Sexual Dimorphism in Cardiometabolic Diseases: From Development to Senescence and Therapeutic Approaches
Abstract
1. Introduction
2. Cardiovascular Disease
2.1. Sexual Dimorphism in Cardiovascular Health
2.2. Sexual Dimorphism in Cardiovascular Disease
2.2.1. Prevalence, Clinical Signs, and Risk Factors
2.2.2. Progression and Outcome
3. Metabolic Syndrome
3.1. Obesity and Dyslipidaemia
3.1.1. Obesity
3.1.2. Adipose Tissue
3.1.3. Leptin and Dyslipidaemia
3.2. Insulin Resistance and Dysglycaemia
3.3. Non-Alcoholic Fatty Liver Disease
3.4. Obstructive Sleep Apnoea
3.5. Polycystic Ovary Syndrome
4. Developmental Origins of Cardiometabolic Diseases
4.1. Preterm Birth
4.2. Intrauterine Growth Restriction
4.3. Sexual Dimorphism in Cardiometabolic Disorders Related to Low-Birth-Weight Individuals
4.4. Macrosomia and Large for Gestational Age
4.5. Sexual Dimorphism in Cardiometabolic Disorders Related to Individuals with Macrosomia
5. Mechanisms Potentially Involved in Sexual Dimorphism in Cardiometabolic Diseases
5.1. Genetic Factors
5.1.1. Y and X Chromosome-Specific Genes
5.1.2. Dosage and Compensation of Gene Expression and X-Inactivation Escape
5.2. Epigenetic Regulation
5.2.1. Epigenetic Marks and Their Influence
5.2.2. DNA Methylation
5.2.3. Long Non-Coding RNA
5.2.4. MicroRNAs
5.3. Maternal Nutrition
5.3.1. Maternal Overnutrition
5.3.2. Maternal Undernutrition
5.4. The Placenta and Embryogenesis
5.4.1. The Placenta
5.4.2. Embryogenesis
5.5. Sex Hormones
5.5.1. Effects of Oestrogens in Females
5.5.2. Effects of Oestrogens in Males
5.5.3. Effects of Androgens in Males
5.5.4. Effects of Androgens in Females
5.6. Microbiota
5.7. Cellular Metabolism and Mitochondria
5.8. Oxidative Stress
5.8.1. Reactive Oxygen Species and Source of Production
5.8.2. Antioxidant Defences
5.8.3. Oxidative Stress During the Prenatal Period
5.9. Programmed Cell Death
5.9.1. Cellular Senescence
5.9.2. Apoptosis
5.9.3. Autophagy
5.10. Inflammation
5.10.1. Immune Cells
5.10.2. Inflammasome and Cytokine Profile
5.10.3. Other Inflammatory Markers
5.11. Endothelial Dysfunction
5.11.1. Endothelium and Nitric Oxide
5.11.2. Hydrogen Sulphide
5.11.3. Sexual Dimorphism and Endothelial Function
5.11.4. Endothelial Progenitor Cells
5.12. Circadian Rhythm
5.13. Lifestyle
5.13.1. Stress
5.13.2. Sedentary Lifestyle/Physical Activity
5.13.3. Socioeconomic Status
5.13.4. Cigarette Smoking
5.13.5. Alcohol Consumption
5.13.6. Environmental Pollution Exposure
6. Therapeutic Options
6.1. Adverse Drug Reactions in Women and Dosage Adaptation
6.2. Antihypertensive Treatment
6.3. Cardiovascular Disease Treatments
6.3.1. Aspirin
6.3.2. Statins
6.3.3. Digitalis
6.4. Oxidative Stress Management
6.4.1. Antioxidant Compounds
6.4.2. Vitamin D
6.4.3. Dietary Antioxidant Capacity
6.5. Nutrition
6.5.1. Mediterranean Diet
6.5.2. Vegetarian Diet
6.5.3. Caloric Restriction
6.6. Cell Therapies
6.6.1. Mesenchymal Stem Cells
6.6.2. Induced Pluripotent Stem Cell Regenerative Therapy
6.7. Hormone Replacement Therapy
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Glossary
References
- Tokatli, M.R.; Sisti, L.G.; Marziali, E.; Nachira, L.; Rossi, M.F.; Amantea, C.; Moscato, U.; Malorni, W. Hormones and Sex-Specific Medicine in Human Physiopathology. Biomolecules 2022, 12, 413. [Google Scholar] [CrossRef]
- Prendergast, B.J.; Onishi, K.G.; Zucker, I. Female Mice Liberated for Inclusion in Neuroscience and Biomedical Research. Neurosci. Biobehav. Rev. 2014, 40, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Regitz-Zagrosek, V.; Kararigas, G. Mechanistic Pathways of Sex Differences in Cardiovascular Disease. Physiol. Rev. 2017, 97, 1–37. [Google Scholar] [CrossRef]
- Lassek, W.D.; Gaulin, S.J.C. Substantial but Misunderstood Human Sexual Dimorphism Results Mainly From Sexual Selection on Males and Natural Selection on Females. Front. Psychol. 2022, 13, 859931. [Google Scholar] [CrossRef]
- Regitz-Zagrosek, V.; Gebhard, C. Gender Medicine: Effects of Sex and Gender on Cardiovascular Disease Manifestation and Outcomes. Nat. Rev. Cardiol. 2023, 20, 236–247. [Google Scholar] [CrossRef]
- Coronado, M.J.; Fairweather, D.; Bruno, K.A. Sex Determines Cardiac Myocyte Stretch and Relaxation. Circ. Cardiovasc. Genet. 2017, 10, e001950. [Google Scholar] [CrossRef]
- Ventura-Clapier, R.; Dworatzek, E.; Seeland, U.; Kararigas, G.; Arnal, J.-F.; Brunelleschi, S.; Carpenter, T.C.; Erdmann, J.; Franconi, F.; Giannetta, E.; et al. Sex in Basic Research: Concepts in the Cardiovascular Field. Cardiovasc. Res. 2017, 113, 711–724. [Google Scholar] [CrossRef]
- Deegan, D.F.; Nigam, P.; Engel, N. Sexual Dimorphism of the Heart: Genetics, Epigenetics, and Development. Front. Cardiovasc. Med. 2021, 8, 668252. [Google Scholar] [CrossRef] [PubMed]
- Colafella, K.M.M.; Denton, K.M. Sex-Specific Differences in Hypertension and Associated Cardiovascular Disease. Nat. Rev. Nephrol. 2018, 14, 185–201. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, B.; Zhao, R.; Zhang, S.; Yu, X.; Li, Y. The Influence of Sex on Cardiac Physiology and Cardiovascular Diseases. J. Cardiovasc. Trans. Res. 2020, 13, 3–13. [Google Scholar] [CrossRef]
- Kerkhof, P.L.M.; Peace, R.A.; Heyndrickx, G.R.; Meijboom, L.J.; Sprengers, R.W.; Handly, N. Heart Function Analysis in Cardiac Patients with Focus on Sex-Specific Aspects. In Sex-Specific Analysis of Cardiovascular Function; Kerkhof, P.L.M., Miller, V.M., Eds.; Advances in Experimental Medicine and Biology; Springer International Publishing: Cham, Switzerland, 2018; pp. 361–377. ISBN 978-3-319-77932-4. [Google Scholar]
- Merz, A.A.; Cheng, S. Sex Differences in Cardiovascular Ageing. Heart 2016, 102, 825–831. [Google Scholar] [CrossRef] [PubMed]
- Greiten, L.E.; Holditch, S.J.; Arunachalam, S.P.; Miller, V.M. Should There Be Sex-Specific Criteria for the Diagnosis and Treatment of Heart Failure? J. Cardiovasc. Trans. Res. 2014, 7, 139–155. [Google Scholar] [CrossRef] [PubMed]
- Ventura-Clapier, R.; Piquereau, J.; Garnier, A.; Mericskay, M.; Lemaire, C.; Crozatier, B. Gender Issues in Cardiovascular Diseases. Focus on Energy Metabolism. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2020, 1866, 165722. [Google Scholar] [CrossRef]
- Dean, J.; Cruz, S.D.; Mehta, P.K.; Merz, C.N.B. Coronary Microvascular Dysfunction: Sex-Specific Risk, Diagnosis, and Therapy. Nat. Rev. Cardiol. 2015, 12, 406–414. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.; Kim, A.; Ebinger, J.E.; Niiranen, T.J.; Claggett, B.L.; Bairey Merz, C.N.; Cheng, S. Sex Differences in Blood Pressure Trajectories Over the Life Course. JAMA Cardiol. 2020, 5, 19–26. [Google Scholar] [CrossRef]
- Gerdts, E.; Sudano, I.; Brouwers, S.; Borghi, C.; Bruno, R.M.; Ceconi, C.; Cornelissen, V.; Diévart, F.; Ferrini, M.; Kahan, T.; et al. Sex Differences in Arterial Hypertension: A Scientific Statement from the ESC Council on Hypertension, the European Association of Preventive Cardiology, Association of Cardiovascular Nursing and Allied Professions, the ESC Council for Cardiology Practice, and the ESC Working Group on Cardiovascular Pharmacotherapy. Eur. Heart J. 2022, 43, 4777–4788. [Google Scholar] [CrossRef]
- Meloni, A.; Cadeddu, C.; Cugusi, L.; Donataccio, M.P.; Deidda, M.; Sciomer, S.; Gallina, S.; Vassalle, C.; Moscucci, F.; Mercuro, G.; et al. Gender Differences and Cardiometabolic Risk: The Importance of the Risk Factors. Int. J. Mol. Sci. 2023, 24, 1588. [Google Scholar] [CrossRef]
- Ortiz-Huidobro, R.I.; Velasco, M.; Larqué, C.; Escalona, R.; Hiriart, M. Molecular Insulin Actions Are Sexually Dimorphic in Lipid Metabolism. Front. Endocrinol. 2021, 12, 690484. [Google Scholar] [CrossRef]
- Argulian, E.; Patel, A.D.; Abramson, J.L.; Kulkarni, A.; Champney, K.; Palmer, S.; Weintraub, W.; Wenger, N.K.; Vaccarino, V. Gender Differences in Short-Term Cardiovascular Outcomes After Percutaneous Coronary Interventions. Am. J. Cardiol. 2006, 98, 48–53. [Google Scholar] [CrossRef]
- Benjamin, E.J.; Blaha, M.J.; Chiuve, S.E.; Cushman, M.; Das, S.R.; Deo, R.; De Ferranti, S.D.; Floyd, J.; Fornage, M.; Gillespie, C.; et al. Heart Disease and Stroke Statistics—2017 Update: A Report From the American Heart Association. Circulation 2017, 135, e146–e603. [Google Scholar] [CrossRef]
- Gažová, A.; Leddy, J.J.; Rexová, M.; Hlivák, P.; Hatala, R.; Kyselovič, J. Predictive Value of CHA2DS2-VASc Scores Regarding the Risk of Stroke and All-Cause Mortality in Patients with Atrial Fibrillation (CONSORT Compliant). Medicine 2019, 98, e16560. [Google Scholar] [CrossRef] [PubMed]
- Podesser, B.K.; Jain, M.; Ngoy, S.; Apstein, C.S.; Eberli, F.R. Unveiling Gender Differences in Demand Ischemia: A Study in a Rat Model of Genetic Hypertension. Eur. J. Cardio-Thorac. Surg. 2007, 31, 298–304. [Google Scholar] [CrossRef]
- Bubb, K.J.; Khambata, R.S.; Ahluwalia, A. Sexual Dimorphism in Rodent Models of Hypertension and Atherosclerosis. Br. J. Pharmacol. 2012, 167, 298–312. [Google Scholar] [CrossRef]
- Simoncini, S.; Coppola, H.; Rocca, A.; Bachmann, I.; Guillot, E.; Zippo, L.; Dignat-George, F.; Sabatier, F.; Bedel, R.; Wilson, A.; et al. Endothelial Colony-Forming Cells Dysfunctions Are Associated with Arterial Hypertension in a Rat Model of Intrauterine Growth Restriction. Int. J. Mol. Sci. 2021, 22, 10159. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.T.; James, S.K.; Wang, T.Y. A review of Sex-Specific Benefits and Risks of Antithrombotic Therapy in Acute Coronary Syndrome. Eur. Heart J. 2017, 38, 165–171. [Google Scholar] [CrossRef]
- Kvandova, M.; Puzserova, A.; Balis, P. Sexual Dimorphism in Cardiometabolic Diseases: The Role of AMPK. Int. J. Mol. Sci. 2023, 24, 11986. [Google Scholar] [CrossRef]
- Gerdts, E.; Regitz-Zagrosek, V. Sex Differences in Cardiometabolic Disorders. Nat. Med. 2019, 25, 1657–1666. [Google Scholar] [CrossRef]
- Wilson, P.W.F.; D’Agostino, R.B.; Sullivan, L.; Parise, H.; Kannel, W.B. Overweight and Obesity as Determinants of Cardiovascular Risk: The Framingham Experience. Arch. Intern. Med. 2002, 162, 1867–1872. [Google Scholar] [CrossRef]
- Lee, M.-J.; Fried, S.K. Sex-Dependent Depot Differences in Adipose Tissue Development and Function; Role of Sex Steroids. JOMES 2017, 26, 172–180. [Google Scholar] [CrossRef]
- Boulet, N.; Briot, A.; Galitzky, J.; Bouloumié, A. The Sexual Dimorphism of Human Adipose Depots. Biomedicines 2022, 10, 2615. [Google Scholar] [CrossRef]
- Liu, J.; Butler, K.R.; Buxbaum, S.G.; Sung, J.H.; Campbell, B.W.; Taylor, H.A. Leptinemia and Its Association with Stroke and Coronary Heart Disease in the Jackson Heart Study. Clin. Endocrinol. 2010, 72, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Wright, A.K.; Kontopantelis, E.; Emsley, R.; Buchan, I.; Mamas, M.A.; Sattar, N.; Ashcroft, D.M.; Rutter, M.K. Cardiovascular Risk and Risk Factor Management in Type 2 Diabetes Mellitus. Circulation 2019, 139, 2742–2753. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre, P.; Staels, B. Hepatic Sexual Dimorphism—Implications for Non-Alcoholic Fatty Liver Disease. Nat. Rev. Endocrinol. 2021, 17, 662–670. [Google Scholar] [CrossRef]
- Khalid, Y.S.; Dasu, N.R.; Suga, H.; Dasu, K.N.; Reja, D.; Shah, A.; McMahon, D.; Levine, A. Increased Cardiovascular Events and Mortality in Females with NAFLD: A Meta-Analysis. Am. J. Cardiovasc. Dis. 2020, 10, 258–271. [Google Scholar] [PubMed]
- Peppard, P.E.; Young, T.; Barnet, J.H.; Palta, M.; Hagen, E.W.; Hla, K.M. Increased Prevalence of Sleep-Disordered Breathing in Adults. Am. J. Epidemiol. 2013, 177, 1006–1014. [Google Scholar] [CrossRef]
- Lin, C.M.; Davidson, T.M.; Ancoli-Israel, S. Gender Differences in Obstructive Sleep Apnea and Treatment Implications. Sleep. Med. Rev. 2008, 12, 481–496. [Google Scholar] [CrossRef]
- Young, T.; Hutton, R.; Finn, L.; Badr, S.; Palta, M. The Gender Bias in Sleep Apnea Diagnosis. Are Women Missed Because They Have Different Symptoms? Arch. Intern. Med. 1996, 156, 2445–2451. [Google Scholar]
- Rubino, F.; Cummings, D.E.; Eckel, R.H.; Cohen, R.V.; Wilding, J.P.H.; Brown, W.A.; Stanford, F.C.; Batterham, R.L.; Farooqi, I.S.; Farpour-Lambert, N.J.; et al. Definition and Diagnostic Criteria of Clinical Obesity. Lancet Diabetes Endocrinol. 2025, 13, 221–262. [Google Scholar] [CrossRef]
- The Lancet Public Health. Tackling Obesity Seriously: The Time Has Come. Lancet Public Health 2018, 3, e153. [Google Scholar] [CrossRef]
- Link, J.C.; Reue, K. Genetic Basis for Sex Differences in Obesity and Lipid Metabolism. Annu. Rev. Nutr. 2017, 37, 225–245. [Google Scholar] [CrossRef]
- Parks, B.W.; Sallam, T.; Mehrabian, M.; Psychogios, N.; Hui, S.T.; Norheim, F.; Castellani, L.W.; Rau, C.D.; Pan, C.; Phun, J.; et al. Genetic Architecture of Insulin Resistance in the Mouse. Cell Metab. 2015, 21, 334–347. [Google Scholar] [CrossRef] [PubMed]
- Rosen, E.D.; Spiegelman, B.M. What We Talk About When We Talk About Fat. Cell 2014, 156, 20–44. [Google Scholar] [CrossRef] [PubMed]
- Uhrbom, M.; Muhl, L.; Genové, G.; Liu, J.; Palmgren, H.; Alexandersson, I.; Karlsson, F.; Zhou, A.-X.; Lunnerdal, S.; Gustafsson, S.; et al. Adipose Stem Cells Are Sexually Dimorphic Cells with Dual Roles as Preadipocytes and Resident Fibroblasts. Nat. Commun. 2024, 15, 7643. [Google Scholar] [CrossRef] [PubMed]
- MacCannell, A.D.V.; Futers, T.S.; Whitehead, A.; Moran, A.; Witte, K.K.; Roberts, L.D. Sexual Dimorphism in Adipose Tissue Mitochondrial Function and Metabolic Flexibility in Obesity. Int. J. Obes. 2021, 45, 1773–1781. [Google Scholar] [CrossRef]
- Wannamethee, S.G.; Tchernova, J.; Whincup, P.; Lowe, G.D.O.; Kelly, A.; Rumley, A.; Wallace, A.M.; Sattar, N. Plasma Leptin: Associations with Metabolic, Inflammatory and Haemostatic Risk Factors for Cardiovascular Disease. Atherosclerosis 2007, 191, 418–426. [Google Scholar] [CrossRef]
- Poetsch, M.S.; Strano, A.; Guan, K. Role of Leptin in Cardiovascular Diseases. Front. Endocrinol. 2020, 11, 354. [Google Scholar] [CrossRef]
- Lau, E.S.; Paniagua, S.M.; Guseh, J.S.; Bhambhani, V.; Zanni, M.V.; Courchesne, P.; Lyass, A.; Larson, M.G.; Levy, D.; Ho, J.E. Sex Differences in Circulating Biomarkers of Cardiovascular Disease. J. Am. Coll. Cardiol. 2019, 74, 1543–1553. [Google Scholar] [CrossRef]
- Lagou, V.; Mägi, R.; Hottenga, J.-J.; Grallert, H.; Perry, J.R.B.; Bouatia-Naji, N.; Marullo, L.; Rybin, D.; Jansen, R.; Min, J.L.; et al. Sex-Dimorphic Genetic Effects and Novel Loci for Fasting Glucose and Insulin Variability. Nat. Commun. 2021, 12, 24. [Google Scholar] [CrossRef]
- Choi, J.H.; Sohn, W.; Cho, Y.K. The Effect of Moderate Alcohol Drinking in Nonalcoholic Fatty Liver Disease. Clin. Mol. Hepatol. 2020, 26, 662–669. [Google Scholar] [CrossRef]
- Khan, R.S.; Bril, F.; Cusi, K.; Newsome, P.N. Modulation of Insulin Resistance in Nonalcoholic Fatty Liver Disease. Hepatology 2019, 70, 711–724. [Google Scholar] [CrossRef]
- Zarghamravanbakhsh, P.; Frenkel, M.; Poretsky, L. Metabolic Causes and Consequences of Nonalcoholic Fatty Liver Disease (NAFLD). Metab. Open 2021, 12, 100149. [Google Scholar] [CrossRef]
- Lin, S.; Huang, J.; Wang, M.; Kumar, R.; Liu, Y.; Liu, S.; Wu, Y.; Wang, X.; Zhu, Y. Comparison of MAFLD and NAFLD Diagnostic Criteria in Real World. Liver Int. 2020, 40, 2082–2089. [Google Scholar] [CrossRef] [PubMed]
- Tietjens, J.R.; Claman, D.; Kezirian, E.J.; De Marco, T.; Mirzayan, A.; Sadroonri, B.; Goldberg, A.N.; Long, C.; Gerstenfeld, E.P.; Yeghiazarians, Y. Obstructive Sleep Apnea in Cardiovascular Disease: A Review of the Literature and Proposed Multidisciplinary Clinical Management Strategy. JAHA 2019, 8, e010440. [Google Scholar] [CrossRef] [PubMed]
- Gami, A. Obstructive Sleep Apnoea, Metabolic Syndrome, and Cardiovascular Outcomes. Eur. Heart J. 2004, 25, 709–711. [Google Scholar] [CrossRef]
- Vgontzas, A.N.; Bixler, E.O.; Chrousos, G.P. Sleep Apnea Is a Manifestation of the Metabolic Syndrome. Sleep. Med. Rev. 2005, 9, 211–224. [Google Scholar] [CrossRef] [PubMed]
- Lavie, L.; Polotsky, V. Cardiovascular Aspects in Obstructive Sleep Apnea Syndrome—Molecular Issues, Hypoxia and Cytokine Profiles. Respiration 2009, 78, 361–370. [Google Scholar] [CrossRef]
- Pialoux, V.; Hanly, P.J.; Foster, G.E.; Brugniaux, J.V.; Beaudin, A.E.; Hartmann, S.E.; Pun, M.; Duggan, C.T.; Poulin, M.J. Effects of Exposure to Intermittent Hypoxia on Oxidative Stress and Acute Hypoxic Ventilatory Response in Humans. Am. J. Respir. Crit. Care Med. 2009, 180, 1002–1009. [Google Scholar] [CrossRef]
- Lavie, L. Oxidative Stress—A Unifying Paradigm in Obstructive Sleep Apnea and Comorbidities. Progress. Cardiovasc. Dis. 2009, 51, 303–312. [Google Scholar] [CrossRef]
- Polotsky, V.Y.; Li, J.; Punjabi, N.M.; Rubin, A.E.; Smith, P.L.; Schwartz, A.R.; O’Donnell, C.P. Intermittent Hypoxia Increases Insulin Resistance in Genetically Obese Mice. J. Physiol. 2003, 552, 253–264. [Google Scholar] [CrossRef]
- Li, J.; Nanayakkara, A.; Jun, J.; Savransky, V.; Polotsky, V.Y. Effect of Deficiency in SREBP Cleavage-Activating Protein on Lipid Metabolism during Intermittent Hypoxia. Physiol. Genom. 2007, 31, 273–280. [Google Scholar] [CrossRef]
- Li, J.; Savransky, V.; Nanayakkara, A.; Smith, P.L.; O’Donnell, C.P.; Polotsky, V.Y. Hyperlipidemia and Lipid Peroxidation Are Dependent on the Severity of Chronic Intermittent Hypoxia. J. Appl. Physiol. 2007, 102, 557–563. [Google Scholar] [CrossRef]
- Escobar-Morreale, H.F. Polycystic Ovary Syndrome: Definition, Aetiology, Diagnosis and Treatment. Nat. Rev. Endocrinol. 2018, 14, 270–284. [Google Scholar] [CrossRef]
- Chen, W.; Pang, Y. Metabolic Syndrome and PCOS: Pathogenesis and the Role of Metabolites. Metabolites 2021, 11, 869. [Google Scholar] [CrossRef] [PubMed]
- Lujan, M.E.; Chizen, D.R.; Pierson, R.A. Diagnostic Criteria for Polycystic Ovary Syndrome: Pitfalls and Controversies. J. Obstet. Gynaecol. Can. 2008, 30, 671–679. [Google Scholar] [CrossRef]
- Wild, R.A.; Carmina, E.; Diamanti-Kandarakis, E.; Dokras, A.; Escobar-Morreale, H.F.; Futterweit, W.; Lobo, R.; Norman, R.J.; Talbott, E.; Dumesic, D.A. Assessment of Cardiovascular Risk and Prevention of Cardiovascular Disease in Women with the Polycystic Ovary Syndrome: A Consensus Statement by the Androgen Excess and Polycystic Ovary Syndrome (AE-PCOS) Society. J. Clin. Endocrinol. Metab. 2010, 95, 2038–2049. [Google Scholar] [CrossRef] [PubMed]
- Bil, E.; Dilbaz, B.; Cirik, D.A.; Ozelci, R.; Ozkaya, E.; Dilbaz, S. Metabolic Syndrome and Metabolic Risk Profile According to Polycystic Ovary Syndrome Phenotype. J. Obstet. Gynaecol. 2016, 42, 837–843. [Google Scholar] [CrossRef]
- Diamanti-Kandarakis, E.; Papavassiliou, A.G. Molecular Mechanisms of Insulin Resistance in Polycystic Ovary Syndrome. Trends Mol. Med. 2006, 12, 324–332. [Google Scholar] [CrossRef]
- Carmina, E.; Koyama, T.; Chang, L.; Stanczyk, F.Z.; Lobo, R.A. Does Ethnicity Influence the Prevalence of Adrenal Hyperandrogenism and Insulin Resistance in Polycystic Ovary Syndrome? Am. J. Obstet. Gynecol. 1992, 167, 1807–1812. [Google Scholar] [CrossRef]
- Lumeng, C.N.; Saltiel, A.R. Inflammatory Links between Obesity and Metabolic Disease. J. Clin. Investig. 2011, 121, 2111–2117. [Google Scholar] [CrossRef]
- Cao, H. Adipocytokines in Obesity and Metabolic Disease. J. Endocrinol. 2014, 220, T47–T59. [Google Scholar] [CrossRef]
- Yilmaz, B.; Vellanki, P.; Ata, B.; Yildiz, B.O. Metabolic Syndrome, Hypertension, and Hyperlipidemia in Mothers, Fathers, Sisters, and Brothers of Women with Polycystic Ovary Syndrome: A Systematic Review and Meta-Analysis. Fertil. Steril. 2018, 109, 356–364.e32. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, B.; Vellanki, P.; Ata, B.; Yildiz, B.O. Diabetes Mellitus and Insulin Resistance in Mothers, Fathers, Sisters, and Brothers of Women with Polycystic Ovary Syndrome: A Systematic Review and Meta-Analysis. Fertil. Steril. 2018, 110, 523–533.e14. [Google Scholar] [CrossRef] [PubMed]
- Crisosto, N.; Echiburú, B.; Maliqueo, M.; Luchsinger, M.; Rojas, P.; Recabarren, S.; Sir-Petermann, T. Reproductive and Metabolic Features during Puberty in Sons of Women with Polycystic Ovary Syndrome. Endocr. Connect. 2017, 6, 607–613. [Google Scholar] [CrossRef]
- Recabarren, S.E.; Smith, R.; Rios, R.; Maliqueo, M.; Echiburú, B.; Codner, E.; Cassorla, F.; Rojas, P.; Sir-Petermann, T. Metabolic Profile in Sons of Women with Polycystic Ovary Syndrome. J. Clin. Endocrinol. Metab. 2008, 93, 1820–1826. [Google Scholar] [CrossRef]
- Hsu, C.-N.; Hou, C.-Y.; Hsu, W.-H.; Tain, Y.-L. Early-Life Origins of Metabolic Syndrome: Mechanisms and Preventive Aspects. Int. J. Mol. Sci. 2021, 22, 11872. [Google Scholar] [CrossRef] [PubMed]
- Osmond, C.; Barker, D.J.; Winter, P.D.; Fall, C.H.; Simmonds, S.J. Early Growth and Death from Cardiovascular Disease in Women. BMJ 1993, 307, 1519–1524. [Google Scholar] [CrossRef]
- Joung, K.E.; Lee, J.; Kim, J.H. Long-Term Metabolic Consequences of Intrauterine Growth Restriction. Curr. Pediatr. Rep. 2020, 8, 45–55. [Google Scholar] [CrossRef]
- Juan, J.; Yang, H. Early Life 1000 Days: Opportunities for Preventing Adult Diseases. Chin. Med. J. 2022, 135, 516–518. [Google Scholar] [CrossRef]
- Velazquez, M.A.; Fleming, T.P.; Watkins, A.J. Periconceptional Environment and the Developmental Origins of Disease. J. Endocrinol. 2019, 242, T33–T49. [Google Scholar] [CrossRef]
- Blackmore, H.L.; Ozanne, S.E. Maternal Diet-Induced Obesity and Offspring Cardiovascular Health. J. Dev. Orig. Health Dis. 2013, 4, 338–347. [Google Scholar] [CrossRef]
- Ohuma, E.O.; Moller, A.-B.; Bradley, E.; Chakwera, S.; Hussain-Alkhateeb, L.; Lewin, A.; Okwaraji, Y.B.; Mahanani, W.R.; Johansson, E.W.; Lavin, T.; et al. National, Regional, and Global Estimates of Preterm Birth in 2020, with Trends from 2010: A Systematic Analysis. Lancet 2023, 402, 1261–1271. [Google Scholar] [CrossRef] [PubMed]
- Goldenberg, R.L.; Culhane, J.F.; Iams, J.D.; Romero, R. Epidemiology and Causes of Preterm Birth. Lancet 2008, 371, 75–84. [Google Scholar] [CrossRef]
- Natarajan, G.; Shankaran, S. Short- and Long-Term Outcomes of Moderate and Late Preterm Infants. Am. J. Perinatol. 2016, 33, 305–317. [Google Scholar] [CrossRef] [PubMed]
- Crump, C.; Sundquist, J.; Winkleby, M.A.; Sundquist, K. Preterm Birth and Risk of Chronic Kidney Disease from Childhood into Mid-Adulthood: National Cohort Study. BMJ 2019, 365, l1346. [Google Scholar] [CrossRef] [PubMed]
- Sipola-Leppänen, M.; Vääräsmäki, M.; Tikanmäki, M.; Matinolli, H.-M.; Miettola, S.; Hovi, P.; Wehkalampi, K.; Ruokonen, A.; Sundvall, J.; Pouta, A.; et al. Cardiometabolic Risk Factors in Young Adults Who Were Born Preterm. Am. J. Epidemiol. 2015, 181, 861–873. [Google Scholar] [CrossRef]
- Hovi, P.; Andersson, S.; Eriksson, J.G.; Järvenpää, A.-L.; Strang-Karlsson, S.; Mäkitie, O.; Kajantie, E. Glucose Regulation in Young Adults with Very Low Birth Weight. N. Engl. J. Med. 2007, 356, 2053–2063. [Google Scholar] [CrossRef]
- Hovi, P.; Vohr, B.; Ment, L.R.; Doyle, L.W.; McGarvey, L.; Morrison, K.M.; Evensen, K.A.I.; van der Pal, S.; Grunau, R.E.; APIC Adults Born Preterm International Collaboration; et al. Blood Pressure in Young Adults Born at Very Low Birth Weight. Hypertension 2016, 68, 880–887. [Google Scholar] [CrossRef]
- Markopoulou, P.; Papanikolaou, E.; Analytis, A.; Zoumakis, E.; Siahanidou, T. Preterm Birth as a Risk Factor for Metabolic Syndrome and Cardiovascular Disease in Adult Life: A Systematic Review and Meta-Analysis. J. Pediatr. 2019, 210, 69–80.e5. [Google Scholar] [CrossRef]
- Yzydorczyk, C.; Armengaud, J.B.; Peyter, A.C.; Chehade, H.; Cachat, F.; Juvet, C.; Siddeek, B.; Simoncini, S.; Sabatier, F.; Dignat-George, F.; et al. Endothelial Dysfunction in Individuals Born after Fetal Growth Restriction: Cardiovascular and Renal Consequences and Preventive Approaches. J. Dev. Orig. Health Dis. 2017, 8, 448–464. [Google Scholar] [CrossRef]
- Sharma, D.; Shastri, S.; Sharma, P. Intrauterine Growth Restriction: Antenatal and Postnatal Aspects. Clin. Med. Insights Pediatr. 2016, 10, CMPed.S40070. [Google Scholar] [CrossRef]
- Crispi, F.; Miranda, J.; Gratacós, E. Long-Term Cardiovascular Consequences of Fetal Growth Restriction: Biology, Clinical Implications, and Opportunities for Prevention of Adult Disease. Am. J. Obstet. Gynecol. 2018, 218, S869–S879. [Google Scholar] [CrossRef] [PubMed]
- Hales, C.N.; Barker, D.J.; Clark, P.M.; Cox, L.J.; Fall, C.; Osmond, C.; Winter, P.D. Fetal and Infant Growth and Impaired Glucose Tolerance at Age 64. BMJ 1991, 303, 1019–1022. [Google Scholar] [CrossRef] [PubMed]
- Funaki, S.; Ogawa, K.; Ozawa, N.; Okamoto, A.; Morisaki, N.; Sago, H. Differences in Pregnancy Complications and Outcomes by Fetal Gender among Japanese Women: A Multicenter Cross-Sectional Study. Sci. Rep. 2020, 10, 18810. [Google Scholar] [CrossRef] [PubMed]
- Barker, D.J.P.; Osmond, C.; Winter, P.D.; Margetts, B.; Simmonds, S.J. Weight in Infancy and Death from Ischaemic Heart Disease. Lancet 1989, 334, 577–580. [Google Scholar] [CrossRef]
- Dasgupta, S.; Gill, A. Hypotension in the Very Low Birthweight Infant: The Old, the New, and the Uncertain. Arch. Dis. Child. Fetal Neonatal Ed. 2003, 88, F450–F454. [Google Scholar] [CrossRef]
- Seri, I.; Tan, R.; Evans, J. Cardiovascular Effects of Hydrocortisone in Preterm Infants With Pressor-Resistant Hypotension. Pediatrics 2001, 107, 1070–1074. [Google Scholar] [CrossRef]
- Evans, N.; Kluckow, M.; Simmons, M.; Osborn, D. Which to Measure, Systemic or Organ Blood Flow? Middle Cerebral Artery and Superior Vena Cava Flow in Very Preterm Infants. Arch. Dis. Child. Fetal Neonatal Ed. 2002, 87, F181–F184. [Google Scholar] [CrossRef]
- Dyson, R.M.; Palliser, H.K.; Lakkundi, A.; de Waal, K.; Latter, J.L.; Clifton, V.L.; Wright, I.M.R. Early Microvascular Changes in the Preterm Neonate: A Comparative Study of the Human and Guinea Pig. Physiol. Rep. 2014, 2, e12145. [Google Scholar] [CrossRef]
- Stark, M.J.; Clifton, V.L.; Wright, I.M.R. Sex-Specific Differences in Peripheral Microvascular Blood Flow in Preterm Infants. Pediatr. Res. 2008, 63, 415–419. [Google Scholar] [CrossRef]
- Hille, E.T.M.; Weisglas-Kuperus, N.; Van Goudoever, J.B.; Jacobusse, G.W.; Ens-Dokkum, M.H.; De Groot, L.; Wit, J.M.; Geven, W.B.; Kok, J.H.; De Kleine, M.J.K.; et al. Functional Outcomes and Participation in Young Adulthood for Very Preterm and Very Low Birth Weight Infants: The Dutch Project on Preterm and Small for Gestational Age Infants at 19 Years of Age. Pediatrics 2007, 120, e587–e595. [Google Scholar] [CrossRef]
- Goldstein, D.S.; Eisenhofer, G.; Kopin, I.J. Sources and Significance of Plasma Levels of Catechols and Their Metabolites in Humans. J. Pharmacol. Exp. Ther. 2003, 305, 800–811. [Google Scholar] [CrossRef] [PubMed]
- Gabory, A.; Attig, L.; Junien, C. Sexual Dimorphism in Environmental Epigenetic Programming. Mol. Cell. Endocrinol. 2009, 304, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Al Salmi, I.; Hoy, W.E.; Kondalsamy-Chennakesavan, S.; Wang, Z.; Gobe, G.C.; Barr, E.L.M.; Shaw, J.E. Disorders of Glucose Regulation in Adults and Birth Weight: Results from the Australian Diabetes, Obesity and Lifestyle (AusDiab) Study. Diabetes Care 2008, 31, 159–164. [Google Scholar] [CrossRef]
- Hallan, S.; Euser, A.M.; Irgens, L.M.; Finken, M.J.J.; Holmen, J.; Dekker, F.W. Effect of Intrauterine Growth Restriction on Kidney Function at Young Adult Age: The Nord Trøndelag Health (HUNT 2) Study. Am. J. Kidney Dis. 2008, 51, 10–20. [Google Scholar] [CrossRef]
- Poston, L.; Caleyachetty, R.; Cnattingius, S.; Corvalán, C.; Uauy, R.; Herring, S.; Gillman, M.W. Preconceptional and Maternal Obesity: Epidemiology and Health Consequences. Lancet Diabetes Endocrinol. 2016, 4, 1025–1036. [Google Scholar] [CrossRef] [PubMed]
- Asplund, C.A.; Seehusen, D.A.; Callahan, T.L.; Olsen, C. Percentage Change in Antenatal Body Mass Index as a Predictor of Neonatal Macrosomia. Ann. Fam. Med. 2008, 6, 550–554. [Google Scholar] [CrossRef]
- Nkwabong, E.; Nzalli Tangho, G.R. Risk Factors for Macrosomia. J. Obstet. Gynaecol. India 2015, 65, 226–229. [Google Scholar] [CrossRef]
- Szmyd, B.; Biedrzycka, M.; Karuga, F.F.; Rogut, M.; Strzelecka, I.; Respondek-Liberska, M. Interventricular Septal Thickness as a Diagnostic Marker of Fetal Macrosomia. J. Clin. Med. 2021, 10, 949. [Google Scholar] [CrossRef]
- Whincup, P.H.; Kaye, S.J.; Owen, C.G.; Huxley, R.; Cook, D.G.; Anazawa, S.; Barrett-Connor, E.; Bhargava, S.K.; Birgisdottir, B.E.; Carlsson, S.; et al. Birth Weight and Risk of Type 2 Diabetes: A Systematic Review. JAMA 2008, 300, 2886–2897. [Google Scholar] [CrossRef]
- Kereliuk, S.M.; Dolinsky, V.W. Recent Experimental Studies of Maternal Obesity, Diabetes during Pregnancy and the Developmental Origins of Cardiovascular Disease. Int. J. Mol. Sci. 2022, 23, 4467. [Google Scholar] [CrossRef]
- Skilton, M.R.; Siitonen, N.; Würtz, P.; Viikari, J.S.A.; Juonala, M.; Seppälä, I.; Laitinen, T.; Lehtimäki, T.; Taittonen, L.; Kähönen, M.; et al. High Birth Weight Is Associated With Obesity and Increased Carotid Wall Thickness in Young Adults: The Cardiovascular Risk in Young Finns Study. ATVB 2014, 34, 1064–1068. [Google Scholar] [CrossRef]
- Hermann, G.M.; Dallas, L.M.; Haskell, S.E.; Roghair, R.D. Neonatal Macrosomia Is an Independent Risk Factor for Adult Metabolic Syndrome. Neonatology 2010, 98, 238–244. [Google Scholar] [CrossRef]
- Ehret, G.B.; Caulfield, M.J. Genes for Blood Pressure: An Opportunity to Understand Hypertension. Eur. Heart J. 2013, 34, 951–961. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, J.-G. Genome-Wide Association Studies of Hypertension and Several Other Cardiovascular Diseases. Pulse 2019, 6, 169–186. [Google Scholar] [CrossRef] [PubMed]
- Imes, C.C.; Lewis, F.M. Family History of Cardiovascular Disease (CVD), Perceived CVD Risk, and Health-Related Behavior: A Review of the Literature. J. Cardiovasc. Nurs. 2014, 29, 108–129. [Google Scholar] [CrossRef]
- Ferreira, C.; Trindade, F.; Ferreira, R.; Neves, J.S.; Leite-Moreira, A.; Amado, F.; Santos, M.; Nogueira-Ferreira, R. Sexual Dimorphism in Cardiac Remodeling: The Molecular Mechanisms Ruled by Sex Hormones in the Heart. J. Mol. Med. 2022, 100, 245–267. [Google Scholar] [CrossRef] [PubMed]
- Huby, R.D.J.; Glaves, P.; Jackson, R. The Incidence of Sexually Dimorphic Gene Expression Varies Greatly between Tissues in the Rat. PLoS ONE 2014, 9, e115792. [Google Scholar] [CrossRef]
- Tower, J. Mitochondrial Maintenance Failure in Aging and Role of Sexual Dimorphism. Arch. Biochem. Biophys. 2015, 576, 17–31. [Google Scholar] [CrossRef]
- Regitz-Zagrosek, V.; Lehmkuhl, E.; Weickert, M.O. Gender Differences in the Metabolic Syndrome and Their Role for Cardiovascular Disease. Clin. Res. Cardiol. 2006, 95, 147. [Google Scholar] [CrossRef]
- Del Pinto, R.; Ferri, C. Inflammation-Accelerated Senescence and the Cardiovascular System: Mechanisms and Perspectives. Int. J. Mol. Sci. 2018, 19, 3701. [Google Scholar] [CrossRef]
- Gabory, A.; Roseboom, T.J.; Moore, T.; Moore, L.G.; Junien, C. Placental Contribution to the Origins of Sexual Dimorphism in Health and Diseases: Sex Chromosomes and Epigenetics. Biol. Sex. Differ. 2013, 4, 5. [Google Scholar] [CrossRef]
- Thej, C.; Kishore, R. Epigenetic Regulation of Sex Dimorphism in Cardiovascular Health. Can. J. Physiol. Pharmacol. 2024, 102, 498–510. [Google Scholar] [CrossRef]
- Webster, A.L.H.; Yan, M.S.-C.; Marsden, P.A. Epigenetics and Cardiovascular Disease. Can. J. Cardiol. 2013, 29, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Lau, E.S.; Binek, A.; Parker, S.J.; Shah, S.H.; Zanni, M.V.; Van Eyk, J.E.; Ho, J.E. Sexual Dimorphism in Cardiovascular Biomarkers: Clinical and Research Implications. Circ. Res. 2022, 130, 578–592. [Google Scholar] [CrossRef]
- Qi, S.; Al Mamun, A.; Ngwa, C.; Romana, S.; Ritzel, R.; Arnold, A.P.; McCullough, L.D.; Liu, F. X Chromosome Escapee Genes Are Involved in Ischemic Sexual Dimorphism through Epigenetic Modification of Inflammatory Signals. J. Neuroinflamm. 2021, 18, 70. [Google Scholar] [CrossRef]
- Asllanaj, E.; Zhang, X.; Ochoa Rosales, C.; Nano, J.; Bramer, W.M.; Portilla-Fernandez, E.; Braun, K.V.E.; Gonzalez-Jaramillo, V.; Ahrens, W.; Ikram, A.; et al. Sexually Dimorphic DNA-Methylation in Cardiometabolic Health: A Systematic Review. Maturitas 2020, 135, 6–26. [Google Scholar] [CrossRef]
- Ransohoff, J.D.; Wei, Y.; Khavari, P.A. The Functions and Unique Features of Long Intergenic Non-Coding RNA. Nat. Rev. Mol. Cell Biol. 2018, 19, 143–157. [Google Scholar] [CrossRef]
- Jusic, A.; Salgado-Somoza, A.; Paes, A.B.; Stefanizzi, F.M.; Martínez-Alarcón, N.; Pinet, F.; Martelli, F.; Devaux, Y.; Robinson, E.L.; Novella, S. Approaching Sex Differences in Cardiovascular Non-Coding RNA Research. Int. J. Mol. Sci. 2020, 21, 4890. [Google Scholar] [CrossRef]
- Lee, J.T.; Bartolomei, M.S. X-Inactivation, Imprinting, and Long Noncoding RNAs in Health and Disease. Cell 2013, 152, 1308–1323. [Google Scholar] [CrossRef]
- Johnson, E.K.; Matkovich, S.J.; Nerbonne, J.M. Regional Differences in mRNA and lncRNA Expression Profiles in Non-Failing Human Atria and Ventricles. Sci. Rep. 2018, 8, 13919. [Google Scholar] [CrossRef]
- Lalem, T.; Zhang, L.; Scholz, M.; Burkhardt, R.; Saccheti, V.; Teren, A.; Thiery, J.; Devaux, Y. Cyclin Dependent Kinase Inhibitor 1 C Is a Female-Specific Marker of Left Ventricular Function after Acute Myocardial Infarction. Int. J. Cardiol. 2019, 274, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Bi, Y.; Wang, Y.; Sun, X. Recent Advances of LncRNA H19 in Diabetes. Horm. Metab. Res. 2022, 54, 212–219. [Google Scholar] [CrossRef]
- Yue, Y.; Yue, Y.; Fan, Z.; Meng, Y.; Wen, C.; An, Y.; Yao, Y.; Li, X. The Long Noncoding RNA Lnc-H19 Is Important for Endurance Exercise by Maintaining Slow Muscle Fiber Types. J. Biol. Chem. 2023, 299, 105281. [Google Scholar] [CrossRef]
- Alfaifi, M.; Verma, A.K.; Alshahrani, M.Y.; Joshi, P.C.; Alkhathami, A.G.; Ahmad, I.; Hakami, A.R.; Beg, M.M.A. Assessment of Cell-Free Long Non-Coding RNA-H19 and miRNA-29a, miRNA-29b Expression and Severity of Diabetes. Diabetes Metab. Syndr. Obes. 2020, 13, 3727–3737. [Google Scholar] [CrossRef]
- Geng, T.; Liu, Y.; Xu, Y.; Jiang, Y.; Zhang, N.; Wang, Z.; Carmichael, G.G.; Taylor, H.S.; Li, D.; Huang, Y. H19 lncRNA Promotes Skeletal Muscle Insulin Sensitivity in Part by Targeting AMPK. Diabetes 2018, 67, 2183–2198. [Google Scholar] [CrossRef]
- Lundsgaard, A.-M.; Kiens, B. Gender Differences in Skeletal Muscle Substrate Metabolism—Molecular Mechanisms and Insulin Sensitivity. Front. Endocrinol. 2014, 5, 195. [Google Scholar] [CrossRef] [PubMed]
- Lapikova-Bryhinska, T.; Ministrini, S.; Puspitasari, Y.M.; Kraler, S.; Mohamed, S.A.; Costantino, S.; Paneni, F.; Khetsuriani, M.; Bengs, S.; Liberale, L.; et al. Long Non-Coding RNAs H19 and NKILA Are Associated with the Risk of Death and Lacunar Stroke in the Elderly Population. Eur. J. Intern. Med. 2024, 123, 94–101. [Google Scholar] [CrossRef]
- O’Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front. Endocrinol. 2018, 9, 402. [Google Scholar] [CrossRef] [PubMed]
- Dudink, E.; Florijn, B.; Weijs, B.; Duijs, J.; Luermans, J.; Peeters, F.; Schurgers, L.; Wildberger, J.; Schotten, U.; Bijkerk, R.; et al. Vascular Calcification and Not Arrhythmia in Idiopathic Atrial Fibrillation Associates with Sex Differences in Diabetic Microvascular Injury miRNA Profiles. MIRNA 2019, 8, 127–134. [Google Scholar] [CrossRef]
- Howard, E.W.; Yang, X. microRNA Regulation in Estrogen Receptor-Positive Breast Cancer and Endocrine Therapy. Biol. Proced. Online 2018, 20, 17. [Google Scholar] [CrossRef]
- Wang, Y.-T.; Tsai, P.-C.; Liao, Y.-C.; Hsu, C.-Y.; Juo, S.-H.H. Circulating microRNAs Have a Sex-Specific Association with Metabolic Syndrome. J. Biomed. Sci. 2013, 20, 72. [Google Scholar] [CrossRef] [PubMed]
- Selvamani, A.; Williams, M.H.; Miranda, R.C.; Sohrabji, F. Circulating miRNA Profiles Provide a Biomarker for Severity of Stroke Outcomes Associated with Age and Sex in a Rat Model. Clin. Sci. 2014, 127, 77–89. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, M.; Kawasaki, T.; Matsuda, T.; Arai, T.; Gojo, S.; Takeuchi, J.K. Correction: Sexual Dimorphisms of mRNA and miRNA in Human/Murine Heart Disease. PLoS ONE 2020, 15, e0229750. [Google Scholar] [CrossRef] [PubMed]
- Desai, M.; Ross, M.G. Maternal-Infant Nutrition and Development Programming of Offspring Appetite and Obesity. Nutr. Rev. 2020, 78, 25–31. [Google Scholar] [CrossRef]
- De Paula Simino, L.A.; De Fante, T.; Figueiredo Fontana, M.; Oliveira Borges, F.; Torsoni, M.A.; Milanski, M.; Velloso, L.A.; Souza Torsoni, A. Lipid Overload during Gestation and Lactation Can Independently Alter Lipid Homeostasis in Offspring and Promote Metabolic Impairment after New Challenge to High-Fat Diet. Nutr. Metab. 2017, 14, 16. [Google Scholar] [CrossRef]
- Huang, Y.; Osorio Mendoza, J.; Li, M.; Jin, Z.; Li, B.; Wu, Y.; Togo, J.; Speakman, J.R. Impact of Graded Maternal Dietary Fat Content on Offspring Susceptibility to High-fat Diet in Mice. Obesity 2021, 29, 2055–2067. [Google Scholar] [CrossRef]
- Fraser, A.; Tilling, K.; Macdonald-Wallis, C.; Sattar, N.; Brion, M.-J.; Benfield, L.; Ness, A.; Deanfield, J.; Hingorani, A.; Nelson, S.M.; et al. Association of Maternal Weight Gain in Pregnancy With Offspring Obesity and Metabolic and Vascular Traits in Childhood. Circulation 2010, 121, 2557–2564. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, A.; Zhou, W.; Li, B.; Zhang, L.; Rudolf, A.M.; Jin, Z.; Hambly, C.; Wang, G.; Speakman, J.R. Maternal Dietary Fat during Lactation Shapes Single Nucleus Transcriptomic Profile of Postnatal Offspring Hypothalamus in a Sexually Dimorphic Manner in Mice. Nat. Commun. 2024, 15, 2382. [Google Scholar] [CrossRef]
- Sasson, I.E.; Vitins, A.P.; Mainigi, M.A.; Moley, K.H.; Simmons, R.A. Pre-Gestational vs Gestational Exposure to Maternal Obesity Differentially Programs the Offspring in Mice. Diabetologia 2015, 58, 615–624. [Google Scholar] [CrossRef]
- Ludwig, D.S.; Peterson, K.E.; Gortmaker, S.L. Relation between Consumption of Sugar-Sweetened Drinks and Childhood Obesity: A Prospective, Observational Analysis. Lancet 2001, 357, 505–508. [Google Scholar] [CrossRef]
- Elliott, S.S.; Keim, N.L.; Stern, J.S.; Teff, K.; Havel, P.J. Fructose, Weight Gain, and the Insulin Resistance Syndrome. Am. J. Clin. Nutr. 2002, 76, 911–922. [Google Scholar] [CrossRef] [PubMed]
- Gracner, T.; Boone, C.; Gertler, P.J. Exposure to Sugar Rationing in the First 1000 Days of Life Protected against Chronic Disease. Science 2024, 386, 1043–1048. [Google Scholar] [CrossRef]
- Andres, A.; Hull, H.R.; Shankar, K.; Casey, P.H.; Cleves, M.A.; Badger, T.M. Longitudinal Body Composition of Children Born to Mothers with Normal Weight, Overweight, and Obesity. Obesity 2015, 23, 1252–1258. [Google Scholar] [CrossRef]
- Oostvogels, A.J.J.M.; Hof, M.H.P.; Gademan, M.G.J.; Roseboom, T.J.; Stronks, K.; Vrijkotte, T.G.M. Does Maternal Pre-Pregnancy Overweight or Obesity Influence Offspring’s Growth Patterns from Birth up to 7 Years? The ABCD-Study. Early Hum. Dev. 2017, 113, 62–70. [Google Scholar] [CrossRef]
- Krishnaveni, G.V.; Veena, S.R.; Hill, J.C.; Kehoe, S.; Karat, S.C.; Fall, C.H.D. Intrauterine Exposure to Maternal Diabetes Is Associated With Higher Adiposity and Insulin Resistance and Clustering of Cardiovascular Risk Markers in Indian Children. Diabetes Care 2010, 33, 402–404. [Google Scholar] [CrossRef]
- Kruse, M.; Seki, Y.; Vuguin, P.M.; Du, X.Q.; Fiallo, A.; Glenn, A.S.; Singer, S.; Breuhahn, K.; Katz, E.B.; Charron, M.J. High-Fat Intake During Pregnancy and Lactation Exacerbates High-Fat Diet-Induced Complications in Male Offspring in Mice. Endocrinology 2013, 154, 3565–3576. [Google Scholar] [CrossRef] [PubMed]
- Nivoit, P.; Morens, C.; Van Assche, F.A.; Jansen, E.; Poston, L.; Remacle, C.; Reusens, B. Established Diet-Induced Obesity in Female Rats Leads to Offspring Hyperphagia, Adiposity and Insulin Resistance. Diabetologia 2009, 52, 1133–1142. [Google Scholar] [CrossRef]
- Tobiansky, D.J.; Kachkovski, G.V.; Enos, R.T.; Schmidt, K.L.; Murphy, E.A.; Floresco, S.B.; Soma, K.K. Maternal Sucrose Consumption Alters Behaviour and Steroids in Adult Rat Offspring. J. Endocrinol. 2021, 251, 161–180. [Google Scholar] [CrossRef]
- Bleker, L.S.; de Rooij, S.R.; Painter, R.C.; Ravelli, A.C.; Roseboom, T.J. Cohort Profile: The Dutch Famine Birth Cohort (DFBC)—A Prospective Birth Cohort Study in the Netherlands. BMJ Open 2021, 11, e042078. [Google Scholar] [CrossRef]
- Ravelli, A.C.; van Der Meulen, J.H.; Osmond, C.; Barker, D.J.; Bleker, O.P. Obesity at the Age of 50 y in Men and Women Exposed to Famine Prenatally. Am. J. Clin. Nutr. 1999, 70, 811–816. [Google Scholar] [CrossRef]
- Lumey, L.; Stein, A.D.; Kahn, H.S.; Romijn, J. Lipid Profiles in Middle-Aged Men and Women after Famine Exposure during Gestation: The Dutch Hunger Winter Families Study1234. Am. J. Clin. Nutr. 2009, 89, 1737–1743. [Google Scholar] [CrossRef]
- Loria, A.S.; Goulopoulou, S.; Bourque, S.L.; Davidge, S.T. Sex Differences in Developmental Origins of Cardiovascular Disease. In Sex Differences in Cardiovascular Physiology and Pathophysiology; Elsevier: Amsterdam, The Netherlands, 2019; pp. 253–289. ISBN 978-0-12-813197-8. [Google Scholar]
- Keshavjee, B.; Lambelet, V.; Coppola, H.; Viertl, D.; Prior, J.O.; Kappeler, L.; Armengaud, J.-B.; Chouraqui, J.-P.; Chehade, H.; Vanderriele, P.-E.; et al. Stress-Induced Premature Senescence Related to Oxidative Stress in the Developmental Programming of Nonalcoholic Fatty Liver Disease in a Rat Model of Intrauterine Growth Restriction. Antioxidants 2022, 11, 1695. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Arzapalo, P.Y.; Rodríguez-Rodríguez, P.; Ramiro-Cortijo, D.; Gil-Ortega, M.; Somoza, B.; De Pablo, Á.L.L.; González, M.D.C.; Arribas, S.M. Fetal Undernutrition Induces Resistance Artery Remodeling and Stiffness in Male and Female Rats Independent of Hypertension. Biomedicines 2020, 8, 424. [Google Scholar] [CrossRef] [PubMed]
- Lewis, R.M.; Desoye, G. Placental Lipid and Fatty Acid Transfer in Maternal Overnutrition. Ann. Nutr. Metab. 2017, 70, 228–231. [Google Scholar] [CrossRef]
- Rasool, A.; Mahmoud, T.; Mathyk, B.; Kaneko-Tarui, T.; Roncari, D.; White, K.O.; O’Tierney-Ginn, P. Obesity Downregulates Lipid Metabolism Genes in First Trimester Placenta. Sci. Rep. 2022, 12, 19368. [Google Scholar] [CrossRef] [PubMed]
- Wankhade, U.D.; Zhong, Y.; Kang, P.; Alfaro, M.; Chintapalli, S.V.; Thakali, K.M.; Shankar, K. Enhanced Offspring Predisposition to Steatohepatitis with Maternal High-Fat Diet Is Associated with Epigenetic and Microbiome Alterations. PLoS ONE 2017, 12, e0175675. [Google Scholar] [CrossRef]
- Muralimanoharan, S.; Li, C.; Nakayasu, E.S.; Casey, C.P.; Metz, T.O.; Nathanielsz, P.W.; Maloyan, A. Sexual Dimorphism in the Fetal Cardiac Response to Maternal Nutrient Restriction. J. Mol. Cell Cardiol. 2017, 108, 181–193. [Google Scholar] [CrossRef]
- Woodman, A.G.; Noble, R.M.N.; Panahi, S.; Gragasin, F.S.; Bourque, S.L. Perinatal Iron Deficiency Combined with a High Salt Diet in Adulthood Causes Sex-Dependent Vascular Dysfunction in Rats. J. Physiol. 2019, 597, 4715–4728. [Google Scholar] [CrossRef]
- Ghidini, A.; Salafia, C.M. Gender Differences of Placental Dysfunction in Severe Prematurity. BJOG 2005, 112, 140–144. [Google Scholar] [CrossRef]
- Khong, Y.; Brosens, I. Defective Deep Placentation. Best Pract. Res. Clin. Obstet. Gynaecol. 2011, 25, 301–311. [Google Scholar] [CrossRef]
- Simpson, E.R.; Davis, S.R. Minireview: Aromatase and the Regulation of Estrogen Biosynthesis—Some New Perspectives. Endocrinology 2001, 142, 4589–4594. [Google Scholar] [CrossRef]
- Lista, P.; Straface, E.; Brunelleschi, S.; Franconi, F.; Malorni, W. On the Role of Autophagy in Human Diseases: A Gender Perspective. J. Cell. Mol. Med. 2011, 15, 1443–1457. [Google Scholar] [CrossRef] [PubMed]
- Nettleship, J.E.; Jones, T.H.; Channer, K.S.; Jones, R.D. Physiological Testosterone Replacement Therapy Attenuates Fatty Streak Formation and Improves High-Density Lipoprotein Cholesterol in the Tfm Mouse. Circulation 2007, 116, 2427–2434. [Google Scholar] [CrossRef] [PubMed]
- Koller, A. Perspectives: Microvascular Endothelial Dysfunction and Gender. Eur. Heart J. Suppl. 2014, 16, A16–A19. [Google Scholar] [CrossRef] [PubMed]
- Sharma, G.; Prossnitz, E.R. G-Protein-Coupled Estrogen Receptor (GPER) and Sex-Specific Metabolic Homeostasis. Adv. Exp. Med. Biol. 2017, 1043, 427–453. [Google Scholar] [CrossRef]
- Mauvais-Jarvis, F.; Clegg, D.J.; Hevener, A.L. The Role of Estrogens in Control of Energy Balance and Glucose Homeostasis. Endocr. Rev. 2013, 34, 309–338. [Google Scholar] [CrossRef]
- Faustini-Fustini, M.; Rochira, V.; Carani, C. Oestrogen Deficiency in Men: Where Are We Today? Eur. J. Endocrinol. 1999, 140, 111–129. [Google Scholar] [CrossRef]
- Pottelbergh, I.V.; Braeckman, L.; Bacquer, D.D.; Backer, G.D.; Kaufman, J.M. Differential Contribution of Testosterone and Estradiol in the Determination of Cholesterol and Lipoprotein Profile in Healthy Middle-Aged Men. Atherosclerosis 2003, 166, 95–102. [Google Scholar] [CrossRef]
- Muller, M.; Van Der Schouw, Y.T.; Thijssen, J.H.H.; Grobbee, D.E. Endogenous Sex Hormones and Cardiovascular Disease in Men. J. Clin. Endocrinol. Metab. 2003, 88, 5076–5086. [Google Scholar] [CrossRef]
- Sader, M.A.; McCredie, R.J.; Griffiths, K.A.; Wishart, S.M.; Handelsman, D.J.; Celermajer, D.S. Oestradiol Improves Arterial Endothelial Function in Healthy Men Receiving Testosterone. Clin. Endocrinol. 2001, 54, 175–181. [Google Scholar] [CrossRef]
- Rosano, G.M.C.; Sheiban, I.; Massaro, R.; Pagnotta, P.; Marazzi, G.; Vitale, C.; Mercuro, G.; Volterrani, M.; Aversa, A.; Fini, M. Low Testosterone Levels Are Associated with Coronary Artery Disease in Male Patients with Angina. Int. J. Impot. Res. 2007, 19, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Connelly, P.J.; Casey, H.; Montezano, A.C.; Touyz, R.M.; Delles, C. Sex Steroids Receptors, Hypertension, and Vascular Ageing. J. Hum. Hypertens. 2022, 36, 120–125. [Google Scholar] [CrossRef]
- Ding, E.L.; Song, Y.; Malik, V.S.; Liu, S. Sex Differences of Endogenous Sex Hormones and Risk of Type 2 DiabetesA Systematic Review and Meta-Analysis. JAMA 2006, 295, 1288–1299. [Google Scholar] [CrossRef] [PubMed]
- Laughlin, G.A.; Barrett-Connor, E.; Bergstrom, J. Low Serum Testosterone and Mortality in Older Men. J. Clin. Endocrinol. Metab. 2008, 93, 68–75. [Google Scholar] [CrossRef]
- Haffner, S.M.; Valdez, R.A.; Mykkänen, L.; Stern, M.P.; Katz, M.S. Decreased Testosterone and Dehydroepiandrosterone Sulfate Concentrations Are Associated with Increased Insulin and Glucose Concentrations in Nondiabetic Men. Metab. Clin. Exp. 1994, 43, 599–603. [Google Scholar] [CrossRef]
- Stellato, R.K.; Feldman, H.A.; Hamdy, O.; Horton, E.S.; McKinlay, J.B. Testosterone, Sex Hormone-Binding Globulin, and the Development of Type 2 Diabetes in Middle-Aged Men: Prospective Results from the Massachusetts Male Aging Study. Diabetes Care 2000, 23, 490–494. [Google Scholar] [CrossRef]
- English, K. Men with Coronary Artery Disease Have Lower Levels of Androgens than Men with Normal Coronary Angiograms. Eur. Heart J. 2000, 21, 890–894. [Google Scholar] [CrossRef] [PubMed]
- Seidell, J.C.; Björntorp, P.; Sjöström, L.; Kvist, H.; Sannerstedt, R. Visceral Fat Accumulation in Men Is Positively Associated with Insulin, Glucose, and C-Peptide Levels, but Negatively with Testosterone Levels. Metab. Clin. Exp. 1990, 39, 897–901. [Google Scholar] [CrossRef]
- Creatsa, M.; Armeni, E.; Stamatelopoulos, K.; Rizos, D.; Georgiopoulos, G.; Kazani, M.; Alexandrou, A.; Dendrinos, S.; Augoulea, A.; Papamichael, C.; et al. Circulating Androgen Levels Are Associated with Subclinical Atherosclerosis and Arterial Stiffness in Healthy Recently Menopausal Women. Metab. Clin. Exp. 2012, 61, 193–201. [Google Scholar] [CrossRef]
- Bernini, G.P.; Sgro’, M.; Moretti, A.; Argenio, G.F.; Barlascini, C.O.; Cristofani, R.; Salvetti, A. Endogenous Androgens and Carotid Intimal-Medial Thickness in Women. J. Clin. Endocrinol. Metab. 1999, 84, 2008–2012. [Google Scholar] [CrossRef]
- Stanhewicz, A.E.; Wenner, M.M.; Stachenfeld, N.S. Sex Differences in Endothelial Function Important to Vascular Health and Overall Cardiovascular Disease Risk across the Lifespan. Am. J. Physiol. Heart Circ. Physiol. 2018, 315, H1569–H1588. [Google Scholar] [CrossRef] [PubMed]
- Ley, R.E.; Turnbaugh, P.J.; Klein, S.; Gordon, J.I. Human Gut Microbes Associated with Obesity. Nature 2006, 444, 1022–1023. [Google Scholar] [CrossRef] [PubMed]
- Wexler, H.M. Bacteroides: The Good, the Bad, and the Nitty-Gritty. Clin. Microbiol. Rev. 2007, 20, 593–621. [Google Scholar] [CrossRef]
- den Besten, G.; van Eunen, K.; Groen, A.K.; Venema, K.; Reijngoud, D.-J.; Bakker, B.M. The Role of Short-Chain Fatty Acids in the Interplay between Diet, Gut Microbiota, and Host Energy Metabolism. J. Lipid Res. 2013, 54, 2325–2340. [Google Scholar] [CrossRef] [PubMed]
- Hollister, E.B.; Gao, C.; Versalovic, J. Compositional and Functional Features of the Gastrointestinal Microbiome and Their Effects on Human Health. Gastroenterology 2014, 146, 1449–1458. [Google Scholar] [CrossRef]
- Mariat, D.; Firmesse, O.; Levenez, F.; Guimarăes, V.; Sokol, H.; Doré, J.; Corthier, G.; Furet, J.-P. The Firmicutes/Bacteroidetes Ratio of the Human Microbiota Changes with Age. BMC Microbiol. 2009, 9, 123. [Google Scholar] [CrossRef]
- Indiani, C.M.D.S.P.; Rizzardi, K.F.; Castelo, P.M.; Ferraz, L.F.C.; Darrieux, M.; Parisotto, T.M. Childhood Obesity and Firmicutes/Bacteroidetes Ratio in the Gut Microbiota: A Systematic Review. Child. Obes. 2018, 14, 501–509. [Google Scholar] [CrossRef]
- Kasai, C.; Sugimoto, K.; Moritani, I.; Tanaka, J.; Oya, Y.; Inoue, H.; Tameda, M.; Shiraki, K.; Ito, M.; Takei, Y.; et al. Comparison of the Gut Microbiota Composition between Obese and Non-Obese Individuals in a Japanese Population, as Analyzed by Terminal Restriction Fragment Length Polymorphism and next-Generation Sequencing. BMC Gastroenterol. 2015, 15, 100. [Google Scholar] [CrossRef]
- Yang, T.; Santisteban, M.M.; Rodriguez, V.; Li, E.; Ahmari, N.; Carvajal, J.M.; Zadeh, M.; Gong, M.; Qi, Y.; Zubcevic, J.; et al. Gut Dysbiosis Is Linked to Hypertension. Hypertension 2015, 65, 1331–1340. [Google Scholar] [CrossRef]
- Dominianni, C.; Sinha, R.; Goedert, J.J.; Pei, Z.; Yang, L.; Hayes, R.B.; Ahn, J. Sex, Body Mass Index, and Dietary Fiber Intake Influence the Human Gut Microbiome. PLoS ONE 2015, 10, e0124599. [Google Scholar] [CrossRef]
- Li, M.; Wang, B.; Zhang, M.; Rantalainen, M.; Wang, S.; Zhou, H.; Zhang, Y.; Shen, J.; Pang, X.; Zhang, M.; et al. Symbiotic Gut Microbes Modulate Human Metabolic Phenotypes. Proc. Natl. Acad. Sci. USA 2008, 105, 2117–2122. [Google Scholar] [CrossRef] [PubMed]
- Haro, C.; Rangel-Zúñiga, O.A.; Alcalá-Díaz, J.F.; Gómez-Delgado, F.; Pérez-Martínez, P.; Delgado-Lista, J.; Quintana-Navarro, G.M.; Landa, B.B.; Navas-Cortés, J.A.; Tena-Sempere, M.; et al. Intestinal Microbiota Is Influenced by Gender and Body Mass Index. PLoS ONE 2016, 11, e0154090. [Google Scholar] [CrossRef]
- Yurkovetskiy, L.; Burrows, M.; Khan, A.A.; Graham, L.; Volchkov, P.; Becker, L.; Antonopoulos, D.; Umesaki, Y.; Chervonsky, A.V. Gender Bias in Autoimmunity Is Influenced by Microbiota. Immunity 2013, 39, 400–412. [Google Scholar] [CrossRef]
- Markle, J.G.M.; Frank, D.N.; Mortin-Toth, S.; Robertson, C.E.; Feazel, L.M.; Rolle-Kampczyk, U.; Von Bergen, M.; McCoy, K.D.; Macpherson, A.J.; Danska, J.S. Sex Differences in the Gut Microbiome Drive Hormone-Dependent Regulation of Autoimmunity. Science 2013, 339, 1084–1088. [Google Scholar] [CrossRef]
- Insenser, M.; Murri, M.; Del Campo, R.; Martínez-García, M.Á.; Fernández-Durán, E.; Escobar-Morreale, H.F. Gut Microbiota and the Polycystic Ovary Syndrome: Influence of Sex, Sex Hormones, and Obesity. J. Clin. Endocrinol. Metab. 2018, 103, 2552–2562. [Google Scholar] [CrossRef]
- Thackray, V.G. Sex, Microbes, and Polycystic Ovary Syndrome. Trends Endocrinol. Metab. 2019, 30, 54–65. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Hwang, Y.-J.; Shin, M.-J.; Yi, H. Difference in the Gut Microbiome between Ovariectomy-Induced Obesity and Diet-Induced Obesity. J. Microbiol. Biotechnol. 2017, 27, 2228–2236. [Google Scholar] [CrossRef]
- Harada, N.; Hanaoka, R.; Horiuchi, H.; Kitakaze, T.; Mitani, T.; Inui, H.; Yamaji, R. Castration Influences Intestinal Microflora and Induces Abdominal Obesity in High-Fat Diet-Fed Mice. Sci. Rep. 2016, 6, 23001. [Google Scholar] [CrossRef]
- Spinelli, J.B.; Haigis, M.C. The Multifaceted Contributions of Mitochondria to Cellular Metabolism. Nat. Cell Biol. 2018, 20, 745–754. [Google Scholar] [CrossRef]
- Gemmell, N.J.; Metcalf, V.J.; Allendorf, F.W. Mother’s Curse: The Effect of mtDNA on Individual Fitness and Population Viability. Trends Ecol. Evol. 2004, 19, 238–244. [Google Scholar] [CrossRef]
- Wang, S.; Guo, J.; Liu, X.; Tian, W.; Zhang, Y.; Wang, Y.; Liu, Y.; E, M.; Fang, S. Sexual Dimorphism in Mitochondrial Dysfunction and Diabetes Mellitus: Evidence from a Population-Based Cohort Study. Diabetol. Metab. Syndr. 2023, 15, 114. [Google Scholar] [CrossRef]
- Ribeiro, R.F.; Ronconi, K.S.; Morra, E.A.; Do Val Lima, P.R.; Porto, M.L.; Vassallo, D.V.; Figueiredo, S.G.; Stefanon, I. Sex Differences in the Regulation of Spatially Distinct Cardiac Mitochondrial Subpopulations. Mol. Cell Biochem. 2016, 419, 41–51. [Google Scholar] [CrossRef]
- Yzydorczyk, C.; Mitanchez, D.; Buffat, C.; Ligi, I.; Grandvuillemin, I.; Boubred, F.; Simeoni, U. Stress Oxydant Chez l’enfant Prématuré: Causes, Biomarqueurs et Possibilités Thérapeutiques. Arch. Pédiatrie 2015, 22, 1047–1055. [Google Scholar] [CrossRef] [PubMed]
- Peyter, A.-C.; Armengaud, J.-B.; Guillot, E.; Yzydorczyk, C. Endothelial Progenitor Cells Dysfunctions and Cardiometabolic Disorders: From Mechanisms to Therapeutic Approaches. Int. J. Mol. Sci. 2021, 22, 6667. [Google Scholar] [CrossRef] [PubMed]
- Kander, M.C.; Cui, Y.; Liu, Z. Gender Difference in Oxidative Stress: A New Look at the Mechanisms for Cardiovascular Diseases. J. Cell Mol. Med. 2017, 21, 1024–1032. [Google Scholar] [CrossRef]
- Ide, T.; Tsutsui, H.; Ohashi, N.; Hayashidani, S.; Suematsu, N.; Tsuchihashi, M.; Tamai, H.; Takeshita, A. Greater Oxidative Stress in Healthy Young Men Compared With Premenopausal Women. Arterioscler. Thromb. Vasc. Biol. 2002, 22, 438–442. [Google Scholar] [CrossRef] [PubMed]
- Martínez de Toda, I.; González-Sánchez, M.; Díaz-Del Cerro, E.; Valera, G.; Carracedo, J.; Guerra-Pérez, N. Sex Differences in Markers of Oxidation and Inflammation. Implications for Ageing. Mech. Ageing Dev. 2023, 211, 111797. [Google Scholar] [CrossRef]
- Miquel, J.; Economos, A.C.; Fleming, J.; Johnson, J.E. Mitochondrial Role in Cell Aging. Exp. Gerontol. 1980, 15, 575–591. [Google Scholar] [CrossRef]
- Barja, G. Updating the Mitochondrial Free Radical Theory of Aging: An Integrated View, Key Aspects, and Confounding Concepts. Antioxid. Redox Signal 2013, 19, 1420–1445. [Google Scholar] [CrossRef]
- Balaban, R.S.; Nemoto, S.; Finkel, T. Mitochondria, Oxidants, and Aging. Cell 2005, 120, 483–495. [Google Scholar] [CrossRef]
- Vermot, A.; Petit-Härtlein, I.; Smith, S.M.E.; Fieschi, F. NADPH Oxidases (NOX): An Overview from Discovery, Molecular Mechanisms to Physiology and Pathology. Antioxidants 2021, 10, 890. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.A.; Drummond, G.R.; Mast, A.E.; Schmidt, H.H.H.W.; Sobey, C.G. Effect of Gender on NADPH-Oxidase Activity, Expression, and Function in the Cerebral Circulation: Role of Estrogen. Stroke 2007, 38, 2142–2149. [Google Scholar] [CrossRef]
- Wong, P.S.; Randall, M.D.; Roberts, R.E. Sex Differences in the Role of NADPH Oxidases in Endothelium-Dependent Vasorelaxation in Porcine Isolated Coronary Arteries. Vasc. Pharmacol. 2015, 72, 83–92. [Google Scholar] [CrossRef]
- De Silva, T.M.; Broughton, B.R.S.; Drummond, G.R.; Sobey, C.G.; Miller, A.A. Gender Influences Cerebral Vascular Responses to Angiotensin II Through Nox2-Derived Reactive Oxygen Species. Stroke 2009, 40, 1091–1097. [Google Scholar] [CrossRef] [PubMed]
- Pierce, J.P.; Kievits, J.; Graustein, B.; Speth, R.C.; Iadecola, C.; Milner, T.A. SEX Differences in the Subcellular Distribution of At1 Receptors and Nadph Oxidase Subunits in the Dendrites of C1 Neurons in the Rat Rostral Ventrolateral Medulla. Neuroscience 2009, 163, 329–338. [Google Scholar] [CrossRef]
- Chen, Y.; Ji, L.-L.; Liu, T.-Y.; Wang, Z.-T. Evaluation of Gender-Related Differences in Various Oxidative Stress Enzymes in Mice. Chin. J. Physiol. 2011, 54, 385–390. [Google Scholar] [CrossRef]
- Barp, J.; Araújo, A.S.R.; Fernandes, T.R.G.; Rigatto, K.V.; Llesuy, S.; Belló-Klein, A.; Singal, P. Myocardial Antioxidant and Oxidative Stress Changes Due to Sex Hormones. Braz. J. Med. Biol. Res. 2002, 35, 1075–1081. [Google Scholar] [CrossRef] [PubMed]
- Vina, J.; Gambini, J.; Lopez-Grueso, R.; Abdelaziz, K.M.; Jove, M.; Borras, C. Females Live Longer than Males: Role of Oxidative Stress. Curr. Pharm. Des. 2011, 17, 3959–3965. [Google Scholar] [CrossRef]
- Liang, Q.; Sheng, Y.; Jiang, P.; Ji, L.; Xia, Y.; Min, Y.; Wang, Z. The Gender-Dependent Difference of Liver GSH Antioxidant System in Mice and Its Influence on Isoline-Induced Liver Injury. Toxicology 2011, 280, 61–69. [Google Scholar] [CrossRef]
- Massafra, C.; Gioia, D.; De Felice, C.; Muscettola, M.; Longini, M.; Buonocore, G. Gender-Related Differences in Erythrocyte Glutathione Peroxidase Activity in Healthy Subjects. Clin. Endocrinol. 2002, 57, 663–667. [Google Scholar] [CrossRef]
- Bellanti, F.; Matteo, M.; Rollo, T.; De Rosario, F.; Greco, P.; Vendemiale, G.; Serviddio, G. Sex Hormones Modulate Circulating Antioxidant Enzymes: Impact of Estrogen Therapy. Redox Biol. 2013, 1, 340–346. [Google Scholar] [CrossRef]
- Fatima, Q.; Amin, S.; Kawa, I.A.; Jeelani, H.; Manzoor, S.; Rizvi, S.M.; Rashid, F. Evaluation of Antioxidant Defense Markers in Relation to Hormonal and Insulin Parameters in Women with Polycystic Ovary Syndrome (PCOS): A Case-Control Study. Diabetes Metab. Syndr. Clin. Res. Rev. 2019, 13, 1957–1961. [Google Scholar] [CrossRef]
- Taravati, A.; Tohidi, F. Comprehensive Analysis of Oxidative Stress Markers and Antioxidants Status in Preeclampsia. Taiwan J. Obstet. Gynecol. 2018, 57, 779–790. [Google Scholar] [CrossRef]
- Díaz-Castro, J.; Florido, J.; Kajarabille, N.; Prados, S.; de Paco, C.; Ocon, O.; Pulido-Moran, M.; Ochoa, J.J. A New Approach to Oxidative Stress and Inflammatory Signaling during Labour in Healthy Mothers and Neonates. Oxid. Med. Cell Longev. 2015, 2015, 178536. [Google Scholar] [CrossRef] [PubMed]
- Af, M. The Biliverdin-Bilirubin Antioxidant Cycle of Cellular Protection: Missing a Wheel? Free Radic. Biol. Med. 2010, 49, 814–820. [Google Scholar] [CrossRef]
- Oral, E.; Gezer, A.; Çagdas, A.; Pakkal, N. Oxytocin Infusion in Labor: The Effect Different Indications and the Use of Different Diluents on Neonatal Bilirubin Levels. Arch. Gynecol. Obstet. 2003, 267, 117–120. [Google Scholar] [CrossRef]
- Carter, C.S. Sex Differences in Oxytocin and Vasopressin: Implications for Autism Spectrum Disorders? Behav. Brain Res. 2007, 176, 170–186. [Google Scholar] [CrossRef]
- Diaz-Castro, J.; Pulido-Moran, M.; Moreno-Fernandez, J.; Kajarabille, N.; De Paco, C.; Garrido-Sanchez, M.; Prados, S.; Ochoa, J.J. Gender Specific Differences in Oxidative Stress and Inflammatory Signaling in Healthy Term Neonates and Their Mothers. Pediatr. Res. 2016, 80, 595–601. [Google Scholar] [CrossRef]
- Georgakopoulou, E.; Tsimaratou, K.; Evangelou, K.; Fernandez, M.-P.; Zoumpourlis, V.; Trougakos, I.; Kletsas, D.; Bartek, J.; Serrano, M.; Gorgoulis, V. Specific Lipofuscin Staining as a Novel Biomarker to Detect Replicative and Stress-Induced Senescence. A Method Applicable in Cryo-Preserved and Archival Tissues. Aging 2012, 5, 37–50. [Google Scholar]
- Campisi, J.; d’Adda di Fagagna, F. Cellular Senescence: When Bad Things Happen to Good Cells. Nat. Rev. Mol. Cell Biol. 2007, 8, 729–740. [Google Scholar] [CrossRef]
- Campisi, J. Aging, Cellular Senescence, and Cancer. Annu. Rev. Physiol. 2013, 75, 685–705. [Google Scholar] [CrossRef]
- Martin-Ruiz, C.; Saretzki, G.; Petrie, J.; Ladhoff, J.; Jeyapalan, J.; Wei, W.; Sedivy, J.; Zglinicki, T. von Stochastic Variation in Telomere Shortening Rate Causes Heterogeneity of Human Fibroblast Replicative Life Span *. J. Biol. Chem. 2004, 279, 17826–17833. [Google Scholar] [CrossRef] [PubMed]
- Chang, E.; Harley, C.B. Telomere Length and Replicative Aging in Human Vascular Tissues. Proc. Natl. Acad. Sci. USA 1995, 92, 11190–11194. [Google Scholar] [CrossRef]
- Xu, C.; Wang, Z.; Su, X.; Da, M.; Yang, Z.; Duan, W.; Mo, X. Association between Leucocyte Telomere Length and Cardiovascular Disease in a Large General Population in the United States. Sci. Rep. 2020, 10, 80. [Google Scholar] [CrossRef] [PubMed]
- Vassallo, P.F.; Simoncini, S.; Ligi, I.; Chateau, A.-L.; Bachelier, R.; Robert, S.; Morere, J.; Fernandez, S.; Guillet, B.; Marcelli, M.; et al. Accelerated Senescence of Cord Blood Endothelial Progenitor Cells in Premature Neonates Is Driven by SIRT1 Decreased Expression. Blood 2014, 123, 2116–2126. [Google Scholar] [CrossRef]
- Tanno, M.; Sakamoto, J.; Miura, T.; Shimamoto, K.; Horio, Y. Nucleocytoplasmic Shuttling of the NAD+-Dependent Histone Deacetylase SIRT1 *. J. Biol. Chem. 2007, 282, 6823–6832. [Google Scholar] [CrossRef] [PubMed]
- Grootaert, M.O.J.; Moulis, M.; Roth, L.; Martinet, W.; Vindis, C.; Bennett, M.R.; De Meyer, G.R.Y. Vascular Smooth Muscle Cell Death, Autophagy and Senescence in Atherosclerosis. Cardiovasc. Res. 2018, 114, 622–634. [Google Scholar] [CrossRef]
- Coppé, J.-P.; Desprez, P.-Y.; Krtolica, A.; Campisi, J. The Senescence-Associated Secretory Phenotype: The Dark Side of Tumor Suppression. Annu. Rev. Pathol. 2010, 5, 99–118. [Google Scholar] [CrossRef]
- Rall-Scharpf, M.; Friedl, T.W.P.; Biechonski, S.; Denkinger, M.; Milyavsky, M.; Wiesmüller, L. Sex-Specific Differences in DNA Double-Strand Break Repair of Cycling Human Lymphocytes during Aging. Aging 2021, 13, 21066–21089. [Google Scholar] [CrossRef]
- Yousefzadeh, M.J.; Zhao, J.; Bukata, C.; Wade, E.A.; McGowan, S.J.; Angelini, L.A.; Bank, M.P.; Gurkar, A.U.; McGuckian, C.A.; Calubag, M.F.; et al. Tissue Specificity of Senescent Cell Accumulation during Physiologic and Accelerated Aging of Mice. Aging Cell 2020, 19, e13094. [Google Scholar] [CrossRef]
- Waskar, M.; Landis, G.N.; Shen, J.; Curtis, C.; Tozer, K.; Abdueva, D.; Skvortsov, D.; Tavaré, S.; Tower, J. Drosophila Melanogaster P53 Has Developmental Stage-Specific and Sex-Specific Effects on Adult Life Span Indicative of Sexual Antagonistic Pleiotropy. Aging 2009, 1, 903–936. [Google Scholar] [PubMed]
- Gardner, M.; Bann, D.; Wiley, L.; Cooper, R.; Hardy, R.; Nitsch, D.; Martin-Ruiz, C.; Shiels, P.; Sayer, A.A.; Barbieri, M.; et al. Gender and Telomere Length: Systematic Review and Meta-Analysis. Exp. Gerontol. 2014, 51, 15–27. [Google Scholar] [CrossRef]
- Coviello-McLaughlin, G.M.; Prowse, K.R. Telomere Length Regulation during Postnatal Development and Ageing in Mus Spretus. Nucleic Acids Res. 1997, 25, 3051–3058. [Google Scholar] [CrossRef] [PubMed]
- Lansdorp, P.M. Sex Differences in Telomere Length, Lifespan, and Embryonic Dyskerin Levels. Aging Cell 2022, 21, e13614. [Google Scholar] [CrossRef] [PubMed]
- Barcena de Arellano, M.L.; Pozdniakova, S.; Kühl, A.A.; Baczko, I.; Ladilov, Y.; Regitz-Zagrosek, V. Sex Differences in the Aging Human Heart: Decreased Sirtuins, pro-Inflammatory Shift and Reduced Anti-Oxidative Defense. Aging 2019, 11, 1918–1933. [Google Scholar] [CrossRef]
- Sasaki, Y.; Ikeda, Y.; Miyauchi, T.; Uchikado, Y.; Akasaki, Y.; Ohishi, M. Estrogen-SIRT1 Axis Plays a Pivotal Role in Protecting Arteries Against Menopause-Induced Senescence and Atherosclerosis. J. Atheroscler. Thromb. 2020, 27, 47–59. [Google Scholar] [CrossRef]
- Olivetti, G.; Abbi, R.; Quaini, F.; Kajstura, J.; Cheng, W.; Nitahara, J.A.; Quaini, E.; Loreto, C.D.; Beltrami, C.A.; Krajewski, S.; et al. Apoptosis in the Failing Human Heart. N. Engl. J. Med. 1997, 336, 1131–1141. [Google Scholar] [CrossRef]
- Elmore, S. Apoptosis: A Review of Programmed Cell Death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef]
- Olivetti, G.; Giordano, G.; Corradi, D.; Melissari, M.; Lagrasta, C.; Gambert, S.R.; Anversa, P. Gender Differences and Aging: Effects on the Human Heart. J. Am. Coll. Cardiol. 1995, 26, 1068–1079. [Google Scholar] [CrossRef]
- Kessler, E.L.; Rivaud, M.R.; Vos, M.A.; van Veen, T.A.B. Sex-Specific Influence on Cardiac Structural Remodeling and Therapy in Cardiovascular Disease. Biol. Sex. Differ. 2019, 10, 7. [Google Scholar] [CrossRef]
- Meisse, D.; Van de Casteele, M.; Beauloye, C.; Hainault, I.; Kefas, B.A.; Rider, M.H.; Foufelle, F.; Hue, L. Sustained Activation of AMP-Activated Protein Kinase Induces c-Jun N-Terminal Kinase Activation and Apoptosis in Liver Cells. FEBS Lett. 2002, 526, 38–42. [Google Scholar] [CrossRef] [PubMed]
- Rogers, N.H.; Witczak, C.A.; Hirshman, M.F.; Goodyear, L.J.; Greenberg, A.S. Estradiol Stimulates Akt, AMPK and TBC1D1/4, But Not Glucose Uptake in Rat Soleus. Biochem. Biophys. Res. Commun. 2009, 382, 646–650. [Google Scholar] [CrossRef]
- Troncoso, M.F.; Pavez, M.; Wilson, C.; Lagos, D.; Duran, J.; Ramos, S.; Barrientos, G.; Silva, P.; Llanos, P.; Basualto-Alarcón, C.; et al. Testosterone Activates Glucose Metabolism through AMPK and Androgen Signaling in Cardiomyocyte Hypertrophy. Biol. Res. 2021, 54, 3. [Google Scholar] [CrossRef]
- Ishii, N.; Matsumura, T.; Kinoshita, H.; Motoshima, H.; Kojima, K.; Tsutsumi, A.; Kawasaki, S.; Yano, M.; Senokuchi, T.; Asano, T.; et al. Activation of AMP-Activated Protein Kinase Suppresses Oxidized Low-Density Lipoprotein-Induced Macrophage Proliferation. J. Biol. Chem. 2009, 284, 34561–34569. [Google Scholar] [CrossRef] [PubMed]
- Capano, M.; Crompton, M. Bax Translocates to Mitochondria of Heart Cells during Simulated Ischaemia: Involvement of AMP-Activated and P38 Mitogen-Activated Protein Kinases. Biochem. J. 2006, 395, 57–64. [Google Scholar] [CrossRef]
- Russell, R.R.; Li, J.; Coven, D.L.; Pypaert, M.; Zechner, C.; Palmeri, M.; Giordano, F.J.; Mu, J.; Birnbaum, M.J.; Young, L.H. AMP-Activated Protein Kinase Mediates Ischemic Glucose Uptake and Prevents Postischemic Cardiac Dysfunction, Apoptosis, and Injury. J. Clin. Investig. 2004, 114, 495–503. [Google Scholar] [CrossRef]
- Guo, S.; Yao, Q.; Ke, Z.; Chen, H.; Wu, J.; Liu, C. Resveratrol Attenuates High Glucose-Induced Oxidative Stress and Cardiomyocyte Apoptosis through AMPK. Mol. Cell. Endocrinol. 2015, 412, 85–94. [Google Scholar] [CrossRef]
- Vijay, V.; Han, T.; Moland, C.L.; Kwekel, J.C.; Fuscoe, J.C.; Desai, V.G. Sexual Dimorphism in the Expression of Mitochondria-Related Genes in Rat Heart at Different Ages. PLoS ONE 2015, 10, e0117047. [Google Scholar] [CrossRef]
- Kroemer, G.; Levine, B. Autophagic Cell Death: The Story of a Misnomer. Nat. Rev. Mol. Cell Biol. 2008, 9, 1004–1010. [Google Scholar] [CrossRef]
- Karantza-Wadsworth, V.; Patel, S.; Kravchuk, O.; Chen, G.; Mathew, R.; Jin, S.; White, E. Autophagy Mitigates Metabolic Stress and Genome Damage in Mammary Tumorigenesis. Genes Dev. 2007, 21, 1621–1635. [Google Scholar] [CrossRef]
- Young, A.R.J.; Narita, M.; Ferreira, M.; Kirschner, K.; Sadaie, M.; Darot, J.F.J.; Tavaré, S.; Arakawa, S.; Shimizu, S.; Watt, F.M.; et al. Autophagy Mediates the Mitotic Senescence Transition. Genes Dev. 2009, 23, 798–803. [Google Scholar] [CrossRef]
- Bjornsti, M.-A.; Houghton, P.J. The Tor Pathway: A Target for Cancer Therapy. Nat. Rev. Cancer 2004, 4, 335–348. [Google Scholar] [CrossRef] [PubMed]
- Johnson, S.C.; Rabinovitch, P.S.; Kaeberlein, M. mTOR Is a Key Modulator of Ageing and Age-Related Disease. Nature 2013, 493, 338–345. [Google Scholar] [CrossRef]
- Hua, Y.; Zhang, Y.; Ceylan-Isik, A.F.; Wold, L.E.; Nunn, J.M.; Ren, J. Chronic Akt Activation Accentuates Aging-Induced Cardiac Hypertrophy and Myocardial Contractile Dysfunction: Role of Autophagy. Basic Res. Cardiol. 2011, 106, 1173–1191. [Google Scholar] [CrossRef]
- Saxton, R.A.; Sabatini, D.M. mTOR Signaling in Growth, Metabolism, and Disease. Cell 2017, 168, 960–976. [Google Scholar] [CrossRef] [PubMed]
- Hamacher-Brady, A.; Brady, N.R.; Gottlieb, R.A. Enhancing Macroautophagy Protects against Ischemia/Reperfusion Injury in Cardiac Myocytes. J. Biol. Chem. 2006, 281, 29776–29787. [Google Scholar] [CrossRef]
- Du, X.-J.; Fang, L.; Kiriazis, H. Sex Dimorphism in Cardiac Pathophysiology: Experimental Findings, Hormonal Mechanisms, and Molecular Mechanisms. Pharmacol. Ther. 2006, 111, 434–475. [Google Scholar] [CrossRef] [PubMed]
- Gottlieb, R.A.; Mentzer, R.M. Autophagy During Cardiac Stress: Joys and Frustrations of Autophagy. Annu. Rev. Physiol. 2010, 72, 45–59. [Google Scholar] [CrossRef]
- Ma, X.; Liu, H.; Foyil, S.R.; Godar, R.J.; Weinheimer, C.J.; Diwan, A. Autophagy Is Impaired in Cardiac Ischemia-Reperfusion Injury. Autophagy 2012, 8, 1394–1396. [Google Scholar] [CrossRef]
- Chen, C.; Hu, L.-X.; Dong, T.; Wang, G.-Q.; Wang, L.-H.; Zhou, X.-P.; Jiang, Y.; Murao, K.; Lu, S.-Q.; Chen, J.-W.; et al. Apoptosis and Autophagy Contribute to Gender Difference in Cardiac Ischemia–Reperfusion Induced Injury in Rats. Life Sci. 2013, 93, 265–270. [Google Scholar] [CrossRef]
- Le, T.Y.L.; Ashton, A.W.; Mardini, M.; Stanton, P.G.; Funder, J.W.; Handelsman, D.J.; Mihailidou, A.S. Role of Androgens in Sex Differences in Cardiac Damage During Myocardial Infarction. Endocrinology 2014, 155, 568–575. [Google Scholar] [CrossRef] [PubMed]
- Gürgen, D.; Kusch, A.; Klewitz, R.; Hoff, U.; Catar, R.; Hegner, B.; Kintscher, U.; Luft, F.C.; Dragun, D. Sex-Specific mTOR Signaling Determines Sexual Dimorphism in Myocardial Adaptation in Normotensive DOCA-Salt Model. Hypertension 2013, 61, 730–736. [Google Scholar] [CrossRef]
- Zhe-Wei, S.; Li-Sha, G.; Yue-Chun, L. The Role of Necroptosis in Cardiovascular Disease. Front. Pharmacol. 2018, 9, 721. [Google Scholar] [CrossRef]
- Zhaolin, Z.; Guohua, L.; Shiyuan, W.; Zuo, W. Role of Pyroptosis in Cardiovascular Disease. Cell Prolif. 2019, 52, e12563. [Google Scholar] [CrossRef]
- Wu, X.; Li, Y.; Zhang, S.; Zhou, X. Ferroptosis as a Novel Therapeutic Target for Cardiovascular Disease. Theranostics 2021, 11, 3052–3059. [Google Scholar] [CrossRef] [PubMed]
- Medzhitov, R. Inflammation 2010: New Adventures of an Old Flame. Cell 2010, 140, 771–776. [Google Scholar] [CrossRef]
- Ferrero-Miliani, L.; Nielsen, O.H.; Andersen, P.S.; Girardin, S.E. Chronic Inflammation: Importance of NOD2 and NALP3 in Interleukin-1β Generation. Clin. Exp. Immunol. 2007, 147, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Hong, Y.; Huang, H. Triptolide Attenuates Inflammatory Response in Membranous Glomerulo-Nephritis Rat via Downregulation of NF-κB Signaling Pathway. Kidney Blood Press. Res. 2016, 41, 901–910. [Google Scholar] [CrossRef]
- Chen, Y.-Q.; Zhou, H.-M.; Chen, F.-F.; Liu, Y.-P.; Han, L.; Song, M.; Wang, Z.-H.; Zhang, W.; Shang, Y.-Y.; Zhong, M. Testosterone Ameliorates Vascular Aging via the Gas6/Axl Signaling Pathway. Aging 2020, 12, 16111–16125. [Google Scholar] [CrossRef]
- Marriott, I.; Huet-Hudson, Y.M. Sexual Dimorphism in Innate Immune Responses to Infectious Organisms. Immunol. Res. 2006, 34, 177–192. [Google Scholar] [CrossRef]
- Desai, M.K.; Brinton, R.D. Autoimmune Disease in Women: Endocrine Transition and Risk Across the Lifespan. Front. Endocrinol. 2019, 10, 265. [Google Scholar] [CrossRef]
- Groß, O.; Yazdi, A.S.; Thomas, C.J.; Masin, M.; Heinz, L.X.; Guarda, G.; Quadroni, M.; Drexler, S.K.; Tschopp, J. Inflammasome Activators Induce Interleukin-1α Secretion via Distinct Pathways with Differential Requirement for the Protease Function of Caspase-1. Immunity 2012, 36, 388–400. [Google Scholar] [CrossRef] [PubMed]
- Sheedy, F.J.; Moore, K.J. IL-1 Signaling in Atherosclerosis: Sibling Rivalry. Nat. Immunol. 2013, 14, 1030–1032. [Google Scholar] [CrossRef]
- Tedgui, A.; Mallat, Z. Cytokines in Atherosclerosis: Pathogenic and Regulatory Pathways. Physiol. Rev. 2006, 86, 515–581. [Google Scholar] [CrossRef] [PubMed]
- Wani, K.; AlHarthi, H.; Alghamdi, A.; Sabico, S.; Al-Daghri, N.M. Role of NLRP3 Inflammasome Activation in Obesity-Mediated Metabolic Disorders. Int. J. Environ. Res. Public Health 2021, 18, 511. [Google Scholar] [CrossRef]
- Man, J.J.; Beckman, J.A.; Jaffe, I.Z. Sex as a Biological Variable in Atherosclerosis. Circ. Res. 2020, 126, 1297–1319. [Google Scholar] [CrossRef] [PubMed]
- Al-Daghri, N.M.; Wani, K.; AlHarthi, H.; Alghamdi, A.; Alnaami, A.M.; Yakout, S.M. Sex-Specific Signature in the Circulating NLRP3 Levels of Saudi Adults with Metabolic Syndrome. J. Clin. Med. 2021, 10, 3288. [Google Scholar] [CrossRef]
- Dhanraj, P.; van Heerden, M.B.; Pepper, M.S.; Ambele, M.A. Sexual Dimorphism in Changes That Occur in Tissues, Organs and Plasma during the Early Stages of Obesity Development. Biology 2021, 10, 717. [Google Scholar] [CrossRef]
- Fisman, E.Z.; Tenenbaum, A. Adiponectin: A Manifold Therapeutic Target for Metabolic Syndrome, Diabetes, and Coronary Disease? Cardiovasc. Diabetol. 2014, 13, 103. [Google Scholar] [CrossRef]
- Ai, M.; Otokozawa, S.; Asztalos, B.F.; White, C.C.; Demissie-Banjaw, S.; Cupples, L.A.; Nakajima, K.; Wilson, P.W.; Schaefer, E.J. Adiponectin: An Independent Risk Factor for Coronary Heart Disease in the Framingham Offspring Study. Atherosclerosis 2011, 217, 543–548. [Google Scholar] [CrossRef]
- Hanley, A.J.G.; Bowden, D.; Wagenknecht, L.E.; Balasubramanyam, A.; Langfeld, C.; Saad, M.F.; Rotter, J.I.; Guo, X.; Chen, Y.-D.I.; Bryer-Ash, M.; et al. Associations of Adiponectin with Body Fat Distribution and Insulin Sensitivity in Nondiabetic Hispanics and African-Americans. J. Clin. Endocrinol. Metab. 2007, 92, 2665–2671. [Google Scholar] [CrossRef]
- Nishizawa, H.; Shimomura, I.; Kishida, K.; Maeda, N.; Kuriyama, H.; Nagaretani, H.; Matsuda, M.; Kondo, H.; Furuyama, N.; Kihara, S.; et al. Androgens Decrease Plasma Adiponectin, an Insulin-Sensitizing Adipocyte-Derived Protein. Diabetes 2002, 51, 2734–2741. [Google Scholar] [CrossRef] [PubMed]
- Sieminska, L.; Wojciechowska, C.; Niedziolka, D.; Marek, B.; Kos-Kudla, B.; Kajdaniuk, D.; Nowak, M. Effect of Postmenopause and Hormone Replacement Therapy on Serum Adiponectin Levels. Metabolism 2005, 54, 1610–1614. [Google Scholar] [CrossRef] [PubMed]
- Moshage, H.J.; Roelofs, H.M.J.; Van Pelt, J.F.; Hazenberg, B.P.C.; Van Leeuwen, M.A.; Limburg, P.C.; Aarden, L.A.; Yap, S.H. The Effect of Interleukin-1, Interleukin-6 and Its Interrelationship on the Synthesis of Serum Amyloid A and C-Reactive Protein in Primary Cultures of Adult Human Hepatocytes. Biochem. Biophys. Res. Commun. 1988, 155, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Gender and C-Reactive Protein: Data from the Multiethnic Study of Atherosclerosis (MESA) Cohort—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/16923436/ (accessed on 3 September 2024).
- Garcia, V.P.; Rocha, H.N.M.; Sales, A.R.K.; Rocha, N.G.; Nóbrega, A.C.L.D. Sex Differences in High Sensitivity C-Reactive Protein in Subjects with Risk Factors of Metabolic Syndrome. Arq. Bras. Cardiol. 2016, 106, 182–187. [Google Scholar] [CrossRef]
- Eggers, K.M.; Lindhagen, L.; Baron, T.; Erlinge, D.; Hjort, M.; Jernberg, T.; Johnston, N.; Marko-Varga, G.; Rezeli, M.; Spaak, J.; et al. Sex-Differences in Circulating Biomarkers during Acute Myocardial Infarction: An Analysis from the SWEDEHEART Registry. PLoS ONE 2021, 16, e0249830. [Google Scholar] [CrossRef]
- Gao, Z.; Chen, Z.; Sun, A.; Deng, X. Gender Differences in Cardiovascular Disease. Med. Nov. Technol. Devices 2019, 4, 100025. [Google Scholar] [CrossRef]
- Förstermann, U.; Sessa, W.C. Nitric Oxide Synthases: Regulation and Function. Eur. Heart J. 2012, 33, 829–837. [Google Scholar] [CrossRef]
- Incalza, M.A.; D’Oria, R.; Natalicchio, A.; Perrini, S.; Laviola, L.; Giorgino, F. Oxidative Stress and Reactive Oxygen Species in Endothelial Dysfunction Associated with Cardiovascular and Metabolic Diseases. Vasc. Pharmacol. 2018, 100, 1–19. [Google Scholar] [CrossRef]
- Hashimoto, M.; Akishita, M.; Eto, M.; Ishikawa, M.; Kozaki, K.; Toba, K.; Sagara, Y.; Taketani, Y.; Orimo, H.; Ouchi, Y. Modulation of Endothelium-Dependent Flow-Mediated Dilatation of the Brachial Artery by Sex and Menstrual Cycle. Circulation 1995, 92, 3431–3435. [Google Scholar] [CrossRef]
- Wang, L.; Ahn, Y.J.; Asmis, R. Sexual Dimorphism in Glutathione Metabolism and Glutathione-Dependent Responses. Redox Biol. 2020, 31, 101410. [Google Scholar] [CrossRef] [PubMed]
- Kolluru, G.K.; Shen, X.; Bir, S.C.; Kevil, C.G. Hydrogen Sulfide Chemical Biology: Pathophysiological Roles and Detection. Nitric Oxide 2013, 35, 5–20. [Google Scholar] [CrossRef]
- King, A.L.; Polhemus, D.J.; Bhushan, S.; Otsuka, H.; Kondo, K.; Nicholson, C.K.; Bradley, J.M.; Islam, K.N.; Calvert, J.W.; Tao, Y.-X.; et al. Hydrogen Sulfide Cytoprotective Signaling Is Endothelial Nitric Oxide Synthase-Nitric Oxide Dependent. Proc. Natl. Acad. Sci. USA 2014, 111, 3182–3187. [Google Scholar] [CrossRef] [PubMed]
- Kolluru, G.K.; Bir, S.C.; Yuan, S.; Shen, X.; Pardue, S.; Wang, R.; Kevil, C.G. Cystathionine γ-Lyase Regulates Arteriogenesis through NO-Dependent Monocyte Recruitment. Cardiovasc. Res. 2015, 107, 590–600. [Google Scholar] [CrossRef]
- Zhang, Y.; Jing, M.; Cai, C.; Zhu, S.; Zhang, C.; Wang, Q.; Zhai, Y.; Ji, X.; Wu, D. Role of Hydrogen Sulphide in Physiological and Pathological Angiogenesis. Cell Prolif. 2023, 56, e13374. [Google Scholar] [CrossRef] [PubMed]
- Wallace, J.L.; Wang, R. Hydrogen Sulfide-Based Therapeutics: Exploiting a Unique but Ubiquitous Gasotransmitter. Nat. Rev. Drug Discov. 2015, 14, 329–345. [Google Scholar] [CrossRef]
- Zeiher, A.M.; Drexler, H.; Saurbier, B.; Just, H. Endothelium-Mediated Coronary Blood Flow Modulation in Humans. Effects of Age, Atherosclerosis, Hypercholesterolemia, and Hypertension. J. Clin. Investig. 1993, 92, 652–662. [Google Scholar] [CrossRef] [PubMed]
- Egashira, K.; Inou, T.; Hirooka, Y.; Kai, H.; Sugimachi, M.; Suzuki, S.; Kuga, T.; Urabe, Y.; Takeshita, A. Effects of Age on Endothelium-Dependent Vasodilation of Resistance Coronary Artery by Acetylcholine in Humans. Circulation 1993, 88, 77–81. [Google Scholar] [CrossRef]
- Guo, X.; Razandi, M.; Pedram, A.; Kassab, G.; Levin, E.R. Estrogen Induces Vascular Wall Dilation: Mediation through Kinase Signaling to Nitric Oxide and Estrogen Receptors Alpha and Beta. J. Biol. Chem. 2005, 280, 19704–19710. [Google Scholar] [CrossRef]
- Haynes, M.P.; Sinha, D.; Russell, K.S.; Collinge, M.; Fulton, D.; Morales-Ruiz, M.; Sessa, W.C.; Bender, J.R. Membrane Estrogen Receptor Engagement Activates Endothelial Nitric Oxide Synthase via the PI3-Kinase-Akt Pathway in Human Endothelial Cells. Circ. Res. 2000, 87, 677–682. [Google Scholar] [CrossRef]
- Miner, J.A.; Martini, E.R.; Smith, M.M.; Brunt, V.E.; Kaplan, P.F.; Halliwill, J.R.; Minson, C.T. Short-Term Oral Progesterone Administration Antagonizes the Effect of Transdermal Estradiol on Endothelium-Dependent Vasodilation in Young Healthy Women. Am. J. Physiol.-Heart Circ. Physiol. 2011, 301, H1716–H1722. [Google Scholar] [CrossRef]
- Adler, T.E.; Usselman, C.W.; Takamata, A.; Stachenfeld, N.S. Blood Pressure Predicts Endothelial Function and the Effects of Ethinyl Estradiol Exposure in Young Women. Am. J. Physiol.-Heart Circ. Physiol. 2018, 315, H925–H933. [Google Scholar] [CrossRef] [PubMed]
- Sudhir, K.; Ko, E.; Zellner, C.; Wong, H.E.; Hutchison, S.J.; Chou, T.M.; Chatterjee, K. Physiological Concentrations of Estradiol Attenuate Endothelin 1-Induced Coronary Vasoconstriction In Vivo. Circulation 1997, 96, 3626–3632. [Google Scholar] [CrossRef] [PubMed]
- Scioli, M.G.; Storti, G.; D’Amico, F.; Rodríguez Guzmán, R.; Centofanti, F.; Doldo, E.; Céspedes Miranda, E.M.; Orlandi, A. Oxidative Stress and New Pathogenetic Mechanisms in Endothelial Dysfunction: Potential Diagnostic Biomarkers and Therapeutic Targets. J. Clin. Med. 2020, 9, 1995. [Google Scholar] [CrossRef]
- Yzydorczyk, C.; Gobeil, F.; Cambonie, G.; Lahaie, I.; Lê, N.L.O.; Samarani, S.; Ahmad, A.; Lavoie, J.C.; Oligny, L.L.; Pladys, P.; et al. Exaggerated Vasomotor Response to ANG II in Rats with Fetal Programming of Hypertension Associated with Exposure to a Low-Protein Diet during Gestation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006, 291, R1060–R1068. [Google Scholar] [CrossRef] [PubMed]
- Nickenig, G.; Bäumer, A.T.; Grohè, C.; Kahlert, S.; Strehlow, K.; Rosenkranz, S.; Stäblein, A.; Beckers, F.; Smits, J.F.M.; Daemen, M.J.A.P.; et al. Estrogen Modulates AT 1 Receptor Gene Expression In Vitro and In Vivo. Circulation 1998, 97, 2197–2201. [Google Scholar] [CrossRef]
- Mirabito, K.M.; Hilliard, L.M.; Head, G.A.; Widdop, R.E.; Denton, K.M. Pressor Responsiveness to Angiotensin II in Female Mice Is Enhanced with Age: Role of the Angiotensin Type 2 Receptor. Biol. Sex. Differ. 2014, 5, 13. [Google Scholar] [CrossRef]
- Zhang, Y.; Davidge, S.T. Effect of Estrogen Replacement on Vasoconstrictor Responses in Rat Mesenteric Arteries. Hypertension 1999, 34, 1117–1122. [Google Scholar] [CrossRef]
- Ferrer, M.; Meyer, M.; Osol, G. Estrogen Replacement Increases Beta-Adrenoceptor-Mediated Relaxation of Rat Mesenteric Arteries. J. Vasc. Res. 1996, 33, 124–131. [Google Scholar] [CrossRef]
- Kimura, M.; Sudhir, K.; Jones, M.; Simpson, E.; Jefferis, A.-M.; Chin-Dusting, J.P.F. Impaired Acetylcholine-Induced Release of Nitric Oxide in the Aorta of Male Aromatase-Knockout Mice: Regulation of Nitric Oxide Production by Endogenous Sex Hormones in Males. Circ. Res. 2003, 93, 1267–1271. [Google Scholar] [CrossRef]
- Lew, R.; Komesaroff, P.; Williams, M.; Dawood, T.; Sudhir, K. Endogenous Estrogens Influence Endothelial Function in Young Men. Circ. Res. 2003, 93, 1127–1133. [Google Scholar] [CrossRef] [PubMed]
- Lucas-Herald, A.K.; Alves-Lopes, R.; Montezano, A.C.; Ahmed, S.F.; Touyz, R.M. Genomic and Non-Genomic Effects of Androgens in the Cardiovascular System: Clinical Implications. Clin. Sci. 2017, 131, 1405–1418. [Google Scholar] [CrossRef] [PubMed]
- Wenner, M.M.; Taylor, H.S.; Stachenfeld, N.S. Androgens Influence Microvascular Dilation in PCOS through ET-A and ET-B Receptors. Am. J. Physiol.-Endocrinol. Metab. 2013, 305, E818–E825. [Google Scholar] [CrossRef]
- Medina, R.J.; Barber, C.L.; Sabatier, F.; Dignat-George, F.; Melero-Martin, J.M.; Khosrotehrani, K.; Ohneda, O.; Randi, A.M.; Chan, J.K.Y.; Yamaguchi, T.; et al. Endothelial Progenitors: A Consensus Statement on Nomenclature. Stem Cells Transl. Med. 2017, 6, 1316–1320. [Google Scholar] [CrossRef]
- Asahara, T.; Kawamoto, A.; Masuda, H. Concise Review: Circulating Endothelial Progenitor Cells for Vascular Medicine. Stem Cells 2011, 29, 1650–1655. [Google Scholar] [CrossRef]
- Hill, J.M.; Zalos, G.; Halcox, J.P.J.; Schenke, W.H.; Waclawiw, M.A.; Quyyumi, A.A.; Finkel, T. Circulating Endothelial Progenitor Cells, Vascular Function, and Cardiovascular Risk. N. Engl. J. Med. 2003, 348, 593–600. [Google Scholar] [CrossRef]
- Bakogiannis, C.; Tousoulis, D.; Androulakis, E.; Briasoulis, A.; Papageorgiou, N.; Vogiatzi, G.; Kampoli, A.-M.; Charakida, M.; Siasos, G.; Latsios, G.; et al. Circulating Endothelial Progenitor Cells as Biomarkers for Prediction of Cardiovascular Outcomes. Curr. Med. Chem. 2012, 19, 2597–2604. [Google Scholar] [CrossRef]
- Berezin, A.E.; Kremzer, A.A. Circulating Endothelial Progenitor Cells as Markers for Severity of Ischemic Chronic Heart Failure. J. Card. Fail. 2014, 20, 438–447. [Google Scholar] [CrossRef] [PubMed]
- Berezin, A.E. The Endothelial Progenitor Cell Dysfunction in Hypertension: The Diagnostic and Predictive Values. Vessel Plus 2018, 2, 22. [Google Scholar] [CrossRef]
- Souza, L.V.; De Meneck, F.; Oliveira, V.; Higa, E.M.; Akamine, E.H.; Franco, M. do C. Detrimental Impact of Low Birth Weight on Circulating Number and Functional Capacity of Endothelial Progenitor Cells in Healthy Children: Role of Angiogenic Factors. J. Pediatr. 2019, 206, 72–77.e1. [Google Scholar] [CrossRef]
- Ligi, I.; Simoncini, S.; Tellier, E.; Grandvuillemin, I.; Marcelli, M.; Bikfalvi, A.; Buffat, C.; Dignat-George, F.; Anfosso, F.; Simeoni, U. Altered Angiogenesis in Low Birth Weight Individuals: A Role for Anti-Angiogenic Circulating Factors. J. Matern. Fetal Neonatal Med. 2014, 27, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Ligi, I.; Simoncini, S.; Tellier, E.; Vassallo, P.F.; Sabatier, F.; Guillet, B.; Lamy, E.; Sarlon, G.; Quemener, C.; Bikfalvi, A.; et al. A Switch toward Angiostatic Gene Expression Impairs the Angiogenic Properties of Endothelial Progenitor Cells in Low Birth Weight Preterm Infants. Blood 2011, 118, 1699–1709. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Wang, H.; Hu, S. Effects of Estrogen on Diverse Stem Cells and Relevant Intracellular Mechanisms. Sci. China Life Sci. 2010, 53, 542–547. [Google Scholar] [CrossRef] [PubMed]
- Rousseau, A.; Ayoubi, F.; Deveaux, C.; Charbit, B.; Delmau, C.; Christin-Maitre, S.; Jaillon, P.; Uzan, G.; Simon, T. Impact of Age and Gender Interaction on Circulating Endothelial Progenitor Cells in Healthy Subjects. Fertil. Steril. 2010, 93, 843–846. [Google Scholar] [CrossRef]
- Iwakura, A.; Luedemann, C.; Shastry, S.; Hanley, A.; Kearney, M.; Aikawa, R.; Isner, J.M.; Asahara, T.; Losordo, D.W. Estrogen-Mediated, Endothelial Nitric Oxide Synthase–Dependent Mobilization of Bone Marrow–Derived Endothelial Progenitor Cells Contributes to Reendothelialization After Arterial Injury. Circulation 2003, 108, 3115–3121. [Google Scholar] [CrossRef]
- Hamada, H.; Kim, M.K.; Iwakura, A.; Ii, M.; Thorne, T.; Qin, G.; Asai, J.; Tsutsumi, Y.; Sekiguchi, H.; Silver, M.; et al. Estrogen Receptors α and β Mediate Contribution of Bone Marrow–Derived Endothelial Progenitor Cells to Functional Recovery After Myocardial Infarction. Circulation 2006, 114, 2261–2270. [Google Scholar] [CrossRef]
- Campesi, I.; Capobianco, G.; Dessole, S.; Occhioni, S.; Montella, A.; Franconi, F. Estrogenic Compounds Have Divergent Effects on Human Endothelial Progenitor Cell Migration According to Sex of the Donor. J. Vasc. Res. 2015, 52, 273–278. [Google Scholar] [CrossRef]
- Schmidt-Lucke, C.; Rössig, L.; Fichtlscherer, S.; Vasa, M.; Britten, M.; Kämper, U.; Dimmeler, S.; Zeiher, A.M. Reduced Number of Circulating Endothelial Progenitor Cells Predicts Future Cardiovascular Events: Proof of Concept for the Clinical Importance of Endogenous Vascular Repair. Circulation 2005, 111, 2981–2987. [Google Scholar] [CrossRef]
- Lam, Y.T.; Hsu, C.J.; Simpson, P.J.; Dunn, L.L.; Chow, R.W.; Chan, K.H.; Yong, A.S.C.; Yu, Y.; Sieveking, D.P.; Lecce, L.; et al. Androgens Stimulate EPC-Mediated Neovascularization and Are Associated with Increased Coronary Collateralization. Endocrinology 2020, 161, bqaa043. [Google Scholar] [CrossRef]
- Albrecht, U. Timing to Perfection: The Biology of Central and Peripheral Circadian Clocks. Neuron 2012, 74, 246–260. [Google Scholar] [CrossRef]
- Rabinovich-Nikitin, I.; Lieberman, B.; Martino, T.A.; Kirshenbaum, L.A. Circadian-Regulated Cell Death in Cardiovascular Diseases. Circulation 2019, 139, 965–980. [Google Scholar] [CrossRef]
- Škrlec, I.; Milić, J.; Steiner, R. The Impact of the Circadian Genes CLOCK and ARNTL on Myocardial Infarction. J. Clin. Med. 2020, 9, 484. [Google Scholar] [CrossRef] [PubMed]
- Škrlec, I.; Milić, J.; Heffer, M.; Wagner, J.; Peterlin, B. Circadian Clock Genes and Circadian Phenotypes in Patients with Myocardial Infarction. Adv. Med. Sci. 2019, 64, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Vetter, C.; Devore, E.E.; Wegrzyn, L.R.; Massa, J.; Speizer, F.E.; Kawachi, I.; Rosner, B.; Stampfer, M.J.; Schernhammer, E.S. Association Between Rotating Night Shift Work and Risk of Coronary Heart Disease Among Women. JAMA 2016, 315, 1726–1734. [Google Scholar] [CrossRef]
- Ayas, N.T.; Taylor, C.M.; Laher, I. Cardiovascular Consequences of Obstructive Sleep Apnea. Curr. Opin. Cardiol. 2016, 31, 599–605. [Google Scholar] [CrossRef]
- Anderson, S.T.; FitzGerald, G.A. Sexual Dimorphism in Body Clocks. Science 2020, 369, 1164–1165. [Google Scholar] [CrossRef] [PubMed]
- Yuan, R.K.; Zitting, K.-M.; Wang, W.; Buxton, O.M.; Williams, J.S.; Duffy, J.F.; Czeisler, C.A. Fasting Blood Triglycerides Vary with Circadian Phase in Both Young and Older People. Physiol. Rep. 2020, 8, e14453. [Google Scholar] [CrossRef]
- Sole, M.J.; Martino, T.A. Diurnal Physiology: Core Principles with Application to the Pathogenesis, Diagnosis, Prevention, and Treatment of Myocardial Hypertrophy and Failure. J. Appl. Physiol. 2009, 107, 1318–1327. [Google Scholar] [CrossRef]
- Muller, J.E.; Stone, P.H.; Turi, Z.G.; Rutherford, J.D.; Czeisler, C.A.; Parker, C.; Poole, W.K.; Passamani, E.; Roberts, R.; Robertson, T.; et al. Circadian Variation in the Frequency of Onset of Acute Myocardial Infarction. N. Engl. J. Med. 1985, 313, 1315–1322. [Google Scholar] [CrossRef]
- Espino, J. Role of Melatonin on Diabetes-Related Metabolic Disorders. World J. Diabetes 2011, 2, 82. [Google Scholar] [CrossRef]
- Kondratov, R.V.; Kondratova, A.A.; Gorbacheva, V.Y.; Vykhovanets, O.V.; Antoch, M.P. Early Aging and Age-Related Pathologies in Mice Deficient in BMAL1, the Core Componentof the Circadian Clock. Genes Dev. 2006, 20, 1868–1873. [Google Scholar] [CrossRef] [PubMed]
- Musiek, E.S.; Lim, M.M.; Yang, G.; Bauer, A.Q.; Qi, L.; Lee, Y.; Roh, J.H.; Ortiz-Gonzalez, X.; Dearborn, J.T.; Culver, J.P.; et al. Circadian Clock Proteins Regulate Neuronal Redox Homeostasis and Neurodegeneration. J. Clin. Investig. 2013, 123, 5389–5400. [Google Scholar] [CrossRef]
- Zhdanova, I.V.; Tucci, V. Melatonin, Circadian Rhythms, and Sleep. Curr. Treat. Opt. Neurol. 2003, 5, 225–229. [Google Scholar] [CrossRef] [PubMed]
- Hardeland, R.; Coto-Montes, A.; Poeggeler, B. Circadian Rhythms, Oxidative Stress, and Antioxidative Defense Mechanisms. Chronobiol. Int. 2003, 20, 921–962. [Google Scholar] [CrossRef]
- Russcher, M.; Koch, B.; Nagtegaal, E.; van der Putten, K.; ter Wee, P.; Gaillard, C. The Role of Melatonin Treatment in Chronic Kidney Disease. Front. Biosci. 2012, 17, 2644–2656. [Google Scholar] [CrossRef]
- Nordholm, A.; Egstrand, S.; Gravesen, E.; Mace, M.L.; Morevati, M.; Olgaard, K.; Lewin, E. Circadian Rhythm of Activin A and Related Parameters of Mineral Metabolism in Normal and Uremic Rats. Pflug. Arch. 2019, 471, 1079–1094. [Google Scholar] [CrossRef]
- Zeman, M.; Dulková, K.; Bada, V.; Herichová, I. Plasma Melatonin Concentrations in Hypertensive Patients with the Dipping and Non-Dipping Blood Pressure Profile. Life Sci. 2005, 76, 1795–1803. [Google Scholar] [CrossRef] [PubMed]
- Borghi, C.; Cicero, A.F.G. Nutraceuticals with a Clinically Detectable Blood Pressure-lowering Effect: A Review of Available Randomized Clinical Trials and Their Meta-analyses. Br. J. Clin. Pharmacol. 2017, 83, 163–171. [Google Scholar] [CrossRef]
- Scheer, F.A.J.L.; Van Montfrans, G.A.; van Someren, E.J.W.; Mairuhu, G.; Buijs, R.M. Daily Nighttime Melatonin Reduces Blood Pressure in Male Patients with Essential Hypertension. Hypertension 2004, 43, 192–197. [Google Scholar] [CrossRef]
- Crnko, S.; Du Pré, B.C.; Sluijter, J.P.G.; Van Laake, L.W. Circadian Rhythms and the Molecular Clock in Cardiovascular Biology and Disease. Nat. Rev. Cardiol. 2019, 16, 437–447. [Google Scholar] [CrossRef]
- Qian, J.; Morris, C.J.; Caputo, R.; Wang, W.; Garaulet, M.; Scheer, F.A.J.L. Sex Differences in the Circadian Misalignment Effects on Energy Regulation. Proc. Natl. Acad. Sci. USA 2019, 116, 23806–23812. [Google Scholar] [CrossRef] [PubMed]
- Boivin, D.B.; Shechter, A.; Boudreau, P.; Begum, E.A.; Ng Ying-Kin, N.M.K. Diurnal and Circadian Variation of Sleep and Alertness in Men vs. Naturally Cycling Women. Proc. Natl. Acad. Sci. USA 2016, 113, 10980–10985. [Google Scholar] [CrossRef]
- Eastman, C.I.; Tomaka, V.A.; Crowley, S.J. Sex and Ancestry Determine the Free-running Circadian Period. J. Sleep. Res. 2017, 26, 547–550. [Google Scholar] [CrossRef] [PubMed]
- Lindberg, E.; Janson, C.; Gislason, T.; Björnsson, E.; Hetta, J.; Boman, G. Sleep Disturbances in a Young Adult Population: Can Gender Differences Be Explained by Differences in Psychological Status? Sleep 1997, 20, 381–387. [Google Scholar] [CrossRef]
- Cusmano, D.M.; Hadjimarkou, M.M.; Mong, J.A. Gonadal Steroid Modulation of Sleep and Wakefulness in Male and Female Rats Is Sexually Differentiated and Neonatally Organized by Steroid Exposure. Endocrinology 2014, 155, 204–214. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.A.; Kryger, M.H. Women and Sleep. J. Womens Health 2008, 17, 1189–1190. [Google Scholar] [CrossRef]
- Škrlec, I.; Talapko, J.; Juzbašić, M.; Steiner, R. Sex Differences in Circadian Clock Genes and Myocardial Infarction Susceptibility. J. Cardiovasc. Dev. Dis. 2021, 8, 53. [Google Scholar] [CrossRef]
- Zhao, R.; Li, D.; Zuo, P.; Bai, R.; Zhou, Q.; Fan, J.; Li, C.; Wang, L.; Yang, X. Influences of Age, Gender, and Circadian Rhythm on Deceleration Capacity in Subjects without Evident Heart Diseases. Ann. Noninvasive Electrocardiol. 2015, 20, 158–166. [Google Scholar] [CrossRef]
- Forman, D.E.; Cittadini, A.; Azhar, G.; Douglas, P.S.; Wei, J.Y. Cardiac Morphology and Function in Senescent Rats: Gender-Related Differences. J. Am. Coll. Cardiol. 1997, 30, 1872–1877. [Google Scholar] [CrossRef]
- Alibhai, F.J.; Reitz, C.J.; Peppler, W.T.; Basu, P.; Sheppard, P.; Choleris, E.; Bakovic, M.; Martino, T.A. Female ClockΔ19/Δ19 Mice Are Protected from the Development of Age-Dependent Cardiomyopathy. Cardiovasc. Res. 2018, 114, 259–271. [Google Scholar] [CrossRef]
- Park, S.-E.; So, W.-Y.; Kang, Y.-S.; Yang, J.-H. Relationship between Perceived Stress, Obesity, and Hypertension in Korean Adults and Older Adults. Healthcare 2023, 11, 2271. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.L.; Makarem, N.; Shimbo, D.; Aggarwal, B. Gender Differences in Associations Between Stress and Cardiovascular Risk Factors and Outcomes. Gend. Genome 2018, 2, 111–122. [Google Scholar] [CrossRef]
- Rosmond, R.; Dallman, M.F.; Björntorp, P. Stress-Related Cortisol Secretion in Men: Relationships with Abdominal Obesity and Endocrine, Metabolic and Hemodynamic Abnormalities 1. J. Clin. Endocrinol. Metab. 1998, 83, 1853–1859. [Google Scholar] [CrossRef] [PubMed]
- Björntorp, P. Do Stress Reactions Cause Abdominal Obesity and Comorbidities? Obes. Rev. 2001, 2, 73–86. [Google Scholar] [CrossRef]
- Nylén, E. Age, Race, Sex and Cardiorespiratory Fitness: Implications for Prevention and Management of Cardiometabolic Disease in Individuals with Diabetes Mellitus. Rev. Cardiovasc. Med. 2024, 25, 263. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.; Gulati, M.; Huang, T.Y.; Kwan, A.C.; Ouyang, D.; Ebinger, J.E.; Casaletto, K.; Moreau, K.L.; Skali, H.; Cheng, S. Sex Differences in Association of Physical Activity With All-Cause and Cardiovascular Mortality. J. Am. Coll. Cardiol. 2024, 83, 783–793. [Google Scholar] [CrossRef]
- Bei, Y.; Wang, L.; Ding, R.; Che, L.; Fan, Z.; Gao, W.; Liang, Q.; Lin, S.; Liu, S.; Lu, X.; et al. Animal Exercise Studies in Cardiovascular Research: Current Knowledge and Optimal Design—A Position Paper of the Committee on Cardiac Rehabilitation, Chinese Medical Doctors’ Association. J. Sport Health Sci. 2021, 10, 660–674. [Google Scholar] [CrossRef]
- Broom, D. Gender in/and/of Health Inequalities. Aust. J. Soc. Issues 2008, 43, 11–28. [Google Scholar] [CrossRef]
- Finkelstein, E.A.; Ruhm, C.J.; Kosa, K.M. ECONOMIC CAUSES AND CONSEQUENCES OF OBESITY. Annu. Rev. Public Health 2005, 26, 239–257. [Google Scholar] [CrossRef]
- Loucks, E.B.; Sullivan, L.M.; Hayes, L.J.; D’Agostino, R.B., Sr.; Larson, M.G.; Vasan, R.S.; Benjamin, E.J.; Berkman, L.F. Association of Educational Level with Inflammatory Markers in the Framingham Offspring Study. Am. J. Epidemiol. 2006, 163, 622–628. [Google Scholar] [CrossRef]
- Thurston, R.C.; Kubzansky, L.D.; Kawachi, I.; Berkman, L.F. Is the Association between Socioeconomic Position and Coronary Heart Disease Stronger in Women than in Men? Am. J. Epidemiol. 2005, 162, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Vélez, M.P.; Alvarado, B.; Lord, C.; Zunzunegui, M.-V. Life Course Socioeconomic Adversity and Age at Natural Menopause in Women from Latin America and the Caribbean. Menopause 2010, 17, 552–559. [Google Scholar] [PubMed]
- Higgins, S.T.; Kurti, A.N.; Redner, R.; White, T.J.; Gaalema, D.E.; Roberts, M.E.; Doogan, N.J.; Tidey, J.W.; Miller, M.E.; Stanton, C.A.; et al. A Literature Review on Prevalence of Gender Differences and Intersections with Other Vulnerabilities to Tobacco Use in the United States, 2004–2014. Prev. Med. 2015, 80, 89–100. [Google Scholar] [CrossRef]
- Huxley, R.R.; Woodward, M. Cigarette Smoking as a Risk Factor for Coronary Heart Disease in Women Compared with Men: A Systematic Review and Meta-Analysis of Prospective Cohort Studies. Lancet 2011, 378, 1297–1305. [Google Scholar] [CrossRef]
- Hiltunen, M.O.; Ylä-Herttuala, S. DNA Methylation, Smooth Muscle Cells, and Atherogenesis. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 1750–1753. [Google Scholar] [CrossRef]
- Burkman, R.; Schlesselman, J.J.; Zieman, M. Safety Concerns and Health Benefits Associated with Oral Contraception. Am. J. Obstet. Gynecol. 2004, 190, S5–S22. [Google Scholar] [CrossRef] [PubMed]
- Dayal, S.; Lentz, S.R. ADMA and Hyperhomocysteinemia. Vasc. Med. 2005, 10 (Suppl. S1), S27–S33. [Google Scholar] [CrossRef]
- Galan, P.; Viteri, F.E.; Bertrais, S.; Czernichow, S.; Faure, H.; Arnaud, J.; Ruffieux, D.; Chenal, S.; Arnault, N.; Favier, A.; et al. Serum Concentrations of β-Carotene, Vitamins C and E, Zinc and Selenium Are Influenced by Sex, Age, Diet, Smoking Status, Alcohol Consumption and Corpulence in a General French Adult Population. Eur. J. Clin. Nutr. 2005, 59, 1181–1190. [Google Scholar] [CrossRef]
- Klatsky, A.L. Alcohol and Cardiovascular Diseases: Where Do We Stand Today? J. Intern. Med. 2015, 278, 238–250. [Google Scholar] [CrossRef]
- Lankester, J.; Zanetti, D.; Ingelsson, E.; Assimes, T.L. Alcohol Use and Cardiometabolic Risk in the UK Biobank: A Mendelian Randomization Study. PLoS ONE 2020, 16, e0255801. [Google Scholar] [CrossRef]
- Cramer, J.; Jørgensen, J.T.; Hoffmann, B.; Loft, S.; Bräuner, E.V.; Prescott, E.; Ketzel, M.; Hertel, O.; Brandt, J.; Jensen, S.S.; et al. Long-Term Exposure to Air Pollution and Incidence of Myocardial Infarction: A Danish Nurse Cohort Study. Environ. Health Perspect. 2020, 128, 057003. [Google Scholar] [CrossRef]
- Duan, C.; Talbott, E.; Brooks, M.; Park, S.K.; Broadwin, R.; Matthews, K.; Barinas-Mitchell, E. Five-Year Exposure to PM2.5 and Ozone and Subclinical Atherosclerosis in Late Midlife Women: The Study of Women’s Health Across the Nation. Int. J. Hyg. Environ. Health 2019, 222, 168–176. [Google Scholar] [CrossRef]
- Lipsett, M.J.; Ostro, B.D.; Reynolds, P.; Goldberg, D.; Hertz, A.; Jerrett, M.; Smith, D.F.; Garcia, C.; Chang, E.T.; Bernstein, L. Long-Term Exposure to Air Pollution and Cardiorespiratory Disease in the California Teachers Study Cohort. Am. J. Respir. Crit. Care Med. 2011, 184, 828–835. [Google Scholar] [CrossRef] [PubMed]
- Cho, E.; Kang, Y.; Cho, Y. Effects of Fine Particulate Matter on Cardiovascular Disease Morbidity: A Study on Seven Metropolitan Cities in South Korea. Int. J. Public Health 2022, 67, 1604389. [Google Scholar] [CrossRef]
- Kang, S.-H.; Heo, J.; Oh, I.-Y.; Kim, J.; Lim, W.-H.; Cho, Y.; Choi, E.-K.; Yi, S.-M.; Do Shin, S.; Kim, H.; et al. Ambient Air Pollution and Out-of-Hospital Cardiac Arrest. Int. J. Cardiol. 2016, 203, 1086–1092. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.-S.; Yang, P.-S.; Lee, J.; Yu, H.T.; Kim, T.-H.; Uhm, J.-S.; Pak, H.-N.; Lee, M.-H.; Joung, B. Long-Term Exposure of Fine Particulate Matter Air Pollution and Incident Atrial Fibrillation in the General Population: A Nationwide Cohort Study. Int. J. Cardiol. 2019, 283, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Satyjeet, F.; Naz, S.; Kumar, V.; Aung, N.H.; Bansari, K.; Irfan, S.; Rizwan, A. Psychological Stress as a Risk Factor for Cardiovascular Disease: A Case-Control Study. Cureus 2020, 12, e10757. [Google Scholar] [CrossRef]
- Sinha, R.; Jastreboff, A.M. Stress as a Common Risk Factor for Obesity and Addiction. Biol. Psychiatry 2013, 73, 827–835. [Google Scholar] [CrossRef]
- Yao, B.; Meng, L.; Hao, M.; Zhang, Y.; Gong, T.; Guo, Z. Chronic Stress: A Critical Risk Factor for Atherosclerosis. J. Int. Med. Res. 2019, 47, 1429–1440. [Google Scholar] [CrossRef]
- Chu, B.; Marwaha, K.; Sanvictores, T.; Awosika, A.O.; Ayers, D. Physiology, Stress Reaction. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Park, J.H.; Moon, J.H.; Kim, H.J.; Kong, M.H.; Oh, Y.H. Sedentary Lifestyle: Overview of Updated Evidence of Potential Health Risks. Korean J. Fam. Med. 2020, 41, 365–373. [Google Scholar] [CrossRef]
- Warren, T.Y.; Barry, V.; Hooker, S.P.; Sui, X.; Church, T.S.; Blair, S.N. Sedentary Behaviors Increase Risk of Cardiovascular Disease Mortality in Men. Med. Sci. Sports Exerc. 2010, 42, 879–885. [Google Scholar] [CrossRef]
- Young, D.R.; Reynolds, K.; Sidell, M.; Brar, S.; Ghai, N.R.; Sternfeld, B.; Jacobsen, S.J.; Slezak, J.M.; Caan, B.; Quinn, V.P. Effects of Physical Activity and Sedentary Time on the Risk of Heart Failure. Circ. Heart Fail. 2014, 7, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Prince, S.A.; Roberts, K.C.; Melvin, A.; Butler, G.P.; Thompson, W. Gender and Education Differences in Sedentary Behaviour in Canada: An Analysis of National Cross-Sectional Surveys. BMC Public Health 2020, 20, 1170. [Google Scholar] [CrossRef] [PubMed]
- Nystoriak, M.A.; Bhatnagar, A. Cardiovascular Effects and Benefits of Exercise. Front. Cardiovasc. Med. 2018, 5, 135. [Google Scholar] [CrossRef]
- Edwards, E.S.; Sackett, S.C. Psychosocial Variables Related to Why Women Are Less Active than Men and Related Health Implications: Supplementary Issue: Health Disparities in Women. Clin. Med. Insights Women’s Health 2016, 9, CMWH.S34668. [Google Scholar] [CrossRef]
- Mielke, G.I.; da Silva, I.C.M.; Kolbe-Alexander, T.L.; Brown, W.J. Shifting the Physical Inactivity Curve Worldwide by Closing the Gender Gap. Sports Med. 2018, 48, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Kaminsky, L.A.; German, C.; Imboden, M.; Ozemek, C.; Peterman, J.E.; Brubaker, P.H. The Importance of Healthy Lifestyle Behaviors in the Prevention of Cardiovascular Disease. Prog. Cardiovasc. Dis. 2022, 70, 8–15. [Google Scholar] [CrossRef]
- Zhuo, C.; Zhao, J.; Chen, M.; Lu, Y. Physical Activity and Risks of Cardiovascular Diseases: A Mendelian Randomization Study. Front. Cardiovasc. Med. 2021, 8, 722154. [Google Scholar] [CrossRef]
- Feng, R.; Wang, L.; Li, Z.; Yang, R.; Liang, Y.; Sun, Y.; Yu, Q.; Ghartey-Kwansah, G.; Sun, Y.; Wu, Y.; et al. A Systematic Comparison of Exercise Training Protocols on Animal Models of Cardiovascular Capacity. Life Sci. 2019, 217, 128–140. [Google Scholar] [CrossRef]
- Boylan, J.M.; Cundiff, J.M.; Matthews, K.A. Socioeconomic Status and Cardiovascular Responses to Standardized Stressors: A Systematic Review and Meta-Analysis. Psychosom. Med. 2018, 80, 278–293. [Google Scholar] [CrossRef]
- Gallo, L.C.; Barger, S.D.; Fortmann, A.L.; Shivpuri, S. Socioeconomic Status and Cardiovascular Disease. In Handbook of Cardiovascular Behavioral Medicine; Waldstein, S.R., Kop, W.J., Suarez, E.C., Lovallo, W.R., Katzel, L.I., Eds.; Springer: New York, NY, USA, 2022; pp. 231–263. ISBN 978-0-387-85960-6. [Google Scholar]
- Jenkins, K.R.; Ofstedal, M.B. The Association between Socioeconomic Status and Cardiovascular Risk Factors among Middle-Aged and Older Men and Women. Women Health 2014, 54, 15–34. [Google Scholar] [CrossRef]
- Hiscock, R.; Bauld, L.; Amos, A.; Fidler, J.A.; Munafò, M. Socioeconomic Status and Smoking: A Review. Ann. N. Y. Acad. Sci. 2012, 1248, 107–123. [Google Scholar] [CrossRef] [PubMed]
- Pampel, F.C.; Krueger, P.M.; Denney, J.T. Socioeconomic Disparities in Health Behaviors. Annu. Rev. Sociol. 2010, 36, 349–370. [Google Scholar] [CrossRef]
- Grittner, U.; Kuntsche, S.; Graham, K.; Bloomfield, K. Social Inequalities and Gender Differences in the Experience of Alcohol-Related Problems. Alcohol. Alcohol. 2012, 47, 597–605. [Google Scholar] [CrossRef] [PubMed]
- Layte, R.; Whelan, C.T. Explaining Social Class Inequalities in Smoking: The Role of Education, Self-Efficacy, and Deprivation. Eur. Sociol. Rev. 2009, 25, 399–410. [Google Scholar] [CrossRef]
- Samet, J.M. Tobacco Smoking. Thorac. Surg. Clin. 2013, 23, 103–112. [Google Scholar] [CrossRef]
- Amram, D.L.; Zagà, V.; Serafini, A.; Cattaruzza, M.S. Tobacco smoking and gender differences: Epidemiological aspects. Tabaccologia 2023, 21, 27–35. [Google Scholar] [CrossRef]
- Yanbaeva, D.G.; Dentener, M.A.; Creutzberg, E.C.; Wesseling, G.; Wouters, E.F.M. Systemic Effects of Smoking. Chest 2007, 131, 1557–1566. [Google Scholar] [CrossRef] [PubMed]
- Arnson, Y.; Shoenfeld, Y.; Amital, H. Effects of Tobacco Smoke on Immunity, Inflammation and Autoimmunity. J. Autoimmun. 2010, 34, J258–J265. [Google Scholar] [CrossRef]
- Poredos, P.; Poredos, A.V.; Gregoric, I. Endothelial Dysfunction and Its Clinical Implications. Angiology 2021, 72, 604–615. [Google Scholar] [CrossRef]
- Edirisinghe, I.; Rahman, I. Cigarette Smoke–Mediated Oxidative Stress, Shear Stress, and Endothelial Dysfunction: Role of VEGFR2. Ann. N. Y. Acad. Sci. 2010, 1203, 66–72. [Google Scholar] [CrossRef]
- Philibert, R.A.; Beach, S.R.H.; Brody, G.H. Demethylation of the Aryl Hydrocarbon Receptor Repressor as a Biomarker for Nascent Smokers. Epigenetics 2012, 7, 1331–1338. [Google Scholar] [CrossRef] [PubMed]
- Seo, Y.-S.; Park, J.-M.; Kim, J.-H.; Lee, M.-Y. Cigarette Smoke-Induced Reactive Oxygen Species Formation: A Concise Review. Antioxidants 2023, 12, 1732. [Google Scholar] [CrossRef] [PubMed]
- Salminen, A.; Kaarniranta, K.; Kauppinen, A. Crosstalk between Oxidative Stress and SIRT1: Impact on the Aging Process. Int. J. Mol. Sci. 2013, 14, 3834–3859. [Google Scholar] [CrossRef] [PubMed]
- Ross, R. Atherosclerosis—An Inflammatory Disease. N. Engl. J. Med. 1999, 340, 115–126. [Google Scholar] [CrossRef]
- Van Der Bom, J.G.; Heckbert, S.R.; Lumley, T.; Holmes, C.E.; Cushman, M.; Folsom, A.R.; Rosendaal, F.R.; Psaty, B.M. Platelet Count and the Risk for Thrombosis and Death in the Elderly. J. Thromb. Haemost. 2009, 7, 399–405. [Google Scholar] [CrossRef]
- Abraham, M.; Alramadhan, S.; Iniguez, C.; Duijts, L.; Jaddoe, V.W.V.; Den Dekker, H.T.; Crozier, S.; Godfrey, K.M.; Hindmarsh, P.; Vik, T.; et al. A Systematic Review of Maternal Smoking during Pregnancy and Fetal Measurements with Meta-Analysis. PLoS ONE 2017, 12, e0170946. [Google Scholar] [CrossRef]
- Philips, E.M.; Santos, S.; Trasande, L.; Aurrekoetxea, J.J.; Barros, H.; von Berg, A.; Bergström, A.; Bird, P.K.; Brescianini, S.; Ní Chaoimh, C.; et al. Changes in Parental Smoking during Pregnancy and Risks of Adverse Birth Outcomes and Childhood Overweight in Europe and North America: An Individual Participant Data Meta-Analysis of 229,000 Singleton Births. PLoS Med. 2020, 17, e1003182. [Google Scholar] [CrossRef]
- Cajachagua-Torres, K.N.; El Marroun, H.; Reiss, I.K.M.; Jaddoe, V.W.V. Maternal Preconception and Pregnancy Tobacco and Cannabis Use in Relation to Placental Developmental Markers: A Population-Based Study. Reprod. Toxicol. 2022, 110, 70–77. [Google Scholar] [CrossRef]
- Ayonrinde, O.T.; Ayonrinde, O.A.; Rooyen, D.V.; Tait, R.; Dunn, M.; Mehta, S.; White, S.; Ayonrinde, O.K. Association between Gestational Cannabis Exposure and Maternal, Perinatal, Placental, and Childhood Outcomes. J. Dev. Orig. Health Dis. 2021, 12, 694–703. [Google Scholar] [CrossRef]
- de Jonge, L.L.; Harris, H.R.; Rich-Edwards, J.W.; Willett, W.C.; Forman, M.R.; Jaddoe, V.W.V.; Michels, K.B. Parental Smoking in Pregnancy and the Risks of Adult-Onset Hypertension. Hypertension 2013, 61, 494–500. [Google Scholar] [CrossRef] [PubMed]
- Harris, H.R.; Willett, W.C.; Michels, K.B. Parental Smoking during Pregnancy and Risk of Overweight and Obesity in the Daughter. Int. J. Obes. 2013, 37, 1356–1363. [Google Scholar] [CrossRef]
- Xiao, D.; Xu, Z.; Huang, X.; Longo, L.D.; Yang, S.; Zhang, L. Prenatal Gender-Related Nicotine Exposure Increases Blood Pressure Response to Angiotensin II in Adult Offspring. Hypertension 2008, 51, 1239–1247. [Google Scholar] [CrossRef]
- Synergistic Effects of Prenatal Nicotine Exposure and Post-Weaning High-Fat Diet on Hypercholesterolaemia in Rat Offspring of Different Sexes—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/30549443/ (accessed on 4 September 2024).
- He, X.; Lu, J.; Dong, W.; Jiao, Z.; Zhang, C.; Yu, Y.; Zhang, Z.; Wang, H.; Xu, D. Prenatal Nicotine Exposure Induces HPA Axis-Hypersensitivity in Offspring Rats via the Intrauterine Programming of up-Regulation of Hippocampal GAD67. Arch. Toxicol. 2017, 91, 3927–3943. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Liblik, K.; Haseeb, S.; Lopez-Santi, R.; Baranchuk, A. Women and Alcohol: Limitations in the Cardiovascular Guidelines. Curr. Probl. Cardiol. 2023, 48, 101200. [Google Scholar] [CrossRef]
- Biddinger, K.J.; Emdin, C.A.; Haas, M.E.; Wang, M.; Hindy, G.; Ellinor, P.T.; Kathiresan, S.; Khera, A.V.; Aragam, K.G. Association of Habitual Alcohol Intake With Risk of Cardiovascular Disease. JAMA Netw. Open 2022, 5, e223849. [Google Scholar] [CrossRef]
- White, A. Gender Differences in the Epidemiology of Alcohol Use and Related Harms in the United States. Alcohol Res. Curr. Rev. 2020, 40, 1. [Google Scholar] [CrossRef]
- Charness, M.E.; Riley, E.P.; Sowell, E.R. Drinking During Pregnancy and the Developing Brain: Is Any Amount Safe? Trends Cogn. Sci. 2016, 20, 80–82. [Google Scholar] [CrossRef]
- Murray, C.J.L.; Aravkin, A.Y.; Zheng, P.; Abbafati, C.; Abbas, K.M.; Abbasi-Kangevari, M.; Abd-Allah, F.; Abdelalim, A.; Abdollahi, M.; Abdollahpour, I.; et al. Global Burden of 87 Risk Factors in 204 Countries and Territories, 1990–2019: A Systematic Analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1223–1249. [Google Scholar] [CrossRef]
- Hadley, M.B.; Vedanthan, R.; Fuster, V. Air Pollution and Cardiovascular Disease: A Window of Opportunity. Nat. Rev. Cardiol. 2018, 15, 193–194. [Google Scholar] [CrossRef]
- Al-Kindi, S.G.; Brook, R.D.; Biswal, S.; Rajagopalan, S. Environmental Determinants of Cardiovascular Disease: Lessons Learned from Air Pollution. Nat. Rev. Cardiol. 2020, 17, 656–672. [Google Scholar] [CrossRef]
- Oberdorster, G.; Ferin, J.; Lehnert, B.E. Correlation between Particle Size, In Vivo Particle Persistence, and Lung Injury. Environ. Health Perspect. 1994, 102, 173. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jalali, S.; Karbakhsh, M.; Momeni, M.; Taheri, M.; Amini, S.; Mansourian, M.; Sarrafzadegan, N. Long-Term Exposure to PM2.5 and Cardiovascular Disease Incidence and Mortality in an Eastern Mediterranean Country: Findings Based on a 15-Year Cohort Study. Environ. Health 2021, 20, 112. [Google Scholar] [CrossRef] [PubMed]
- Alexeeff, S.E.; Liao, N.S.; Liu, X.; Van Den Eeden, S.K.; Sidney, S. Long-Term PM2.5 Exposure and Risks of Ischemic Heart Disease and Stroke Events: Review and Meta-Analysis. JAHA 2021, 10, e016890. [Google Scholar] [CrossRef] [PubMed]
- Rajagopalan, S.; Brook, R.D.; Salerno, P.R.V.O.; Bourges-Sevenier, B.; Landrigan, P.; Nieuwenhuijsen, M.J.; Munzel, T.; Deo, S.V.; Al-Kindi, S. Air Pollution Exposure and Cardiometabolic Risk. Lancet Diabetes Endocrinol. 2024, 12, 196–208. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Eum, K.-D.; Kazemiparkouhi, F.; Li, C.; Manjourides, J.; Pavlu, V.; Suh, H. The Impact of Long-Term PM2.5 Exposure on Specific Causes of Death: Exposure-Response Curves and Effect Modification among 53 Million U.S. Medicare Beneficiaries. Environ. Health 2020, 19, 20. [Google Scholar] [CrossRef] [PubMed]
- O’Toole, T.E.; Hellmann, J.; Wheat, L.; Haberzettl, P.; Lee, J.; Conklin, D.J.; Bhatnagar, A.; Pope, C.A. Episodic Exposure to Fine Particulate Air Pollution Decreases Circulating Levels of Endothelial Progenitor Cells. Circ. Res. 2010, 107, 200–203. [Google Scholar] [CrossRef] [PubMed]
- Schieber, M.; Chandel, N.S. ROS Function in Redox Signaling and Oxidative Stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef]
- Liao, M.; Braunstein, Z.; Rao, X. Sex Differences in Particulate Air Pollution-Related Cardiovascular Diseases: A Review of Human and Animal Evidence. Sci. Total Environ. 2023, 884, 163803. [Google Scholar] [CrossRef]
- Xi, Y.; Richardson, D.B.; Kshirsagar, A.V.; Wade, T.J.; Flythe, J.E.; Whitsel, E.A.; Peterson, G.C.; Wyatt, L.H.; Rappold, A.G. Effects of Short-Term Ambient PM2.5 Exposure on Cardiovascular Disease Incidence and Mortality among U.S. Hemodialysis Patients: A Retrospective Cohort Study. Environ. Health 2022, 21, 33. [Google Scholar] [CrossRef]
- Liu, C.; Chan, K.H.; Lv, J.; Lam, H.; Newell, K.; Meng, X.; Liu, Y.; Chen, R.; Kartsonaki, C.; Wright, N.; et al. Long-Term Exposure to Ambient Fine Particulate Matter and Incidence of Major Cardiovascular Diseases: A Prospective Study of 0.5 Million Adults in China. Environ. Sci. Technol. 2022, 56, 13200–13211. [Google Scholar] [CrossRef] [PubMed]
- Balmes, J.R. Household Air Pollution from Domestic Combustion of Solid Fuels and Health. J. Allergy Clin. Immunol. 2019, 143, 1979–1987. [Google Scholar] [CrossRef]
- Matz, C.; Stieb, D.; Davis, K.; Egyed, M.; Rose, A.; Chou, B.; Brion, O. Effects of Age, Season, Gender and Urban-Rural Status on Time-Activity: Canadian Human Activity Pattern Survey 2 (CHAPS 2). Int. J. Environ. Res. Public Health 2014, 11, 2108–2124. [Google Scholar] [CrossRef] [PubMed]
- Lévi, F.A.; Okyar, A.; Hadadi, E.; Innominato, P.F.; Ballesta, A. Circadian Regulation of Drug Responses: Toward Sex-Specific and Personalized Chronotherapy. Annu. Rev. Pharmacol. Toxicol. 2024, 64, 89–114. [Google Scholar] [CrossRef]
- Maddur, H. Alcohol and Liver Function in Women. Alcohol Res. Curr. Rev. 2020, 40, arcr.v40.2.10. [Google Scholar] [CrossRef]
- Kacevska, M.; Robertson, G.R.; Clarke, S.J.; Liddle, C. Inflammation and CYP3A4-Mediated Drug Metabolism in Advanced Cancer: Impact and Implications for Chemotherapeutic Drug Dosing. Expert Opin. Drug Metab. Toxicol. 2008, 4, 137–149. [Google Scholar] [CrossRef]
- Zhao, M.; Ma, J.; Li, M.; Zhang, Y.; Jiang, B.; Zhao, X.; Huai, C.; Shen, L.; Zhang, N.; He, L.; et al. Cytochrome P450 Enzymes and Drug Metabolism in Humans. Int. J. Mol. Sci. 2021, 22, 12808. [Google Scholar] [CrossRef]
- Gorski, J.C.; Jones, D.R.; Haehner-Daniels, B.D.; Hamman, M.A.; O’Mara, E.M.; Hall, S.D. The Contribution of Intestinal and Hepatic CYP3A to the Interaction between Midazolam and Clarithromycin. Clin. Pharmacol. Ther. 1998, 64, 133–143. [Google Scholar] [CrossRef]
- Soldin, O.P.; Chung, S.H.; Mattison, D.R. Sex Differences in Drug Disposition. BioMed Res. Int. 2011, 2011, 187103. [Google Scholar] [CrossRef]
- Ueno, K.; Sato, H. Sex-Related Differences in Pharmacokinetics and Pharmacodynamics of Anti-Hypertensive Drugs. Hypertens. Res. 2012, 35, 245–250. [Google Scholar] [CrossRef]
- Layton, A.T.; Sullivan, J.C. Recent Advances in Sex Differences in Kidney Function. Am. J. Physiol.-Ren. Physiol. 2019, 316, F328–F331. [Google Scholar] [CrossRef] [PubMed]
- Nolin, T.D. A Synopsis of Clinical Pharmacokinetic Alterations in Advanced CKD. Semin. Dial. 2015, 28, 325–329. [Google Scholar] [CrossRef]
- Rosano, G.M.C.; Lewis, B.; Agewall, S.; Wassmann, S.; Vitale, C.; Schmidt, H.; Drexel, H.; Patak, A.; Torp-Pedersen, C.; Kjeldsen, K.P.; et al. Gender Differences in the Effect of Cardiovascular Drugs: A Position Document of the Working Group on Pharmacology and Drug Therapy of the ESC. Eur. Heart J. 2015, 36, 2677–2680. [Google Scholar] [CrossRef]
- Bots, S.H.; den Ruijter, H.M. Recommended Heart Failure Medications and Adverse Drug Reactions in Women. Circulation 2019, 139, 1469–1471. [Google Scholar] [CrossRef]
- Laborante, R.; Borovac, J.A.; Galli, M.; Rodolico, D.; Ciliberti, G.; Restivo, A.; Cappannoli, L.; Arcudi, A.; Vergallo, R.; Zito, A.; et al. Gender-Differences in Antithrombotic Therapy across the Spectrum of Ischemic Heart Disease: Time to Tackle the Yentl Syndrome? Front. Cardiovasc. Med. 2022, 9, 1009475. [Google Scholar] [CrossRef]
- Ljungman, C.; Kahan, T.; Schiöler, L.; Hjerpe, P.; Wettermark, B.; Boström, K.B.; Manhem, K. Antihypertensive Treatment and Control According to Gender, Education, Country of Birth and Psychiatric Disorder: The Swedish Primary Care Cardiovascular Database (SPCCD). J. Hum. Hypertens. 2015, 29, 385–393. [Google Scholar] [CrossRef]
- Wallentin, F.; Wettermark, B.; Kahan, T. Drug Treatment of Hypertension in Sweden in Relation to Sex, Age, and Comorbidity. J. Clin. Hypertens. 2018, 20, 106–114. [Google Scholar] [CrossRef]
- Gu, Q.; Burt, V.L.; Paulose-Ram, R.; Dillon, C.F. Gender Differences in Hypertension Treatment, Drug Utilization Patterns, and Blood Pressure Control among US Adults with Hypertension: Data from the National Health and Nutrition Examination Survey 1999–2004. Am. J. Hypertens. 2008, 21, 789–798. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, A.; Beevers, D.G.; Bulpitt, C.; Butler, A.; Coles, E.C.; Hunt, D.; Munro-Faure, A.D.; Newson, R.B.; O’Riordan, P.W.; Petrie, J.C. Beta Adrenoceptor Blockade Is Associated with Increased Survival in Male but Not Female Hypertensive Patients: A Report from the DHSS Hypertension Care Computing Project (DHCCP). J. Hum. Hypertens. 1988, 2, 219–227. [Google Scholar]
- Wassertheil-Smoller, S.; Psaty, B.; Greenland, P.; Oberman, A.; Kotchen, T.; Mouton, C.; Black, H.; Aragaki, A.; Trevisan, M. Association between Cardiovascular Outcomes and Antihypertensive Drug Treatment in Older Women. JAMA 2004, 292, 2849–2859. [Google Scholar] [CrossRef]
- Turnbull, F.; Woodward, M.; Neal, B.; Barzi, F.; Ninomiya, T.; Chalmers, J.; Perkovic, V.; Li, N.; MacMahon, S. Blood Pressure Lowering Treatment Trialists’ Collaboration Do Men and Women Respond Differently to Blood Pressure-Lowering Treatment? Results of Prospectively Designed Overviews of Randomized Trials. Eur. Heart J. 2008, 29, 2669–2680. [Google Scholar] [CrossRef] [PubMed]
- Guirguis-Blake, J.M.; Evans, C.V.; Senger, C.A.; O’Connor, E.A.; Whitlock, E.P. Aspirin for the Primary Prevention of Cardiovascular Events: A Systematic Evidence Review for the U.S. Preventive Services Task Force. Ann. Intern. Med. 2016, 164, 804–813. [Google Scholar] [CrossRef]
- Yerman, T.; Gan, W.Q.; Sin, D.D. The Influence of Gender on the Effects of Aspirin in Preventing Myocardial Infarction. BMC Med. 2007, 5, 29. [Google Scholar] [CrossRef]
- Länne, T.; Ahlgren, Å.R. Increased Arterial Stiffness in IDDM Women—Methodological Considerations. Diabetologia 1996, 39, 871–872. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.-H.; Lee, P.-Y.; Ng, W.; Tse, H.-F.; Lau, C.-P. Aspirin Resistance Is Associated with a High Incidence of Myonecrosis after Non-Urgent Percutaneous Coronary Intervention despite Clopidogrel Pretreatment. J. Am. Coll. Cardiol. 2004, 43, 1122–1126. [Google Scholar] [CrossRef]
- Gum, P.A.; Kottke-Marchant, K.; Poggio, E.D.; Gurm, H.; Welsh, P.A.; Brooks, L.; Sapp, S.K.; Topol, E.J. Profile and Prevalence of Aspirin Resistance in Patients with Cardiovascular Disease. Am. J. Cardiol. 2001, 88, 230–235. [Google Scholar] [CrossRef]
- Hu, J.; Norman, M.; Wallensteen, M.; Gennser, G. Increased Large Arterial Stiffness and Impaired Acetylcholine Induced Skin Vasodilatation in Women with Previous Gestational Diabetes Mellitus. Br. J. Obstet. Gynaecol. 1998, 105, 1279–1287. [Google Scholar] [CrossRef] [PubMed]
- Pepine, C.J.; Kerensky, R.A.; Lambert, C.R.; Smith, K.M.; Von Mering, G.O.; Sopko, G.; Bairey Merz, C.N. Some Thoughts on the Vasculopathy of Women With Ischemic Heart Disease. J. Am. Coll. Cardiol. 2006, 47, S30–S35. [Google Scholar] [CrossRef]
- Kelton, J.G.; Hirsh, J.; Carter, C.J.; Buchanan, M.R. Sex Differences in the Antithrombotic Effects of Aspirin. Blood 1978, 52, 1073–1076. [Google Scholar] [CrossRef]
- Heckmann, N.D.; Piple, A.S.; Wang, J.C.; Richardson, M.K.; Mayfield, C.K.; Oakes, D.A.; Christ, A.B.; Lieberman, J.R. Aspirin for Venous Thromboembolic Prophylaxis Following Total Hip and Total Knee Arthroplasty: An Analysis of Safety and Efficacy Accounting for Surgeon Selection Bias. J. Arthroplast. 2023, 38, S412–S419.e1. [Google Scholar] [CrossRef]
- Johnson, M.; Ramey, E.; Ramwell, P.W. Androgen-Mediated Sensitivity in Platelet Aggregation. Am. J. Physiol. 1977, 232, H381–H385. [Google Scholar] [CrossRef] [PubMed]
- Tuñón, J.; Egido, J. Endothelial Dysfunction, Inflammation, and Statins: New Evidence. Rev. Española Cardiol. 2004, 57, 903–905. [Google Scholar] [CrossRef]
- Zhou, Q.; Liao, J.K. Statins and Cardiovascular Diseases: From Cholesterol Lowering to Pleiotropy. Curr. Pharm. Des. 2009, 15, 467–478. [Google Scholar] [CrossRef]
- Hunt, N.B.; Emmens, J.E.; Irawati, S.; de Vos, S.; Bos, J.H.J.; Wilffert, B.; Hak, E.; de Boer, R.A. Sex Disparities in the Effect of Statins on Lipid Parameters: The PharmLines Initiative. Medicine 2022, 101, e28394. [Google Scholar] [CrossRef] [PubMed]
- Karlson, B.W.; Palmer, M.K.; Nicholls, S.J.; Barter, P.J.; Lundman, P. Effects of Age, Gender and Statin Dose on Lipid Levels: Results from the VOYAGER Meta-Analysis Database. Atherosclerosis 2017, 265, 54–59. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Y.; Ou, Y.; Yang, M.; Yuan, J.; He, Q.; Li, Y.; Mi, N.; Xie, P.; Li, W.; et al. Gender-Specific Association between the Regular Use of Statins and the Risk of Irritable Bowel Syndrome: A Population-Based Prospective Cohort Study. Front. Pharmacol. 2023, 13, 1044542. [Google Scholar] [CrossRef]
- Nanna, M.G.; Wang, T.Y.; Xiang, Q.; Goldberg, A.C.; Robinson, J.G.; Roger, V.L.; Virani, S.S.; Wilson, P.W.F.; Louie, M.J.; Koren, A.; et al. Sex Differences in the Use of Statins in Community Practice. Circ. Cardiovasc. Qual. Outcomes 2019, 12, e005562. [Google Scholar] [CrossRef]
- Watanabe, A.M. Digitalis and the Autonomic Nervous System. J. Am. Coll. Cardiol. 1985, 5, 35A–42A. [Google Scholar] [CrossRef]
- Rathore, S.S.; Wang, Y.; Krumholz, H.M. Sex-Based Differences in the Effect of Digoxin for the Treatment of Heart Failure. N. Engl. J. Med. 2002, 347, 1403–1411. [Google Scholar] [CrossRef]
- Sullivan, K.; Doumouras, B.S.; Santema, B.T.; Walsh, M.N.; Douglas, P.S.; Voors, A.A.; Van Spall, H.G.C. Sex-Specific Differences in Heart Failure: Pathophysiology, Risk Factors, Management, and Outcomes. Can. J. Cardiol. 2021, 37, 560–571. [Google Scholar] [CrossRef]
- Vaziri, N.D.; Ni, Z.; Oveisi, F.; Trnavsky-Hobbs, D.L. Effect of Antioxidant Therapy on Blood Pressure and NO Synthase Expression in Hypertensive Rats. Hypertension 2000, 36, 957–964. [Google Scholar] [CrossRef] [PubMed]
- Cambonie, G.; Comte, B.; Yzydorczyk, C.; Ntimbane, T.; Germain, N.; Lê, N.L.O.; Pladys, P.; Gauthier, C.; Lahaie, I.; Abran, D.; et al. Antenatal Antioxidant Prevents Adult Hypertension, Vascular Dysfunction, and Microvascular Rarefaction Associated with in Utero Exposure to a Low-Protein Diet. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2007, 292, R1236–R1245. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, K.A.; Yuan Yuan, D.; Nawaz, W.; Ze, H.; Zhuo, C.X.; Talal, B.; Taleb, A.; Mais, E.; Qilong, D. Antioxidant Therapy for Management of Oxidative Stress Induced Hypertension. Free Radic. Res. 2017, 51, 428–438. [Google Scholar] [CrossRef]
- Mazzali, M.; Hughes, J.; Kim, Y.G.; Jefferson, J.A.; Kang, D.H.; Gordon, K.L.; Lan, H.Y.; Kivlighn, S.; Johnson, R.J. Elevated Uric Acid Increases Blood Pressure in the Rat by a Novel Crystal-Independent Mechanism. Hypertension 2001, 38, 1101–1106. [Google Scholar] [CrossRef]
- Mancia, G.; Kreutz, R.; Brunström, M.; Burnier, M.; Grassi, G.; Januszewicz, A.; Muiesan, M.L.; Tsioufis, K.; Agabiti-Rosei, E.; Algharably, E.A.E.; et al. 2023 ESH Guidelines for the Management of Arterial Hypertension The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension: Endorsed by the International Society of Hypertension (ISH) and the European Renal Association (ERA). J. Hypertens. 2023, 41, 1874–2071. [Google Scholar] [CrossRef] [PubMed]
- Whelton, P.K.; Carey, R.M.; Aronow, W.S.; Casey, D.E.; Collins, K.J.; Dennison Himmelfarb, C.; DePalma, S.M.; Gidding, S.; Jamerson, K.A.; Jones, D.W.; et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 2018, 71, 1269–1324. [Google Scholar] [CrossRef]
- McGreevy, C.; Williams, D. New Insights about Vitamin D and Cardiovascular Disease: A Narrative Review. Ann. Intern. Med. 2011, 155, 820–826. [Google Scholar] [CrossRef]
- Tamez, H.; Kalim, S.; Thadhani, R.I. Does Vitamin D Modulate Blood Pressure? Curr. Opin. Nephrol. Hypertens. 2013, 22, 204–209. [Google Scholar] [CrossRef]
- Anderson, J.L.; May, H.T.; Horne, B.D.; Bair, T.L.; Hall, N.L.; Carlquist, J.F.; Lappé, D.L.; Muhlestein, J.B. Intermountain Heart Collaborative (IHC) Study Group Relation of Vitamin D Deficiency to Cardiovascular Risk Factors, Disease Status, and Incident Events in a General Healthcare Population. Am. J. Cardiol. 2010, 106, 963–968. [Google Scholar] [CrossRef]
- Lips, P.; Eekhoff, M.; Van Schoor, N.; Oosterwerff, M.; De Jongh, R.; Krul-Poel, Y.; Simsek, S. Vitamin D and Type 2 Diabetes. J. Steroid Biochem. Mol. Biol. 2017, 173, 280–285. [Google Scholar] [CrossRef]
- Wiseman, H. Vitamin D Is a Membrane Antioxidant. Ability to Inhibit Iron-Dependent Lipid Peroxidation in Liposomes Compared to Cholesterol, Ergosterol and Tamoxifen and Relevance to Anticancer Action. FEBS Lett. 1993, 326, 285–288. [Google Scholar] [CrossRef]
- Polidoro, L.; Properzi, G.; Marampon, F.; Gravina, G.L.; Festuccia, C.; Di Cesare, E.; Scarsella, L.; Ciccarelli, C.; Zani, B.M.; Ferri, C. Vitamin D Protects Human Endothelial Cells from H₂O₂ Oxidant Injury through the Mek/Erk-Sirt1 Axis Activation. J. Cardiovasc. Transl. Res. 2013, 6, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Uberti, F.; Lattuada, D.; Morsanuto, V.; Nava, U.; Bolis, G.; Vacca, G.; Squarzanti, D.F.; Cisari, C.; Molinari, C. Vitamin D Protects Human Endothelial Cells From Oxidative Stress Through the Autophagic and Survival Pathways. J. Clin. Endocrinol. Metab. 2014, 99, 1367–1374. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, N.; Amani, R.; Hajiani, E.; Cheraghian, B. Does Vitamin D Improve Liver Enzymes, Oxidative Stress, and Inflammatory Biomarkers in Adults with Non-Alcoholic Fatty Liver Disease? A Randomized Clinical Trial. Endocrine 2014, 47, 70–80. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, P. Association of CYP19A1 and CYP1A2 Genetic Polymorphisms with Type 2 Diabetes Mellitus Risk in the Chinese Han Population. Lipids Health Dis. 2020, 19, 187. [Google Scholar] [CrossRef] [PubMed]
- Shimodaira, M.; Nakayama, T.; Sato, N.; Saito, K.; Morita, A.; Sato, I.; Takahashi, T.; Soma, M.; Izumi, Y. Association Study of Aromatase Gene (CYP19A1) in Essential Hypertension. Int. J. Med. Sci. 2008, 5, 29–35. [Google Scholar] [CrossRef]
- Ning, Z.; Song, S.; Miao, L.; Zhang, P.; Wang, X.; Liu, J.; Hu, Y.; Xu, Y.; Zhao, T.; Liang, Y.; et al. High Prevalence of Vitamin D Deficiency in Urban Health Checkup Population. Clin. Nutr. 2016, 35, 859–863. [Google Scholar] [CrossRef]
- de Oliveira, C.L.; Cureau, F.V.; Cople-Rodrigues, C.D.S.; Giannini, D.T.; Bloch, K.V.; Kuschnir, M.C.C.; de Carvalho, K.M.B.; Schaan, B.D. Prevalence and Factors Associated with Hypovitaminosis D in Adolescents from a Sunny Country: Findings from the ERICA Survey. J. Steroid Biochem. Mol. Biol. 2020, 199, 105609. [Google Scholar] [CrossRef]
- Zhu, X.-L.; Chen, Z.-H.; Li, Y.; Yang, P.-T.; Liu, L.; Wu, L.-X.; Wang, Y.-Q. Associations of Vitamin D with Novel and Traditional Anthropometric Indices According to Age and Sex: A Cross-Sectional Study in Central Southern China. Eat. Weight. Disord. 2020, 25, 1651–1661. [Google Scholar] [CrossRef]
- Verdoia, M.; Schaffer, A.; Barbieri, L.; Di Giovine, G.; Marino, P.; Suryapranata, H.; De Luca, G. Novara Atherosclerosis Study Group (NAS) Impact of Gender Difference on Vitamin D Status and Its Relationship with the Extent of Coronary Artery Disease. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 464–470. [Google Scholar] [CrossRef]
- Pál, É.; Hadjadj, L.; Fontányi, Z.; Monori-Kiss, A.; Lippai, N.; Horváth, E.M.; Magyar, A.; Horváth, E.; Monos, E.; Nádasy, G.L.; et al. Gender, Hyperandrogenism and Vitamin D Deficiency Related Functional and Morphological Alterations of Rat Cerebral Arteries. PLoS ONE 2019, 14, e0216951. [Google Scholar] [CrossRef]
- Han, B.; Li, Q.; Wang, N.; Chen, Y.; Zhu, C.; Chen, Y.; Xia, F.; Cang, Z.; Lu, M.; Meng, Y.; et al. Sexual Dimorphism for the Association between Vitamin D and Insulin Resistance in Chinese People. Int. J. Endocrinol. 2018, 2018, 1–6. [Google Scholar] [CrossRef]
- Rautiainen, S.; Levitan, E.B.; Orsini, N.; Åkesson, A.; Morgenstern, R.; Mittleman, M.A.; Wolk, A. Total Antioxidant Capacity from Diet and Risk of Myocardial Infarction: A Prospective Cohort of Women. Am. J. Med. 2012, 125, 974–980. [Google Scholar] [CrossRef]
- Rautiainen, S.; Levitan, E.B.; Mittleman, M.A.; Wolk, A. Total Antioxidant Capacity of Diet and Risk of Heart Failure: A Population-Based Prospective Cohort of Women. Am. J. Med. 2013, 126, 494–500. [Google Scholar] [CrossRef] [PubMed]
- Rautiainen, S.; Larsson, S.; Virtamo, J.; Wolk, A. Total Antioxidant Capacity of Diet and Risk of Stroke: A Population-Based Prospective Cohort of Women. Stroke 2012, 43, 335–340. [Google Scholar] [CrossRef]
- Abbasalizad Farhangi, M.; Najafi, M. Dietary Total Antioxidant Capacity (TAC) among Candidates for Coronary Artery Bypass Grafting (CABG) Surgery: Emphasis to Possible Beneficial Role of TAC on Serum Vitamin D. PLoS ONE 2018, 13, e0208806. [Google Scholar] [CrossRef]
- Jayedi, A.; Rashidy-Pour, A.; Parohan, M.; Zargar, M.S.; Shab-Bidar, S. Dietary and Circulating Vitamin C, Vitamin E, β-Carotene and Risk of Total Cardiovascular Mortality: A Systematic Review and Dose-Response Meta-Analysis of Prospective Observational Studies. Public Health Nutr. 2019, 22, 1872–1887. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.K.; Mehra, N.K.; Swarnakar, N.K. Role of Antioxidants for the Treatment of Cardiovascular Diseases: Challenges and Opportunities. Curr. Pharm. Des. 2015, 21, 4441–4455. [Google Scholar] [CrossRef]
- Cook, N.R. A Randomized Factorial Trial of Vitamins C and E and Beta Carotene in the Secondary Prevention of Cardiovascular Events in Women: Results From the Women’s Antioxidant Cardiovascular Study. Arch. Intern. Med. 2007, 167, 1610. [Google Scholar] [CrossRef]
- Osganian, S.K.; Stampfer, M.J.; Rimm, E.; Spiegelman, D.; Hu, F.B.; Manson, J.E.; Willett, W.C. Vitamin C and Risk of Coronary Heart Disease in Women. J. Am. Coll. Cardiol. 2003, 42, 246–252. [Google Scholar] [CrossRef]
- Klipstein-Grobusch, K.; den Breeijen, J.H.; Grobbee, D.E.; Boeing, H.; Hofman, A.; Witteman, J.C. Dietary Antioxidants and Peripheral Arterial Disease: The Rotterdam Study. Am. J. Epidemiol. 2001, 154, 145–149. [Google Scholar] [CrossRef] [PubMed]
- Ismail, N.M.; Harun, A.; Yusof, A.A.; Zaiton, Z.; Marzuki, A. Role of Vitamin e on Oxidative Stress in Smokers. Malays. J. Med. Sci. 2002, 9, 34–42. [Google Scholar] [PubMed]
- Crawford, R.S.; Kirk, E.A.; Rosenfeld, M.E.; LeBoeuf, R.C.; Chait, A. Dietary Antioxidants Inhibit Development of Fatty Streak Lesions in the LDL Receptor-Deficient Mouse. Arterioscler. Thromb. Vasc. Biol. 1998, 18, 1506–1513. [Google Scholar] [CrossRef]
- Amom, Z.; Zakaria, Z.; Mohamed, J.; Azlan, A.; Bahari, H.; Taufik Hidayat Baharuldin, M.; Aris Moklas, M.; Osman, K.; Asmawi, Z.; Kamal Nik Hassan, M. Lipid Lowering Effect of Antioxidant Alpha-Lipoic Acid in Experimental Atherosclerosis. J. Clin. Biochem. Nutr. 2008, 43, 88–94. [Google Scholar] [CrossRef]
- Colao, A.; Vetrani, C.; Muscogiuri, G.; Barrea, L.; Tricopoulou, A.; Soldati, L.; Piscitelli, P. UNESCO Chair on Health Education and Sustainable Development “Planeterranean” Diet: Extending Worldwide the Health Benefits of Mediterranean Diet Based on Nutritional Properties of Locally Available Foods. J. Transl. Med. 2022, 20, 232. [Google Scholar] [CrossRef] [PubMed]
- Mattioli, A.V.; Pennella, S.; Pedrazzi, P.; Farinetti, A. Gender Differences in Adherence to Mediterranean Diet and Risk of Atrial Fibrillation. JHC 2015, 1, 4–13. [Google Scholar] [CrossRef]
- Barrea, L.; Verde, L.; Suárez, R.; Frias-Toral, E.; Vásquez, C.A.; Colao, A.; Savastano, S.; Muscogiuri, G. Sex-Differences in Mediterranean Diet: A Key Piece to Explain Sex-Related Cardiovascular Risk in Obesity? A Cross-Sectional Study. J. Transl. Med. 2024, 22, 44. [Google Scholar] [CrossRef]
- Bédard, A.; Tchernof, A.; Lamarche, B.; Corneau, L.; Dodin, S.; Lemieux, S. Effects of the Traditional Mediterranean Diet on Adiponectin and Leptin Concentrations in Men and Premenopausal Women: Do Sex Differences Exist? Eur. J. Clin. Nutr. 2014, 68, 561–566. [Google Scholar] [CrossRef]
- Soldevila-Domenech, N.; Pastor, A.; Sala-Vila, A.; Lázaro, I.; Boronat, A.; Muñoz, D.; Castañer, O.; Fagundo, B.; Corella, D.; Fernández-Aranda, F.; et al. Sex Differences in Endocannabinoids during 3 Years of Mediterranean Diet Intervention: Association with Insulin Resistance and Weight Loss in a Population with Metabolic Syndrome. Front. Nutr. 2022, 9, 1076677. [Google Scholar] [CrossRef]
- van den Brink, A.C.; Brouwer-Brolsma, E.M.; Berendsen, A.A.M.; van de Rest, O. The Mediterranean, Dietary Approaches to Stop Hypertension (DASH), and Mediterranean-DASH Intervention for Neurodegenerative Delay (MIND) Diets Are Associated with Less Cognitive Decline and a Lower Risk of Alzheimer’s Disease-A Review. Adv. Nutr. 2019, 10, 1040–1065. [Google Scholar] [CrossRef]
- Aminianfar, A.; Hassanzadeh Keshteli, A.; Esmaillzadeh, A.; Adibi, P. Association between Adherence to MIND Diet and General and Abdominal Obesity: A Cross-Sectional Study. Nutr. J. 2020, 19, 15. [Google Scholar] [CrossRef] [PubMed]
- Arjmand, G.; Abbas-Zadeh, M.; Fardaei, M.; Eftekhari, M.H. The Effect of Short-Term Mediterranean-DASH Intervention for Neurodegenerative Delay (MIND) Diet on Hunger Hormones, Anthropometric Parameters, and Brain Structures in Middle-Aged Overweight and Obese Women: A Randomized Controlled Trial. Iran. J. Med. Sci. 2022, 47, 422. [Google Scholar] [CrossRef] [PubMed]
- Bonyadi, N.; Dolatkhah, N.; Salekzamani, Y.; Hashemian, M. Effect of Berry-Based Supplements and Foods on Cognitive Function: A Systematic Review. Sci. Rep. 2022, 12, 3239. [Google Scholar] [CrossRef]
- Chan, R.; Yu, B.; Leung, J.; Lee, J.S.-W.; Woo, J. Association of Dietary Patterns with Serum High-Sensitivity C-Reactive Protein Level in Community-Dwelling Older Adults. Clin. Nutr. ESPEN 2019, 31, 38–47. [Google Scholar] [CrossRef]
- Vučić Lovrenčić, M.; Gerić, M.; Košuta, I.; Dragičević, M.; Garaj-Vrhovac, V.; Gajski, G. Sex-Specific Effects of Vegetarian Diet on Adiponectin Levels and Insulin Sensitivity in Healthy Non-Obese Individuals. Nutrition 2020, 79–80, 110862. [Google Scholar] [CrossRef]
- Adams, M.; Sabaté, J. Sexual Dimorphism in Cardiovascular Disease Risk and Risk Factors Among Vegetarians: An Exploration of the Potential Mechanisms. Curr. Atheroscler. Rep. 2019, 21, 35. [Google Scholar] [CrossRef]
- Halvorsen, R.E.; Elvestad, M.; Molin, M.; Aune, D. Fruit and Vegetable Consumption and the Risk of Type 2 Diabetes: A Systematic Review and Dose–Response Meta-Analysis of Prospective Studies. BMJ Nutr. Prev. Health 2021, 4, 519. [Google Scholar] [CrossRef]
- Le Couteur, D.G.; Solon-Biet, S.; Cogger, V.C.; Mitchell, S.J.; Senior, A.; De Cabo, R.; Raubenheimer, D.; Simpson, S.J. The Impact of Low-Protein High-Carbohydrate Diets on Aging and Lifespan. Cell. Mol. Life Sci. 2016, 73, 1237–1252. [Google Scholar] [CrossRef]
- Fontana, L.; Klein, S.; Holloszy, J.O. Effects of Long-Term Calorie Restriction and Endurance Exercise on Glucose Tolerance, Insulin Action, and Adipokine Production. Age 2010, 32, 97–108. [Google Scholar] [CrossRef]
- Villareal, D.T.; Kotyk, J.J.; Armamento-Villareal, R.C.; Kenguva, V.; Seaman, P.; Shahar, A.; Wald, M.J.; Kleerekoper, M.; Fontana, L. Reduced Bone Mineral Density Is Not Associated with Significantly Reduced Bone Quality in Men and Women Practicing Long-Term Calorie Restriction with Adequate Nutrition. Aging Cell 2011, 10, 96–102. [Google Scholar] [CrossRef]
- Fontana, L.; Villareal, D.T.; Weiss, E.P.; Racette, S.B.; Steger-May, K.; Klein, S.; Holloszy, J.O. Calorie Restriction or Exercise: Effects on Coronary Heart Disease Risk Factors. A Randomized, Controlled Trial. Am. J. Physiol.-Endocrinol. Metab. 2007, 293, E197–E202. [Google Scholar] [CrossRef] [PubMed]
- Weiss, E.P.; Fontana, L. Caloric Restriction: Powerful Protection for the Aging Heart and Vasculature. Am. J. Physiol. Heart Circ. Physiol. 2011, 301, H1205–H1219. [Google Scholar] [CrossRef]
- Weiss, E.P.; Racette, S.B.; Villareal, D.T.; Fontana, L.; Steger-May, K.; Schechtman, K.B.; Klein, S.; Holloszy, J.O. Improvements in Glucose Tolerance and Insulin Action Induced by Increasing Energy Expenditure or Decreasing Energy Intake: A Randomized Controlled Trial. Am. J. Clin. Nutr. 2006, 84, 1033–1042. [Google Scholar] [CrossRef] [PubMed]
- Kane, A.E.; Sinclair, D.A.; Mitchell, J.R.; Mitchell, S.J. Sex Differences in the Response to Dietary Restriction in Rodents. Curr. Opin. Physiol. 2018, 6, 28–34. [Google Scholar] [CrossRef]
- Tower, J. Sex-Specific Gene Expression and Life Span Regulation. Trends Endocrinol. Metab. 2017, 28, 735–747. [Google Scholar] [CrossRef] [PubMed]
- Austad, S.N.; Bartke, A. Sex Differences in Longevity and in Responses to Anti-Aging Interventions: A Mini-Review. Gerontology 2015, 62, 40–46. [Google Scholar] [CrossRef]
- Ferland, G.; Doucet, I.; Mainville, D. Phylloquinone and Menaquinone-4 Tissue Distribution at Different Life Stages in Male and Female Sprague–Dawley Rats Fed Different VK Levels Since Weaning or Subjected to a 40% Calorie Restriction since Adulthood. Nutrients 2016, 8, 141. [Google Scholar] [CrossRef]
- Li, J.; Hu, S.; Cheng, K. Engineering Better Stem Cell Therapies for Treating Heart Diseases. Ann. Transl. Med. 2020, 8, 569. [Google Scholar] [CrossRef]
- Nesselmann, C.; Ma, N.; Bieback, K.; Wagner, W.; Ho, A.; Konttinen, Y.T.; Zhang, H.; Hinescu, M.E.; Steinhoff, G. Mesenchymal Stem Cells and Cardiac Repair. J. Cell. Mol. Med. 2008, 12, 1795–1810. [Google Scholar] [CrossRef]
- Mihai, M.C.; Popa, M.A.; Șuică, V.I.; Antohe, F.; Jackson, E.K.; Leeners, B.; Simionescu, M.; Dubey, R.K. Proteomic Analysis of Estrogen-Mediated Enhancement of Mesenchymal Stem Cell-Induced Angiogenesis In Vivo. Cells 2021, 10, 2181. [Google Scholar] [CrossRef]
- Nakada, D.; Oguro, H.; Levi, B.P.; Ryan, N.; Kitano, A.; Saitoh, Y.; Takeichi, M.; Wendt, G.R.; Morrison, S.J. Oestrogen Increases Haematopoietic Stem-Cell Self-Renewal in Females and during Pregnancy. Nature 2014, 505, 555–558. [Google Scholar] [CrossRef] [PubMed]
- Lock, R.; Al Asafen, H.; Fleischer, S.; Tamargo, M.; Zhao, Y.; Radisic, M.; Vunjak-Novakovic, G. A Framework for Developing Sex-Specific Engineered Heart Models. Nat. Rev. Mater. 2022, 7, 295–313. [Google Scholar] [CrossRef] [PubMed]
- Youssef, A.A.; Ross, E.G.; Bolli, R.; Pepine, C.J.; Leeper, N.J.; Yang, P.C. The Promise and Challenge of Induced Pluripotent Stem Cells for Cardiovascular Applications. JACC Basic Transl. Sci. 2016, 1, 510–523. [Google Scholar] [CrossRef] [PubMed]
- Kissinger, D. Hormone Replacement Therapy Perspectives. Front. Glob. Womens Health 2024, 5, 1397123. [Google Scholar] [CrossRef]
- Lobo, R.A. Hormone-Replacement Therapy: Current Thinking. Nat. Rev. Endocrinol. 2017, 13, 220–231. [Google Scholar] [CrossRef]
- Bath, P.M.W.; Gray, L.J. Association between Hormone Replacement Therapy and Subsequent Stroke: A Meta-Analysis. BMJ 2005, 330, 342. [Google Scholar] [CrossRef]
- Cho, L.; Kaunitz, A.M.; Faubion, S.S.; Hayes, S.N.; Lau, E.S.; Pristera, N.; Scott, N.; Shifren, J.L.; Shufelt, C.L.; Stuenkel, C.A.; et al. Rethinking Menopausal Hormone Therapy: For Whom, What, When, and How Long? Circulation 2023, 147, 597–610. [Google Scholar] [CrossRef]


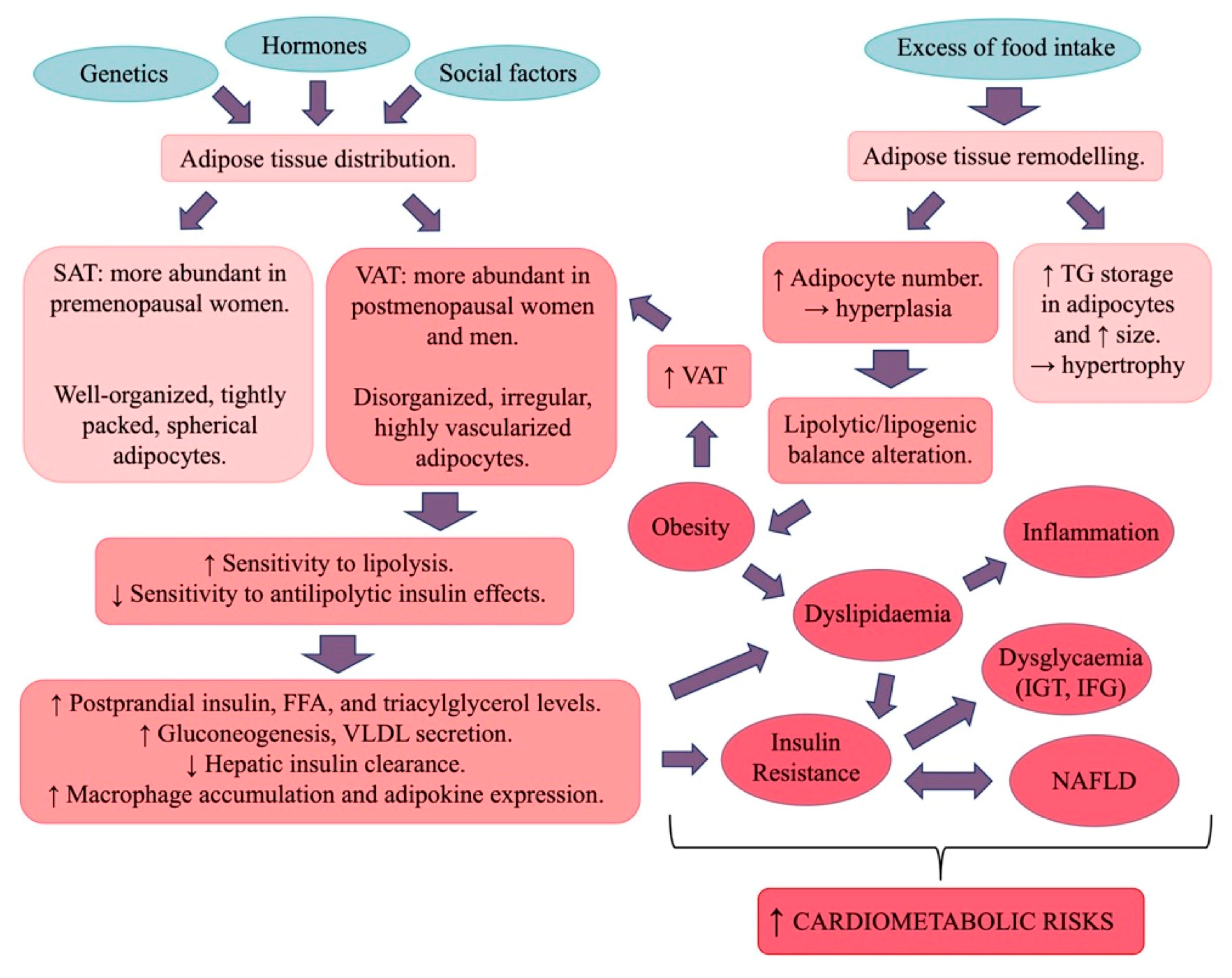
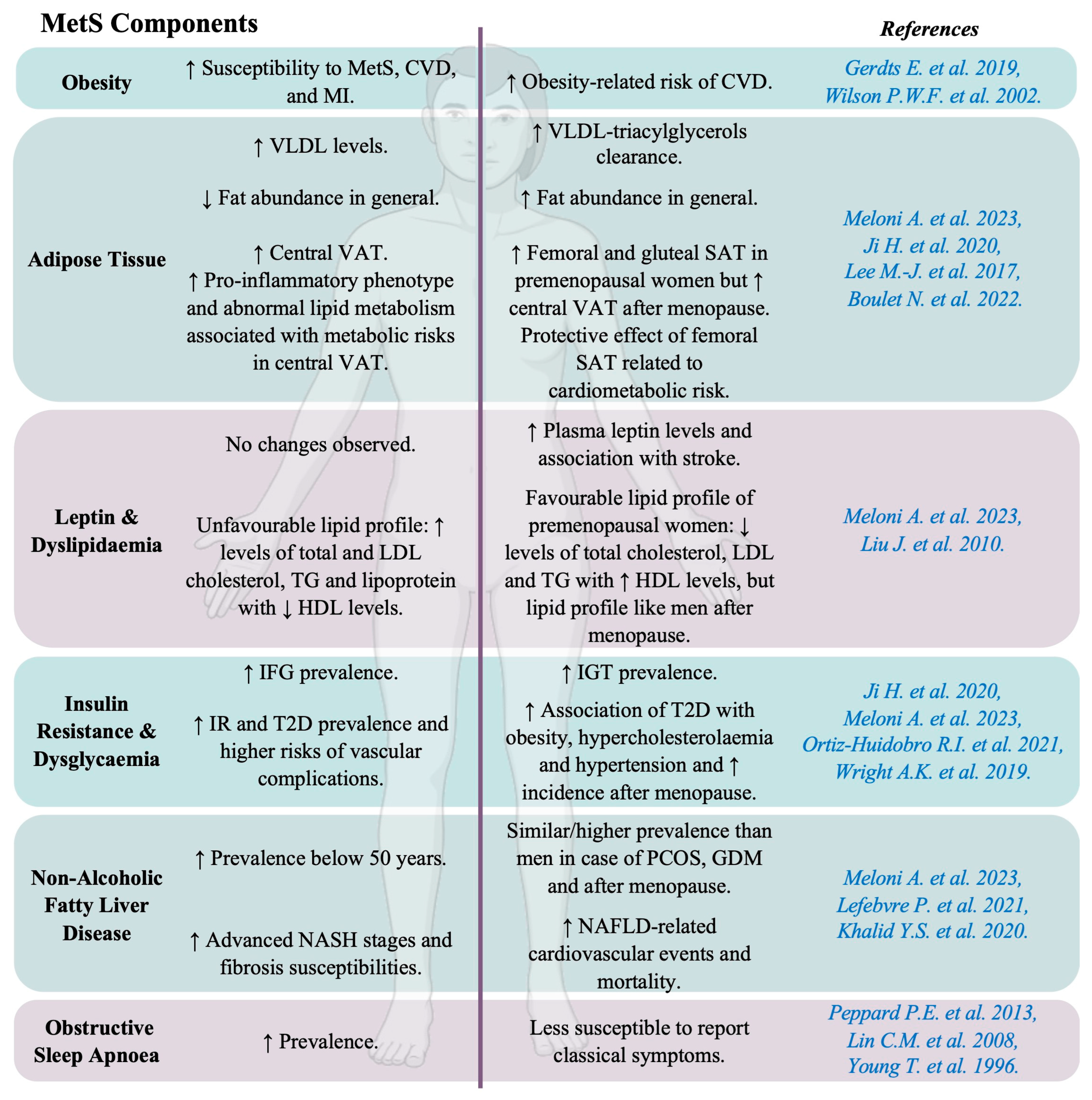



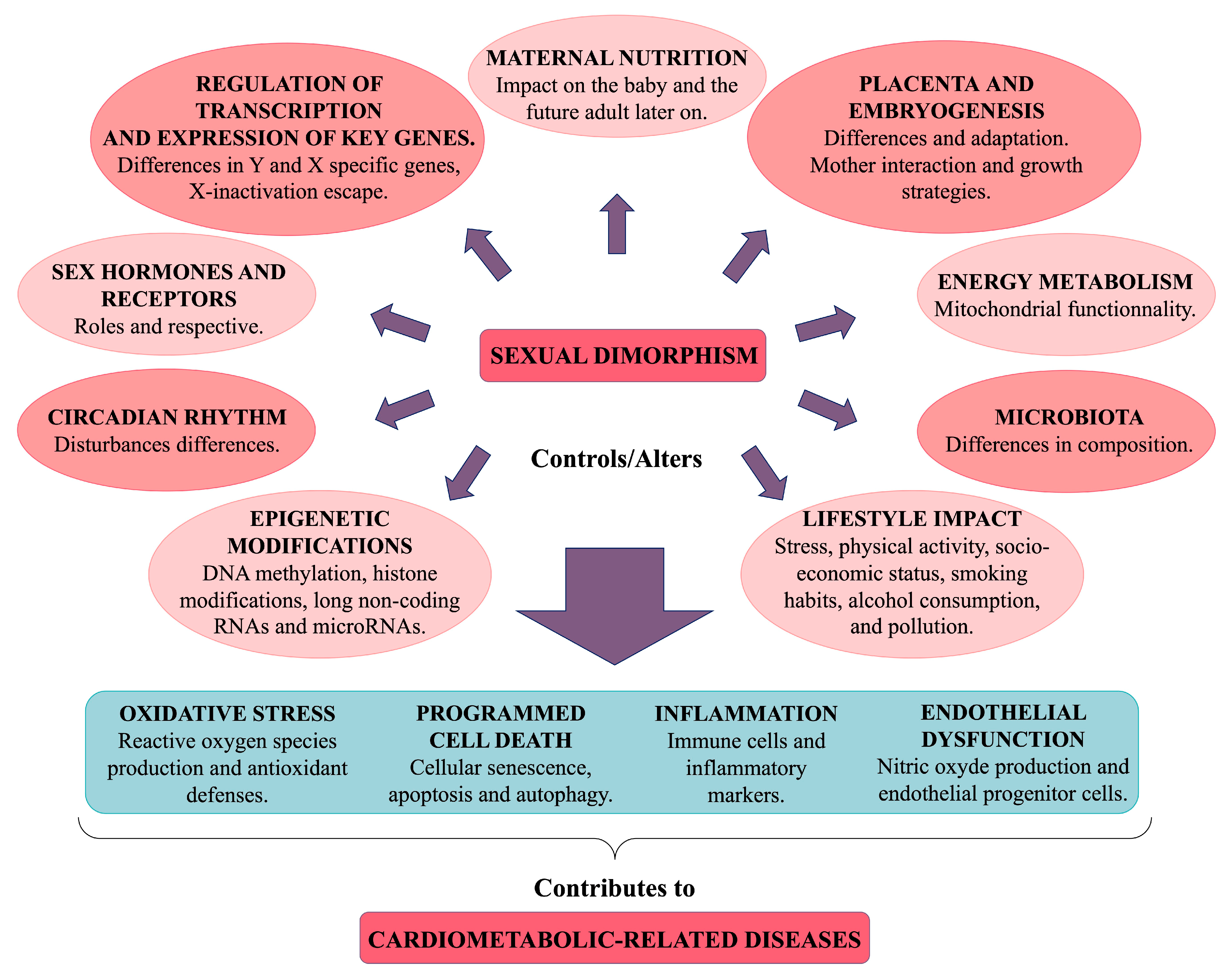
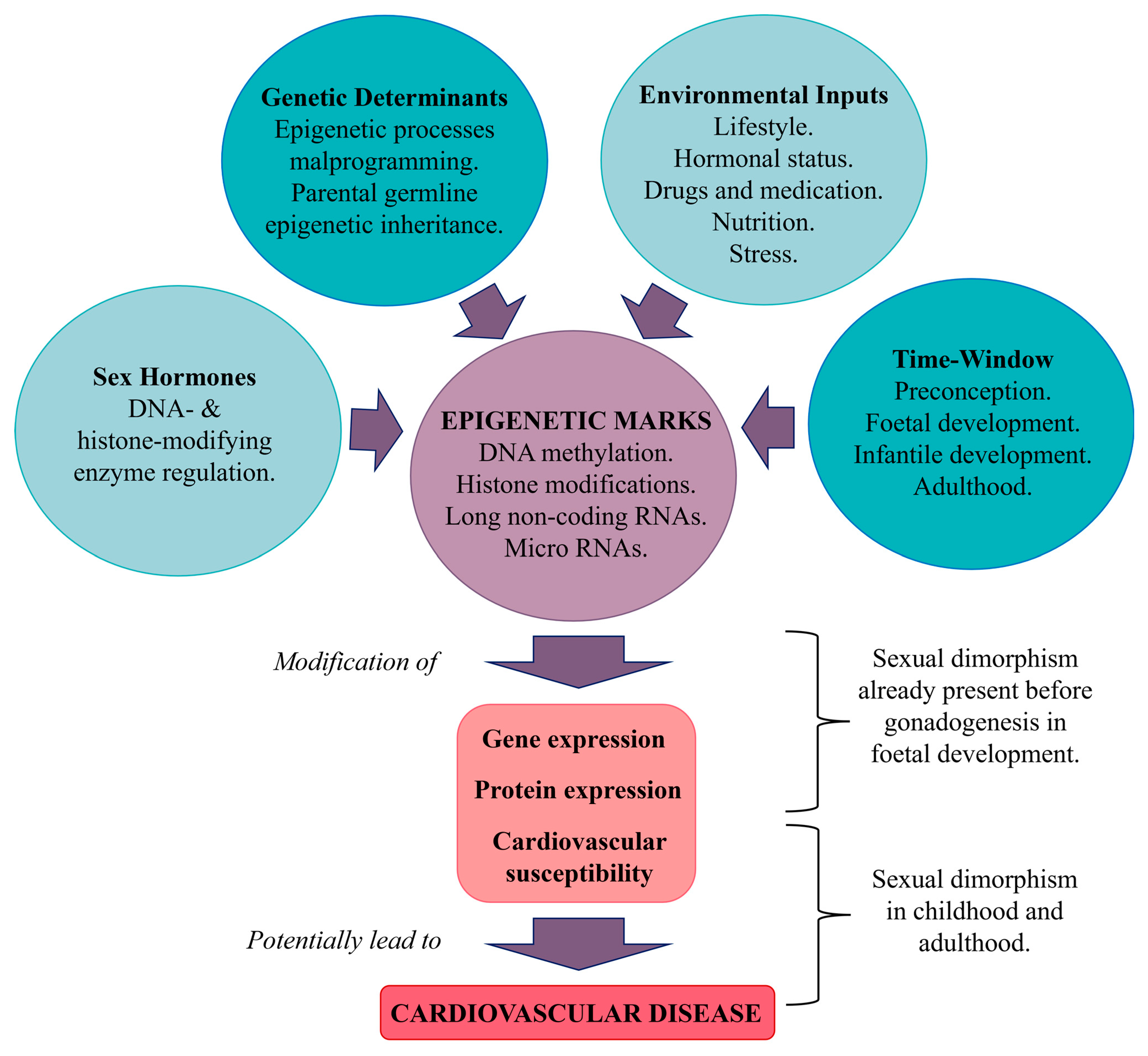
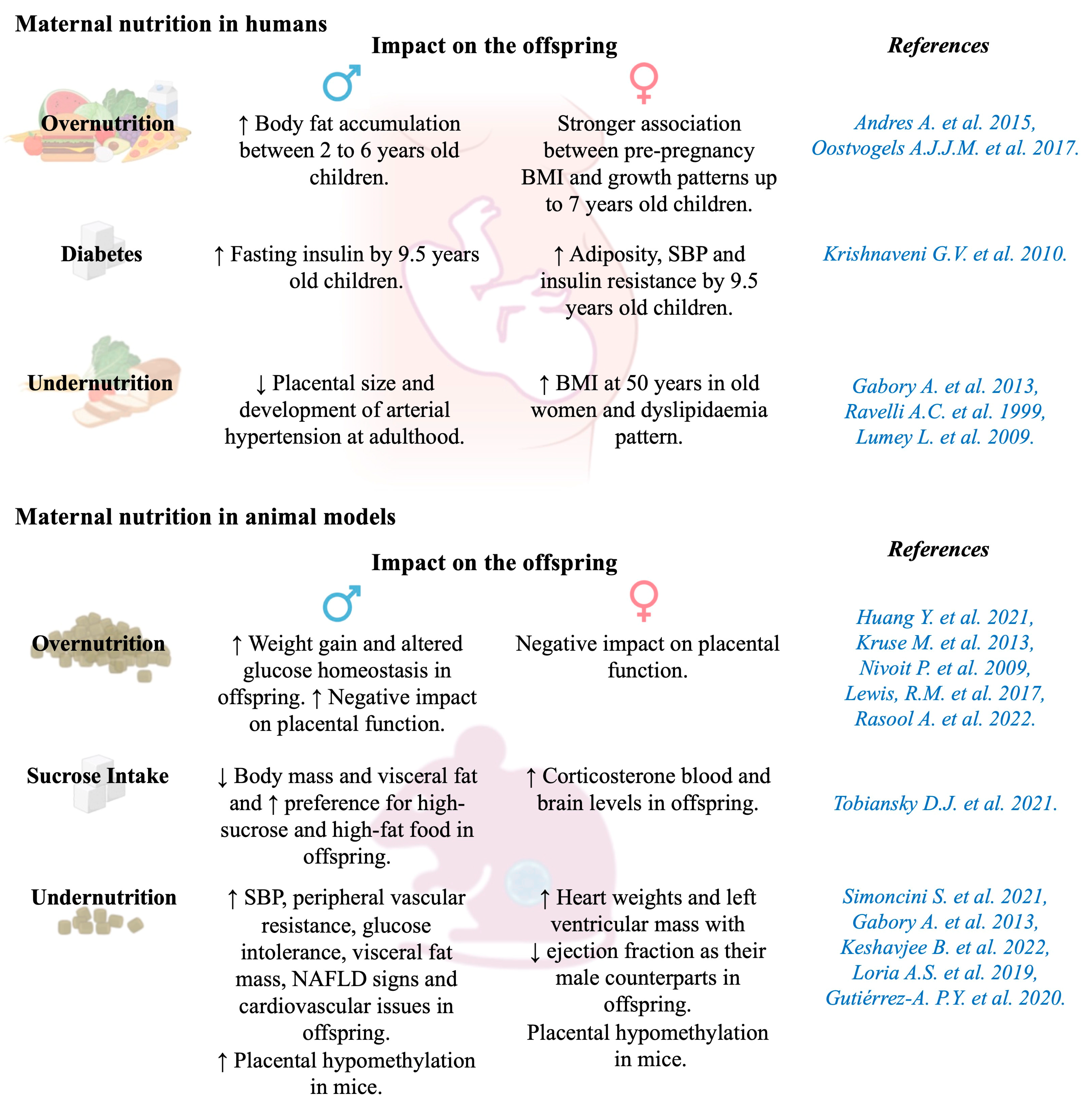

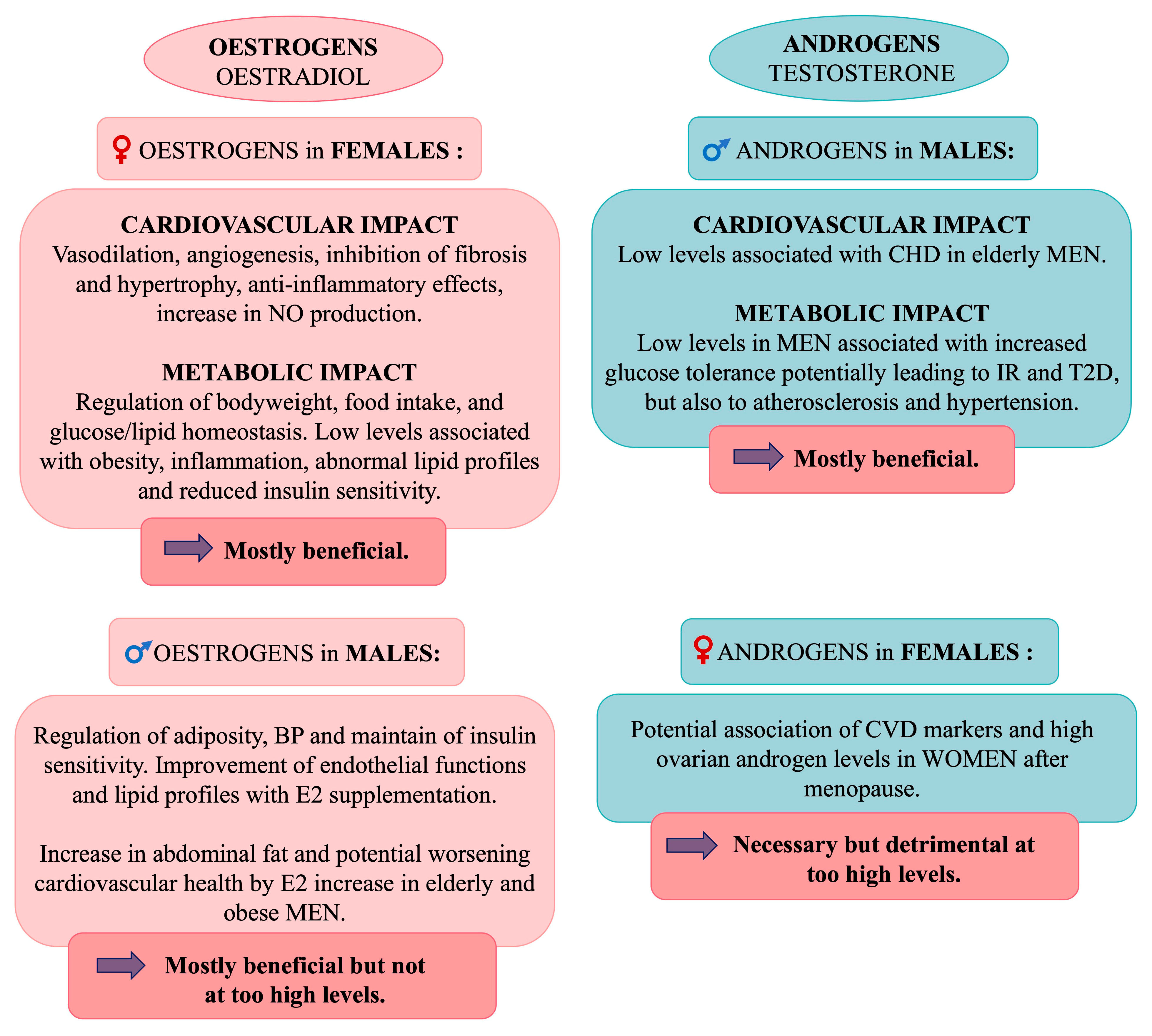


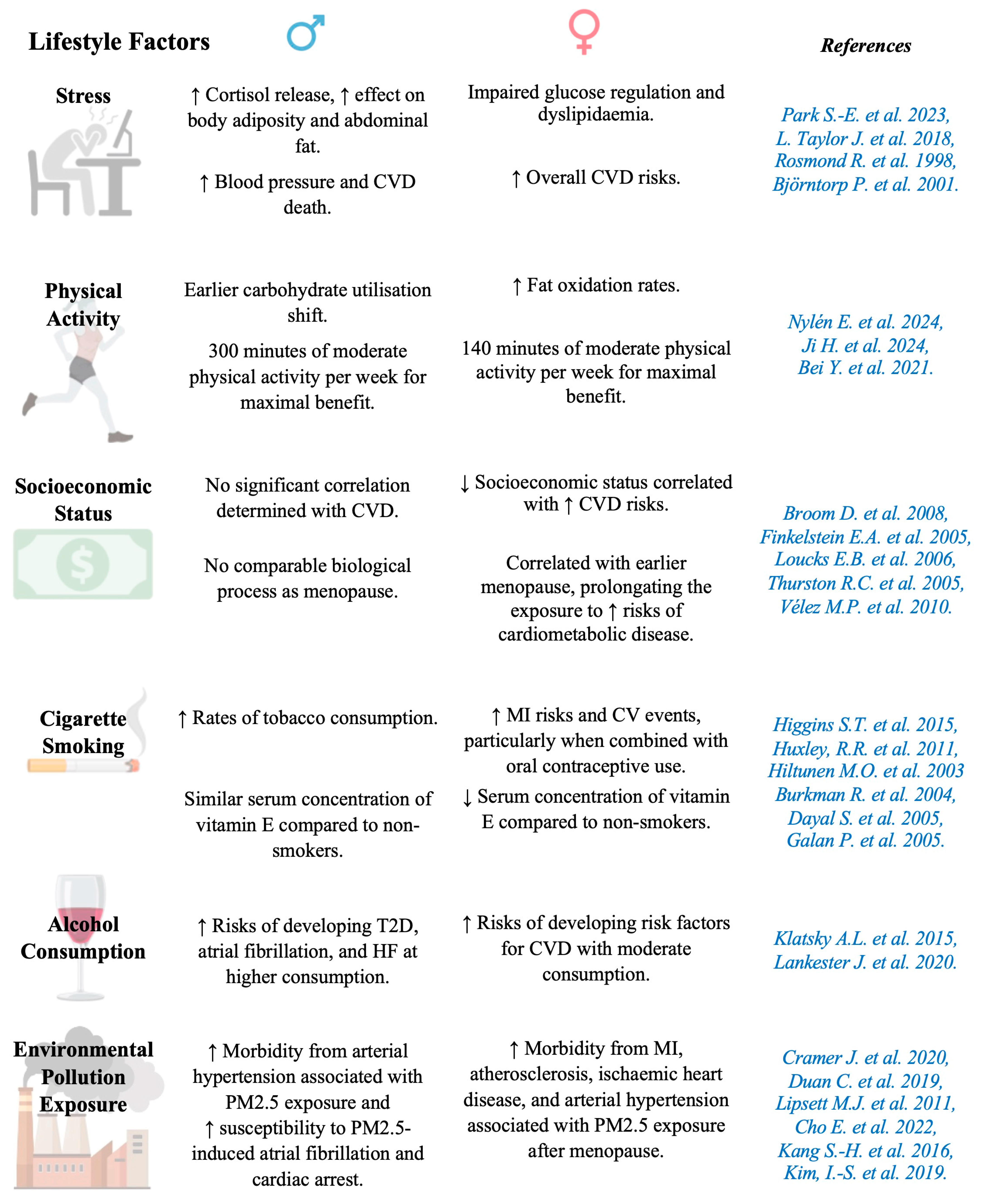
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chevalley, T.; Dübi, M.; Fumeaux, L.; Merli, M.S.; Sarre, A.; Schaer, N.; Simeoni, U.; Yzydorczyk, C. Sexual Dimorphism in Cardiometabolic Diseases: From Development to Senescence and Therapeutic Approaches. Cells 2025, 14, 467. https://doi.org/10.3390/cells14060467
Chevalley T, Dübi M, Fumeaux L, Merli MS, Sarre A, Schaer N, Simeoni U, Yzydorczyk C. Sexual Dimorphism in Cardiometabolic Diseases: From Development to Senescence and Therapeutic Approaches. Cells. 2025; 14(6):467. https://doi.org/10.3390/cells14060467
Chicago/Turabian StyleChevalley, Thea, Marion Dübi, Laurent Fumeaux, Maria Serena Merli, Alexandre Sarre, Natacha Schaer, Umberto Simeoni, and Catherine Yzydorczyk. 2025. "Sexual Dimorphism in Cardiometabolic Diseases: From Development to Senescence and Therapeutic Approaches" Cells 14, no. 6: 467. https://doi.org/10.3390/cells14060467
APA StyleChevalley, T., Dübi, M., Fumeaux, L., Merli, M. S., Sarre, A., Schaer, N., Simeoni, U., & Yzydorczyk, C. (2025). Sexual Dimorphism in Cardiometabolic Diseases: From Development to Senescence and Therapeutic Approaches. Cells, 14(6), 467. https://doi.org/10.3390/cells14060467


.png)




