The Sensory Input from the External Cuneate Nucleus and Central Cervical Nucleus to the Cerebellum Refines Forelimb Movements
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Viral Vectors
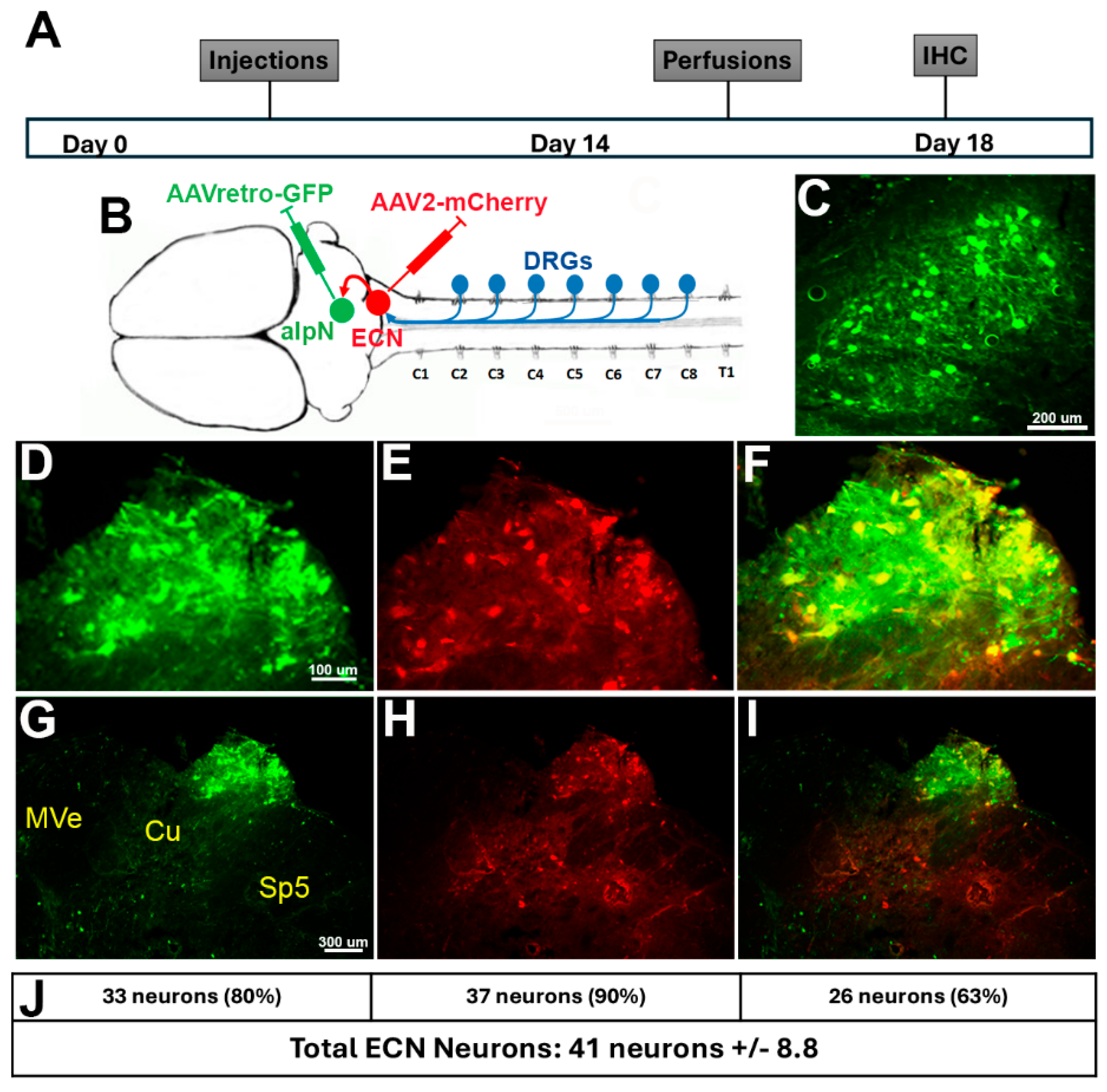
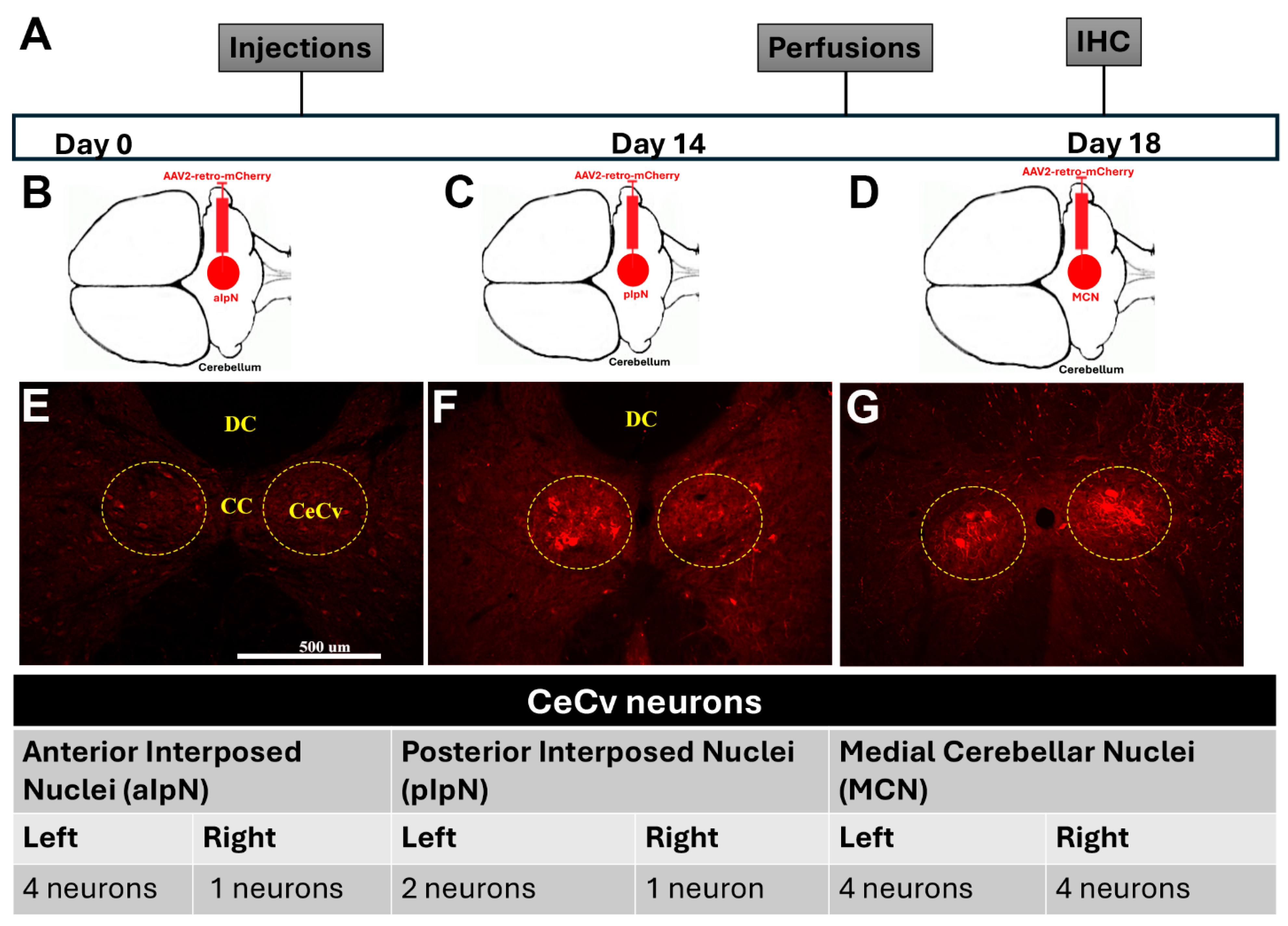
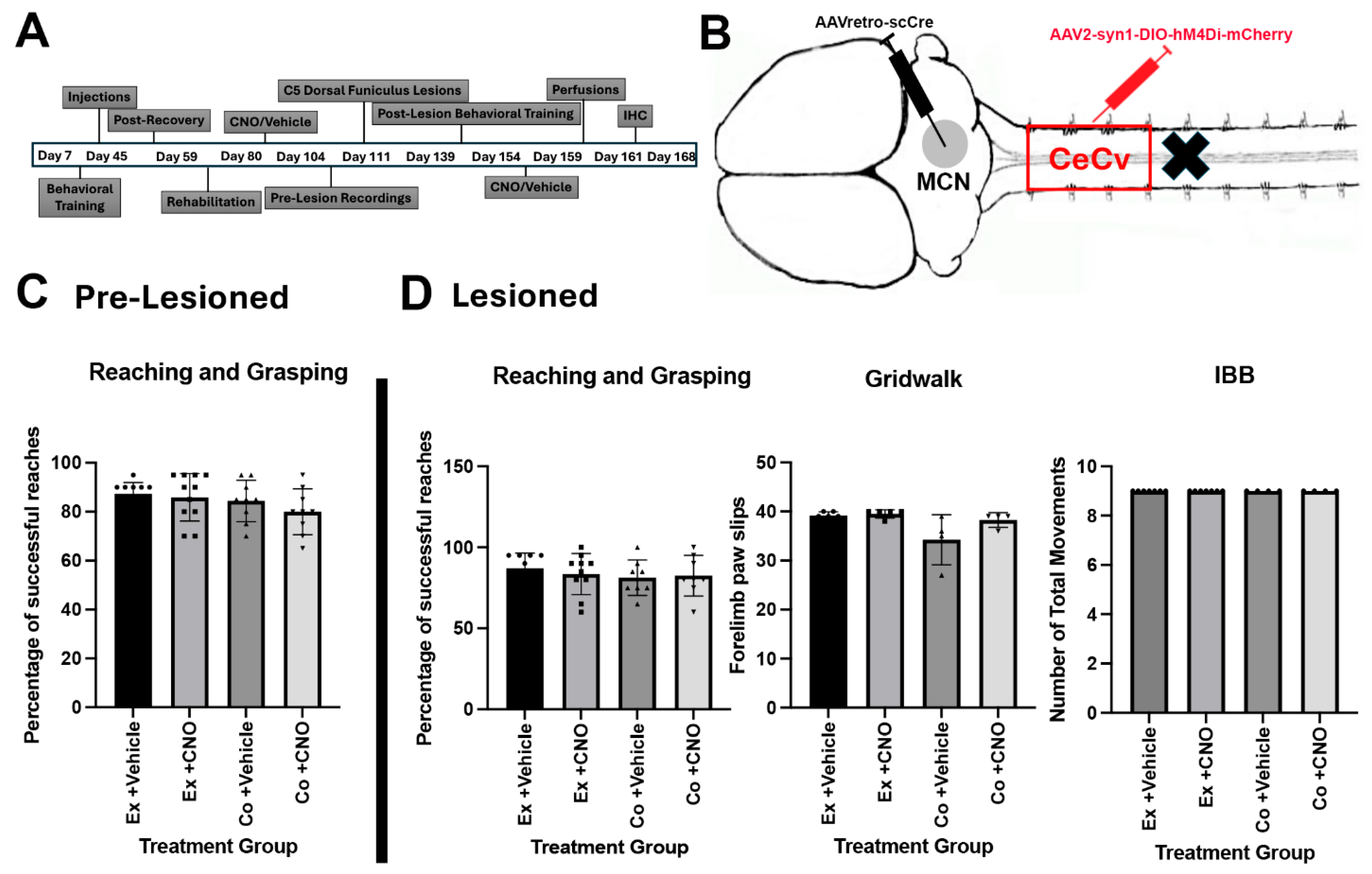
2.3. Surgical Procedures
2.4. Behavioral Training and Assessment
2.5. Video Recordings
2.6. Tissue Processing and Histology
2.7. Statistics
3. Results—Sensory Input Is Involved in Forelimb Function
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Azim, E.; Fink, A.J.; Jessell, T.M. Internal and External Feedback Circuits for Skilled Forelimb Movement. Cold Spring Harb. Symp. Quant. Biol. 2014, 79, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Azim, E.; Ekerot, C.F.; Alstermark, B. Direct and indirect spino-cerebellar pathways: Shared ideas but different functions in motor control. Front. Comput. Neurosci. 2015, 9, 75. [Google Scholar] [CrossRef] [PubMed]
- Shadmehr, R.; Smith, M.A.; Krakauer, J.W. Error Correction, Sensory Prediction, and Adaptation in Motor Control. Annu. Rev. Neurosci. 2010, 33, 89–108. [Google Scholar] [CrossRef]
- Brownstone, R.M.; Bui, T.V.; Stifani, N. Spinal circuits for motor learning. Curr. Opin. Neurobiol. 2015, 33, 166–173. [Google Scholar] [CrossRef]
- Beitzel, C.S.; Houck, B.D.; Lewis, S.M.; Person, A.L. Rubrocerebellar Feedback Loop Isolates the Interposed Nucleus as an Independent Processor of Corollary Discharge Information in Mice. J. Neurosci. 2017, 37, 10085–10096. [Google Scholar] [CrossRef]
- Filli, L.; Engmann, A.K.; Zörner, B.; Weinmann, O.; Moraitis, T.; Gullo, M.; Kasper, H.; Schneider, R.; Schwab, M.E. Bridging the Gap: A Reticulo-Propriospinal Detour Bypassing an Incomplete Spinal Cord Injury. J. Neurosci. 2014, 34, 13399–13410. [Google Scholar] [CrossRef]
- Gonzalez Castro, L.N.; Monsen, C.B.; Smith, M.A. The binding of learning to action in motor adaptation. PLoS Comput. Biol. 2011, 7, e1002052. [Google Scholar] [CrossRef]
- Low, J.S.T.; John, L.; Tracey, D.J. Nucleus Z in the rat: Spinal afferents from collaterals of dorsal spinocerebellar tract neurons. J. Comp. Neurol. 1986, 243, 510–526. [Google Scholar] [CrossRef]
- Yuengert, R.; Hori, K.; Kibodeaux, E.E.; McClellan, J.X.; Morales, J.E.; Huang, T.P.; Neul, J.L.; Lai, H.C. Origin of a Non-Clarke’s Column Division of the Dorsal Spinocerebellar Tract and the Role of Caudal Proprioceptive Neurons in Motor Function. Cell Rep. 2015, 13, 1258–1271. [Google Scholar] [CrossRef]
- Chalif, J.I.; Martínez-Silva, L.; Pagiazitis, J.G.; Murray, A.; Mentis, G.Z. Control of mammalian locomotion by ventral spinocerebellar tract neurons. Cell 2022, 185, 328–344.e26. [Google Scholar] [CrossRef]
- Brooks, J.X.; Cullen, K.E. Predictive Sensing: The Role of Motor Signals in Sensory Processing. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2019, 4, 842–850. [Google Scholar] [CrossRef] [PubMed]
- Grünbaum, T.; Christensen, M.S. The functional role of conscious sensation of movement. Neurosci. Biobehav. Rev. 2024, 164, 105813. [Google Scholar] [CrossRef] [PubMed]
- Takeoka, A.; Vollenweider, I.; Courtine, G.; Arber, S. Muscle spindle feedback directs locomotor recovery and circuit reorganization after Spinal Cord Injury. Cell 2014, 159, 1626–1639. [Google Scholar] [CrossRef]
- Takeoka, A.; Arber, S. Functional local proprioceptive feedback circuits initiate and maintain locomotor recovery after Spinal Cord Injury. Cell Rep. 2019, 27, 71–85. [Google Scholar] [CrossRef]
- Oscarsson, Q. Functional Organization of the Spino- and Cuneocerebellar Tracts. Physiol. Rev. 1965, 45, 495–522. [Google Scholar] [CrossRef]
- Rahimi-Balaei, M.; Afsharinezhad, P.; Bailey, K.; Buchok, M.; Yeganeh, B.; Marzban, H. Embryonic stages in cerebellar afferent development. Cerebellum Ataxias 2015, 2, 7. [Google Scholar] [CrossRef]
- Loutit, A.J.; Vickery, R.M.; Potas, J.R. Functional organization and connectivity of the dorsal column nuclei complex reveals a sensorimotor integration and distribution hub. J. Comp. Neurol. 2020, 529, 187–220. [Google Scholar] [CrossRef]
- Sheikh, I.S.; Keefe, K.M.; Sterling, N.A.; Junker, I.P.; Li, C.; Chen, J.; Xu, X.-M.; Kirby, L.G.; Smith, G.M. Compensatory adaptation of parallel motor pathways promotes skilled forelimb recovery after spinal cord injury. iScience 2024, 27, 111371. [Google Scholar] [CrossRef]
- Krashes, M.J.; Koda, S.; Ye, C.; Rogan, S.C.; Adams, A.C.; Cusher, D.S.; Maratos-Flier, E.; Roth, B.L.; Lowell, B.B. Rapid, reversible activation of AgRP neurons drives feeding behavior in mice. J. Clin. Investig. 2011, 121, 1424–1428. [Google Scholar] [CrossRef]
- Klein, A.; Dunnett, S.B. Analysis of skilled forelimb movement in rats: The single pellet reaching test and staircase test. Curr. Protoc. Neurosci. 2012, 58, 8–28. [Google Scholar] [CrossRef]
- Gensel, J.C.; Tovar, C.A.; Hamers, F.P.; Deibert, R.J.; Beattie, M.S.; Bresnahan, J.C. Behavioral and histological characterization of unilateral cervical spinal cord contusion injury in rats. J. Neurotrauma. 2006, 23, 36–54. [Google Scholar] [CrossRef] [PubMed]
- Irvine, K.-A.; Ferguson, A.R.; Mitchell, K.D.; Beattie, S.B.; Beattie, M.S.; Bresnahan, J.C. A Novel Method for Assessing Proximal and Distal Forelimb Function in the Rat: The Irvine, Beatties and Bresnahan (IBB) Forelimb Scale. J. Vis. Exp. 2010, 46, e2246. [Google Scholar] [CrossRef]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates, 7th ed.; Academic Press: New York, NY, USA, 2014. [Google Scholar]
- Ebner, F.F.; Kaas, J.H. Somatosensory system. In Rat Nervous System, 4th ed.; 2015; Chapter 24; pp. 675–701. [Google Scholar] [CrossRef]
- Matsushita, M.; Ikeda, M. The central cervical nucleus as cell origin of a spinocerebellar tract arising from the cervical cord: A study in the cat using horseradish peroxidase. Brain Research 1975, 100, 412–417. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.H.; Cooper, S.E.; Hacking, A.; Ghez, C. Differential Effects of Deep Cerebellar Nuclei Inactivation on Reaching and Adaptive Control. J. Neurophysiol. 2000, 83, 1886–1899. [Google Scholar] [CrossRef]
- Uemura, Y.; Haque, T.; Sato, F.; Tsutsumi, Y.; Ohara, H.; Oka, A.; Furuta, T.; Bae, Y.C.; Yamashiro, T.; Tachibana, Y.; et al. Proprioceptive thalamus receiving forelimb and neck muscle spindle inputs via the external cuneate nucleus in the rat. Brain Struct. Funct. 2020, 225, 2177–2192. [Google Scholar] [CrossRef]
- Boivie, J.; Boman, K. Termination of a separate (proprioceptive?) cuneothalamic tract from external cuneate nucleus in monkey. Brain Res. 1981, 224, 235–246. [Google Scholar] [CrossRef]
- Gerrits, N.M.; Voogd, J.; Nas, W.S.C. Cerebellar and olivary projections of the external and rostral internal cuneate nuclei in the cat. Exp. Brain Res. 1985, 57, 239–255. [Google Scholar] [CrossRef]
- Jasmin, L.; Courville, J. Distribution of external cuneate nucleus afferents to the cerebellum: I. Notes on the projections from the main cuneate and other adjacent nuclei. an experimental study with radioactive tracers in the cat. J. Comp. Neurol. 1987, 261, 481–496. [Google Scholar] [CrossRef]
- Hirai, N.; Hongo, T.; Sasaki, S. Cerebellar projection and input organizations of the spinocerebellar tract arising from the central cervical nucleus in the cat. Brain Res. 1978, 157, 341–345. [Google Scholar] [CrossRef]
- Hirai, N.; Hongo, T.; Sasaki, S.; Yamashita, M.; Yoshida, K. Neck muscle afferent input to spinocerebellar tract cells of the central cervical nucleus in the cat. Exp. Brain Res. 1984, 55, 286–300. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, M.; Yaginuma, H. Projections from the central cervical nucleus to the cerebellar nuclei in the rat, studied by anterograde axonal tracing. J. Comp. Neurol. 1995, 353, 234–246. [Google Scholar] [CrossRef] [PubMed]
- Alstermark, B.; Ekerot, C.F. The lateral reticular nucleus; integration of descending and ascending systems regulating voluntary forelimb movements. Front. Comput. Neurosci. 2015, 9, 102. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.S.; Sugihara, I.; Shinoda, Y. Projection patterns of single mossy fibers originating from the lateral reticular nucleus in the rat cerebellar cortex and nuclei. J. Comp. Neurol. 1999, 411, 97–118. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eneanya, C.; Smith, G.M. The Sensory Input from the External Cuneate Nucleus and Central Cervical Nucleus to the Cerebellum Refines Forelimb Movements. Cells 2025, 14, 589. https://doi.org/10.3390/cells14080589
Eneanya C, Smith GM. The Sensory Input from the External Cuneate Nucleus and Central Cervical Nucleus to the Cerebellum Refines Forelimb Movements. Cells. 2025; 14(8):589. https://doi.org/10.3390/cells14080589
Chicago/Turabian StyleEneanya, Chidubem, and George M. Smith. 2025. "The Sensory Input from the External Cuneate Nucleus and Central Cervical Nucleus to the Cerebellum Refines Forelimb Movements" Cells 14, no. 8: 589. https://doi.org/10.3390/cells14080589
APA StyleEneanya, C., & Smith, G. M. (2025). The Sensory Input from the External Cuneate Nucleus and Central Cervical Nucleus to the Cerebellum Refines Forelimb Movements. Cells, 14(8), 589. https://doi.org/10.3390/cells14080589






