Heat Preconditioning of Nanofat Does Not Improve Its Vascularization Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Anesthesia
2.3. Generation and Heat Preconditioning of Nanofat
2.4. Ex Vivo Analysis of Nanofat
2.5. Seeding of Dermal Substitutes with Nanofat
2.6. Dorsal Skinfold Chamber Model
2.7. Intravital Fluorescence Microscopy
2.8. Histology and Immunohistochemistry of In Vivo Samples
2.9. Statistical Analysis
3. Results
3.1. Ex Vivo Characterization of Control and Heat-Preconditioned Nanofat
3.2. In Vivo Microscopy of Nanofat-Seeded Implants
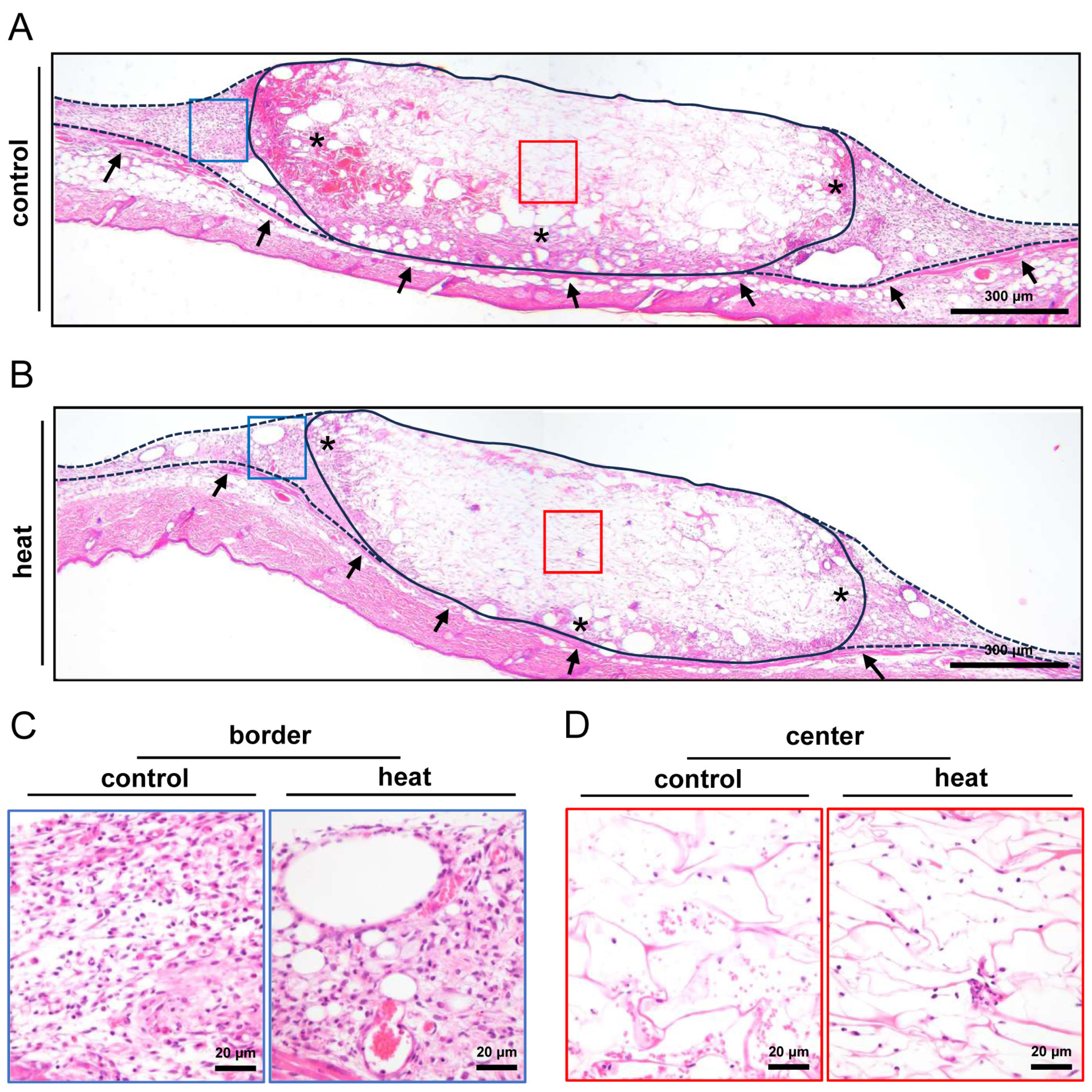
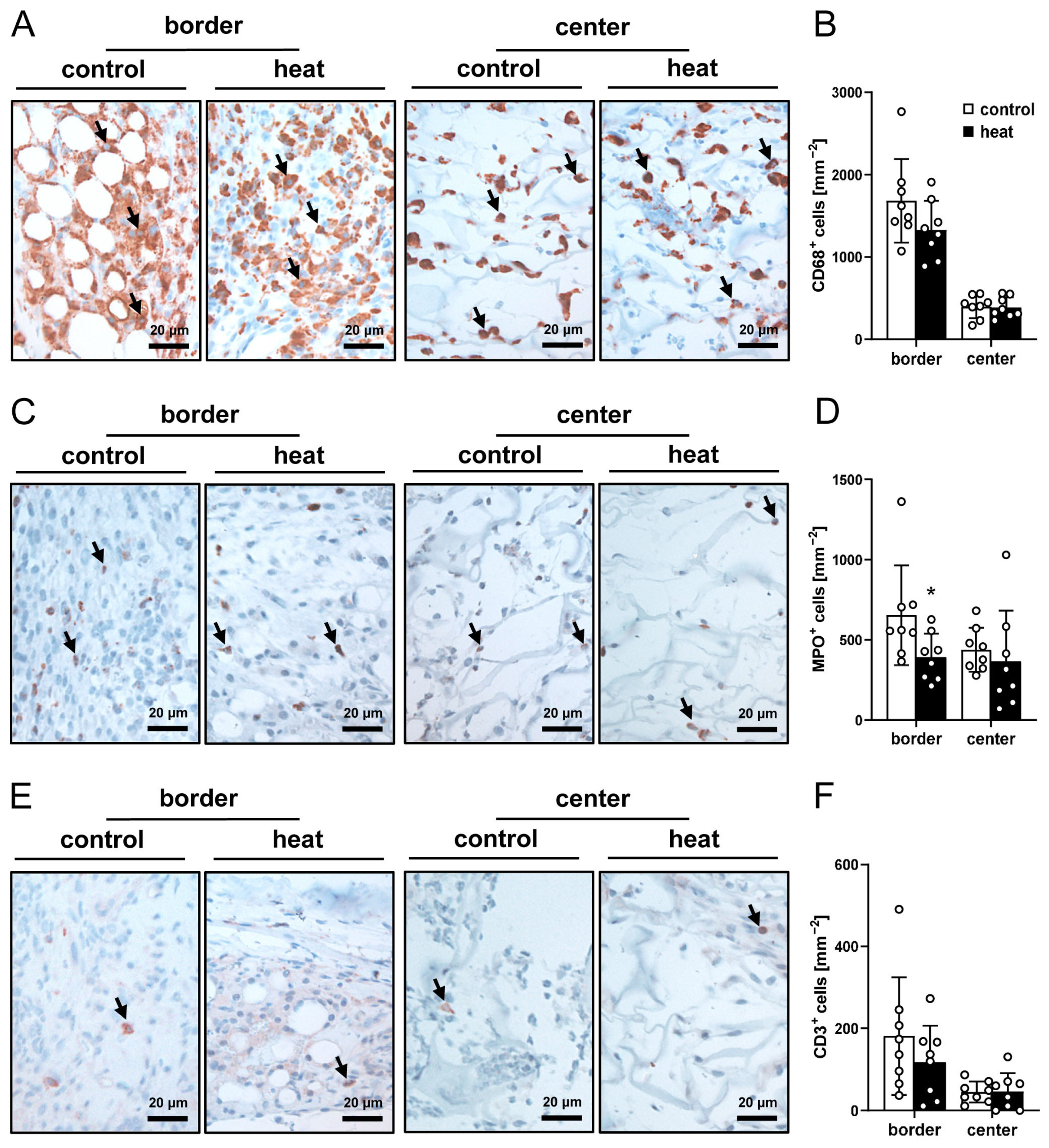
3.3. Histological and Immunohistochemical Analysis of Nanofat-Seeded Implants
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ASC | Adipose-derived stem cell |
| Casp-3+ | Caspase-3-positive |
| Col | Collagen |
| FITC | Fluorescein isothiocyanate |
| GFP | Green fluorescent protein |
| HE | Hematoxylin-eosin |
| HO-1 | Heme-oxygenase-1 |
| HSP | Heat shock protein |
| LYVE | Vessel endothelial hyaluronan receptor |
| MPO | Myeloperoxidase |
| NIH | National Institutes of Health |
| PBS | Phosphate-buffered saline |
| qRT-PCR | Quantitative real-time polymerase chain reaction |
| RBC | Red blood cell |
| ROI | Region of interest |
| VEGF | Vascular endothelial growth factor |
References
- Tonnard, P.; Verpaele, A.; Peeters, G.; Hamdi, M.; Cornelissen, M.; Declercq, H. Nanofat grafting: Basic research and clinical applications. Plast. Reconstr. Surg. 2013, 132, 1017–1026. [Google Scholar] [CrossRef] [PubMed]
- Jeyaraman, M.; Muthu, S.; Sharma, S.; Ganta, C.; Ranjan, R.; Jha, S.K. Nanofat: A therapeutic paradigm in regenerative medicine. World J. Stem Cells 2021, 13, 1733–1746. [Google Scholar] [CrossRef] [PubMed]
- Weinzierl, A.; Harder, Y.; Schmauss, D.; Menger, M.D.; Laschke, M.W. Boosting Tissue Vascularization: Nanofat as a Potential Source of Functional Microvessel Segments. Front. Bioeng. Biotechnol. 2022, 10, 820835. [Google Scholar] [CrossRef] [PubMed]
- Uyulmaz, S.; Sanchez Macedo, N.; Rezaeian, F.; Giovanoli, P.; Lindenblatt, N. Nanofat Grafting for Scar Treatment and Skin Quality Improvement. Aesthet. Surg. J. 2018, 38, 421–428. [Google Scholar] [CrossRef]
- La Padula, S.; Ponzo, M.; Lombardi, M.; Iazzetta, V.; Errico, C.; Polverino, G.; Russo, F.; D’Andrea, L.; Hersant, B.; Meningaud, J.P.; et al. Nanofat in Plastic Reconstructive, Regenerative, and Aesthetic Surgery: A Review of Advancements in Face-Focused Applications. J. Clin. Med. 2023, 12, 4351. [Google Scholar] [CrossRef]
- Limido, E.; Weinzierl, A.; Ampofo, E.; Harder, Y.; Menger, M.D.; Laschke, M.W. Nanofat Accelerates and Improves the Vascularization, Lymphatic Drainage and Healing of Full-Thickness Murine Skin Wounds. Int. J. Mol. Sci. 2024, 25, 851. [Google Scholar] [CrossRef] [PubMed]
- Surowiecka, A.; Strużyna, J. Adipose-Derived Stem Cells for Facial Rejuvenation. J. Pers. Med. 2022, 12, 117. [Google Scholar] [CrossRef]
- Bonomi, F.; Limido, E.; Weinzierl, A.; Ampofo, E.; Harder, Y.; Menger, M.D.; Laschke, M.W. Nanofat Improves Vascularization and Tissue Integration of Dermal Substitutes without Affecting Their Biocompatibility. J. Funct. Biomater. 2024, 15, 294. [Google Scholar] [CrossRef]
- Frueh, F.S.; Später, T.; Lindenblatt, N.; Calcagni, M.; Giovanoli, P.; Scheuer, C.; Menger, M.D.; Laschke, M.W. Adipose Tissue- Derived Microvascular Fragments Improve Vascularization, Lymphangiogenesis, and Integration of Dermal Skin Substitutes. J. Investig. Dermatol. 2017, 137, 217–227. [Google Scholar] [CrossRef]
- Hiasa, G.; Hamada, M.; Ikeda, S.; Hiwada, K. Ischemic preconditioning and lipopolysaccharide attenuate nuclear factor-kappaB activation and gene expression of inflammatory cytokines in the ischemia-reperfused rat heart. Jpn. Circ. J. 2001, 65, 984–990. [Google Scholar] [CrossRef]
- Mitchell, J.R.; Verweij, M.; Brand, K.; van de Ven, M.; Goemaere, N.; van den Engel, S.; Chu, T.; Forrer, F.; Müller, C.; de Jong, M.; et al. Short-term dietary restriction and fasting precondition against ischemia reper-fusion injury in mice. Aging Cell 2010, 9, 40–53. [Google Scholar] [CrossRef] [PubMed]
- Bonomi, F.; Limido, E.; Weinzierl, A.; Harder, Y.; Menger, M.D.; Laschke, M.W. Preconditioning Strategies for Improving the Outcome of Fat Grafting. Tissue Eng. Part B Rev. 2024; epub ahead of print. [Google Scholar] [CrossRef]
- Harder, Y.; Contaldo, C.; Klenk, J.; Banic, A.; Jakob, S.M.; Erni, D. Improved skin flap survival after local heat preconditioning in pigs. J. Surg. Res. 2004, 119, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Harder, Y.; Amon, M.; Schramm, R.; Georgi, M.; Banic, A.; Erni, D.; Menger, M.D. Heat shock preconditioning reduces ischemic tissue necrosis by heat shock protein (HSP)-32-mediated improvement of the microcirculation rather than induction of ischemic tolerance. Ann. Surg. 2005, 242, 869–878. [Google Scholar] [CrossRef]
- Contaldo, C.; Harder, Y.; Plock, J.; Banic, A.; Jakob, S.M.; Erni, D. The influence of local and systemic preconditioning on oxygenation, metabolism and survival in critically ischaemic skin flaps in pigs. J. Plast. Reconstr. Aesthet. Surg. 2007, 60, 1182–1192. [Google Scholar] [CrossRef]
- Mehta, S.; Rolph, R.; Cornelius, V.; Harder, Y.; Farhadi, J. Local heat preconditioning in skin sparing mastectomy: A pilot study. J. Plast. Reconstr. Aesthet. Surg. 2013, 66, 1676–1682. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.E.; Kim, Y.J.; Kim, Y.W.; Cheon, Y.W. Heating pretreatment of the recipient site enhances survival of transplanted fat in a mouse model. Plast. Reconstr. Surg. 2023, 152, 787–795. [Google Scholar] [CrossRef]
- Calderwood, S.K.; Gong, J. Heat Shock Proteins Promote Cancer: It’s a Protection Racket. Trends Biochem. Sci. 2016, 41, 311–323. [Google Scholar] [CrossRef]
- Maines, M.D. The heme oxygenase system: A regulator of second messenger gases. Annu. Rev. Pharmacol. Toxicol. 1997, 37, 517–554. [Google Scholar] [CrossRef]
- Stone, J.R.; Marletta, M.A. Soluble guanylate cyclase from bovine lung: Activation with nitric oxide and carbon monoxide and spectral characterization of the ferrous and ferric states. Biochemistry 1994, 33, 5636–5640. [Google Scholar] [CrossRef]
- Infanger, M.; Schmidt, O.; Kossmehl, P.; Grad, S.; Ertel, W.; Grimm, D. Vascular endothelial growth factor serum level is strongly enhanced after burn injury and correlated with local and general tissue edema. Burns 2004, 30, 305–311. [Google Scholar] [CrossRef]
- Kevelaitis, E.; Patel, A.P.; Oubenaissa, A.; Peynet, J.; Mouas, C.; Yellon, D.M.; Menasché, P. Backtable heat-enhanced preconditioning: A simple and effective means of improving function of heart transplants. Ann. Thorac. Surg. 2001, 72, 107–113. [Google Scholar] [CrossRef]
- Kelty, J.D.; Noseworthy, P.A.; Feder, M.E.; Robertson, R.M.; Ramirez, J.M. Thermal preconditioning and heat-shock protein 72 preserve synaptic transmission during thermal stress. J. Neurosci. 2002, 22, RC193. [Google Scholar] [CrossRef]
- McCormick, P.H.; Chen, G.; Tlerney, S.; Kelly, C.J.; Bouchier-Hayes, D.J. Clinically relevant thermal preconditioning attenuates ischemia-reperfusion injury. J. Surg. Res. 2003, 109, 24–30. [Google Scholar] [CrossRef]
- Gerazova-Efremova, K.; Dinevska-Kjovkarovska, S.; Miova, B. Heat-Shock Protein 70-Mediated Heat Preconditioning Attenuates Hepatic Carbohydrate and Oxidative Disturbances in Rats with Type 1 Diabetes. Can. J. Diabetes 2019, 43, 345–353. [Google Scholar] [CrossRef]
- Belity, T.; Horowitz, M.; Hoffman, J.R.; Epstein, Y.; Bruchim, Y.; Todder, D.; Cohen, H. Heat-Stress Preconditioning Attenuates Behavioral Responses to Psychological Stress: The Role of HSP-70 in Modulating Stress Responses. Int. J. Mol. Sci. 2022, 23, 4129. [Google Scholar] [CrossRef]
- Xu, H.; Takashi, E.; Liang, J.; Chen, Y.; Yuan, Y.; Fan, J. Effect of Heat Shock Preconditioning on Pressure Injury Prevention via Hsp27 Upregulation in Rat Models. Int. J. Mol. Sci. 2022, 23, 8955. [Google Scholar] [CrossRef]
- Matsumoto, K.; Honda, K.; Kobayashi, N. Protective effect of heat preconditioning of rat liver graft resulting in improved transplant survival. Transplantation 2001, 71, 862–868. [Google Scholar] [CrossRef]
- Watanabe, J.; Kushihata, F.; Matsumoto, K.; Honda, K.; Matsuda, S.; Kobayashi, N. Downregulation of Cytokine Release by Heat Pre- conditioning of Livers Used for Transplantation in Rats. Dig. Dis. Sci. 2005, 50, 1823–1828. [Google Scholar] [CrossRef]
- Mehta, S.; Cro, S.C.; Coomber, B.; Rolph, R.; Cornelius, V.; Farhadi, J. A randomised controlled feasibility trial to evaluate local heat preconditioning on wound healing after reconstructive breast surgery: The preHEAT trial. Pilot Feasibility Stud. 2019, 5, 5. [Google Scholar] [CrossRef]
- Shaik, S.; Hayes, D.; Gimble, J.; Devireddy, R. Inducing Heat Shock Proteins Enhances the Stemness of Frozen-Thawed Adipose Tissue-Derived Stem Cells. Stem Cells Dev. 2017, 26, 608–616. [Google Scholar] [CrossRef]
- Kim, W.K.; Kim, W.H.; Kweon, O.K.; Kang, B.J. Intravenous Administration of Heat Shock-Treated MSCs Can Improve Neuroprotection and Neuroregeneration in Canine Spinal Cord Injury Model. Animals 2020, 10, 2164. [Google Scholar] [CrossRef]
- Kim, W.K.; Kim, W.H.; Kweon, O.K.; Kang, B.J. Heat-Shock Proteins Can Potentiate the Therapeutic Ability of Cryopreserved Mesenchymal Stem Cells for the Treatment of Acute Spinal Cord Injury in Dogs. Stem Cell Rev. Rep. 2022, 18, 1461–1477. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.K.; Kang, B.J. Transplantation of Heat-Shock Preconditioned Neural Stem/Progenitor Cells Combined with RGD-Functionalised Hydrogel Promotes Spinal Cord Functional Recovery in a Rat Hemi-Transection Model. Stem Cell Rev. Rep. 2024, 20, 283–300. [Google Scholar] [CrossRef]
- Sun, J.; Liao, J.K. Induction of angiogenesis by heat shock protein 90 mediated by protein kinase Akt and endothelial nitric oxide synthase. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 2238–2244. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.K.; Na, H.J.; Lee, W.R.; Jeoung, M.H.; Lee, S. Heat shock protein 70-1A is a novel angiogenic regulator. Biochem. Biophys. Res. Commun. 2016, 469, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Moya, A.; Larochette, N.; Paquet, J.; Deschepper, M.; Bensidhoum, M.; Izzo, V.; Kroemer, G.; Petite, H.; Logeart-Avramoglou, D. Quiescence Preconditioned Human Multipotent Stromal Cells Adopt a Metabolic Profile Favorable for Enhanced Survival under Ischemia. Stem Cells 2017, 35, 181–196. [Google Scholar] [CrossRef]
- Alekseenko, L.L.; Shilina, M.A.; Lyublinskaya, O.G.; Kornienko, J.S.; Anatskaya, O.V.; Vinogradov, A.E.; Grinchuk, T.M.; Fridlyanskaya, I.I.; Nikolsky, N.N. Quiescent Human Mesenchymal Stem Cells Are More Resistant to Heat Stress than Cycling Cells. Stem Cells Int. 2018, 2018, 3753547. [Google Scholar] [CrossRef]
- Schutte, B.; Ramaekers, F.C. Molecular switches that govern the balance between proliferation and apoptosis. Prog. Cell Cycle Res. 2000, 4, 207–217. [Google Scholar] [CrossRef]
- Cho, I.J.; Lui, P.P.; Obajdin, J.; Riccio, F.; Stroukov, W.; Willis, T.L.; Spagnoli, F.; Watt, F.M. Mechanisms, Hallmarks, and Implications of Stem Cell Quiescence. Stem Cell Rep. 2019, 12, 1190–1200. [Google Scholar] [CrossRef]
- Van Velthoven, C.T.J.; Rando, T.A. Stem Cell Quiescence: Dynamism, Restraint, and Cellular Idling. Cell Stem Cell 2019, 24, 213–225. [Google Scholar] [CrossRef]
- Lee, P.J.; Alam, J.; Wiegand, G.W.; Choi, A.M. Overexpression of heme oxygenase-1 in human pulmonary epithelial cells results in cell growth arrest and increased resistance to hyperoxia. Proc. Natl. Acad. Sci. USA 1996, 93, 10393–10398. [Google Scholar] [CrossRef] [PubMed]
- Linguaggiato, P.; Gonzalez-Michaca, L.; Croatt, A.J.; Haggard, J.J.; Alam, J.; Nath, K.A. Cellular overexpression of heme oxygenase-1 up-regulates p21 and confers resistance to apoptosis. Kidney Int. 2001, 60, 2181–2191. [Google Scholar] [CrossRef] [PubMed]
- Karimian, A.; Ahmadi, Y.; Yousefi, B. Multiple functions of p21 in cell cycle, apoptosis and transcriptional regulation after DNA damage. DNA Repair 2016, 42, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; He, H.; Cui, Y.; Yu, S.; Li, S.; Afedo, S.Y.; Wang, Y.; Bai, X.; He, J. Regulatory effects of HIF-1α and HO-1 in hypoxia-induced proliferation of pulmonary arterial smooth muscle cells in yak. Cell Signal 2021, 87, 110140. [Google Scholar] [CrossRef]
- Karamanos, N.K.; Theocharis, A.D.; Piperigkou, Z.; Manou, D.; Passi, A.; Skandalis, S.S.; Vynios, D.H.; Orian-Rousseau, V.; Ricard-Blum, S.; Schmelzer, C.E.H.; et al. A guide to the composition and functions of the extracellular matrix. FEBS J. 2021, 288, 6850–6912. [Google Scholar] [CrossRef]
- Gouarderes, S.; Mingotaud, A.F.; Vicendo, P.; Gibot, L. Vascular and extracellular matrix remodeling by physical approaches to improve drug delivery at the tumor site. Expert. Opin. Drug Deliv. 2020, 17, 1703–1726. [Google Scholar] [CrossRef]
- DiPietro, L.A. Angiogenesis and wound repair: When enough is enough. J. Leukoc. Biol. 2016, 100, 979–984. [Google Scholar] [CrossRef]
- Sorg, H.; Sorg, C.G.G. Skin Wound Healing: Of Players, Patterns, and Processes. Eur. Surg. Res. 2023, 64, 141–157. [Google Scholar] [CrossRef]
- Zhong, X.; Yan, W.; He, X.; Ni, Y. Improved fat graft viability by delayed fat flap with ischaemic pretreatment. J. Plast. Reconstr. Aesthet. Surg. 2009, 62, 526–531. [Google Scholar] [CrossRef]
- Gassman, A.A.; Lewis, M.S.; Bradley, J.P.; Lee, J.C. Remote ischemic preconditioning improves the viability of donor lipoaspirate during murine fat transfer. Plast. Reconstr. Surg. 2015, 136, 495–502. [Google Scholar] [CrossRef]





| Protein | Expression (% of Control) |
|---|---|
| Pro-angiogenic | |
| HGF | 564 |
| KC/CXCL1/CINC-1/GRO-alpha | 330 |
| KGF/FGF-7 | 306 |
| DLL4 | 299 |
| ET-1 | 231 |
| GM-CSF | 211 |
| PD-ECGF | 207 |
| AR | 164 |
| Proliferin | 157 |
| FGF acid/FGF-1/ECGF/HBGF-1 | 155 |
| Coagulator Factor III/TF | 147 |
| EGF | 125 |
| VEGF/VPF | 125 |
| IGFBP-3 | 116 |
| MMP-3 | 109 |
| Ang-1 | 108 |
| PIGF-2 | 104 |
| MIP-1alpha | 98 |
| OPN | 98 |
| Leptin/OB | 92 |
| IL-10/CSIF | 87 |
| ANG | 84 |
| PDGF-AA | 84 |
| Cyr61/CCN1, IGFBP10 | 78 |
| FGF basic/FGF-22 | 71 |
| MMP-9 | 68 |
| IL-1beta | 55 |
| Fractalkine/CX3CL1 | 53 |
| IGFBP-1 | 52 |
| IL-1alpha | 45 |
| VEGF B/VRF | 45 |
| SDF-1/CXCL12 | 43 |
| MCP-1/CCL2 | 33 |
| MMP-8 | 27 |
| NOV/CCN3/IGFBP-9 | 25 |
| CXCL 16 | 22 |
| HB-EGF | 21 |
| Endoglin/CD105 | 19 |
| IGFBP-2 | 6 |
| Anti-angiogenic | |
| PTX3/TSG-14 | 637 |
| ADAMTS1 | 204 |
| Serpin F1/PEDF | 204 |
| DPP IV/CD26 | 195 |
| Ang-3 | 166 |
| PRL | 160 |
| IP-10/CXCL 10/CRG-2 | 151 |
| PDFG-AB/BB | 148 |
| Endostatin/Collagen VIII | 148 |
| TIMP-1 | 111 |
| CXCL4/PF4 | 69 |
| Serpin E1/PAI-1 | 33 |
| TIMP-4 | 30 |
| TSP-2 | 16 |
| d0 | d3 | d6 | d10 | d14 | |
|---|---|---|---|---|---|
| diameter (µm): | |||||
| border: control | - | - | 20.0 ± 4.5 | 19.4 ± 1.1 | 15.2 ± 0.9 |
| heat | - | - | 15.5 ± 2.4 | 17.2 ± 0.7 | 12.1 ± 0.8 * |
| center: control | - | - | - | - | 20.9 ± 4.6 |
| heat | - | - | - | - | - |
| centerline RBC velocity (µm/s): | |||||
| border: control | - | - | 58.0 ± 17.4 | 96.1 ± 18.2 | 170.4 ± 14.9 |
| heat | - | - | 69.3 ± 11.3 | 134.7 ± 31.2 | 181.8 ± 28.8 |
| center: control | - | - | - | - | 131.8 ± 113.0 |
| heat | - | - | - | - | - |
| shear rate (s−1): | |||||
| border: control | - | - | 23.0 ± 4.2 | 43.5 ± 8.4 | 113.6 ± 19.2 |
| heat | - | - | 39.3 ± 13.2 | 69.3 ± 15.2 | 126.4 ± 31.9 |
| center: control | - | - | - | - | 63.8 ± 57.5 |
| heat | - | - | - | - | - |
| volumetric blood flow (pL/s): | |||||
| border: control | - | - | 15.7 ± 10.7 | 20.1 ± 5.0 | 20.4 ± 3.1 |
| heat | - | - | 7.8 ± 1.3 | 30.6 ± 10.7 | 15.4 ± 2.5 |
| center: control | - | - | - | - | 21.7 ± 15.5 |
| heat | - | - | - | - | - |
| d0 | d3 | d6 | d10 | d14 | |
|---|---|---|---|---|---|
| diameter (µm): | |||||
| control | 42.5 ± 2.3 | 38.7 ± 1.4 | 37.8 ± 1.6 | 35.1 ± 1.4 | 33.0 ± 2.0 |
| heat | 36.6 ± 1.4 | 34.4 ± 1.3 | 37.0 ± 1.3 | 36.2 ± 1.3 | 38.9 ± 1.3 * |
| centerline RBC velocity (µm/s): | |||||
| control | 516.0 ± 61.1 | 522.9 ± 71.7 | 608.2 ± 75.3 | 512.8 ± 74.2 | 440.5 ± 125.0 |
| heat | 651.0 ± 97.6 | 674.0 ± 42.7 | 667.6 ± 86.1 | 658.1 ± 103.3 | 714.9 ± 109.2 |
| shear rate (s−1): | |||||
| control | 97.3 ± 8.7 | 98.3 ± 10.6 | 134.9 ± 20.1 | 112.5 ± 13.4 | 101.4 ± 28.0 |
| heat | 144.0 ± 22.4 | 157.3 ± 11.2 * | 146.2 ± 20.2 | 148.7 ± 22.4 | 152.1 ± 25.0 |
| volumetric blood flow (pL/s): | |||||
| control | 510.9 ± 110.6 | 450.8 ± 86.7 | 438.5 ± 66.9 | 347.2 ± 69.1 | 292.5 ± 86.3 |
| heat | 445.2 ± 72.4 | 423.8 ± 42.2 | 481.8 ± 68.8 | 436.7 ± 79.4 | 553.8 ± 76.8 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonomi, F.; Limido, E.; Weinzierl, A.; Bickelmann, C.; Ampofo, E.; Harder, Y.; Menger, M.D.; Laschke, M.W. Heat Preconditioning of Nanofat Does Not Improve Its Vascularization Properties. Cells 2025, 14, 581. https://doi.org/10.3390/cells14080581
Bonomi F, Limido E, Weinzierl A, Bickelmann C, Ampofo E, Harder Y, Menger MD, Laschke MW. Heat Preconditioning of Nanofat Does Not Improve Its Vascularization Properties. Cells. 2025; 14(8):581. https://doi.org/10.3390/cells14080581
Chicago/Turabian StyleBonomi, Francesca, Ettore Limido, Andrea Weinzierl, Caroline Bickelmann, Emmanuel Ampofo, Yves Harder, Michael D. Menger, and Matthias W. Laschke. 2025. "Heat Preconditioning of Nanofat Does Not Improve Its Vascularization Properties" Cells 14, no. 8: 581. https://doi.org/10.3390/cells14080581
APA StyleBonomi, F., Limido, E., Weinzierl, A., Bickelmann, C., Ampofo, E., Harder, Y., Menger, M. D., & Laschke, M. W. (2025). Heat Preconditioning of Nanofat Does Not Improve Its Vascularization Properties. Cells, 14(8), 581. https://doi.org/10.3390/cells14080581









