Beyond Infection: The Role of Secreted Viral Proteins in Pathogenesis, Disease Severity and Diagnostic Applications
Abstract
1. Introduction
2. Exploiting the Cellular Machinery: Pathways Used by Secreted Viral Proteins
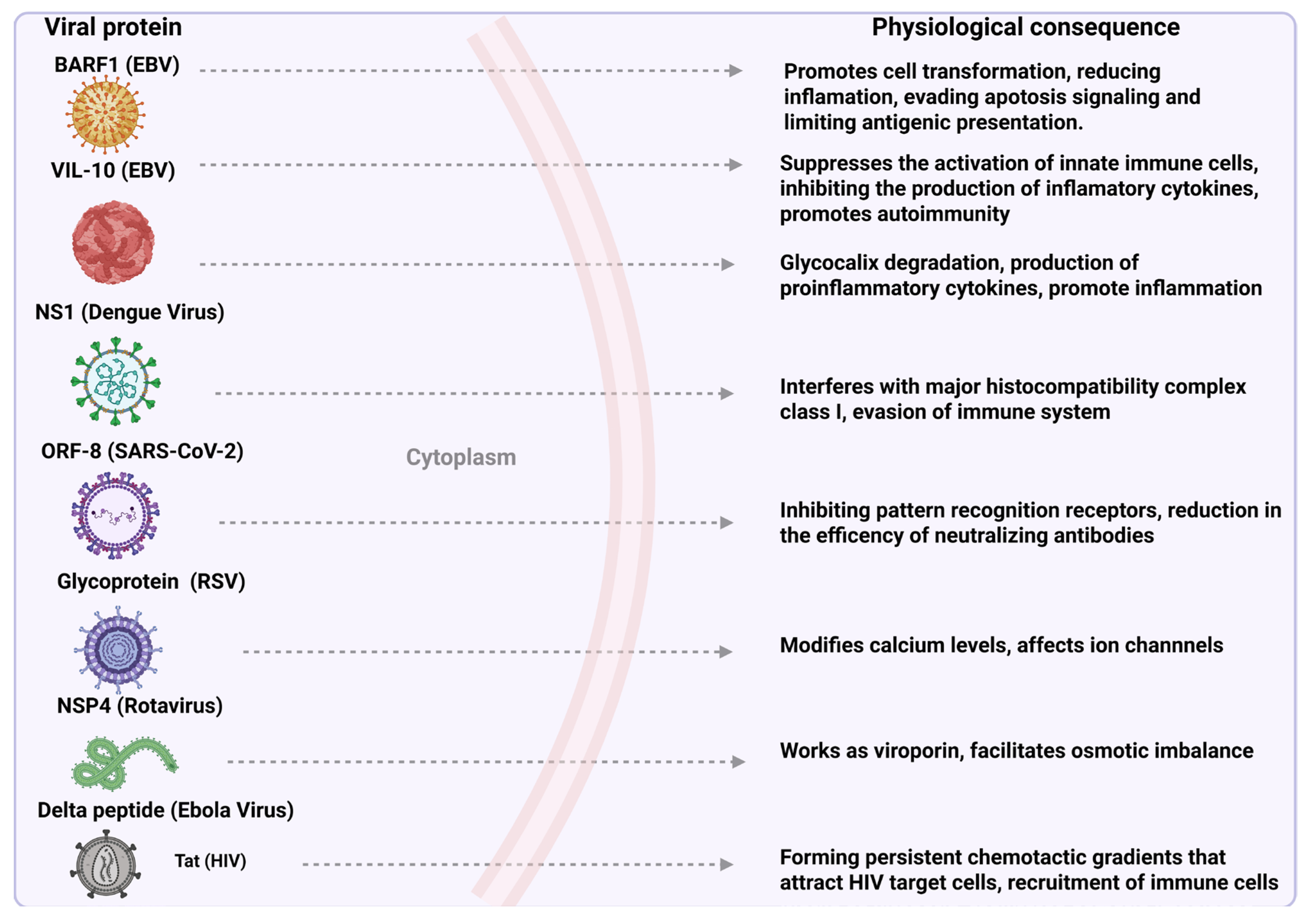
3. Viral Secreted Proteins: Mechanisms and Relevance to Pathogenesis
3.1. Epstein–Barr Virus (EBV)
- BamHI-A Rightward Frame 1 (BARF1)
- Viral Interleukin 10 (vIL-10 Protein)
3.2. Dengue Virus
- Nonstructural 1 Protein (NS1)
3.3. Rotavirus
- Nonstructural Protein 4 (NSP4)
3.4. Human Immunodeficiency Virus (HIV)
- Tat Protein (Tat)
3.5. Respiratory Syncytial Virus (RSV)
- Glycoprotein (G)
3.6. SARS-CoV-2
- Open Reading Frame 8 (ORF8)
- Nonstructural Protein 2 (NSP2)
3.7. Ebola Virus
Delta Peptide (Δ-Peptide)
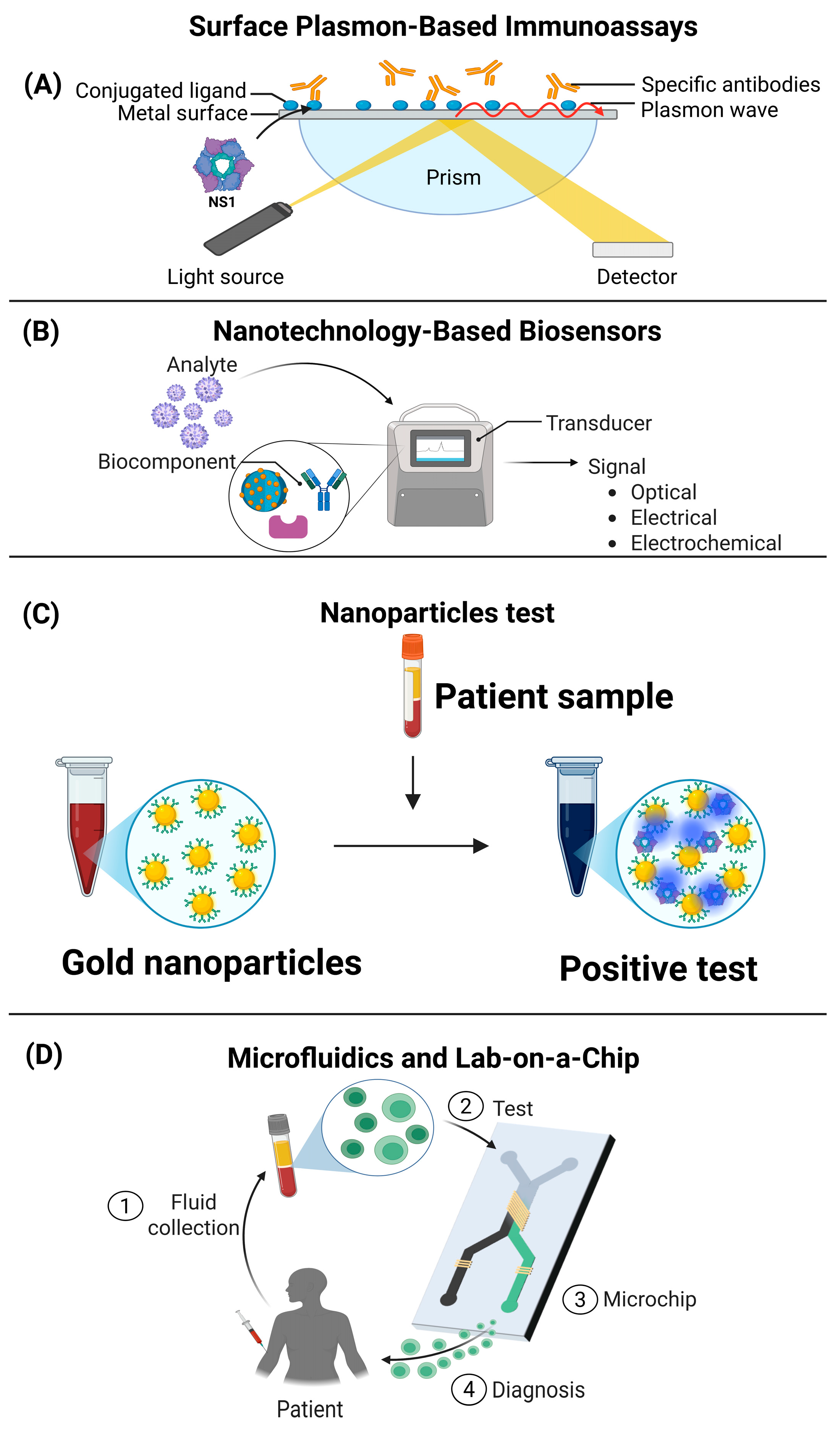
4. Emerging Technologies for Viral Diagnostics Based on Secreted Proteins
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schatz, M.; Tong, P.B.V.; Beaumelle, B. Unconventional secretion of viral proteins. Semin. Cell Dev. Biol. 2018, 83, 8–11. [Google Scholar] [CrossRef] [PubMed]
- Nickel, W.; Rabouille, C. Mechanisms of regulated unconventional protein secretion. Nat. Rev. Mol. Cell Biol. 2009, 10, 148–155, Erratum in Nat. Rev. Mol. Cell Biol. 2009, 10, 234. [Google Scholar] [CrossRef] [PubMed]
- Simon, F.; Thoma-Kress, A.K. Intercellular Transport of Viral Proteins. Results Probl. Cell Differ. 2024, 73, 435–474. [Google Scholar] [CrossRef] [PubMed]
- Sepúlveda, C.S.; García, C.C.; Damonte, E.B. Determining the Virus Life-Cycle Stage Blocked by an Antiviral. Methods Mol. Biol. 2018, 1604, 371–392. [Google Scholar] [CrossRef] [PubMed]
- Rabouille, C.; Kondylis, V. TANGOing along the protein secretion pathway. Genome Biol. 2006, 7, 213. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rosales Ramirez, R.; Ludert, J.E. The dengue virus nonstructural protein 1 (Ns1) is secreted from mosquito cells in association with the intracellular cholesterol transporter chaperone caveolin complex. J. Virol. 2019, 93, e01985-18. [Google Scholar] [CrossRef]
- Mompeán, M.; Treviño, M.Á.; Laurents, D.V. Partial structure, dampened mobility, and modest impact of a His tag in the SARS-CoV-2 Nsp2 C-terminal region. Eur. Biophys. J. 2021, 50, 1129–1137. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Safadi, D.E.; Lebeau, G.; Lagrave, A.; Mélade, J.; Grondin, L.; Rosanaly, S.; Begue, F.; Hoareau, M.; Veeren, B.; Roche, M.; et al. Extracellular Vesicles Are Conveyors of the NS1 Toxin during Dengue Virus and Zika Virus Infection. Viruses 2023, 15, 364. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zheng, N.; Liu, S.; Chen, J.; Xu, Y.; Cao, W.; Lin, J.; Lu, G.; Zhang, G. SARS-CoV-2 NSP2 as a Potential Delivery Vehicle for Proteins. Mol. Pharm. 2024, 21, 1149–1159. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Yang, Z.; Li, J. Exosomes and SARS-CoV-2 infection. Front. Immunol. 2024, 15, 1467109. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Peng, Y.; Yang, Y.; Li, Y.; Shi, T.; Luan, Y.; Yin, C. Exosome and virus infection. Front. Immunol. 2023, 14, 1154217. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lam, S.M.; Huang, X.; Shui, G. Neurological aspects of SARS-CoV-2 infection: Lipoproteins and exosomes as Trojan horses. Trends Endocrinol. Metab. 2022, 33, 554–568. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chahar, H.S.; Bao, X.; Casola, A. Exosomes and Their Role in the Life Cycle and Pathogenesis of RNA Viruses. Viruses 2015, 7, 3204–3225. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mizuno-Yamasaki, E.; Rivera-Molina, F.; Novick, P. GTPase networks in membrane traffic. Annu. Rev. Biochem. 2012, 81, 637–659. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Reyes-Ruiz, J.M.; Osuna-Ramos, J.F.; De Jesús-González, L.A.; Hurtado-Monzón, A.M.; Farfan-Morales, C.N.; Cervantes-Salazar, M.; Bolaños, J.; Cigarroa-Mayorga, O.E.; Martín-Martínez, E.S.; Medina, F.; et al. Isolation and characterization of exosomes released from mosquito cells infected with dengue virus. Virus. Res. 2019, 266, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Dash, P.K.; Kumari, T.; Guo, M.L.; Ghosh, J.K.; Buch, S.J.; Tripathi, R.K. HIV-1 Nef hijacks both exocytic and endocytic pathways of host intracellular trafficking through differential regulation of Rab GTPases. Biol. Cell 2022, 114, 276–292. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zeng, C.Q.; Morris, A.P.; Estes, M.K. A functional NSP4 enterotoxin peptide secreted from rotavirus-infected cells. J. Virol. 2000, 74, 11663–11670. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rayne, F.; Debaisieux, S.; Yezid, H.; Lin, Y.L.; Mettling, C.; Konate, K.; Chazal, N.; Arold, S.T.; Pugnière, M.; Sanchez, F.; et al. Phosphatidylinositol-(4,5)-bisphosphate enables efficient secretion of HIV-1 Tat by infected T-cells. EMBO J. 2010, 29, 1348–1362. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, J.Y.; Liao, C.H.; Wang, Q.; Tan, Y.J.; Luo, R.; Qiu, Y.; Ge, X.Y. The ORF6, ORF8 and nucleocapsid proteins of SARS-CoV-2 inhibit type I interferon signaling pathway. Virus. Res. 2020, 286, 198074. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tarbouriech, N.; Ruggiero, F.; de Turenne-Tessier, M.; Ooka, T.; Burmeister, W.P. Structure of the Epstein-Barr virus oncogene BARF1. J. Mol. Biol. 2006, 359, 667–678. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Liu, Z.; Zeng, B.; Hu, G.; Gan, R. Epstein-Barr virus-associated gastric cancer: A distinct subtype. Cancer Lett. 2020, 495, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Hoebe, E.K.; Hutajulu, S.H.; van Beek, J.; Stevens, S.J.; Paramita, D.K.; Greijer, A.E.; Middeldorp, J.M. Purified hexameric Epstein-Barr virus-encoded BARF1 protein for measuring anti-BARF1 antibody responses in nasopharyngeal carcinoma patients. Clin. Vaccine Immunol. 2011, 18, 298–304. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hoebe, E.K.; Le Large, T.Y.; Greijer, A.E.; Middeldorp, J.M. BamHI-A rightward frame 1, an Epstein-Barr virus-encoded oncogene and immune modulator. Rev. Med. Virol. 2013, 23, 367–383. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shim, A.H.; Chang, R.A.; Chen, X.; Longnecker, R.; He, X. Multipronged attenuation of macrophage-colony stimulating factor signaling by Epstein-Barr virus BARF1. Proc. Natl. Acad. Sci. USA 2012, 109, 12962–12967. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, Y.; Xu, M.; Zhang, X.; Chu, F.; Zhou, T. MAPK/c-Jun signaling pathway contributes to the upregulation of the anti-apoptotic proteins Bcl-2 and Bcl-xL induced by Epstein-Barr virus-encoded BARF1 in gastric carcinoma cells. Oncol. Lett. 2018, 15, 7537–7544. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Seto, E.; Yang, L.; Middeldorp, J.; Sheen, T.S.; Chen, J.Y.; Fukayama, M.; Eizuru, Y.; Ooka, T.; Takada, K. Epstein-Barr virus (EBV)-encoded BARF1 gene is expressed in nasopharyngeal carcinoma and EBV-associated gastric carcinoma tissues in the absence of lytic gene expression. J. Med. Virol. 2005, 76, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Tsao, S.W.; Ooka, T.; Nicholls, J.M.; Cheung, H.W.; Fu, S.; Wong, Y.C.; Wang, X. Anti-apoptotic role of BARF1 in gastric cancer cells. Cancer Lett. 2006, 238, 90–103, Erratum in Cancer Lett. 2015, 369, 427. [Google Scholar] [CrossRef] [PubMed]
- Sakka, E.; Zur Hausen, A.; Houali, K.; Liu, H.; Fiorini, S.; Ooka, T. Cellular localization of BARF1 oncoprotein and its cell stimulating activity in human epithelial cell. Virus. Res. 2013, 174, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.S.; Kim, D.H.; Roh, J.K.; Middeldorp, J.M.; Kim, Y.S.; Kim, S.; Han, S.; Kim, C.W.; Lee, B.L.; Kim, W.H.; et al. Epstein-Barr virus-encoded BARF1 promotes proliferation of gastric carcinoma cells through regulation of NF-κB. J. Virol. 2013, 87, 10515–10523. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mosser, D.M.; Zhang, X. Interleukin-10: New perspectives on an old cytokine. Immunol. Rev. 2008, 226, 205–218. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yoon, S.I.; Jones, B.C.; Logsdon, N.J.; Walter, M.R. Same structure, different function crystal structure of the Epstein-Barr virus IL-10 bound to the soluble IL-10R1 chain. Structure 2005, 13, 551–564. [Google Scholar] [CrossRef] [PubMed]
- Jog, N.R.; Chakravarty, E.F.; Guthridge, J.M.; James, J.A. Epstein Barr Virus Interleukin 10 Suppresses Anti-inflammatory Phenotype in Human Monocytes. Front. Immunol. 2018, 9, 2198. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ren, Y.X.; Yang, J.; Sun, R.M.; Zhang, L.J.; Zhao, L.F.; Li, B.Z.; Li, L.; Long, H.T.; Sun, Q.M.; Huang, Y.C.; et al. Viral IL-10 down-regulates the “MHC-I antigen processing operon” through the NF-κB signaling pathway in nasopharyngeal carcinoma cells. Cytotechnology 2016, 68, 2625–2636. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ren, Y.; Yang, J.; Li, M.; Huang, N.; Chen, Y.; Wu, X.; Wang, X.; Qiu, S.; Wang, H.; Li, X. Viral IL-10 promotes cell proliferation and cell cycle progression via JAK2/STAT3 signaling pathway in nasopharyngeal carcinoma cells. Biotechnol. Appl. Biochem. 2020, 67, 929–938. [Google Scholar] [CrossRef] [PubMed]
- Muller, D.A.; Young, P.R. The flavivirus NS1 protein: Molecular and structural biology, immunology, role in pathogenesis and application as a diagnostic biomarker. Antiviral Res. 2013, 98, 192–208. [Google Scholar] [CrossRef] [PubMed]
- Silva, T.; Gomes, L.; Jeewandara, C.; Ogg, G.S.; Malavige, G.N. Dengue NS1 induces phospholipase A2 enzyme activity, prostaglandins, and inflammatory cytokines in monocytes. Antiviral. Res. 2022, 202, 105312. [Google Scholar] [CrossRef] [PubMed]
- Perera, D.R.; Ranadeva, N.D.; Sirisena, K.; Wijesinghe, K.J. Roles of NS1 Protein in Flavivirus Pathogenesis. ACS Infect. Dis. 2024, 10, 20–56. [Google Scholar] [CrossRef] [PubMed]
- Gutsche, I.; Coulibaly, F.; Voss, J.E.; Salmon, J.; d’Alayer, J.; Ermonval, M.; Larquet, E.; Charneau, P.; Krey, T.; Mégret, F.; et al. Secreted dengue virus nonstructural protein NS1 is an atypical barrel-shaped high-density lipoprotein. Proc. Natl. Acad. Sci. USA 2011, 108, 8003–8808. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, S.; He, Y.; Wu, Z.; Wang, M.; Jia, R.; Zhu, D.; Liu, M.; Zhao, X.; Yang, Q.; Wu, Y.; et al. Secretory pathways and multiple functions of nonstructural protein 1 in flavivirus infection. Front. Immunol. 2023, 14, 1205002. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Scaturro, P.; Cortese, M.; Chatel-Chaix, L.; Fischl, W.; Bartenschlager, R. Dengue Virus Non-structural Protein 1 Modulates Infectious Particle Production via Interaction with the Structural Proteins. PLoS Pathog. 2015, 11, e1005277. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Benfrid, S.; Park, K.H.; Dellarole, M.; Voss, J.E.; Tamietti, C.; Pehau-Arnaudet, G.; Raynal, B.; Brûlé, S.; England, P.; Zhang, X.; et al. Dengue virus NS1 protein conveys pro-inflammatory signals by docking onto high-density lipoproteins. EMBO Rep. 2022, 23, e53600. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Puerta-Guardo, H.; Glasner, D.R.; Espinosa, D.A.; Biering, S.B.; Patana, M.; Ratnasiri, K.; Wang, C.; Beatty, P.R.; Harris, E. Flavivirus NS1 Triggers Tissue-Specific Vascular Endothelial Dysfunction Reflecting Disease Tropism. Cell Rep. 2019, 26, 1598–1613.e8. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chan, K.W.K.; Watanabe, S.; Jin, J.Y.; Pompon, J.; Teng, D.; Alonso, S.; Vijaykrishna, D.; Halstead, S.B.; Marzinek, J.K.; Bond, P.J.; et al. A T164S mutation in the dengue virus NS1 protein is associated with greater disease severity in mice. Sci. Transl. Med. 2019, 11, eaat7726. [Google Scholar] [CrossRef] [PubMed]
- Conde, J.N.; da Silva, E.M.; Allonso, D.; Coelho, D.R.; Andrade, I.D.S.; de Medeiros, L.N.; Menezes, J.L.; Barbosa, A.S.; Mohana-Borges, R. Inhibition of the Membrane Attack Complex by Dengue Virus NS1 through Interaction with Vitronectin and Terminal Complement Proteins. J. Virol. 2016, 90, 9570–9581. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Avirutnan, P.; Punyadee, N.; Noisakran, S.; Komoltri, C.; Thiemmeca, S.; Auethavornanan, K.; Jairungsri, A.; Kanlaya, R.; Tangthawornchaikul, N.; Puttikhunt, C.; et al. Vascular leakage in severe dengue virus infections: A potential role for the nonstructural viral protein NS1 and complement. J. Infect. Dis. 2006, 193, 1078–1088. [Google Scholar] [CrossRef] [PubMed]
- Puerta-Guardo, H.; Biering, S.B.; de Sousa, F.T.G.; Shu, J.; Glasner, D.R.; Li, J.; Blanc, S.F.; Beatty, P.R.; Harris, E. Flavivirus NS1 Triggers Tissue-Specific Disassembly of Intercellular Junctions Leading to Barrier Dysfunction and Vascular Leak in a GSK-3β-Dependent Manner. Pathogens 2022, 11, 615. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Youn, S.; Cho, H.; Fremont, D.H.; Diamond, M.S. A short n-terminal peptide motif on flavivirus nonstructural protein Ns1 modulates cellular targeting and immune recognition. J. Virol. 2010, 84, 9516–9532. [Google Scholar] [CrossRef]
- Patro, A.R.K.; Mohanty, S.; Prusty, B.K.; Singh, D.K.; Gaikwad, S.; Saswat, T.; Chattopadhyay, S.; Das, B.K.; Tripathy, R.; Ravindran, B. Cytokine Signature Associated with Disease Severity in Dengue. Viruses 2019, 11, 34. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Alcon, S.; Talarmin, A.; Debruyne, M.; Falconar, A.; Deubel, V.; Flamand, M. Enzyme-linked immunosorbent assay specific to Dengue virus type 1 nonstructural protein NS1 reveals circulation of the antigen in the blood during the acute phase of disease in patients experiencing primary or secondary infections. J. Clin. Microbiol. 2002, 40, 376–381. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Watterson, D.; Modhiran, N.; Young, P.R. The many faces of the flavivirus NS1 protein offer a multitude of options for inhibitor design. Antivir. Res. 2016, 130, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Ramappa, R.; Pamidimukkala, K.; Rao, C.D.; Suguna, K. New tetrameric forms of the rotavirus NSP4 with antiparallel helices. Arch. Virol. 2018, 163, 1531–1547. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H. Rotavirus Replication: Gaps of Knowledge on Virus Entry and Morphogenesis. Tohoku J. Exp. Med. 2019, 248, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Nurdin, J.A.; Kotaki, T.; Kawagishi, T.; Sato, S.; Yamasaki, M.; Nouda, R.; Minami, S.; Kanai, Y.; Kobayashi, T. N-Glycosylation of Rotavirus NSP4 Protein Affects Viral Replication and Pathogenesis. J. Virol. 2023, 97, e0186122. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bugarcic, A.; Taylor, J.A. Rotavirus nonstructural glycoprotein NSP4 is secreted from the apical surfaces of polarized epithelial cells. J. Virol. 2006, 80, 12343–12349. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gonzalez-Ochoa, G.; Flores-Mendoza, L.K.; Icedo-Garcia, R.; Gomez-Flores, R.; Tamez-Guerra, P. Modulation of rotavirus severe gastroenteritis by the combination of probiotics and prebiotics. Arch. Microbiol. 2017, 199, 953–961. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ousingsawat, J.; Mirza, M.; Tian, Y.; Roussa, E.; Schreiber, R.; Cook, D.I.; Kunzelmann, K. Rotavirus toxin NSP4 induces diarrhea by activation of TMEM16A and inhibition of Na+ absorption. Pflug. Arch. 2011, 461, 579–589. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Zeng, C.Q.; Ball, J.M.; Estes, M.K.; Morris, A.P. The rotavirus enterotoxin NSP4 mobilizes intracellular calcium in human intestinal cells by stimulating phospholipase C-mediated inositol 1,4,5-trisphosphate production. Proc. Natl. Acad. Sci. USA 1997, 94, 3960–3965. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yeom, J.S.; Kim, Y.S.; Jun, J.S.; Do, H.J.; Park, J.S.; Seo, J.H.; Park, E.S.; Lim, J.Y.; Woo, H.O.; Park, C.H.; et al. NSP4 antibody levels in rotavirus gastroenteritis patients with seizures. Eur. J. Paediatr. Neurol. 2017, 21, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.; Perry, J.L.; Dosey, T.L.; Delcour, A.H.; Hyser, J.M. The Rotavirus NSP4 Viroporin Domain is a Calcium-conducting Ion Channel. Sci. Rep. 2017, 7, 43487. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chang-Graham, A.L.; Perry, J.L.; Strtak, A.C.; Ramachandran, N.K.; Criglar, J.M.; Philip, A.A.; Patton, J.T.; Estes, M.K.; Hyser, J.M. Rotavirus Calcium Dysregulation Manifests as Dynamic Calcium Signaling in the Cytoplasm and Endoplasmic Reticulum. Sci. Rep. 2019, 9, 10822. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Afchangi, A.; Jalilvand, S.; Arashkia, A.; Latifi, T.; Farahmand, M.; Abolghasem Shirazi, M.M.; Mousavi Nasab, S.D.; Marashi, S.M.; Roohvand, F.; Shoja, Z. Co-administration of rotavirus nanospheres VP6 and NSP4 proteins enhanced the anti-NSP4 humoral responses in immunized mice. Microb. Pathog. 2022, 163, 105405. [Google Scholar] [CrossRef] [PubMed]
- Hyser, J.M.; Estes, M.K. Pathophysiological Consequences of Calcium-Conducting Viroporins. Annu. Rev. Virol. 2015, 2, 473–496. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Alshaghdali, K.; Saeed, M.; Kamal, M.A.; Saeed, A. Interaction of Ectodomain of Respiratory Syncytial Virus G Protein with TLR2/TLR6 Heterodimer: An In vitro and In silico Approach to Decipher the Role of RSV G Protein in Pro-inflammatory Response against the Virus. Curr. Pharm. Des. 2021, 27, 4464–4476. [Google Scholar] [CrossRef] [PubMed]
- Zeitler, M.; Steringer, J.P.; Müller, H.M.; Mayer, M.P.; Nickel, W. HIV-Tat Protein Forms Phosphoinositide-dependent Membrane Pores Implicated in Unconventional Protein Secretion. J. Biol. Chem. 2015, 290, 21976–21984. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cao, H.; Wu, J.; Luan, N.; Wang, Y.; Lin, K.; Liu, C. Evaluation of a bivalent recombinant vaccine candidate targeting norovirus and rotavirus: Antibodies to rotavirus NSP4 exert antidiarrheal effects without virus neutralization. J. Med. Virol. 2022, 94, 3847–3856. [Google Scholar] [CrossRef] [PubMed]
- Kurnaeva, M.A.; Sheval, E.V.; Musinova, Y.R.; Vassetzky, Y.S. Tat basic domain: A “Swiss army knife” of HIV-1 Tat? Rev. Med. Virol. 2019, 29, e2031. [Google Scholar] [CrossRef] [PubMed]
- Salfeld, J.; Göttlinger, H.G.; Sia, R.A.; Park, R.E.; Sodroski, J.G.; Haseltine, W.A. A tripartite HIV-1 tat-env-rev fusion protein. EMBO J. 1990, 9, 965–970. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, C.; Mori, L.P.; Lyu, S.; Bronson, R.; Getzler, A.J.; Pipkin, M.E.; Valente, S.T. The chaperone protein p32 stabilizes HIV-1 Tat and strengthens the p-TEFb/RNAPII/TAR complex promoting HIV transcription elongation. Proc. Natl. Acad. Sci. USA 2023, 120, e2217476120. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Faust, T.B.; Binning, J.M.; Gross, J.D.; Frankel, A.D. Making Sense of Multifunctional Proteins: Human Immunodeficiency Virus Type 1 Accessory and Regulatory Proteins and Connections to Transcription. Annu. Rev. Virol. 2017, 4, 241–260. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cafaro, A.; Schietroma, I.; Sernicola, L.; Belli, R.; Campagna, M.; Mancini, F.; Farcomeni, S.; Pavone-Cossut, M.R.; Borsetti, A.; Monini, P.; et al. Role of HIV-1 Tat Protein Interactions with Host Receptors in HIV Infection and Pathogenesis. Int. J. Mol. Sci. 2024, 25, 1704. [Google Scholar] [CrossRef]
- Cafaro, A.; Barillari, G.; Moretti, S.; Palladino, C.; Tripiciano, A.; Falchi, M.; Picconi, O.; Pavone Cossut, M.R.; Campagna, M.; Arancio, A.; et al. HIV-1 Tat Protein Enters Dysfunctional Endothelial Cells via Integrins and Renders Them Permissive to Virus Replication. Int. J. Mol. Sci. 2020, 22, 317. [Google Scholar] [CrossRef] [PubMed]
- Ensoli, B.; Moretti, S.; Borsetti, A.; Maggiorella, M.T.; Buttò, S.; Picconi, O.; Tripiciano, A.; Sgadari, C.; Monini, P.; Cafaro, A. New insights into pathogenesis point to HIV-1 Tat as a key vaccine target. Arch. Virol. 2021, 166, 2955–2974. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Urbinati, C.; Milanesi, M.; Lauro, N.; Bertelli, C.; David, G.; D’Ursi, P.; Rusnati, M.; Chiodelli, P. HIV-1 Tat and Heparan Sulfate Proteoglycans Orchestrate the Setup of in Cis and in Trans Cell-Surface Interactions Functional to Lymphocyte Trans-Endothelial Migration. Molecules 2021, 26, 7488. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Antell, G.C.; Dampier, W.; Aiamkitsumrit, B.; Nonnemacher, M.R.; Jacobson, J.M.; Pirrone, V.; Zhong, W.; Kercher, K.; Passic, S.; Williams, J.W.; et al. Utilization of HIV-1 envelope V3 to identify X4- and R5-specific Tat and LTR sequence signatures. Retrovirology 2016, 13, 32. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tripiciano, A.; Picconi, O.; Moretti, S.; Sgadari, C.; Cafaro, A.; Francavilla, V.; Arancio, A.; Paniccia, G.; Campagna, M.; Pavone-Cossut, M.R.; et al. Anti-Tat immunity defines CD4+ T-cell dynamics in people living with HIV on long-term cART. EBioMedicine 2021, 66, 103306. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hamza, A.; Shafat, Z.; Parray, Z.A.; Hisamuddin, M.; Khan, W.H.; Ahmed, A.; Almajhdi, F.N.; Farrag, M.A.; Mohammed, A.A.; Islam, A.; et al. Structural Characterization and Binding Studies of the Ectodomain G Protein of Respiratory Syncytial Virus Reveal the Crucial Role of pH with Possible Implications in Host-Pathogen Interactions. ACS Omega 2021, 6, 10403–10414. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liang, B.; Kabatova, B.; Kabat, J.; Dorward, D.W.; Liu, X.; Surman, S.; Liu, X.; Moseman, A.P.; Buchholz, U.J.; Collins, P.L.; et al. Effects of Alterations to the CX3C Motif and Secreted Form of Human Respiratory Syncytial Virus (RSV) G Protein on Immune Responses to a Parainfluenza Virus Vector Expressing the RSV G Protein. J. Virol. 2019, 93, e02043-18. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Escribano-Romero, E.; Rawling, J.; García-Barreno, B.; Melero, J.A. The soluble form of human respiratory syncytial virus attachment protein differs from the membrane-bound form in its oligomeric state but is still capable of binding to cell surface proteoglycans. J. Virol. 2004, 78, 3524–3532. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Anderson, J.; Do, L.A.H.; van Kasteren, P.B.; Licciardi, P.V. The role of respiratory syncytial virus G protein in immune cell infection and pathogenesis. EBioMedicine 2024, 107, 105318. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hamza, A.; Samad, A.; Parray, Z.A.; Ara, S.; Ahmed, A.; Almajhdi, F.N.; Hussain, T.; Islam, A.; Parveen, S. Mutation in the CX3C Motif of G Protein Disrupts Its Interaction with Heparan Sulfate: A Calorimetric, Spectroscopic, and Molecular Docking Study. Int. J. Mol. Sci. 2022, 23, 1950. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- De Graaff, P.M.; Heidema, J.; Poelen, M.C.; van Dijk, M.E.; Lukens, M.V.; van Gestel, S.P.; Reinders, J.; Rozemuller, E.; Tilanus, M.; Hoogerhout, P.; et al. HLA-DP4 presents an immunodominant peptide from the RSV G protein to CD4 T cells. Virology 2004, 326, 220–230. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Teng, M.N.; Whitehead, S.S.; Collins, P.L. Contribution of the respiratory syncytial virus G glycoprotein and its secreted and membrane-bound forms to virus replication in vitro and in vivo. Virology 2001, 289, 283–296. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.Q.; Zhou, Y.; Wang, Q.L.; Chen, J.; Chen, H.; Xie, H.H.; Li, L. Conciliatory Anti-Allergic Decoction Attenuates Pyroptosis in RSV-Infected Asthmatic Mice and Lipopolysaccharide (LPS)-Induced 16HBE Cells by Inhibiting TLR3/NLRP3/NF-κB/IRF3 Signaling Pathway. J. Immunol. Res. 2022, 2022, 1800401. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bukreyev, A.; Serra, M.E.; Laham, F.R.; Melendi, G.A.; Kleeberger, S.R.; Collins, P.L.; Polack, F.P. The Cysteine-Rich Region and Secreted Form of the Attachment G Glycoprotein of Respiratory Syncytial Virus Enhance the Cytotoxic T-Lymphocyte Response despite Lacking Major Histocompatibility Complex Class I-Restricted Epitopes. J. Virol. 2006, 80, 5854–5861. [Google Scholar] [CrossRef]
- Caidi, H.; Miao, C.; Thornburg, N.J.; Tripp, R.A.; Anderson, L.J.; Haynes, L.M. Anti-respiratory syncytial virus (RSV) G monoclonal antibodies reduce lung inflammation and viral lung titers when delivered therapeutically in a BALB/c mouse model. Antivir. Res. 2018, 154, 149–157. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Flower, T.G.; Buffalo, C.Z.; Hooy, R.M.; Allaire, M.; Ren, X.; Hurley, J.H. Structure of SARS-CoV-2 ORF8, a rapidly evolving immune evasion protein. Proc. Natl. Acad. Sci. USA 2021, 118, e2021785118. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vinjamuri, S.; Li, L.; Bouvier, M. SARS-CoV-2 ORF8: One protein, seemingly one structure, and many functions. Front. Immunol. 2022, 13, 1035559. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, X.; Wang, W.; Wang, T.; Wang, J.; Jiang, Y.; Wang, X.; Qiu, Z.; Feng, N.; Sun, W.; Li, C.; et al. SARS-CoV-2 ORF8 Protein Induces Endoplasmic Reticulum Stress-like Responses and Facilitates Virus Replication by Triggering Calnexin: An Unbiased Study. J. Virol. 2023, 97, e0001123. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lin, X.; Fu, B.; Xiong, Y.; Xing, N.; Xue, W.; Guo, D.; Zaky, M.; Pavani, K.; Kunec, D.; Trimpert, J.; et al. Unconventional secretion of unglycosylated ORF8 is critical for the cytokine storm during SARS-CoV-2 infection. PLoS Pathog. 2023, 19, e1011128. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ponde, N.O.; Shoger, K.E.; Khatun, M.S.; Sarkar, M.K.; Dey, I.; Taylor, T.C.; Cisney, R.N.; Arunkumar, S.P.; Gudjonsson, J.E.; Kolls, J.K.; et al. SARS-CoV-2 ORF8 Mediates Signals in Macrophages and Monocytes through MyD88 Independently of the IL-17 Receptor. J. Immunol. 2023, 211, 252–260. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lin, X.; Fu, B.; Yin, S.; Li, Z.; Liu, H.; Zhang, H.; Xing, N.; Wang, Y.; Xue, W.; Xiong, Y.; et al. ORF8 contributes to cytokine storm during SARS-CoV-2 infection by activating IL-17 pathway. iScience 2021, 24, 102293. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kumar, J.; Dhyani, S.; Kumar, P.; Sharma, N.R.; Ganguly, S. SARS-CoV-2-encoded ORF8 protein possesses complement inhibitory properties. J. Biol. Chem. 2023, 299, 102930. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rodriguez-Rodriguez, B.A.; Ciabattoni, G.O.; Duerr, R.; Valero-Jimenez, A.M.; Yeung, S.T.; Crosse, K.M.; Schinlever, A.R.; Bernard-Raichon, L.; Rodriguez Galvan, J.; McGrath, M.E.; et al. A neonatal mouse model characterizes transmissibility of SARS-CoV-2 variants and reveals a role for ORF8. Nat. Commun. 2023, 14, 3026. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, C.; Zheng, W.; Huang, X.; Bell, E.W.; Zhou, X.; Zhang, Y. Protein Structure and Sequence Reanalysis of 2019-nCoV Genome Refutes Snakes as Its Intermediate Host and the Unique Similarity between Its Spike Protein Insertions and HIV-1. J. Proteome Res. 2020, 19, 1351–1360. [Google Scholar] [CrossRef]
- Kohyama, M.; Suzuki, T.; Nakai, W.; Ono, C.; Matsuoka, S.; Iwatani, K.; Liu, Y.; Sakai, Y.; Nakagawa, A.; Tomii, K.; et al. SARS-CoV-2 ORF8 is a viral cytokine regulating immune responses. Int. Immunol. 2023, 35, 43–52. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ma, J.; Chen, Y.; Wu, W.; Chen, Z. Structure and Function of N-Terminal Zinc Finger Domain of SARS-CoV-2 NSP2. Virol Sin. 2021, 36, 1104–1112. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xu, Z.; Choi, J.H.; Dai, D.L.; Luo, J.; Ladak, R.J.; Li, Q.; Wang, Y.; Zhang, C.; Wiebe, S.; Liu, A.C.H.; et al. SARS-CoV-2 impairs interferon production via NSP2-induced repression of mRNA translation. Proc. Natl. Acad. Sci. USA 2022, 119, e2204539119. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jain, S.; Martynova, E.; Rizvanov, A.; Khaiboullina, S.; Baranwal, M. Structural and Functional Aspects of Ebola Virus Proteins. Pathogens 2021, 10, 1330. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Volchkova, V.A.; Klenk, H.D.; Volchkov, V.E. Delta-peptide is the carboxy-terminal cleavage fragment of the nonstructural small glycoprotein sGP of Ebola virus. Virology 1999, 265, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Melnik, L.I.; Guha, S.; Ghimire, J.; Smither, A.R.; Beddingfield, B.J.; Hoffmann, A.R.; Sun, L.; Ungerleider, N.A.; Baddoo, M.C.; Flemington, E.K.; et al. Ebola virus delta peptide is an enterotoxin. Cell Rep. 2022, 38, 110172. [Google Scholar] [CrossRef] [PubMed]
- Melnik, L.I.; Garry, R.F. Enterotoxigenic Escherichia coli Heat-Stable Toxin and Ebola Virus Delta Peptide: Similarities and Differences. Pathogens 2022, 11, 170. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pokhrel, R.; Pavadai, E.; Gerstman, B.S.; Chapagain, P.P. Membrane pore formation and ion selectivity of the Ebola virus delta peptide. Phys. Chem. Chem. Phys. 2019, 21, 5578–5585. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Melnik, L.I.; Komin, A.; Wiedman, G.; Fuselier, T.; Morris, C.F.; Starr, C.G.; Searson, P.C.; Gallaher, W.R.; Hristova, K.; et al. Ebola Virus Delta Peptide Is a Viroporin. J. Virol. 2017, 91, e00438-17. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Guha, S.; Ghimire, J.; Wu, E.; Wimley, W.C. Mechanistic Landscape of Membrane-Permeabilizing Peptides. Chem. Rev. 2019, 119, 6040–6085. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, J.; Eagles, D.A.; Tucker, I.J.; Pereira Schmidt, A.C.; Deplazes, E. Secondary structure propensities of the Ebola delta peptide E40 in solution and model membrane environments. Biophys. Chem. 2024, 314, 107318. [Google Scholar] [CrossRef] [PubMed]
- Lucas, A.; McFadden, G. Secreted immunomodulatory viral proteins as novel biotherapeutics. J. Immunol. 2004, 173, 4765–4774. [Google Scholar] [CrossRef] [PubMed]
- Durán, A.; González, A.; Delgado, L.; Mosquera, J.; Valero, N. Serum level of C-reactive protein is not a parameter to determine the difference between viral and atypical bacterial infections. J. Med. Virol. 2016, 88, 351–355. [Google Scholar] [CrossRef] [PubMed]
- Shaaban, A.; Du, Y.C. An Optical Universal Plasmon-Based Biosensor for Virus Detection. J. Med. Biol. Eng. 2023, 28, 1–8. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Alquraan, L.; Alzoubi, K.H.; Rababa’h, S.Y. Mutations of SARS-CoV-2 and their impact on disease diagnosis and severity. Inform. Med. Unlocked. 2023, 39, 101256. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Beckham, J.D.; Tyler, K.L. Arbovirus Infections. Neuroinfectious Dis. 2015, 21, 1599–1611. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Varghese, J.; De Silva, I.; Millar, D.S. Latest Advances in Arbovirus Diagnostics. Microorganisms 2023, 11, 1159. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kasbergen, L.M.R.; Nieuwenhuijse, D.F.; de Bruin, E.; Sikkema, R.S.; Koopmans, M.P.G. The increasing complexity of arbovirus serology: An in-depth systematic review on cross-reactivity. PLoS Negl. Trop. Dis. 2023, 17, e0011651. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ceconi, M.; Ariën, K.K.; Delputte, P. Diagnosing arthropod-borne flaviviruses: Non-structural protein 1 (NS1) as a biomarker. Trends Microbiol. 2024, 32, 678–696. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Manske, M.K.; Ruan, G.J.; Witter, T.L.; Nowakowski, K.E.; Abeykoon, J.P.; Tang, X.; Yu, Y.; Gwin, K.A.; Wu, A.; et al. Secreted ORF8 induces monocytic pro-inflammatory cytokines through NLRP3 pathways in patients with severe COVID-19. iScience 2023, 26, 106929. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Widoretno Sjahrurachman, A.; Dewi, B.E.; Lischer, K.; Pratami, D.K.; Flamandita, D.; Sahlan, M. Surface plasmon resonance analysis for detecting non-structural protein 1 of dengue virus in Indonesia. Saudi J. Biol. Sci. 2020, 27, 1931–1937. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wei, H.; Zhang, C.; Du, X.; Zhang, Z. Research progress of biosensors for detection of SARS-CoV-2 variants based on ACE2. Talanta 2023, 251, 123813. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, Y.; Zhang, L.; Wei, W.; Zhao, H.; Zhou, Z.; Zhang, Y.; Liu, S. Colorimetric detection of influenza A virus using antibody-functionalized gold nanoparticles. Analyst 2015, 140, 3989–3995. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Liu, G.; La, M.; Liu, L. Colorimetric and Electrochemical Methods for the Detection of SARS-CoV-2 Main Protease by Peptide-Triggered Assembly of Gold Nanoparticles. Molecules 2022, 27, 615. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Asghari, A.; Wang, C.; Yoo, K.M.; Rostamian, A.; Xu, X.; Shin, J.D.; Dalir, H.; Chen, R.T. Fast, accurate, point-of-care COVID-19 pandemic diagnosis enabled through advanced lab-on-chip optical biosensors: Opportunities and challenges. Appl. Phys. Rev. 2021, 8, 031313. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herrera-Moro Huitron, L.; Cruz-Holguin, V.J.; Ulloa-Aguilar, J.M.; De Jesús-González, L.A.; Osuna-Ramos, J.F.; Guzmán-Huerta, M.; de León-Bautista, M.P.; León-Reyes, G.; García-Cordero, J.; Cedillo-Barrón, L.; et al. Beyond Infection: The Role of Secreted Viral Proteins in Pathogenesis, Disease Severity and Diagnostic Applications. Cells 2025, 14, 624. https://doi.org/10.3390/cells14090624
Herrera-Moro Huitron L, Cruz-Holguin VJ, Ulloa-Aguilar JM, De Jesús-González LA, Osuna-Ramos JF, Guzmán-Huerta M, de León-Bautista MP, León-Reyes G, García-Cordero J, Cedillo-Barrón L, et al. Beyond Infection: The Role of Secreted Viral Proteins in Pathogenesis, Disease Severity and Diagnostic Applications. Cells. 2025; 14(9):624. https://doi.org/10.3390/cells14090624
Chicago/Turabian StyleHerrera-Moro Huitron, Luis, Víctor Javier Cruz-Holguin, José Manuel Ulloa-Aguilar, Luis Adrián De Jesús-González, Juan Fidel Osuna-Ramos, Mario Guzmán-Huerta, Mercedes Piedad de León-Bautista, Guadalupe León-Reyes, Julio García-Cordero, Leticia Cedillo-Barrón, and et al. 2025. "Beyond Infection: The Role of Secreted Viral Proteins in Pathogenesis, Disease Severity and Diagnostic Applications" Cells 14, no. 9: 624. https://doi.org/10.3390/cells14090624
APA StyleHerrera-Moro Huitron, L., Cruz-Holguin, V. J., Ulloa-Aguilar, J. M., De Jesús-González, L. A., Osuna-Ramos, J. F., Guzmán-Huerta, M., de León-Bautista, M. P., León-Reyes, G., García-Cordero, J., Cedillo-Barrón, L., Cerna-Cortes, J. F., & León-Juárez, M. (2025). Beyond Infection: The Role of Secreted Viral Proteins in Pathogenesis, Disease Severity and Diagnostic Applications. Cells, 14(9), 624. https://doi.org/10.3390/cells14090624







