A Personalized 14-3-3 Disease-Targeting Workflow Yields Repositioning Drug Candidates
Abstract
1. Introduction
2. Materials and Methods
2.1. Peptides
2.2. Cloning and Plasmid Constructs
2.3. Cell Culture
2.4. Expression of 14-3-3γ in N2a Cell
2.5. Coimmunoprecipitation Assays
2.6. Immunofluorescence
2.7. Recombinant Protein Production and Purification
2.8. In Vitro BRET Assay
2.9. High-Throughput Screening and Hit Validation
3. Results
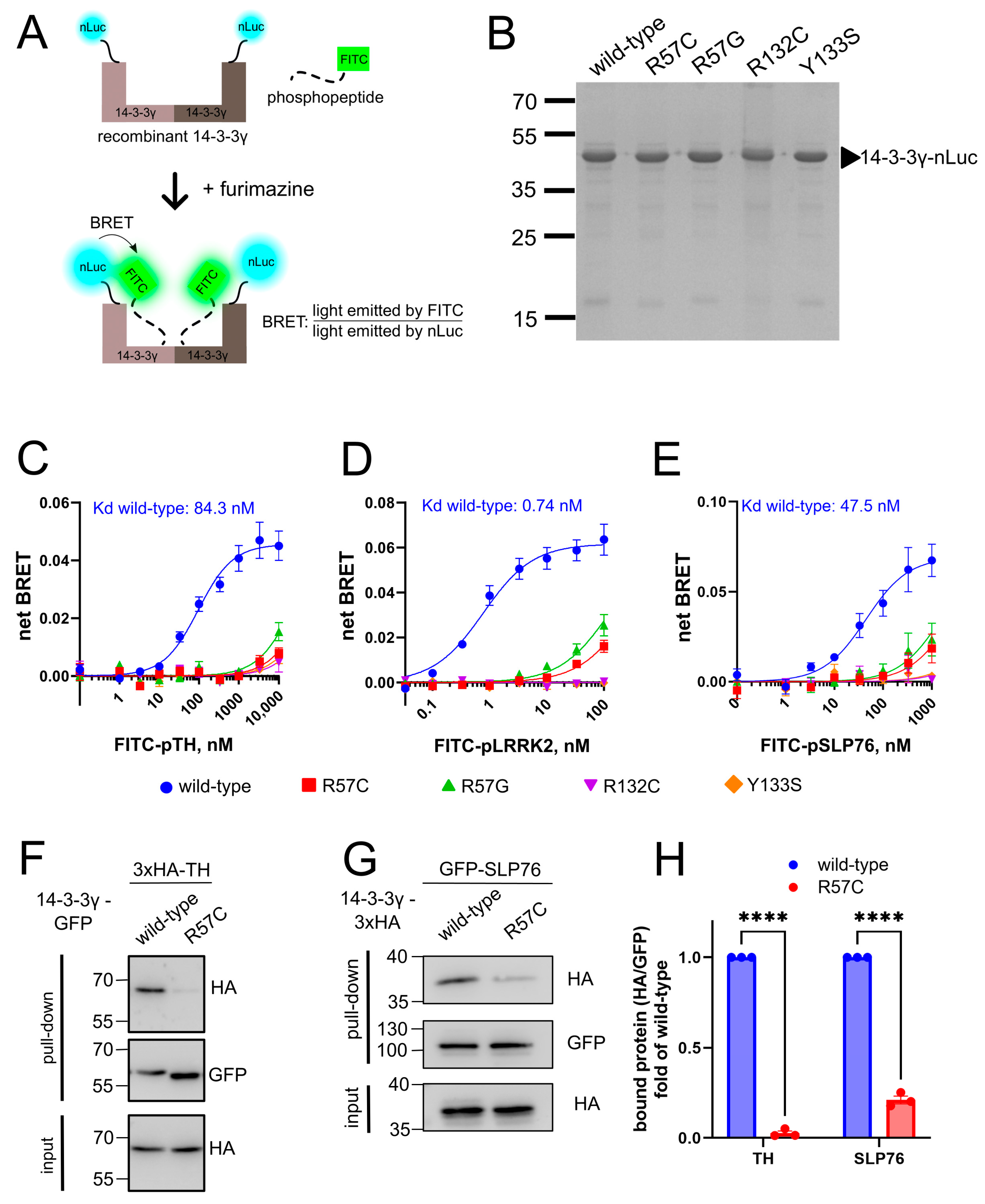
3.1. Mutations Affecting R57, R132, and Y133 Display Normal Cellular Expressions but Aberrant Localizations
3.2. Mutations Affecting R57, R132, and Y133 Fail to Interact with the Binding Partners In Vitro and in Cells
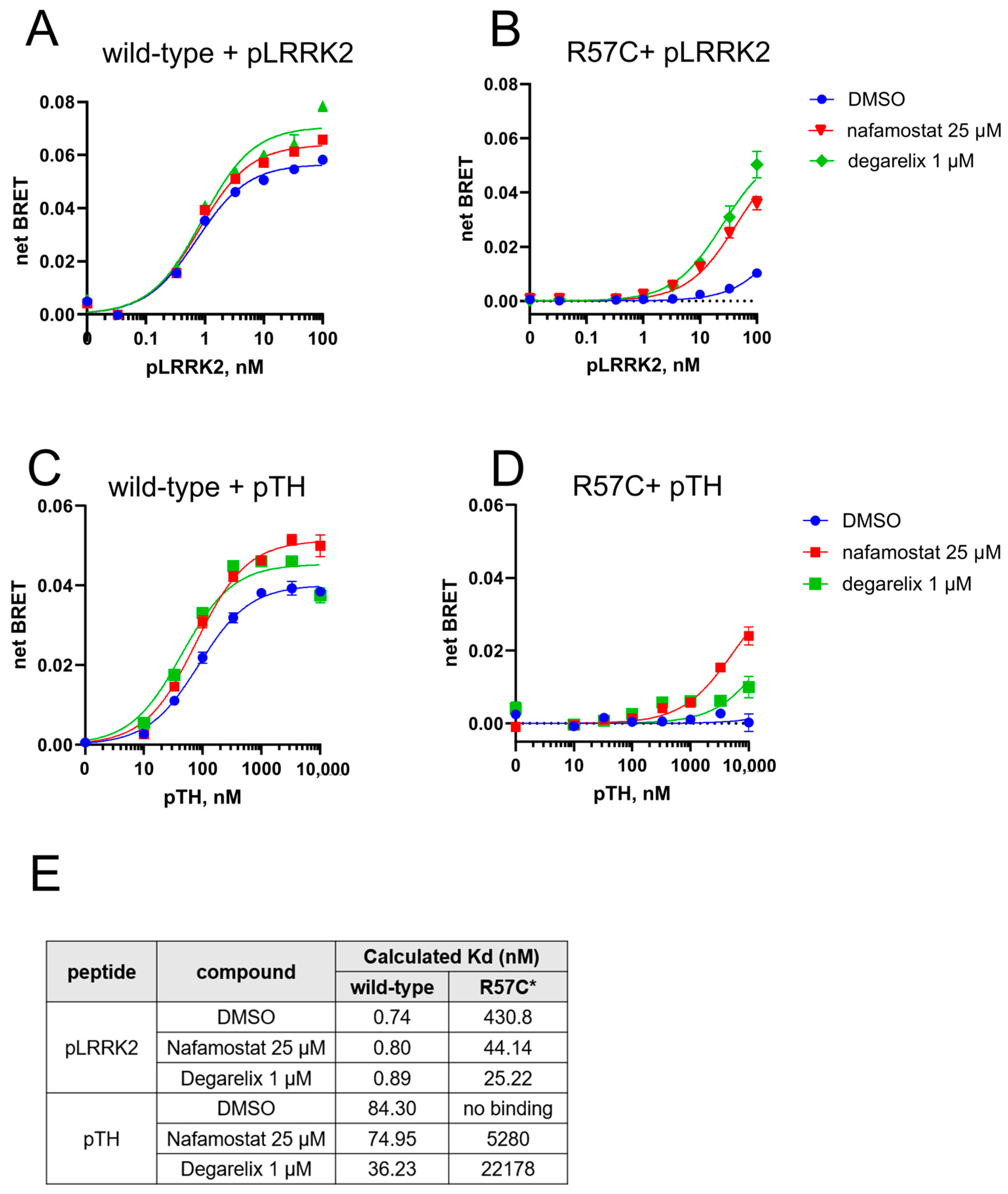
3.3. High-Throughput Screening to Find Repositioning Drug Candidates Restoring the Phosphopeptide Binding by Pathogenic 14-3-3γ Variants
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aitken, A. 14-3-3 proteins: A historic overview. Semin. Cancer Biol. 2006, 16, 162–172. [Google Scholar] [CrossRef]
- Skoulakis, E.M.C.; Davis, R.L. 14-3-3 proteins in neuronal development and function. Mol. Neurobiol. 1998, 16, 269–284. [Google Scholar] [CrossRef]
- Obsilova, V.; Obsil, T. Structural insights into the functional roles of 14-3-3 proteins. Front. Mol. Biosci. 2022, 9, 1016071. [Google Scholar] [CrossRef]
- Aghazadeh, Y.; Papadopoulos, V. The role of the 14-3-3 protein family in health, disease, and drug development. Drug Discov. Today 2016, 21, 278–287. [Google Scholar] [CrossRef]
- Ramocki, M.B.; Bartnik, M.; Szafranski, P.; Kołodziejska, K.E.; Xia, Z.; Bravo, J.; Miller, G.S.; Rodriguez, D.L.; Williams, C.A.; Bader, P.I.; et al. Recurrent distal 7q11.23 deletion including HIP1 and YWHAG identified in patients with intellectual disabilities, epilepsy, and neurobehavioral problems. Am. J. Hum. Genet. 2010, 87, 857–865. [Google Scholar] [CrossRef]
- Guella, I.; McKenzie, M.B.; Evans, D.M.; Buerki, S.E.; Toyota, E.B.; Van Allen, M.I.; Suri, M.; Elmslie, F.; Simon, M.E.H.; van Gassen, K.L.I.; et al. De Novo Mutations in YWHAG Cause Early-Onset Epilepsy. Am. J. Hum. Genet. 2017, 101, 300–310. [Google Scholar] [CrossRef]
- Ye, X.G.; Liu, Z.G.; Wang, J.; Dai, J.M.; Qiao, P.X.; Gao, P.M.; Liao, W.P. YWHAG Mutations Cause Childhood Myoclonic Epilepsy and Febrile Seizures: Molecular Sub-regional Effect and Mechanism. Front. Genet. 2021, 12, 632466. [Google Scholar] [CrossRef]
- Cetica, V.; Pisano, T.; Lesca, G.; Marafi, D.; Licchetta, L.; Riccardi, F.; Mei, D.; Chung, H.B.; Bayat, A.; Balasubramanian, M.; et al. Clinical and molecular characterization of patients with YWHAG-related epilepsy. Epilepsia 2024, 65, 1439–1450. [Google Scholar] [CrossRef]
- Iodice, A.; Giannelli, C.; Soli, F.; Riva, A.; Striano, P. Myoclonic epilepsy of infancy related to YWHAG gene mutation: Towards a better phenotypic characterization. Seizure 2022, 94, 161–164. [Google Scholar] [CrossRef]
- Sedláčková, L.; Štěrbová, K.; Vlčková, M.; Maulisová, A.; Laššuthová, P. A novel variant in YWHAG further supports phenotype of developmental and epileptic encephalopathy. Am. J. Med. Genet. A 2021, 185, 1363–1365. [Google Scholar] [CrossRef]
- Yi, Z.; Song, Z.; Xue, J.; Yang, C.; Li, F.; Pan, H.; Feng, X.; Zhang, Y.; Pan, H. A heterozygous missense variant in the YWHAG gene causing developmental and epileptic encephalopathy 56 in a Chinese family. BMC Med. Genom. 2022, 15, 216. [Google Scholar] [CrossRef]
- Amato, M.E.; Balsells, S.; Martorell, L.; Alcalá San Martín, A.; Ansell, K.; Børresen, M.L.; Johnson, H.; Korff, C.; Garcia-Tarodo, S.; Lefranc, J.; et al. Developmental and epileptic encephalopathy 56 due to YWHAG variants: 12 new cases and review of the literature. Eur. J. Paediatr. Neurol. 2024, 53, 63–72. [Google Scholar] [CrossRef]
- Yang, X.; Lee, W.H.; Sobott, F.; Papagrigoriou, E.; Robinson, C.V.; Grossmann, J.G.; Sundström, M.; Doyle, D.A.; Elkins, J.M. Structural basis for protein-protein interactions in the 14-3-3 protein family. Proc. Natl. Acad. Sci. USA 2006, 103, 17237–17242. [Google Scholar] [CrossRef]
- Halskau, Ø., Jr.; Ying, M.; Baumann, A.; Kleppe, R.; Rodriguez-Larrea, D.; Almås, B.; Haavik, J.; Martinez, A. Three-way interaction between 14-3-3 proteins, the N-terminal region of tyrosine hydroxylase, and negatively charged membranes. J. Biol. Chem. 2009, 284, 32758–32769. [Google Scholar] [CrossRef]
- Soini, L.; Leysen, S.; Davis, J.; Westwood, M.; Ottmann, C. The 14-3-3/SLP76 protein-protein interaction in T-cell receptor signalling: A structural and biophysical characterization. FEBS Lett. 2021, 595, 404–414. [Google Scholar] [CrossRef]
- Stevers, L.M.; de Vries, R.M.; Doveston, R.G.; Milroy, L.G.; Brunsveld, L.; Ottmann, C. Structural interface between LRRK2 and 14-3-3 protein. Biochem. J. 2017, 474, 1273–1287. [Google Scholar] [CrossRef]
- Wang, B.; Yang, H.; Liu, Y.C.; Jelinek, T.; Zhang, L.; Ruoslahti, E.; Fu, H. Isolation of high-affinity peptide antagonists of 14-3-3 proteins by phage display. Biochemistry 1999, 38, 12499–12504. [Google Scholar] [CrossRef]
- Teyra, J.; Kelil, A.; Jain, S.; Helmy, M.; Jajodia, R.; Hooda, Y.; Gu, J.; D’Cruz, A.A.; Nicholson, S.E.; Min, J.; et al. Large-scale survey and database of high affinity ligands for peptide recognition modules. Mol. Syst. Biol. 2020, 16, e9310. [Google Scholar] [CrossRef]
- Koval, A.; Larasati, Y.A.; Savitsky, M.; Solis, G.P.; Good, J.M.; Quinodoz, M.; Rivolta, C.; Superti-Furga, A.; Katanaev, V.L. In-depth molecular profiling of an intronic GNAO1 mutant as the basis for personalized high-throughput drug screening. Med 2023, 4, 311–325.e7. [Google Scholar] [CrossRef] [PubMed]
- Larasati, Y.A.; Thiel, M.; Koval, A.; Silachev, D.N.; Koy, A.; Katanaev, V.L. Zinc for GNAO1 encephalopathy: Preclinical profiling and a clinical case. Med 2025, 6, 100495. [Google Scholar] [CrossRef] [PubMed]
- Solis, G.P.; Bilousov, O.; Koval, A.; Luchtenborg, A.M.; Lin, C.; Katanaev, V.L. Golgi-Resident Galphao Promotes Protrusive Membrane Dynamics. Cell 2017, 170, 939–955. [Google Scholar] [CrossRef] [PubMed]
- Solis, G.P.; Koval, A.; Valnohova, J.; Kazemzadeh, A.; Savitsky, M.; Katanaev, V.L. Neomorphic Gαo mutations gain interaction with Ric8 proteins in GNAO1 encephalopathies. J. Clin. Investig. 2024, 134, e172057. [Google Scholar] [CrossRef]
- Solis, G.P.; Kazemzadeh, A.; Abrami, L.; Valnohova, J.; Alvarez, C.; van der Goot, F.G.; Katanaev, V.L. Local and substrate-specific S-palmitoylation determines subcellular localization of Gαo. Nat. Commun. 2022, 13, 2072. [Google Scholar] [CrossRef]
- Zhang, J.H.; Chung, T.D.; Oldenburg, K.R. A Simple Statistical Parameter for Use in Evaluation and Validation of High Throughput Screening Assays. J. Biomol. Screen 1999, 4, 67–73. [Google Scholar] [CrossRef]
- Larasati, Y.A.; Savitsky, M.; Koval, A.; Solis, G.P.; Valnohova, J.; Katanaev, V.L. Restoration of the GTPase activity and cellular interactions of Gα(o) mutants by Zn(2+) in GNAO1 encephalopathy models. Sci. Adv. 2022, 8, eabn9350. [Google Scholar] [CrossRef]
- Du, Y.; Khuri, F.R.; Fu, H. A homogenous luminescent proximity assay for 14-3-3 interactions with both phosphorylated and nonphosphorylated client peptides. Curr. Chem. Genom. 2008, 2, 40–47. [Google Scholar] [CrossRef][Green Version]
- Shen, Y.H.; Godlewski, J.; Bronisz, A.; Zhu, J.; Comb, M.J.; Avruch, J.; Tzivion, G. Significance of 14-3-3 self-dimerization for phosphorylation-dependent target binding. Mol. Biol. Cell 2003, 14, 4721–4733. [Google Scholar] [CrossRef]
- van Hemert, M.J.; Niemantsverdriet, M.; Schmidt, T.; Backendorf, C.; Spaink, H.P. Isoform-specific differences in rapid nucleocytoplasmic shuttling cause distinct subcellular distributions of 14-3-3 sigma and 14-3-3 zeta. J. Cell Sci. 2004, 117, 1411–1420. [Google Scholar] [CrossRef]
- Chaudhri, M.; Scarabel, M.; Aitken, A. Mammalian and yeast 14-3-3 isoforms form distinct patterns of dimers in vivo. Biochem. Biophys. Res. Commun. 2003, 300, 679–685. [Google Scholar] [CrossRef]
- Mukhopadhyay, A.; Sehgal, L.; Bose, A.; Gulvady, A.; Senapati, P.; Thorat, R.; Basu, S.; Bhatt, K.; Hosing, A.S.; Balyan, R.; et al. 14-3-3γ Prevents Centrosome Amplification and Neoplastic Progression. Sci. Rep. 2016, 6, 26580. [Google Scholar] [CrossRef]
- Ghorbani, S.; Szigetvari, P.D.; Haavik, J.; Kleppe, R. Serine 19 phosphorylation and 14-3-3 binding regulate phosphorylation and dephosphorylation of tyrosine hydroxylase on serine 31 and serine 40. J. Neurochem. 2020, 152, 29–47. [Google Scholar] [CrossRef]
- Skjevik, A.A.; Mileni, M.; Baumann, A.; Halskau, O.; Teigen, K.; Stevens, R.C.; Martinez, A. The N-terminal sequence of tyrosine hydroxylase is a conformationally versatile motif that binds 14-3-3 proteins and membranes. J. Mol. Biol. 2014, 426, 150–168. [Google Scholar] [CrossRef][Green Version]
- Daubner, S.C.; Le, T.; Wang, S. Tyrosine hydroxylase and regulation of dopamine synthesis. Arch. Biochem. Biophys. 2011, 508, 1–12. [Google Scholar] [CrossRef]
- Paisán-Ruíz, C.; Nath, P.; Washecka, N.; Gibbs, J.R.; Singleton, A.B. Comprehensive analysis of LRRK2 in publicly available Parkinson’s disease cases and neurologically normal controls. Hum. Mutat. 2008, 29, 485–490. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani, S.; Fossbakk, A.; Jorge-Finnigan, A.; Flydal, M.I.; Haavik, J.; Kleppe, R. Regulation of tyrosine hydroxylase is preserved across different homo- and heterodimeric 14-3-3 proteins. Amino Acids 2016, 48, 1221–1229. [Google Scholar] [CrossRef]
- Manschwetus, J.T.; Wallbott, M.; Fachinger, A.; Obergruber, C.; Pautz, S.; Bertinetti, D.; Schmidt, S.H.; Herberg, F.W. Binding of the Human 14-3-3 Isoforms to Distinct Sites in the Leucine-Rich Repeat Kinase 2. Front. Neurosci. 2020, 14, 302. [Google Scholar] [CrossRef]
- Sengupta, A.; Liriano, J.; Bienkiewicz, E.A.; Miller, B.G.; Frederich, J.H. Probing the 14-3-3 Isoform-Specificity Profile of Protein-Protein Interactions Stabilized by Fusicoccin A. ACS Omega 2020, 5, 25029–25035. [Google Scholar] [CrossRef]
- Hjalte, J.; Hossain, S.; Hugerth, A.; Sjögren, H.; Wahlgren, M.; Larsson, P.; Lundberg, D. Aggregation Behavior of Structurally Similar Therapeutic Peptides Investigated by (1)H NMR and All-Atom Molecular Dynamics Simulations. Mol. Pharm. 2022, 19, 904–917. [Google Scholar] [CrossRef]
- Oda, M.; Ino, Y.; Nakamura, K.; Kuramoto, S.; Shimamura, K.; Iwaki, M.; Fujii, S. Pharmacological Studies on 6-Amidino-2-Naphthyl[4-(4,5-dihydro-1H-imidazol-2-yl) amino] Benzoate Dimethane Sulfonate (FUT-187). I: Inhibitory Activities on Various Kinds of Enzymes In Vitro and Anticomplement Activity In Vivo. Jpn. J. Pharmacol. 1990, 52, 23–34. [Google Scholar] [CrossRef]
- Scheffer, I.E.; Zuberi, S.; Mefford, H.C.; Guerrini, R.; McTague, A. Developmental and epileptic encephalopathies. Nat. Rev. Dis. Primers 2024, 10, 61. [Google Scholar] [CrossRef] [PubMed]
- Katanaev, V.L.; Valnohova, J.; Silachev, D.N.; Larasati, Y.; Koval, A. Pediatric GNAO1 encephalopathies: From molecular etiology of the disease to drug discovery. Neural Regen. Res. 2023, 18, 2188–2189. [Google Scholar] [CrossRef]
- Silachev, D.; Koval, A.; Savitsky, M.; Padmasola, G.; Quairiaux, C.; Thorel, F.; Katanaev, V.L. Mouse models characterize GNAO1 encephalopathy as a neurodevelopmental disorder leading to motor anomalies: From a severe G203R to a milder C215Y mutation. Acta Neuropathol. Commun. 2022, 10, 9. [Google Scholar] [CrossRef] [PubMed]
- Benedetti, M.C.; D’Andrea, T.; Colantoni, A.; Silachev, D.; de Turris, V.; Boussadia, Z.; Babenko, V.A.; Volovikov, E.A.; Belikova, L.; Bogomazova, A.N.; et al. Cortical neurons obtained from patient-derived iPSCs with GNAO1 p.G203R variant show altered differentiation and functional properties. Heliyon 2024, 10, e26656. [Google Scholar] [CrossRef]
- Savitsky, M.; Solis, G.P.; Kryuchkov, M.; Katanaev, V.L. Humanization of Drosophila Gαo to Model GNAO1 Paediatric Encephalopathies. Biomedicines 2020, 8, 395. [Google Scholar] [CrossRef]
- Lasa-Aranzasti, A.; Larasati, Y.A.; da Silva Cardoso, J.; Solis, G.P.; Koval, A.; Cazurro-Gutiérrez, A.; Ortigoza-Escobar, J.D.; Miranda, M.C.; De la Casa-Fages, B.; Moreno-Galdó, A.; et al. Clinical and Molecular Profiling in GNAO1 Permits Phenotype-Genotype Correlation. Mov. Disord. 2024, 39, 1578–1591. [Google Scholar] [CrossRef]
- Tekin, I.; Roskoski, R., Jr.; Carkaci-Salli, N.; Vrana, K.E. Complex molecular regulation of tyrosine hydroxylase. J. Neural Transm. 2014, 121, 1451–1481. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Kortholt, A. LRRK2 Structure-Based Activation Mechanism and Pathogenesis. Biomolecules 2023, 13, 612. [Google Scholar] [CrossRef]
- Taymans, J.-M.; Fell, M.; Greenamyre, T.; Hirst, W.D.; Mamais, A.; Padmanabhan, S.; Peter, I.; Rideout, H.; Thaler, A. Perspective on the current state of the LRRK2 field. npj Park. Dis. 2023, 9, 104. [Google Scholar] [CrossRef]
- Muda, K.; Bertinetti, D.; Gesellchen, F.; Hermann, J.S.; von Zweydorf, F.; Geerlof, A.; Jacob, A.; Ueffing, M.; Gloeckner, C.J.; Herberg, F.W. Parkinson-related LRRK2 mutation R1441C/G/H impairs PKA phosphorylation of LRRK2 and disrupts its interaction with 14-3-3. Proc. Natl. Acad. Sci. USA 2014, 111, E34–E43. [Google Scholar] [CrossRef]
- Nichols, R.J.; Dzamko, N.; Morrice, N.A.; Campbell, D.G.; Deak, M.; Ordureau, A.; Macartney, T.; Tong, Y.; Shen, J.; Prescott, A.R.; et al. 14-3-3 binding to LRRK2 is disrupted by multiple Parkinson’s disease-associated mutations and regulates cytoplasmic localization. Biochem. J. 2010, 430, 393–404. [Google Scholar] [CrossRef]
- Zhao, J.; Molitor, T.P.; Langston, J.W.; Nichols, R.J. LRRK2 dephosphorylation increases its ubiquitination. Biochem. J. 2015, 469, 107–120. [Google Scholar] [CrossRef] [PubMed]
- Gerber, K.J.; Squires, K.E.; Hepler, J.R. 14-3-3γ binds regulator of G protein signaling 14 (RGS14) at distinct sites to inhibit the RGS14:Gα(i)-AlF(4)(-) signaling complex and RGS14 nuclear localization. J. Biol. Chem. 2018, 293, 14616–14631. [Google Scholar] [CrossRef]
- Brunet, A.; Kanai, F.; Stehn, J.; Xu, J.; Sarbassova, D.; Frangioni, J.V.; Dalal, S.N.; DeCaprio, J.A.; Greenberg, M.E.; Yaffe, M.B. 14-3-3 transits to the nucleus and participates in dynamic nucleocytoplasmic transport. J. Cell Biol. 2002, 156, 817–828. [Google Scholar] [CrossRef] [PubMed]
- Al-Horani, R.A.; Desai, U.R. Recent advances on plasmin inhibitors for the treatment of fibrinolysis-related disorders. Med. Res. Rev. 2014, 34, 1168–1216. [Google Scholar] [CrossRef]
- Aoyama, T.; Sasaki, H.; Shibuya, M.; Suzuki, Y. Spectrofluorometric Determination of FUT-175 (Nafamstat Mesilate) in Blood Based on Trypsin-Inhibitory Activity. Chem. Pharm. Bull. 1985, 33, 2142–2144. [Google Scholar] [CrossRef][Green Version]
- Cao, Y.G.; Zhang, M.; Yu, D.; Shao, J.P.; Chen, Y.C.; Liu, X.Q. A method for quantifying the unstable and highly polar drug nafamostat mesilate in human plasma with optimized solid-phase extraction and ESI-MS detection: More accurate evaluation for pharmacokinetic study. Anal. Bioanal. Chem. 2008, 391, 1063–1071. [Google Scholar] [CrossRef]
- Frampton, J.E.; Lyseng-Williamson, K.A. Degarelix. Drugs 2009, 69, 1967–1976. [Google Scholar] [CrossRef] [PubMed]
- Shore, N.D. Experience with degarelix in the treatment of prostate cancer. Ther. Adv. Urol. 2013, 5, 11–24. [Google Scholar] [CrossRef]
- Zhanel, G.G.; Schweizer, F.; Karlowsky, J.A. Oritavancin: Mechanism of action. Clin. Infect. Dis. 2012, 54 (Suppl. S3), S214–S219. [Google Scholar] [CrossRef]
- Saravolatz, L.D.; Stein, G.E. Oritavancin: A Long-Half-Life Lipoglycopeptide. Clin. Infect. Dis. 2015, 61, 627–632. [Google Scholar] [CrossRef]
- Morice, A.H.; Sever, P.S. Vasoactive intestinal peptide as a bronchodilator in severe asthma. Peptides 1986, 7 (Suppl. S1), 279–280. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Pal, S.; Dewan, B. The impact of aviptadil as an add-on to the standard of care in acute respiratory distress syndrome. Eur. Respir. J. 2024, 64, PA2620. [Google Scholar] [CrossRef]
- Chakraborty, D.; Choudhury, S.; Lahiry, S. Aviptadil: Class Effect of a Synthetic Vasoactive Intestinal Peptide as a Treatment Option in Patients with COVID-19 with Severe Respiratory Failure. EMJ Microbiol. Infect. Dis. 2022. [Google Scholar] [CrossRef]
- Uwagawa, T.; Li, Z.; Chang, Z.; Xia, Q.; Peng, B.; Sclabas, G.M.; Ishiyama, S.; Hung, M.C.; Evans, D.B.; Abbruzzese, J.L.; et al. Mechanisms of synthetic serine protease inhibitor (FUT-175)-mediated cell death. Cancer 2007, 109, 2142–2153. [Google Scholar] [CrossRef] [PubMed]
- Lemaire, S.; Mingeot-Leclercq, M.P.; Tulkens, P.M.; Van Bambeke, F. Study of macrophage functions in murine J774 cells and human activated THP-1 cells exposed to oritavancin, a lipoglycopeptide with high cellular accumulation. Antimicrob. Agents Chemother. 2014, 58, 2059–2066. [Google Scholar] [CrossRef]
- Chen, T.; Wang, J.; Li, C.; Zhang, W.; Zhang, L.; An, L.; Pang, T.; Shi, X.; Liao, H. Nafamostat mesilate attenuates neuronal damage in a rat model of transient focal cerebral ischemia through thrombin inhibition. Sci. Rep. 2014, 4, 5531. [Google Scholar] [CrossRef]
- Liu, Y.; Li, C.; Wang, J.; Fang, Y.; Sun, H.; Tao, X.; Zhou, X.-F.; Liao, H. Nafamostat Mesilate Improves Neurological Outcome and Axonal Regeneration after Stroke in Rats. Mol. Neurobiol. 2017, 54, 4217–4231. [Google Scholar] [CrossRef]
- Gerber, J.; Smirnov, A.; Wellmer, A.; Ragheb, J.; Prange, J.; Schütz, E.; Wettich, K.; Kalich, S.; Nau, R. Activity of LY333328 in experimental meningitis caused by a Streptococcus pneumoniae strain susceptible to penicillin. Antimicrob. Agents Chemother. 2001, 45, 2169–2172. [Google Scholar] [CrossRef]
- Cabellos, C.; Fernàndez, A.; Maiques Jose, M.; Tubau, F.; Ardanuy, C.; Viladrich Pedro, F.; Liñares, J.; Gudiol, F. Experimental Study of LY333328 (Oritavancin), Alone and in Combination, in Therapy of Cephalosporin-Resistant Pneumococcal Meningitis. Antimicrob. Agents Chemother. 2003, 47, 1907–1911. [Google Scholar] [CrossRef]
- Mathias, K.; Machado, R.S.; Stork, S.; Dos Santos, D.; Joaquim, L.; Generoso, J.; Danielski, L.G.; Barichello, T.; Prophiro, J.S.; Petronilho, F. Blood-brain barrier permeability in the ischemic stroke: An update. Microvasc. Res. 2024, 151, 104621. [Google Scholar] [CrossRef]
- Yang, R.; Wang, J.; Wang, F.; Zhang, H.; Tan, C.; Chen, H.; Wang, X. Blood-Brain Barrier Integrity Damage in Bacterial Meningitis: The Underlying Link, Mechanisms, and Therapeutic Targets. Int. J. Mol. Sci. 2023, 24, 2852. [Google Scholar] [CrossRef] [PubMed]
- Janes, J.; Young, M.E.; Chen, E.; Rogers, N.H.; Burgstaller-Muehlbacher, S.; Hughes, L.D.; Love, M.S.; Hull, M.V.; Kuhen, K.L.; Woods, A.K.; et al. The ReFRAME library as a comprehensive drug repurposing library and its application to the treatment of cryptosporidiosis. Proc. Natl. Acad. Sci. USA 2018, 115, 10750–10755. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cao, Y.; Chen, Y.; Cheng, H.; Liu, Z.; Wang, M.; Feng, Y.; Fei, B.; Cui, K.; Huang, Z. YWHAG promotes colorectal cancer progression by regulating the CTTN-Wnt/β-catenin signaling axis. Med. Oncol. 2024, 41, 100. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Chuang, S.M.; Yang, M.F.; Liao, J.W.; Yu, S.L.; Chen, J.J. A novel function of YWHAZ/β-catenin axis in promoting epithelial-mesenchymal transition and lung cancer metastasis. Mol. Cancer Res. 2012, 10, 1319–1331. [Google Scholar] [CrossRef]
- Huang, X.Y.; Ke, A.W.; Shi, G.M.; Zhang, X.; Zhang, C.; Shi, Y.H.; Wang, X.Y.; Ding, Z.B.; Xiao, Y.S.; Yan, J.; et al. αB-crystallin complexes with 14-3-3ζ to induce epithelial-mesenchymal transition and resistance to sorafenib in hepatocellular carcinoma. Hepatology 2013, 57, 2235–2247. [Google Scholar] [CrossRef]


| Peptide | Peptide Sequence 1 | Reference |
|---|---|---|
| FITC-pTH | MPTPDATTPQAKGFRRAVpSELDAKQAEAIMSPRFIGRRQSLIE | [14] |
| FITC-ppLRRK2 | QRHSNpSLGPIFDGSGGGSGIKARASpSSPVILVGTHLD | [15] |
| FITC-pSLP76 | FPQSApSLPPYFS | [16] |
| R18 peptide | PHCVPRDLSWLDLEANMCLP | [17] |
| Target Plasmid | Source Plasmid | Linearization Sites | Primers for Fragment Amplification |
|---|---|---|---|
| 14-3-3γ-GFP or 14-3-3γ-HA | pEGFP-N1 or p3xHA-N1 | EcoRI | Fwd:CCCGCGGTACCGTCGACTGCAGATTGTTACCCTCACCGCCATCG Rev:CAGATCTCGAGCTCAAGCTTCGATGGTGGACCGTGAACAACTGG |
| 14-3-3γ-Nluc | pET23a_GNAO1 [19,20] | NcoI, EcoRI | Nluc fragment: Fwd:CGATGGCGGTGAGGGTAACAATGGTGGAGGCGGGACGCGTTCTG Rev:CGCAAGCTTGTCGACGGAGCTCGTCACAGAATGCGTTCGCACAGCCGC 14-3-3γ fragment: Fwd:GATCTCACCATCACCATCACCATGTGGACCGTGAACAACTGGTGC Rev: ATTGTTACCCTCACCGCCATCG |
| Plasmid | Primers |
|---|---|
| 14-3-3γ-R57C-GFP and 14-3-3γ-R57C-3xHA | Fwd: GTTGGCGCTCGTTGCAGCTCTTGGCGCGTTATTAGTTCC Rev: GCCAAGAGCTGCAACGAGCGCCAACCACATTCTTATATG |
| 14-3-3γ-R57G-GFP and 14-3-3γ-R57G-3xHA | Fwd: GTTGGCGCTCGTGGCAGCTCTTGGCGCGTTATTAGTTCC Rev: GCCAAGAGCTGCCACGAGCGCCAACCACATTCTTATATG |
| 14-3-3γ-R132C-GFP and 14-3-3γ- R132C-3xHA | Fwd: GGGCGATTATTACTGTTATCTGGCAGAAGTGGCTACCG Rev: TGCCAGATAACAGTAATAATCGCCCTTCATTTTCAGG |
| 14-3-3γ-Y133S-GFP and 14-3-3γ-Y133S-3xHA | Fwd: GATTATTACCGTTCTCTGGCAGAAGTGGCTACCGGTG Rev: ACTTCTGCCAGAGAACGGTAATAATCGCCCTTCATTTTC |
| 3xHA-TH | Fwd: AAGTCCGGAATGCCCACCCCC Rev: TGGGATCCCTAGCCAATGGCA |
| GFP-SLP76 | Fwd: AAGGAATTCATGGCACTGAGG Rev: TGGGATCCGTTGGGTACCCT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Larasati, Y.A.; Solis, G.P.; Koval, A.; Korff, C.; Katanaev, V.L. A Personalized 14-3-3 Disease-Targeting Workflow Yields Repositioning Drug Candidates. Cells 2025, 14, 559. https://doi.org/10.3390/cells14080559
Larasati YA, Solis GP, Koval A, Korff C, Katanaev VL. A Personalized 14-3-3 Disease-Targeting Workflow Yields Repositioning Drug Candidates. Cells. 2025; 14(8):559. https://doi.org/10.3390/cells14080559
Chicago/Turabian StyleLarasati, Yonika A., Gonzalo P. Solis, Alexey Koval, Christian Korff, and Vladimir L. Katanaev. 2025. "A Personalized 14-3-3 Disease-Targeting Workflow Yields Repositioning Drug Candidates" Cells 14, no. 8: 559. https://doi.org/10.3390/cells14080559
APA StyleLarasati, Y. A., Solis, G. P., Koval, A., Korff, C., & Katanaev, V. L. (2025). A Personalized 14-3-3 Disease-Targeting Workflow Yields Repositioning Drug Candidates. Cells, 14(8), 559. https://doi.org/10.3390/cells14080559







