Nicotinamide Inhibits CD4+ T-Cell Activation and Function
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Flow Cytometry
2.3. qPCR
2.4. Luminex
2.5. RNA-Sequencing
2.6. Glucose Uptake Assay
2.7. ROS Measurement
2.8. Seahorse Assay
2.9. Proliferation Assay
2.10. Statistics
3. Results
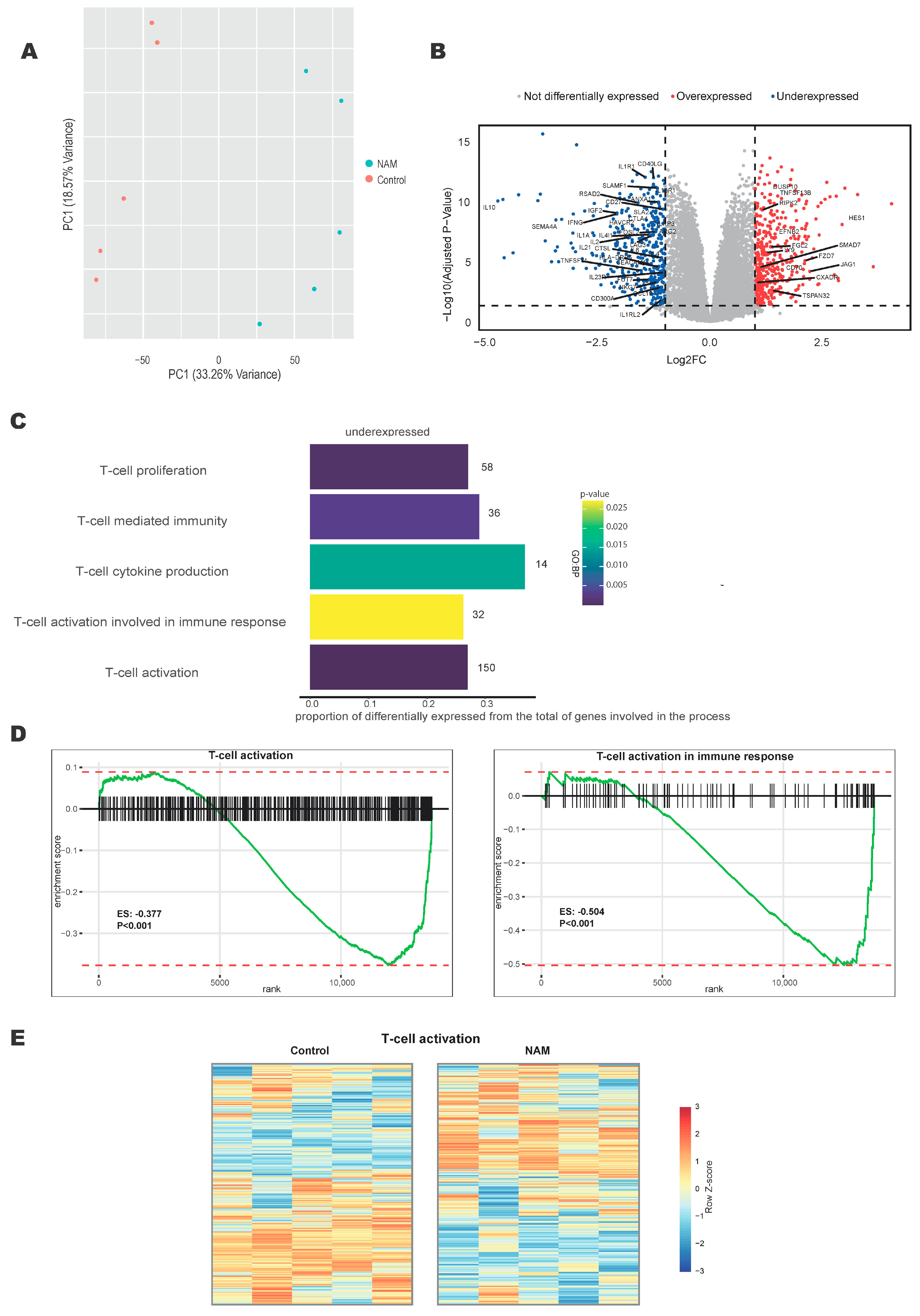
3.1. NAM Regulates T-Cell Activation Pathways
3.2. NAM Inhibits the Expression of T-Cell Surface Activation Markers
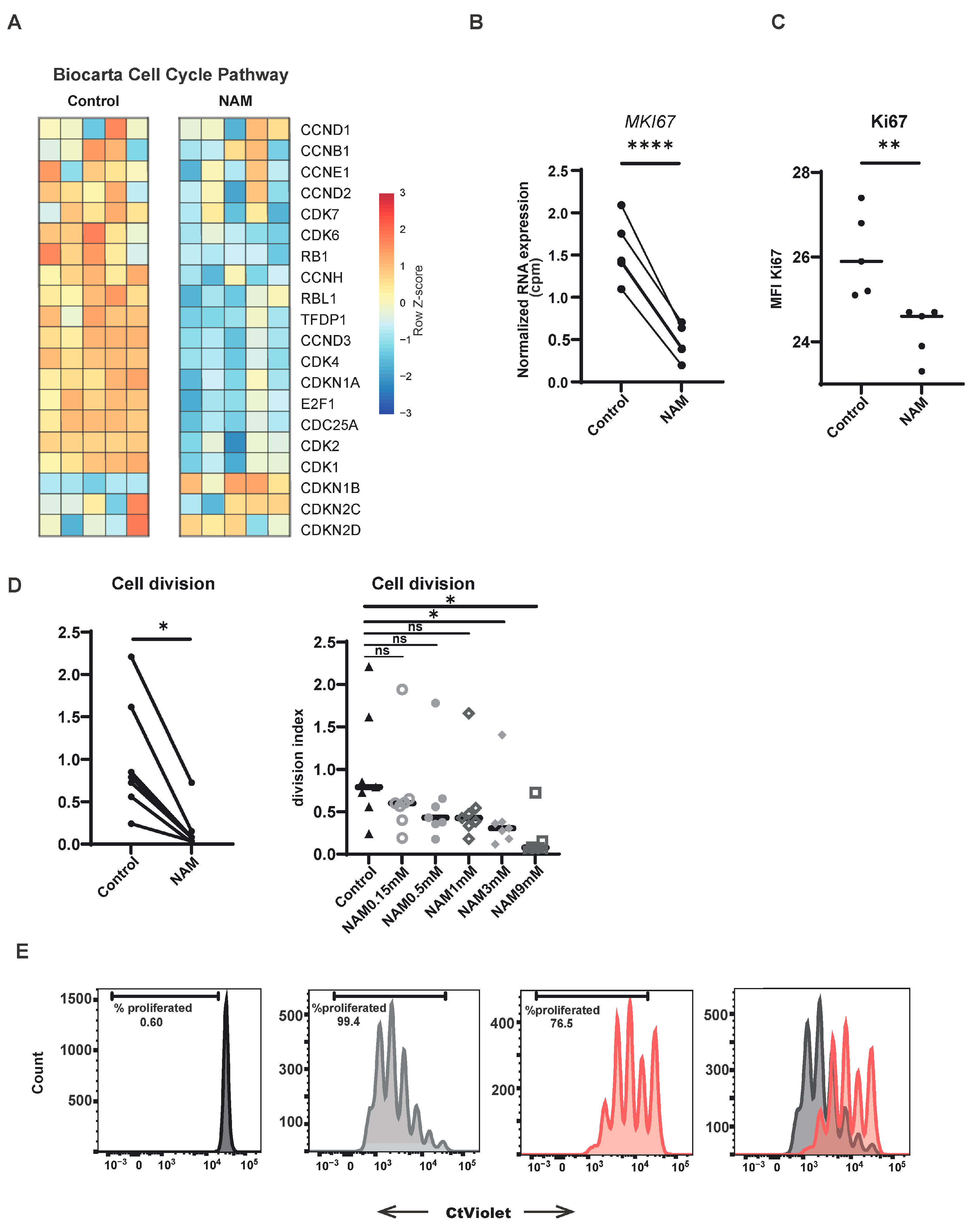
3.3. NAM Inhibits CD4+ T-Cell Proliferation
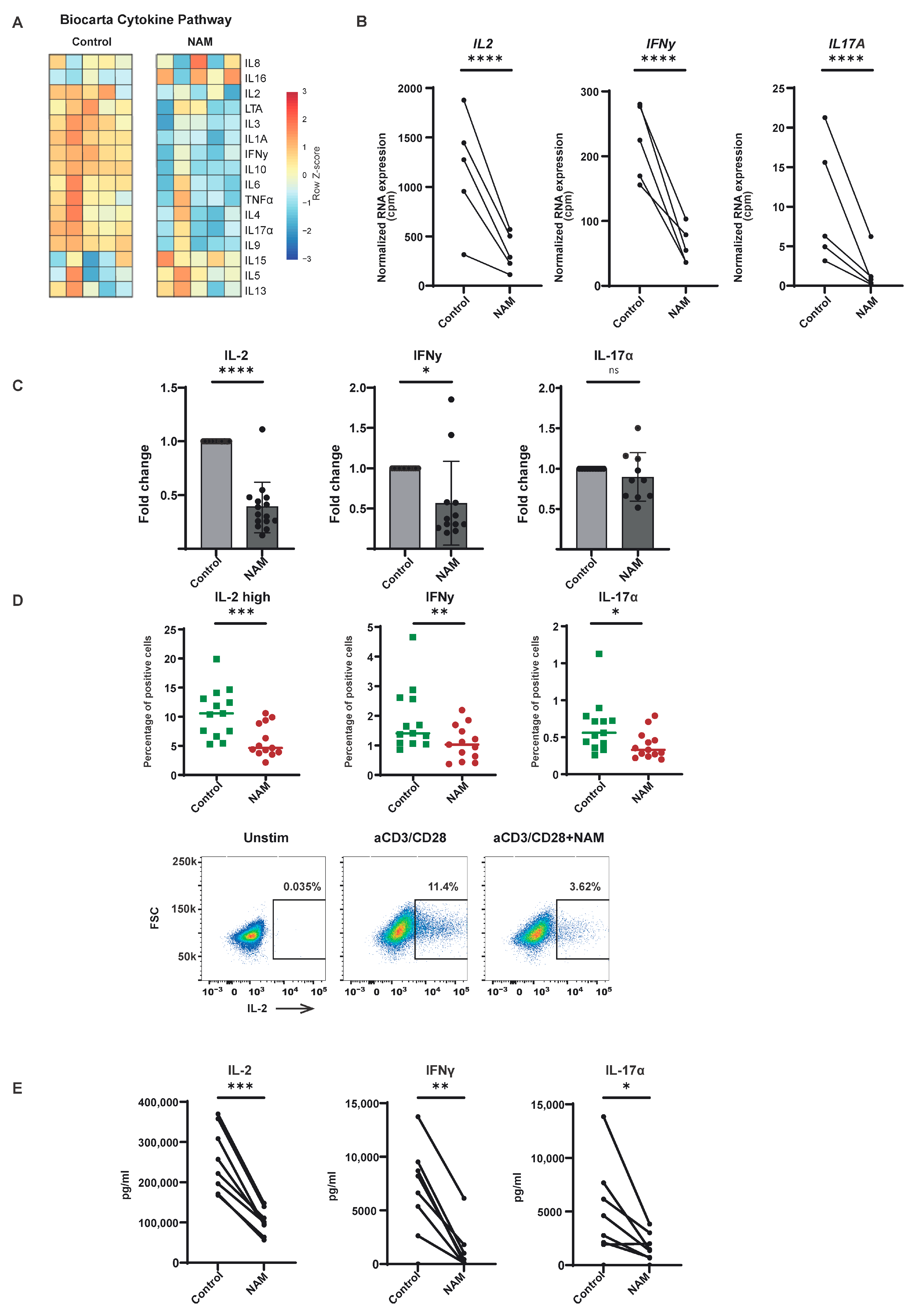
3.4. NAM Reduces Pro-Inflammatory Cytokine Production in TCR Activated CD4+ T-Cells
3.5. NAM Impairs Metabolic Processes Essential for T-Cell Activation
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Song, Y.; Li, J.; Wu, Y. Evolving Understanding of Autoimmune Mechanisms and New Therapeutic Strategies of Autoimmune Disorders. Signal Transduct. Target. Ther. 2024, 9, 263. [Google Scholar] [PubMed]
- Speiser, D.E.; Chijioke, O.; Schaeuble, K.; Münz, C. CD4+ T Cells in Cancer. Nat. Cancer 2023, 4, 317–329. [Google Scholar] [CrossRef] [PubMed]
- Attfield, K.E.; Jensen, L.T.; Kaufmann, M.; Friese, M.A.; Fugger, L. The Immunology of Multiple Sclerosis. Nat. Rev. Immunol. 2022, 22, 734–750. [Google Scholar] [CrossRef]
- Maschmeyer, P.; Chang, H.D.; Cheng, Q.; Mashreghi, M.F.; Hiepe, F.; Alexander, T.; Radbruch, A. Immunological Memory in Rheumatic Inflammation—A Roadblock to Tolerance Induction. Nat. Rev. Rheumatol. 2021, 17, 291–305. [Google Scholar] [CrossRef]
- Tenbrock, K.; Rauen, T. T Cell Dysregulation in SLE. Clin. Immunol. 2022, 239, 109031. [Google Scholar] [CrossRef]
- Lees, J.R. Targeting Antigen Presentation in Autoimmunity. Cell. Immunol. 2019, 339, 4–9. [Google Scholar] [CrossRef] [PubMed]
- Nijhuis, L.; Peeters, J.G.C.; Vastert, S.J.; Van Loosdregt, J. Restoring T Cell Tolerance, Exploring the Potential of Histone Deacetylase Inhibitors for the Treatment of Juvenile Idiopathic Arthritis. Front. Immunol. 2019, 10, 151. [Google Scholar] [CrossRef]
- Alenzi, F.Q. Effect of Nicotinamide on Experimental Induced Diabetes. Iran. J. Allergy Asthma Immunol. 2009, 8, 11–18. [Google Scholar]
- Hoffer, A.; Saskatoon, S. Hoffer: Nicotinic acid and arthritis 235 treatment of arthritis by nicotinic acid and nicotinamide. Can. Med. Assoc. J. 1959, 81, 235–238. [Google Scholar] [PubMed] [PubMed Central]
- Kaneko, S.; Wang, J.; Kaneko, M.; Yiu, G.; Hurrell, J.M.; Chitnis, T.; Khoury, S.J.; He, Z. Protecting Axonal Degeneration by Increasing Nicotinamide Adenine Dinucleotide Levels in Experimental Autoimmune Encephalomyelitis Models. J. Neurosci. 2006, 26, 9794–9804. [Google Scholar] [CrossRef]
- Kamal, M.; Al Abbasy, A.J.; Al Muslemani, A.; Bener, A. Effect of Nicotinamide on Newly Diagnosed Type 1 Diabetic Children. Acta Pharmacol. Sin. 2006, 27, 724–727. [Google Scholar] [CrossRef][Green Version]
- Olmos, P.R.; Hodgson, M.I.; Maiz, A.; Manrique, M.; De Valdés, M.D.; Foncea, R.; Acosta, A.M.; Emmerich, M.V.; Velasco, S.; Muñiz, O.P.; et al. Nicotinamide Protected First-Phase Insulin Response (FPIR) and Prevented Clinical Disease in First-Degree Relatives of Type-1 Diabetics. Diabetes Res. Clin. Pract. 2006, 71, 320–333. [Google Scholar] [CrossRef]
- Hiromatsu, Y.; Sato, M.; Yamada, K.; Nonaka, K.; Hiromatsu, Y. Inhibitory Effects of Nicotinamide on Recombinant Human Interferon-Gamma-Induced Intercellular Adhesion Molecule-1 (ICAM-1) and HLA-DR Antigen Expression on Cultured Human Endothelial Cells. Immunol. Lett. 1991, 31, 35–39. [Google Scholar]
- Beier, U.H.; Wang, L.; Bhatti, T.R.; Liu, Y.; Han, R.; Ge, G.; Hancock, W.W. Sirtuin-1 Targeting Promotes Foxp3+ T-Regulatory Cell Function and Prolongs Allograft Survival. Mol. Cell. Biol. 2011, 31, 1022–1029. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.W.; Kang, S.G.; Ryu, J.K.; Schilling, B.; Fei, M.; Lee, I.S.; Kehasse, A.; Shirakawa, K.; Yokoyama, M.; Schnölzer, M.; et al. SIRT1 Deacetylates RORγt and Enhances Th17 Cell Generation. J. Exp. Med. 2015, 212, 607–617. [Google Scholar] [CrossRef]
- Saenz, L.; Lozano, J.J.; Valdor, R.; Baroja-Mazo, A.; Ramirez, P.; Parrilla, P.; Aparicio, P.; Sumoy, L.; Yélamos, J. Transcriptional Regulation by Poly(ADP-Ribose) Polymerase-1 during T Cell Activation. BMC Genom. 2008, 9, 171. [Google Scholar] [CrossRef]
- Rosado, M.M.; Bennici, E.; Novelli, F.; Pioli, C. Beyond DNA Repair, the Immunological Role of PARP-1 and Its Siblings. Immunology 2013, 139, 428–437. [Google Scholar]
- Nasta, F.; Laudisi, F.; Sambucci, M.; Rosado, M.M.; Pioli, C. Increased Foxp3+ Regulatory T Cells in Poly(ADP-Ribose) Polymerase-1 Deficiency. J. Immunol. 2010, 184, 3470–3477. [Google Scholar] [CrossRef]
- Niederer, F.; Ospelt, C.; Brentano, F.; Hottiger, M.O.; Gay, R.E.; Gay, S.; Detmar, M.; Kyburz, D. SIRT1 Overexpression in the Rheumatoid Arthritis Synovium Contributes to Proinflammatory Cytokine Production and Apoptosis Resistance. Ann. Rheum. Dis. 2011, 70, 1866–1873. [Google Scholar] [CrossRef]
- Van Loosdregt, J.; Vercoulen, Y.; Guichelaar, T.; Gent, Y.Y.J.; Beekman, J.M.; Van Beekum, O.; Brenkman, A.B.; Hijnen, D.-J.; Mutis, T.; Kalkhoven, E.; et al. Regulation of Treg Functionality by Acetylation-Mediated Foxp3 Protein Stabilization. Blood J. Am. Soc. Hematol. 2010, 115, 965–974. [Google Scholar] [CrossRef]
- Agliano, F.; Karginov, T.A.; Ménoret, A.; Provatas, A.; Vella, A.T. Nicotinamide Breaks Effector CD8 T Cell Responses by Targeting MTOR Signaling. iScience 2022, 25, 103932. [Google Scholar] [CrossRef] [PubMed]
- Alavi, S.; Al Emran, A.; Tseng, H.Y.; Tiffen, J.C.; McGuire, H.M.; Hersey, P. Nicotinamide Inhibits T Cell Exhaustion and Increases Differentiation of CD8 Effector T Cells. Cancers 2022, 14, 323. [Google Scholar] [CrossRef] [PubMed]
- De Jager, W.; Prakken, B.J.; Bijlsma, J.W.J.; Kuis, W.; Rijkers, G.T. Improved Multiplex Immunoassay Performance in Human Plasma and Synovial Fluid Following Removal of Interfering Heterophilic Antibodies. J. Immunol. Methods 2005, 300, 124–135. [Google Scholar] [CrossRef] [PubMed]
- Scholman, R.C.; Giovannone, B.; Hiddingh, S.; Meerding, J.M.; Malvar Fernandez, B.; van Dijk, M.E.A.; Tempelman, M.J.; Prakken, B.J.; de Jager, W. Effect of Anticoagulants on 162 Circulating Immune Related Proteins in Healthy Subjects. Cytokine 2018, 106, 114–124. [Google Scholar] [CrossRef]
- Smyth, G.K. Linear Models and Empirical Bayes Methods for Assessing Differential Expression in Microarray Experiments. Stat. Appl. Genet. Mol. Biol. 2004, 3, 1. [Google Scholar] [CrossRef]
- Wu, D.; Smyth, G.K. Camera: A Competitive Gene Set Test Accounting for Inter-Gene Correlation. Nucleic Acids Res. 2012, 40, e133. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple. J. R. Stat. Soc. Ser. B Methodol. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Kolberg, L.; Raudvere, U.; Kuzmin, I.; Vilo, J.; Peterson, H. Gprofiler2—An R Package for Gene List Functional Enrichment Analysis and Namespace Conversion Toolset g:Profiler. F1000Res 2020, 9, 709. [Google Scholar] [CrossRef]
- Korotkevich, G.; Sukhov, V.; Budin, N.; Shpak, B.; Artyomov, M.N.; Sergushichev, A. Fast Gene Set Enrichment Analysis. bioRxiv 2016, 060012. [Google Scholar] [CrossRef]
- Shipkova, M.; Wieland, E. Surface Markers of Lymphocyte Activation and Markers of Cell Proliferation. Clin. Chim. Acta 2012, 413, 1338–1349. [Google Scholar] [CrossRef]
- Reddy, M.; Eirikis, E.; Davis, C.; Davis, H.M.; Prabhakar, U. Comparative Analysis of Lymphocyte Activation Marker Expression and Cytokine Secretion Profile in Stimulated Human Peripheral Blood Mononuclear Cell Cultures: An in Vitro Model to Monitor Cellular Immune Function. J. Immunol. Methods 2004, 293, 127–142. [Google Scholar] [CrossRef] [PubMed]
- Cibrián, D.; Sánchez-Madrid, F. CD69: From Activation Marker to Metabolic Gatekeeper. Eur. J. Immunol. 2017, 47, 946–953. [Google Scholar] [CrossRef] [PubMed]
- González-Amaro, R.; Cortés, J.R.; Sánchez-Madrid, F.; Martín, P. Is CD69 an Effective Brake to Control Inflammatory Diseases? Trends Mol. Med. 2013, 19, 625–632. [Google Scholar] [CrossRef]
- Sharpe, A.H.; Pauken, K.E. The Diverse Functions of the PD1 Inhibitory Pathway. Nat. Rev. Immunol. 2018, 18, 153–167. [Google Scholar] [CrossRef]
- Dimeloe, S.; Burgener, A.V.; Grählert, J.; Hess, C. T-Cell Metabolism Governing Activation, Proliferation and Differentiation; a Modular View. Immunology 2017, 150, 35–44. [Google Scholar] [CrossRef]
- Frauwirth, K.A.; Riley, J.L.; Harris, M.H.; Parry, R.V.; Rathmell, J.C.; Plas, D.R.; Elstrom, R.L.; June, C.H.; Thompson, C.B. The CD28 Signaling Pathway Regulates Glucose Metabolism Ability of Resting Cells to Take up and Utilize Nutrients at Levels Sufficient to Maintain Viability (Rathmell et al. in Fat and Muscle Cells Insulin Induces Glucose Uptake in Excess of That Required to Maintain. Immunity 2002, 16, 769–777. [Google Scholar] [PubMed]
- Mocholi, E.; Russo, L.; Gopal, K.; Ramstead, A.G.; Hochrein, S.M.; Vos, H.R.; Geeven, G.; Adegoke, A.O.; Hoekstra, A.; van Es, R.M.; et al. Pyruvate Metabolism Controls Chromatin Remodeling during CD4+ T Cell Activation. Cell Rep. 2023, 42, 112583. [Google Scholar] [CrossRef]
- Peng, H.Y.; Lucavs, J.; Ballard, D.; Das, J.K.; Kumar, A.; Wang, L.; Ren, Y.; Xiong, X.; Song, J. Metabolic Reprogramming and Reactive Oxygen Species in T Cell Immunity. Front. Immunol. 2021, 12, 652687. [Google Scholar] [CrossRef]
- Bettenworth, D.; Nowacki, T.M.; Ross, M.; Kyme, P.; Schwammbach, D.; Kerstiens, L.; Thoennissen, G.B.; Bokemeyer, C.; Hengst, K.; Berdel, W.E.; et al. Nicotinamide Treatment Ameliorates the Course of Experimental Colitis Mediated by Enhanced Neutrophil-Specific Antibacterial Clearance. Mol. Nutr. Food Res. 2014, 58, 1474–1490. [Google Scholar] [CrossRef]
- Frye, R.A. Characterization of Five Human CDNAs with Homology to the Yeast SIR2 Gene: Sir2-like Proteins (Sirtuins) Metabolize NAD and May Have Protein ADP-Ribosyltransferase Activity. Biochem. Biophys. Res. Commun. 1999, 260, 273–279. [Google Scholar] [CrossRef]
- Gupte, R.; Liu, Z.; Kraus, W.L. PARPs and ADP-Ribosylation: Recent Advances Linking Molecular Functions to Biological Outcomes. Genes Dev. 2017, 31, 101–126. [Google Scholar] [CrossRef] [PubMed]
- Hwang, E.S.; Song, S.B. Nicotinamide Is an Inhibitor of SIRT1 in Vitro, but Can Be a Stimulator in Cells. Cell. Mol. Life Sci. 2017, 74, 3347–3362. [Google Scholar] [CrossRef] [PubMed]
- Rajman, L.; Chwalek, K.; Sinclair, D.A. Therapeutic Potential of NAD-Boosting Molecules: The In Vivo Evidence. Cell Metab. 2018, 27, 529–547. [Google Scholar] [PubMed]
- Ansari, H.R.; Raghava, G.P. Identification of NAD Interacting Residues in Proteins. BMC Bioinform. 2010, 11, 160. [Google Scholar] [CrossRef]
- Hunt, S.V.; Jamison, A.; Malhotra, R. Oral Nicotinamide for Non-Melanoma Skin Cancers: A Review. Eye 2023, 37, 823–829. [Google Scholar]
- Yiasemides, E.; Sivapirabu, G.; Halliday, G.M.; Park, J.; Damian, D.L. Oral Nicotinamide Protects against Ultraviolet Radiation-Induced Immunosuppression in Humans. Carcinogenesis 2009, 30, 101–105. [Google Scholar] [CrossRef]
- Crinò, A.; Schiaffini, R.; Manfrini, S.; Mesturino, C.; Visalli, N.; Beretta Anguissola, G.; Suraci, C.; Pitocco, D.; Spera, S.; Corbi, S.; et al. A Randomized Trial of Nicotinamide and Vitamin E in Children with Recent Onset Type 1 Diabetes (IMDIAB IX). Eur. J. Endocrinol. 2004, 150, 719–724. [Google Scholar] [CrossRef]
- Pitocco, D.; Crinò, A.; Di Stasio, E.; Manfrini, S.; Guglielmi, C.; Spera, S.; Beretta Anguissola, G.; Visalli, N.; Suraci, C.; Matteoli, M.C.; et al. The Effects of Calcitriol and Nicotinamide on Residual Pancreatic β-Cell Function in Patients with Recent-Onset Type 1 Diabetes (IMDIAB XI). Diabet. Med. 2006, 23, 920–923. [Google Scholar] [CrossRef]
- Pozzilli, P.; Browne, P.D.; Kolb, H. Meta-Analysis of Nicotinamide Treatment in Patients With Recent-Onset IDDM. Diabetes Care 1996, 19, 1357–1363. [Google Scholar]
- Gale, E.A.; European Nicotinamide Diabetes Intervention Trial (ENDIT) Group. European Nicotinamide Diabetes Intervention Trial (ENDIT): A Randomised Controlled Trial of Intervention before the Onset of Type 1 Diabetes. Lancet 2004, 363, 925–931. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nijhuis, L.; Bodelόn, A.; Scholman, R.C.; Houtzager, I.; Sijbers, L.J.P.M.; Mocholi, E.; Picavet, L.W.; Calis, J.J.A.; Mokry, M.; Vastert, S.J.; et al. Nicotinamide Inhibits CD4+ T-Cell Activation and Function. Cells 2025, 14, 560. https://doi.org/10.3390/cells14080560
Nijhuis L, Bodelόn A, Scholman RC, Houtzager I, Sijbers LJPM, Mocholi E, Picavet LW, Calis JJA, Mokry M, Vastert SJ, et al. Nicotinamide Inhibits CD4+ T-Cell Activation and Function. Cells. 2025; 14(8):560. https://doi.org/10.3390/cells14080560
Chicago/Turabian StyleNijhuis, Lotte, Alejandra Bodelόn, Rianne C. Scholman, Isabelle Houtzager, Lyanne J. P. M. Sijbers, Enric Mocholi, Lucas W. Picavet, Jorg J. A. Calis, Michal Mokry, Sebastiaan J. Vastert, and et al. 2025. "Nicotinamide Inhibits CD4+ T-Cell Activation and Function" Cells 14, no. 8: 560. https://doi.org/10.3390/cells14080560
APA StyleNijhuis, L., Bodelόn, A., Scholman, R. C., Houtzager, I., Sijbers, L. J. P. M., Mocholi, E., Picavet, L. W., Calis, J. J. A., Mokry, M., Vastert, S. J., & van Loosdregt, J. (2025). Nicotinamide Inhibits CD4+ T-Cell Activation and Function. Cells, 14(8), 560. https://doi.org/10.3390/cells14080560





