Sirtuins and Gut Microbiota: Dynamics in Health and a Journey from Metabolic Dysfunction to Hepatocellular Carcinoma
Abstract
:1. Background
2. Overview of Sirtuins and Their Biological Functions
2.1. Sirtuins Classification and Subcellular Localization
2.2. Role of Sirtuins in Cellular Homeostasis and Metabolism
3. The Role of Gut Microbiota in Host Health and Disease
4. Interrelationship Between Sirtuins and Gut Microbiota: A Bidirectional Perspective
4.1. Influence of Sirtuins on Gut Microbiota Composition
4.1.1. SIRT1
4.1.2. SIRT2
4.1.3. SIRT3
4.1.4. SIRT4
4.1.5. SIRT5-7
4.2. Impact of Gut Microbiota on Sirtuin Activity
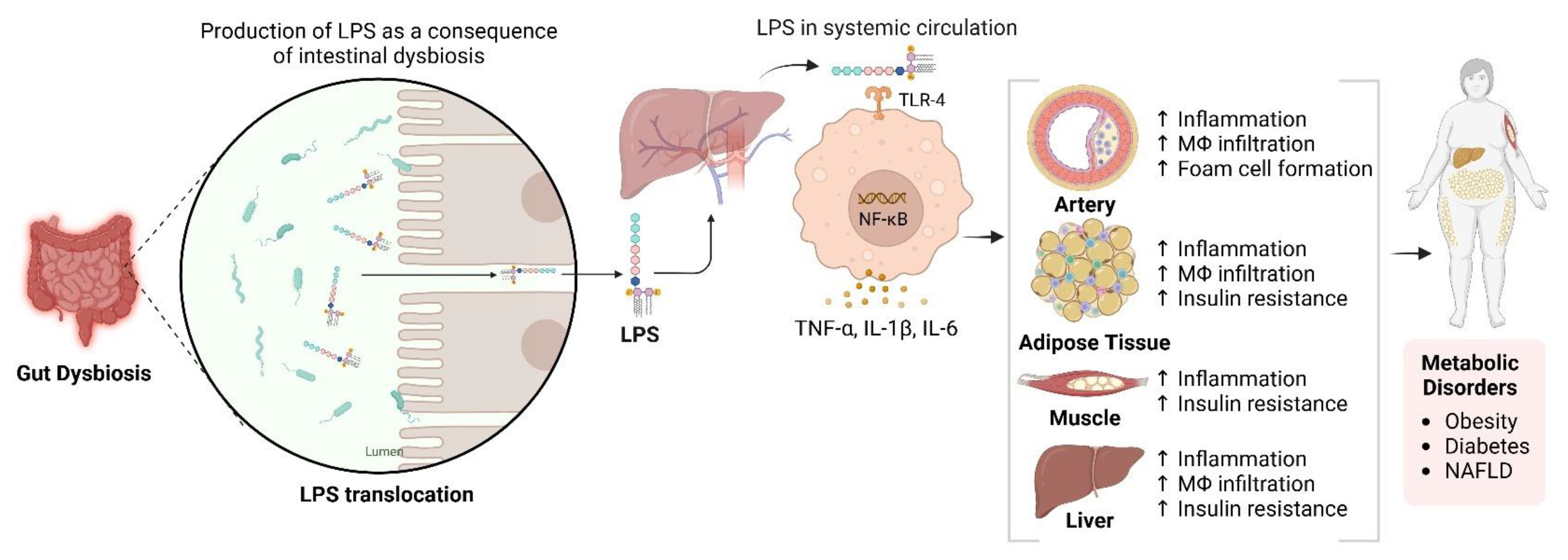
5. Role of Sirtuins and Gut Microbiota in Non-Alcoholic Fatty Liver Disease (NAFLD)
6. Role of Sirtuins and Gut Microbiota in Hepatocellular Carcinoma (HCC)
| Disease | Composition Change | References | |
|---|---|---|---|
| Increase | Decrease | ||
| NAFLD | Streptococcus, Megasphaera, Enterobacteriaceae, Streptococcus, Gallibacterium | Bacillus and Lactococcus, Pseudomonas, Faecalibacterium prausnitzii, Catenibacterium, Rikenellaceae, Mogibacterium, Peptostreptococcaceae | [215] |
| Firmicutes (Streptococcus mitis and Roseburia inulinivorans) and Bacteroidetes (Barnesiella intestinihominis and Bacteroides uniformis) | Bacteroidetes (Prevotella sp. CAG 520, Prevotella sp. AM42 24, Butyricimonas virosa, and Odoribacter splanchnicus), Proteobacteria (Escherichia coli), Lentisphaerae (Victivallis vadensis), and Firmicutes (Holdemanella biformis, Dorea longicatena, Allisonella histaminiformans, and Blautia obeum) | [216] | |
| Bacteroidetes, Proteobacteria, Bacteroides, Alistipes, Verrucomicrobia, Faecalibaculum, Helicobacter, Epsilonbacteraeota | Muribaculaceae, Lactobacillus | [217] | |
| HCC | Escherichia coli | [218] | |
| Proteobacteria, Desulfococcus, Enterobacter, Prevotella, Veillonella | Cetobacterium | [219] | |
| Bacteroides | Akkermansia, Bifidobacterium | [220] | |
| Neisseria, Enterobacteriaceae, Veillonella, Limnobacter | Enterococcus, Phyllobacterium, lostridium, Ruminococcus, Coprococcus | [221] | |
| Proteobacteria, Enterobacteriaceae, Bacteroides xylanisolvens, B. caecimuris, Ruminococcus gnavus, Clostridium bolteae, Veillonella parvula | Erysipelotrichaceae, Oscillospiraceae | [222] | |
| Klebsiella, Haemophilus | Alistipes, Phascolarctobacterium, Ruminococcus | [181] | |
7. Interventions Targeting Sirtuins and Gut Microbiota
7.1. Sirtuin Activators
7.1.1. Resveratrol
7.1.2. Pterostilbene
7.1.3. E1231
7.1.4. Quercetin
7.1.5. Nicotinamide Riboside (NR)
7.1.6. Berberine
7.1.7. Yinchen Linggui Zhugan Decoction (YLZD)
7.1.8. The Tangshen Formula (TSF)
7.1.9. Curcumin
7.1.10. Dihydromyricetin
7.2. Sirtuin Inhibitors
7.3. Gene Editing Approaches
7.4. Small Molecule Targeting
8. Gut Microbiota-Based Interventions: Probiotics, Prebiotics, and Synbiotics
9. Future Directions
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wu, Q.; Zhang, T.-N.; Chen, H.-h.; Yu, X.-F.; Lv, J.; Liu, Y.-Y.; Liu, Y.; Zheng, G.; Zhao, J.; Wei, Y.-F.; et al. The sirtuin family in health and disease. Signal Transduct. Target. Ther. 2022, 7, 402. [Google Scholar]
- Bhatt, V.; Tiwari, A.K. Sirtuins, a key regulator of ageing and age-related neurodegenerative diseases. Int. J. Neurosci. 2022, 133, 1167–1192. [Google Scholar] [CrossRef] [PubMed]
- Ceballos, M.P.; Quiroga, A.D.; Palma, N.F. Role of sirtuins in hepatocellular carcinoma progression and multidrug resistance: Mechanistical and pharmacological perspectives. Biochem. Pharmacol. 2023, 212, 115573. [Google Scholar] [CrossRef] [PubMed]
- Afzaal, M.; Saeed, F.; Shah, Y.A.; Hussain, M.; Rabail, R.; Socol, C.T.; Hassoun, A.; Pateiro, M.; Lorenzo, J.M.; Rusu, A.V.; et al. Human gut microbiota in health and disease: Unveiling the relationship. Front. Microbiol. 2022, 13, 999001. [Google Scholar] [CrossRef]
- Kim, S.; Seo, S.-U.; Kweon, M.-N. Gut microbiota-derived metabolites tune host homeostasis fate. Semin. Immunopathol. 2024, 46, 1–15. [Google Scholar] [CrossRef]
- Li, X.; Du, Y.; Xue, C.; Kang, X.; Sun, C.; Peng, H.; Fang, L.; Han, Y.; Xu, X.; Zhao, C. SIRT2 Deficiency Aggravates Diet-Induced Nonalcoholic Fatty Liver Disease through Modulating Gut Microbiota and Metabolites. Int. J. Mol. Sci. 2023, 24, 8970. [Google Scholar] [CrossRef]
- Sharma, A.; Mahur, P.; Muthukumaran, J.; Singh, A.K.; Jain, M. Shedding light on structure, function and regulation of human sirtuins: A comprehensive review. 3 Biotech 2022, 13, 1–15. [Google Scholar] [CrossRef]
- Afzaal, A.; Rehman, K.; Kamal, S.; Akash, M.S.H. Versatile role of sirtuins in metabolic disorders: From modulation of mitochondrial function to therapeutic interventions. J. Biochem. Mol. Toxicol. 2022, 36, e23047. [Google Scholar] [CrossRef]
- D’Angelo, S.; Mele, E.; Di Filippo, F.; Viggiano, A.; Meccariello, R. Sirt1 Activity in the Brain: Simultaneous Effects on Energy Homeostasis and Reproduction. Int. J. Environ. Res. Public Health 2021, 18, 1243. [Google Scholar] [CrossRef]
- Zhang, Y.; Long, X.; Ruan, X.; Wei, Q.; Zhang, L.; Wo, L.; Huang, D.; Lin, L.; Wang, D.; Xia, L.; et al. SIRT2-mediated deacetylation and deubiquitination of C/EBPβ prevents ethanol-induced liver injury. Cell Discov. 2021, 7, 1–17. [Google Scholar] [CrossRef]
- Lambona, C.; Zwergel, C.; Valente, S.; Mai, A. SIRT3 Activation a Promise in Drug Development? New Insights into SIRT3 Biology and Its Implications on the Drug Discovery Process. J. Med. Chem. 2024, 67, 1662–1689. [Google Scholar] [CrossRef] [PubMed]
- Tao, Z.; Jin, Z.; Wu, J.; Cai, G.; Yu, X. Sirtuin family in autoimmune diseases. Front. Immunol. 2023, 14, 1186231. [Google Scholar] [CrossRef] [PubMed]
- Su, S.; Ndiaye, M.A.; Singh, C.K.; Ahmad, N. Mitochondrial Sirtuins in Skin and Skin Cancers. Photochem. Photobiol. 2020, 96, 973–980. [Google Scholar] [CrossRef] [PubMed]
- Poljšak, B.; Kovač, V.; Špalj, S.; Milisav, I. The Central Role of the NAD+ Molecule in the Development of Aging and the Prevention of Chronic Age-Related Diseases: Strategies for NAD+ Modulation. Int. J. Mol. Sci. 2023, 24, 2959. [Google Scholar] [CrossRef]
- Kosciuk, T.; Wang, M.; Hong, J.Y.; Lin, H. Updates on the epigenetic roles of sirtuins. Curr. Opin. Chem. Biol. 2019, 51, 18–29. [Google Scholar] [CrossRef]
- Zulkifli, N.D.; Zulkifle, N. Insight from sirtuins interactome: Topological prominence and multifaceted roles of SIRT1 in modulating immunity, aging and cancer. Genom. Inform. 2023, 21, e23. [Google Scholar] [CrossRef]
- Lagunas-Rangel, F.A. Current role of mammalian sirtuins in DNA repair. DNA Repair 2019, 80, 85–92. [Google Scholar] [CrossRef]
- Roos, W.P.; Krumm, A. The multifaceted influence of histone deacetylases on DNA damage signalling and DNA repair. Nucleic Acids Res. 2016, 44, 10017–10030. [Google Scholar] [CrossRef]
- Chang, A.R.; Ferrer, C.M.; Mostoslavsky, R. SIRT6, a Mammalian Deacylase with Multitasking Abilities. Physiol. Rev. 2020, 100, 145–169. [Google Scholar] [CrossRef]
- Carter, R.J.; Parsons, J.L. Base Excision Repair, a Pathway Regulated by Posttranslational Modifications. Mol. Cell. Biol. 2016, 36, 1426–1437. [Google Scholar] [CrossRef]
- Alves-Fernandes, D.K.; Jasiulionis, M.G. The Role of SIRT1 on DNA Damage Response and Epigenetic Alterations in Cancer. Int. J. Mol. Sci. 2019, 20, 3153. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Cao, N.; Fenech, M.; Wang, X. Role of Sirtuins in Maintenance of Genomic Stability: Relevance to Cancer and Healthy Aging. DNA Cell Biol. 2016, 35, 542–575. [Google Scholar] [CrossRef]
- O’Callaghan, C.; Vassilopoulos, A. Sirtuins at the crossroads of stemness, aging, and cancer. Aging Cell 2017, 16, 1208–1218. [Google Scholar] [CrossRef] [PubMed]
- Goes, J.; Viana, M.; Sampaio, L.; Dias, R.; Oliveira, R.; Cavalcante, C.; Borges, D.; Magalhães, S.; Pinheiro, R.; Junior, H. Differential Gene Expression of Sirtuins (Sirt1 to Sirt7) Reveals Potential Roles In Myelodysplastic Neoplasm Pathobiology. Hematol. Transfus. Cell Ther. 2023, 45, S432. [Google Scholar] [CrossRef]
- Chojdak-Łukasiewicz, J.; Bizoń, A.; Waliszewska-Prosół, M.; Piwowar, A.; Budrewicz, S.; Pokryszko-Dragan, A. Role of Sirtuins in Physiology and Diseases of the Central Nervous System. Biomedicines 2022, 10, 2434. [Google Scholar] [CrossRef]
- Hong, Y.A.; Kim, J.E.; Jo, M.J.; Ko, G.J. The Role of Sirtuins in Kidney Diseases. Int. J. Mol. Sci. 2020, 21, 6686. [Google Scholar] [CrossRef]
- Ding, Y.-N.; Wang, H.-Y.; Chen, X.-F.; Tang, X.; Chen, H.-Z. Roles of Sirtuins in Cardiovascular Diseases: Mechanisms and Therapeutics. Circ. Res. 2025, 136, 524–550. [Google Scholar] [CrossRef]
- Kumari, P.; Tarighi, S.; Braun, T.; Ianni, A. SIRT7 Acts as a Guardian of Cellular Integrity by Controlling Nucleolar and Extra-Nucleolar Functions. Genes 2021, 12, 1361. [Google Scholar] [CrossRef]
- Maissan, P.; Mooij, E.J.; Barberis, M. Sirtuins-Mediated System-Level Regulation of Mammalian Tissues at the Interface between Metabolism and Cell Cycle: A Systematic Review. Biology 2021, 10, 194. [Google Scholar] [CrossRef]
- Vazquez, B.N.; Vaquero, A.; Schindler, K. Sirtuins in female meiosis and in reproductive longevity. Mol. Reprod. Dev. 2020, 87, 1175–1187. [Google Scholar] [CrossRef]
- Kratz, E.M.; Kokot, I.; Dymicka-Piekarska, V.; Piwowar, A. Sirtuins—The New Important Players in Women’s Gynecological Health. Antioxidants 2021, 10, 84. [Google Scholar] [CrossRef] [PubMed]
- Hamaidi, I.; Kim, S. Sirtuins are crucial regulators of T cell metabolism and functions. Exp. Mol. Med. 2022, 54, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Watroba, M.; Szukiewicz, D. Sirtuins at the Service of Healthy Longevity. Front. Physiol. 2021, 12, 724506. [Google Scholar] [CrossRef] [PubMed]
- Zhao, E.; Hou, J.; Ke, X.; Abbas, M.N.; Kausar, S.; Zhang, L.; Cui, H. The Roles of Sirtuin Family Proteins in Cancer Progression. Cancers 2019, 11, 1949. [Google Scholar] [CrossRef]
- Carafa, V.; Altucci, L.; Nebbioso, A. Dual Tumor Suppressor and Tumor Promoter Action of Sirtuins in Determining Malignant Phenotype. Front. Pharmacol. 2019, 10, 38. [Google Scholar] [CrossRef]
- Zhu, S.; Dong, Z.; Ke, X.; Hou, J.; Zhao, E.; Zhang, K.; Wang, F.; Yang, L.; Xiang, Z.; Cui, H. The roles of sirtuins family in cell metabolism during tumor development. Semin. Cancer Biol. 2019, 57, 59–71. [Google Scholar] [CrossRef]
- Yin, J.-Y.; Lu, X.-T.; Hou, M.-L.; Cao, T.; Tian, Z. Sirtuin1-p53: A potential axis for cancer therapy. Biochem. Pharmacol. 2023, 212, 115543. [Google Scholar] [CrossRef]
- Chen, G.; Huang, P.; Hu, C. The role of SIRT2 in cancer: A novel therapeutic target. Int. J. Cancer 2020, 147, 3297–3304. [Google Scholar] [CrossRef]
- Onyiba, C.I.; Scarlett, C.J.; Weidenhofer, J. The Mechanistic Roles of Sirtuins in Breast and Prostate Cancer. Cancers 2022, 14, 5118. [Google Scholar] [CrossRef]
- George, J.; Ahmad, N. Mitochondrial Sirtuins in Cancer: Emerging Roles and Therapeutic Potential. Cancer Res. 2016, 76, 2500–2506. [Google Scholar] [CrossRef]
- Fiorentino, F.; Carafa, V.; Favale, G.; Altucci, L.; Mai, A.; Rotili, D. The Two-Faced Role of SIRT6 in Cancer. Cancers 2021, 13, 1156. [Google Scholar] [CrossRef] [PubMed]
- Karbasforooshan, H.; Hayes, A.W.; Mohammadzadeh, N.; Zirak, M.R.; Karimi, G. The possible role of Sirtuins and microRNAs in hepatocellular carcinoma therapy. Cell Cycle 2020, 19, 3209–3221. [Google Scholar] [CrossRef] [PubMed]
- Farcas, M.; Gavrea, A.-A.; Gulei, D.; Ionescu, C.; Irimie, A.; Catana, C.S.; Berindan-Neagoe, I. SIRT1 in the Development and Treatment of Hepatocellular Carcinoma. Front. Nutr. 2019, 6, 148. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.; Yao, H.; Caito, S.; Hwang, J.-W.; Arunachalam, G.; Rahman, I. Regulation of SIRT1 in cellular functions: Role of polyphenols. Arch. Biochem. Biophys. 2010, 501, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Li, X. SIRT1 and energy metabolism. Acta Biochim. Biophys. Sin. 2013, 45, 51–60. [Google Scholar] [CrossRef]
- Zhang, L.; Kim, S.; Ren, X. The Clinical Significance of SIRT2 in Malignancies: A Tumor Suppressor or an Oncogene? Front. Oncol. 2020, 10, 1721. [Google Scholar] [CrossRef]
- Hong, J.Y.; Lin, H. Sirtuin Modulators in Cellular and Animal Models of Human Diseases. Front. Pharmacol. 2021, 12, 735044. [Google Scholar] [CrossRef]
- Yang, W.; Chen, W.; Su, H.; Li, R.; Song, C.; Wang, Z.; Yang, L. Recent advances in the development of histone deacylase SIRT2 inhibitors. RSC Adv. 2020, 10, 37382–37390. [Google Scholar] [CrossRef]
- Zhu, C.; Dong, X.; Wang, X.; Zheng, Y.; Qiu, J.; Peng, Y.; Xu, J.; Chai, Z.; Liu, C. Multiple Roles of SIRT2 in Regulating Physiological and Pathological Signal Transduction. Genet. Res. 2022, 2022, 1–14. [Google Scholar] [CrossRef]
- Bharathi, S.S.; Zhang, Y.; Mohsen, A.-W.; Uppala, R.; Balasubramani, M.; Schreiber, E.; Uechi, G.; Beck, M.E.; Rardin, M.J.; Vockley, J.; et al. Sirtuin 3 (SIRT3) Protein Regulates Long-chain Acyl-CoA Dehydrogenase by Deacetylating Conserved Lysines Near the Active Site. J. Biol. Chem. 2013, 288, 33837–33847. [Google Scholar] [CrossRef]
- Shen, Y.; Wu, Q.; Shi, J.; Zhou, S. Regulation of SIRT3 on mitochondrial functions and oxidative stress in Parkinson’s disease. Biomed. Pharmacother. 2020, 132, 110928. [Google Scholar] [CrossRef] [PubMed]
- Sebaa, R.; Johnson, J.; Pileggi, C.; Norgren, M.; Xuan, J.; Sai, Y.; Tong, Q.; Krystkowiak, I.; Bondy-Chorney, E.; Davey, N.E.; et al. SIRT3 controls brown fat thermogenesis by deacetylation regulation of pathways upstream of UCP1. Mol. Metab. 2019, 25, 35–49. [Google Scholar] [CrossRef] [PubMed]
- Kincaid, B.; Bossy-Wetzel, E. Forever young: SIRT3 a shield against mitochondrial meltdown, aging, and neurodegeneration. Front. Aging Neurosci. 2013, 5, 48. [Google Scholar] [CrossRef] [PubMed]
- Hallows, W.C.; Yu, W.; Smith, B.C.; Devries, M.K.; Ellinger, J.J.; Someya, S.; Shortreed, M.R.; Prolla, T.; Markley, J.L.; Smith, L.M.; et al. Sirt3 promotes the urea cycle and fatty acid oxidation during dietary restriction. Mol. Cell 2011, 41, 139–149. [Google Scholar] [CrossRef]
- Zhu, Y.; Yan, Y.; Principe, D.R.; Zou, X.; Vassilopoulos, A.; Gius, D. SIRT3 and SIRT4 are mitochondrial tumor suppressor proteins that connect mitochondrial metabolism and carcinogenesis. Cancer Metab. 2014, 2, 15. [Google Scholar] [CrossRef]
- Wang, C.; Liu, Y.; Zhu, Y.; Kong, C. Functions of mammalian SIRT4 in cellular metabolism and research progress in human cancer (Review). Oncol. Lett. 2020, 20, 1. [Google Scholar] [CrossRef]
- Mathias, R.A.; Greco, T.M.; Oberstein, A.; Budayeva, H.G.; Chakrabarti, R.; Rowland, E.A.; Kang, Y.; Shenk, T.; Cristea, I.M. Sirtuin 4 Is a Lipoamidase Regulating Pyruvate Dehydrogenase Complex Activity. Cell 2014, 159, 1615–1625. [Google Scholar] [CrossRef]
- Tomaselli, D.; Steegborn, C.; Mai, A.; Rotili, D. Sirt4: A Multifaceted Enzyme at the Crossroads of Mitochondrial Metabolism and Cancer. Front. Oncol. 2020, 10, 474. [Google Scholar] [CrossRef]
- Yang, X.; Liu, B.; Zhu, W.; Luo, J. SIRT5, functions in cellular metabolism with a multiple enzymatic activities. Sci. China Life Sci. 2015, 58, 912–914. [Google Scholar] [CrossRef]
- Fabbrizi, E.; Fiorentino, F.; Carafa, V.; Altucci, L.; Mai, A.; Rotili, D. Emerging Roles of SIRT5 in Metabolism, Cancer, and SARS-CoV-2 Infection. Cells 2023, 12, 852. [Google Scholar] [CrossRef]
- Polletta, L.; Vernucci, E.; Carnevale, I.; Arcangeli, T.; Rotili, D.; Palmerio, S.; Steegborn, C.; Nowak, T.; Schutkowski, M.; Pellegrini, L.; et al. SIRT5 regulation of ammonia-induced autophagy and mitophagy. Autophagy 2015, 11, 253–270. [Google Scholar] [CrossRef] [PubMed]
- Nahálková, J. A new view on functions of the lysine demalonylase activity of SIRT5. Life Sci. 2023, 320, 121572. [Google Scholar] [CrossRef] [PubMed]
- Pederson, N.J.; Diehl, K.L. DNA stimulates SIRT6 to mono-ADP-ribosylate proteins within histidine repeats. bioRxiv 2024. [Google Scholar] [CrossRef]
- Zhong, L.; D’Urso, A.; Toiber, D.; Sebastian, C.; Henry, R.E.; Vadysirisack, D.D.; Guimaraes, A.; Marinelli, B.; Wikstrom, J.D.; Nir, T.; et al. The Histone Deacetylase Sirt6 Regulates Glucose Homeostasis via Hif1α. Cell 2010, 140, 280–293. [Google Scholar] [CrossRef]
- Fiorentino, F.; Mai, A.; Rotili, D. Emerging Therapeutic Potential of SIRT6 Modulators. J. Med. Chem. 2021, 64, 9732–9758. [Google Scholar] [CrossRef]
- Blank, M.F.; Grummt, I. The seven faces of SIRT7. Transcription 2017, 8, 67–74. [Google Scholar] [CrossRef]
- Kiran, S.; Anwar, T.; Kiran, M.; Ramakrishna, G. Sirtuin 7 in cell proliferation, stress and disease: Rise of the Seventh Sirtuin! Cell. Signal. 2015, 27, 673–682. [Google Scholar] [CrossRef]
- Lagunas-Rangel, F.A. SIRT7 in the aging process. Cell. Mol. Life Sci. 2022, 79, 297. [Google Scholar] [CrossRef]
- Matijašić, M.; Meštrović, T.; Čipčić Paljetak, H.; Perić, M.; Barešić, A.; Verbanac, D. Gut Microbiota beyond Bacteria—Mycobiome, Virome, Archaeome, and Eukaryotic Parasites in IBD. Int. J. Mol. Sci. 2020, 21, 2668. [Google Scholar] [CrossRef]
- Vemuri, R.; Shankar, E.M.; Chieppa, M.; Eri, R.; Kavanagh, K. Beyond Just Bacteria: Functional Biomes in the Gut Ecosystem Including Virome, Mycobiome, Archaeome and Helminths. Microorganisms 2020, 8, 483. [Google Scholar] [CrossRef]
- Garcia-Bonete, M.J.; Rajan, A.; Suriano, F.; Layunta, E. The Underrated Gut Microbiota Helminths, Bacteriophages, Fungi, and Archaea. Life 2023, 13, 1765. [Google Scholar] [CrossRef] [PubMed]
- Sasso, J.M.; Ammar, R.M.; Tenchov, R.; Lemmel, S.; Kelber, O.; Grieswelle, M.; Zhou, Q.A. Gut Microbiome–Brain Alliance: A Landscape View into Mental and Gastrointestinal Health and Disorders. ACS Chem. Neurosci. 2023, 14, 1717–1763. [Google Scholar] [CrossRef] [PubMed]
- Abenavoli, L.; Giubilei, L.; Procopio, A.C.; Spagnuolo, R.; Luzza, F.; Boccuto, L.; Scarpellini, E. Gut Microbiota in Non-Alcoholic Fatty Liver Disease Patients with Inflammatory Bowel Diseases: A Complex Interplay. Nutrients 2022, 14, 5323. [Google Scholar] [CrossRef] [PubMed]
- Barko, P.C.; McMichael, M.A.; Swanson, K.S.; Williams, D.A. The Gastrointestinal Microbiome: A Review. J. Vet. Intern. Med. 2017, 32, 9–25. [Google Scholar] [CrossRef]
- Pickard, J.M.; Zeng, M.Y.; Caruso, R.; Núñez, G. Gut microbiota: Role in pathogen colonization, immune responses, and inflammatory disease. Immunol. Rev. 2017, 279, 70–89. [Google Scholar] [CrossRef]
- Srivastava, A.; Prabhakar, M.R.; Mohanty, A.; Meena, S.S. Influence of gut microbiome on the human physiology. Syst. Microbiol. Biomanufacturing 2021, 2, 217–231. [Google Scholar] [CrossRef]
- Garcia-Gutierrez, E.; Mayer, M.J.; Cotter, P.D.; Narbad, A. Gut microbiota as a source of novel antimicrobials. Gut Microbes 2018, 10, 1–21. [Google Scholar] [CrossRef]
- Rowland, I.; Gibson, G.; Heinken, A.; Scott, K.; Swann, J.; Thiele, I.; Tuohy, K. Gut microbiota functions: Metabolism of nutrients and other food components. Eur. J. Nutr. 2018, 57, 1–24. [Google Scholar] [CrossRef]
- Levy, M.; Blacher, E.; Elinav, E. Microbiome, metabolites and host immunity. Curr. Opin. Microbiol. 2017, 35, 8–15. [Google Scholar] [CrossRef]
- Fung, T.C.; Olson, C.A.; Hsiao, E.Y. Interactions between the microbiota, immune and nervous systems in health and disease. Nat. Neurosci. 2017, 20, 145–155. [Google Scholar] [CrossRef]
- Gasaly, N.; de Vos, P.; Hermoso, M.A. Impact of Bacterial Metabolites on Gut Barrier Function and Host Immunity: A Focus on Bacterial Metabolism and Its Relevance for Intestinal Inflammation. Front. Immunol. 2021, 12, 658354. [Google Scholar] [CrossRef] [PubMed]
- Postler, T.S.; Ghosh, S. Understanding the Holobiont: How Microbial Metabolites Affect Human Health and Shape the Immune System. Cell Metab. 2017, 26, 110–130. [Google Scholar] [CrossRef] [PubMed]
- Louis, P.; Flint, H.J. Formation of propionate and butyrate by the human colonic microbiota. Environ. Microbiol. 2017, 19, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Blaak, E.E.; Canfora, E.E.; Theis, S.; Frost, G.; Groen, A.K.; Mithieux, G.; Nauta, A.; Scott, K.; Stahl, B.; Van Harsselaar, J.; et al. Short chain fatty acids in human gut and metabolic health. Benef. Microbes 2020, 11, 411–455. [Google Scholar] [CrossRef]
- Parada Venegas, D.; De La Fuente, M.K.; Landskron, G.; González, M.J.; Quera, R.; Dijkstra, G.; Harmsen, H.J.M.; Faber, K.N.; Hermoso, M.A. Short Chain Fatty Acids (SCFAs)-Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases. Front. Immunol. 2019, 10, 277. [Google Scholar] [CrossRef]
- Martin-Gallausiaux, C.; Marinelli, L.; Blottière, H.M.; Larraufie, P.; Lapaque, N. SCFA: Mechanisms and functional importance in the gut. Proc. Nutr. Soc. 2021, 80, 37–49. [Google Scholar] [CrossRef]
- He, J.; Zhang, P.; Shen, L.; Niu, L.; Tan, Y.; Chen, L.; Zhao, Y.; Bai, L.; Hao, X.; Li, X.; et al. Short-Chain Fatty Acids and Their Association with Signalling Pathways in Inflammation, Glucose and Lipid Metabolism. Int. J. Mol. Sci. 2020, 21, 6356. [Google Scholar] [CrossRef]
- Ramirez-Perez, O.L.; Cruz-Ramon, V.C.; Chinchilla-López, P.; Méndez-Sánchez, N. The Role of the Gut Microbiota in Bile Acid Metabolism. Ann. Hepatol. 2017, 16 (Suppl. 1), s15–s20. [Google Scholar] [CrossRef]
- Oliphant, K.; Allen-Vercoe, E. Macronutrient metabolism by the human gut microbiome: Major fermentation by-products and their impact on host health. Microbiome 2019, 7, 91. [Google Scholar] [CrossRef]
- Moszak, M.; Szulińska, M.; Bogdański, P. You Are What You Eat—The Relationship between Diet, Microbiota, and Metabolic Disorders—A Review. Nutrients 2020, 12, 1096. [Google Scholar] [CrossRef]
- Anwar, H.; Iftikhar, A.; Muzaffar, H.; Almatroudi, A.; Allemailem, K.S.; Navaid, S.; Saleem, S.; Khurshid, M. Biodiversity of Gut Microbiota: Impact of Various Host and Environmental Factors. BioMed Res. Int. 2021, 2021, 5575245. [Google Scholar] [CrossRef] [PubMed]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What Is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xu, B.; Chen, H.; Yang, F.; Huang, J.; Jiao, X.; Zhang, Y. Environmental factors and gut microbiota: Toward better conservation of deer species. Front. Microbiol. 2023, 14, 1136413. [Google Scholar] [CrossRef] [PubMed]
- Rothschild, D.; Weissbrod, O.; Barkan, E.; Kurilshikov, A.; Korem, T.; Zeevi, D.; Costea, P.I.; Godneva, A.; Kalka, I.N.; Bar, N.; et al. Environment dominates over host genetics in shaping human gut microbiota. Nature 2018, 555, 210–215. [Google Scholar] [CrossRef]
- Kurilshikov, A.; Wijmenga, C.; Fu, J.; Zhernakova, A. Host Genetics and Gut Microbiome: Challenges and Perspectives. Trends Immunol. 2017, 38, 633–647. [Google Scholar] [CrossRef]
- Jeong, S. Factors influencing development of the infant microbiota: From prenatal period to early infancy. Clin. Exp. Pediatr. 2021, 65, 438–447. [Google Scholar] [CrossRef]
- Hasan, N.; Yang, H. Factors affecting the composition of the gut microbiota, and its modulation. PeerJ 2019, 7, e7502. [Google Scholar] [CrossRef]
- Kriss, M.; Hazleton, K.Z.; Nusbacher, N.M.; Martin, C.G.; A Lozupone, C. Low diversity gut microbiota dysbiosis: Drivers, functional implications and recovery. Curr. Opin. Microbiol. 2018, 44, 34–40. [Google Scholar] [CrossRef]
- Heravi, F.S. Gut Microbiota and Autoimmune Diseases: Mechanisms, Treatment, Challenges, and Future Recommendations. Curr. Clin. Microbiol. Rep. 2024, 11, 18–33. [Google Scholar] [CrossRef]
- Weiss, G.A.; Hennet, T. Mechanisms and consequences of intestinal dysbiosis. Cell. Mol. Life Sci. 2017, 74, 2959–2977. [Google Scholar] [CrossRef]
- Willighagen, E. Defining Dysbiosis for a Cluster of Chronic Diseases. Sci. Rep. 2019, 9, 1–11. [Google Scholar]
- Jalandra, R.; Dhar, R.; Pethusamy, K.; Sharma, M.; Karmakar, S. Dysbiosis: Gut feeling. F1000Research 2022, 11, 911. [Google Scholar] [CrossRef]
- Hou, K.; Wu, Z.; Chen, X.-Y.; Wang, J.-Q.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.B.; Wei, L.; Li, J.; et al. Microbiota in health and diseases. Signal Transduct. Target. Ther. 2022, 7, 135. [Google Scholar] [CrossRef] [PubMed]
- Kho, Z.Y.; Lal, S.K. The Human Gut Microbiome—A Potential Controller of Wellness and Disease. Front. Microbiol. 2018, 9, 1835. [Google Scholar] [CrossRef]
- Meng, X.; Zhang, G.; Cao, H.; Yu, D.; Fang, X.; Vos, W.M.; Wu, H. Gut dysbacteriosis and intestinal disease: Mechanism and treatment. J. Appl. Microbiol. 2020, 129, 787–805. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X.-L.; Zhou, M.; Kang, C.; Lang, H.-D.; Chen, M.-T.; Hui, S.-C.; Wang, B.; Mi, M.-T. Crosstalk between gut microbiota and Sirtuin-3 in colonic inflammation and tumorigenesis. Exp. Mol. Med. 2018, 50, 1–11. [Google Scholar] [CrossRef]
- Caron, A.Z.; He, X.; Mottawea, W.; Seifert, E.L.; Jardine, K.; Dewar-Darch, D.; Cron, G.O.; Harper, M.; Stintzi, A.; McBurney, M.W. The SIRT1 deacetylase protects mice against the symptoms of metabolic syndrome. FASEB J. 2013, 28, 1306–1316. [Google Scholar] [CrossRef]
- Vikram, A.; Kim, Y.-R.; Kumar, S.; Li, Q.; Kassan, M.; Jacobs, J.S.; Irani, K. Vascular microRNA-204 is remotely governed by the microbiome and impairs endothelium-dependent vasorelaxation by downregulating Sirtuin1. Nat. Commun. 2016, 7, 12565. [Google Scholar] [CrossRef]
- Wellman, A.S.; Metukuri, M.R.; Kazgan, N.; Xu, X.; Xu, Q.; Ren, N.S.; Czopik, A.; Shanahan, M.T.; Kang, A.; Chen, W.; et al. Intestinal Epithelial Sirtuin 1 Regulates Intestinal Inflammation During Aging in Mice by Altering the Intestinal Microbiota. Gastroenterology 2017, 153, 772–786. [Google Scholar] [CrossRef]
- Munteanu, C.; Onose, G.; Poștaru, M.; Turnea, M.; Rotariu, M.; Galaction, A.I. Hydrogen Sulfide and Gut Microbiota: Their Synergistic Role in Modulating Sirtuin Activity and Potential Therapeutic Implications for Neurodegenerative Diseases. Pharmaceuticals 2024, 17, 1480. [Google Scholar] [CrossRef]
- Hou, D.; Yu, T.; Lu, X.; Hong, J.Y.; Yang, M.; Zi, Y.; Ho, T.T.; Lin, H. Sirt2 inhibition improves gut epithelial barrier integrity and protects mice from colitis. Proc. Natl. Acad. Sci. USA 2024, 121, 2319833121. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Xie, Y.; Li, G.; Zhang, T.; Sui, Y.; Zhao, Z.; Zhang, Y.; Yang, W.; Geng, X.; Xue, D.; et al. Gut microbiota-derived nicotinamide mononucleotide alleviates acute pancreatitis by activating pancreatic SIRT3 signalling. Br. J. Pharmacol. 2022, 180, 647–666. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Hui, S.; Lang, H.; Zhou, M.; Zhang, Y.; Kang, C.; Zeng, X.; Zhang, Q.; Yi, L.; Mi, M. SIRT3 Deficiency Promotes High-Fat Diet-Induced Nonalcoholic Fatty Liver Disease in Correlation with Impaired Intestinal Permeability through Gut Microbial Dysbiosis. Mol. Nutr. Food Res. 2018, 63, e1800612. [Google Scholar] [CrossRef] [PubMed]
- Knop, M.; Treitz, C.; Bettendorf, S.; Bossen, J.; von Frieling, J.; Doms, S.; Bruchhaus, I.; Kühnlein, R.P.; Baines, J.F.; Tholey, A.; et al. A mitochondrial sirtuin shapes the intestinal microbiota by controlling lysozyme expression. bioRxiv 2023. [Google Scholar] [CrossRef]
- Tucker, S.A.; Hu, S.-H.; Vyas, S.; Park, A.; Joshi, S.; Inal, A.; Lam, T.; Tan, E.; Haigis, K.M.; Haigis, M.C. SIRT4 loss reprograms intestinal nucleotide metabolism to support proliferation following perturbation of homeostasis. Cell Rep. 2024, 43, 113975. [Google Scholar] [CrossRef]
- Sun, R.; Zhang, Z.; Bao, R.; Guo, X.; Gu, Y.; Yang, W.; Wei, J.; Chen, X.; Tong, L.; Meng, J.; et al. Loss of SIRT5 promotes bile acid-induced immunosuppressive microenvironment and hepatocarcinogenesis. J. Hepatol. 2022, 77, 453–466. [Google Scholar] [CrossRef]
- Xu, K.; Guo, Y.; Wang, Y.; Ren, Y.; Low, V.; Cho, S.; Ping, L.; Peng, K.; Li, X.; Qiu, Y.; et al. Decreased Enterobacteriaceae translocation due to gut microbiota remodeling mediates the alleviation of premature aging by a high-fat diet. Aging Cell 2022, 22, 13760. [Google Scholar] [CrossRef]
- Kim, S.; Byun, J.; Jung, S.; Kim, B.; Lee, K.; Jeon, H.; Lee, J.; Choi, H.; Kim, E.; Jeen, Y.; et al. Sirtuin 7 Inhibitor Attenuates Colonic Mucosal Immune Activation in Mice—Potential Therapeutic Target in Inflammatory Bowel Disease. Biomedicines 2022, 10, 2693. [Google Scholar] [CrossRef]
- Caruso, R.; Marafini, I.; Franzè, E.; Stolfi, C.; Zorzi, F.; Monteleone, I.; Caprioli, F.; Colantoni, A.; Sarra, M.; Sedda, S.; et al. Defective expression of SIRT1 contributes to sustain inflammatory pathways in the gut. Mucosal Immunol. 2014, 7, 1467–1479. [Google Scholar] [CrossRef]
- Lytvynenko, A.; Voznesenskaya, T.; Janchij, R. SIRT1 Is A Regulator Of Autophagy In Intestinal Cells. Fiziolohichnyĭ zhurnal 2020, 66, 97–103. [Google Scholar] [CrossRef]
- Ma, Y.; Xu, C.; Wang, W.; Sun, L.; Yang, S.; Lu, D.; Liu, Y.; Yang, H. Role of SIRT1 in the protection of intestinal epithelial barrier under hypoxia and its mechanism. Chin. J. Gastrointest. Surg. 2014, 17, 602–606. [Google Scholar]
- Markandey, M.; Aditya, B.; Edward, I.N.; Saurabh, K.; Simon, T.; Fiona, P.; Ahuja, V. Gut microbiota: Sculptors of the intestinal stem cell niche in health and inflammatory bowel disease. Gut Microbes 2021, 13, 1990827. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhou, Y.; Rychahou, P.; Weiss, H.L.; Lee, E.Y.; Perry, C.L.; Barrett, T.A.; Wang, Q.; Evers, B.M. SIRT2 Contributes to the Regulation of Intestinal Cell Proliferation and Differentiation. Cell. Mol. Gastroenterol. Hepatol. 2020, 10, 43–57. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Cao, Y.; Zheng, Q.; Tu, J.; Zhou, W.; He, J.; Zhong, J.; Chen, Y.; Wang, J.; Cai, R.; et al. SENP1-Sirt3 Signaling Controls Mitochondrial Protein Acetylation and Metabolism. Mol. Cell 2019, 75, 823–834.e5. [Google Scholar] [CrossRef]
- Nasrin, N.; Wu, X.; Fortier, E.; Feng, Y.; Bare’, O.C.; Chen, S.; Ren, X.; Wu, Z.; Streeper, R.S.; Bordone, L. SIRT4 Regulates Fatty Acid Oxidation and Mitochondrial Gene Expression in Liver and Muscle Cells. J. Biol. Chem. 2010, 285, 31995–32002. [Google Scholar] [CrossRef]
- Bringman-Rodenbarger, L.R.; Guo, A.H.; Lyssiotis, C.A.; Lombard, D.B. Emerging Roles for SIRT5 in Metabolism and Cancer. Antioxidants Redox Signal. 2018, 28, 677–690. [Google Scholar] [CrossRef]
- Xiong, X.; Yang, C.; He, W.-Q.; Yu, J.; Xin, Y.; Zhang, X.; Huang, R.; Ma, H.; Xu, S.; Li, Z.; et al. Sirtuin 6 maintains epithelial STAT6 activity to support intestinal tuft cell development and type 2 immunity. Nat. Commun. 2022, 13, 1–16. [Google Scholar] [CrossRef]
- Xu, K.; Guo, Y.; Ping, L.; Qiu, Y.; Liu, Q.; Li, Z.; Wang, Z. Protective Effects of SIRT6 Overexpression against DSS-Induced Colitis in Mice. Cells 2020, 9, 1513. [Google Scholar] [CrossRef]
- Lerrer, B.; Gertler, A.A.; Cohen, H.Y. The complex role of SIRT6 in carcinogenesis. Carcinog. 2015, 37, 108–118. [Google Scholar] [CrossRef]
- Liu, X.; Li, C.; Li, Q.; Chang, H.-C.; Tang, Y.-C. SIRT7 Facilitates CENP-A Nucleosome Assembly and Suppresses Intestinal Tumorigenesis. iScience 2020, 23, 101461. [Google Scholar] [CrossRef]
- Haslberger, A.; Lilja, S.; Bäck, H.; Stoll, C.; Mayer, A.; Pointner, A.; Hippe, B.; Krammer, U. Increased Sirtuin expression, senescence regulating miRNAs, mtDNA, and Bifidobateria correlate with wellbeing and skin appearance after Sirtuin- activating drink. Bioact. Compd. Health Dis. 2021, 4, 45. [Google Scholar] [CrossRef]
- Salazar, J.; Durán, P.; Díaz, M.P.; Chacín, M.; Santeliz, R.; Mengual, E.; Gutiérrez, E.; León, X.; Díaz, A.; Bernal, M.; et al. Exploring the Relationship between the Gut Microbiota and Ageing: A Possible Age Modulator. Int. J. Environ. Res. Public Health 2023, 20, 5845. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.; Mach, N. The Crosstalk between the Gut Microbiota and Mitochondria during Exercise. Front. Physiol. 2017, 8, 319. [Google Scholar] [CrossRef] [PubMed]
- Gong, S.; Feng, Y.; Zeng, Y.; Zhang, H.; Pan, M.; He, F.; Wu, R.; Chen, J.; Lu, J.; Zhang, S.; et al. Gut microbiota accelerates cisplatin-induced acute liver injury associated with robust inflammation and oxidative stress in mice. J. Transl. Med. 2021, 19, 1–13. [Google Scholar] [CrossRef]
- Singh, R.; Chandrashekharappa, S.; Bodduluri, S.R.; Baby, B.V.; Hegde, B.; Kotla, N.G.; Hiwale, A.A.; Saiyed, T.; Patel, P.; Vijay-Kumar, M.; et al. Enhancement of the gut barrier integrity by a microbial metabolite through the Nrf2 pathway. Nat. Commun. 2019, 10, 1–18. [Google Scholar] [CrossRef]
- Yang, M.; Massad, K.; Kimchi, E.T.; Staveley-O’carroll, K.F.; Li, G. Gut microbiota and metabolite interface-mediated hepatic inflammation. Immunometabolism 2024, 6, e00037. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, X.; Lv, A.; Fan, S.; Zhang, J. Saccharomyces Boulardii Modulates Necrotizing Enterocolitis in Neonatal Mice by Regulating the Sirtuin 1/NF-κB Pathway and the Intestinal Microbiota. Mol. Med. Rep. 2020, 22, 671–680. [Google Scholar] [CrossRef]
- Estes, C.; Razavi, H.; Loomba, R.; Younossi, Z.; Sanyal, A.J. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology 2018, 67, 123–133. [Google Scholar] [CrossRef]
- EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J. Hepatol. 2016, 64, 1388–1402. [CrossRef]
- Tiniakos, D.G.; Vos, M.B.; Brunt, E.M. Nonalcoholic Fatty Liver Disease: Pathology and Pathogenesis. Annu. Rev. Pathol. Mech. Dis. 2010, 5, 145–171. [Google Scholar] [CrossRef]
- Grander, C.; Grabherr, F.; Moschen, A.R.; Tilg, H. Non-Alcoholic Fatty Liver Disease: Cause or Effect of Metabolic Syndrome. Visc. Med. 2016, 32, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Paschos, P.; Paletas, K. Non alcoholic fatty liver disease two-hit process: Multifactorial character of the second hit. Hippokratia 2009, 13, 128. [Google Scholar]
- Jang, H.R.; Lee, H.-Y. Mechanisms linking gut microbial metabolites to insulin resistance. World J. Diabetes 2021, 12, 730–744. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Tang, X.; Chen, H.-Z. Sirtuins and Insulin Resistance. Front. Endocrinol. 2018, 9, 748. [Google Scholar] [CrossRef]
- Wang, Y.-J.; Paneni, F.; Stein, S.; Matter, C.M. Modulating Sirtuin Biology and Nicotinamide Adenine Diphosphate Metabolism in Cardiovascular Disease—From Bench to Bedside. Front. Physiol. 2021, 12, 755060. [Google Scholar] [CrossRef]
- Chandramowlishwaran, P.; Vijay, A.; Abraham, D.; Li, G.; Mwangi, S.M.; Srinivasan, S. Role of Sirtuins in Modulating Neurodegeneration of the Enteric Nervous System and Central Nervous System. Front. Neurosci. 2020, 14, 614331. [Google Scholar] [CrossRef]
- Ghosh-Swaby, O.R.; Reichelt, A.C.; Sheppard, P.A.; Davies, J.; Bussey, T.J.; Saksida, L.M. Metabolic hormones mediate cognition. Front. Neuroendocr. 2022, 66, 101009. [Google Scholar] [CrossRef]
- Zeng, Y.; Fang, Q.; Chen, J.; Wang, Y.; Liu, X.; Zhang, X.; Shi, Y.; Zhan, H.; Zhong, X.; Yao, M.; et al. Melatonin Improves Mitochondrial Dysfunction and Attenuates Neuropathic Pain by Regulating SIRT1 in Dorsal Root Ganglions. Neuroscience 2023, 534, 29–40. [Google Scholar] [CrossRef]
- Naaz, S.; Mishra, S.; Pal, P.K.; Chattopadhyay, A.; Das, A.R.; Bandyopadhyay, D. Activation of SIRT1/PGC 1α/SIRT3 pathway by melatonin provides protection against mitochondrial dysfunction in isoproterenol induced myocardial injury. Heliyon 2020, 6, e05159. [Google Scholar] [CrossRef]
- Fujitsuka, N.; Asakawa, A.; Morinaga, A.; Amitani, M.S.; Amitani, H.; Katsuura, G.; Sawada, Y.; Sudo, Y.; Uezono, Y.; Mochiki, E.; et al. Increased ghrelin signaling prolongs survival in mouse models of human aging through activation of sirtuin1. Mol. Psychiatry 2016, 21, 1613–1623. [Google Scholar] [CrossRef]
- Song, N.-Y.; Lee, Y.-H.; Na, H.-K.; Baek, J.-H.; Surh, Y.-J. Leptin induces SIRT1 expression through activation of NF-E2-related factor 2: Implications for obesity-associated colon carcinogenesis. Biochem. Pharmacol. 2018, 153, 282–291. [Google Scholar] [CrossRef] [PubMed]
- Marwarha, G.; Raza, S.; Meiers, C.; Ghribi, O. RETRACTED: Leptin attenuates BACE1 expression and amyloid-β genesis via the activation of SIRT1 signaling pathway. Biochim. Biophys. Acta BBA Mol. Basis Dis. 2014, 1842, 1587–1595. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, T. Age-Associated Weight Gain, Leptin, and SIRT1: A Possible Role for Hypothalamic SIRT1 in the Prevention of Weight Gain and Aging through Modulation of Leptin Sensitivity. Front. Endocrinol. 2015, 6, 109. [Google Scholar] [CrossRef] [PubMed]
- Chaudhry, T.S.; Senapati, S.G.; Gadam, S.; Mannam, H.P.S.S.; Voruganti, H.V.; Abbasi, Z.; Abhinav, T.; Challa, A.B.; Pallipamu, N.; Bheemisetty, N.; et al. The Impact of Microbiota on the Gut–Brain Axis: Examining the Complex Interplay and Implications. J. Clin. Med. 2023, 12, 5231. [Google Scholar] [CrossRef]
- Wu, T.; Liu, Y.-H.; Fu, Y.-C.; Liu, X.-M.; Zhou, X.-H. Direct evidence of sirtuin downregulation in the liver of non-alcoholic fatty liver disease patients. Ann. Clin. Lab. Sci. 2014, 44, 410–418. [Google Scholar]
- Bruce, K.D.; Szczepankiewicz, D.; Sihota, K.K.; Ravindraanandan, M.; Thomas, H.; Lillycrop, K.A.; Burdge, G.C.; Hanson, M.A.; Byrne, C.D.; Cagampang, F.R. Altered cellular redox status, sirtuin abundance and clock gene expression in a mouse model of developmentally primed NASH. Biochim. Biophys. Acta BBA Mol. Cell Biol. Lipids 2016, 1861, 584–593. [Google Scholar] [CrossRef]
- Canfora, E.E.; Meex, R.C.R.; Venema, K.; Blaak, E.E. Gut microbial metabolites in obesity, NAFLD and T2DM. Nat. Rev. Endocrinol. 2019, 15, 261–273. [Google Scholar] [CrossRef]
- Aron-Wisnewsky, J.; Vigliotti, C.; Witjes, J.; Le, P.; Holleboom, A.G.; Verheij, J.; Nieuwdorp, M.; Clément, K. Gut microbiota and human NAFLD: Disentangling microbial signatures from metabolic disorders. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 279–297. [Google Scholar] [CrossRef]
- Loo, T.M.; Kamachi, F.; Watanabe, Y.; Yoshimoto, S.; Kanda, H.; Arai, Y.; Nakajima-Takagi, Y.; Iwama, A.; Koga, T.; Sugimoto, Y. Gut microbiota promotes obesity-associated liver cancer through PGE2-mediated suppression of antitumor immunity. Cancer Discov. 2017, 7, 522–538. [Google Scholar] [CrossRef]
- Bo, T.; Shao, S.; Wu, D.; Niu, S.; Zhao, J.; Gao, L. Relative variations of gut microbiota in disordered cholesterol metabolism caused by high-cholesterol diet and host genetics. Microbiologyopen 2017, 6, e00491. [Google Scholar] [CrossRef]
- Zhang, X.; Coker, O.O.; Chu, E.S.; Fu, K.; Lau, H.C.H.; Wang, Y.-X.; Chan, A.W.H.; Wei, H.; Yang, X.; Sung, J.J.Y.; et al. Dietary cholesterol drives fatty liver-associated liver cancer by modulating gut microbiota and metabolites. Gut 2020, 70, 761–774. [Google Scholar] [CrossRef] [PubMed]
- Takaki, A.; Kawai, D.; Yamamoto, K. Multiple Hits, Including Oxidative Stress, as Pathogenesis and Treatment Target in Non-Alcoholic Steatohepatitis (NASH). Int. J. Mol. Sci. 2013, 14, 20704–20728. [Google Scholar] [CrossRef] [PubMed]
- Henao-Mejia, J.; Elinav, E.; Jin, C.; Hao, L.; Mehal, W.Z.; Strowig, T.; Thaiss, C.A.; Kau, A.L.; Eisenbarth, S.C.; Jurczak, M.J.; et al. Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature 2012, 482, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Wiest, R.; Albillos, A.; Trauner, M.; Bajaj, J.S.; Jalan, R. Targeting the gut-liver axis in liver disease. J. Hepatol. 2017, 67, 1084–1103. [Google Scholar] [CrossRef]
- Zhou, D.; Pan, Q.; Xin, F.-Z.; Zhang, R.-N.; He, C.-X.; Chen, G.-Y.; Liu, C.; Chen, Y.-W.; Fan, J.-G. Sodium butyrate attenuates high-fat diet-induced steatohepatitis in mice by improving gut microbiota and gastrointestinal barrier. World J. Gastroenterol. 2017, 23, 60–75. [Google Scholar] [CrossRef]
- Kang, C.; Wang, B.; Kaliannan, K.; Wang, X.; Lang, H.; Hui, S.; Huang, L.; Zhang, Y.; Zhou, M.; Chen, M.; et al. Gut Microbiota Mediates the Protective Effects of Dietary Capsaicin against Chronic Low-Grade Inflammation and Associated Obesity Induced by High-Fat Diet. mBio 2017, 8, e00470-17. [Google Scholar] [CrossRef]
- Muccioli, G.G.; Naslain, D.; Bäckhed, F.; Reigstad, C.S.; Lambert, D.M.; Delzenne, N.M.; Cani, P.D. The endocannabinoid system links gut microbiota to adipogenesis. Mol. Syst. Biol. 2010, 6, 392. [Google Scholar] [CrossRef]
- Lam, Y.Y.; Ha, C.W.Y.; Campbell, C.R.; Mitchell, A.J.; Dinudom, A.; Oscarsson, J.; Cook, D.I.; Hunt, N.H.; Caterson, I.D.; Holmes, A.J.; et al. Increased Gut Permeability and Microbiota Change Associate with Mesenteric Fat Inflammation and Metabolic Dysfunction in Diet-Induced Obese Mice. PLoS ONE 2012, 7, e34233. [Google Scholar] [CrossRef]
- Everard, A.; Lazarevic, V.; Gaïa, N.; Johansson, M.; Ståhlman, M.; Bäckhed, F.; Delzenne, N.M.; Schrenzel, J.; Francois, P.; Cani, P.D. Microbiome of prebiotic-treated mice reveals novel targets involved in host response during obesity. ISME J. 2014, 8, 2116–2130. [Google Scholar] [CrossRef]
- Villanueva, A. Hepatocellular Carcinoma. N. Engl. J. Med. 2019, 380, 1450–1462. [Google Scholar] [CrossRef]
- Akinyemiju, T.; Abera, S.; Ahmed, M.; Alam, N.; Alemayohu, M.A.; Allen, C.; Al-Raddadi, R.; Alvis-Guzman, N.; Amoako, Y.; Artaman, A.; et al. The Burden of Primary Liver Cancer and Underlying Etiologies from 1990 to 2015 at the Global, Regional, and National Level: Results from the Global Burden of Disease Study 2015. JAMA Oncol. 2017, 3, 1683–1691. [Google Scholar] [PubMed]
- Jindal, A.; Thadi, A.; Shailubhai, K. Hepatocellular Carcinoma: Etiology and Current and Future Drugs. J. Clin. Exp. Hepatol. 2019, 9, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.J.; Cheung, R.; Ahmed, A. Nonalcoholic steatohepatitis is the most rapidly growing indication for liver transplantation in patients with hepatocellular carcinoma in the U.S. Hepatology 2014, 59, 2188–2195. [Google Scholar] [CrossRef] [PubMed]
- An, L.; Wirth, U.; Koch, D.; Schirren, M.; Drefs, M.; Koliogiannis, D.; Niess, H.; Andrassy, J.; Guba, M.; Bazhin, A.V.; et al. Metabolic Role of Autophagy in the Pathogenesis and Development of NAFLD. Metabolites 2023, 13, 101. [Google Scholar] [CrossRef]
- Itoh, M.; Suganami, T.; Nakagawa, N.; Tanaka, M.; Yamamoto, Y.; Kamei, Y.; Terai, S.; Sakaida, I.; Ogawa, Y. Melanocortin 4 Receptor–Deficient Mice as a Novel Mouse Model of Nonalcoholic Steatohepatitis. Am. J. Pathol. 2011, 179, 2454–2463. [Google Scholar] [CrossRef]
- Wang, J.; Xie, G. Bile Acid–microbiota Crosstalk in Gastrointestinal Inflammation and Carcinogenesis. Nat. Rev. Gastroenterol. Hepatol. 2017, 15, 111–128. [Google Scholar]
- Milosevic, I.; Vujovic, A.; Barac, A.; Djelic, M.; Korac, M.; Radovanovic Spurnic, A.; Gmizic, I.; Stevanovic, O.; Djordjevic, V.; Lekic, N.; et al. Gut-Liver Axis, Gut Microbiota, and Its Modulation in the Management of Liver Diseases: A Review of the Literature. Int. J. Mol. Sci. 2019, 20, 395. [Google Scholar] [CrossRef]
- Yu, L.; Li, Y.; Song, S.; Zhang, Y.; Wang, Y.; Wang, H.; Yang, Z.; Wang, Y. The dual role of sirtuins in cancer: Biological functions and implications. Front. Oncol. 2024, 14, 1384928. [Google Scholar] [CrossRef]
- Ling, S.; Li, J.; Shan, Q.; Dai, H.; Lu, D.; Wen, X.; Song, P.; Xie, H.; Zhou, L.; Liu, J.; et al. USP22 mediates the multidrug resistance of hepatocellular carcinoma via the SIRT1/AKT/MRP1 signaling pathway. Mol. Oncol. 2017, 11, 682–695. [Google Scholar] [CrossRef]
- Hao, C.; Zhu, P.-X.; Yang, X.; Han, Z.-P.; Jiang, J.-H.; Zong, C.; Zhang, X.-G.; Liu, W.-T.; Zhao, Q.-D.; Fan, T.-T.; et al. Overexpression of SIRT1 promotes metastasis through epithelial-mesenchymal transition in hepatocellular carcinoma. BMC Cancer 2014, 14, 978. [Google Scholar] [CrossRef]
- Ren, Z.; Li, A.; Jiang, J.; Zhou, L.; Yu, Z.; Lu, H.; Xie, H.; Chen, X.; Shao, L.; Zhang, R.; et al. Gut microbiome analysis as a tool towards targeted non-invasive biomarkers for early hepatocellular carcinoma. Gut 2018, 68, 1014–1023. [Google Scholar] [CrossRef] [PubMed]
- Pant, K.; Yadav, A.K.; Gupta, P.; Islam, R.; Saraya, A.; Venugopal, S.K. Butyrate induces ROS-mediated apoptosis by modulating miR-22/SIRT-1 pathway in hepatic cancer cells. Redox Biol. 2017, 12, 340–349. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yang, Y.; Yang, T.; Liu, Y.; Li, A.; Fu, S.; Wu, M.; Pan, Z.; Zhou, W. microRNA-22, downregulated in hepatocellular carcinoma and correlated with prognosis, suppresses cell proliferation and tumourigenicity. Br. J. Cancer 2010, 103, 1215–1220. [Google Scholar] [CrossRef] [PubMed]
- Qian, L.; Zhang, Y.; Wang, G.; Li, B.; Zhou, H.; Qiu, J.; Qin, L. MicroRNA-29 Regulates Tumor Progression and Survival Through miR-29a-SIRT1-Wnt/β-catenin Pathway in Hepatocellular Carcinoma. Biochem. Genet. 2023. preprint. [Google Scholar] [CrossRef]
- Wang, X.; Ling, S.; Wu, W.; Shan, Q.; Liu, P.; Wang, C.; Wei, X.; Ding, W.; Teng, X.; Xu, X. Ubiquitin-Specific Protease 22/Silent Information Regulator 1 Axis Plays a Pivotal Role in the Prognosis and 5-Fluorouracil Resistance in Hepatocellular Carcinoma. Dig. Dis. Sci. 2019, 65, 1064–1073. [Google Scholar]
- Xiong, H.; Ni, Z.; He, J.; Jiang, S.; Li, X.; Gong, W.; Zheng, L.; Chen, S.; Li, B.; Zhang, N.; et al. LncRNA HULC triggers autophagy via stabilizing Sirt1 and attenuates the chemosensitivity of HCC cells. Oncogene 2017, 36, 3528–3540. [Google Scholar] [CrossRef]
- Zhou, Z.-Q.; Guan, J.; Chen, S.; Sun, J.; Zhang, Z. Sirtuin 1 Protects the Mitochondria in Hepatocellular Carcinoma Cells via Suppressing Hypoxia-Induced Factor-1 Alpha Expression. Mol. Cell. Biochem. 2022. preprint. [Google Scholar] [CrossRef]
- Li, Y.; Xu, S.; Li, J.; Zheng, L.; Feng, M.; Wang, X.; Han, K.; Pi, H.; Li, M.; Huang, X.; et al. SIRT1 facilitates hepatocellular carcinoma metastasis by promoting PGC-1α-mediated mitochondrial biogenesis. Oncotarget 2016, 7, 29255–29274. [Google Scholar] [CrossRef]
- Herranz, D.; Muñoz-Martin, M.; Cañamero, M.; Mulero, F.; Martinez-Pastor, B.; Fernandez-Capetillo, O.; Serrano, M. Sirt1 improves healthy ageing and protects from metabolic syndrome-associated cancer. Nat. Commun. 2010, 1, 1–8. [Google Scholar] [CrossRef]
- Chen, H.-C.; Jeng, Y.-M.; Yuan, R.-H.; Hsu, H.-C.; Chen, Y.-L. SIRT1 Promotes Tumorigenesis and Resistance to Chemotherapy in Hepatocellular Carcinoma and its Expression Predicts Poor Prognosis. Ann. Surg. Oncol. 2011, 19, 2011–2019. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, B.; Wong, N.; Lo, A.W.; To, K.F.; Chan, A.W.; Ng, M.H.; Ho, C.Y.; Cheng, S.H.; Lai, P.B.; et al. Sirtuin 1 is upregulated in a subset of hepatocellular carcinomas where it is essential for telomere maintenance and tumor cell growth. Cancer Res. 2011, 71, 4138–4149. [Google Scholar] [CrossRef] [PubMed]
- Tanos, B.; Rodriguez-Boulan, E. The epithelial polarity program: Machineries involved and their hijacking by cancer. Oncogene 2008, 27, 6939–6957. [Google Scholar] [CrossRef] [PubMed]
- Portmann, S.; Fahrner, R.; Lechleiter, A.; Keogh, A.; Overney, S.; Laemmle, A.; Mikami, K.; Montani, M.; Tschan, M.P.; Candinas, D.; et al. Antitumor Effect of SIRT1 Inhibition in Human HCC Tumor Models In Vitro and In Vivo. Mol. Cancer Ther. 2013, 12, 499–508. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Chan, A.W.; To, K.-F.; Chen, W.; Zhang, Z.; Ren, J.; Song, C.; Cheung, Y.-S.; Lai, P.B.; Cheng, S.-H.; et al. SIRT2 overexpression in hepatocellular carcinoma mediates epithelial to mesenchymal transition by protein kinase B/glycogen synthase kinase-3β/β-catenin signaling. Hepatology 2013, 57, 2287–2298. [Google Scholar] [CrossRef]
- Huang, S.; Zhao, Z.; Tang, D.; Zhou, Q.; Li, Y.; Zhou, L.; Yin, Y.; Wang, Y.; Pan, Y.; Dorfman, R.G.; et al. Downregulation of SIRT2 Inhibits Invasion of Hepatocellular Carcinoma by Inhibiting Energy Metabolism. Transl. Oncol. 2017, 10, 917–927. [Google Scholar] [CrossRef]
- Song, C.-L.; Tang, H.; Ran, L.-K.; Ko, B.C.B.; Zhang, Z.-Z.; Chen, X.; Ren, J.-H.; Tao, N.-N.; Li, W.-Y.; Huang, A.-L.; et al. Sirtuin 3 inhibits hepatocellular carcinoma growth through the glycogen synthase kinase-3β/BCL2-associated X protein-dependent apoptotic pathway. Oncogene 2015, 35, 631–641. [Google Scholar] [CrossRef]
- Zeng, X.; Wang, N.; Zhai, H.; Wang, R.; Wu, J.; Pu, W. SIRT3 functions as a tumor suppressor in hepatocellular carcinoma. Tumor Biol. 2017, 39, 1010428317691178. [Google Scholar] [CrossRef]
- Liu, Y.; Cheng, W.; Yin, X.-M.; Jiang, B. The expression of SIRT3 in primary hepatocellular carcinoma and the mechanism of its tumor suppressing effects. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 978–998. [Google Scholar]
- Zhang, Y.-Y.; Zhou, L.-M. Sirt3 inhibits hepatocellular carcinoma cell growth through reducing Mdm2-mediated p53 degradation. Biochem. Biophys. Res. Commun. 2012, 423, 26–31. [Google Scholar] [CrossRef]
- Tao, N.-N.; Zhou, H.-Z.; Tang, H.; Cai, X.-F.; Zhang, W.-L.; Ren, J.-H.; Zhou, L.; Chen, X.; Chen, K.; Li, W.-Y.; et al. Sirtuin 3 enhanced drug sensitivity of human hepatoma cells through glutathione S-transferase pi 1/JNK signaling pathway. Oncotarget 2016, 7, 50117–50130. [Google Scholar] [CrossRef]
- Wang, Y.; Du, L.; Liang, X.; Meng, P.; Bi, L.; Wang, C.; Tang, B. Sirtuin 4 Depletion Promotes Hepatocellular Carcinoma Tumorigenesis Through Regulating Adenosine-Monophosphate–Activated Protein Kinase Alpha/Mammalian Target of Rapamycin Axis in Mice. Hepatology 2019, 69, 1614–1631. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.-Y.; Wong, D.K.-H.; Seto, W.-K.; Mak, L.-Y.; Cheung, T.-T.; Yuen, M.-F. Tumor suppressive role of mitochondrial sirtuin 4 in induction of G2/M cell cycle arrest and apoptosis in hepatitis B virus-related hepatocellular carcinoma. Cell Death Discov. 2021, 7, 88. [Google Scholar]
- Zhang, R.; Wang, C.; Tian, Y.; Yao, Y.; Mao, J.; Wang, H.; Li, Z.; Xu, Y.; Ye, M.; Wang, L. SIRT5 Promotes Hepatocellular Carcinoma Progression by Regulating Mitochondrial Apoptosis. J. Cancer 2019, 10, 3871–3882. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.; Xi, L.; Liu, Y.; Liu, R.; Wu, Z.; Jian, Z. SIRT5 promotes cell proliferation and invasion in hepatocellular carcinoma by targeting E2F1. Mol. Med. Rep. 2017, 17, 342–349. [Google Scholar] [CrossRef]
- Lee, N.; Ryu, H.G.; Kwon, J.-H.; Kim, D.-K.; Kim, S.R.; Wang, H.J.; Kim, K.-T.; Choi, K.Y. SIRT6 Depletion Suppresses Tumor Growth by Promoting Cellular Senescence Induced by DNA Damage in HCC. PLoS ONE 2016, 11, e0165835. [Google Scholar] [CrossRef]
- Zhang, Z.-G.; Qin, C.-Y. Sirt6 suppresses hepatocellular carcinoma cell growth via inhibiting the extracellular signal-regulated kinase signaling pathway. Mol. Med. Rep. 2013, 9, 882–888. [Google Scholar] [CrossRef]
- Zhang, C.; Yu, Y.; Huang, Q.; Tang, K. SIRT6 regulates the proliferation and apoptosis of hepatocellular carcinoma via the ERK1/2 signaling pathway. Mol. Med. Rep. 2019, 20, 1575–1582. [Google Scholar]
- Ran, L.-K.; Chen, Y.; Zhang, Z.-Z.; Tao, N.-N.; Ren, J.-H.; Zhou, L.; Tang, H.; Chen, X.; Chen, K.; Li, W.-Y.; et al. SIRT6 Overexpression Potentiates Apoptosis Evasion in Hepatocellular Carcinoma via BCL2-Associated X Protein–Dependent Apoptotic Pathway. Clin. Cancer Res. 2016, 22, 3372–3382. [Google Scholar] [CrossRef]
- Wang, M.; Lan, L.; Yang, F.; Jiang, S.; Xu, H.; Zhang, C.; Zhou, G.; Xia, H.; Xia, J. Hepatic SIRT6 deficit promotes liver tumorigenesis in the mice models. Genes Dis. 2020, 9, 789–796. [Google Scholar] [CrossRef]
- Kim, J.K.; Noh, J.H.; Jung, K.H.; Eun, J.W.; Bae, H.J.; Kim, M.G.; Chang, Y.G.; Shen, Q.; Park, W.S.; Lee, J.Y.; et al. Sirtuin7 oncogenic potential in human hepatocellular carcinoma and its regulation by the tumor suppressors MiR-125a-5p and MiR-125b. Hepatology 2013, 57, 1055–1067. [Google Scholar] [CrossRef]
- Kim, Y.; Jung, K.-Y.; Kim, Y.H.; Xu, P.; Kang, B.E.; Jo, Y.; Pandit, N.; Kwon, J.; Gariani, K.; Gariani, J.; et al. Inhibition of SIRT7 Overcomes Sorafenib Acquired Resistance by Suppressing ERK1/2 Phosphorylation via the DDX3X-mediated NLRP3 Inflammasome in Hepatocellular Carcinoma. Drug Resist. Updates 2024, 73, 101054. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Ding, C.; Yu, T.; Liu, B.; Tang, W.; Wang, Z.; Tang, X.; Liang, G.; Peng, J.; Zhang, X.; et al. SIRT7 Promotes Hippo/YAP Activation and Cancer Cell Proliferation in Hepatocellular Carcinoma via Suppressing MST1. Cancer Sci. 2024, 115, 1209–1223. [Google Scholar] [CrossRef] [PubMed]
- Okubo, H.; Nakatsu, Y.; Kushiyama, A.; Yamamotoya, T.; Matsunaga, Y.; Inoue, M.-K.; Fujishiro, M.; Sakoda, H.; Ohno, H.; Yoneda, M.; et al. Gut Microbiota as a Therapeutic Target for Metabolic Disorders. Curr. Med. Chem. 2018, 25, 984–1001. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Moens de Hase, E.; Van Hul, M. Gut Microbiota and Host Metabolism: From Proof of Concept to Therapeutic Intervention. Microorganisms 2021, 9, 1302. [Google Scholar] [CrossRef]
- Caussy, C.; Hsu, C.; Lo, M.T.; Liu, A.; Bettencourt, R.; Ajmera, V.H.; Bassirian, S.; Hooker, J.; Sy, E.; Richards, L.; et al. Link between gut-microbiome derived metabolite and shared gene-effects with hepatic steatosis and fibrosis in NAFLD. Hepatology 2018, 68, 918–932. [Google Scholar]
- Zeybel, M.; Arif, M.; Li, X.; Altay, O.; Yang, H.; Shi, M.; Akyildiz, M.; Saglam, B.; Gonenli, M.G.; Yigit, B.; et al. Multiomics Analysis Reveals the Impact of Microbiota on Host Metabolism in Hepatic Steatosis. Adv. Sci. 2022, 9, 2104373. [Google Scholar] [CrossRef]
- Liu, L.; Fu, Q.; Li, T.; Shao, K.; Zhu, X.; Cong, Y.; Zhao, X. Gut microbiota and butyrate contribute to nonalcoholic fatty liver disease in premenopause due to estrogen deficiency. PLoS ONE 2022, 17, e0262855. [Google Scholar] [CrossRef]
- Grąt, M.; Wronka, K.; Krasnodębski, M.; Masior, Ł.; Lewandowski, Z.; Kosińska, I.; Grąt, K.; Stypułkowski, J.; Rejowski, S.; Wasilewicz, M.; et al. Profile of Gut Microbiota Associated with the Presence of Hepatocellular Cancer in Patients with Liver Cirrhosis. Transplant. Proc. 2016, 48, 1687–1691. [Google Scholar] [CrossRef]
- Ni, J.; Huang, R.; Zhou, H.; Xu, X.; Li, Y.; Cao, P.; Zhong, K.; Ge, M.; Chen, X.; Hou, B.; et al. Analysis of the Relationship Between the Degree of Dysbiosis in Gut Microbiota and Prognosis at Different Stages of Primary Hepatocellular Carcinoma. Front. Microbiol. 2019, 10, 1458. [Google Scholar] [CrossRef]
- Ponziani, F.R.; Bhoori, S.; Castelli, C.; Putignani, L.; Rivoltini, L.; Del Chierico, F.; Sanguinetti, M.; Morelli, D.; Sterbini, F.P.; Petito, V.; et al. Hepatocellular Carcinoma Is Associated with Gut Microbiota Profile and Inflammation in Nonalcoholic Fatty Liver Disease. Hepatology 2019, 69, 107–120. [Google Scholar] [CrossRef]
- Zheng, R.; Wang, G.; Pang, Z.; Ran, N.; Gu, Y.; Guan, X.; Yuan, Y.; Zuo, X.; Pan, H.; Zheng, J.; et al. Liver cirrhosis contributes to the disorder of gut microbiota in patients with hepatocellular carcinoma. Cancer Med. 2020, 9, 4232–4250. [Google Scholar] [CrossRef] [PubMed]
- Behary, J.; Amorim, N.; Jiang, X.-T.; Raposo, A.; Gong, L.; McGovern, E.; Ibrahim, R.; Chu, F.; Stephens, C.; Jebeili, H.; et al. Gut microbiota impact on the peripheral immune response in non-alcoholic fatty liver disease related hepatocellular carcinoma. Nat. Commun. 2021, 12, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.; Sinclair, D.A.; Ellis, J.L.; Steegborn, C. Sirtuin activators and inhibitors: Promises, achievements, and challenges. Pharmacol. Ther. 2018, 188, 140–154. [Google Scholar] [CrossRef] [PubMed]
- Manjula, R.; Anuja, K.; Alcain, F.J. SIRT1 and SIRT2 Activity Control in Neurodegenerative Diseases. Front. Pharmacol. 2021, 11, 585821. [Google Scholar] [CrossRef]
- Tian, C.; Huang, R.; Xiang, M. SIRT1: Harnessing multiple pathways to hinder NAFLD. Pharmacol. Res. 2024, 203, 107155. [Google Scholar] [CrossRef]
- Lakhan, S.E.; Kirchgessner, A. Gut microbiota and sirtuins in obesity-related inflammation and bowel dysfunction. J. Transl. Med. 2011, 9, 202. [Google Scholar] [CrossRef]
- Bayram, H.M.; Majoo, F.M.; Ozturkcan, A. Polyphenols in the prevention and treatment of non-alcoholic fatty liver disease: An update of preclinical and clinical studies. Clin. Nutr. ESPEN 2021, 44, 1–14. [Google Scholar] [CrossRef]
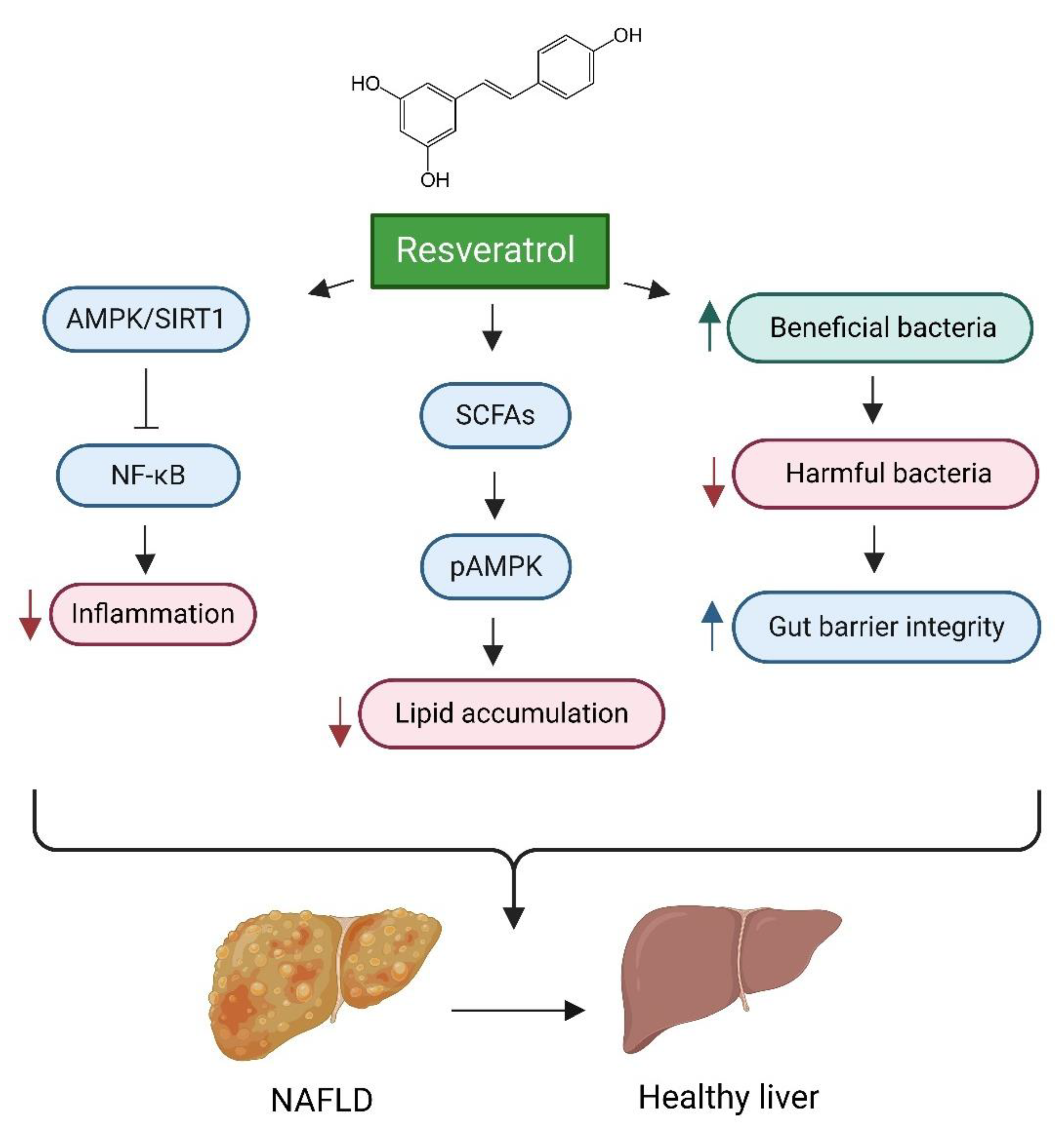
- Wang, P.; Wang, J.; Li, D.; Ke, W.; Chen, F.; Hu, X. Targeting the gut microbiota with resveratrol: A demonstration of novel evidence for the management of hepatic steatosis. J. Nutr. Biochem. 2020, 81, 108363. [Google Scholar] [CrossRef]
- Du, F.; Huang, R.; Lin, D.; Wang, Y.; Yang, X.; Huang, X.; Zheng, B.; Chen, Z.; Huang, Y.; Wang, X.; et al. Resveratrol Improves Liver Steatosis and Insulin Resistance in Non-alcoholic Fatty Liver Disease in Association with the Gut Microbiota. Front. Microbiol. 2021, 12, 611323. [Google Scholar] [CrossRef]
- Prakash, V.; Bose, C.; Sunilkumar, D.; Cherian, R.M.; Thomas, S.S.; Nair, B.G. Resveratrol as a Promising Nutraceutical: Implications in Gut Microbiota Modulation, Inflammatory Disorders, and Colorectal Cancer. Int. J. Mol. Sci. 2024, 25, 3370. [Google Scholar] [CrossRef]
- Karabekir, S.C.; Ozgorgulu, A. Possible protective effects of resveratrol in hepatocellular carcinoma. Iran. J. Basic Med. Sci. 2020, 23, 71–78. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Li, Y.; Deng, X.; Xiao, X.; Zeng, J. Integrative evidence construction for resveratrol treatment of nonalcoholic fatty liver disease: Preclinical and clinical meta-analyses. Front. Pharmacol. 2023, 14, 1230783. [Google Scholar] [CrossRef]
- Tian, Y.; Ma, J.; Wang, W.; Zhang, L.; Xu, J.; Wang, K.; Li, D. Resveratrol supplement inhibited the NF-κB inflammation pathway through activating AMPKα-SIRT1 pathway in mice with fatty liver. Mol. Cell. Biochem. 2016, 422, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.-Y.; Ding, X.-Q.; Gu, T.-T.; Guo, W.-J.; Jiao, R.-Q.; Song, L.; Sun, Y.; Pan, Y.; Kong, L.-D. Pterostilbene improves hepatic lipid accumulation via the MiR-34a/Sirt1/SREBP-1 pathway in fructose-fed rats. J. Agric. Food Chem. 2020, 68, 1436–1446. [Google Scholar] [CrossRef]
- Estrela, J.M.; Ortega, A.; Mena, S.; Rodriguez, M.L.; Asensi, M. Pterostilbene: Biomedical applications. Crit. Rev. Clin. Lab. Sci. 2013, 50, 65–78. [Google Scholar] [CrossRef]
- Gómez-Zorita, S.; Fernández-Quintela, A.; Lasa, A.; Aguirre, L.; Rimando, A.M.; Portillo, M.P. Pterostilbene, a Dimethyl Ether Derivative of Resveratrol, Reduces Fat Accumulation in Rats Fed an Obesogenic Diet. J. Agric. Food Chem. 2014, 62, 8371–8378. [Google Scholar] [CrossRef]
- Wang, P.; Sang, S. Metabolism and pharmacokinetics of resveratrol and pterostilbene. BioFactors 2018, 44, 16–25. [Google Scholar] [CrossRef]
- Han, J.; Li, S.; Wang, W.; Jiang, X.; Liu, C.; Lei, L.; Li, Y.; Sheng, R.; Zhang, Y.; Wu, Y.; et al. SIRT1 Activator E1231 Alleviates Nonalcoholic Fatty Liver Disease by Regulating Lipid Metabolism. Curr. Issues Mol. Biol. 2023, 45, 5052–5070. [Google Scholar] [CrossRef]
- Vidyashankar, S.; Varma, R.S.; Patki, P.S. Quercetin ameliorate insulin resistance and up-regulates cellular antioxidants during oleic acid induced hepatic steatosis in HepG2 cells. Toxicol. Vitr. 2013, 27, 945–953. [Google Scholar] [CrossRef]
- Cao, P.; Wang, Y.; Zhang, C.; Sullivan, M.A.; Chen, W.; Jing, X.; Yu, H.; Li, F.; Wang, Q.; Zhou, Z.; et al. Quercetin ameliorates nonalcoholic fatty liver disease (NAFLD) via the promotion of AMPK-mediated hepatic mitophagy. J. Nutr. Biochem. 2023, 120, 109414. [Google Scholar] [CrossRef]
- Ying, H.-Z.; Liu, Y.-H.; Yu, B.; Wang, Z.-Y.; Zang, J.-N.; Yu, C.-H. Dietary quercetin ameliorates nonalcoholic steatohepatitis induced by a high-fat diet in gerbils. Food Chem. Toxicol. 2013, 52, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Liu, J.; Mei, G.; Chen, H.; Peng, S.; Zhao, Y.; Yao, P.; Tang, Y. Quercetin and non-alcoholic fatty liver disease: A review based on experimental data and bioinformatic analysis. Food Chem. Toxicol. 2021, 154, 112314. [Google Scholar] [CrossRef]
- Pham, T.X.; Bae, M.; Kim, M.-B.; Lee, Y.; Hu, S.; Kang, H.; Park, Y.-K.; Lee, J.-Y. Nicotinamide riboside, an NAD+ precursor, attenuates the development of liver fibrosis in a diet-induced mouse model of liver fibrosis. Biochim. Biophys. Acta BBA Mol. Basis Dis. 2019, 1865, 2451–2463. [Google Scholar] [CrossRef]
- Longo, L.; de Castro, J.M.; Keingeski, M.B.; Rampelotto, P.H.; Stein, D.J.; Guerreiro, G.T.S.; de Souza, V.E.G.; Cerski, C.T.S.; Uribe-Cruz, C.; Torres, I.L.; et al. Nicotinamide riboside and dietary restriction effects on gut microbiota and liver inflammatory and morphologic markers in cafeteria diet–induced obesity in rats. Nutrition 2023, 110, 112019. [Google Scholar] [CrossRef]
- Han, X.; Bao, X.; Lou, Q.; Xie, X.; Zhang, M.; Zhou, S.; Guo, H.; Jiang, G.; Shi, Q. Nicotinamide riboside exerts protective effect against aging-induced NAFLD-like hepatic dysfunction in mice. PeerJ 2019, 7, e7568. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, X.; Zhang, L.; Sun, D.; Ma, Y.; Bai, Y.; Bai, X.; Liang, X.; Liang, H. Nicotinamide Riboside Ameliorates Fructose-Induced Lipid Metabolism Disorders in Mice by Activating Browning of WAT, and May Be Also Related to the Regulation of Gut Microbiota. Nutrients 2024, 16, 3920. [Google Scholar] [CrossRef]
- Pang, N.; Hu, Q.; Zhou, Y.; Xiao, Y.; Li, W.; Ding, Y.; Chen, Y.; Ye, M.; Pei, L.; Li, Q.; et al. Nicotinamide Adenine Dinucleotide Precursor Suppresses Hepatocellular Cancer Progression in Mice. Nutrients 2023, 15, 1447. [Google Scholar] [CrossRef]
- Lin, X.; Zhang, J.; Chu, Y.; Nie, Q.; Zhang, J. Berberine prevents NAFLD and HCC by modulating metabolic disorders. Pharmacol. Ther. 2024, 254, 108593. [Google Scholar] [CrossRef]
- Dai, Y.; Zhu, W.; Zhou, J.; Shen, T. The combination of berberine and evodiamine ameliorates high-fat diet-induced non-alcoholic fatty liver disease associated with modulation of gut microbiota in rats. Braz. J. Med. Biol. Res. 2022, 55, e12096. [Google Scholar] [CrossRef]
- Shu, X.; Li, M.; Cao, Y.; Li, C.; Zhou, W.; Ji, G.; Zhang, L. Berberine Alleviates Non-alcoholic Steatohepatitis Through Modulating Gut Microbiota Mediated Intestinal FXR Activation. Front. Pharmacol. 2021, 12, 750826. [Google Scholar] [CrossRef]
- Ionita-Radu, F.; Patoni, C.; Nancoff, A.S.; Marin, F.-S.; Gaman, L.; Bucurica, A.; Socol, C.; Jinga, M.; Dutu, M.; Bucurica, S. Berberine Effects in Pre-Fibrotic Stages of Non-Alcoholic Fatty Liver Disease—Clinical and Pre-Clinical Overview and Systematic Review of the Literature. Int. J. Mol. Sci. 2024, 25, 4201. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Tian, G.; Zhuang, Z.; Chen, J.; You, N.; Zhuo, L.; Liang, B.; Song, Y.; Zang, S.; Liu, J.; et al. Berberine prevents non-alcoholic steatohepatitis-derived hepatocellular carcinoma by inhibiting inflammation and angiogenesis in mice. Am. J. Transl. Res. 2019, 11, 2668–2682. [Google Scholar] [PubMed]
- Jiang, H.; Mao, T.; Sun, Z.; Shi, L.; Han, X.; Zhang, Y.; Zhang, X.; Wang, J.; Hu, J.; Zhang, L.; et al. Yinchen Linggui Zhugan decoction ameliorates high fat diet-induced nonalcoholic fatty liver disease by modulation of SIRT1/Nrf2 signaling pathway and gut microbiota. Front. Microbiol. 2022, 13, 1001778. [Google Scholar] [CrossRef]
- Wang, S.; Li, X.; Zhang, B.; Li, Y.; Chen, K.; Qi, H.; Gao, M.; Rong, J.; Liu, L.; Wan, Y.; et al. Tangshen formula targets the gut microbiota to treat non-alcoholic fatty liver disease in HFD mice: A 16S rRNA and non-targeted metabolomics analyses. Biomed. Pharmacother. 2024, 173, 116405. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, H.; Li, X.; Li, N.; Wang, Q.; Liu, Y.; Liang, Q.; Shao, Z.; Zhang, N.; Zhao, T.; et al. Tangshen Formula Alleviates Hepatic Steatosis by Inducing Autophagy Through the AMPK/SIRT1 Pathway. Front. Physiol. 2019, 10, 494. [Google Scholar] [CrossRef]
- Kong, Q.; Zhang, B.-X.; Zhang, H.-J.; Yan, M.-H.; Li, P. Experimental study of Tangshen formulain improved lipid metabolism and phenotypic switch of macrophage in db/db mice. China J. Chin. Mater. Medica 2014, 41, 1693–1698. [Google Scholar] [CrossRef]
- Rahmani, S.; Asgary, S.; Askari, G.; Keshvari, M.; Hatamipour, M.; Feizi, A.; Sahebkar, A. Treatment of Non-alcoholic Fatty Liver Disease with Curcumin: A Randomized Placebo-controlled Trial. Phytotherapy Res. 2016, 30, 1540–1548. [Google Scholar] [CrossRef]
- Amel Zabihi, N.; Pirro, M.; Johnston, T.P.; Sahebkar, A. Is there a role for curcumin supplementation in the treatment of non-alcoholic fatty liver disease? The data suggest yes. Curr. Pharm. Des. 2017, 23, 969–982. [Google Scholar]
- Lee, D.E.; Lee, S.J.; Kim, S.J.; Lee, H.-S.; Kwon, O.-S. Curcumin Ameliorates Nonalcoholic Fatty Liver Disease through Inhibition of O-GlcNAcylation. Nutrients 2019, 11, 2702. [Google Scholar] [CrossRef]
- Du, S.; Zhu, X.; Zhou, N.; Zheng, W.; Zhou, W.; Li, X. Curcumin alleviates hepatic steatosis by improving mitochondrial function in postnatal overfed rats and fatty L02 cells through the SIRT3 pathway. Food Funct. 2022, 13, 2155–2171. [Google Scholar] [CrossRef]
- Gong, H.; Xu, H.; Li, M.; Zhang, D. Molecular mechanism and therapeutic significance of dihydromyricetin in nonalcoholic fatty liver disease. Eur. J. Pharmacol. 2022, 935, 175325. [Google Scholar] [CrossRef] [PubMed]
- Kang, L.; Ma, X.; Yu, F.; Xu, L.; Lang, L. Dihydromyricetin Alleviates Non-Alcoholic Fatty Liver Disease by Modulating Gut Microbiota and Inflammatory Signaling Pathways. J. Microbiol. Biotechnol. 2024, 34, 2637–2647. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Yang, J.; Hu, O.; Huang, J.; Ran, L.; Chen, M.; Zhang, Y.; Zhou, X.; Zhu, J.; Zhang, Q.; et al. Dihydromyricetin Ameliorates Nonalcoholic Fatty Liver Disease by Improving Mitochondrial Respiratory Capacity and Redox Homeostasis Through Modulation of SIRT3 Signaling. Antioxidants Redox Signal. 2019, 30, 163–183. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Chen, Z.; Zhang, C.; Xu, X.; Jin, J.; Zhan, W.; Han, T.; Wang, J. Dihydromyricetin ameliorates oleic acid-induced lipid accumulation in L02 and HepG2 cells by inhibiting lipogenesis and oxidative stress. Life Sci. 2016, 157, 131–139. [Google Scholar] [CrossRef]
- Bahar, A.N.; Keskin-Aktan, A.; Akarca-Dizakar, S.Ö.; Sonugür, G.; Akbulut, K.G. AGK2, a SIRT2 inhibitor, ameliorates D-galactose-induced liver fibrosis by inhibiting fibrogenic factors. J. Biochem. Mol. Toxicol. 2024, 38, e70000. [Google Scholar] [CrossRef]
- Hoffmann, G.; Breitenbücher, F.; Schuler, M.; Ehrenhofer-Murray, A.E. A Novel Sirtuin 2 (SIRT2) Inhibitor with p53-dependent Pro-apoptotic Activity in Non-small Cell Lung Cancer. J. Biol. Chem. 2014, 289, 5208–5216. [Google Scholar] [CrossRef]
- Wang, T.; Xu, Z.; Lu, Y.; Shi, J.; Liu, W.; Zhang, C.; Jiang, Z.; Qi, B.; Bai, L. Recent Progress on the Discovery of Sirt2 Inhibitors for the Treatment of Various Cancers. Curr. Top. Med. Chem. 2019, 19, 1051–1058. [Google Scholar] [CrossRef]
- Kaya, S.G.; Eren, G. Selective inhibition of SIRT2: A disputable therapeutic approach in cancer therapy. Bioorganic Chem. 2023, 143, 107038. [Google Scholar] [CrossRef]
- Palaz, F.; Ozsoz, M.; Zarrinpar, A.; Sahin, I. CRISPR in Targeted Therapy and Adoptive T Cell Immunotherapy for Hepatocellular Carcinoma. J. Hepatocell. Carcinoma 2024, 11, 975–995. [Google Scholar] [CrossRef]
- He, L.; Li, Z.; Su, D.; Du, H.; Zhang, K.; Zhang, W.; Wang, S.; Xie, F.; Qiu, Y.; Ma, S.; et al. Tumor Microenvironment-Responsive Nanocapsule Delivery CRISPR/Cas9 to Reprogram the Immunosuppressive Microenvironment in Hepatoma Carcinoma. Adv. Sci. 2024, 11, e2403858. [Google Scholar] [CrossRef]
- Yu, S.; Zhao, R.; Zhang, B.; Lai, C.; Li, L.; Shen, J.; Tan, X.; Shao, J. Research progress and application of the CRISPR/Cas9 gene-editing technology based on hepatocellular carcinoma. Asian J. Pharm. Sci. 2023, 18, 100828. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Meng, X.; Huang, C.; Li, J. Emerging role of silent information regulator 1 (SIRT1) in hepatocellular carcinoma: A potential therapeutic target. Tumor Biol. 2015, 36, 4063–4074. [Google Scholar] [CrossRef] [PubMed]
- Kamal, M.A.; Mandour, Y.M.; El-Aziz, M.K.A.; Stein, U.; El Tayebi, H.M. Small Molecule Inhibitors for Hepatocellular Carcinoma: Advances and Challenges. Molecules 2022, 27, 5537. [Google Scholar] [CrossRef]
- Binarci, B.; Kılıç, E.; Doğan, T.; Çetin-Atalay, R.; Kahraman, D.; Baytaş, S.N. Design, Synthesis, and Evaluation of Novel Indole-Based Small Molecules as Sirtuin Inhibitors with Anticancer Activities. Drug Dev. Res. 2024, 85, e70008. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Su, J.; Wang, H.; Chen, J.; Tian, Y.; Zhang, J.; Feng, T.; Di, L.; Lu, X.; Sheng, H.; et al. Discovery of Novel PROTAC SIRT6 Degraders with Potent Efficacy against Hepatocellular Carcinoma. J. Med. Chem. 2024, 67, 17319–17349. [Google Scholar] [CrossRef]
- Holmes, A.; Finger, C.; Morales-Scheihing, D.; Lee, J.; McCullough, L.D. Gut dysbiosis and age-related neurological diseases; an innovative approach for therapeutic interventions. Transl. Res. 2020, 226, 39–56. [Google Scholar] [CrossRef]
- Markowiak, P.; Śliżewska, K. Effects of Probiotics, Prebiotics, and Synbiotics on Human Health. Nutrients 2017, 9, 1021. [Google Scholar] [CrossRef]
- Manzoor, S.; Wani, S.M.; Mir, S.A.; Rizwan, D. Role of probiotics and prebiotics in mitigation of different diseases. Nutrition 2022, 96, 111602. [Google Scholar] [CrossRef]
- Upasana. Application of Probiotics, Prebiotics and Synbiotics in Maintaining Gut Health. Indian J. Nutr. Diet. 2022, 59, 388–397. [Google Scholar]
- Yadav, M.K.; Kumari, I.; Singh, B.; Sharma, K.K.; Tiwari, S.K. Probiotics, prebiotics and synbiotics: Safe options for next-generation therapeutics. Appl. Microbiol. Biotechnol. 2022, 106, 505–521. [Google Scholar] [CrossRef]
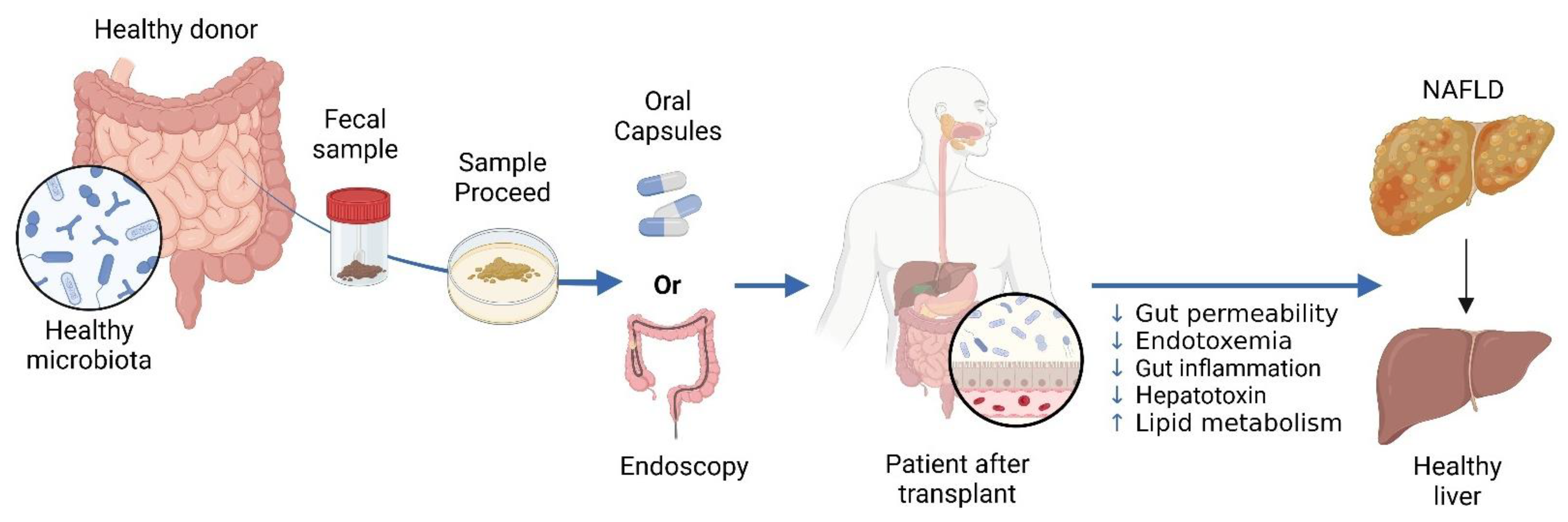
- Shao, T.; Hsu, R.; Hacein-Bey, C.; Zhang, W.; Gao, L.; Kurth, M.J.; Zhao, H.; Shuai, Z.; Leung, P.S.C. The Evolving Landscape of Fecal Microbial Transplantation. Clin. Rev. Allergy Immunol. 2023, 65, 101–120. [Google Scholar] [CrossRef] [PubMed]
- Abenavoli, L.; Maurizi, V.; Rinninella, E.; Tack, J.; Di Berardino, A.; Santori, P.; Rasetti, C.; Procopio, A.C.; Boccuto, L.; Scarpellini, E. Fecal Microbiota Transplantation in NAFLD Treatment. Medicina 2022, 58, 1559. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.-X.; Cheng, S.-L.; Liu, Y.-H.; Li, Y.; Zhang, R.; Li, N.-N.; Li, Z. Fecal microbiota transplantation for treatment of non-alcoholic fatty liver disease: Mechanism, clinical evidence, and prospect. World J. Gastroenterol. 2024, 30, 833–842. [Google Scholar] [CrossRef] [PubMed]


| Sirtuin | Class | Type of Activity | Acyl Substrates | Cellular Function | Target Substrates | Metabolic Role | Biological Role | References |
|---|---|---|---|---|---|---|---|---|
| SIRT1 | I | Deacetylase | Remove acetyl and long chain fatty acyl group from Lysine | Chromatin structure development, mitochondrial biogenesis | NF-κB, p53, FOXO1, FOXO3, TORC2, PGC-1α, PPAR-γ, SREBP, LXR, FXR, LKB1 | Fatty acid oxidation, cholesterol and bile acid homeostasis | Cell survival and metabolism | [44,45] |
| SIRT2 | I | Deacetylase | Remove of acetyl, long-chain fatty acyl, 4-oxononanoyl, and benzoyl groups | Neurodegeneration, cell cycle control, cell motility | α-Tubulin, p53, p300, NF-κB FOXO1, FOXO3, HIF1α, PEPCK | Lipid metabolism, glucose homeostasis | Cell cycle regulation, tumour suppression/promotion, metabolism | [46,47,48,49] |
| SIRT3 | I | Deacetylase | Remove acetyl and long-chain fatty acyl groups from lysine | Protection against oxidative stress, regulation of mitochondrial function and metabolism, ATP production | IDH2, LCAD, AceCS2, MnSOD, Ku70, HMGCS2, OTC, subunits of the ETC (complexes I–III and ATP synthase) | Fatty acid oxidation, amino acid metabolism, urea cycle promotion, and ketone body formation | Thermogenesis, oxidative stress resistance, tumour suppression | [50,51,52,53,54,55] |
| SIRT4 | Class II | Mono-ADP-ribosyl transferase activity, Deacetylase, Lipoamidase | Remove lipoyl, biotinyl, hydroxymethylglutaryl, 3-methylglutaryl and 3-methylglutaconyl groups | Regulation of mitochondrial metabolism | GDH, MCD, MTP-α, PDH, MCCC, ANT2, ANT3, IDE | Glucose metabolism, fatty acid oxidation, amino acid catabolism | Insulin secretion, metabolic homeostasis, tumour suppression | [56,57,58] |
| SIRT5 | Class III | Deacetylase, Desuccinylase, Deglutarylase, Demalonylase | Remove succinyl, glutaryl, and malonyl groups | Regulation of mitochondrial metabolism and ammonia detoxification | CPS1, GLUD1, UOX, GDH, IDH2, SDHA | Urea cycle and TCA cycle regulation, fatty acid and amino acid metabolism | Cellular energy homeostasis, and metabolism | [59,60,61,62] |
| SIRT6 | Class IV | Deacetylase, Mono-ADP-ribosyl transferase activity | Remove acetyl and long-chain fatty acyl groups | DNA repair, telomeric preservation | PARP1, TNFα, NF-κB, GCN5, PPARα HIF1α TRF2 | Regulation of glucose and lipid metabolism | Genomic stability, glucose homeostasis, inflammation control | [63,64,65] |
| SIRT7 | Class IV | Deacetylase | Remove acetyl groups | rDNA transcription, ribosome biogenesis, cell proliferation, DNA repair, cellular senescence | RNA Pol I, PAF53, U3–55k, GABPβ1, H3K18, H3K122, NPM1 | Lipid metabolism | Cell cycle regulation, tumour promotion, ageing, metabolic homeostasis | [66,67,68] |
| Sirtuin | Roles of Sirtuins in Gut Health | References |
|---|---|---|
| SIRT1 | Maintains intestinal epithelial barrier integrity, regulates inflammation, and modulates autophagy, potentially influencing gut microbiota composition and diversity. | [107,119,120,121] |
| SIRT2 | Regulates intestinal epithelial cell proliferation and differentiation, impacting the gut environment and reducing inflammation, facilitating better host-microbiota interactions. | [111,122,123] |
| SIRT3 | Enhances mitochondrial function in intestinal cells, regulates oxidative stress, and maintains gut barrier homeostasis; deficiency leads to microbial dysbiosis and impaired permeability. | [110,113,124] |
| SIRT4 | Modulates amino acid metabolism in intestinal cells, potentially influencing nutrient availability for gut microbiota. | [115,125] |
| SIRT5 | Regulates cellular homeostasis and various metabolic pathways in intestinal cells, potentially influencing nutrient availability for gut microbiota. | [60,126] |
| SIRT6 | Maintains intestinal epithelial barrier integrity, mitigates inflammation, and enhances favourable immune responses; may affect gut microbiota composition and diversity. | [127,128,129] |
| SIRT7 | Maintains intestinal homeostasis and modulates inflammation; potentially affecting gut microbiota composition. | [118,130] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhra, M.; Elahi, M.A.; Tariq, A.; Abu-Zaid, A.; Yaqinuddin, A. Sirtuins and Gut Microbiota: Dynamics in Health and a Journey from Metabolic Dysfunction to Hepatocellular Carcinoma. Cells 2025, 14, 466. https://doi.org/10.3390/cells14060466
Zhra M, Elahi MA, Tariq A, Abu-Zaid A, Yaqinuddin A. Sirtuins and Gut Microbiota: Dynamics in Health and a Journey from Metabolic Dysfunction to Hepatocellular Carcinoma. Cells. 2025; 14(6):466. https://doi.org/10.3390/cells14060466
Chicago/Turabian StyleZhra, Mahmoud, Muhammad Affan Elahi, Aamira Tariq, Ahmed Abu-Zaid, and Ahmed Yaqinuddin. 2025. "Sirtuins and Gut Microbiota: Dynamics in Health and a Journey from Metabolic Dysfunction to Hepatocellular Carcinoma" Cells 14, no. 6: 466. https://doi.org/10.3390/cells14060466
APA StyleZhra, M., Elahi, M. A., Tariq, A., Abu-Zaid, A., & Yaqinuddin, A. (2025). Sirtuins and Gut Microbiota: Dynamics in Health and a Journey from Metabolic Dysfunction to Hepatocellular Carcinoma. Cells, 14(6), 466. https://doi.org/10.3390/cells14060466





