Perivascular Adipocytes’ Adipogenesis Is Defined by Their Anatomical Location in the Descending Thoracic Aorta
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Adipocyte Progenitors Isolation and Culture
2.3. Adipogenesis Experiments
2.4. Angiotensin II and Yoda1 In Vitro Experiments
2.5. RNA Isolation and Purification
2.6. RNA-Seq Analyses
2.7. Enrichment Analysis
2.8. Statistical Analysis
3. Results
3.1. AP and LP Adipocyte Progenitor Cells Exhibit Different Adipogenic Potential
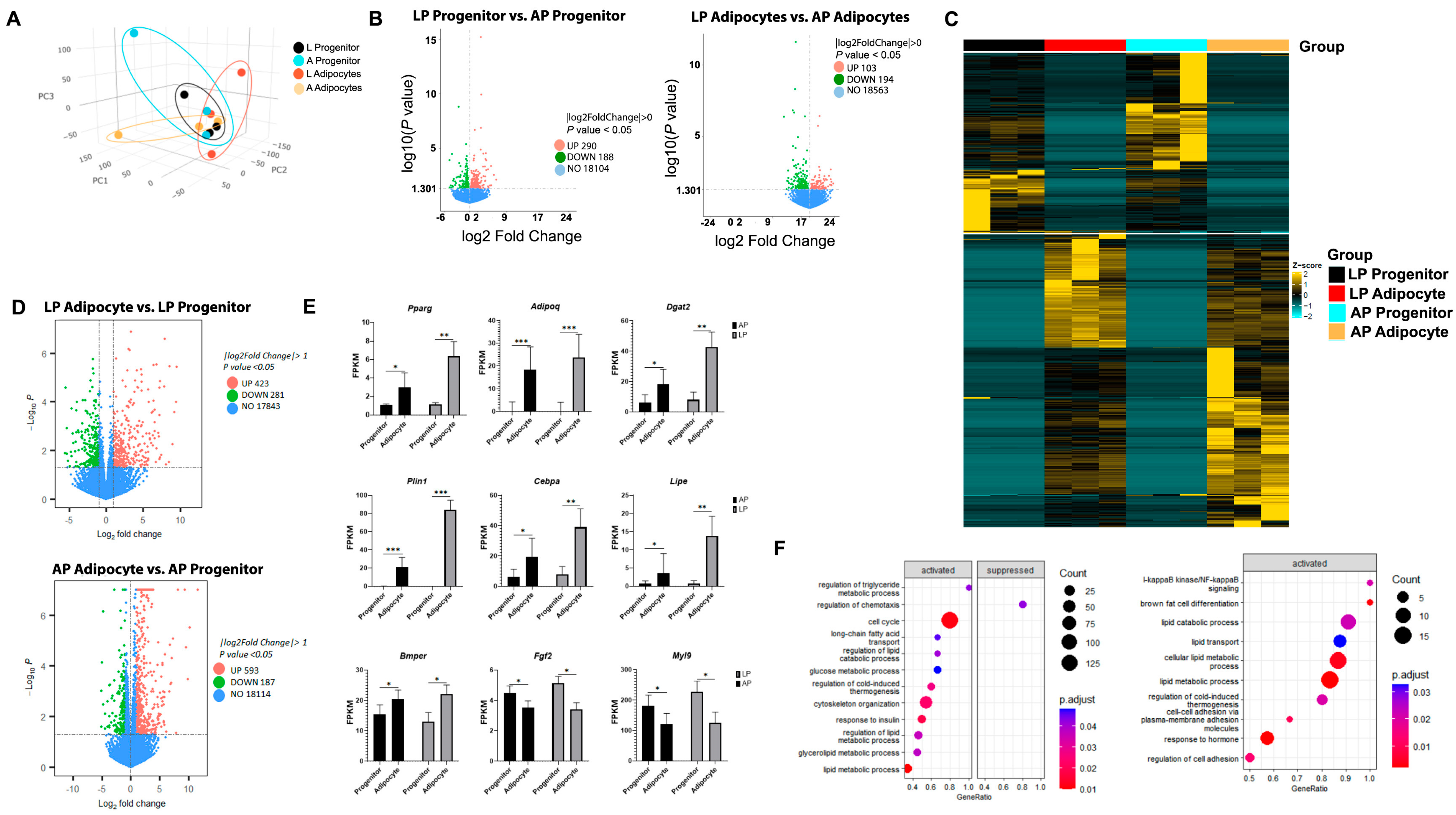
3.2. Thoracic Aorta AP and LP Adipocyte Progenitor Cells and Adipocytes Present Divergent Transcriptome Profiles
3.3. Adipogenesis Activates Specific Gene Pathways in Thoracic Aorta AP and LP Adipocyte Progenitor Cells and Adipocytes
3.4. Adipogenesis Alters Gene Expression of Collagens and Integrins in Thoracic Aorta AP and LP Adipocyte Progenitor Cells and Adipocytes
3.5. Thoracic Aorta AP and LP Adipocytes’ Responses to Angiotensin-II and Mechanosensor Stimulation Differ
4. Discussion
4.1. Thoracic Aorta AP and LP Adipocytes’ Ontogeny Impact Their Function
4.2. AP and LP Adipocytes in the Thoracic Aorta: Transcriptional Differences in ECM Genes
4.3. Thoracic Aorta AP and LP Adipocytes’ Anatomical Location Influences Responses to Vasomodulators
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pearson, A.C.; Guo, R.; Orsinelli, D.A.; Binkley, P.F.; Pasierski, T.J. Transesophageal echocardiographic assessment of the effects of age, gender, and hypertension on thoracic aortic wall size, thickness, and stiffness. Am. Heart J. 1994, 128, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Craiem, D.; Chironi, G.; Casciaro, M.E.; Redheuil, A.; Mousseaux, E.; Simon, A. Three-dimensional evaluation of thoracic aorta enlargement and unfolding in hypertensive men using non-contrast computed tomography. J. Hum. Hypertens. 2013, 27, 504–509. [Google Scholar] [CrossRef]
- Hayashi, K.; Mani, V.; Nemade, A.; Aguiar, S.; Postley, J.E.; Fuster, V.; Fayad, Z.A. Variations in atherosclerosis and remodeling patterns in aorta and carotids. J. Cardiovasc. Magn. Reson. 2010, 12, 10. [Google Scholar] [CrossRef] [PubMed]
- Ye, M.; Ruan, C.-C.; Fu, M.; Xu, L.; Chen, D.; Zhu, M.; Zhu, D.; Gao, P. Developmental and functional characteristics of the thoracic aorta perivascular adipocyte. Cell. Mol. Life Sci. 2018, 76, 777–789. [Google Scholar] [CrossRef]
- Vargas, D.; López, C.; Acero, E.; Benitez, E.; Wintaco, A.; Camacho, J.; Carreño, M.; Umaña, J.; Jimenez, D.; Díaz, S.; et al. Thermogenic capacity of human periaortic adipose tissue is transformed by body weight. PLoS ONE 2018, 13, e0194269. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Chiba, S.; Moriwaki, C.; Kitamura, H.; Ina, K.; Aosa, T.; Tomonari, K.; Gotoh, K.; Masaki, T.; Katsuragi, I.; et al. A Clinical Approach to Brown Adipose Tissue in the Para-Aortic Area of the Human Thorax. PLoS ONE 2015, 10, e0122594. [Google Scholar] [CrossRef]
- Tran, K.-V.; Fitzgibbons, T.; Min, S.Y.; DeSouza, T.; Corvera, S. Distinct adipocyte progenitor cells are associated with regional phenotypes of perivascular aortic fat in mice. Mol. Metab. 2018, 9, 199–206. [Google Scholar] [CrossRef]
- Ghaben, A.L.; Scherer, P.E. Adipogenesis and metabolic health. Nat. Rev. Mol. Cell Biol. 2019, 20, 242–258. [Google Scholar] [CrossRef]
- Saxton, S.; Ryding, K.; Withers, S.; Heagerty, A. Beta3-adrenoceptor mediated adiponectin secretion is essential in modulation of vascular tone by perivascular adipose tissue. J. Hypertens. 2018, 36, e26. [Google Scholar] [CrossRef]
- US Nuclear Regulatory Commission. Committee for the Update of the Guide for the Care and Use of Laboratory Animals. Guide for the Care and Use of Laboratory Animals, 8th ed.; The National Academies Press: Cambridge, MA, USA, 2011. [Google Scholar]
- Myers, M.N.; Abou-Rjeileh, U.; Chirivi, M.; Parales-Girón, J.; Lock, A.L.; Tam, J.; Zachut, M.; Contreras, G.A. Cannabinoid-1 receptor activation modulates lipid mobilization and adipogenesis in the adipose tissue of dairy cows. J. Dairy Sci. 2023, 106, 3650–3661. [Google Scholar] [CrossRef]
- Rendon, C.J.; Flood, E.; Thompson, J.M.; Chirivi, M.; Watts, S.W.; Contreras, G.A. PIEZO1 mechanoreceptor activation reduces adipogenesis in perivascular adipose tissue preadipocytes. Front. Endocrinol. 2022, 13, 995499. [Google Scholar] [CrossRef]
- Tieu, B.C.; Ju, X.; Lee, C.; Sun, H.; Lejeune, W.; Recinos, I.A.; Brasier, A.R.; Tilton, R.G. Aortic adventitial fibroblasts participate in angiotensin-induced vascular wall inflammation and remodeling. J. Vasc. Res. 2010, 48, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef]
- Liao, Y.; Smyth, G.K.; Shi, W. feature Counts: An efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 2014, 30, 923–930. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Wu, T.; Hu, E.; Xu, S.; Chen, M.; Guo, P.; Dai, Z.; Feng, T.; Zhou, L.; Tang, W.; Zhan, L.; et al. clusterProfiler 4.0: A universal enrichment tool for interpreting omics data. Innovation 2021, 2, 100141. [Google Scholar] [CrossRef] [PubMed]
- Tchkonia, T.; Thomou, T.; Zhu, Y.; Karagiannides, I.; Pothoulakis, C.; Jensen, M.D.; Kirkland, J.L. Mechanisms and metabolic implications of regional differences among fat depots. Cell Metab. 2013, 17, 644–656. [Google Scholar] [CrossRef]
- Li, L.; Miano, J.M.; Cserjesi, P.; Olson, E.N. SM22 alpha, a marker of adult smooth muscle, is expressed in multiple myogenic lineages during embryogenesis. Circ. Res. 1996, 78, 188–195. [Google Scholar] [CrossRef]
- Elsafadi, M.; Manikandan, M.; Dawud, R.A.; Alajez, N.M.; Hamam, R.; Alfayez, M.; Kassem, M.; Aldahmash, A.; Mahmood, A. Transgelin is a TGFβ-inducible gene that regulates osteoblastic and adipogenic differentiation of human skeletal stem cells through actin cytoskeleston organization. Cell Death Dis. 2016, 7, e2321. [Google Scholar] [CrossRef]
- Chang, L.; Villacorta, L.; Li, R.; Hamblin, M.; Xu, W.; Dou, C.; Zhang, J.; Wu, J.; Zeng, R.; Chen, Y.E. Loss of perivascular adipose tissue on peroxisome proliferator-activated receptor-γ deletion in smooth muscle cells impairs intravascular thermoregulation and enhances atherosclerosis. Circulation 2012, 126, 1067–1078. [Google Scholar] [CrossRef]
- Brinkley, T.E.; Leng, X.; Chughtai, H.L.; Nicklas, B.J.; Kritchevsky, S.B.; Ding, J.; Kitzman, D.W.; Hundley, W.G. Periaortic fat and cardiovascular risk: A comparison of high-risk older adults and age-matched healthy controls. Int. J. Obes. 2014, 38, 1397–1402. [Google Scholar] [CrossRef] [PubMed]
- Burl, R.B.; Rondini, E.A.; Wei, H.; Pique-Regi, R.; Granneman, J.G. Deconstructing cold-induced brown adipocyte neogenesis in mice. eLife 2022, 11, 80167. [Google Scholar] [CrossRef]
- Rendon, C.J.; Sempere, L.; Lauver, A.; Watts, S.W.; Contreras, G.A. Anatomical location, sex, and age modulate adipocyte progenitor populations in perivascular adipose tissues. Front. Physiol. 2024, 15, 1411218. [Google Scholar] [CrossRef]
- Garritson, J.D.; Zhang, J.; Achenbach, A.; Ferhat, M.; Eich, E.; Stubben, C.J.; Martinez, P.L.; Ibele, A.R.; Hilgendorf, K.I.; Boudina, S. BMPER is a marker of adipose progenitors and adipocytes and a positive modulator of adipogenesis. Commun. Biol. 2023, 6, 1–13. [Google Scholar] [CrossRef]
- Uezumi, A.; Ito, T.; Morikawa, D.; Shimizu, N.; Yoneda, T.; Segawa, M.; Yamaguchi, M.; Ogawa, R.; Matev, M.M.; Miyagoe-Suzuki, Y.; et al. Fibrosis and adipogenesis originate from a common mesenchymal progenitor in skeletal muscle. J. Cell Sci. 2011, 124 Pt 21, 3654–3664. [Google Scholar] [CrossRef] [PubMed]
- Watts, S.W.; Thompson, J.M.; Bhattacharya, S.; Panda, V.; Terrian, L.; Contreras, A.; Nault, R. Integrins play a role in stress relaxation of perivascular adipose tissue. Pharmacol. Res. 2024, 206, 107269. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Tintut, Y.; Demer, L.L.; Vazquez-Padron, R.I.; Bendeck, M.P.; Hsu, J.J. Collagen VIII in vascular diseases. Matrix Biol. 2024, 133, 64–76. [Google Scholar] [CrossRef]
- Buras, E.D.; Woo, M.-S.; Verma, R.K.; Kondisetti, S.H.; Davis, C.S.; Claflin, D.R.; Converso-Baran, K.; Michele, D.E.; Brooks, S.V.; Chun, T.-H. Thrombospondin-1 promotes fibro-adipogenic stromal expansion and contractile dysfunction of the diaphragm in obesity. J. Clin. Investig. 2024, 9, 175047. [Google Scholar] [CrossRef]
- Kong, P.; Gonzalez-Quesada, C.; Li, N.; Cavalera, M.; Lee, D.-W.; Frangogiannis, N.G. Thrombospondin-1 regulates adiposity and metabolic dysfunction in diet-induced obesity enhancing adipose inflammation and stimulating adipocyte proliferation. Am. J. Physiol. Endocrinol. Metab. 2013, 305, E439–E450. [Google Scholar] [CrossRef]
- Gutierrez, L.S.; Gutierrez, J. Thrombospondin 1 in Metabolic Diseases. Front. Endocrinol. 2021, 12, 638536. [Google Scholar] [CrossRef]
- Wilson, C.; Thompson, J.M.; Terrian, L.; Lauver, A.D.; Flood, E.D.; Fink, G.D.; Sather, L.; Bhattacharya, S.; Contreras, G.A.; Watts, S.W. Perivascular Adipose Tissue Remodels Only after Elevation of Blood Pressure in the Dahl SS Rat Fed a High-Fat Diet. J. Vasc. Res. 2023, 61, 26–37. [Google Scholar] [CrossRef]
- Morandi, E.M.; Verstappen, R.; Zwierzina, M.E.; Geley, S.; Pierer, G.; Ploner, C. ITGAV and ITGA5 diversely regulate proliferation and adipogenic differentiation of human adipose derived stem cells. Sci. Rep. 2016, 6, 28889. [Google Scholar] [CrossRef] [PubMed]
- Villa-Diaz, L.G.; Kim, J.K.; Laperle, A.; Palecek, S.P.; Krebsbach, P.H. Inhibition of Focal Adhesion Kinase Signaling by Integrin α6β1 Supports Human Pluripotent Stem Cell Self-Renewal. Stem Cells 2016, 34, 1753–1764. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Ojeda, F.J.; Wang, J.; Bäcker, T.; Krueger, M.; Zamani, S.; Rosowski, S.; Gruber, T.; Onogi, Y.; Feuchtinger, A.; Schulz, T.J.; et al. Active integrins regulate white adipose tissue insulin sensitivity and brown fat thermogenesis. Mol. Metab. 2021, 45, 101147. [Google Scholar] [CrossRef] [PubMed]
- Reggio, S.; Rouault, C.; Poitou, C.; Bichet, J.-C.; Prifti, E.; Bouillot, J.-L.; Rizkalla, S.; Lacasa, D.; Tordjman, J.; Clément, K. Increased Basement Membrane Components in Adipose Tissue During Obesity: Links with TGFβ and Metabolic Phenotypes. J. Clin. Endocrinol. Metab. 2016, 101, 2578–2587. [Google Scholar] [CrossRef]
- Li, Y.; Wang, W.; Liu, Z.; Liu, G.; Li, X. Blockade of ITGA2/3/5 Promotes Adipogenic Differentiation of Human Adipose-derived Mesenchymal Stem Cells. Cell Biochem. Biophys. 2024, 83, 1105–1111. [Google Scholar] [CrossRef]
- Thelen, K.; Ayala-Lopez, N.; Watts, S.W.; Contreras, G.A. Expansion and Adipogenesis Induction of Adipocyte Progenitors from Perivascular Adipose Tissue Isolated by Magnetic Activated Cell Sorting. J. Vis. Exp. 2017, 124, 55818. [Google Scholar]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Contreras, G.A.; Rendon, C.J.; Shadowens, A.; Chirivi, M.; Salcedo-Tacuma, D.; Lauver, D.A.; Watts, S.W. Perivascular Adipocytes’ Adipogenesis Is Defined by Their Anatomical Location in the Descending Thoracic Aorta. Cells 2025, 14, 579. https://doi.org/10.3390/cells14080579
Contreras GA, Rendon CJ, Shadowens A, Chirivi M, Salcedo-Tacuma D, Lauver DA, Watts SW. Perivascular Adipocytes’ Adipogenesis Is Defined by Their Anatomical Location in the Descending Thoracic Aorta. Cells. 2025; 14(8):579. https://doi.org/10.3390/cells14080579
Chicago/Turabian StyleContreras, G. Andres, C. Javier Rendon, Alyssa Shadowens, Miguel Chirivi, David Salcedo-Tacuma, D. Adam Lauver, and Stephanie W. Watts. 2025. "Perivascular Adipocytes’ Adipogenesis Is Defined by Their Anatomical Location in the Descending Thoracic Aorta" Cells 14, no. 8: 579. https://doi.org/10.3390/cells14080579
APA StyleContreras, G. A., Rendon, C. J., Shadowens, A., Chirivi, M., Salcedo-Tacuma, D., Lauver, D. A., & Watts, S. W. (2025). Perivascular Adipocytes’ Adipogenesis Is Defined by Their Anatomical Location in the Descending Thoracic Aorta. Cells, 14(8), 579. https://doi.org/10.3390/cells14080579







