Phosphoinositides in Retinal Function and Disease
Abstract
:1. Introduction
2. Chemical Structures of Phosphoinositides
3. Phosphatidylinositol Content in the Retina and RPE
4. Content of Minor Phosphoinositides in the Retina and RPE
Comparison to Other Tissues and Cell Types
5. Importance of PI Generally
Importance of Phosphoinositides for Dynamics and Functions of Membranes in Retina and RPE
6. Membrane Trafficking in Retina and RPE
7. Features of the Retina that Make It Ideal for Studies of Phosphoinositide Regulation In Vivo
8. Importance of Phosphoinositides for Membrane Trafficking in Retina
9. Retinal Cilia and Phosphoinositides
Phosphoinositides and the BBSome
10. Autophagy and other Stress Responses Involving Redirection of Membrane Traffic and Phosphoinositides
11. Evidence for Effects of Light on Phosphoinositide Metabolism
Light Regulation of PI-3 Kinase
12. PI(4,5)P2 and Phospholipase C in Intrinsically Photosensitive Ganglion Cells
13. Studies of PI Metabolism in the RPE
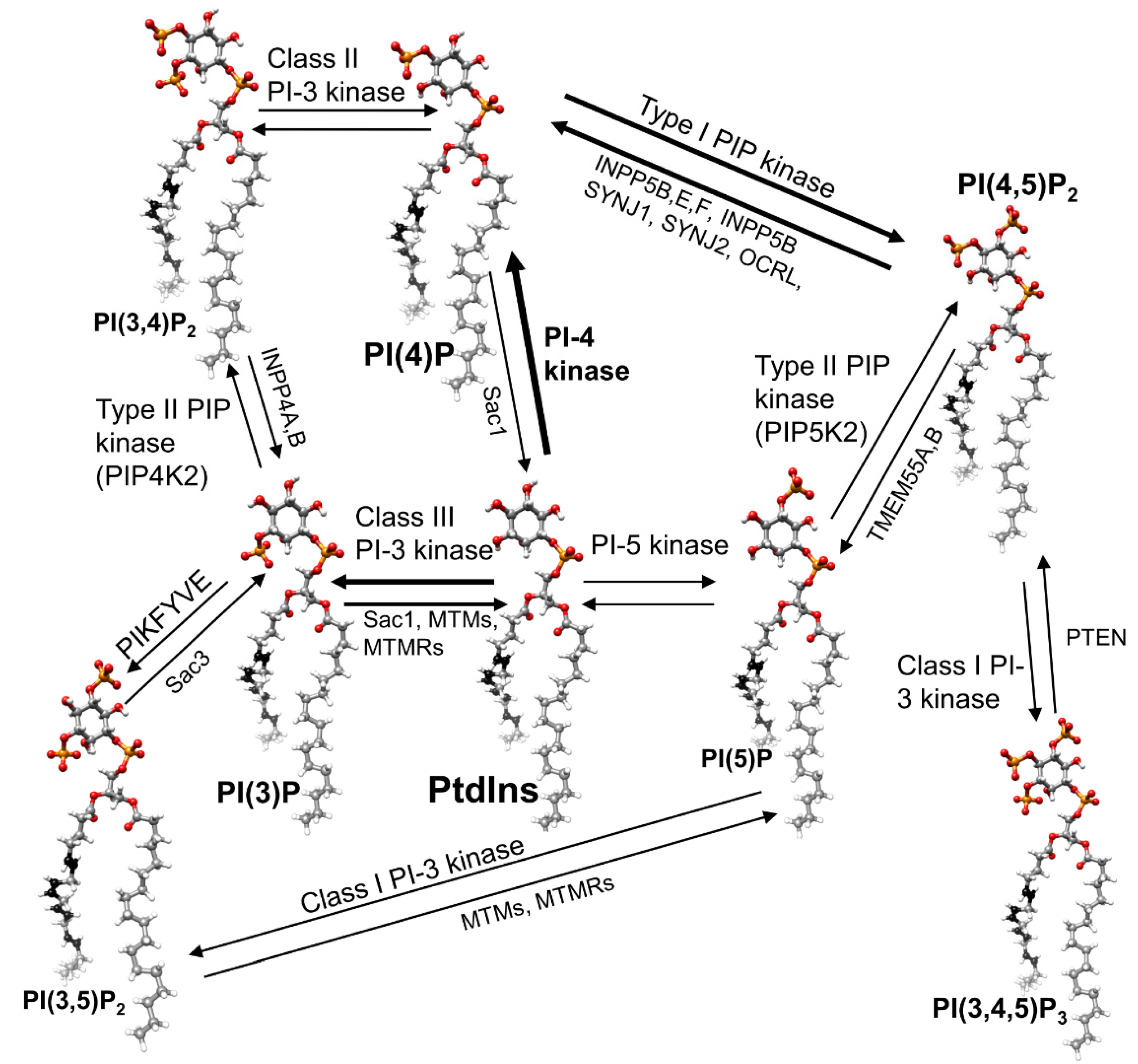
14. Phosphoinositide Kinases and Phosphatase
14.1. Kinases
14.2. Phosphatases
15. Retinal Phenotypes of Genetic Defects in Genes Related to Phosphoinositide Metabolism and Signaling
15.1. Phosphatases and Inherited Retinopathies
15.2. PI3NM3
15.3. PI Binding Proteins
16. Conclusions
Funding
Conflicts of Interest
References
- Phan, T.K.; Williams, S.A.; Bindra, G.K.; Lay, F.T.; Poon, I.K.H.; Hulett, M.D. Phosphoinositides: Multipurpose cellular lipids with emerging roles in cell death. Cell Death Differ. 2019, 26, 781–793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dickson, E.J.; Hille, B. Understanding phosphoinositides: Rare, dynamic, and essential membrane phospholipids. Biochem. J. 2019, 476, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Schink, K.O.; Tan, K.W.; Stenmark, H. Phosphoinositides in Control of Membrane Dynamics. Annu. Rev. Cell Dev. Biol. 2016, 32, 143–171. [Google Scholar] [CrossRef] [PubMed]
- Balla, T. Phosphoinositides: Tiny lipids with giant impact on cell regulation. Physiol. Rev. 2013, 93, 1019–1137. [Google Scholar] [CrossRef]
- Maffucci, T. An introduction to phosphoinositides. Curr. Top. Microbiol. Immunol. 2012, 362, 1–42. [Google Scholar] [CrossRef]
- Brockerhoff, S.E. Phosphoinositides and photoreceptors. Mol. Neurobiol. 2011, 44, 420–425. [Google Scholar] [CrossRef] [Green Version]
- Broekhuyse, R.M. Phospholipids in tissues of the eye. I. Isolation, characterization and quantitative analysis by two-dimensional thin-layer chromatography of diacyl and vinyl-ether phospholipids. Biochim. Biophys. Acta 1968, 152, 307–315. [Google Scholar] [CrossRef]
- Anderson, R.E.; Feldman, L.S.; Feldman, G.L. Lipids of ocular tissues. II. The phospholipids of mature bovine and rabbit whole retina. Biochim. Biophys. Acta 1970, 202, 367–373. [Google Scholar] [CrossRef]
- Anderson, R.E. Lipids of ocular tissues. IV. A comparison of the phospholipids from the retina of six mammalian species. Exp. Eye Res. 1970, 10, 339–344. [Google Scholar] [CrossRef]
- Anderson, R.E.; Maude, M.B.; Zimmerman, W. Lipids of ocular tissues--X. Lipid composition of subcellular fractions of bovine retina. Vision Res. 1975, 15, 1087–1090. [Google Scholar] [CrossRef]
- Anderson, R.E.; Maude, M.B. Phospholipids of bovine outer segments. Biochemistry 1970, 9, 3624–3628. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, N.C.; Fleischer, S.; McConnell, D.G. Lipid composition of bovine retinal outer segment fragments. Biochim. Biophys. Acta 1970, 211, 10–19. [Google Scholar] [CrossRef]
- Anderson, R.E.; Risk, M. Lipids of ocular tissues. IX. The phospholipids of frog photoreceptor membranes. Vision Res. 1974, 14, 129–131. [Google Scholar] [CrossRef]
- Anderson, R.E.; Lissandrello, P.M.; Maude, M.B.; Matthes, M.T. Lipids of bovine retinal pigment epithelium. Exp. Eye Res. 1976, 23, 149–157. [Google Scholar] [CrossRef]
- Balla, T. Inositol-lipid binding motifs: Signal integrators through protein-lipid and protein-protein interactions. J. Cell Sci. 2005, 118, 2093–2104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, S.; Thapa, N.; Tan, X.; Hedman, A.C.; Anderson, R.A. PIP kinases define PI4,5P(2)signaling specificity by association with effectors. Biochim. Biophys. Acta 2015, 1851, 711–723. [Google Scholar] [CrossRef] [Green Version]
- Lemmon, M.A. Membrane recognition by phospholipid-binding domains. Nat. Rev. Mol. Cell Biol. 2008, 9, 99–111. [Google Scholar] [CrossRef]
- Anderson, R.E.; Maude, M.B.; Kelleher, P.A. Metabolism of phosphatidylinositol in the frog retina. Biochim. Biophys. Acta 1980, 620, 236–246. [Google Scholar] [CrossRef]
- Choe, H.G.; Ghalayini, A.J.; Anderson, R.E. Phosphoinositide metabolism in frog rod outer segments. Exp. Eye Res. 1990, 51, 167–176. [Google Scholar] [CrossRef]
- Gehm, B.D.; Mc Connell, D.G. Phosphoinositide synthesis in bovine rod outer segments. Biochemistry 1990, 29, 5442–5446. [Google Scholar] [CrossRef]
- Rodriguez de Turco, E.B.; Gordon, W.C.; Bazan, N.G. Light stimulates in vivo inositol lipid turnover in frog retinal pigment epithelial cells at the onset of shedding and phagocytosis of photoreceptor membranes. Exp. Eye Res. 1992, 55, 719–725. [Google Scholar] [CrossRef]
- He, F.; Agosto, M.A.; Anastassov, I.A.; Tse, D.Y.; Wu, S.M.; Wensel, T.G. Phosphatidylinositol-3-phosphate is light-regulated and essential for survival in retinal rods. Sci. Rep. 2016, 6, 26978. [Google Scholar] [CrossRef]
- Stephens, L.R.; Jackson, T.R.; Hawkins, P.T. Agonist-stimulated synthesis of phosphatidylinositol(3,4,5)-trisphosphate: A new intracellular signalling system? Biochim. Biophys. Acta 1993, 1179, 27–75. [Google Scholar] [CrossRef]
- Bui, H.H.; Sanders, P.E.; Bodenmiller, D.; Kuo, M.S.; Donoho, G.P.; Fischl, A.S. Direct analysis of PI(3,4,5)P3 using liquid chromatography electrospray ionization tandem mass spectrometry. Anal. Biochem. 2018, 547, 66–76. [Google Scholar] [CrossRef]
- Kadamur, G.; Ross, E.M. Mammalian phospholipase C. Annu. Rev. Physiol. 2013, 75, 127–154. [Google Scholar] [CrossRef] [Green Version]
- Trebak, M.; Lemonnier, L.; Smyth, J.T.; Vazquez, G.; Putney, J.W., Jr. Phospholipase C-coupled receptors and activation of TRPC channels. Handb Exp. Pharmacol. 2007, 593–614. [Google Scholar]
- Wang, L.; Budolfson, K.; Wang, F. Pik3c3 deletion in pyramidal neurons results in loss of synapses, extensive gliosis and progressive neurodegeneration. Neuroscience 2011, 172, 427–442. [Google Scholar] [CrossRef] [Green Version]
- Zhou, X.; Wang, L.; Hasegawa, H.; Amin, P.; Han, B.X.; Kaneko, S.; He, Y.; Wang, F. Deletion of PIK3C3/Vps34 in sensory neurons causes rapid neurodegeneration by disrupting the endosomal but not the autophagic pathway. Proc. Natl. Acad. Sci. USA 2010, 107, 9424–9429. [Google Scholar] [CrossRef] [Green Version]
- Carnero, A.; Blanco-Aparicio, C.; Renner, O.; Link, W.; Leal, J.F. The PTEN/PI3K/AKT signalling pathway in cancer, therapeutic implications. Curr. Cancer Drug Targets 2008, 8, 187–198. [Google Scholar] [CrossRef]
- Carpentier, S.; N’Kuli, F.; Grieco, G.; Van Der Smissen, P.; Janssens, V.; Emonard, H.; Bilanges, B.; Vanhaesebroeck, B.; Gaide Chevronnay, H.P.; Pierreux, C.E.; et al. Class III phosphoinositide 3-kinase/VPS34 and dynamin are critical for apical endocytic recycling. Traffic 2013, 14, 933–948. [Google Scholar] [CrossRef]
- Dall’Armi, C.; Devereaux, K.A.; Di Paolo, G. The role of lipids in the control of autophagy. Curr. Biol. 2013, 23, R33–R45. [Google Scholar] [CrossRef] [Green Version]
- Lenoir, M.; Overduin, M. PtdIns(4)P signalling and recognition systems. Adv. Exp. Med. Biol. 2013, 991, 59–83. [Google Scholar] [CrossRef]
- McLaughlin, S.; Wang, J.; Gambhir, A.; Murray, D. PIP(2) and proteins: Interactions, organization, and information flow. Annu. Rev. Biophys. Biomol. Struct. 2002, 31, 151–175. [Google Scholar] [CrossRef] [Green Version]
- Falkenburger, B.H.; Jensen, J.B.; Dickson, E.J.; Suh, B.C.; Hille, B. Phosphoinositides: Lipid regulators of membrane proteins. J. Physiol. 2010, 588, 3179–3185. [Google Scholar] [CrossRef]
- Hilgemann, D.W.; Feng, S.; Nasuhoglu, C. The complex and intriguing lives of PIP2 with ion channels and transporters. Sci. STKE 2001, 2001, re19. [Google Scholar] [CrossRef]
- Fukami, K.; Inanobe, S.; Kanemaru, K.; Nakamura, Y. Phospholipase C is a key enzyme regulating intracellular calcium and modulating the phosphoinositide balance. Prog. Lipid Res. 2010, 49, 429–437. [Google Scholar] [CrossRef]
- Vines, C.M. Phospholipase C. Adv. Exp. Med. Biol. 2012, 740, 235–254. [Google Scholar] [CrossRef]
- Shewan, A.; Eastburn, D.J.; Mostov, K. Phosphoinositides in cell architecture. Cold Spring Harb. Perspect. Biol. 2011, 3, a004796. [Google Scholar] [CrossRef] [Green Version]
- Yin, H.L.; Janmey, P.A. Phosphoinositide regulation of the actin cytoskeleton. Annu. Rev. Physiol. 2003, 65, 761–789. [Google Scholar] [CrossRef]
- Hille, B.; Dickson, E.J.; Kruse, M.; Vivas, O.; Suh, B.C. Phosphoinositides regulate ion channels. Biochim. Biophys. Acta 2015, 1851, 844–856. [Google Scholar] [CrossRef]
- Bielas, S.L.; Silhavy, J.L.; Brancati, F.; Kisseleva, M.V.; Al-Gazali, L.; Sztriha, L.; Bayoumi, R.A.; Zaki, M.S.; Abdel-Aleem, A.; Rosti, R.O.; et al. Mutations in INPP5E, encoding inositol polyphosphate-5-phosphatase E, link phosphatidyl inositol signaling to the ciliopathies. Nat. Genet. 2009, 41, 1032–1036. [Google Scholar] [CrossRef] [Green Version]
- Jacoby, M.; Cox, J.J.; Gayral, S.; Hampshire, D.J.; Ayub, M.; Blockmans, M.; Pernot, E.; Kisseleva, M.V.; Compere, P.; Schiffmann, S.N.; et al. INPP5E mutations cause primary cilium signaling defects, ciliary instability and ciliopathies in human and mouse. Nat. Genet. 2009, 41, 1027–1031. [Google Scholar] [CrossRef] [Green Version]
- Saar, K.; Al-Gazali, L.; Sztriha, L.; Rueschendorf, F.; Nur, E.K.M.; Reis, A.; Bayoumi, R. Homozygosity mapping in families with Joubert syndrome identifies a locus on chromosome 9q34.3 and evidence for genetic heterogeneity. Am. J. Hum. Genet. 1999, 65, 1666–1671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- George, A.A.; Hayden, S.; Holzhausen, L.C.; Ma, E.Y.; Suzuki, S.C.; Brockerhoff, S.E. Synaptojanin 1 is required for endolysosomal trafficking of synaptic proteins in cone photoreceptor inner segments. PLoS ONE 2014, 9, e84394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holzhausen, L.C.; Lewis, A.A.; Cheong, K.K.; Brockerhoff, S.E. Differential role for synaptojanin 1 in rod and cone photoreceptors. J. Comp. Neurol. 2009, 517, 633–644. [Google Scholar] [CrossRef] [Green Version]
- Van Epps, H.A.; Hayashi, M.; Lucast, L.; Stearns, G.W.; Hurley, J.B.; De Camilli, P.; Brockerhoff, S.E. The zebrafish nrc mutant reveals a role for the polyphosphoinositide phosphatase synaptojanin 1 in cone photoreceptor ribbon anchoring. J. Neurosci. 2004, 24, 8641–8650. [Google Scholar] [CrossRef] [Green Version]
- Hagstrom, S.A.; North, M.A.; Nishina, P.L.; Berson, E.L.; Dryja, T.P. Recessive mutations in the gene encoding the tubby-like protein TULP1 in patients with retinitis pigmentosa. Nat. Genet. 1998, 18, 174–176. [Google Scholar] [CrossRef]
- Jacobson, S.G.; Cideciyan, A.V.; Huang, W.C.; Sumaroka, A.; Roman, A.J.; Schwartz, S.B.; Luo, X.; Sheplock, R.; Dauber, J.M.; Swider, M.; et al. TULP1 mutations causing early-onset retinal degeneration: Preserved but insensitive macular cones. Invest. Ophthalmol. Vis. Sci. 2014, 55, 5354–5364. [Google Scholar] [CrossRef] [Green Version]
- He, F.; Nichols, R.M.; Kailasam, L.; Wensel, T.G.; Agosto, M.A. Critical Role for Phosphatidylinositol-3 Kinase Vps34/PIK3C3 in ON-Bipolar Cells. Invest. Ophthalmol. Vis. Sci. 2019, 60, 2861–2874. [Google Scholar] [CrossRef] [Green Version]
- Hammond, G.R.; Hong, Y. Phosphoinositides and Membrane Targeting in Cell Polarity. Cold Spring Harb. Perspect. Biol. 2018, 10. [Google Scholar] [CrossRef]
- Mayinger, P. Phosphoinositides and vesicular membrane traffic. Biochim. Biophys. Acta 2012, 1821, 1104–1113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chuang, J.Z.; Zhao, Y.; Sung, C.H. SARA-regulated vesicular targeting underlies formation of the light-sensing organelle in mammalian rods. Cell 2007, 130, 535–547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deretic, D.; Traverso, V.; Parkins, N.; Jackson, F.; Rodriguez de Turco, E.B.; Ransom, N. Phosphoinositides, ezrin/moesin, and rac1 regulate fusion of rhodopsin transport carriers in retinal photoreceptors. Mol. Biol. Cell 2004, 15, 359–370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spencer, W.J.; Lewis, T.R.; Phan, S.; Cady, M.A.; Serebrovskaya, E.O.; Schneider, N.F.; Kim, K.Y.; Cameron, L.A.; Skiba, N.P.; Ellisman, M.H.; et al. Photoreceptor disc membranes are formed through an Arp2/3-dependent lamellipodium-like mechanism. Proc. Natl. Acad. Sci. USA 2019. [Google Scholar] [CrossRef]
- Bucki, R.; Wang, Y.H.; Yang, C.; Kandy, S.K.; Fatunmbi, O.; Bradley, R.; Pogoda, K.; Svitkina, T.; Radhakrishnan, R.; Janmey, P.A. Lateral distribution of phosphatidylinositol 4,5-bisphosphate in membranes regulates formin- and ARP2/3-mediated actin nucleation. J. Biol. Chem. 2019, 294, 4704–4722. [Google Scholar] [CrossRef] [Green Version]
- Daste, F.; Walrant, A.; Holst, M.R.; Gadsby, J.R.; Mason, J.; Lee, J.E.; Brook, D.; Mettlen, M.; Larsson, E.; Lee, S.F.; et al. Control of actin polymerization via the coincidence of phosphoinositides and high membrane curvature. J. Cell Biol. 2017, 216, 3745–3765. [Google Scholar] [CrossRef]
- Ivanovic, I.; Anderson, R.E.; Le, Y.Z.; Fliesler, S.J.; Sherry, D.M.; Rajala, R.V. Deletion of the p85alpha regulatory subunit of phosphoinositide 3-kinase in cone photoreceptor cells results in cone photoreceptor degeneration. Invest. Ophthalmol. Vis. Sci. 2011, 52, 3775–3783. [Google Scholar] [CrossRef] [Green Version]
- Xu, W.; Jin, M.; Huang, W.; Wang, H.; Hu, R.; Li, J.; Cao, Y. Apical PtdIns(4,5)P2 is required for ciliogenesis and suppression of polycystic kidney disease. FASEB J. 2019, 33, 2848–2857. [Google Scholar] [CrossRef] [Green Version]
- Phua, S.C.; Nihongaki, Y.; Inoue, T. Autonomy declared by primary cilia through compartmentalization of membrane phosphoinositides. Curr. Opin. Cell Biol. 2018, 50, 72–78. [Google Scholar] [CrossRef]
- Park, J.; Lee, N.; Kavoussi, A.; Seo, J.T.; Kim, C.H.; Moon, S.J. Ciliary Phosphoinositide Regulates Ciliary Protein Trafficking in Drosophila. Cell Rep. 2015, 13, 2808–2816. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Gonzalo, F.R.; Phua, S.C.; Roberson, E.C.; Garcia, G., 3rd; Abedin, M.; Schurmans, S.; Inoue, T.; Reiter, J.F. Phosphoinositides Regulate Ciliary Protein Trafficking to Modulate Hedgehog Signaling. Dev. Cell 2015, 34, 400–409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vieira, O.V.; Gaus, K.; Verkade, P.; Fullekrug, J.; Vaz, W.L.; Simons, K. FAPP2, cilium formation, and compartmentalization of the apical membrane in polarized Madin-Darby canine kidney (MDCK) cells. Proc. Natl. Acad. Sci. USA 2006, 103, 18556–18561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nachury, M.V.; Seeley, E.S.; Jin, H. Trafficking to the ciliary membrane: How to get across the periciliary diffusion barrier? Annu. Rev. Cell Dev. Biol. 2010, 26, 59–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, N.; West, C.C.; Murga-Zamalloa, C.A.; Sun, L.; Anderson, R.M.; Wells, C.D.; Weinreb, R.N.; Travers, J.B.; Khanna, H.; Sun, Y. OCRL localizes to the primary cilium: A new role for cilia in Lowe syndrome. Hum. Mol. Genet. 2012, 21, 3333–3344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coon, B.G.; Hernandez, V.; Madhivanan, K.; Mukherjee, D.; Hanna, C.B.; Barinaga-Rementeria Ramirez, I.; Lowe, M.; Beales, P.L.; Aguilar, R.C. The Lowe syndrome protein OCRL1 is involved in primary cilia assembly. Hum. Mol. Genet. 2012, 21, 1835–1847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Humbert, M.C.; Weihbrecht, K.; Searby, C.C.; Li, Y.; Pope, R.M.; Sheffield, V.C.; Seo, S. ARL13B, PDE6D, and CEP164 form a functional network for INPP5E ciliary targeting. Proc. Natl. Acad. Sci. USA 2012, 109, 19691–19696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, H.; White, S.R.; Shida, T.; Schulz, S.; Aguiar, M.; Gygi, S.P.; Bazan, J.F.; Nachury, M.V. The conserved Bardet-Biedl syndrome proteins assemble a coat that traffics membrane proteins to cilia. Cell 2010, 141, 1208–1219. [Google Scholar] [CrossRef] [Green Version]
- Nachury, M.V.; Loktev, A.V.; Zhang, Q.; Westlake, C.J.; Peranen, J.; Merdes, A.; Slusarski, D.C.; Scheller, R.H.; Bazan, J.F.; Sheffield, V.C.; et al. A core complex of BBS proteins cooperates with the GTPase Rab8 to promote ciliary membrane biogenesis. Cell 2007, 129, 1201–1213. [Google Scholar] [CrossRef] [Green Version]
- Chinskey, N.D.; Zheng, Q.D.; Zacks, D.N. Control of Photoreceptor Autophagy after Retinal Detachment: The Switch from Survival to Death. Invest. Ophthalmol. Vis. Sci. 2014, 55, 688–695. [Google Scholar] [CrossRef] [Green Version]
- Reme, C.; Drinker, C.K.; Aeberhard, B. Modification of autophagic degradation by medium- and illumination conditions in frog visual cells in vitro. Doc. Ophthalmol. 1984, 56, 377–383. [Google Scholar] [CrossRef]
- Reme, C. Autophagy in rods and cones of the vertebrate retina. Dev. Ophthalmol. 1981, 4, 101–148. [Google Scholar]
- Reme, C.E.; Sulser, M. Diurnal variation of autophagy in rod visual cells in the rat. Albrecht Von Graefes Arch. Klin. Exp. Ophthalmol. 1977, 203, 261–270. [Google Scholar] [CrossRef]
- Chen, Y.; Sawada, O.; Kohno, H.; Le, Y.Z.; Subauste, C.; Maeda, T.; Maeda, A. Autophagy protects the retina from light-induced degeneration. J. Biol. Chem. 2013, 288, 7506–7518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Besirli, C.G.; Chinskey, N.D.; Zheng, Q.D.; Zacks, D.N. Autophagy activation in the injured photoreceptor inhibits fas-mediated apoptosis. Invest. Ophthalmol. Vis. Sci. 2011, 52, 4193–4199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- George, A.A.; Hayden, S.; Stanton, G.R.; Brockerhoff, S.E. Arf6 and the 5’phosphatase of synaptojanin 1 regulate autophagy in cone photoreceptors. Bioessays 2016, 38 (Suppl. 1), S119–S135. [Google Scholar] [CrossRef]
- Ghalayini, A.; Anderson, R.E. Phosphatidylinositol 4,5-bisphosphate: Light-mediated breakdown in the vertebrate retina. Biochem. Biophys. Res. Commun. 1984, 124, 503–506. [Google Scholar] [CrossRef]
- Anderson, R.E.; Maude, M.B.; Pu, G.A.; Hollyfield, J.G. Effect of light on the metabolism of lipids in the rat retina. J. Neurochem. 1985, 44, 773–778. [Google Scholar] [CrossRef] [PubMed]
- Tarver, A.P.; Anderson, R.E. Phospholipase C activity and substrate specificity in frog photoreceptors. Exp. Eye Res. 1988, 46, 29–35. [Google Scholar] [CrossRef]
- Ghalayini, A.J.; Tarver, A.P.; Mackin, W.M.; Koutz, C.A.; Anderson, R.E. Identification and immunolocalization of phospholipase C in bovine rod outer segments. J. Neurochem. 1991, 57, 1405–1412. [Google Scholar] [CrossRef]
- Das, N.D.; Yoshioka, T.; Samuelson, D.; Shichi, H. Immunocytochemical localization of phosphatidylinositol-4,5-bisphosphate in dark- and light-adapted rat retinas. Cell Struct. Funct. 1986, 11, 53–63. [Google Scholar] [CrossRef] [Green Version]
- Das, N.D.; Yoshioka, T.; Samuelson, D.; Cohen, R.J.; Shichi, H. Immunochemical evidence for the light-regulated modulation of phosphatidylinositol-4,5-bisphosphate in rat photoreceptor cells. Cell Struct. Funct. 1987, 12, 471–481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panfoli, I.; Morelli, A.; Pepe, I. Calcium ion-regulated phospholipase C activity in bovine rod outer segments. Biochem. Biophys. Res. Commun. 1990, 173, 283–288. [Google Scholar] [CrossRef]
- Grigorjev, I.V.; Grits, A.I.; Artamonov, I.D.; Baranova, L.A.; Volotovski, I.D. betagamma-Transducin stimulates hydrolysis and synthesis of phosphatidylinositol 4,5-bisphosphate in bovine rod outer segment membranes. Biochim. Biophys. Acta 1996, 1310, 131–136. [Google Scholar] [CrossRef] [Green Version]
- Van Rooijen, L.A.; Bazan, N.G. The inositide cycle in bovine photoreceptor membranes. Life Sci. 1986, 38, 1685–1693. [Google Scholar] [CrossRef]
- Millar, F.A.; Fisher, S.C.; Muir, C.A.; Edwards, E.; Hawthorne, J.N. Polyphosphoinositide hydrolysis in response to light stimulation of rat and chick retina and retinal rod outer segments. Biochim. Biophys. Acta 1988, 970, 205–211. [Google Scholar] [CrossRef]
- He, F.; Mao, M.; Wensel, T.G. Enhancement of phototransduction g protein-effector interactions by phosphoinositides. J. Biol. Chem. 2004, 279, 8986–8990. [Google Scholar] [CrossRef] [Green Version]
- Womack, K.B.; Gordon, S.E.; He, F.; Wensel, T.G.; Lu, C.C.; Hilgemann, D.W. Do phosphatidylinositides modulate vertebrate phototransduction? J. Neurosci. 2000, 20, 2792–2799. [Google Scholar] [CrossRef]
- Gross, O.P.; Pugh, E.N., Jr.; Burns, M.E. Spatiotemporal cGMP dynamics in living mouse rods. Biophys J. 2012, 102, 1775–1784. [Google Scholar] [CrossRef] [Green Version]
- Peng, Y.W.; Rhee, S.G.; Yu, W.P.; Ho, Y.K.; Schoen, T.; Chader, G.J.; Yau, K.W. Identification of components of a phosphoinositide signaling pathway in retinal rod outer segments. Proc. Natl. Acad. Sci. USA 1997, 94, 1995–2000. [Google Scholar] [CrossRef] [Green Version]
- Orisme, W.; Li, J.; Goldmann, T.; Bolch, S.; Wolfrum, U.; Smith, W.C. Light-dependent translocation of arrestin in rod photoreceptors is signaled through a phospholipase C cascade and requires ATP. Cell Signal. 2010, 22, 447–456. [Google Scholar] [CrossRef] [Green Version]
- Guo, X.; Ghalayini, A.J.; Chen, H.; Anderson, R.E. Phosphatidylinositol 3-kinase in bovine photoreceptor rod outer segments. Invest. Ophthalmol. Vis. Sci. 1997, 38, 1873–1882. [Google Scholar] [PubMed]
- Guo, X.X.; Huang, Z.; Bell, M.W.; Chen, H.; Anderson, R.E. Tyrosine phosphorylation is involved in phosphatidylinositol 3-kinase activation in bovine rod outer segments. Mol. Vis. 2000, 6, 216–221. [Google Scholar] [PubMed]
- Rajala, R.V.; McClellan, M.E.; Chan, M.D.; Tsiokas, L.; Anderson, R.E. Interaction of the retinal insulin receptor beta-subunit with the p85 subunit of phosphoinositide 3-kinase. Biochemistry 2004, 43, 5637–5650. [Google Scholar] [CrossRef] [PubMed]
- Rajala, R.V.; Anderson, R.E. Light regulation of the insulin receptor in the retina. Mol. Neurobiol. 2003, 28, 123–138. [Google Scholar] [CrossRef]
- Rajala, R.V.; McClellan, M.E.; Ash, J.D.; Anderson, R.E. In vivo regulation of phosphoinositide 3-kinase in retina through light-induced tyrosine phosphorylation of the insulin receptor beta-subunit. J. Biol. Chem. 2002, 277, 43319–43326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajala, R.V.; Ranjo-Bishop, M.; Wang, Y.; Rajala, A.; Anderson, R.E. The p110alpha isoform of phosphoinositide 3-kinase is essential for cone photoreceptor survival. Biochimie 2015, 112, 35–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ivanovic, I.; Allen, D.T.; Dighe, R.; Le, Y.Z.; Anderson, R.E.; Rajala, R.V. Phosphoinositide 3-kinase signaling in retinal rod photoreceptors. Invest. Ophthalmol. Vis. Sci. 2011, 52, 6355–6362. [Google Scholar] [CrossRef]
- Detwiler, P.B. Phototransduction in Retinal Ganglion Cells. Yale J. Biol. Med. 2018, 91, 49–52. [Google Scholar]
- Graham, D.M.; Wong, K.Y.; Shapiro, P.; Frederick, C.; Pattabiraman, K.; Berson, D.M. Melanopsin ganglion cells use a membrane-associated rhabdomeric phototransduction cascade. J. Neurophysiol. 2008, 99, 2522–2532. [Google Scholar] [CrossRef]
- Hardie, R.C. Photosensitive TRPs. Handb. Exp. Pharmacol. 2014, 223, 795–826. [Google Scholar] [CrossRef]
- Isoldi, M.C.; Rollag, M.D.; Castrucci, A.M.; Provencio, I. Rhabdomeric phototransduction initiated by the vertebrate photopigment melanopsin. Proc. Natl. Acad. Sci. USA 2005, 102, 1217–1221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, Z.; Yue, W.W.S.; Chen, L.; Sheng, Y.; Yau, K.W. Cyclic-Nucleotide- and HCN-Channel-Mediated Phototransduction in Intrinsically Photosensitive Retinal Ganglion Cells. Cell 2018, 175, 652–664 e612. [Google Scholar] [CrossRef] [Green Version]
- Montell, C. Drosophila visual transduction. Trends Neurosci. 2012, 35, 356–363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xue, T.; Do, M.T.; Riccio, A.; Jiang, Z.; Hsieh, J.; Wang, H.C.; Merbs, S.L.; Welsbie, D.S.; Yoshioka, T.; Weissgerber, P.; et al. Melanopsin signalling in mammalian iris and retina. Nature 2011, 479, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Arendt, D. Evolution of eyes and photoreceptor cell types. Int. J. Dev. Biol. 2003, 47, 563–571. [Google Scholar] [PubMed]
- Kuriyama, S.; Ohuchi, T.; Yoshimura, N.; Honda, Y. Growth factor-induced cytosolic calcium ion transients in cultured human retinal pigment epithelial cells. Invest. Ophthalmol. Vis. Sci. 1991, 32, 2882–2890. [Google Scholar]
- Osborne, N.N.; FitzGibbon, F.; Schwartz, G. Muscarinic acetylcholine receptor-mediated phosphoinositide turnover in cultured human retinal pigment epithelium cells. Vision Res. 1991, 31, 1119–1127. [Google Scholar] [CrossRef]
- Feldman, E.L.; Randolph, A.E.; Johnston, G.C.; DelMonte, M.A.; Greene, D.A. Receptor-coupled phosphoinositide hydrolysis in human retinal pigment epithelium. J. Neurochem. 1991, 56, 2094–2100. [Google Scholar] [CrossRef]
- York, N.; Halbach, P.; Chiu, M.A.; Bird, I.M.; Pillers, D.M.; Pattnaik, B.R. Oxytocin (OXT)-stimulated inhibition of Kir7.1 activity is through PIP2-dependent Ca(2+) response of the oxytocin receptor in the retinal pigment epithelium in vitro. Cell Signal. 2017, 37, 93–102. [Google Scholar] [CrossRef]
- Heth, C.A.; Marescalchi, P.A. Inositol triphosphate generation in cultured rat retinal pigment epithelium. Invest. Ophthalmol. Vis. Sci. 1994, 35, 409–416. [Google Scholar]
- D’Cruz, P.M.; Yasumura, D.; Weir, J.; Matthes, M.T.; Abderrahim, H.; LaVail, M.M.; Vollrath, D. Mutation of the receptor tyrosine kinase gene Mertk in the retinal dystrophic RCS rat. Hum. Mol. Genet. 2000, 9, 645–651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Treins, C.; Giorgetti-Peraldi, S.; Murdaca, J.; Semenza, G.L.; Van Obberghen, E. Insulin stimulates hypoxia-inducible factor 1 through a phosphatidylinositol 3-kinase/target of rapamycin-dependent signaling pathway. J. Biol. Chem. 2002, 277, 27975–27981. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geraldes, P.; Yagi, K.; Ohshiro, Y.; He, Z.; Maeno, Y.; Yamamoto-Hiraoka, J.; Rask-Madsen, C.; Chung, S.W.; Perrella, M.A.; King, G.L. Selective regulation of heme oxygenase-1 expression and function by insulin through IRS1/phosphoinositide 3-kinase/Akt-2 pathway. J. Biol. Chem. 2008, 283, 34327–34336. [Google Scholar] [CrossRef] [Green Version]
- Mwaikambo, B.R.; Yang, C.; Chemtob, S.; Hardy, P. Hypoxia up-regulates CD36 expression and function via hypoxia-inducible factor-1- and phosphatidylinositol 3-kinase-dependent mechanisms. J. Biol. Chem. 2009, 284, 26695–26707. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.M.; Wang, Y.S.; Zhang, J.; Li, Y.; Xu, J.F.; Zhu, J.; Zhao, W.; Chu, D.K.; Wiedemann, P. Role of PI3K/Akt and MEK/ERK in mediating hypoxia-induced expression of HIF-1alpha and VEGF in laser-induced rat choroidal neovascularization. Invest. Ophthalmol. Vis. Sci. 2009, 50, 1873–1879. [Google Scholar] [CrossRef]
- Kim, D.I.; Lim, S.K.; Park, M.J.; Han, H.J.; Kim, G.Y.; Park, S.H. The involvement of phosphatidylinositol 3-kinase/Akt signaling in high glucose-induced downregulation of GLUT-1 expression in ARPE cells. Life Sci. 2007, 80, 626–632. [Google Scholar] [CrossRef]
- Qin, D.; Zhang, G.M.; Xu, X.; Wang, L.Y. The PI3K/Akt signaling pathway mediates the high glucose-induced expression of extracellular matrix molecules in human retinal pigment epithelial cells. J. Diabetes Res. 2015, 2015, 920280. [Google Scholar] [CrossRef]
- Ferguson, T.A.; Green, D.R. Autophagy and phagocytosis converge for better vision. Autophagy 2014, 10, 165–167. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.Y.; Zhao, H.; Martinez, J.; Doggett, T.A.; Kolesnikov, A.V.; Tang, P.H.; Ablonczy, Z.; Chan, C.C.; Zhou, Z.; Green, D.R.; et al. Noncanonical autophagy promotes the visual cycle. Cell 2013, 154, 365–376. [Google Scholar] [CrossRef] [Green Version]
- Muniz-Feliciano, L.; Doggett, T.A.; Zhou, Z.; Ferguson, T.A. RUBCN/rubicon and EGFR regulate lysosomal degradative processes in the retinal pigment epithelium (RPE) of the eye. Autophagy 2017, 13, 2072–2085. [Google Scholar] [CrossRef] [Green Version]
- Shaw, J.D.; Hama, H.; Sohrabi, F.; DeWald, D.B.; Wendland, B. PtdIns(3,5)P2 is required for delivery of endocytic cargo into the multivesicular body. Traffic 2003, 4, 479–490. [Google Scholar] [CrossRef]
- Ketel, K.; Krauss, M.; Nicot, A.S.; Puchkov, D.; Wieffer, M.; Muller, R.; Subramanian, D.; Schultz, C.; Laporte, J.; Haucke, V. A phosphoinositide conversion mechanism for exit from endosomes. Nature 2016, 529, 408–412. [Google Scholar] [CrossRef]
- Isobe, Y.; Nigorikawa, K.; Tsurumi, G.; Takemasu, S.; Takasuga, S.; Kofuji, S.; Hazeki, K. PIKfyve accelerates phagosome acidification through activation of TRPML1 while arrests aberrant vacuolation independent of the Ca2+ channel. J. Biochem. 2019, 165, 75–84. [Google Scholar] [CrossRef]
- Ikonomov, O.C.; Sbrissa, D.; Shisheva, A. Localized PtdIns 3,5-P2 synthesis to regulate early endosome dynamics and fusion. Am. J. Physiol. Cell Physiol. 2006, 291, C393–C404. [Google Scholar] [CrossRef] [Green Version]
- Dong, X.P.; Shen, D.; Wang, X.; Dawson, T.; Li, X.; Zhang, Q.; Cheng, X.; Zhang, Y.; Weisman, L.S.; Delling, M.; et al. PI(3,5)P(2) controls membrane trafficking by direct activation of mucolipin Ca(2+) release channels in the endolysosome. Nat. Commun. 2010, 1, 38. [Google Scholar] [CrossRef] [Green Version]
- Sasaki, T.; Takasuga, S.; Sasaki, J.; Kofuji, S.; Eguchi, S.; Yamazaki, M.; Suzuki, A. Mammalian phosphoinositide kinases and phosphatases. Prog. Lipid Res. 2009, 48, 307–343. [Google Scholar] [CrossRef]
- Brown, J.R.; Auger, K.R. Phylogenomics of phosphoinositide lipid kinases: Perspectives on the evolution of second messenger signaling and drug discovery. BMC Evol. Biol. 2011, 11, 4. [Google Scholar] [CrossRef] [Green Version]
- Azadi, S.; Brush, R.S.; Anderson, R.E.; Rajala, R.V. Class I Phosphoinositide 3-Kinase Exerts a Differential Role on Cell Survival and Cell Trafficking in Retina. Adv. Exp. Med. Biol. 2016, 854, 363–369. [Google Scholar] [CrossRef]
- Sakagami, H.; Katsumata, O.; Hara, Y.; Tamaki, H.; Fukaya, M. Preferential localization of type I phosphatidylinositol 4-phosphate 5-kinase gamma at the periactive zone of mouse photoreceptor ribbon synapses. Brain Res. 2014, 1586, 23–33. [Google Scholar] [CrossRef]
- Wenk, M.R.; Pellegrini, L.; Klenchin, V.A.; Di Paolo, G.; Chang, S.; Daniell, L.; Arioka, M.; Martin, T.F.; De Camilli, P. PIP kinase Igamma is the major PI(4,5)P(2) synthesizing enzyme at the synapse. Neuron 2001, 32, 79–88. [Google Scholar] [CrossRef] [Green Version]
- Wright, B.D.; Loo, L.; Street, S.E.; Ma, A.; Taylor-Blake, B.; Stashko, M.A.; Jin, J.; Janzen, W.P.; Frye, S.V.; Zylka, M.J. The lipid kinase PIP5K1C regulates pain signaling and sensitization. Neuron 2014, 82, 836–847. [Google Scholar] [CrossRef] [Green Version]
- Di Paolo, G.; Moskowitz, H.S.; Gipson, K.; Wenk, M.R.; Voronov, S.; Obayashi, M.; Flavell, R.; Fitzsimonds, R.M.; Ryan, T.A.; De Camilli, P. Impaired PtdIns(4,5)P2 synthesis in nerve terminals produces defects in synaptic vesicle trafficking. Nature 2004, 431, 415–422. [Google Scholar] [CrossRef]
- Huang, Z.; Guo, X.X.; Chen, S.X.; Alvarez, K.M.; Bell, M.W.; Anderson, R.E. Regulation of type II phosphatidylinositol phosphate kinase by tyrosine phosphorylation in bovine rod outer segments. Biochemistry 2001, 40, 4550–4559. [Google Scholar] [CrossRef]
- Zhao, L.; Chen, Y.; Bajaj, A.O.; Eblimit, A.; Xu, M.; Soens, Z.T.; Wang, F.; Ge, Z.; Jung, S.Y.; He, F.; et al. Integrative subcellular proteomic analysis allows accurate prediction of human disease-causing genes. Genome Res. 2016, 26, 660–669. [Google Scholar] [CrossRef] [Green Version]
- Prosseda, P.P.; Luo, N.; Wang, B.; Alvarado, J.A.; Hu, Y.; Sun, Y. Loss of OCRL increases ciliary PI(4,5)P2 in Lowe oculocerebrorenal syndrome. J. Cell Sci. 2017, 130, 3447–3454. [Google Scholar] [CrossRef] [Green Version]
- Attree, O.; Olivos, I.M.; Okabe, I.; Bailey, L.C.; Nelson, D.L.; Lewis, R.A.; McInnes, R.R.; Nussbaum, R.L. The Lowe’s oculocerebrorenal syndrome gene encodes a protein highly homologous to inositol polyphosphate-5-phosphatase. Nature 1992, 358, 239–242. [Google Scholar] [CrossRef]
- Shim, H.; Wu, C.; Ramsamooj, S.; Bosch, K.N.; Chen, Z.; Emerling, B.M.; Yun, J.; Liu, H.; Choo-Wing, R.; Yang, Z.; et al. Deletion of the gene Pip4k2c, a novel phosphatidylinositol kinase, results in hyperactivation of the immune system. Proc. Natl. Acad. Sci. USA 2016, 113, 7596–7601. [Google Scholar] [CrossRef] [Green Version]
- Young, R.W. The renewal of rod and cone outer segments in the rhesus monkey. J. Cell Biol. 1971, 49, 303–318. [Google Scholar] [CrossRef] [Green Version]
- Adams, N.A.; Awadein, A.; Toma, H.S. The retinal ciliopathies. Ophthalmic Genet. 2007, 28, 113–125. [Google Scholar] [CrossRef]
- Wheway, G.; Parry, D.A.; Johnson, C.A. The role of primary cilia in the development and disease of the retina. Organogenesis 2013, 10, 69–85. [Google Scholar] [CrossRef] [Green Version]
- Gal, A.; Li, Y.; Thompson, D.A.; Weir, J.; Orth, U.; Jacobson, S.G.; Apfelstedt-Sylla, E.; Vollrath, D. Mutations in MERTK, the human orthologue of the RCS rat retinal dystrophy gene, cause retinitis pigmentosa. Nat. Genet. 2000, 26, 270–271. [Google Scholar] [CrossRef]
- Marmorstein, A.D.; Johnson, A.A.; Bachman, L.A.; Andrews-Pfannkoch, C.; Knudsen, T.; Gilles, B.J.; Hill, M.; Gandhi, J.K.; Marmorstein, L.Y.; Pulido, J.S. Mutant Best1 Expression and Impaired Phagocytosis in an iPSC Model of Autosomal Recessive Bestrophinopathy. Sci Rep. 2018, 8, 4487. [Google Scholar] [CrossRef]
- Strauss, O.; Reichhart, N.; Gomez, N.M.; Muller, C. Contribution of Ion Channels in Calcium Signaling Regulating Phagocytosis: MaxiK, Cav1.3 and Bestrophin-1. Adv. Exp. Med. Biol. 2016, 854, 739–744. [Google Scholar] [CrossRef]
- Muller, C.; Mas Gomez, N.; Ruth, P.; Strauss, O. CaV1.3 L-type channels, maxiK Ca(2+)-dependent K(+) channels and bestrophin-1 regulate rhythmic photoreceptor outer segment phagocytosis by retinal pigment epithelial cells. Cell Signal. 2014, 26, 968–978. [Google Scholar] [CrossRef]
- Xiao, Q.; Hartzell, H.C.; Yu, K. Bestrophins and retinopathies. Pflugers Arch. 2010, 460, 559–569. [Google Scholar] [CrossRef] [Green Version]
- Strauss, O. The retinal pigment epithelium in visual function. Physiol. Rev. 2005, 85, 845–881. [Google Scholar] [CrossRef] [Green Version]
- Luo, N.; Lu, J.; Sun, Y. Evidence of a role of inositol polyphosphate 5-phosphatase INPP5E in cilia formation in zebrafish. Vision Res. 2012, 75, 98–107. [Google Scholar] [CrossRef] [Green Version]
- Travaglini, L.; Brancati, F.; Silhavy, J.; Iannicelli, M.; Nickerson, E.; Elkhartoufi, N.; Scott, E.; Spencer, E.; Gabriel, S.; Thomas, S.; et al. Phenotypic spectrum and prevalence of INPP5E mutations in Joubert syndrome and related disorders. Eur. J. Hum. Genet. 2013, 21, 1074–1078. [Google Scholar] [CrossRef] [Green Version]
- Plotnikova, O.V.; Seo, S.; Cottle, D.L.; Conduit, S.; Hakim, S.; Dyson, J.M.; Mitchell, C.A.; Smyth, I.M. INPP5E interacts with AURKA, linking phosphoinositide signaling to primary cilium stability. J. Cell Sci. 2015, 128, 364–372. [Google Scholar] [CrossRef] [Green Version]
- Xu, W.; Jin, M.; Hu, R.; Wang, H.; Zhang, F.; Yuan, S.; Cao, Y. The Joubert Syndrome Protein Inpp5e Controls Ciliogenesis by Regulating Phosphoinositides at the Apical Membrane. J. Am. Soc. Nephrol. 2017, 28, 118–129. [Google Scholar] [CrossRef]
- Verstreken, P.; Koh, T.W.; Schulze, K.L.; Zhai, R.G.; Hiesinger, P.R.; Zhou, Y.; Mehta, S.Q.; Cao, Y.; Roos, J.; Bellen, H.J. Synaptojanin is recruited by endophilin to promote synaptic vesicle uncoating. Neuron 2003, 40, 733–748. [Google Scholar] [CrossRef] [Green Version]
- Chi, Y.; Zhou, B.; Wang, W.Q.; Chung, S.K.; Kwon, Y.U.; Ahn, Y.H.; Chang, Y.T.; Tsujishita, Y.; Hurley, J.H.; Zhang, Z.Y. Comparative mechanistic and substrate specificity study of inositol polyphosphate 5-phosphatase Schizosaccharomyces pombe Synaptojanin and SHIP2. J. Biol. Chem. 2004, 279, 44987–44995. [Google Scholar] [CrossRef] [Green Version]
- Chang-Ileto, B.; Frere, S.G.; Chan, R.B.; Voronov, S.V.; Roux, A.; Di Paolo, G. Synaptojanin 1-mediated PI(4,5)P2 hydrolysis is modulated by membrane curvature and facilitates membrane fission. Dev. Cell 2011, 20, 206–218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lev, S.; Hernandez, J.; Martinez, R.; Chen, A.; Plowman, G.; Schlessinger, J. Identification of a novel family of targets of PYK2 related to Drosophila retinal degeneration B (rdgB) protein. Mol. Cell Biol. 1999, 19, 2278–2288. [Google Scholar] [CrossRef] [Green Version]
- Tian, D.; Lev, S. Cellular and developmental distribution of human homologues of the Drosophilia rdgB protein in the rat retina. Invest. Ophthalmol. Vis. Sci. 2002, 43, 1946–1953. [Google Scholar]
- Kohn, L.; Kadzhaev, K.; Burstedt, M.S.I.; Haraldsson, S.; Hallberg, B.; Sandgren, O.; Golovleva, I. Mutation in the PYK2-binding domain of PITPNM3 causes autosomal dominant cone dystrophy (CORD5) in two Swedish families. Eur. J. Hum. Genet. 2007, 15, 664–671. [Google Scholar] [CrossRef]
- Kohn, L.; Kadzhaev, K.; Burstedt, M.S.I.; Haraldsson, S.; Sandgren, O.; Golovleva, I. Mutation in the PYK2-binding domain of PITPNM3 causes autosomal dominant cone dystrophy (CORD5) in two Swedish families. Rencent Adv. Exp. Med. Biol. 2008, 613, 229–234. [Google Scholar] [CrossRef]
- Kohn, L.; Kohl, S.; Bowne, S.J.; Sullivan, L.S.; Kellner, U.; Daiger, S.P.; Sandgren, O.; Golovleva, I. PITPNM3 is an uncommon cause of cone and cone-rod dystrophies. Ophthalmic Genet. 2010, 31, 139–140. [Google Scholar] [CrossRef] [Green Version]
- Bakhoum, M.F.; Sengillo, J.D.; Cui, X.; Tsang, S.H. Autoimmune retinopathy in a patient with a missense mutation in PITPNM3. Retin Cases Brief. Rep. 2018, 12 (Suppl. 1), S72–S75. [Google Scholar] [CrossRef]
- Lin, Z.; Li, W.; Zhang, H.; Wu, W.; Peng, Y.; Zeng, Y.; Wan, Y.; Wang, J.; Ouyang, N. CCL18/PITPNM3 enhances migration, invasion, and EMT through the NF-kappaB signaling pathway in hepatocellular carcinoma. Tumour Biol. 2016, 37, 3461–3468. [Google Scholar] [CrossRef]
- Ile, K.E.; Kassen, S.; Cao, C.; Vihtehlic, T.; Shah, S.D.; Mousley, C.J.; Alb, J.G., Jr.; Huijbregts, R.P.; Stearns, G.W.; Brockerhoff, S.E.; et al. Zebrafish class 1 phosphatidylinositol transfer proteins: PITPbeta and double cone cell outer segment integrity in retina. Traffic 2010, 11, 1151–1167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, B.; Hawes, N.L.; Hurd, R.E.; Davisson, M.T.; Nusinowitz, S.; Heckenlively, J.R. Retinal degeneration mutants in the mouse. Vision Res. 2002, 42, 517–525. [Google Scholar] [CrossRef] [Green Version]
- Borman, A.D.; Pearce, L.R.; Mackay, D.S.; Nagel-Wolfrum, K.; Davidson, A.E.; Henderson, R.; Garg, S.; Waseem, N.H.; Webster, A.R.; Plagnol, V.; et al. A homozygous mutation in the TUB gene associated with retinal dystrophy and obesity. Hum. Mutat. 2014, 35, 289–293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hagstrom, S.A.; Watson, R.F.; Pauer, G.J.; Grossman, G.H. Tulp1 is involved in specific photoreceptor protein transport pathways. Adv. Exp. Med. Biol. 2012, 723, 783–789. [Google Scholar] [CrossRef] [PubMed]
- Xi, Q.; Pauer, G.J.; Traboulsi, E.I.; Hagstrom, S.A. Mutation screen of the TUB gene in patients with retinitis pigmentosa and Leber congenital amaurosis. Exp. Eye Res. 2006, 83, 569–573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xi, Q.; Pauer, G.J.; West, K.A.; Crabb, J.W.; Hagstrom, S.A. Retinal degeneration caused by mutations in TULP1. Adv. Exp. Med. Biol. 2003, 533, 303–308. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Jackson, P.K. The tubby family proteins. Genome Biol. 2011, 12, 225. [Google Scholar] [CrossRef] [Green Version]
- Hagstrom, S.A.; Duyao, M.; North, M.A.; Li, T. Retinal degeneration in tulp1-/- mice: Vesicular accumulation in the interphotoreceptor matrix. Invest. Ophthalmol. Vis. Sci. 1999, 40, 2795–2802. [Google Scholar]
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wensel, T.G. Phosphoinositides in Retinal Function and Disease. Cells 2020, 9, 866. https://doi.org/10.3390/cells9040866
Wensel TG. Phosphoinositides in Retinal Function and Disease. Cells. 2020; 9(4):866. https://doi.org/10.3390/cells9040866
Chicago/Turabian StyleWensel, Theodore G. 2020. "Phosphoinositides in Retinal Function and Disease" Cells 9, no. 4: 866. https://doi.org/10.3390/cells9040866
APA StyleWensel, T. G. (2020). Phosphoinositides in Retinal Function and Disease. Cells, 9(4), 866. https://doi.org/10.3390/cells9040866




