Novel Thyroid Hormone Receptor-β Agonist TG68 Exerts Anti-Inflammatory, Lipid-Lowering and Anxiolytic Effects in a High-Fat Diet (HFD) Mouse Model of Obesity
Abstract
:1. Introduction
2. Materials and Methods
2.1. In Vitro Study
2.1.1. Cell Cultures and Reagents
2.1.2. MTT Assay for Cell Viability
2.1.3. Inflammatory Response in LPS/TNFα-Treated HMC3 Cells
2.1.4. Inflammatory Response in HMC3 Cells Exposed to Aβ25–35
2.2. In Vivo Study
2.2.1. Animals
2.2.2. Biochemical Testing and Tissues Analysis
- Analysis of serum triglycerides, cholesterol, and glucose
- Gene expression analysis
- Inflammatory Response in HFD mice
2.2.3. Behavioral Testing
2.3. Statistical Analysis
3. Results
3.1. In Vitro Experiments
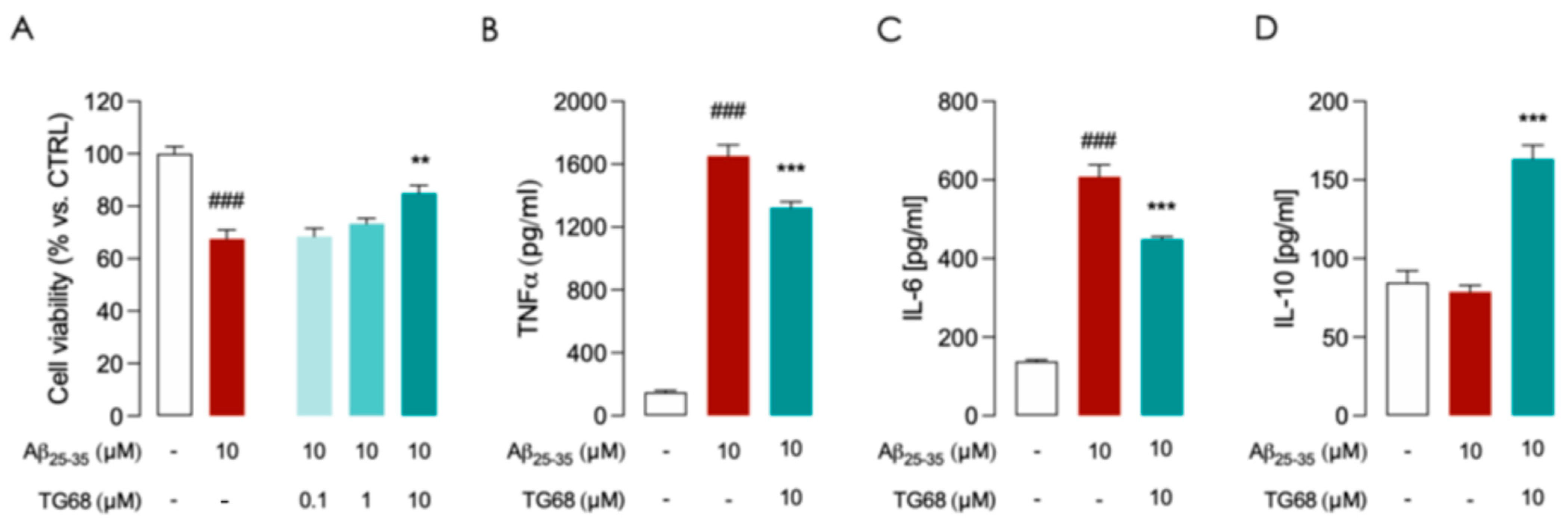
TG68 Prevents LPS/TNFα and Aβ-Induced Inflammatory Activation in HMC3 Cells
3.2. In Vivo Experiments
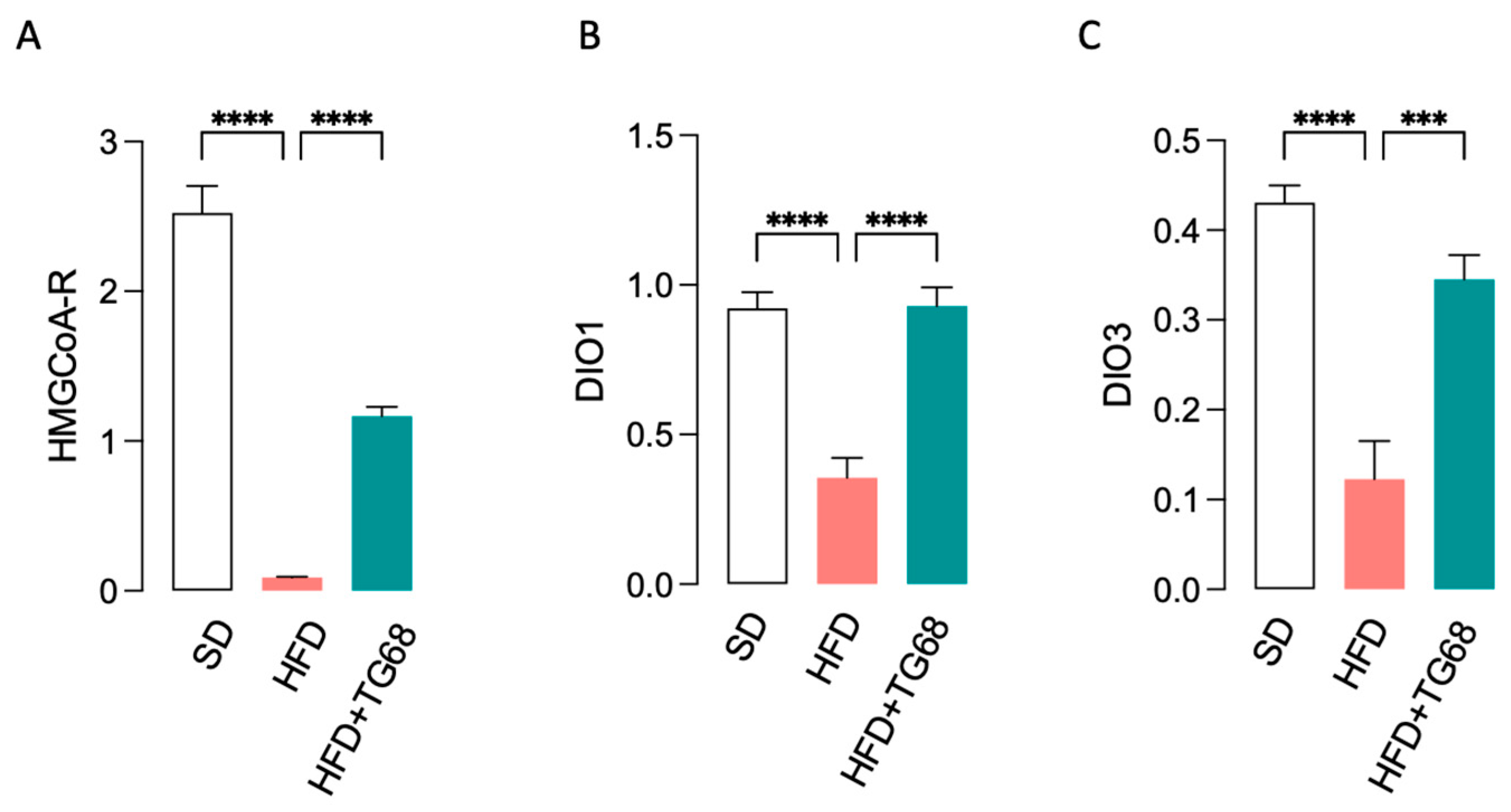
3.2.1. In Diet-Induced Obese Mice TG68 Treatment Causes a Significant Reduction in Body Weight (BW) and Blood Lipids and Glucose Levels
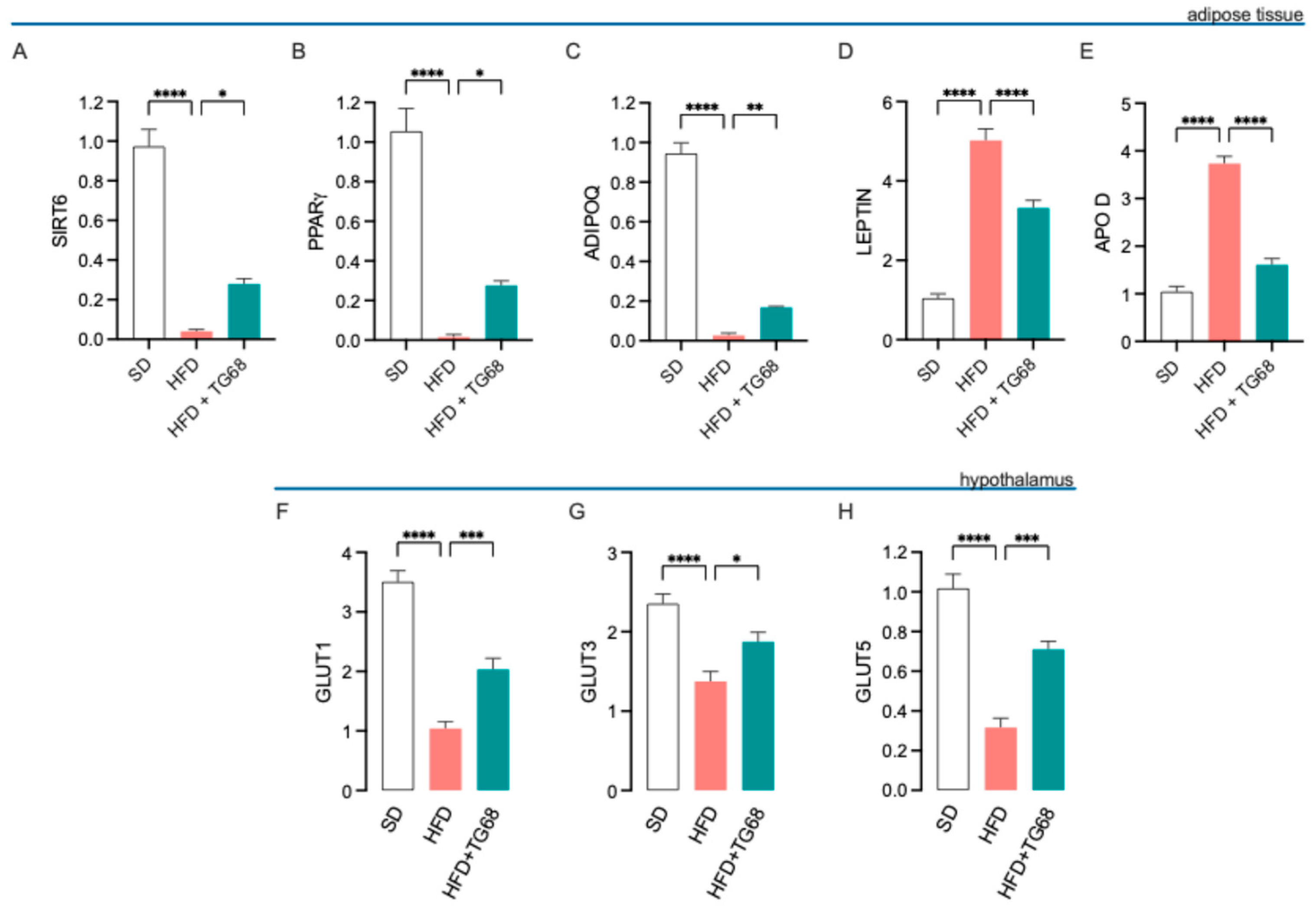
3.2.2. TG68 Counteracts Obesity-Induced Transcriptional Changes in Adipose Tissue and Hypothalamus
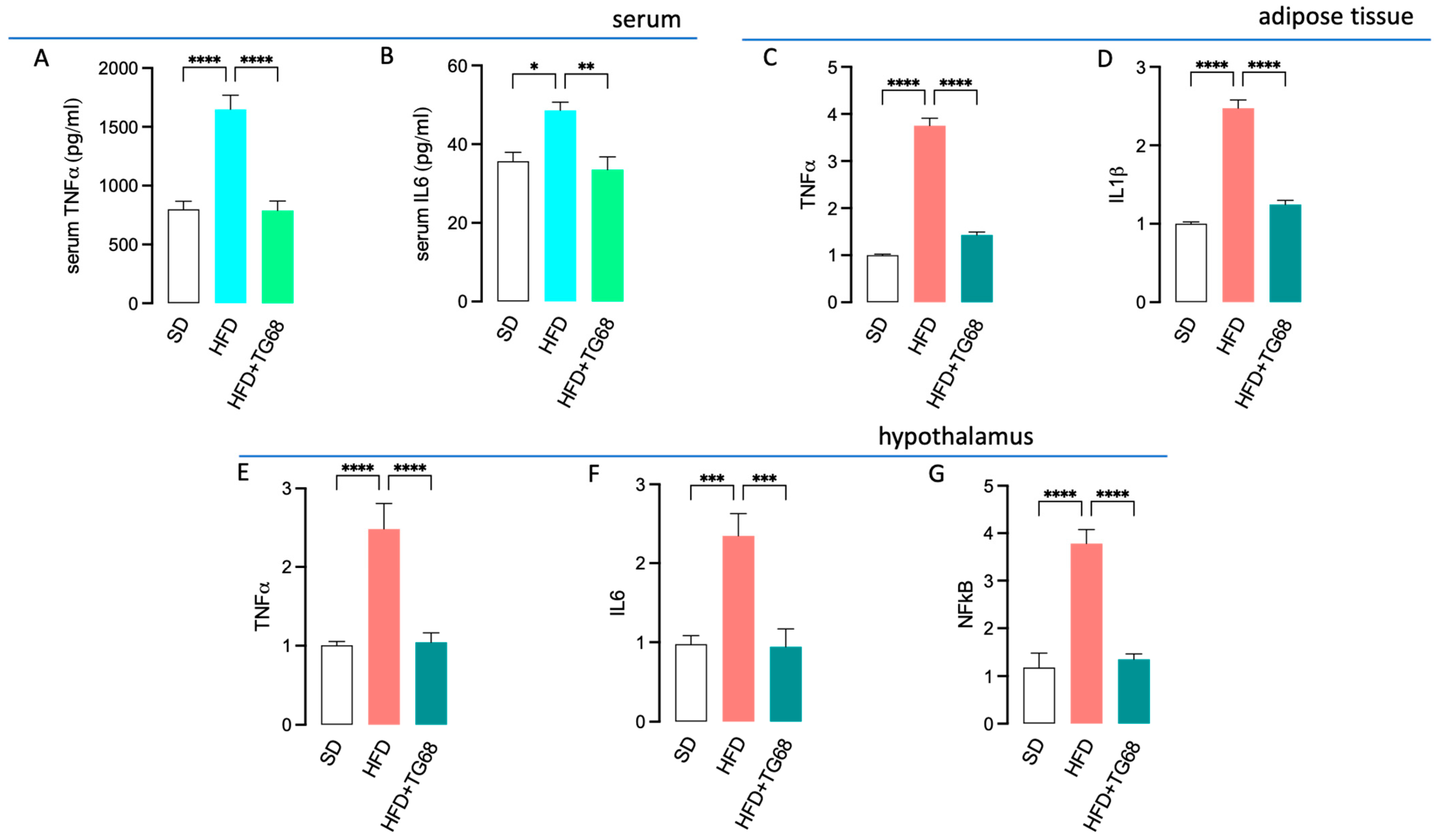
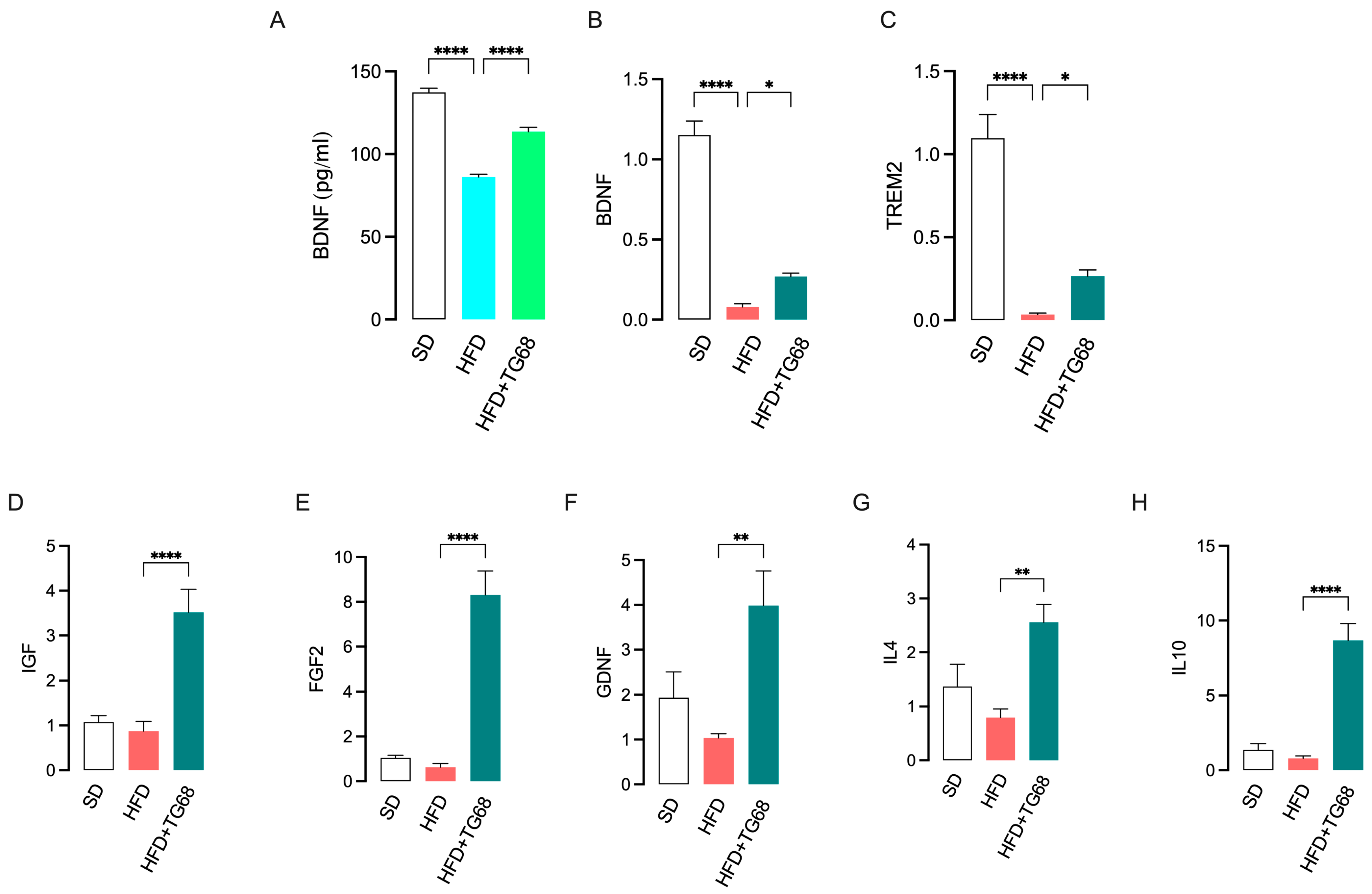
3.2.3. TG68 Limits Systemic Inflammation and Attenuates the Transcriptional Changes Induced by HFD on the Hypothalamus of Obese Mice
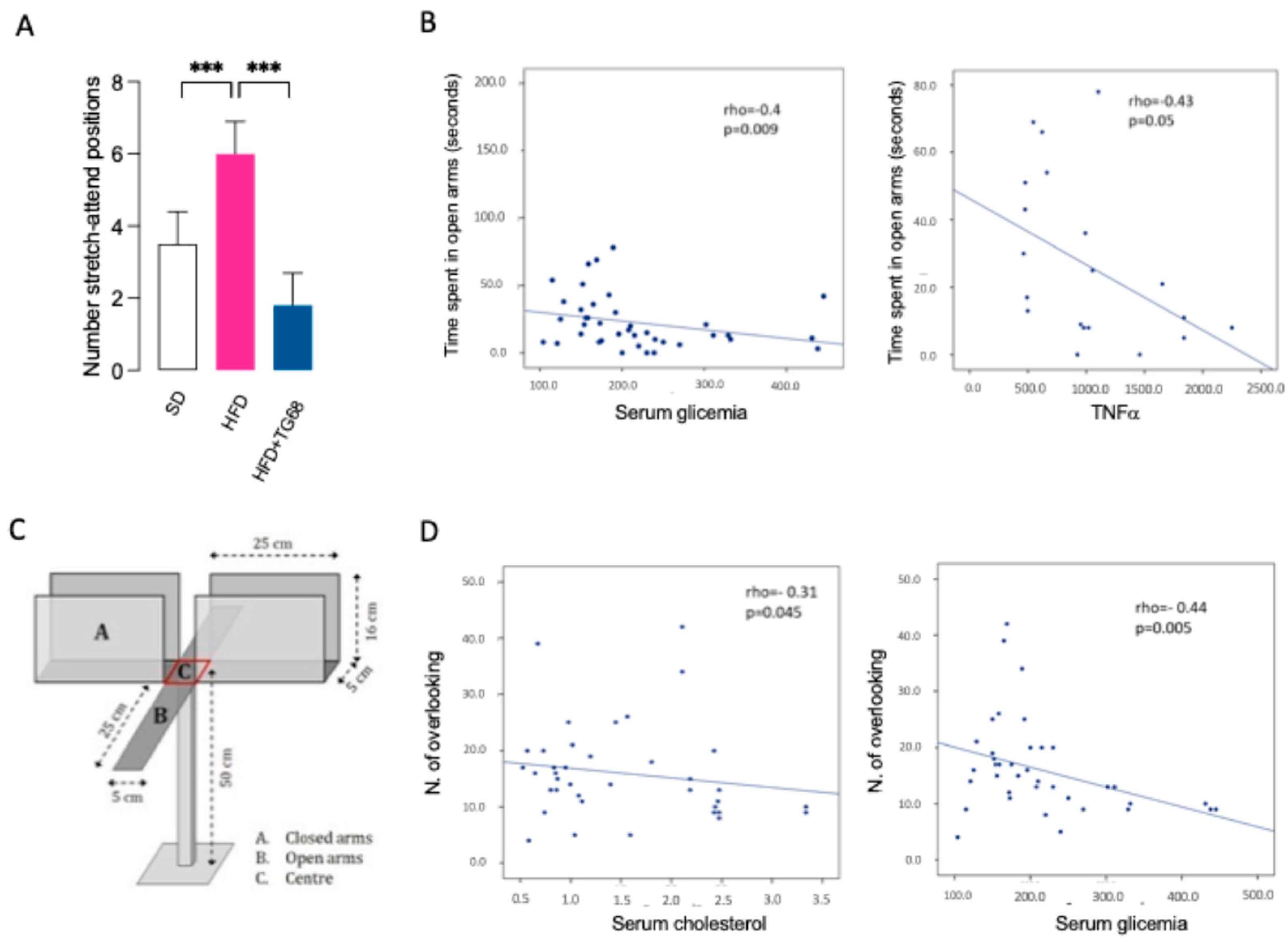
3.2.4. TG68 Improves Anxiety-like Behavior Induced by HFD
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Locke, A.E.; Kahali, B.; Berndt, S.I.; Justice, A.E.; Pers, T.H.; Day, F.R.; Powell, C.; Vedantam, S.; Buchkovich, M.L.; Yang, J.; et al. Genetic studies of body mass index yield new insights for obesity biology. Nature 2015, 518, 197–206. [Google Scholar] [CrossRef]
- Kueck, P.J.; Morris, J.K.; Stanford, J.A. Current Perspectives: Obesity and Neurodegeneration—Links and Risks. Degener. Neurol. Neuromuscul. Dis. 2023, 13, 111–129. [Google Scholar] [CrossRef]
- Tobeh, N.S.; Bruce, K.D. Emerging Alzheimer’s disease therapeutics: Promising insights from lipid metabolism and microglia-focused interventions. Front. Aging Neurosci. 2023, 15, 1259012. [Google Scholar] [CrossRef] [PubMed]
- Baechle, J.J.; Chen, N.; Makhijani, P.; Winer, S.; Furman, D.; Winer, D.A. Chronic inflammation and the hallmarks of aging. Mol. Metab. 2023, 74, 101755. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Zhang, D.; Zeng, Y.; Huang, T.Y.; Xu, H.; Zhao, Y. Molecular and cellular mechanisms underlying the pathogenesis of Alzheimer’s disease. Mol. Neurodegener. 2020, 15, 40. [Google Scholar] [CrossRef]
- Song, M.; Bai, Y.; Song, F. High-fat diet and neuroinflammation: The role of mitochondria. Pharmacol. Res. 2025, 212, 107615. [Google Scholar] [CrossRef] [PubMed]
- Le Moli, R.; Vella, V.; Tumino, D.; Piticchio, T.; Naselli, A.; Belfiore, A.; Frasca, F. Inflammasome activation as a link between obesity and thyroid disorders: Implications for an integrated clinical management. Front. Endocrinol. 2022, 13, 959276. [Google Scholar] [CrossRef]
- Yen, P.M. Physiological and Molecular Basis of Thyroid Hormone Action. Physiol. Rev. 2001, 81, 1097–1142. [Google Scholar] [CrossRef]
- Sepúlveda, P.; Ferreira, A.F.F.; Sandoval, C.; Bergoc, G.; Moreno, A.C.R.; Nunes, M.T.; Torrão, A.D.S. Thyroid Hormone Supplementation Restores Cognitive Deficit, Insulin Signaling, and Neuroinflammation in the Hippocampus of a Sporadic Alzheimer’s-like Disease Rat Model. Cells 2024, 13, 1793. [Google Scholar] [CrossRef]
- Cooke, G.E.; Mullally, S.; Correia, N.; O’Mara, S.M.; Gibney, J. Hippocampal Volume Is Decreased in Adults with Hypothyroidism. Thyroid 2014, 24, 433–440. [Google Scholar] [CrossRef]
- Lasa, M.; Contreras-Jurado, C. Thyroid hormones act as modulators of inflammation through their nuclear receptors. Front. Endocrinol. 2022, 13, 937099. [Google Scholar] [CrossRef] [PubMed]
- Chamas, L.; Seugnet, I.; Poirier, R.; Clerget-Froidevaux, M.-S.; Enderlin, V. A Fine Regulation of the Hippocampal Thyroid Signalling Protects Hypothyroid Mice against Glial Cell Activation. Int. J. Mol. Sci. 2022, 23, 11938. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.-T.; Yen, T.-L.; Chen, Y.-H.; Jan, J.-S.; Teng, R.-D.; Yang, C.-H.; Sun, J.-M. Aging-Associated Thyroid Dysfunction Contributes to Oxidative Stress and Worsened Functional Outcomes Following Traumatic Brain Injury. Antioxidants 2023, 12, 217. [Google Scholar] [CrossRef] [PubMed]
- Głombik, K.; Detka, J.; Bobula, B.; Bąk, J.; Kusek, M.; Tokarski, K.; Budziszewska, B. Contribution of Hypothyroidism to Cognitive Impairment and Hippocampal Synaptic Plasticity Regulation in an Animal Model of Depression. Int. J. Mol. Sci. 2021, 22, 1599. [Google Scholar] [CrossRef]
- Bavarsad, K.; Hosseini, M.; Hadjzadeh, M.; Sahebkar, A. The effects of thyroid hormones on memory impairment and Alzheimer’s disease. J. Cell. Physiol. 2019, 234, 14633–14640. [Google Scholar] [CrossRef]
- Chaalal, A.; Poirier, R.; Blum, D.; Laroche, S.; Enderlin, V. Thyroid Hormone Supplementation Restores Spatial Memory, Hippocampal Markers of Neuroinflammation, Plasticity-Related Signaling Molecules, and β-Amyloid Peptide Load in Hypothyroid Rats. Mol. Neurobiol. 2019, 56, 722–735. [Google Scholar] [CrossRef]
- AlAnazi, F.H.; Al-kuraishy, H.M.; Alexiou, A.; Papadakis, M.; Ashour, M.H.M.; Alnaaim, S.A.; Elhussieny, O.; Saad, H.M.; Batiha, G.E.-S. Primary Hypothyroidism and Alzheimer’s Disease: A Tale of Two. Cell Mol. Neurobiol. 2023, 43, 3405–3416. [Google Scholar] [CrossRef]
- Lazar, M.A. Thyroid Hormone Receptors: Multiple Forms, Multiple Possibilities*. Endocr. Rev. 1993, 14, 184–193. [Google Scholar] [CrossRef]
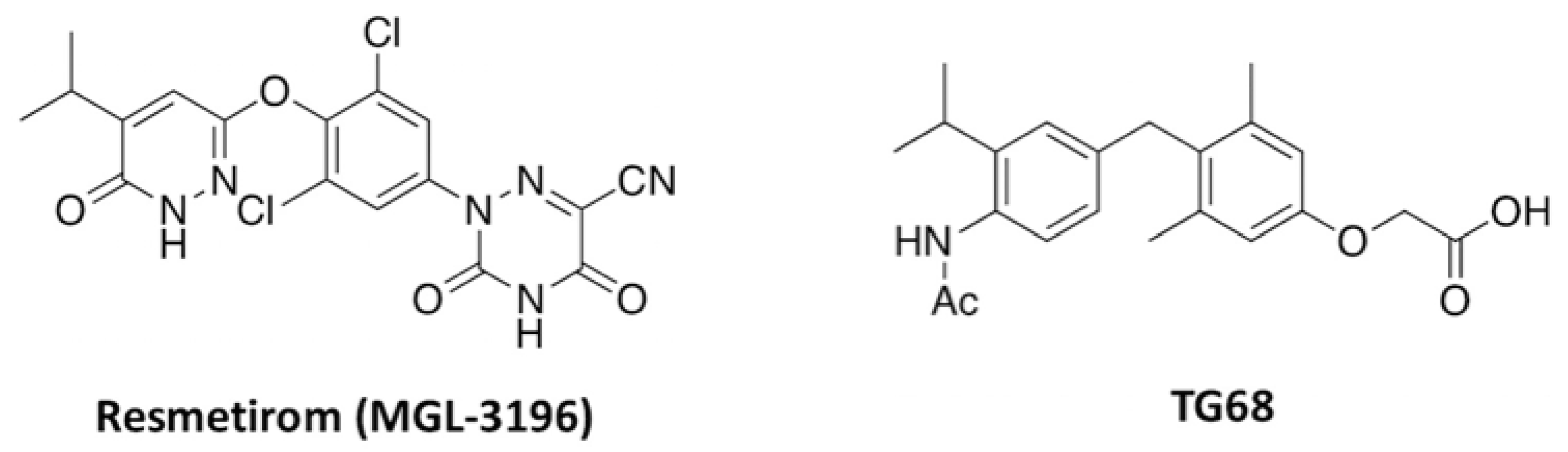
- Saponaro, F.; Sestito, S.; Runfola, M.; Rapposelli, S.; Chiellini, G. Selective Thyroid Hormone Receptor-Beta (TRβ) Agonists: New Perspectives for the Treatment of Metabolic and Neurodegenerative Disorders. Front. Med. 2020, 7, 331. [Google Scholar] [CrossRef]
- Zucchi, R. Thyroid Hormone Analogues: An Update. Thyroid 2020, 30, 1099–1105. [Google Scholar] [CrossRef]
- Moreno, M.; De Lange, P.; Lombardi, A.; Silvestri, E.; Lanni, A.; Goglia, F. Metabolic Effects of Thyroid Hormone Derivatives. Thyroid 2008, 18, 239–253. [Google Scholar] [CrossRef]
- Columbano, A.; Chiellini, G.; Kowalik, M.A. GC-1: A Thyromimetic With Multiple Therapeutic Applications in Liver Disease. Gene Expr. 2017, 17, 265–275. [Google Scholar] [CrossRef]
- Jakobsson, T.; Vedin, L.-L.; Parini, P. Potential Role of Thyroid Receptor β Agonists in the Treatment of Hyperlipidemia. Drugs 2017, 77, 1613–1621. [Google Scholar] [CrossRef] [PubMed]
- Runfola, M.; Sestito, S.; Bellusci, L.; La Pietra, V.; D’Amore, V.M.; Kowalik, M.A.; Chiellini, G.; Gul, S.; Perra, A.; Columbano, A.; et al. Design, synthesis and biological evaluation of novel TRβ selective agonists sustained by ADME-toxicity analysis. Eur. J. Med. Chem. 2020, 188, 112006. [Google Scholar] [CrossRef] [PubMed]
- Caddeo, A.; Kowalik, M.A.; Serra, M.; Runfola, M.; Bacci, A.; Rapposelli, S.; Columbano, A.; Perra, A. TG68, a Novel Thyroid Hormone Receptor-β Agonist for the Treatment of NAFLD. Int. J. Mol. Sci. 2021, 22, 13105. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Xie, H.; Shan, H.; Zheng, Z.; Li, G.; Li, M.; Hong, L. Development of Thyroid Hormones and Synthetic Thyromimetics in Non-Alcoholic Fatty Liver Disease. Int. J. Mol. Sci. 2022, 23, 1102. [Google Scholar] [CrossRef]
- Kuchay, M.S.; Isaacs, S.; Misra, A. Intrahepatic hypothyroidism in MASLD: Role of liver-specific thyromimetics including resmetirom. Diabetes Metab. Syndr. Clin. Res. Rev. 2024, 18, 103034. [Google Scholar] [CrossRef]
- Harrison, S.A.; Bedossa, P.; Guy, C.D.; Schattenberg, J.M.; Loomba, R.; Taub, R.; Labriola, D.; Moussa, S.E.; Neff, G.W.; Rinella, M.E.; et al. A Phase 3, Randomized, Controlled Trial of Resmetirom in NASH with Liver Fibrosis. N. Engl. J. Med. 2024, 390, 497–509. [Google Scholar] [CrossRef]
- Lazarus, J.V.; Ivancovsky Wajcman, D.; Mark, H.E.; Younossi, Z.M.; Kopka, C.J.; Cohen, N.; Bansal, M.B.; Betel, M.; Brennan, P.N. Opportunities and challenges following approval of resmetirom for MASH liver disease. Nat. Med. 2024, 30, 3402–3405. [Google Scholar] [CrossRef]
- Scanlan, T.S.; Yoshihara, H.A.; Nguyen, N.H.; Chiellini, G. Selective thyromimetics: Tissue-selective thyroid hormone analogs. Curr. Opin. Drug Discov. Devel. 2001, 4, 614–622. [Google Scholar]
- Baldassarro, V.A.; Quadalti, C.; Runfola, M.; Manera, C.; Rapposelli, S.; Calzà, L. Synthetic Thyroid Hormone Receptor-β Agonists Promote Oligodendrocyte Precursor Cell Differentiation in the Presence of Inflammatory Challenges. Pharmaceuticals 2023, 16, 1207. [Google Scholar] [CrossRef]
- Kim, B.; Ko, Y.H.; Runfola, M.; Rapposelli, S.; Ortore, G.; Chiellini, G.; Kim, J.H. Diphenyl-Methane Based Thyromimetic Inhibitors for Transthyretin Amyloidosis. Int. J. Mol. Sci. 2021, 22, 3488. [Google Scholar] [CrossRef] [PubMed]
- Asano, Y. Chaacteristics of Open Field Behavior of Wistar and Sprague-Dawley Rats. Exp. Anim. 1986, 35, 505–508. [Google Scholar] [CrossRef] [PubMed]
- Fruh, S.M. Obesity: Risk factors, complications, and strategies for sustainable long-term weight management. J. Am. Assoc. Nurse Pract. 2017, 29, S3–S14. [Google Scholar] [CrossRef] [PubMed]
- Block, M.L.; Zecca, L.; Hong, J.-S. Microglia-mediated neurotoxicity: Uncovering the molecular mechanisms. Nat. Rev. Neurosci. 2007, 8, 57–69. [Google Scholar] [CrossRef]
- Pérez, S.; Rius-Pérez, S. Macrophage Polarization and Reprogramming in Acute Inflammation: A Redox Perspective. Antioxidants 2022, 11, 1394. [Google Scholar] [CrossRef]
- Cai, Z.; Hussain, M.D.; Yan, L.-J. Microglia, neuroinflammation, and beta-amyloid protein in Alzheimer’s disease. Int. J. Neurosci. 2014, 124, 307–321. [Google Scholar] [CrossRef]
- Shi, S.; Liang, D.; Chen, Y.; Xie, Y.; Wang, Y.; Wang, L.; Wang, Z.; Qiao, Z. Gx-50 reduces β-amyloid-induced TNF-α, IL-1β, NO, and PGE2 expression and inhibits NF-κB signaling in a mouse model of Alzheimer’s disease. Eur. J. Immunol. 2016, 46, 665–676. [Google Scholar] [CrossRef]
- De A Boleti, A.P.; De O Cardoso, P.H.; Frihling, B.E.F.; Silva, P.S.E.; De Moraes, L.F.R.N.; Migliolo, L. Adipose tissue, systematic inflammation, and neurodegenerative diseases. Neural Regen. Res. 2023, 18, 38. [Google Scholar] [CrossRef]
- Friesen, J.A.; Rodwell, V.W. The 3-hydroxy-3-methylglutaryl coenzyme-A (HMG-CoA) reductases. Genome Biol. 2004, 5, 248. [Google Scholar] [CrossRef]
- Wu, M.; Liao, M.; Huang, R.; Chen, C.; Tian, T.; Wang, H.; Li, J.; Li, J.; Sun, Y.; Wu, C.; et al. Hippocampal overexpression of TREM2 ameliorates high fat diet induced cognitive impairment and modulates phenotypic polarization of the microglia. Genes. Dis. 2020, 9, 401–414. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xu, H.; Wang, H.; Yang, K.; Luan, J.; Wang, S. TREM2: Potential therapeutic targeting of microglia for Alzheimer’s disease. Biomed. Pharmacother. 2023, 165, 115218. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, S.; Pallati, P.K.; Rai, V.; Sharma, P.; Agrawal, D.K.; Nandipati, K.C. Increased expression of triggering receptor expressed on myeloid cells-1 in the population with obesity and insulin resistance. Obesity 2017, 25, 527–538. [Google Scholar] [CrossRef]
- Ferrara, S.J.; Chaudhary, P.; DeBell, M.J.; Marracci, G.; Miller, H.; Calkins, E.; Pocius, E.; Napier, B.A.; Emery, B.; Bourdette, D.; et al. TREM2 is thyroid hormone regulated making the TREM2 pathway druggable with ligands for thyroid hormone receptor. Cell Chem. Biol. 2022, 29, 239–248.e4. [Google Scholar] [CrossRef]
- Wiens, K.R.; Wasti, N.; Ulloa, O.O.; Klegeris, A. Diversity of Microglia-Derived Molecules with Neurotrophic Properties That Support Neurons in the Central Nervous System and Other Tissues. Molecules 2024, 29, 5525. [Google Scholar] [CrossRef]
- Jurga, A.M.; Paleczna, M.; Kuter, K.Z. Overview of General and Discriminating Markers of Differential Microglia Phenotypes. Front. Cell. Neurosci. 2020, 14, 198. [Google Scholar] [CrossRef]
- Li, Y.; Cheng, Y.; Zhou, Y.; Du, H.; Zhang, C.; Zhao, Z.; Chen, Y.; Zhou, Z.; Mei, J.; Wu, W.; et al. High fat diet-induced obesity leads to depressive and anxiety-like behaviors in mice via AMPK/mTOR-mediated autophagy. Exp. Neurol. 2022, 348, 113949. [Google Scholar] [CrossRef] [PubMed]
- Seibenhener, M.L.; Wooten, M.C. Use of the Open Field Maze to Measure Locomotor and Anxiety-like Behavior in Mice. JoVE 2015, 96, 52434. [Google Scholar] [CrossRef]
- Holly, K.S.; Orndorff, C.O.; Murray, T.A. MATSAP: An automated analysis of stretch-attend posture in rodent behavioral experiments. Sci. Rep. 2016, 6, 31286. [Google Scholar] [CrossRef]
- Komada, M.; Takao, K.; Miyakawa, T. Elevated Plus Maze for Mice. JoVE 2008, 22, 1088. [Google Scholar] [CrossRef]
- Duffy, C.M.; Hofmeister, J.J.; Nixon, J.P.; Butterick, T.A. High fat diet increases cognitive decline and neuroinflammation in a model of orexin loss. Neurobiol. Learn. Mem. 2019, 157, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Kullmann, S.; Kleinridders, A.; Small, D.M.; Fritsche, A.; Häring, H.-U.; Preissl, H.; Heni, M. Central nervous pathways of insulin action in the control of metabolism and food intake. Lancet Diabetes Endocrinol. 2020, 8, 524–534. [Google Scholar] [CrossRef] [PubMed]
- Talbot, K.; Wang, H.-Y.; Kazi, H.; Han, L.-Y.; Bakshi, K.P.; Stucky, A.; Fuino, R.L.; Kawaguchi, K.R.; Samoyedny, A.J.; Wilson, R.S.; et al. Demonstrated brain insulin resistance in Alzheimer’s disease patients is associated with IGF-1 resistance, IRS-1 dysregulation, and cognitive decline. J. Clin. Investig. 2012, 122, 1316–1338. [Google Scholar] [CrossRef] [PubMed]
- Galizzi, G.; Palumbo, L.; Amato, A.; Conigliaro, A.; Nuzzo, D.; Terzo, S.; Caruana, L.; Picone, P.; Alessandro, R.; Mulè, F.; et al. Altered insulin pathway compromises mitochondrial function and quality control both in in vitro and in vivo model systems. Mitochondrion 2021, 60, 178–188. [Google Scholar] [CrossRef]
- Salas-Venegas, V.; Flores-Torres, R.P.; Rodríguez-Cortés, Y.M.; Rodríguez-Retana, D.; Ramírez-Carreto, R.J.; Concepción-Carrillo, L.E.; Pérez-Flores, L.J.; Alarcón-Aguilar, A.; López-Díazguerrero, N.E.; Gómez-González, B.; et al. The Obese Brain: Mechanisms of Systemic and Local Inflammation, and Interventions to Reverse the Cognitive Deficit. Front. Integr. Neurosci. 2022, 16, 798995. [Google Scholar] [CrossRef]
- Valdearcos, M.; Douglass, J.D.; Robblee, M.M.; Dorfman, M.D.; Stifler, D.R.; Bennett, M.L.; Gerritse, I.; Fasnacht, R.; Barres, B.A.; Thaler, J.P.; et al. Microglial Inflammatory Signaling Orchestrates the Hypothalamic Immune Response to Dietary Excess and Mediates Obesity Susceptibility. Cell Metab. 2017, 26, 185–197.e3. [Google Scholar] [CrossRef]
- Skaper, S.D.; Facci, L.; Zusso, M.; Giusti, P. Synaptic Plasticity, Dementia and Alzheimer Disease. CNS Neurol. Disord. Drug Targets 2017, 16, 220–233. [Google Scholar] [CrossRef]
- Guillemot-Legris, O.; Muccioli, G.G. Obesity-Induced Neuroinflammation: Beyond the Hypothalamus. Trends Neurosci. 2017, 40, 237–253. [Google Scholar] [CrossRef]
- Buettner, R.; Schölmerich, J.; Bollheimer, L.C. High-fat Diets: Modeling the Metabolic Disorders of Human Obesity in Rodents. Obesity 2007, 15, 798–808. [Google Scholar] [CrossRef]
- Hersey, M.; Woodruff, J.L.; Maxwell, N.; Sadek, A.T.; Bykalo, M.K.; Bain, I.; Grillo, C.A.; Piroli, G.G.; Hashemi, P.; Reagan, L.P. High-fat diet induces neuroinflammation and reduces the serotonergic response to escitalopram in the hippocampus of obese rats. Brain Behav. Immun. 2021, 96, 63–72. [Google Scholar] [CrossRef]
- Pirozzi, C.; Opallo, N.; Del Piano, F.; Melini, S.; Lama, A. Body and mind: How obesity triggers neuropsychiatric and neurodegenerative disorders. Front. Psychiatry 2025, 15, 1524555. [Google Scholar] [CrossRef]
- Ibeh, S.; Babale, I.; Nwaiwu, J.; Reslan, M.; Mohamed, W.; Goli, M.; Mechref, Y.; Kobeissy, F. The Western Diet Puzzle: Connecting Metabolic Dysfunction to Cognitive and Neurological Consequences. In Nutrition and Psychiatric Disorders; Mohamed, W., Kobeissy, F., Eds.; Nutritional Neurosciences; Springer Nature: Singapore, 2024; pp. 467–483. ISBN 978-981-97-2680-6. [Google Scholar]
- Burokas, A.; Arboleya, S.; Moloney, R.D.; Peterson, V.L.; Murphy, K.; Clarke, G.; Stanton, C.; Dinan, T.G.; Cryan, J.F. Targeting the Microbiota-Gut-Brain Axis: Prebiotics Have Anxiolytic and Antidepressant-like Effects and Reverse the Impact of Chronic Stress in Mice. Biol. Psychiatry 2017, 82, 472–487. [Google Scholar] [CrossRef] [PubMed]
- Taksande, B.; Rathi, N.; Kotpalliwar, S. Parasympathetic overactivity: A manifestation of temporal lobe epilepsy. J. Neurosci. Rural. Pract. 2013, 4, 479–480. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.P.; Koka, S.; Li, C.; Rajagopal, S. Inflammatory Mediators in Obesity. Mediat. Inflamm. 2019, 2019, 9481819. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.-Y.; Kim, C.-H.; Sung, E.-J.; Shin, H.-C.; Shin, W.-J.; Jung, K.-H. The Association between Frailty and Cognition in Elderly Women. Korean J. Fam. Med. 2016, 37, 164. [Google Scholar] [CrossRef]
- Mohamed, A.M.; Habib, M.Z.; Ebeid, M.A.; Abdelraouf, S.M.; El Faramawy, Y.; Aboul-Fotouh, S.; Magdy, Y. Amisulpride alleviates chronic mild stress-induced cognitive deficits: Role of prefrontal cortex microglia and Wnt/β-catenin pathway. Eur. J. Pharmacol. 2020, 885, 173411. [Google Scholar] [CrossRef]
- Obadia, N.; Andrade, G.; Leardini-Tristão, M.; Albuquerque, L.; Garcia, C.; Lima, F.; Daleprane, J.; Castro-Faria-Neto, H.C.; Tibiriçá, E.; Estato, V. TLR4 mutation protects neurovascular function and cognitive decline in high-fat diet-fed mice. J. Neuroinflammation 2022, 19, 104. [Google Scholar] [CrossRef]
- Vogelzangs, N.; Beekman, A.T.F.; De Jonge, P.; Penninx, B.W.J.H. Anxiety disorders and inflammation in a large adult cohort. Transl. Psychiatry 2013, 3, e249. [Google Scholar] [CrossRef]
- Michopoulos, V.; Powers, A.; Gillespie, C.F.; Ressler, K.J.; Jovanovic, T. Inflammation in Fear- and Anxiety-Based Disorders: PTSD, GAD, and Beyond. Neuropsychopharmacology 2017, 42, 254–270. [Google Scholar] [CrossRef]
- Rohleder, N. Stimulation of Systemic Low-Grade Inflammation by Psychosocial Stress. Psychosom. Med. 2014, 76, 181–189. [Google Scholar] [CrossRef]
- Yuan, N.; Chen, Y.; Xia, Y.; Dai, J.; Liu, C. Inflammation-related biomarkers in major psychiatric disorders: A cross-disorder assessment of reproducibility and specificity in 43 meta-analyses. Transl. Psychiatry 2019, 9, 233. [Google Scholar] [CrossRef] [PubMed]
- Sah, A.; Singewald, N. The (neuro)inflammatory system in anxiety disorders and PTSD: Potential treatment targets. Pharmacol. Ther. 2025, 269, 108825. [Google Scholar] [CrossRef] [PubMed]
- Kraeuter, A.-K.; Guest, P.C.; Sarnyai, Z. The Elevated Plus Maze Test for Measuring Anxiety-Like Behavior in Rodents. In Pre-Clinical Models; Guest, P.C., Ed.; Methods in Molecular Biology; Springer: New York, NY, USA, 2019; Volume 1916, pp. 69–74. ISBN 978-1-4939-8993-5. [Google Scholar]
- De Figueiredo Cerqueira, M.M.; Castro, M.M.L.; Vieira, A.A.; Kurosawa, J.A.A.; Amaral Junior, F.L.D.; De Siqueira Mendes, F.D.C.C.; Sosthenes, M.C.K. Comparative analysis between Open Field and Elevated Plus Maze tests as a method for evaluating anxiety-like behavior in mice. Heliyon 2023, 9, e14522. [Google Scholar] [CrossRef] [PubMed]
- Carola, V.; D’Olimpio, F.; Brunamonti, E.; Mangia, F.; Renzi, P. Evaluation of the elevated plus-maze and open-field tests for the assessment of anxiety-related behaviour in inbred mice. Behav. Brain Res. 2002, 134, 49–57. [Google Scholar] [CrossRef]
- Burgos-Robles, A.; Vidal-Gonzalez, I.; Quirk, G.J. Sustained Conditioned Responses in Prelimbic Prefrontal Neurons Are Correlated with Fear Expression and Extinction Failure. J. Neurosci. 2009, 29, 8474–8482. [Google Scholar] [CrossRef]



| Reference Sequence (RefSeq) RNA | Gene Symbol | Primer Sequences (5’–3’) | |
|---|---|---|---|
| NM_009605 | ADIPOQ | (F) | AGATGGCACTCCTGGAGAGAAG |
| (R) | ACATAAGCGGCTTCTCCAGGCT | ||
| NM_007470 | APO D | (F) | GGTGAAGCCAAACAGAGCAACG |
| (R) | CAGGAGTACACGAGGGCATAGT | ||
| NM_007540 | BDNF | (F) | GGCTGACACTTTTGAGCACGTC |
| (R) | CTCCAAAGGCACTTGACTGCTG | ||
| NM_007860 | DIO1 | (F) | GTAGGCAAGGTGCTAATGACGC |
| (R) | ACTGGATGCTGAAGAAGGTGGG | ||
| NM_172119 | DIO3 | (F) | TGCGTATCAGACGACAACCGTC |
| (R) | TGGAAGCCATCAGGTCGGACAA | ||
| NM_184052 | IGF | (F) | GTGGATGCTCTTCAGTTCGTGTG |
| (R) | TCCAGTCTCCTCAGATCACAGC | ||
| NM_008361.4 | IL 1b | (F) | TGGCCTTCCTGATGAGAGCAT |
| (R) | GAAGACACGGATTCCCAGGAA | ||
| NM_008355 | IL 4 | (F) | TCACAGCAACGAAGAACACCA |
| (R) | CAGGCATCGAAAAGCCCGAA | ||
| NM_031168 | IL-6 | (F) | TACCACTTCACAAGTCGGAGGC |
| (R) | CTGCAAGTGCATCATCGTTGTTC | ||
| NM_010548 | IL10 | (F) | CGGGAAGACAATAACTGCACCC |
| (R) | CGGTTAGCAGTATGTTGTCCAGC | ||
| NM_008006 | FGF 2 | (F) | AAGCGGCTCTACTGCAAGAACG |
| (R) | CCTTGATAGACACAACTCCTCTC | ||
| NM_008084 | GAPDH | (F) | ACACCAGTAGACTCCACGACA |
| (R) | ACGGCAAATTCAACGGCACAG | ||
| NM_011400 | GLUT 1 | (F) | GCTTCTCCAACTGGACCTCAAAC |
| (R) | ACGAGGAGCACCGTGAAGATGA | ||
| NM_011401 | GLUT 3 | (F) | CCGCTTCTCATCTCCATTGTCC |
| (R) | CCTGCTCCAATCGTGGCATAGA | ||
| NM_019741 | GLUT 5 | (F) | ATCGCTGCCTTTGGCTCATCCT |
| (R) | GCAGCGTCAAGGTGAAGGACT | ||
| NM_010438 | HMGCoA-R | (F) | CGAGAGGAGGAAGAAAGCAG |
| (R) | CGCAGGCTGAGAAATAGAGG | ||
| NM_008493 | LEPTIN | (F) | AGCCGAGGAGGAGAACAAAG |
| (R) | TGACAGGACAGGAGGAGGA | ||
| NM_010907 | NFkB p65 | (F) | CAGAGCAGTACATGGCTTTG |
| (R) | CAGCAGGTGGAGGAGGAG | ||
| NM_010381 | GDNF | (F) | CCTTCGCGCTGACCAGTGACT |
| (R) | GCCGCTTGTTTATCTGGTGACC | ||
| NM_011146 | PPARg | (F) | AGGAGACGACCTGGATTACC |
| (R) | AGGCAAGTCCAGAGACATTG | ||
| NM_027118 | SIRT 6 | (F) | GAGAGGAAGGAGTGAGGAG |
| (R) | TGGAGTGTAGGAGGAGGAGG | ||
| NM_013693 | TNFa | (F) | GATGATCCGCGACGTGGAG |
| (R) | GAAGTAGACCTGCCCAGTTG | ||
| NM_001271100 | TREM 2 | (F) | GAGCCATCCAGCAGCGT |
| (R) | GAGCCCATCCAGCAGCAG | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Polini, B.; Ricardi, C.; Di Lupo, F.; Runfola, M.; Bacci, A.; Rapposelli, S.; Bizzarri, R.; Scalese, M.; Saponaro, F.; Chiellini, G. Novel Thyroid Hormone Receptor-β Agonist TG68 Exerts Anti-Inflammatory, Lipid-Lowering and Anxiolytic Effects in a High-Fat Diet (HFD) Mouse Model of Obesity. Cells 2025, 14, 580. https://doi.org/10.3390/cells14080580
Polini B, Ricardi C, Di Lupo F, Runfola M, Bacci A, Rapposelli S, Bizzarri R, Scalese M, Saponaro F, Chiellini G. Novel Thyroid Hormone Receptor-β Agonist TG68 Exerts Anti-Inflammatory, Lipid-Lowering and Anxiolytic Effects in a High-Fat Diet (HFD) Mouse Model of Obesity. Cells. 2025; 14(8):580. https://doi.org/10.3390/cells14080580
Chicago/Turabian StylePolini, Beatrice, Caterina Ricardi, Francesca Di Lupo, Massimiliano Runfola, Andrea Bacci, Simona Rapposelli, Ranieri Bizzarri, Marco Scalese, Federica Saponaro, and Grazia Chiellini. 2025. "Novel Thyroid Hormone Receptor-β Agonist TG68 Exerts Anti-Inflammatory, Lipid-Lowering and Anxiolytic Effects in a High-Fat Diet (HFD) Mouse Model of Obesity" Cells 14, no. 8: 580. https://doi.org/10.3390/cells14080580
APA StylePolini, B., Ricardi, C., Di Lupo, F., Runfola, M., Bacci, A., Rapposelli, S., Bizzarri, R., Scalese, M., Saponaro, F., & Chiellini, G. (2025). Novel Thyroid Hormone Receptor-β Agonist TG68 Exerts Anti-Inflammatory, Lipid-Lowering and Anxiolytic Effects in a High-Fat Diet (HFD) Mouse Model of Obesity. Cells, 14(8), 580. https://doi.org/10.3390/cells14080580










