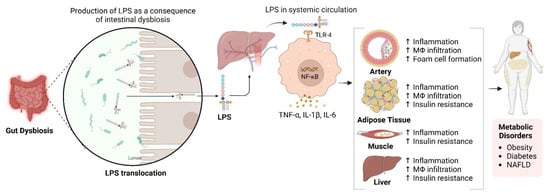
Figure 3.
Impact of Gut Dysbiosis on Inflammation and Metabolic Dysfunction. This figure illustrates the process following gut dysbiosis, which leads to the release of lipopolysaccharides (LPS). These LPS molecules enter the systemic circulation via the portal vein, activating macrophages through Toll-like receptor 4 (TLR4). This activation results in the release of pro-inflammatory cytokines, including TNF-α, IL-1β, and IL-6, contributing to increased inflammation and hepatic insulin resistance. Additionally, activated macrophages infiltrate adipose tissue, exacerbating inflammation and insulin resistance, thereby contributing to metabolic disorders such as obesity, diabetes, and non-alcoholic fatty liver disease (NAFLD). The diagram also indicates the systemic effects of inflammation on various tissues, including the arteries, adipose tissue, muscle, and liver. Created in BioRender. https://BioRender.com/r59u349 (accessed on 12 March 2025).
Chen et al. demonstrated that SIRT3 deficiency worsens NAFLD from a high-fat diet through impaired intestinal permeability linked to gut microbial dysbiosis in SIRT3 knockout mice. The SIRT3KO mice exhibited increased Oscillibacter, Parabacteroides, and Mucispirillum, while Alloprevotella decreased [113]. Prior studies have shown a negative correlation between Oscillibacter levels and the mRNA expression of zonula occludens-1, a protein relevant to gut permeability [166,167,168]. Furthermore, Everard et al. noted increased Mucispirillum and Parabacteroides in humans and mice on a high-fat diet [169]. The elevation of Oscillibacter, Mucispirillum, and Parabacteroides correlates with exacerbated HFD-induced NAFLD in SIRT3KO mice, characterised by diminished tight junction protein expression like ZO-1 and claudins [113]. Thus, SIRT3-mediated intestinal barrier dysfunction, coupled with LPS release from gut microbiome alterations, facilitates NAFLD progression.
6. Role of Sirtuins and Gut Microbiota in Hepatocellular Carcinoma (HCC)
Liver cancer represents a considerable global health issue, with rising rates primarily attributed to hepatocellular carcinoma (HCC), which constitutes about 90% of cases [170,171]. Significant risk factors for HCC development include chronic hepatitis B and C virus infections. Non-viral factors involve environmental carcinogens such as aflatoxin B1, alcohol misuse, and genetic conditions like hemochromatosis and Wilson disease [172]. Importantly, non-alcoholic steatohepatitis (NASH) has emerged as a critical criterion for liver transplantation among HCC patients in the United States [173]. The transition from NAFLD to HCC entails a multifaceted interaction of metabolic variables, inflammation, gut microbiota imbalance, oxidative stress, and aberrant lipid metabolism [174,175,176]. Dysbiosis, has been implicated in the pathogenesis of HCC and its related chronic diseases, which encompass chronic hepatitis B and C, alcoholic liver disease, NAFLD, and NASH [177].
Sirtuins play a complex role in cancer development, including HCC. Their dual nature in cancer biology is evident, as they can function as both promoters and suppressors of tumours, depending on the cellular context and the specific sirtuin involved. This complexity is particularly significant in relation to DNA repair, genomic stability, cell cycle regulation, apoptosis, metabolism, and the oxidative stress response [34,178].
SIRT1 has a critical role in HCC by virtue of promoting tumourigenicity, metastasis, chemoresistance, and heralding a poor prognosis [43,179,180]. The interplay between SIRT1 and intestinal microbiota is complex. Butyrate, a short-chain fatty acid from gut bacteria, is crucial in this relationship. A decrease in butyrate-producing bacteria may harm the intestinal mucosa, possibly leading to HCC development [181]. Pant et al. illustrated that butyrate induces microRNA-22 (miR-22), which downregulates SIRT1 in a concentration-dependent manner, increasing reactive oxygen species (ROS) and apoptosis in hepatic cells exposed to sodium butyrate [182]. MiR-22′s downregulation in human liver cancer cells correlates with enhanced tumourigenicity and cellular proliferation [183]. It is implicated in HCC development via the miR-29a-SIRT1-WNT/β-catenin pathway [184]. Furthermore, overexpression of SIRT1 promotes HCC proliferation and resistance to chemotherapy by promoting autophagy [185,186]. Moreover, SIRT1 protects mitochondrial function of HCC cells by suppressing the expression of hypoxia-induced factor-1 alpha expression and also promotes stem-cell like features in HCC cells [187].
SIRT1 also promotes HCC metastasis by enhancing PGC-1α-mediated mitochondrial biogenesis [188]. Conversely, Herranz et al. reported that SIRT1 overexpression can protect transgenic animals from diethylnitrosamine/high-fat diet-induced liver cancer by mitigating NF-kB-mediated inflammation and averting malignant transformation [189]. This observation contrasts with earlier assertions of SIRT1′s role in promoting HCC and other studies indicating elevated SIRT1 in human HCC samples [190,191]. Malignant cells may utilise survival strategies typically reserved for non-malignant cells [192]. Portmann et al. have demonstrated that inhibiting SIRT1 leads to impaired tumour growth both in vivo and in vitro and this supports the notion that SIRT1 activity in healthy hepatocytes protects against cancer, but after transformation, SIRT1 becomes a protective force for the tumour cells as a survival advantage [193]. However, additional research is essential to elucidate the intricate relationship between butyrate and SIRT1 in the etiology of HCC.
SIRT2 mediates the deacetylation and activation of protein kinase B, impacting the glycogen synthase kinase-3β/β-catenin signalling pathway, which is implicated in epithelial–mesenchymal transition (EMT) [194]. Huang et al. support the tumour-promoting role of SIRT2, revealing that its downregulation hinders energy metabolism and invasion in HCC cells [195]. Further investigation is warranted to elucidate SIRT2′s specific functions in HCC.
SIRT3 plays the role of a tumour suppressor in HCC. SIRT3 is downregulated in HCC tumour tissue compared to adjacent non-cancerous tissues [196,197]. Overexpression of SIRT3 decreases HCC cell proliferation and promotes apoptosis through multiple mechanisms, including the activation of the GSK-3β/Bax signalling pathway [196], upregulation of p53 [198], and reduction in Mdm2-mediated p53 degradation [199]. SIRT3 also decreases GSTP1 levels and activates the JNK signalling pathway [200], which further increases the efficacy of chemotherapeutic agents on HCC cells.
SIRT4 and SIRT5 also play significant roles in HCC. SIRT4 depletion promotes HCC tumour development through the AMPKα/mTOR pathway [201], while overexpression of SIRT4 induces G2/M cell cycle arrest and apoptosis, leading to tumour suppression [202]. SIRT5, on the other hand, is associated with the progression of HCC through its effects on mitochondrial apoptosis pathways [203]. It also promotes HCC cell proliferation and invasion by targeting E2F1 [204].
SIRT6 plays a complex role in HCC. While some studies suggest SIRT6 acts as a tumour promoter by preventing DNA damage and cellular senescence [205], others indicate that it suppresses tumour growth by inhibiting the ERK1/2 pathway [206]. SIRT6 overexpression has been associated with increased HCC cell proliferation and apoptosis evasion [207,208]. However, SIRT6 deficiency in knockout (HKO) mice enhances liver tumourigenesis via ERK1/2 pathway activation. Overexpression of SIRT6, particularly in the HuH7 liver cancer cell line and xenograft mouse model, inhibits tumour growth, suggesting its potential as a therapeutic target for HCC [209]. The contradictory findings highlight the need for further research to clarify SIRT6′s role in HCC and its potential as a therapeutic target [3].
SIRT7 plays a multifaceted role in HCC, with its upregulation noted in numerous patients [210]. It facilitates HCC cell proliferation through ERK1/2 phosphorylation and activation of the RAF/MEK/ERK signalling cascade, promoting tumour growth [211]. Moreover, SIRT7 boosts HCC cell proliferation by inhibiting MST1 and modulating the Hippo/YAP pathway, resulting in enhanced YAP activation [212].
In summary, the interplay between sirtuins and gut microbiota is essential for elucidating the pathogenesis of NAFLD and HCC. Table 3 highlights the microbial changes linked to these diseases, indicating opportunities for specific therapeutic interventions.
Therapeutic approaches aimed at the gut microbiome, including prebiotics, probiotics, and faecal microbiota transplant, appear to hold potential for addressing metabolic conditions and age-related diseases [213]. However, challenges remain in translating research findings into effective clinical applications due to the complex nature of host-microbiota interactions and methodological concerns [214]. Additional research is required to clarify specific mechanisms and to create targeted treatments for different health issues. Subsequent investigations should aim to utilise these findings to formulate of microbiota-targeted therapies to improve disease management and patient prognosis.

Table 3.
Microbiota composition changes in NAFLD and HCC: A summary of human studies.
Table 3.
Microbiota composition changes in NAFLD and HCC: A summary of human studies.
| Disease | Composition Change | References | |
|---|---|---|---|
| Increase | Decrease | ||
| NAFLD | Streptococcus, Megasphaera, Enterobacteriaceae, Streptococcus, Gallibacterium | Bacillus and Lactococcus, Pseudomonas, Faecalibacterium prausnitzii, Catenibacterium, Rikenellaceae, Mogibacterium, Peptostreptococcaceae | [215] |
| Firmicutes (Streptococcus mitis and Roseburia inulinivorans) and Bacteroidetes (Barnesiella intestinihominis and Bacteroides uniformis) | Bacteroidetes (Prevotella sp. CAG 520, Prevotella sp. AM42 24, Butyricimonas virosa, and Odoribacter splanchnicus), Proteobacteria (Escherichia coli), Lentisphaerae (Victivallis vadensis), and Firmicutes (Holdemanella biformis, Dorea longicatena, Allisonella histaminiformans, and Blautia obeum) | [216] | |
| Bacteroidetes, Proteobacteria, Bacteroides, Alistipes, Verrucomicrobia, Faecalibaculum, Helicobacter, Epsilonbacteraeota | Muribaculaceae, Lactobacillus | [217] | |
| HCC | Escherichia coli | [218] | |
| Proteobacteria, Desulfococcus, Enterobacter, Prevotella, Veillonella | Cetobacterium | [219] | |
| Bacteroides | Akkermansia, Bifidobacterium | [220] | |
| Neisseria, Enterobacteriaceae, Veillonella, Limnobacter | Enterococcus, Phyllobacterium, lostridium, Ruminococcus, Coprococcus | [221] | |
| Proteobacteria, Enterobacteriaceae, Bacteroides xylanisolvens, B. caecimuris, Ruminococcus gnavus, Clostridium bolteae, Veillonella parvula | Erysipelotrichaceae, Oscillospiraceae | [222] | |
| Klebsiella, Haemophilus | Alistipes, Phascolarctobacterium, Ruminococcus | [181] | |
7. Interventions Targeting Sirtuins and Gut Microbiota
SIRTs are pivotal in linking health and disease, highlighting their potential for targeting interventions [223]. The healthcare sector increasingly recognises the therapeutic value of manipulating the sirtuin pathway and gut microbiome in disease management [146]. SIRT1 and SIRT2 have emerged as promising targets for therapeutic interventions in various diseases associated with gut dysbiosis, including cancer, neurodegenerative disorders, and metabolic conditions [6,146,224]. However, studies demonstrating the pharmacological utility of modifying other sirtuins in diseases where gut dysbiosis contributes to the underlying mechanisms remain limited.
Current strategies for modulating sirtuin activity in the treatment of hepatocellular carcinoma (HCC) and non-alcoholic fatty liver disease (NAFLD) focus on the activation and inhibition of specific sirtuins, particularly SIRT1 and SIRT2, respectively. These approaches aim to exploit the complex roles of sirtuins in HCC progression, chemoresistance, and the underlying mechanisms of NAFLD. Various strategies, including pharmacological agents, gene editing, and small molecule inhibitors, are being explored to enhance or inhibit sirtuin functions, making this modulation a promising avenue for cancer therapy [3,225].
7.1. Sirtuin Activators
7.1.1. Resveratrol
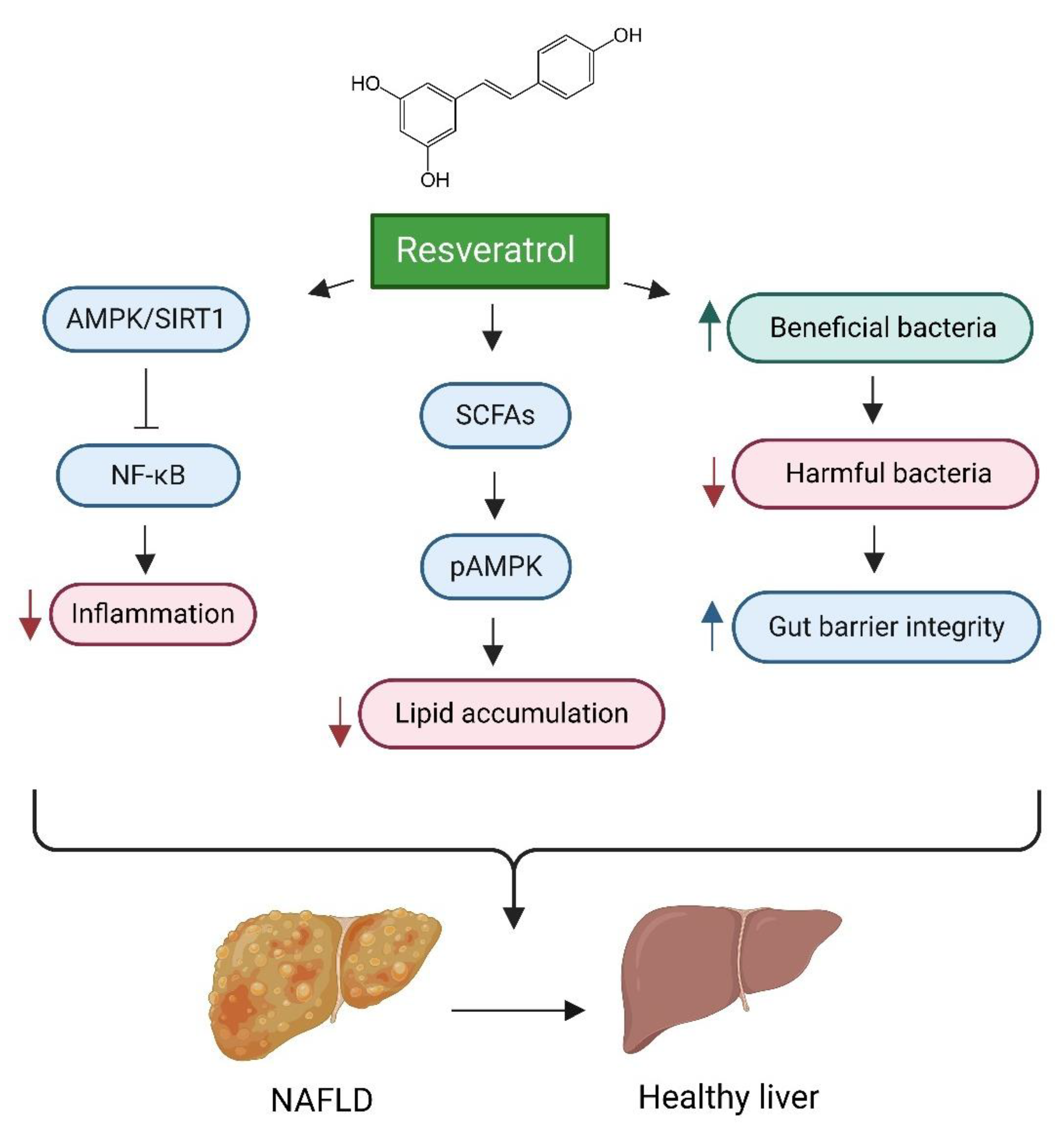
One prominent example of therapeutic intervention is the targeted activation of the SIRT1 pathway [226]. Resveratrol, a polyphenolic compound, has demonstrated efficacy in mitigating the progression of NAFLD in both preclinical and clinical contexts [227]. Resveratrol enhances gut microbiota by promoting the proliferation of beneficial bacteria, thereby improving gut health. This effect is characterised by a decrease in harmful bacteria and an increase in short-chain fatty acid (SCFA)-producing bacteria, which contributes to overall gut microbiota balance and metabolic health [228]. Furthermore, resveratrol alleviates NAFLD by strengthening gut barrier integrity, reducing inflammation, and increasing the production of short-chain fatty acids (SCFAs) [229,230]. Additionally, it exhibits potential protective effects against HCC through the modulation of inflammatory, angiogenic, and oxidative stress pathways [231]. Resveratrol’s proposed mechanism of action involves the modulation of hepatic lipid metabolism and the reduction in oxidative stress, primarily through the activation of the AMPKα/SIRT1 signalling pathway. This activation effectively suppresses the nuclear factor kappa B (NF-κB) inflammatory pathway, resulting in decreased inflammation and reduced hepatic steatosis [232,233]. (see Figure 4).
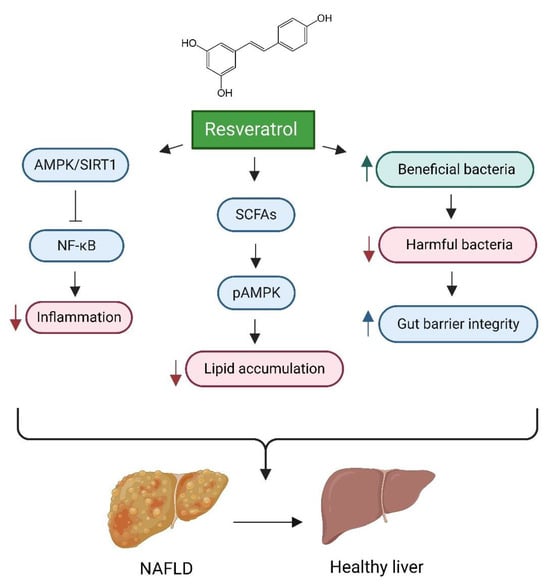
Figure 4.
Impact of Resveratrol on Gut Health and Liver Disease. This figure illustrates the multifaceted mechanisms through which resveratrol mitigates the progression of non-alcoholic fatty liver disease (NAFLD) and improves liver health. Key processes include the enhancement of gut microbiota by promoting beneficial bacterial proliferation, which strengthens gut barrier integrity and overall gut health. Additionally, resveratrol activates the AMPKα/SIRT1 signalling pathway, suppressing the NF-κB inflammatory pathway and leading to decreased inflammation and hepatic steatosis. Furthermore, resveratrol influences hepatic lipid metabolism and reduces lipid accumulation by increasing the production of short-chain fatty acids (SCFAs), which enhances AMPK activation and contributes to decreased lipid accumulation. Created in BioRender. https://BioRender.com/r59u349 (accessed on 12 March 2025).
7.1.2. Pterostilbene
Pterostilbene, a dimethyl ether variant of resveratrol, exhibits encouraging effects against NAFLD and obesity. It diminishes liver fat accumulation by influencing the miR-34a/Sirt1/SREBP-1 pathway in rats fed a fructose diet [234]. Compared to resveratrol, pterostilbene has enhanced bioavailability and metabolic stability [235]. In rats consuming a high-calorie diet, pterostilbene lowers adipose tissue volume, inhibits lipogenesis in fat tissue, and promotes fatty acid oxidation in the liver [236]. From a metabolic perspective, pterostilbene displays greater stability than resveratrol and often shows more potent pharmacological effects [237]. Taken together, these studies indicate that pterostilbene is a promising candidate for addressing NAFLD and obesity, with potential benefits over resveratrol due to its superior bioavailability and metabolic characteristics.
7.1.3. E1231
E1231, treatment activates SIRT1 alleviating NAFLD by regulating lipid metabolism. Moreover, E1231 prevented lipid accumulation and improved mitochondrial function in free fatty acid challenged hepatocytes. E1231 prevented liver injury via regulation of SIRT1 and AMPK-α pathway [238].
7.1.4. Quercetin
Quercetin, a natural flavonoid present in plants, has demonstrated encouraging effects in the treatment of NAFLD. Laboratory studies indicate that quercetin diminishes lipid buildup, lowers inflammatory cytokines, and boosts antioxidant activity in liver cells [239]. Animal studies show that quercetin helps improve NAFLD by triggering AMPK-mediated mitophagy, reducing lipid storage, and alleviating oxidative stress [240]. Supplementing the diet with quercetin in gerbils suffering from high-fat diet-induced NASH led to better lipid profiles, a decline in inflammatory markers, and regulation of Sirt1 and NF-κB p65 expression [241]. Thus, the hepatoprotective properties of quercetin are linked to enhanced fatty acid metabolism, anti-inflammatory and antioxidant effects, and modulation of gut microbiota as well as bile acids [242]. These results emphasise the potential of quercetin as a therapeutic option for NAFLD.
7.1.5. Nicotinamide Riboside (NR)
Nicotinamide riboside (NR), an NAD+ precursor, shows promise in addressing NAFLD and its progression to HCC. NR supplementation reduces hepatic lipid accumulation, inflammation, and fibrosis in various NAFLD models [243,244,245]. It activates SIRT1/AMPK-mediated browning of white adipose tissue and modulates gut microbiota, potentially improving lipid metabolism [246]. Furthermore, NR supplementation alleviated weight loss, reduced metastasis, and extended survival in BALB/c nude (xenograft) and C57BL/6J (allograft) mouse models with HCC by boosting NAD+ levels [247].
7.1.6. Berberine
Berberine, a natural plant alkaloid, has emerged as a promising therapeutic agent for NAFLD and its progression to HCC [248]. It exerts beneficial effects on gut microbiota by promoting the growth of beneficial bacterial populations while reducing pathogenic strains [249,250]. Additionally, berberine enhances lipid metabolism, improves insulin sensitivity, and diminishes inflammation [248,251]. Berberine has beneficial effects on NAFLD through various molecular pathways, including the activation of SIRT3, SIRT1, AMPK, and PPAR-γ, as well as the suppression of the NLRP3 pathway [251]. Additionally, berberine activates intestinal farnesoid X receptor (FXR), resulting in increased expression of fibroblast growth factor 15 (FGF15), which in turn inhibits lipogenesis and the activation of NF-κB pathway in the liver [250]. Furthermore, berberine suppresses the p38 MAPK/ERK-COX2 signalling pathways, thereby reducing inflammation and angiogenesis associated with non-alcoholic steatohepatitis (NASH) and HCC [252].
7.1.7. Yinchen Linggui Zhugan Decoction (YLZD)
Yinchen Linggui Zhugan decoction (YLZD), a traditional Chinese medicine, has demonstrated promise in treating NAFLD by modulating the SIRT1/Nrf2 pathway and gut microbiota. In Sprague Dawley (SD) rat models, YLZD treatment has been shown to reduce NAFLD induced by a high-fat diet, increasing serum and faecal butyric acid levels and total short-chain fatty acids (SCFAs), while promoting a favourable shift in gut microbiota composition towards SCFA-producing bacteria [253].
7.1.8. The Tangshen Formula (TSF)
The Tangshen formula (TSF), a herbal medicine from China, has exhibited encouraging effects in the management of NAFLD. Research indicates that TSF reduces hepatic steatosis and enhances lipid metabolism in several animal models [254,255,256]. Its mechanism of action involves various pathways, such as the modulation of gut microbiota and metabolic profiles [254], the activation of autophagy via the AMPK/SIRT1 pathway [255], and the regulation of macrophage activation and their phenotypic changes [256]. TSF treatment has been found to decrease lipid accumulation, lessen inflammation, and enhance insulin resistance and the integrity of the intestinal barrier [254,255]. Collectively, these findings suggest that TSF is a promising therapeutic option for NAFLD, functioning as a modulator of gut microbiota and metabolic profiles while also affecting hepatic cellular processes to reduce steatosis and related metabolic issues.
7.1.9. Curcumin
Curcumin, a natural polyphenol, appears to be a promising candidate for the treatment of NAFLD. Research indicates that curcumin supplementation can lower liver fat levels, enhance lipid profiles, and reduce insulin resistance in patients with NAFLD [257]. The beneficial effects of curcumin are linked to its properties as an antioxidant, anti-inflammatory agent, and its ability to prevent fat accumulation [258]. From a mechanistic standpoint, curcumin inhibits the O-GlcNAcylation pathway, promoting antioxidant responses in NASH mice [259]. Furthermore, curcumin improves mitochondrial function via the SIRT3 pathway, reducing liver fat accumulation in postnatal overfed rats and fatty L02 cells [260]. These results imply that curcumin may represent an effective therapeutic option for NAFLD, targeting various components of the disease’s underlying mechanisms. Nevertheless, despite substantial experimental support, clinical evidence is still scarce, highlighting the necessity for more human studies to comprehensively determine curcumin’s effectiveness in treating NAFLD.
7.1.10. Dihydromyricetin
Dihydromyricetin (DHM) has demonstrated encouraging effects in the treatment of NAFLD. Research suggests that DHM improves NAFLD by managing lipid and glucose metabolism, diminishing inflammation, and restoring the balance of gut microbiota [261,262]. DHM inhibits inflammatory signalling pathways, specifically TLR4/NF-κB, while promoting the growth of beneficial gut bacteria [262]. Additionally, it boosts mitochondrial function and redox equilibrium through SIRT3-dependent pathways, enhancing both mitochondrial respiratory capacity and antioxidant functions [262,263]. Laboratory studies indicate that DHM shields hepatocytes from lipid build-up and oxidative stress induced by oleic acid by inhibiting lipogenesis and modulating the PPARγ, AMPK, and AKT signalling pathways [264]. These results imply that DHM has the potential to serve as a therapeutic option for NAFLD, targeting various factors involved in the disease’s development, such as lipid metabolism, inflammation, oxidative stress, and gut microbiota imbalance.
7.2. Sirtuin Inhibitors
In the realm of hepatocellular carcinoma (HCC), the pharmaceutical sector has investigated the potential of sirtuin-targeting compounds, including the SIRT2 inhibitor salermide. This compound has demonstrated anti-tumour effects in preclinical models using human liver cancer cell lines, specifically HepG2 and Hep3B. Salermide inhibits SIRT2-mediated deacetylation of key metabolic enzymes, disrupting energy metabolism and reducing cell invasion [195].
AGK-2, a SIRT2 inhibitor, demonstrates an improvement in liver fibrosis induced by D-galactose in a rat model. This effect comes from its ability to inhibit fibrogenic factors, reduce inflammation, and decrease SIRT2 protein expression, suggesting a protective role against liver injury [265]. One study suggests that AK-7, another SIRT2 inhibitor, enhances p53 activity by preventing its deacetylation in non-small cell lung cancer (NSCLC) cells, positioning it as a potential candidate for cancer treatment, particularly for targeting p53-proficient cancers [266]. However, the specific role of AGK-2 and AK-7 in HCC needs further research. Overall, the therapeutic potential of SIRT2 inhibition remains contentious, with some studies suggesting both tumour-promoting and tumour-suppressing effects [267,268].
7.3. Gene Editing Approaches
Gene editing technologies like CRISPR/Cas9 provide innovative strategies to modulate sirtuin genes in HCC. This technology has shown potential in targeting cancer cells and creating engineered T cells for HCC treatment [269]. Additionally, CRISPR/Cas9 can alter the immunosuppressive environment in HCC by targeting genes such as GDF15 [270]. Targeting SIRT1 or combining conventional chemotherapy with SIRT1 inhibitors may improve therapeutic efficacy in HCC [43]. Overall, CRISPR/Cas9 and sirtuin modulation are promising strategies for treating HCC [271,272].
7.4. Small Molecule Targeting
Another strategy involves developing small-molecule inhibitors that target sirtuins. For instance, Binarci et al. have created novel indole-based compounds that effectively inhibit sirtuin activity in HCC cell lines, showing similar effectiveness to established inhibitors like EX-527 [273,274]. This suggests that these small molecules could serve as valuable therapeutic agents in HCC. Furthermore, the use of proteolysis-targeting chimeras (PROTACs) to degrade SIRT6 has shown promise, significantly reducing SIRT6 levels and inhibiting HCC cell proliferation [275]. This innovative method may offer a more precise way to target sirtuin activity compared to traditional inhibitors.
8. Gut Microbiota-Based Interventions: Probiotics, Prebiotics, and Synbiotics
Alongside sirtuin-targeted therapies, the pharmaceutical field is exploring gut microbiota-based interventions for disease management. Strategies addressing dysbiosis encompass FMT, probiotics, prebiotics, and dietary modifications [276]. Probiotics are beneficial live microorganisms, whereas prebiotics are selective substrates for host microorganisms [277]. These components can influence gut microbiota, improve immune response, and alleviate various health conditions, including gastrointestinal disorders, allergies, and infections [278]. The combined application of probiotics and prebiotics, referred to as synbiotics, demonstrates positive effects on the maintenance of a healthy gut microbiome [279]. Probiotics, prebiotics, and synbiotics have exhibited effectiveness in modulating immune responses, treating infections, managing inflammatory bowel diseases, and augmenting cancer treatment modalities [280].
In NAFLD, FMT is a novel strategy to modify gut microbiome and achieve metabolic balance [103]. FMT entails transferring a healthy microbiome to individuals with dysbiosis. The main aim is to replenish the recipient’s gut with beneficial microbes and restore a balanced microbial population [281]. FMT has shown promise in improving intestinal structure and function, enhancing lipid metabolism, reducing insulin resistance, suppressing inflammation, and alleviating NAFLD symptoms [282,283]. (see Figure 5).
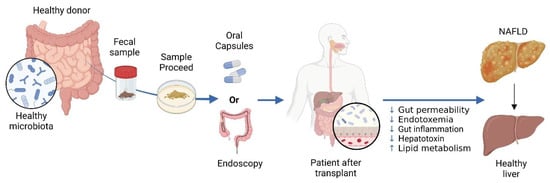
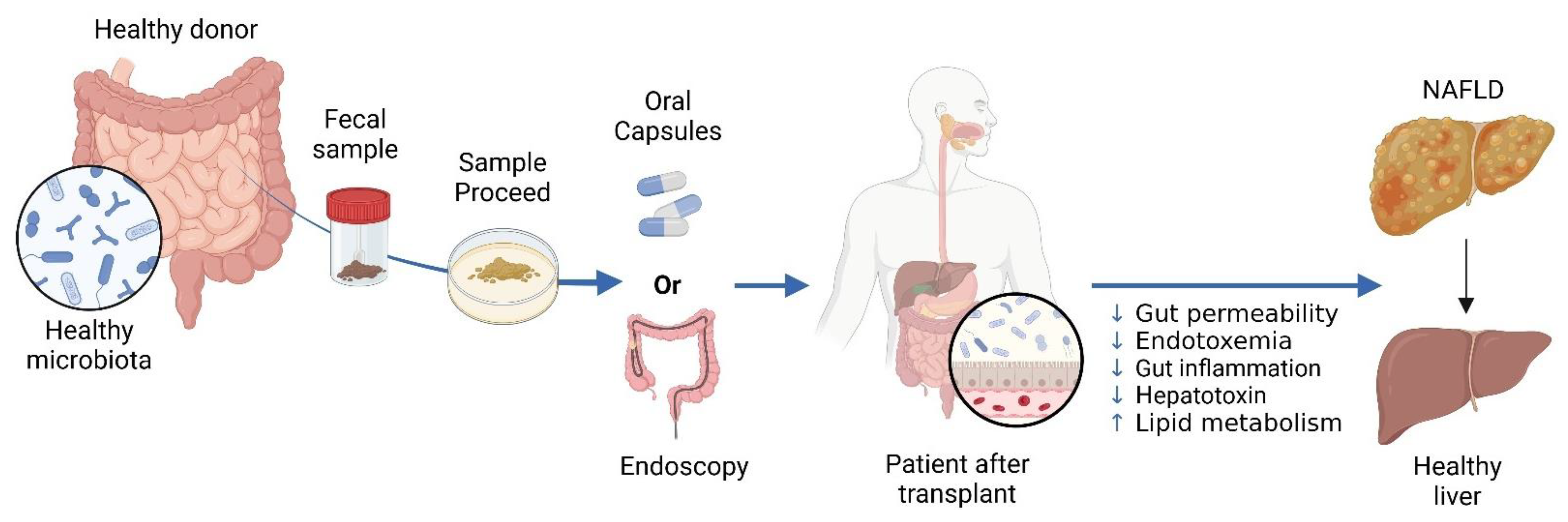
Figure 5.
The Use of Fecal Microbiota Transplantation (FMT) in Treating NAFLD. In FMT, a healthy donor’s stool is processed and administered to a patient with NAFLD. Delivery can be achieved through oral or endoscopic methods. NAFLD is linked to gut dysbiosis and heightened intestinal permeability, which facilitates the transfer of gut-derived factors to the liver, exacerbating the condition. FMT aims to rectify dysbiosis and enhance the gut barrier, with the goal of improving liver health in individuals with NAFLD. Created in BioRender. https://BioRender.com/r59u349 (accessed on 12 March 2025).
By diversifying sirtuin-targeting and gut microbiome-modulating interventions, the pharmaceutical industry seeks to offer enhanced and individualised treatment modalities for various diseases, capitalising on the intricate interactions between these biological pathways and their health ramifications.
9. Future Directions
Future research should concentrate on elucidating the specific mechanisms by which sirtuins influence gut microbiota composition and function, particularly in the context of metabolic disorders and cancer. It is crucial to explore how different sirtuin isoforms modulate gut health and contribute to the progression of non-alcoholic fatty liver disease (NAFLD) and its transition to hepatocellular carcinoma (HCC). Clinical trials assessing the efficacy of selective sirtuin activators or inhibitors in treating metabolic dysfunctions and cancer are essential, as they could provide valuable insights into how targeting sirtuin pathways may benefit patients with NAFLD or liver cancer.
Additionally, investigating the role of gut microbiota-derived metabolites in regulating sirtuin activity could inform therapeutic strategies. Understanding the microbiome profiles associated with these conditions will be vital for developing personalised treatment approaches. Moreover, examining the interplay between sirtuins, gut microbiota, and immune responses in clinical settings may uncover novel approaches to enhance the effectiveness of immunotherapies for liver cancer.
This focused research direction has the potential to advance the understanding of sirtuin–microbiota interactions, ultimately leading to innovative therapies aimed at preventing and treating metabolic disorders and liver cancer.
10. Conclusions
The intricate interplay between sirtuins and gut microbiota is a crucial hub for regulating metabolic health and disease. Sirtuins significantly influence both the composition and functionality of gut microbiota while maintaining the integrity of the intestinal barrier and modulating immune responses. In turn, metabolites produced by gut microbiota can affect sirtuin activity, creating a dynamic feedback loop that impacts overall health.
Given the rising prevalence of non-alcoholic fatty liver disease (NAFLD) and its progression to hepatocellular carcinoma (HCC), understanding the molecular mechanisms underlying these interactions is essential for developing targeted therapeutic interventions. However, the composition of the gut microbiome differs greatly among people, calling for individualised treatment strategies. Although the prospects for personalised treatments based on the microbiome are promising, there are still hurdles to overcome, including enhancing the understanding of the mechanisms, standardising testing methods, and confirming results in larger cohorts. This review highlights the promise of novel strategies that modulate sirtuin and microbiota pathways to improve health outcomes. It emphasises the urgent need for further research into the detailed mechanisms of sirtuin-microbiota interactions and their implications for preventing and treating metabolic disorders and liver cancer. Although there is potential, additional studies are necessary to thoroughly comprehend these complex interactions and create successful interventions, such as tailored microbiome therapies and engineered microbial communities.
Author Contributions
Conceptualization, A.T. and M.Z.; writing—original draft preparation, M.A.E., A.A.-Z. and A.Y.; writing—review and editing, A.T. and M.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable as the manuscript entails literature review of existing data.
Informed Consent Statement
Not applicable as the manuscript harbours literature review of already published research articles.
Data Availability Statement
No new data were created.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Wu, Q.; Zhang, T.-N.; Chen, H.-h.; Yu, X.-F.; Lv, J.; Liu, Y.-Y.; Liu, Y.; Zheng, G.; Zhao, J.; Wei, Y.-F.; et al. The sirtuin family in health and disease. Signal Transduct. Target. Ther. 2022, 7, 402. [Google Scholar]
- Bhatt, V.; Tiwari, A.K. Sirtuins, a key regulator of ageing and age-related neurodegenerative diseases. Int. J. Neurosci. 2022, 133, 1167–1192. [Google Scholar] [CrossRef] [PubMed]
- Ceballos, M.P.; Quiroga, A.D.; Palma, N.F. Role of sirtuins in hepatocellular carcinoma progression and multidrug resistance: Mechanistical and pharmacological perspectives. Biochem. Pharmacol. 2023, 212, 115573. [Google Scholar] [CrossRef] [PubMed]
- Afzaal, M.; Saeed, F.; Shah, Y.A.; Hussain, M.; Rabail, R.; Socol, C.T.; Hassoun, A.; Pateiro, M.; Lorenzo, J.M.; Rusu, A.V.; et al. Human gut microbiota in health and disease: Unveiling the relationship. Front. Microbiol. 2022, 13, 999001. [Google Scholar] [CrossRef]
- Kim, S.; Seo, S.-U.; Kweon, M.-N. Gut microbiota-derived metabolites tune host homeostasis fate. Semin. Immunopathol. 2024, 46, 1–15. [Google Scholar] [CrossRef]
- Li, X.; Du, Y.; Xue, C.; Kang, X.; Sun, C.; Peng, H.; Fang, L.; Han, Y.; Xu, X.; Zhao, C. SIRT2 Deficiency Aggravates Diet-Induced Nonalcoholic Fatty Liver Disease through Modulating Gut Microbiota and Metabolites. Int. J. Mol. Sci. 2023, 24, 8970. [Google Scholar] [CrossRef]
- Sharma, A.; Mahur, P.; Muthukumaran, J.; Singh, A.K.; Jain, M. Shedding light on structure, function and regulation of human sirtuins: A comprehensive review. 3 Biotech 2022, 13, 1–15. [Google Scholar] [CrossRef]
- Afzaal, A.; Rehman, K.; Kamal, S.; Akash, M.S.H. Versatile role of sirtuins in metabolic disorders: From modulation of mitochondrial function to therapeutic interventions. J. Biochem. Mol. Toxicol. 2022, 36, e23047. [Google Scholar] [CrossRef]
- D’Angelo, S.; Mele, E.; Di Filippo, F.; Viggiano, A.; Meccariello, R. Sirt1 Activity in the Brain: Simultaneous Effects on Energy Homeostasis and Reproduction. Int. J. Environ. Res. Public Health 2021, 18, 1243. [Google Scholar] [CrossRef]
- Zhang, Y.; Long, X.; Ruan, X.; Wei, Q.; Zhang, L.; Wo, L.; Huang, D.; Lin, L.; Wang, D.; Xia, L.; et al. SIRT2-mediated deacetylation and deubiquitination of C/EBPβ prevents ethanol-induced liver injury. Cell Discov. 2021, 7, 1–17. [Google Scholar] [CrossRef]
- Lambona, C.; Zwergel, C.; Valente, S.; Mai, A. SIRT3 Activation a Promise in Drug Development? New Insights into SIRT3 Biology and Its Implications on the Drug Discovery Process. J. Med. Chem. 2024, 67, 1662–1689. [Google Scholar] [CrossRef] [PubMed]
- Tao, Z.; Jin, Z.; Wu, J.; Cai, G.; Yu, X. Sirtuin family in autoimmune diseases. Front. Immunol. 2023, 14, 1186231. [Google Scholar] [CrossRef] [PubMed]
- Su, S.; Ndiaye, M.A.; Singh, C.K.; Ahmad, N. Mitochondrial Sirtuins in Skin and Skin Cancers. Photochem. Photobiol. 2020, 96, 973–980. [Google Scholar] [CrossRef] [PubMed]
- Poljšak, B.; Kovač, V.; Špalj, S.; Milisav, I. The Central Role of the NAD+ Molecule in the Development of Aging and the Prevention of Chronic Age-Related Diseases: Strategies for NAD+ Modulation. Int. J. Mol. Sci. 2023, 24, 2959. [Google Scholar] [CrossRef]
- Kosciuk, T.; Wang, M.; Hong, J.Y.; Lin, H. Updates on the epigenetic roles of sirtuins. Curr. Opin. Chem. Biol. 2019, 51, 18–29. [Google Scholar] [CrossRef]
- Zulkifli, N.D.; Zulkifle, N. Insight from sirtuins interactome: Topological prominence and multifaceted roles of SIRT1 in modulating immunity, aging and cancer. Genom. Inform. 2023, 21, e23. [Google Scholar] [CrossRef]
- Lagunas-Rangel, F.A. Current role of mammalian sirtuins in DNA repair. DNA Repair 2019, 80, 85–92. [Google Scholar] [CrossRef]
- Roos, W.P.; Krumm, A. The multifaceted influence of histone deacetylases on DNA damage signalling and DNA repair. Nucleic Acids Res. 2016, 44, 10017–10030. [Google Scholar] [CrossRef]
- Chang, A.R.; Ferrer, C.M.; Mostoslavsky, R. SIRT6, a Mammalian Deacylase with Multitasking Abilities. Physiol. Rev. 2020, 100, 145–169. [Google Scholar] [CrossRef]
- Carter, R.J.; Parsons, J.L. Base Excision Repair, a Pathway Regulated by Posttranslational Modifications. Mol. Cell. Biol. 2016, 36, 1426–1437. [Google Scholar] [CrossRef]
- Alves-Fernandes, D.K.; Jasiulionis, M.G. The Role of SIRT1 on DNA Damage Response and Epigenetic Alterations in Cancer. Int. J. Mol. Sci. 2019, 20, 3153. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Cao, N.; Fenech, M.; Wang, X. Role of Sirtuins in Maintenance of Genomic Stability: Relevance to Cancer and Healthy Aging. DNA Cell Biol. 2016, 35, 542–575. [Google Scholar] [CrossRef]
- O’Callaghan, C.; Vassilopoulos, A. Sirtuins at the crossroads of stemness, aging, and cancer. Aging Cell 2017, 16, 1208–1218. [Google Scholar] [CrossRef] [PubMed]
- Goes, J.; Viana, M.; Sampaio, L.; Dias, R.; Oliveira, R.; Cavalcante, C.; Borges, D.; Magalhães, S.; Pinheiro, R.; Junior, H. Differential Gene Expression of Sirtuins (Sirt1 to Sirt7) Reveals Potential Roles In Myelodysplastic Neoplasm Pathobiology. Hematol. Transfus. Cell Ther. 2023, 45, S432. [Google Scholar] [CrossRef]
- Chojdak-Łukasiewicz, J.; Bizoń, A.; Waliszewska-Prosół, M.; Piwowar, A.; Budrewicz, S.; Pokryszko-Dragan, A. Role of Sirtuins in Physiology and Diseases of the Central Nervous System. Biomedicines 2022, 10, 2434. [Google Scholar] [CrossRef]
- Hong, Y.A.; Kim, J.E.; Jo, M.J.; Ko, G.J. The Role of Sirtuins in Kidney Diseases. Int. J. Mol. Sci. 2020, 21, 6686. [Google Scholar] [CrossRef]
- Ding, Y.-N.; Wang, H.-Y.; Chen, X.-F.; Tang, X.; Chen, H.-Z. Roles of Sirtuins in Cardiovascular Diseases: Mechanisms and Therapeutics. Circ. Res. 2025, 136, 524–550. [Google Scholar] [CrossRef]
- Kumari, P.; Tarighi, S.; Braun, T.; Ianni, A. SIRT7 Acts as a Guardian of Cellular Integrity by Controlling Nucleolar and Extra-Nucleolar Functions. Genes 2021, 12, 1361. [Google Scholar] [CrossRef]
- Maissan, P.; Mooij, E.J.; Barberis, M. Sirtuins-Mediated System-Level Regulation of Mammalian Tissues at the Interface between Metabolism and Cell Cycle: A Systematic Review. Biology 2021, 10, 194. [Google Scholar] [CrossRef]
- Vazquez, B.N.; Vaquero, A.; Schindler, K. Sirtuins in female meiosis and in reproductive longevity. Mol. Reprod. Dev. 2020, 87, 1175–1187. [Google Scholar] [CrossRef]
- Kratz, E.M.; Kokot, I.; Dymicka-Piekarska, V.; Piwowar, A. Sirtuins—The New Important Players in Women’s Gynecological Health. Antioxidants 2021, 10, 84. [Google Scholar] [CrossRef] [PubMed]
- Hamaidi, I.; Kim, S. Sirtuins are crucial regulators of T cell metabolism and functions. Exp. Mol. Med. 2022, 54, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Watroba, M.; Szukiewicz, D. Sirtuins at the Service of Healthy Longevity. Front. Physiol. 2021, 12, 724506. [Google Scholar] [CrossRef] [PubMed]
- Zhao, E.; Hou, J.; Ke, X.; Abbas, M.N.; Kausar, S.; Zhang, L.; Cui, H. The Roles of Sirtuin Family Proteins in Cancer Progression. Cancers 2019, 11, 1949. [Google Scholar] [CrossRef]
- Carafa, V.; Altucci, L.; Nebbioso, A. Dual Tumor Suppressor and Tumor Promoter Action of Sirtuins in Determining Malignant Phenotype. Front. Pharmacol. 2019, 10, 38. [Google Scholar] [CrossRef]
- Zhu, S.; Dong, Z.; Ke, X.; Hou, J.; Zhao, E.; Zhang, K.; Wang, F.; Yang, L.; Xiang, Z.; Cui, H. The roles of sirtuins family in cell metabolism during tumor development. Semin. Cancer Biol. 2019, 57, 59–71. [Google Scholar] [CrossRef]
- Yin, J.-Y.; Lu, X.-T.; Hou, M.-L.; Cao, T.; Tian, Z. Sirtuin1-p53: A potential axis for cancer therapy. Biochem. Pharmacol. 2023, 212, 115543. [Google Scholar] [CrossRef]
- Chen, G.; Huang, P.; Hu, C. The role of SIRT2 in cancer: A novel therapeutic target. Int. J. Cancer 2020, 147, 3297–3304. [Google Scholar] [CrossRef]
- Onyiba, C.I.; Scarlett, C.J.; Weidenhofer, J. The Mechanistic Roles of Sirtuins in Breast and Prostate Cancer. Cancers 2022, 14, 5118. [Google Scholar] [CrossRef]
- George, J.; Ahmad, N. Mitochondrial Sirtuins in Cancer: Emerging Roles and Therapeutic Potential. Cancer Res. 2016, 76, 2500–2506. [Google Scholar] [CrossRef]
- Fiorentino, F.; Carafa, V.; Favale, G.; Altucci, L.; Mai, A.; Rotili, D. The Two-Faced Role of SIRT6 in Cancer. Cancers 2021, 13, 1156. [Google Scholar] [CrossRef] [PubMed]
- Karbasforooshan, H.; Hayes, A.W.; Mohammadzadeh, N.; Zirak, M.R.; Karimi, G. The possible role of Sirtuins and microRNAs in hepatocellular carcinoma therapy. Cell Cycle 2020, 19, 3209–3221. [Google Scholar] [CrossRef] [PubMed]
- Farcas, M.; Gavrea, A.-A.; Gulei, D.; Ionescu, C.; Irimie, A.; Catana, C.S.; Berindan-Neagoe, I. SIRT1 in the Development and Treatment of Hepatocellular Carcinoma. Front. Nutr. 2019, 6, 148. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.; Yao, H.; Caito, S.; Hwang, J.-W.; Arunachalam, G.; Rahman, I. Regulation of SIRT1 in cellular functions: Role of polyphenols. Arch. Biochem. Biophys. 2010, 501, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Li, X. SIRT1 and energy metabolism. Acta Biochim. Biophys. Sin. 2013, 45, 51–60. [Google Scholar] [CrossRef]
- Zhang, L.; Kim, S.; Ren, X. The Clinical Significance of SIRT2 in Malignancies: A Tumor Suppressor or an Oncogene? Front. Oncol. 2020, 10, 1721. [Google Scholar] [CrossRef]
- Hong, J.Y.; Lin, H. Sirtuin Modulators in Cellular and Animal Models of Human Diseases. Front. Pharmacol. 2021, 12, 735044. [Google Scholar] [CrossRef]
- Yang, W.; Chen, W.; Su, H.; Li, R.; Song, C.; Wang, Z.; Yang, L. Recent advances in the development of histone deacylase SIRT2 inhibitors. RSC Adv. 2020, 10, 37382–37390. [Google Scholar] [CrossRef]
- Zhu, C.; Dong, X.; Wang, X.; Zheng, Y.; Qiu, J.; Peng, Y.; Xu, J.; Chai, Z.; Liu, C. Multiple Roles of SIRT2 in Regulating Physiological and Pathological Signal Transduction. Genet. Res. 2022, 2022, 1–14. [Google Scholar] [CrossRef]
- Bharathi, S.S.; Zhang, Y.; Mohsen, A.-W.; Uppala, R.; Balasubramani, M.; Schreiber, E.; Uechi, G.; Beck, M.E.; Rardin, M.J.; Vockley, J.; et al. Sirtuin 3 (SIRT3) Protein Regulates Long-chain Acyl-CoA Dehydrogenase by Deacetylating Conserved Lysines Near the Active Site. J. Biol. Chem. 2013, 288, 33837–33847. [Google Scholar] [CrossRef]
- Shen, Y.; Wu, Q.; Shi, J.; Zhou, S. Regulation of SIRT3 on mitochondrial functions and oxidative stress in Parkinson’s disease. Biomed. Pharmacother. 2020, 132, 110928. [Google Scholar] [CrossRef] [PubMed]
- Sebaa, R.; Johnson, J.; Pileggi, C.; Norgren, M.; Xuan, J.; Sai, Y.; Tong, Q.; Krystkowiak, I.; Bondy-Chorney, E.; Davey, N.E.; et al. SIRT3 controls brown fat thermogenesis by deacetylation regulation of pathways upstream of UCP1. Mol. Metab. 2019, 25, 35–49. [Google Scholar] [CrossRef] [PubMed]
- Kincaid, B.; Bossy-Wetzel, E. Forever young: SIRT3 a shield against mitochondrial meltdown, aging, and neurodegeneration. Front. Aging Neurosci. 2013, 5, 48. [Google Scholar] [CrossRef] [PubMed]
- Hallows, W.C.; Yu, W.; Smith, B.C.; Devries, M.K.; Ellinger, J.J.; Someya, S.; Shortreed, M.R.; Prolla, T.; Markley, J.L.; Smith, L.M.; et al. Sirt3 promotes the urea cycle and fatty acid oxidation during dietary restriction. Mol. Cell 2011, 41, 139–149. [Google Scholar] [CrossRef]
- Zhu, Y.; Yan, Y.; Principe, D.R.; Zou, X.; Vassilopoulos, A.; Gius, D. SIRT3 and SIRT4 are mitochondrial tumor suppressor proteins that connect mitochondrial metabolism and carcinogenesis. Cancer Metab. 2014, 2, 15. [Google Scholar] [CrossRef]
- Wang, C.; Liu, Y.; Zhu, Y.; Kong, C. Functions of mammalian SIRT4 in cellular metabolism and research progress in human cancer (Review). Oncol. Lett. 2020, 20, 1. [Google Scholar] [CrossRef]
- Mathias, R.A.; Greco, T.M.; Oberstein, A.; Budayeva, H.G.; Chakrabarti, R.; Rowland, E.A.; Kang, Y.; Shenk, T.; Cristea, I.M. Sirtuin 4 Is a Lipoamidase Regulating Pyruvate Dehydrogenase Complex Activity. Cell 2014, 159, 1615–1625. [Google Scholar] [CrossRef]
- Tomaselli, D.; Steegborn, C.; Mai, A.; Rotili, D. Sirt4: A Multifaceted Enzyme at the Crossroads of Mitochondrial Metabolism and Cancer. Front. Oncol. 2020, 10, 474. [Google Scholar] [CrossRef]
- Yang, X.; Liu, B.; Zhu, W.; Luo, J. SIRT5, functions in cellular metabolism with a multiple enzymatic activities. Sci. China Life Sci. 2015, 58, 912–914. [Google Scholar] [CrossRef]
- Fabbrizi, E.; Fiorentino, F.; Carafa, V.; Altucci, L.; Mai, A.; Rotili, D. Emerging Roles of SIRT5 in Metabolism, Cancer, and SARS-CoV-2 Infection. Cells 2023, 12, 852. [Google Scholar] [CrossRef]
- Polletta, L.; Vernucci, E.; Carnevale, I.; Arcangeli, T.; Rotili, D.; Palmerio, S.; Steegborn, C.; Nowak, T.; Schutkowski, M.; Pellegrini, L.; et al. SIRT5 regulation of ammonia-induced autophagy and mitophagy. Autophagy 2015, 11, 253–270. [Google Scholar] [CrossRef] [PubMed]
- Nahálková, J. A new view on functions of the lysine demalonylase activity of SIRT5. Life Sci. 2023, 320, 121572. [Google Scholar] [CrossRef] [PubMed]
- Pederson, N.J.; Diehl, K.L. DNA stimulates SIRT6 to mono-ADP-ribosylate proteins within histidine repeats. bioRxiv 2024. [Google Scholar] [CrossRef]
- Zhong, L.; D’Urso, A.; Toiber, D.; Sebastian, C.; Henry, R.E.; Vadysirisack, D.D.; Guimaraes, A.; Marinelli, B.; Wikstrom, J.D.; Nir, T.; et al. The Histone Deacetylase Sirt6 Regulates Glucose Homeostasis via Hif1α. Cell 2010, 140, 280–293. [Google Scholar] [CrossRef]
- Fiorentino, F.; Mai, A.; Rotili, D. Emerging Therapeutic Potential of SIRT6 Modulators. J. Med. Chem. 2021, 64, 9732–9758. [Google Scholar] [CrossRef]
- Blank, M.F.; Grummt, I. The seven faces of SIRT7. Transcription 2017, 8, 67–74. [Google Scholar] [CrossRef]
- Kiran, S.; Anwar, T.; Kiran, M.; Ramakrishna, G. Sirtuin 7 in cell proliferation, stress and disease: Rise of the Seventh Sirtuin! Cell. Signal. 2015, 27, 673–682. [Google Scholar] [CrossRef]
- Lagunas-Rangel, F.A. SIRT7 in the aging process. Cell. Mol. Life Sci. 2022, 79, 297. [Google Scholar] [CrossRef]
- Matijašić, M.; Meštrović, T.; Čipčić Paljetak, H.; Perić, M.; Barešić, A.; Verbanac, D. Gut Microbiota beyond Bacteria—Mycobiome, Virome, Archaeome, and Eukaryotic Parasites in IBD. Int. J. Mol. Sci. 2020, 21, 2668. [Google Scholar] [CrossRef]
- Vemuri, R.; Shankar, E.M.; Chieppa, M.; Eri, R.; Kavanagh, K. Beyond Just Bacteria: Functional Biomes in the Gut Ecosystem Including Virome, Mycobiome, Archaeome and Helminths. Microorganisms 2020, 8, 483. [Google Scholar] [CrossRef]
- Garcia-Bonete, M.J.; Rajan, A.; Suriano, F.; Layunta, E. The Underrated Gut Microbiota Helminths, Bacteriophages, Fungi, and Archaea. Life 2023, 13, 1765. [Google Scholar] [CrossRef] [PubMed]
- Sasso, J.M.; Ammar, R.M.; Tenchov, R.; Lemmel, S.; Kelber, O.; Grieswelle, M.; Zhou, Q.A. Gut Microbiome–Brain Alliance: A Landscape View into Mental and Gastrointestinal Health and Disorders. ACS Chem. Neurosci. 2023, 14, 1717–1763. [Google Scholar] [CrossRef] [PubMed]
- Abenavoli, L.; Giubilei, L.; Procopio, A.C.; Spagnuolo, R.; Luzza, F.; Boccuto, L.; Scarpellini, E. Gut Microbiota in Non-Alcoholic Fatty Liver Disease Patients with Inflammatory Bowel Diseases: A Complex Interplay. Nutrients 2022, 14, 5323. [Google Scholar] [CrossRef] [PubMed]
- Barko, P.C.; McMichael, M.A.; Swanson, K.S.; Williams, D.A. The Gastrointestinal Microbiome: A Review. J. Vet. Intern. Med. 2017, 32, 9–25. [Google Scholar] [CrossRef]
- Pickard, J.M.; Zeng, M.Y.; Caruso, R.; Núñez, G. Gut microbiota: Role in pathogen colonization, immune responses, and inflammatory disease. Immunol. Rev. 2017, 279, 70–89. [Google Scholar] [CrossRef]
- Srivastava, A.; Prabhakar, M.R.; Mohanty, A.; Meena, S.S. Influence of gut microbiome on the human physiology. Syst. Microbiol. Biomanufacturing 2021, 2, 217–231. [Google Scholar] [CrossRef]
- Garcia-Gutierrez, E.; Mayer, M.J.; Cotter, P.D.; Narbad, A. Gut microbiota as a source of novel antimicrobials. Gut Microbes 2018, 10, 1–21. [Google Scholar] [CrossRef]
- Rowland, I.; Gibson, G.; Heinken, A.; Scott, K.; Swann, J.; Thiele, I.; Tuohy, K. Gut microbiota functions: Metabolism of nutrients and other food components. Eur. J. Nutr. 2018, 57, 1–24. [Google Scholar] [CrossRef]
- Levy, M.; Blacher, E.; Elinav, E. Microbiome, metabolites and host immunity. Curr. Opin. Microbiol. 2017, 35, 8–15. [Google Scholar] [CrossRef]
- Fung, T.C.; Olson, C.A.; Hsiao, E.Y. Interactions between the microbiota, immune and nervous systems in health and disease. Nat. Neurosci. 2017, 20, 145–155. [Google Scholar] [CrossRef]
- Gasaly, N.; de Vos, P.; Hermoso, M.A. Impact of Bacterial Metabolites on Gut Barrier Function and Host Immunity: A Focus on Bacterial Metabolism and Its Relevance for Intestinal Inflammation. Front. Immunol. 2021, 12, 658354. [Google Scholar] [CrossRef] [PubMed]
- Postler, T.S.; Ghosh, S. Understanding the Holobiont: How Microbial Metabolites Affect Human Health and Shape the Immune System. Cell Metab. 2017, 26, 110–130. [Google Scholar] [CrossRef] [PubMed]
- Louis, P.; Flint, H.J. Formation of propionate and butyrate by the human colonic microbiota. Environ. Microbiol. 2017, 19, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Blaak, E.E.; Canfora, E.E.; Theis, S.; Frost, G.; Groen, A.K.; Mithieux, G.; Nauta, A.; Scott, K.; Stahl, B.; Van Harsselaar, J.; et al. Short chain fatty acids in human gut and metabolic health. Benef. Microbes 2020, 11, 411–455. [Google Scholar] [CrossRef]
- Parada Venegas, D.; De La Fuente, M.K.; Landskron, G.; González, M.J.; Quera, R.; Dijkstra, G.; Harmsen, H.J.M.; Faber, K.N.; Hermoso, M.A. Short Chain Fatty Acids (SCFAs)-Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases. Front. Immunol. 2019, 10, 277. [Google Scholar] [CrossRef]
- Martin-Gallausiaux, C.; Marinelli, L.; Blottière, H.M.; Larraufie, P.; Lapaque, N. SCFA: Mechanisms and functional importance in the gut. Proc. Nutr. Soc. 2021, 80, 37–49. [Google Scholar] [CrossRef]
- He, J.; Zhang, P.; Shen, L.; Niu, L.; Tan, Y.; Chen, L.; Zhao, Y.; Bai, L.; Hao, X.; Li, X.; et al. Short-Chain Fatty Acids and Their Association with Signalling Pathways in Inflammation, Glucose and Lipid Metabolism. Int. J. Mol. Sci. 2020, 21, 6356. [Google Scholar] [CrossRef]
- Ramirez-Perez, O.L.; Cruz-Ramon, V.C.; Chinchilla-López, P.; Méndez-Sánchez, N. The Role of the Gut Microbiota in Bile Acid Metabolism. Ann. Hepatol. 2017, 16 (Suppl. 1), s15–s20. [Google Scholar] [CrossRef]
- Oliphant, K.; Allen-Vercoe, E. Macronutrient metabolism by the human gut microbiome: Major fermentation by-products and their impact on host health. Microbiome 2019, 7, 91. [Google Scholar] [CrossRef]
- Moszak, M.; Szulińska, M.; Bogdański, P. You Are What You Eat—The Relationship between Diet, Microbiota, and Metabolic Disorders—A Review. Nutrients 2020, 12, 1096. [Google Scholar] [CrossRef]
- Anwar, H.; Iftikhar, A.; Muzaffar, H.; Almatroudi, A.; Allemailem, K.S.; Navaid, S.; Saleem, S.; Khurshid, M. Biodiversity of Gut Microbiota: Impact of Various Host and Environmental Factors. BioMed Res. Int. 2021, 2021, 5575245. [Google Scholar] [CrossRef] [PubMed]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What Is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xu, B.; Chen, H.; Yang, F.; Huang, J.; Jiao, X.; Zhang, Y. Environmental factors and gut microbiota: Toward better conservation of deer species. Front. Microbiol. 2023, 14, 1136413. [Google Scholar] [CrossRef] [PubMed]
- Rothschild, D.; Weissbrod, O.; Barkan, E.; Kurilshikov, A.; Korem, T.; Zeevi, D.; Costea, P.I.; Godneva, A.; Kalka, I.N.; Bar, N.; et al. Environment dominates over host genetics in shaping human gut microbiota. Nature 2018, 555, 210–215. [Google Scholar] [CrossRef]
- Kurilshikov, A.; Wijmenga, C.; Fu, J.; Zhernakova, A. Host Genetics and Gut Microbiome: Challenges and Perspectives. Trends Immunol. 2017, 38, 633–647. [Google Scholar] [CrossRef]
- Jeong, S. Factors influencing development of the infant microbiota: From prenatal period to early infancy. Clin. Exp. Pediatr. 2021, 65, 438–447. [Google Scholar] [CrossRef]
- Hasan, N.; Yang, H. Factors affecting the composition of the gut microbiota, and its modulation. PeerJ 2019, 7, e7502. [Google Scholar] [CrossRef]
- Kriss, M.; Hazleton, K.Z.; Nusbacher, N.M.; Martin, C.G.; A Lozupone, C. Low diversity gut microbiota dysbiosis: Drivers, functional implications and recovery. Curr. Opin. Microbiol. 2018, 44, 34–40. [Google Scholar] [CrossRef]
- Heravi, F.S. Gut Microbiota and Autoimmune Diseases: Mechanisms, Treatment, Challenges, and Future Recommendations. Curr. Clin. Microbiol. Rep. 2024, 11, 18–33. [Google Scholar] [CrossRef]
- Weiss, G.A.; Hennet, T. Mechanisms and consequences of intestinal dysbiosis. Cell. Mol. Life Sci. 2017, 74, 2959–2977. [Google Scholar] [CrossRef]
- Willighagen, E. Defining Dysbiosis for a Cluster of Chronic Diseases. Sci. Rep. 2019, 9, 1–11. [Google Scholar]
- Jalandra, R.; Dhar, R.; Pethusamy, K.; Sharma, M.; Karmakar, S. Dysbiosis: Gut feeling. F1000Research 2022, 11, 911. [Google Scholar] [CrossRef]
- Hou, K.; Wu, Z.; Chen, X.-Y.; Wang, J.-Q.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.B.; Wei, L.; Li, J.; et al. Microbiota in health and diseases. Signal Transduct. Target. Ther. 2022, 7, 135. [Google Scholar] [CrossRef] [PubMed]
- Kho, Z.Y.; Lal, S.K. The Human Gut Microbiome—A Potential Controller of Wellness and Disease. Front. Microbiol. 2018, 9, 1835. [Google Scholar] [CrossRef]
- Meng, X.; Zhang, G.; Cao, H.; Yu, D.; Fang, X.; Vos, W.M.; Wu, H. Gut dysbacteriosis and intestinal disease: Mechanism and treatment. J. Appl. Microbiol. 2020, 129, 787–805. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X.-L.; Zhou, M.; Kang, C.; Lang, H.-D.; Chen, M.-T.; Hui, S.-C.; Wang, B.; Mi, M.-T. Crosstalk between gut microbiota and Sirtuin-3 in colonic inflammation and tumorigenesis. Exp. Mol. Med. 2018, 50, 1–11. [Google Scholar] [CrossRef]
- Caron, A.Z.; He, X.; Mottawea, W.; Seifert, E.L.; Jardine, K.; Dewar-Darch, D.; Cron, G.O.; Harper, M.; Stintzi, A.; McBurney, M.W. The SIRT1 deacetylase protects mice against the symptoms of metabolic syndrome. FASEB J. 2013, 28, 1306–1316. [Google Scholar] [CrossRef]
- Vikram, A.; Kim, Y.-R.; Kumar, S.; Li, Q.; Kassan, M.; Jacobs, J.S.; Irani, K. Vascular microRNA-204 is remotely governed by the microbiome and impairs endothelium-dependent vasorelaxation by downregulating Sirtuin1. Nat. Commun. 2016, 7, 12565. [Google Scholar] [CrossRef]
- Wellman, A.S.; Metukuri, M.R.; Kazgan, N.; Xu, X.; Xu, Q.; Ren, N.S.; Czopik, A.; Shanahan, M.T.; Kang, A.; Chen, W.; et al. Intestinal Epithelial Sirtuin 1 Regulates Intestinal Inflammation During Aging in Mice by Altering the Intestinal Microbiota. Gastroenterology 2017, 153, 772–786. [Google Scholar] [CrossRef]
- Munteanu, C.; Onose, G.; Poștaru, M.; Turnea, M.; Rotariu, M.; Galaction, A.I. Hydrogen Sulfide and Gut Microbiota: Their Synergistic Role in Modulating Sirtuin Activity and Potential Therapeutic Implications for Neurodegenerative Diseases. Pharmaceuticals 2024, 17, 1480. [Google Scholar] [CrossRef]
- Hou, D.; Yu, T.; Lu, X.; Hong, J.Y.; Yang, M.; Zi, Y.; Ho, T.T.; Lin, H. Sirt2 inhibition improves gut epithelial barrier integrity and protects mice from colitis. Proc. Natl. Acad. Sci. USA 2024, 121, 2319833121. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Xie, Y.; Li, G.; Zhang, T.; Sui, Y.; Zhao, Z.; Zhang, Y.; Yang, W.; Geng, X.; Xue, D.; et al. Gut microbiota-derived nicotinamide mononucleotide alleviates acute pancreatitis by activating pancreatic SIRT3 signalling. Br. J. Pharmacol. 2022, 180, 647–666. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Hui, S.; Lang, H.; Zhou, M.; Zhang, Y.; Kang, C.; Zeng, X.; Zhang, Q.; Yi, L.; Mi, M. SIRT3 Deficiency Promotes High-Fat Diet-Induced Nonalcoholic Fatty Liver Disease in Correlation with Impaired Intestinal Permeability through Gut Microbial Dysbiosis. Mol. Nutr. Food Res. 2018, 63, e1800612. [Google Scholar] [CrossRef] [PubMed]
- Knop, M.; Treitz, C.; Bettendorf, S.; Bossen, J.; von Frieling, J.; Doms, S.; Bruchhaus, I.; Kühnlein, R.P.; Baines, J.F.; Tholey, A.; et al. A mitochondrial sirtuin shapes the intestinal microbiota by controlling lysozyme expression. bioRxiv 2023. [Google Scholar] [CrossRef]
- Tucker, S.A.; Hu, S.-H.; Vyas, S.; Park, A.; Joshi, S.; Inal, A.; Lam, T.; Tan, E.; Haigis, K.M.; Haigis, M.C. SIRT4 loss reprograms intestinal nucleotide metabolism to support proliferation following perturbation of homeostasis. Cell Rep. 2024, 43, 113975. [Google Scholar] [CrossRef]
- Sun, R.; Zhang, Z.; Bao, R.; Guo, X.; Gu, Y.; Yang, W.; Wei, J.; Chen, X.; Tong, L.; Meng, J.; et al. Loss of SIRT5 promotes bile acid-induced immunosuppressive microenvironment and hepatocarcinogenesis. J. Hepatol. 2022, 77, 453–466. [Google Scholar] [CrossRef]
- Xu, K.; Guo, Y.; Wang, Y.; Ren, Y.; Low, V.; Cho, S.; Ping, L.; Peng, K.; Li, X.; Qiu, Y.; et al. Decreased Enterobacteriaceae translocation due to gut microbiota remodeling mediates the alleviation of premature aging by a high-fat diet. Aging Cell 2022, 22, 13760. [Google Scholar] [CrossRef]
- Kim, S.; Byun, J.; Jung, S.; Kim, B.; Lee, K.; Jeon, H.; Lee, J.; Choi, H.; Kim, E.; Jeen, Y.; et al. Sirtuin 7 Inhibitor Attenuates Colonic Mucosal Immune Activation in Mice—Potential Therapeutic Target in Inflammatory Bowel Disease. Biomedicines 2022, 10, 2693. [Google Scholar] [CrossRef]
- Caruso, R.; Marafini, I.; Franzè, E.; Stolfi, C.; Zorzi, F.; Monteleone, I.; Caprioli, F.; Colantoni, A.; Sarra, M.; Sedda, S.; et al. Defective expression of SIRT1 contributes to sustain inflammatory pathways in the gut. Mucosal Immunol. 2014, 7, 1467–1479. [Google Scholar] [CrossRef]
- Lytvynenko, A.; Voznesenskaya, T.; Janchij, R. SIRT1 Is A Regulator Of Autophagy In Intestinal Cells. Fiziolohichnyĭ zhurnal 2020, 66, 97–103. [Google Scholar] [CrossRef]
- Ma, Y.; Xu, C.; Wang, W.; Sun, L.; Yang, S.; Lu, D.; Liu, Y.; Yang, H. Role of SIRT1 in the protection of intestinal epithelial barrier under hypoxia and its mechanism. Chin. J. Gastrointest. Surg. 2014, 17, 602–606. [Google Scholar]
- Markandey, M.; Aditya, B.; Edward, I.N.; Saurabh, K.; Simon, T.; Fiona, P.; Ahuja, V. Gut microbiota: Sculptors of the intestinal stem cell niche in health and inflammatory bowel disease. Gut Microbes 2021, 13, 1990827. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhou, Y.; Rychahou, P.; Weiss, H.L.; Lee, E.Y.; Perry, C.L.; Barrett, T.A.; Wang, Q.; Evers, B.M. SIRT2 Contributes to the Regulation of Intestinal Cell Proliferation and Differentiation. Cell. Mol. Gastroenterol. Hepatol. 2020, 10, 43–57. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Cao, Y.; Zheng, Q.; Tu, J.; Zhou, W.; He, J.; Zhong, J.; Chen, Y.; Wang, J.; Cai, R.; et al. SENP1-Sirt3 Signaling Controls Mitochondrial Protein Acetylation and Metabolism. Mol. Cell 2019, 75, 823–834.e5. [Google Scholar] [CrossRef]
- Nasrin, N.; Wu, X.; Fortier, E.; Feng, Y.; Bare’, O.C.; Chen, S.; Ren, X.; Wu, Z.; Streeper, R.S.; Bordone, L. SIRT4 Regulates Fatty Acid Oxidation and Mitochondrial Gene Expression in Liver and Muscle Cells. J. Biol. Chem. 2010, 285, 31995–32002. [Google Scholar] [CrossRef]
- Bringman-Rodenbarger, L.R.; Guo, A.H.; Lyssiotis, C.A.; Lombard, D.B. Emerging Roles for SIRT5 in Metabolism and Cancer. Antioxidants Redox Signal. 2018, 28, 677–690. [Google Scholar] [CrossRef]
- Xiong, X.; Yang, C.; He, W.-Q.; Yu, J.; Xin, Y.; Zhang, X.; Huang, R.; Ma, H.; Xu, S.; Li, Z.; et al. Sirtuin 6 maintains epithelial STAT6 activity to support intestinal tuft cell development and type 2 immunity. Nat. Commun. 2022, 13, 1–16. [Google Scholar] [CrossRef]
- Xu, K.; Guo, Y.; Ping, L.; Qiu, Y.; Liu, Q.; Li, Z.; Wang, Z. Protective Effects of SIRT6 Overexpression against DSS-Induced Colitis in Mice. Cells 2020, 9, 1513. [Google Scholar] [CrossRef]
- Lerrer, B.; Gertler, A.A.; Cohen, H.Y. The complex role of SIRT6 in carcinogenesis. Carcinog. 2015, 37, 108–118. [Google Scholar] [CrossRef]
- Liu, X.; Li, C.; Li, Q.; Chang, H.-C.; Tang, Y.-C. SIRT7 Facilitates CENP-A Nucleosome Assembly and Suppresses Intestinal Tumorigenesis. iScience 2020, 23, 101461. [Google Scholar] [CrossRef]
- Haslberger, A.; Lilja, S.; Bäck, H.; Stoll, C.; Mayer, A.; Pointner, A.; Hippe, B.; Krammer, U. Increased Sirtuin expression, senescence regulating miRNAs, mtDNA, and Bifidobateria correlate with wellbeing and skin appearance after Sirtuin- activating drink. Bioact. Compd. Health Dis. 2021, 4, 45. [Google Scholar] [CrossRef]
- Salazar, J.; Durán, P.; Díaz, M.P.; Chacín, M.; Santeliz, R.; Mengual, E.; Gutiérrez, E.; León, X.; Díaz, A.; Bernal, M.; et al. Exploring the Relationship between the Gut Microbiota and Ageing: A Possible Age Modulator. Int. J. Environ. Res. Public Health 2023, 20, 5845. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.; Mach, N. The Crosstalk between the Gut Microbiota and Mitochondria during Exercise. Front. Physiol. 2017, 8, 319. [Google Scholar] [CrossRef] [PubMed]
- Gong, S.; Feng, Y.; Zeng, Y.; Zhang, H.; Pan, M.; He, F.; Wu, R.; Chen, J.; Lu, J.; Zhang, S.; et al. Gut microbiota accelerates cisplatin-induced acute liver injury associated with robust inflammation and oxidative stress in mice. J. Transl. Med. 2021, 19, 1–13. [Google Scholar] [CrossRef]
- Singh, R.; Chandrashekharappa, S.; Bodduluri, S.R.; Baby, B.V.; Hegde, B.; Kotla, N.G.; Hiwale, A.A.; Saiyed, T.; Patel, P.; Vijay-Kumar, M.; et al. Enhancement of the gut barrier integrity by a microbial metabolite through the Nrf2 pathway. Nat. Commun. 2019, 10, 1–18. [Google Scholar] [CrossRef]
- Yang, M.; Massad, K.; Kimchi, E.T.; Staveley-O’carroll, K.F.; Li, G. Gut microbiota and metabolite interface-mediated hepatic inflammation. Immunometabolism 2024, 6, e00037. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, X.; Lv, A.; Fan, S.; Zhang, J. Saccharomyces Boulardii Modulates Necrotizing Enterocolitis in Neonatal Mice by Regulating the Sirtuin 1/NF-κB Pathway and the Intestinal Microbiota. Mol. Med. Rep. 2020, 22, 671–680. [Google Scholar] [CrossRef]
- Estes, C.; Razavi, H.; Loomba, R.; Younossi, Z.; Sanyal, A.J. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology 2018, 67, 123–133. [Google Scholar] [CrossRef]
- EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J. Hepatol. 2016, 64, 1388–1402. [CrossRef]
- Tiniakos, D.G.; Vos, M.B.; Brunt, E.M. Nonalcoholic Fatty Liver Disease: Pathology and Pathogenesis. Annu. Rev. Pathol. Mech. Dis. 2010, 5, 145–171. [Google Scholar] [CrossRef]
- Grander, C.; Grabherr, F.; Moschen, A.R.; Tilg, H. Non-Alcoholic Fatty Liver Disease: Cause or Effect of Metabolic Syndrome. Visc. Med. 2016, 32, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Paschos, P.; Paletas, K. Non alcoholic fatty liver disease two-hit process: Multifactorial character of the second hit. Hippokratia 2009, 13, 128. [Google Scholar]
- Jang, H.R.; Lee, H.-Y. Mechanisms linking gut microbial metabolites to insulin resistance. World J. Diabetes 2021, 12, 730–744. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Tang, X.; Chen, H.-Z. Sirtuins and Insulin Resistance. Front. Endocrinol. 2018, 9, 748. [Google Scholar] [CrossRef]
- Wang, Y.-J.; Paneni, F.; Stein, S.; Matter, C.M. Modulating Sirtuin Biology and Nicotinamide Adenine Diphosphate Metabolism in Cardiovascular Disease—From Bench to Bedside. Front. Physiol. 2021, 12, 755060. [Google Scholar] [CrossRef]
- Chandramowlishwaran, P.; Vijay, A.; Abraham, D.; Li, G.; Mwangi, S.M.; Srinivasan, S. Role of Sirtuins in Modulating Neurodegeneration of the Enteric Nervous System and Central Nervous System. Front. Neurosci. 2020, 14, 614331. [Google Scholar] [CrossRef]
- Ghosh-Swaby, O.R.; Reichelt, A.C.; Sheppard, P.A.; Davies, J.; Bussey, T.J.; Saksida, L.M. Metabolic hormones mediate cognition. Front. Neuroendocr. 2022, 66, 101009. [Google Scholar] [CrossRef]
- Zeng, Y.; Fang, Q.; Chen, J.; Wang, Y.; Liu, X.; Zhang, X.; Shi, Y.; Zhan, H.; Zhong, X.; Yao, M.; et al. Melatonin Improves Mitochondrial Dysfunction and Attenuates Neuropathic Pain by Regulating SIRT1 in Dorsal Root Ganglions. Neuroscience 2023, 534, 29–40. [Google Scholar] [CrossRef]
- Naaz, S.; Mishra, S.; Pal, P.K.; Chattopadhyay, A.; Das, A.R.; Bandyopadhyay, D. Activation of SIRT1/PGC 1α/SIRT3 pathway by melatonin provides protection against mitochondrial dysfunction in isoproterenol induced myocardial injury. Heliyon 2020, 6, e05159. [Google Scholar] [CrossRef]
- Fujitsuka, N.; Asakawa, A.; Morinaga, A.; Amitani, M.S.; Amitani, H.; Katsuura, G.; Sawada, Y.; Sudo, Y.; Uezono, Y.; Mochiki, E.; et al. Increased ghrelin signaling prolongs survival in mouse models of human aging through activation of sirtuin1. Mol. Psychiatry 2016, 21, 1613–1623. [Google Scholar] [CrossRef]
- Song, N.-Y.; Lee, Y.-H.; Na, H.-K.; Baek, J.-H.; Surh, Y.-J. Leptin induces SIRT1 expression through activation of NF-E2-related factor 2: Implications for obesity-associated colon carcinogenesis. Biochem. Pharmacol. 2018, 153, 282–291. [Google Scholar] [CrossRef] [PubMed]
- Marwarha, G.; Raza, S.; Meiers, C.; Ghribi, O. RETRACTED: Leptin attenuates BACE1 expression and amyloid-β genesis via the activation of SIRT1 signaling pathway. Biochim. Biophys. Acta BBA Mol. Basis Dis. 2014, 1842, 1587–1595. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, T. Age-Associated Weight Gain, Leptin, and SIRT1: A Possible Role for Hypothalamic SIRT1 in the Prevention of Weight Gain and Aging through Modulation of Leptin Sensitivity. Front. Endocrinol. 2015, 6, 109. [Google Scholar] [CrossRef] [PubMed]
- Chaudhry, T.S.; Senapati, S.G.; Gadam, S.; Mannam, H.P.S.S.; Voruganti, H.V.; Abbasi, Z.; Abhinav, T.; Challa, A.B.; Pallipamu, N.; Bheemisetty, N.; et al. The Impact of Microbiota on the Gut–Brain Axis: Examining the Complex Interplay and Implications. J. Clin. Med. 2023, 12, 5231. [Google Scholar] [CrossRef]
- Wu, T.; Liu, Y.-H.; Fu, Y.-C.; Liu, X.-M.; Zhou, X.-H. Direct evidence of sirtuin downregulation in the liver of non-alcoholic fatty liver disease patients. Ann. Clin. Lab. Sci. 2014, 44, 410–418. [Google Scholar]
- Bruce, K.D.; Szczepankiewicz, D.; Sihota, K.K.; Ravindraanandan, M.; Thomas, H.; Lillycrop, K.A.; Burdge, G.C.; Hanson, M.A.; Byrne, C.D.; Cagampang, F.R. Altered cellular redox status, sirtuin abundance and clock gene expression in a mouse model of developmentally primed NASH. Biochim. Biophys. Acta BBA Mol. Cell Biol. Lipids 2016, 1861, 584–593. [Google Scholar] [CrossRef][Green Version]
- Canfora, E.E.; Meex, R.C.R.; Venema, K.; Blaak, E.E. Gut microbial metabolites in obesity, NAFLD and T2DM. Nat. Rev. Endocrinol. 2019, 15, 261–273. [Google Scholar] [CrossRef]
- Aron-Wisnewsky, J.; Vigliotti, C.; Witjes, J.; Le, P.; Holleboom, A.G.; Verheij, J.; Nieuwdorp, M.; Clément, K. Gut microbiota and human NAFLD: Disentangling microbial signatures from metabolic disorders. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 279–297. [Google Scholar] [CrossRef]
- Loo, T.M.; Kamachi, F.; Watanabe, Y.; Yoshimoto, S.; Kanda, H.; Arai, Y.; Nakajima-Takagi, Y.; Iwama, A.; Koga, T.; Sugimoto, Y. Gut microbiota promotes obesity-associated liver cancer through PGE2-mediated suppression of antitumor immunity. Cancer Discov. 2017, 7, 522–538. [Google Scholar] [CrossRef]
- Bo, T.; Shao, S.; Wu, D.; Niu, S.; Zhao, J.; Gao, L. Relative variations of gut microbiota in disordered cholesterol metabolism caused by high-cholesterol diet and host genetics. Microbiologyopen 2017, 6, e00491. [Google Scholar] [CrossRef]
- Zhang, X.; Coker, O.O.; Chu, E.S.; Fu, K.; Lau, H.C.H.; Wang, Y.-X.; Chan, A.W.H.; Wei, H.; Yang, X.; Sung, J.J.Y.; et al. Dietary cholesterol drives fatty liver-associated liver cancer by modulating gut microbiota and metabolites. Gut 2020, 70, 761–774. [Google Scholar] [CrossRef] [PubMed]
- Takaki, A.; Kawai, D.; Yamamoto, K. Multiple Hits, Including Oxidative Stress, as Pathogenesis and Treatment Target in Non-Alcoholic Steatohepatitis (NASH). Int. J. Mol. Sci. 2013, 14, 20704–20728. [Google Scholar] [CrossRef] [PubMed]
- Henao-Mejia, J.; Elinav, E.; Jin, C.; Hao, L.; Mehal, W.Z.; Strowig, T.; Thaiss, C.A.; Kau, A.L.; Eisenbarth, S.C.; Jurczak, M.J.; et al. Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature 2012, 482, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Wiest, R.; Albillos, A.; Trauner, M.; Bajaj, J.S.; Jalan, R. Targeting the gut-liver axis in liver disease. J. Hepatol. 2017, 67, 1084–1103. [Google Scholar] [CrossRef]
- Zhou, D.; Pan, Q.; Xin, F.-Z.; Zhang, R.-N.; He, C.-X.; Chen, G.-Y.; Liu, C.; Chen, Y.-W.; Fan, J.-G. Sodium butyrate attenuates high-fat diet-induced steatohepatitis in mice by improving gut microbiota and gastrointestinal barrier. World J. Gastroenterol. 2017, 23, 60–75. [Google Scholar] [CrossRef]
- Kang, C.; Wang, B.; Kaliannan, K.; Wang, X.; Lang, H.; Hui, S.; Huang, L.; Zhang, Y.; Zhou, M.; Chen, M.; et al. Gut Microbiota Mediates the Protective Effects of Dietary Capsaicin against Chronic Low-Grade Inflammation and Associated Obesity Induced by High-Fat Diet. mBio 2017, 8, e00470-17. [Google Scholar] [CrossRef]
- Muccioli, G.G.; Naslain, D.; Bäckhed, F.; Reigstad, C.S.; Lambert, D.M.; Delzenne, N.M.; Cani, P.D. The endocannabinoid system links gut microbiota to adipogenesis. Mol. Syst. Biol. 2010, 6, 392. [Google Scholar] [CrossRef]
- Lam, Y.Y.; Ha, C.W.Y.; Campbell, C.R.; Mitchell, A.J.; Dinudom, A.; Oscarsson, J.; Cook, D.I.; Hunt, N.H.; Caterson, I.D.; Holmes, A.J.; et al. Increased Gut Permeability and Microbiota Change Associate with Mesenteric Fat Inflammation and Metabolic Dysfunction in Diet-Induced Obese Mice. PLoS ONE 2012, 7, e34233. [Google Scholar] [CrossRef]
- Everard, A.; Lazarevic, V.; Gaïa, N.; Johansson, M.; Ståhlman, M.; Bäckhed, F.; Delzenne, N.M.; Schrenzel, J.; Francois, P.; Cani, P.D. Microbiome of prebiotic-treated mice reveals novel targets involved in host response during obesity. ISME J. 2014, 8, 2116–2130. [Google Scholar] [CrossRef]
- Villanueva, A. Hepatocellular Carcinoma. N. Engl. J. Med. 2019, 380, 1450–1462. [Google Scholar] [CrossRef]
- Akinyemiju, T.; Abera, S.; Ahmed, M.; Alam, N.; Alemayohu, M.A.; Allen, C.; Al-Raddadi, R.; Alvis-Guzman, N.; Amoako, Y.; Artaman, A.; et al. The Burden of Primary Liver Cancer and Underlying Etiologies from 1990 to 2015 at the Global, Regional, and National Level: Results from the Global Burden of Disease Study 2015. JAMA Oncol. 2017, 3, 1683–1691. [Google Scholar] [PubMed]
- Jindal, A.; Thadi, A.; Shailubhai, K. Hepatocellular Carcinoma: Etiology and Current and Future Drugs. J. Clin. Exp. Hepatol. 2019, 9, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.J.; Cheung, R.; Ahmed, A. Nonalcoholic steatohepatitis is the most rapidly growing indication for liver transplantation in patients with hepatocellular carcinoma in the U.S. Hepatology 2014, 59, 2188–2195. [Google Scholar] [CrossRef] [PubMed]
- An, L.; Wirth, U.; Koch, D.; Schirren, M.; Drefs, M.; Koliogiannis, D.; Niess, H.; Andrassy, J.; Guba, M.; Bazhin, A.V.; et al. Metabolic Role of Autophagy in the Pathogenesis and Development of NAFLD. Metabolites 2023, 13, 101. [Google Scholar] [CrossRef]
- Itoh, M.; Suganami, T.; Nakagawa, N.; Tanaka, M.; Yamamoto, Y.; Kamei, Y.; Terai, S.; Sakaida, I.; Ogawa, Y. Melanocortin 4 Receptor–Deficient Mice as a Novel Mouse Model of Nonalcoholic Steatohepatitis. Am. J. Pathol. 2011, 179, 2454–2463. [Google Scholar] [CrossRef]
- Wang, J.; Xie, G. Bile Acid–microbiota Crosstalk in Gastrointestinal Inflammation and Carcinogenesis. Nat. Rev. Gastroenterol. Hepatol. 2017, 15, 111–128. [Google Scholar]
- Milosevic, I.; Vujovic, A.; Barac, A.; Djelic, M.; Korac, M.; Radovanovic Spurnic, A.; Gmizic, I.; Stevanovic, O.; Djordjevic, V.; Lekic, N.; et al. Gut-Liver Axis, Gut Microbiota, and Its Modulation in the Management of Liver Diseases: A Review of the Literature. Int. J. Mol. Sci. 2019, 20, 395. [Google Scholar] [CrossRef]
- Yu, L.; Li, Y.; Song, S.; Zhang, Y.; Wang, Y.; Wang, H.; Yang, Z.; Wang, Y. The dual role of sirtuins in cancer: Biological functions and implications. Front. Oncol. 2024, 14, 1384928. [Google Scholar] [CrossRef]
- Ling, S.; Li, J.; Shan, Q.; Dai, H.; Lu, D.; Wen, X.; Song, P.; Xie, H.; Zhou, L.; Liu, J.; et al. USP22 mediates the multidrug resistance of hepatocellular carcinoma via the SIRT1/AKT/MRP1 signaling pathway. Mol. Oncol. 2017, 11, 682–695. [Google Scholar] [CrossRef]
- Hao, C.; Zhu, P.-X.; Yang, X.; Han, Z.-P.; Jiang, J.-H.; Zong, C.; Zhang, X.-G.; Liu, W.-T.; Zhao, Q.-D.; Fan, T.-T.; et al. Overexpression of SIRT1 promotes metastasis through epithelial-mesenchymal transition in hepatocellular carcinoma. BMC Cancer 2014, 14, 978. [Google Scholar] [CrossRef]
- Ren, Z.; Li, A.; Jiang, J.; Zhou, L.; Yu, Z.; Lu, H.; Xie, H.; Chen, X.; Shao, L.; Zhang, R.; et al. Gut microbiome analysis as a tool towards targeted non-invasive biomarkers for early hepatocellular carcinoma. Gut 2018, 68, 1014–1023. [Google Scholar] [CrossRef] [PubMed]
- Pant, K.; Yadav, A.K.; Gupta, P.; Islam, R.; Saraya, A.; Venugopal, S.K. Butyrate induces ROS-mediated apoptosis by modulating miR-22/SIRT-1 pathway in hepatic cancer cells. Redox Biol. 2017, 12, 340–349. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yang, Y.; Yang, T.; Liu, Y.; Li, A.; Fu, S.; Wu, M.; Pan, Z.; Zhou, W. microRNA-22, downregulated in hepatocellular carcinoma and correlated with prognosis, suppresses cell proliferation and tumourigenicity. Br. J. Cancer 2010, 103, 1215–1220. [Google Scholar] [CrossRef] [PubMed]
- Qian, L.; Zhang, Y.; Wang, G.; Li, B.; Zhou, H.; Qiu, J.; Qin, L. MicroRNA-29 Regulates Tumor Progression and Survival Through miR-29a-SIRT1-Wnt/β-catenin Pathway in Hepatocellular Carcinoma. Biochem. Genet. 2023. preprint. [Google Scholar] [CrossRef]
- Wang, X.; Ling, S.; Wu, W.; Shan, Q.; Liu, P.; Wang, C.; Wei, X.; Ding, W.; Teng, X.; Xu, X. Ubiquitin-Specific Protease 22/Silent Information Regulator 1 Axis Plays a Pivotal Role in the Prognosis and 5-Fluorouracil Resistance in Hepatocellular Carcinoma. Dig. Dis. Sci. 2019, 65, 1064–1073. [Google Scholar]
- Xiong, H.; Ni, Z.; He, J.; Jiang, S.; Li, X.; Gong, W.; Zheng, L.; Chen, S.; Li, B.; Zhang, N.; et al. LncRNA HULC triggers autophagy via stabilizing Sirt1 and attenuates the chemosensitivity of HCC cells. Oncogene 2017, 36, 3528–3540. [Google Scholar] [CrossRef]
- Zhou, Z.-Q.; Guan, J.; Chen, S.; Sun, J.; Zhang, Z. Sirtuin 1 Protects the Mitochondria in Hepatocellular Carcinoma Cells via Suppressing Hypoxia-Induced Factor-1 Alpha Expression. Mol. Cell. Biochem. 2022. preprint. [Google Scholar] [CrossRef]
- Li, Y.; Xu, S.; Li, J.; Zheng, L.; Feng, M.; Wang, X.; Han, K.; Pi, H.; Li, M.; Huang, X.; et al. SIRT1 facilitates hepatocellular carcinoma metastasis by promoting PGC-1α-mediated mitochondrial biogenesis. Oncotarget 2016, 7, 29255–29274. [Google Scholar] [CrossRef]
- Herranz, D.; Muñoz-Martin, M.; Cañamero, M.; Mulero, F.; Martinez-Pastor, B.; Fernandez-Capetillo, O.; Serrano, M. Sirt1 improves healthy ageing and protects from metabolic syndrome-associated cancer. Nat. Commun. 2010, 1, 1–8. [Google Scholar] [CrossRef]
- Chen, H.-C.; Jeng, Y.-M.; Yuan, R.-H.; Hsu, H.-C.; Chen, Y.-L. SIRT1 Promotes Tumorigenesis and Resistance to Chemotherapy in Hepatocellular Carcinoma and its Expression Predicts Poor Prognosis. Ann. Surg. Oncol. 2011, 19, 2011–2019. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, B.; Wong, N.; Lo, A.W.; To, K.F.; Chan, A.W.; Ng, M.H.; Ho, C.Y.; Cheng, S.H.; Lai, P.B.; et al. Sirtuin 1 is upregulated in a subset of hepatocellular carcinomas where it is essential for telomere maintenance and tumor cell growth. Cancer Res. 2011, 71, 4138–4149. [Google Scholar] [CrossRef] [PubMed]
- Tanos, B.; Rodriguez-Boulan, E. The epithelial polarity program: Machineries involved and their hijacking by cancer. Oncogene 2008, 27, 6939–6957. [Google Scholar] [CrossRef] [PubMed]
- Portmann, S.; Fahrner, R.; Lechleiter, A.; Keogh, A.; Overney, S.; Laemmle, A.; Mikami, K.; Montani, M.; Tschan, M.P.; Candinas, D.; et al. Antitumor Effect of SIRT1 Inhibition in Human HCC Tumor Models In Vitro and In Vivo. Mol. Cancer Ther. 2013, 12, 499–508. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Chan, A.W.; To, K.-F.; Chen, W.; Zhang, Z.; Ren, J.; Song, C.; Cheung, Y.-S.; Lai, P.B.; Cheng, S.-H.; et al. SIRT2 overexpression in hepatocellular carcinoma mediates epithelial to mesenchymal transition by protein kinase B/glycogen synthase kinase-3β/β-catenin signaling. Hepatology 2013, 57, 2287–2298. [Google Scholar] [CrossRef]
- Huang, S.; Zhao, Z.; Tang, D.; Zhou, Q.; Li, Y.; Zhou, L.; Yin, Y.; Wang, Y.; Pan, Y.; Dorfman, R.G.; et al. Downregulation of SIRT2 Inhibits Invasion of Hepatocellular Carcinoma by Inhibiting Energy Metabolism. Transl. Oncol. 2017, 10, 917–927. [Google Scholar] [CrossRef]
- Song, C.-L.; Tang, H.; Ran, L.-K.; Ko, B.C.B.; Zhang, Z.-Z.; Chen, X.; Ren, J.-H.; Tao, N.-N.; Li, W.-Y.; Huang, A.-L.; et al. Sirtuin 3 inhibits hepatocellular carcinoma growth through the glycogen synthase kinase-3β/BCL2-associated X protein-dependent apoptotic pathway. Oncogene 2015, 35, 631–641. [Google Scholar] [CrossRef]
- Zeng, X.; Wang, N.; Zhai, H.; Wang, R.; Wu, J.; Pu, W. SIRT3 functions as a tumor suppressor in hepatocellular carcinoma. Tumor Biol. 2017, 39, 1010428317691178. [Google Scholar] [CrossRef]
- Liu, Y.; Cheng, W.; Yin, X.-M.; Jiang, B. The expression of SIRT3 in primary hepatocellular carcinoma and the mechanism of its tumor suppressing effects. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 978–998. [Google Scholar]
- Zhang, Y.-Y.; Zhou, L.-M. Sirt3 inhibits hepatocellular carcinoma cell growth through reducing Mdm2-mediated p53 degradation. Biochem. Biophys. Res. Commun. 2012, 423, 26–31. [Google Scholar] [CrossRef]
- Tao, N.-N.; Zhou, H.-Z.; Tang, H.; Cai, X.-F.; Zhang, W.-L.; Ren, J.-H.; Zhou, L.; Chen, X.; Chen, K.; Li, W.-Y.; et al. Sirtuin 3 enhanced drug sensitivity of human hepatoma cells through glutathione S-transferase pi 1/JNK signaling pathway. Oncotarget 2016, 7, 50117–50130. [Google Scholar] [CrossRef]
- Wang, Y.; Du, L.; Liang, X.; Meng, P.; Bi, L.; Wang, C.; Tang, B. Sirtuin 4 Depletion Promotes Hepatocellular Carcinoma Tumorigenesis Through Regulating Adenosine-Monophosphate–Activated Protein Kinase Alpha/Mammalian Target of Rapamycin Axis in Mice. Hepatology 2019, 69, 1614–1631. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.-Y.; Wong, D.K.-H.; Seto, W.-K.; Mak, L.-Y.; Cheung, T.-T.; Yuen, M.-F. Tumor suppressive role of mitochondrial sirtuin 4 in induction of G2/M cell cycle arrest and apoptosis in hepatitis B virus-related hepatocellular carcinoma. Cell Death Discov. 2021, 7, 88. [Google Scholar]
- Zhang, R.; Wang, C.; Tian, Y.; Yao, Y.; Mao, J.; Wang, H.; Li, Z.; Xu, Y.; Ye, M.; Wang, L. SIRT5 Promotes Hepatocellular Carcinoma Progression by Regulating Mitochondrial Apoptosis. J. Cancer 2019, 10, 3871–3882. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.; Xi, L.; Liu, Y.; Liu, R.; Wu, Z.; Jian, Z. SIRT5 promotes cell proliferation and invasion in hepatocellular carcinoma by targeting E2F1. Mol. Med. Rep. 2017, 17, 342–349. [Google Scholar] [CrossRef]
- Lee, N.; Ryu, H.G.; Kwon, J.-H.; Kim, D.-K.; Kim, S.R.; Wang, H.J.; Kim, K.-T.; Choi, K.Y. SIRT6 Depletion Suppresses Tumor Growth by Promoting Cellular Senescence Induced by DNA Damage in HCC. PLoS ONE 2016, 11, e0165835. [Google Scholar] [CrossRef]
- Zhang, Z.-G.; Qin, C.-Y. Sirt6 suppresses hepatocellular carcinoma cell growth via inhibiting the extracellular signal-regulated kinase signaling pathway. Mol. Med. Rep. 2013, 9, 882–888. [Google Scholar] [CrossRef]
- Zhang, C.; Yu, Y.; Huang, Q.; Tang, K. SIRT6 regulates the proliferation and apoptosis of hepatocellular carcinoma via the ERK1/2 signaling pathway. Mol. Med. Rep. 2019, 20, 1575–1582. [Google Scholar]
- Ran, L.-K.; Chen, Y.; Zhang, Z.-Z.; Tao, N.-N.; Ren, J.-H.; Zhou, L.; Tang, H.; Chen, X.; Chen, K.; Li, W.-Y.; et al. SIRT6 Overexpression Potentiates Apoptosis Evasion in Hepatocellular Carcinoma via BCL2-Associated X Protein–Dependent Apoptotic Pathway. Clin. Cancer Res. 2016, 22, 3372–3382. [Google Scholar] [CrossRef]
- Wang, M.; Lan, L.; Yang, F.; Jiang, S.; Xu, H.; Zhang, C.; Zhou, G.; Xia, H.; Xia, J. Hepatic SIRT6 deficit promotes liver tumorigenesis in the mice models. Genes Dis. 2020, 9, 789–796. [Google Scholar] [CrossRef]
- Kim, J.K.; Noh, J.H.; Jung, K.H.; Eun, J.W.; Bae, H.J.; Kim, M.G.; Chang, Y.G.; Shen, Q.; Park, W.S.; Lee, J.Y.; et al. Sirtuin7 oncogenic potential in human hepatocellular carcinoma and its regulation by the tumor suppressors MiR-125a-5p and MiR-125b. Hepatology 2013, 57, 1055–1067. [Google Scholar] [CrossRef]
- Kim, Y.; Jung, K.-Y.; Kim, Y.H.; Xu, P.; Kang, B.E.; Jo, Y.; Pandit, N.; Kwon, J.; Gariani, K.; Gariani, J.; et al. Inhibition of SIRT7 Overcomes Sorafenib Acquired Resistance by Suppressing ERK1/2 Phosphorylation via the DDX3X-mediated NLRP3 Inflammasome in Hepatocellular Carcinoma. Drug Resist. Updates 2024, 73, 101054. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Ding, C.; Yu, T.; Liu, B.; Tang, W.; Wang, Z.; Tang, X.; Liang, G.; Peng, J.; Zhang, X.; et al. SIRT7 Promotes Hippo/YAP Activation and Cancer Cell Proliferation in Hepatocellular Carcinoma via Suppressing MST1. Cancer Sci. 2024, 115, 1209–1223. [Google Scholar] [CrossRef] [PubMed]
- Okubo, H.; Nakatsu, Y.; Kushiyama, A.; Yamamotoya, T.; Matsunaga, Y.; Inoue, M.-K.; Fujishiro, M.; Sakoda, H.; Ohno, H.; Yoneda, M.; et al. Gut Microbiota as a Therapeutic Target for Metabolic Disorders. Curr. Med. Chem. 2018, 25, 984–1001. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Moens de Hase, E.; Van Hul, M. Gut Microbiota and Host Metabolism: From Proof of Concept to Therapeutic Intervention. Microorganisms 2021, 9, 1302. [Google Scholar] [CrossRef]
- Caussy, C.; Hsu, C.; Lo, M.T.; Liu, A.; Bettencourt, R.; Ajmera, V.H.; Bassirian, S.; Hooker, J.; Sy, E.; Richards, L.; et al. Link between gut-microbiome derived metabolite and shared gene-effects with hepatic steatosis and fibrosis in NAFLD. Hepatology 2018, 68, 918–932. [Google Scholar]
- Zeybel, M.; Arif, M.; Li, X.; Altay, O.; Yang, H.; Shi, M.; Akyildiz, M.; Saglam, B.; Gonenli, M.G.; Yigit, B.; et al. Multiomics Analysis Reveals the Impact of Microbiota on Host Metabolism in Hepatic Steatosis. Adv. Sci. 2022, 9, 2104373. [Google Scholar] [CrossRef]
- Liu, L.; Fu, Q.; Li, T.; Shao, K.; Zhu, X.; Cong, Y.; Zhao, X. Gut microbiota and butyrate contribute to nonalcoholic fatty liver disease in premenopause due to estrogen deficiency. PLoS ONE 2022, 17, e0262855. [Google Scholar] [CrossRef]
- Grąt, M.; Wronka, K.; Krasnodębski, M.; Masior, Ł.; Lewandowski, Z.; Kosińska, I.; Grąt, K.; Stypułkowski, J.; Rejowski, S.; Wasilewicz, M.; et al. Profile of Gut Microbiota Associated with the Presence of Hepatocellular Cancer in Patients with Liver Cirrhosis. Transplant. Proc. 2016, 48, 1687–1691. [Google Scholar] [CrossRef]
- Ni, J.; Huang, R.; Zhou, H.; Xu, X.; Li, Y.; Cao, P.; Zhong, K.; Ge, M.; Chen, X.; Hou, B.; et al. Analysis of the Relationship Between the Degree of Dysbiosis in Gut Microbiota and Prognosis at Different Stages of Primary Hepatocellular Carcinoma. Front. Microbiol. 2019, 10, 1458. [Google Scholar] [CrossRef]
- Ponziani, F.R.; Bhoori, S.; Castelli, C.; Putignani, L.; Rivoltini, L.; Del Chierico, F.; Sanguinetti, M.; Morelli, D.; Sterbini, F.P.; Petito, V.; et al. Hepatocellular Carcinoma Is Associated with Gut Microbiota Profile and Inflammation in Nonalcoholic Fatty Liver Disease. Hepatology 2019, 69, 107–120. [Google Scholar] [CrossRef]
- Zheng, R.; Wang, G.; Pang, Z.; Ran, N.; Gu, Y.; Guan, X.; Yuan, Y.; Zuo, X.; Pan, H.; Zheng, J.; et al. Liver cirrhosis contributes to the disorder of gut microbiota in patients with hepatocellular carcinoma. Cancer Med. 2020, 9, 4232–4250. [Google Scholar] [CrossRef] [PubMed]
- Behary, J.; Amorim, N.; Jiang, X.-T.; Raposo, A.; Gong, L.; McGovern, E.; Ibrahim, R.; Chu, F.; Stephens, C.; Jebeili, H.; et al. Gut microbiota impact on the peripheral immune response in non-alcoholic fatty liver disease related hepatocellular carcinoma. Nat. Commun. 2021, 12, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.; Sinclair, D.A.; Ellis, J.L.; Steegborn, C. Sirtuin activators and inhibitors: Promises, achievements, and challenges. Pharmacol. Ther. 2018, 188, 140–154. [Google Scholar] [CrossRef] [PubMed]
- Manjula, R.; Anuja, K.; Alcain, F.J. SIRT1 and SIRT2 Activity Control in Neurodegenerative Diseases. Front. Pharmacol. 2021, 11, 585821. [Google Scholar] [CrossRef]
- Tian, C.; Huang, R.; Xiang, M. SIRT1: Harnessing multiple pathways to hinder NAFLD. Pharmacol. Res. 2024, 203, 107155. [Google Scholar] [CrossRef]
- Lakhan, S.E.; Kirchgessner, A. Gut microbiota and sirtuins in obesity-related inflammation and bowel dysfunction. J. Transl. Med. 2011, 9, 202. [Google Scholar] [CrossRef]
- Bayram, H.M.; Majoo, F.M.; Ozturkcan, A. Polyphenols in the prevention and treatment of non-alcoholic fatty liver disease: An update of preclinical and clinical studies. Clin. Nutr. ESPEN 2021, 44, 1–14. [Google Scholar] [CrossRef]
- Wang, P.; Wang, J.; Li, D.; Ke, W.; Chen, F.; Hu, X. Targeting the gut microbiota with resveratrol: A demonstration of novel evidence for the management of hepatic steatosis. J. Nutr. Biochem. 2020, 81, 108363. [Google Scholar] [CrossRef]
- Du, F.; Huang, R.; Lin, D.; Wang, Y.; Yang, X.; Huang, X.; Zheng, B.; Chen, Z.; Huang, Y.; Wang, X.; et al. Resveratrol Improves Liver Steatosis and Insulin Resistance in Non-alcoholic Fatty Liver Disease in Association with the Gut Microbiota. Front. Microbiol. 2021, 12, 611323. [Google Scholar] [CrossRef]
- Prakash, V.; Bose, C.; Sunilkumar, D.; Cherian, R.M.; Thomas, S.S.; Nair, B.G. Resveratrol as a Promising Nutraceutical: Implications in Gut Microbiota Modulation, Inflammatory Disorders, and Colorectal Cancer. Int. J. Mol. Sci. 2024, 25, 3370. [Google Scholar] [CrossRef]
- Karabekir, S.C.; Ozgorgulu, A. Possible protective effects of resveratrol in hepatocellular carcinoma. Iran. J. Basic Med. Sci. 2020, 23, 71–78. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Li, Y.; Deng, X.; Xiao, X.; Zeng, J. Integrative evidence construction for resveratrol treatment of nonalcoholic fatty liver disease: Preclinical and clinical meta-analyses. Front. Pharmacol. 2023, 14, 1230783. [Google Scholar] [CrossRef]
- Tian, Y.; Ma, J.; Wang, W.; Zhang, L.; Xu, J.; Wang, K.; Li, D. Resveratrol supplement inhibited the NF-κB inflammation pathway through activating AMPKα-SIRT1 pathway in mice with fatty liver. Mol. Cell. Biochem. 2016, 422, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.-Y.; Ding, X.-Q.; Gu, T.-T.; Guo, W.-J.; Jiao, R.-Q.; Song, L.; Sun, Y.; Pan, Y.; Kong, L.-D. Pterostilbene improves hepatic lipid accumulation via the MiR-34a/Sirt1/SREBP-1 pathway in fructose-fed rats. J. Agric. Food Chem. 2020, 68, 1436–1446. [Google Scholar] [CrossRef]
- Estrela, J.M.; Ortega, A.; Mena, S.; Rodriguez, M.L.; Asensi, M. Pterostilbene: Biomedical applications. Crit. Rev. Clin. Lab. Sci. 2013, 50, 65–78. [Google Scholar] [CrossRef]
- Gómez-Zorita, S.; Fernández-Quintela, A.; Lasa, A.; Aguirre, L.; Rimando, A.M.; Portillo, M.P. Pterostilbene, a Dimethyl Ether Derivative of Resveratrol, Reduces Fat Accumulation in Rats Fed an Obesogenic Diet. J. Agric. Food Chem. 2014, 62, 8371–8378. [Google Scholar] [CrossRef]
- Wang, P.; Sang, S. Metabolism and pharmacokinetics of resveratrol and pterostilbene. BioFactors 2018, 44, 16–25. [Google Scholar] [CrossRef]
- Han, J.; Li, S.; Wang, W.; Jiang, X.; Liu, C.; Lei, L.; Li, Y.; Sheng, R.; Zhang, Y.; Wu, Y.; et al. SIRT1 Activator E1231 Alleviates Nonalcoholic Fatty Liver Disease by Regulating Lipid Metabolism. Curr. Issues Mol. Biol. 2023, 45, 5052–5070. [Google Scholar] [CrossRef]
- Vidyashankar, S.; Varma, R.S.; Patki, P.S. Quercetin ameliorate insulin resistance and up-regulates cellular antioxidants during oleic acid induced hepatic steatosis in HepG2 cells. Toxicol. Vitr. 2013, 27, 945–953. [Google Scholar] [CrossRef]
- Cao, P.; Wang, Y.; Zhang, C.; Sullivan, M.A.; Chen, W.; Jing, X.; Yu, H.; Li, F.; Wang, Q.; Zhou, Z.; et al. Quercetin ameliorates nonalcoholic fatty liver disease (NAFLD) via the promotion of AMPK-mediated hepatic mitophagy. J. Nutr. Biochem. 2023, 120, 109414. [Google Scholar] [CrossRef]
- Ying, H.-Z.; Liu, Y.-H.; Yu, B.; Wang, Z.-Y.; Zang, J.-N.; Yu, C.-H. Dietary quercetin ameliorates nonalcoholic steatohepatitis induced by a high-fat diet in gerbils. Food Chem. Toxicol. 2013, 52, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Liu, J.; Mei, G.; Chen, H.; Peng, S.; Zhao, Y.; Yao, P.; Tang, Y. Quercetin and non-alcoholic fatty liver disease: A review based on experimental data and bioinformatic analysis. Food Chem. Toxicol. 2021, 154, 112314. [Google Scholar] [CrossRef]
- Pham, T.X.; Bae, M.; Kim, M.-B.; Lee, Y.; Hu, S.; Kang, H.; Park, Y.-K.; Lee, J.-Y. Nicotinamide riboside, an NAD+ precursor, attenuates the development of liver fibrosis in a diet-induced mouse model of liver fibrosis. Biochim. Biophys. Acta BBA Mol. Basis Dis. 2019, 1865, 2451–2463. [Google Scholar] [CrossRef]
- Longo, L.; de Castro, J.M.; Keingeski, M.B.; Rampelotto, P.H.; Stein, D.J.; Guerreiro, G.T.S.; de Souza, V.E.G.; Cerski, C.T.S.; Uribe-Cruz, C.; Torres, I.L.; et al. Nicotinamide riboside and dietary restriction effects on gut microbiota and liver inflammatory and morphologic markers in cafeteria diet–induced obesity in rats. Nutrition 2023, 110, 112019. [Google Scholar] [CrossRef]
- Han, X.; Bao, X.; Lou, Q.; Xie, X.; Zhang, M.; Zhou, S.; Guo, H.; Jiang, G.; Shi, Q. Nicotinamide riboside exerts protective effect against aging-induced NAFLD-like hepatic dysfunction in mice. PeerJ 2019, 7, e7568. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, X.; Zhang, L.; Sun, D.; Ma, Y.; Bai, Y.; Bai, X.; Liang, X.; Liang, H. Nicotinamide Riboside Ameliorates Fructose-Induced Lipid Metabolism Disorders in Mice by Activating Browning of WAT, and May Be Also Related to the Regulation of Gut Microbiota. Nutrients 2024, 16, 3920. [Google Scholar] [CrossRef]
- Pang, N.; Hu, Q.; Zhou, Y.; Xiao, Y.; Li, W.; Ding, Y.; Chen, Y.; Ye, M.; Pei, L.; Li, Q.; et al. Nicotinamide Adenine Dinucleotide Precursor Suppresses Hepatocellular Cancer Progression in Mice. Nutrients 2023, 15, 1447. [Google Scholar] [CrossRef]
- Lin, X.; Zhang, J.; Chu, Y.; Nie, Q.; Zhang, J. Berberine prevents NAFLD and HCC by modulating metabolic disorders. Pharmacol. Ther. 2024, 254, 108593. [Google Scholar] [CrossRef]
- Dai, Y.; Zhu, W.; Zhou, J.; Shen, T. The combination of berberine and evodiamine ameliorates high-fat diet-induced non-alcoholic fatty liver disease associated with modulation of gut microbiota in rats. Braz. J. Med. Biol. Res. 2022, 55, e12096. [Google Scholar] [CrossRef]
- Shu, X.; Li, M.; Cao, Y.; Li, C.; Zhou, W.; Ji, G.; Zhang, L. Berberine Alleviates Non-alcoholic Steatohepatitis Through Modulating Gut Microbiota Mediated Intestinal FXR Activation. Front. Pharmacol. 2021, 12, 750826. [Google Scholar] [CrossRef]
- Ionita-Radu, F.; Patoni, C.; Nancoff, A.S.; Marin, F.-S.; Gaman, L.; Bucurica, A.; Socol, C.; Jinga, M.; Dutu, M.; Bucurica, S. Berberine Effects in Pre-Fibrotic Stages of Non-Alcoholic Fatty Liver Disease—Clinical and Pre-Clinical Overview and Systematic Review of the Literature. Int. J. Mol. Sci. 2024, 25, 4201. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Tian, G.; Zhuang, Z.; Chen, J.; You, N.; Zhuo, L.; Liang, B.; Song, Y.; Zang, S.; Liu, J.; et al. Berberine prevents non-alcoholic steatohepatitis-derived hepatocellular carcinoma by inhibiting inflammation and angiogenesis in mice. Am. J. Transl. Res. 2019, 11, 2668–2682. [Google Scholar] [PubMed]
- Jiang, H.; Mao, T.; Sun, Z.; Shi, L.; Han, X.; Zhang, Y.; Zhang, X.; Wang, J.; Hu, J.; Zhang, L.; et al. Yinchen Linggui Zhugan decoction ameliorates high fat diet-induced nonalcoholic fatty liver disease by modulation of SIRT1/Nrf2 signaling pathway and gut microbiota. Front. Microbiol. 2022, 13, 1001778. [Google Scholar] [CrossRef]
- Wang, S.; Li, X.; Zhang, B.; Li, Y.; Chen, K.; Qi, H.; Gao, M.; Rong, J.; Liu, L.; Wan, Y.; et al. Tangshen formula targets the gut microbiota to treat non-alcoholic fatty liver disease in HFD mice: A 16S rRNA and non-targeted metabolomics analyses. Biomed. Pharmacother. 2024, 173, 116405. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, H.; Li, X.; Li, N.; Wang, Q.; Liu, Y.; Liang, Q.; Shao, Z.; Zhang, N.; Zhao, T.; et al. Tangshen Formula Alleviates Hepatic Steatosis by Inducing Autophagy Through the AMPK/SIRT1 Pathway. Front. Physiol. 2019, 10, 494. [Google Scholar] [CrossRef]
- Kong, Q.; Zhang, B.-X.; Zhang, H.-J.; Yan, M.-H.; Li, P. Experimental study of Tangshen formulain improved lipid metabolism and phenotypic switch of macrophage in db/db mice. China J. Chin. Mater. Medica 2014, 41, 1693–1698. [Google Scholar] [CrossRef]
- Rahmani, S.; Asgary, S.; Askari, G.; Keshvari, M.; Hatamipour, M.; Feizi, A.; Sahebkar, A. Treatment of Non-alcoholic Fatty Liver Disease with Curcumin: A Randomized Placebo-controlled Trial. Phytotherapy Res. 2016, 30, 1540–1548. [Google Scholar] [CrossRef]
- Amel Zabihi, N.; Pirro, M.; Johnston, T.P.; Sahebkar, A. Is there a role for curcumin supplementation in the treatment of non-alcoholic fatty liver disease? The data suggest yes. Curr. Pharm. Des. 2017, 23, 969–982. [Google Scholar]
- Lee, D.E.; Lee, S.J.; Kim, S.J.; Lee, H.-S.; Kwon, O.-S. Curcumin Ameliorates Nonalcoholic Fatty Liver Disease through Inhibition of O-GlcNAcylation. Nutrients 2019, 11, 2702. [Google Scholar] [CrossRef]
- Du, S.; Zhu, X.; Zhou, N.; Zheng, W.; Zhou, W.; Li, X. Curcumin alleviates hepatic steatosis by improving mitochondrial function in postnatal overfed rats and fatty L02 cells through the SIRT3 pathway. Food Funct. 2022, 13, 2155–2171. [Google Scholar] [CrossRef]
- Gong, H.; Xu, H.; Li, M.; Zhang, D. Molecular mechanism and therapeutic significance of dihydromyricetin in nonalcoholic fatty liver disease. Eur. J. Pharmacol. 2022, 935, 175325. [Google Scholar] [CrossRef] [PubMed]
- Kang, L.; Ma, X.; Yu, F.; Xu, L.; Lang, L. Dihydromyricetin Alleviates Non-Alcoholic Fatty Liver Disease by Modulating Gut Microbiota and Inflammatory Signaling Pathways. J. Microbiol. Biotechnol. 2024, 34, 2637–2647. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Yang, J.; Hu, O.; Huang, J.; Ran, L.; Chen, M.; Zhang, Y.; Zhou, X.; Zhu, J.; Zhang, Q.; et al. Dihydromyricetin Ameliorates Nonalcoholic Fatty Liver Disease by Improving Mitochondrial Respiratory Capacity and Redox Homeostasis Through Modulation of SIRT3 Signaling. Antioxidants Redox Signal. 2019, 30, 163–183. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Chen, Z.; Zhang, C.; Xu, X.; Jin, J.; Zhan, W.; Han, T.; Wang, J. Dihydromyricetin ameliorates oleic acid-induced lipid accumulation in L02 and HepG2 cells by inhibiting lipogenesis and oxidative stress. Life Sci. 2016, 157, 131–139. [Google Scholar] [CrossRef]
- Bahar, A.N.; Keskin-Aktan, A.; Akarca-Dizakar, S.Ö.; Sonugür, G.; Akbulut, K.G. AGK2, a SIRT2 inhibitor, ameliorates D-galactose-induced liver fibrosis by inhibiting fibrogenic factors. J. Biochem. Mol. Toxicol. 2024, 38, e70000. [Google Scholar] [CrossRef]
- Hoffmann, G.; Breitenbücher, F.; Schuler, M.; Ehrenhofer-Murray, A.E. A Novel Sirtuin 2 (SIRT2) Inhibitor with p53-dependent Pro-apoptotic Activity in Non-small Cell Lung Cancer. J. Biol. Chem. 2014, 289, 5208–5216. [Google Scholar] [CrossRef]
- Wang, T.; Xu, Z.; Lu, Y.; Shi, J.; Liu, W.; Zhang, C.; Jiang, Z.; Qi, B.; Bai, L. Recent Progress on the Discovery of Sirt2 Inhibitors for the Treatment of Various Cancers. Curr. Top. Med. Chem. 2019, 19, 1051–1058. [Google Scholar] [CrossRef]
- Kaya, S.G.; Eren, G. Selective inhibition of SIRT2: A disputable therapeutic approach in cancer therapy. Bioorganic Chem. 2023, 143, 107038. [Google Scholar] [CrossRef]
- Palaz, F.; Ozsoz, M.; Zarrinpar, A.; Sahin, I. CRISPR in Targeted Therapy and Adoptive T Cell Immunotherapy for Hepatocellular Carcinoma. J. Hepatocell. Carcinoma 2024, 11, 975–995. [Google Scholar] [CrossRef]
- He, L.; Li, Z.; Su, D.; Du, H.; Zhang, K.; Zhang, W.; Wang, S.; Xie, F.; Qiu, Y.; Ma, S.; et al. Tumor Microenvironment-Responsive Nanocapsule Delivery CRISPR/Cas9 to Reprogram the Immunosuppressive Microenvironment in Hepatoma Carcinoma. Adv. Sci. 2024, 11, e2403858. [Google Scholar] [CrossRef]
- Yu, S.; Zhao, R.; Zhang, B.; Lai, C.; Li, L.; Shen, J.; Tan, X.; Shao, J. Research progress and application of the CRISPR/Cas9 gene-editing technology based on hepatocellular carcinoma. Asian J. Pharm. Sci. 2023, 18, 100828. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Meng, X.; Huang, C.; Li, J. Emerging role of silent information regulator 1 (SIRT1) in hepatocellular carcinoma: A potential therapeutic target. Tumor Biol. 2015, 36, 4063–4074. [Google Scholar] [CrossRef] [PubMed]
- Kamal, M.A.; Mandour, Y.M.; El-Aziz, M.K.A.; Stein, U.; El Tayebi, H.M. Small Molecule Inhibitors for Hepatocellular Carcinoma: Advances and Challenges. Molecules 2022, 27, 5537. [Google Scholar] [CrossRef]
- Binarci, B.; Kılıç, E.; Doğan, T.; Çetin-Atalay, R.; Kahraman, D.; Baytaş, S.N. Design, Synthesis, and Evaluation of Novel Indole-Based Small Molecules as Sirtuin Inhibitors with Anticancer Activities. Drug Dev. Res. 2024, 85, e70008. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Su, J.; Wang, H.; Chen, J.; Tian, Y.; Zhang, J.; Feng, T.; Di, L.; Lu, X.; Sheng, H.; et al. Discovery of Novel PROTAC SIRT6 Degraders with Potent Efficacy against Hepatocellular Carcinoma. J. Med. Chem. 2024, 67, 17319–17349. [Google Scholar] [CrossRef]
- Holmes, A.; Finger, C.; Morales-Scheihing, D.; Lee, J.; McCullough, L.D. Gut dysbiosis and age-related neurological diseases; an innovative approach for therapeutic interventions. Transl. Res. 2020, 226, 39–56. [Google Scholar] [CrossRef]
- Markowiak, P.; Śliżewska, K. Effects of Probiotics, Prebiotics, and Synbiotics on Human Health. Nutrients 2017, 9, 1021. [Google Scholar] [CrossRef]
- Manzoor, S.; Wani, S.M.; Mir, S.A.; Rizwan, D. Role of probiotics and prebiotics in mitigation of different diseases. Nutrition 2022, 96, 111602. [Google Scholar] [CrossRef]
- Upasana. Application of Probiotics, Prebiotics and Synbiotics in Maintaining Gut Health. Indian J. Nutr. Diet. 2022, 59, 388–397. [Google Scholar]
- Yadav, M.K.; Kumari, I.; Singh, B.; Sharma, K.K.; Tiwari, S.K. Probiotics, prebiotics and synbiotics: Safe options for next-generation therapeutics. Appl. Microbiol. Biotechnol. 2022, 106, 505–521. [Google Scholar] [CrossRef]
- Shao, T.; Hsu, R.; Hacein-Bey, C.; Zhang, W.; Gao, L.; Kurth, M.J.; Zhao, H.; Shuai, Z.; Leung, P.S.C. The Evolving Landscape of Fecal Microbial Transplantation. Clin. Rev. Allergy Immunol. 2023, 65, 101–120. [Google Scholar] [CrossRef] [PubMed]
- Abenavoli, L.; Maurizi, V.; Rinninella, E.; Tack, J.; Di Berardino, A.; Santori, P.; Rasetti, C.; Procopio, A.C.; Boccuto, L.; Scarpellini, E. Fecal Microbiota Transplantation in NAFLD Treatment. Medicina 2022, 58, 1559. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.-X.; Cheng, S.-L.; Liu, Y.-H.; Li, Y.; Zhang, R.; Li, N.-N.; Li, Z. Fecal microbiota transplantation for treatment of non-alcoholic fatty liver disease: Mechanism, clinical evidence, and prospect. World J. Gastroenterol. 2024, 30, 833–842. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).