Oxidized Phospholipids in Healthy and Diseased Lung Endothelium
Abstract
:1. Introduction
2. OxPLs in Health and Disease
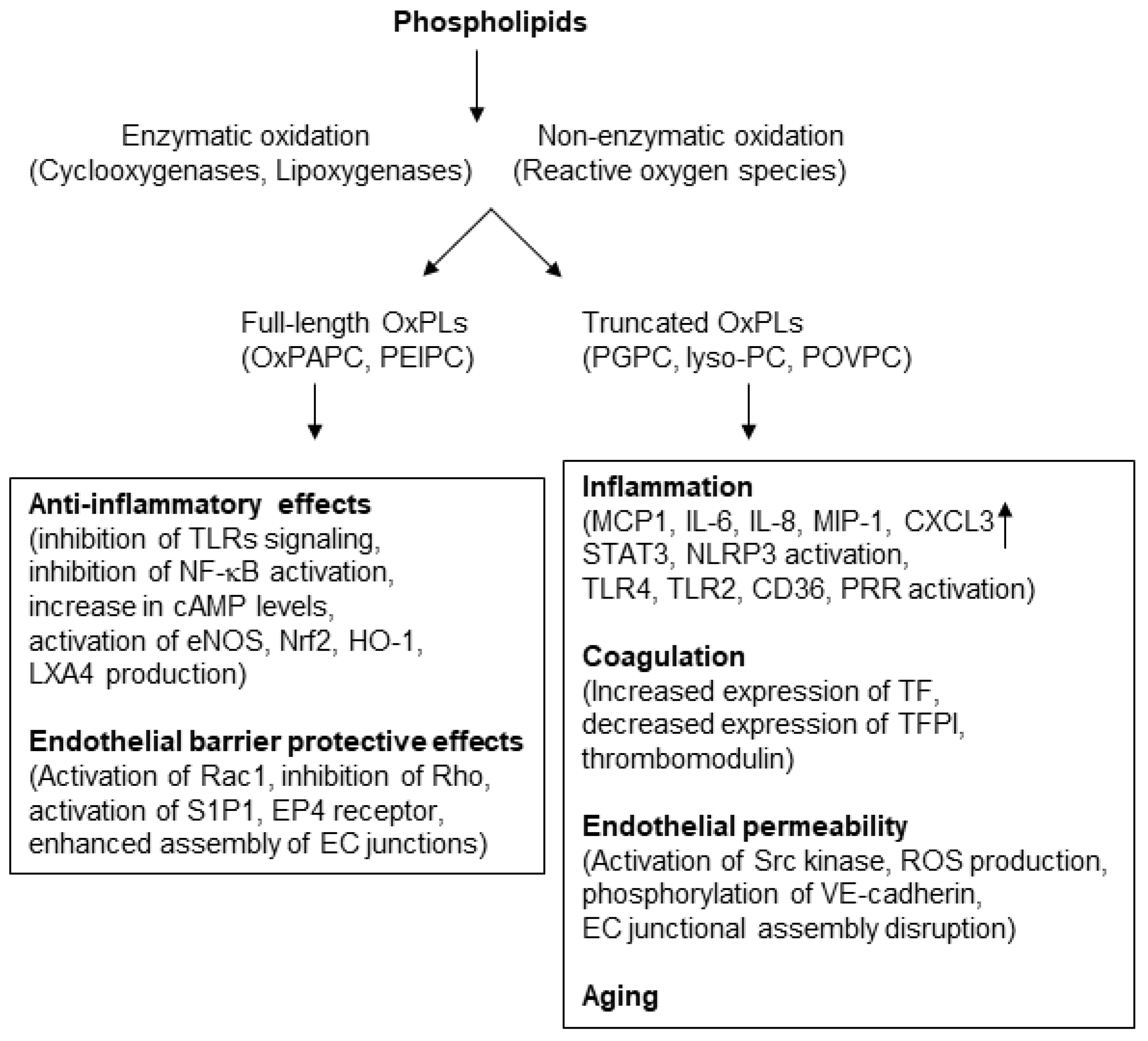
3. Structural Heterogeneity Determines the Differential Effects of OxPLs
4. OxPLs in Inflammation: Role and Mechanisms
5. Anti-Inflammatory Effects of OxPLs and Involved Mechanisms
6. OxPLs in the Positive Regulation of Endothelial Barrier Function
7. OxPLs in Endothelial Dysfunction
8. OxPLs in Atherosclerosis
9. OxPLs in Aging
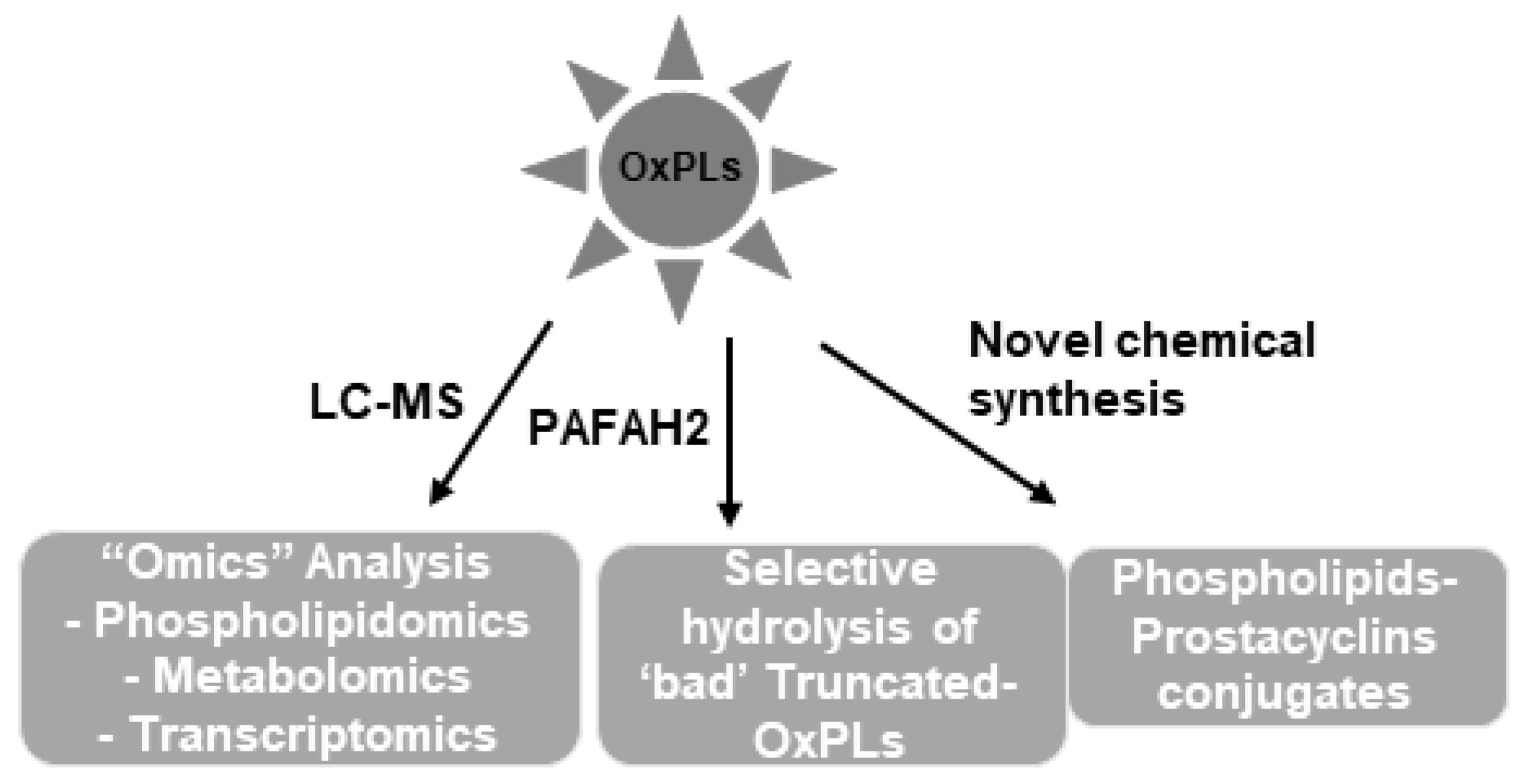
10. Phospholipidomics: A New Era of Structural-Functional Analysis of OxPLs
11. Future Perspectives
12. Summary
Funding
Conflicts of Interest
References
- Bochkov, V.N.; Oskolkova, O.V.; Birukov, K.G.; Levonen, A.L.; Binder, C.J.; Stockl, J. Generation and biological activities of oxidized phospholipids. Antiox. Redox Signal. 2010, 12, 1009–1059. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fruhwirth, G.O.; Loidl, A.; Hermetter, A. Oxidized phospholipids: From molecular properties to disease. Biochim. Biophys. Acta 2007, 1772, 718–736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bochkov, V.; Gesslbauer, B.; Mauerhofer, C.; Philippova, M.; Erne, P.; Oskolkova, O.V. Pleiotropic effects of oxidized phospholipids. Free Radic. Biol. Med. 2017, 111, 6–24. [Google Scholar] [CrossRef] [PubMed]
- Reis, A.; Spickett, C.M. Chemistry of phospholipid oxidation. Biochim. Biophys. Acta 2012, 1818, 2374–2387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anthonymuthu, T.S.; Kenny, E.M.; Lamade, A.M.; Kagan, V.E.; Bayir, H. Oxidized phospholipid signaling in traumatic brain injury. Free Radic. Biol. Med. 2018, 124, 493–503. [Google Scholar] [CrossRef] [PubMed]
- Berliner, J.A.; Leitinger, N.; Tsimikas, S. The role of oxidized phospholipids in atherosclerosis. J. Lipid Res. 2009, 50, S207–S212. [Google Scholar] [CrossRef] [Green Version]
- Chang, M.K.; Binder, C.J.; Torzewski, M.; Witztum, J.L. C-reactive protein binds to both oxidized LDL and apoptotic cells through recognition of a common ligand: Phosphorylcholine of oxidized phospholipids. Proc. Natl. Acad. Sci. USA 2002, 99, 13043–13048. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.; Birukov, K.G.; Romanoski, C.E.; Springstead, J.R.; Lusis, A.J.; Berliner, J.A. Role of phospholipid oxidation products in atherosclerosis. Circ. Res. 2012, 111, 778–799. [Google Scholar] [CrossRef]
- Lichtenstern, C.; Hofer, S.; Mollers, A.; Snyder-Ramos, S.; Spies-Martin, D.; Martin, E.; Schmidt, J.; Motsch, J.; Bardenheuer, H.J.; Weigand, M.A. Lipid peroxidation in acute respiratory distress syndrome and liver failure. J. Surg. Res. 2011, 168, 243–252. [Google Scholar] [CrossRef]
- Nakamura, T.; Henson, P.M.; Murphy, R.C. Occurrence of oxidized metabolites of arachidonic acid esterified to phospholipids in murine lung tissue. Anal. Biochem. 1998, 262, 23–32. [Google Scholar] [CrossRef]
- Qin, J.; Goswami, R.; Balabanov, R.; Dawson, G. Oxidized phosphatidylcholine is a marker for neuroinflammation in multiple sclerosis brain. J. Neurosci. Res. 2007, 85, 977–984. [Google Scholar] [CrossRef] [PubMed]
- Wood, L.G.; Gibson, P.G.; Garg, M.L. Biomarkers of lipid peroxidation, airway inflammation and asthma. Eur. Respir. J. 2003, 21, 177–186. [Google Scholar] [CrossRef] [Green Version]
- Yoshimi, N.; Ikura, Y.; Sugama, Y.; Kayo, S.; Ohsawa, M.; Yamamoto, S.; Inoue, Y.; Hirata, K.; Itabe, H.; Yoshikawa, J.; et al. Oxidized phosphatidylcholine in alveolar macrophages in idiopathic interstitial pneumonias. Lung 2005, 183, 109–121. [Google Scholar] [CrossRef] [PubMed]
- Fu, P.; Birukov, K.G. Oxidized phospholipids in control of inflammation and endothelial barrier. Transl. Res. 2009, 153, 166–176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karki, P.; Birukov, K.G. Lipid mediators in the regulation of endothelial barriers. Tissue Barriers 2018, 6, e1385573. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, C.T.; Price, P.V.; Christman, B.W. Exhaled breath condensate isoprostanes are elevated in patients with acute lung injury or ARDS. Chest 1998, 114, 1653–1659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cracowski, J.L.; Cracowski, C.; Bessard, G.; Pepin, J.L.; Bessard, J.; Schwebel, C.; Stanke-Labesque, F.; Pison, C. Increased lipid peroxidation in patients with pulmonary hypertension. Am. J. Respir. Crit. Care Med. 2001, 164, 1038–1042. [Google Scholar] [CrossRef]
- Montuschi, P.; Corradi, M.; Ciabattoni, G.; Nightingale, J.; Kharitonov, S.A.; Barnes, P.J. Increased 8-isoprostane, a marker of oxidative stress, in exhaled condensate of asthma patients. Am. J. Respir. Crit. Care Med. 1999, 160, 216–220. [Google Scholar] [CrossRef] [Green Version]
- Montuschi, P.; Kharitonov, S.A.; Ciabattoni, G.; Corradi, M.; Van Rensen, L.; Geddes, D.M.; Hodson, M.E.; Barnes, P.J. Exhaled 8-isoprostane as a new non-invasive biomarker of oxidative stress in cystic fibrosis. Thorax 2000, 55, 205–209. [Google Scholar] [CrossRef] [Green Version]
- Pennathur, S.; Bergt, C.; Shao, B.; Byun, J.; Kassim, S.Y.; Singh, P.; Green, P.S.; McDonald, T.O.; Brunzell, J.; Chait, A.; et al. Human atherosclerotic intima and blood of patients with established coronary artery disease contain high density lipoprotein damaged by reactive nitrogen species. J. Biol. Chem. 2004, 279, 42977–42983. [Google Scholar] [CrossRef] [Green Version]
- Kadl, A.; Huber, J.; Gruber, F.; Bochkov, V.N.; Binder, B.R.; Leitinger, N. Analysis of inflammatory gene induction by oxidized phospholipids in vivo by quantitative real-time RT-PCR in comparison with effects of LPS. Vascul. Pharmacol. 2002, 38, 219–227. [Google Scholar] [CrossRef]
- Lee, H.; Shi, W.; Tontonoz, P.; Wang, S.; Subbanagounder, G.; Hedrick, C.C.; Hama, S.; Borromeo, C.; Evans, R.M.; Berliner, J.A.; et al. Role for peroxisome proliferator-activated receptor alpha in oxidized phospholipid-induced synthesis of monocyte chemotactic protein-1 and interleukin-8 by endothelial cells. Circ. Res. 2000, 87, 516–521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reddy, S.T.; Grijalva, V.; Ng, C.; Hassan, K.; Hama, S.; Mottahedeh, R.; Wadleigh, D.J.; Navab, M.; Fogelman, A.M. Identification of genes induced by oxidized phospholipids in human aortic endothelial cells. Vascul. Pharmacol. 2002, 38, 211–218. [Google Scholar] [CrossRef]
- Watson, A.D.; Leitinger, N.; Navab, M.; Faull, K.F.; Horkko, S.; Witztum, J.L.; Palinski, W.; Schwenke, D.; Salomon, R.G.; Sha, W.; et al. Structural identification by mass spectrometry of oxidized phospholipids in minimally oxidized low density lipoprotein that induce monocyte/endothelial interactions and evidence for their presence in vivo. J. Biol. Chem. 1997, 272, 13597–13607. [Google Scholar] [CrossRef] [Green Version]
- Bochkov, V.N.; Mechtcheriakova, D.; Lucerna, M.; Huber, J.; Malli, R.; Graier, W.F.; Hofer, E.; Binder, B.R.; Leitinger, N. Oxidized phospholipids stimulate tissue factor expression in human endothelial cells via activation of ERK/EGR-1 and Ca(++)/NFAT. Blood 2002, 99, 199–206. [Google Scholar] [CrossRef]
- Ohkura, N.; Hiraishi, S.; Itabe, H.; Hamuro, T.; Kamikubo, Y.; Takano, T.; Matsuda, J.; Horie, S. Oxidized phospholipids in oxidized low-density lipoprotein reduce the activity of tissue factor pathway inhibitor through association with its carboxy-terminal region. Antiox. Redox. Signal. 2004, 6, 705–712. [Google Scholar] [CrossRef]
- Gharavi, N.M.; Baker, N.A.; Mouillesseaux, K.P.; Yeung, W.; Honda, H.M.; Hsieh, X.; Yeh, M.; Smart, E.J.; Berliner, J.A. Role of endothelial nitric oxide synthase in the regulation of SREBP activation by oxidized phospholipids. Circ. Res. 2006, 98, 768–776. [Google Scholar] [CrossRef] [Green Version]
- Landar, A.; Zmijewski, J.W.; Dickinson, D.A.; Le Goffe, C.; Johnson, M.S.; Milne, G.L.; Zanoni, G.; Vidari, G.; Morrow, J.D.; Darley-Usmar, V.M. Interaction of electrophilic lipid oxidation products with mitochondria in endothelial cells and formation of reactive oxygen species. Am. J. Physiol. Heart Circ. Physiol. 2006, 290, H1777–H1787. [Google Scholar] [CrossRef] [Green Version]
- Rouhanizadeh, M.; Hwang, J.; Clempus, R.E.; Marcu, L.; Lassegue, B.; Sevanian, A.; Hsiai, T.K. Oxidized-1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphorylcholine induces vascular endothelial superoxide production: Implication of NADPH oxidase. Free Radic. Biol. Med. 2005, 39, 1512–1522. [Google Scholar] [CrossRef] [Green Version]
- Therond, P.; Abella, A.; Laurent, D.; Couturier, M.; Chalas, J.; Legrand, A.; Lindenbaum, A. In vitro study of the cytotoxicity of isolated oxidized lipid low-density lipoproteins fractions in human endothelial cells: Relationship with the glutathione status and cell morphology. Free Radic. Biol. Med. 2000, 28, 585–596. [Google Scholar] [CrossRef]
- Bochkov, V.N.; Kadl, A.; Huber, J.; Gruber, F.; Binder, B.R.; Leitinger, N. Protective role of phospholipid oxidation products in endotoxin-induced tissue damage. Nature 2002, 419, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Li, J.; Yang, L.; Mu, Y.; Xie, W.; Pitt, B.; Li, S. Inhibition of LPS- and CpG DNA-induced TNF-alpha response by oxidized phospholipids. Am. J. Physiol. Lung Cell Mol. Physiol. 2004, 286, L808–L816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Birukov, K.G.; Bochkov, V.N.; Birukova, A.A.; Kawkitinarong, K.; Rios, A.; Leitner, A.; Verin, A.D.; Bokoch, G.M.; Leitinger, N.; Garcia, J.G. Epoxycyclopentenone-containing oxidized phospholipids restore endothelial barrier function via Cdc42 and Rac. Circ. Res. 2004, 95, 892–901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nonas, S.; Miller, I.; Kawkitinarong, K.; Chatchavalvanich, S.; Gorshkova, I.; Bochkov, V.N.; Leitinger, N.; Natarajan, V.; Garcia, J.G.; Birukov, K.G. Oxidized phospholipids reduce vascular leak and inflammation in rat model of acute lung injury. Am. J. Respir. Crit. Care Med. 2006, 173, 1130–1138. [Google Scholar] [CrossRef]
- Ke, Y.; Zebda, N.; Oskolkova, O.; Afonyushkin, T.; Berdyshev, E.; Tian, Y.; Meng, F.; Sarich, N.; Bochkov, V.N.; Wang, J.M.; et al. Anti-Inflammatory Effects of OxPAPC Involve Endothelial Cell-Mediated Generation of LXA4. Circ. Res. 2017, 121, 244–257. [Google Scholar] [CrossRef]
- Meliton, A.Y.; Meng, F.; Tian, Y.; Sarich, N.; Mutlu, G.M.; Birukova, A.A.; Birukov, K.G. Oxidized phospholipids protect against lung injury and endothelial barrier dysfunction caused by heat-inactivated Staphylococcus aureus. Am. J. Physiol. Lung Cell Mol. Physiol. 2015, 308, L550–L562. [Google Scholar] [CrossRef] [Green Version]
- Nonas, S.; Birukova, A.A.; Fu, P.; Xing, J.; Chatchavalvanich, S.; Bochkov, V.N.; Leitinger, N.; Garcia, J.G.; Birukov, K.G. Oxidized phospholipids reduce ventilator-induced vascular leak and inflammation in vivo. Crit. Care 2008, 12, R27. [Google Scholar] [CrossRef] [Green Version]
- Birukova, A.A.; Starosta, V.; Tian, X.; Higginbotham, K.; Koroniak, L.; Berliner, J.A.; Birukov, K.G. Fragmented oxidation products define barrier disruptive endothelial cell response to OxPAPC. Transl. Res. 2013, 161, 495–504. [Google Scholar] [CrossRef] [Green Version]
- Starosta, V.; Wu, T.; Zimman, A.; Pham, D.; Tian, X.; Oskolkova, O.; Bochkov, V.; Berliner, J.A.; Birukova, A.A.; Birukov, K.G. Differential regulation of endothelial cell permeability by high and low doses of oxidized 1-palmitoyl-2-arachidonyl-sn-glycero-3-phosphocholine. Am. J. Respir. Cell Mol. Biol. 2012, 46, 331–341. [Google Scholar] [CrossRef] [Green Version]
- Itoh, T.; Fairall, L.; Amin, K.; Inaba, Y.; Szanto, A.; Balint, B.L.; Nagy, L.; Yamamoto, K.; Schwabe, J.W. Structural basis for the activation of PPARgamma by oxidized fatty acids. Nat. Struct. Mol. Biol. 2008, 15, 924–931. [Google Scholar] [CrossRef]
- Jira, W.; Spiteller, G.; Carson, W.; Schramm, A. Strong increase in hydroxy fatty acids derived from linoleic acid in human low density lipoproteins of atherosclerotic patients. Chem. Phys. Lipids 1998, 91, 1–11. [Google Scholar] [CrossRef]
- Ryman, V.E.; Packiriswamy, N.; Sordillo, L.M. Apoptosis of Endothelial Cells by 13-HPODE Contributes to Impairment of Endothelial Barrier Integrity. Mediators Inflamm. 2016, 2016, 9867138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mavangira, V.; Brown, J.; Gandy, J.C.; Sordillo, L.M. 20-hydroxyeicosatetraenoic acid alters endothelial cell barrier integrity independent of oxidative stress and cell death. Prostaglandins Other Lipid Mediat. 2020, 149, 106425. [Google Scholar] [CrossRef] [PubMed]
- Mabalirajan, U.; Rehman, R.; Ahmad, T.; Kumar, S.; Singh, S.; Leishangthem, G.D.; Aich, J.; Kumar, M.; Khanna, K.; Singh, V.P.; et al. Linoleic acid metabolite drives severe asthma by causing airway epithelial injury. Sci. Rep. 2013, 3, 1349. [Google Scholar] [CrossRef] [Green Version]
- Zarbock, A.; Distasi, M.R.; Smith, E.; Sanders, J.M.; Kronke, G.; Harry, B.L.; Von Vietinghoff, S.; Buscher, K.; Nadler, J.L.; Ley, K. Improved survival and reduced vascular permeability by eliminating or blocking 12/15-lipoxygenase in mouse models of acute lung injury (ALI). J. Immunol. 2009, 183, 4715–4722. [Google Scholar] [CrossRef] [Green Version]
- Othman, A.; Ahmad, S.; Megyerdi, S.; Mussell, R.; Choksi, K.; Maddipati, K.R.; Elmarakby, A.; Rizk, N.; Al-Shabrawey, M. 12/15-Lipoxygenase-derived lipid metabolites induce retinal endothelial cell barrier dysfunction: Contribution of NADPH oxidase. PLoS One 2013, 8, e57254. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Gao, L.; Xiao, L.; Yang, L.; Li, W.; Liu, G.; Chen, L.; Zhang, J. 12(S)-hydroxyeicosatetraenoic acid impairs vascular endothelial permeability by altering adherens junction phosphorylation levels and affecting the binding and dissociation of its components in high glucose-induced vascular injury. J. Diabetes Investig. 2019, 10, 639–649. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, H.; Yan, L.; Du, W.; Zhang, M.; Chen, H.; Zhang, L.; Li, G.; Li, J.; Dong, Y.; et al. MMP-2 and MMP-9 contribute to the angiogenic effect produced by hypoxia/15-HETE in pulmonary endothelial cells. J. Mol. Cell Cardiol. 2018, 121, 36–50. [Google Scholar] [CrossRef]
- Wang, S.; Cao, W.; Xing, H.; Chen, Y.L.; Li, Q.; Shen, T.; Jiang, C.; Zhu, D. Activation of ERK pathway is required for 15-HETE-induced angiogenesis in human umbilical vascular endothelial cells. J. Recept Signal. Transduct. Res. 2016, 36, 225–232. [Google Scholar] [CrossRef]
- Li, Q.; Mao, M.; Qiu, Y.; Liu, G.; Sheng, T.; Yu, X.; Wang, S.; Zhu, D. Key Role of ROS in the Process of 15-Lipoxygenase/15-Hydroxyeicosatetraenoiccid-Induced Pulmonary Vascular Remodeling in Hypoxia Pulmonary Hypertension. PLoS One 2016, 11, e0149164. [Google Scholar] [CrossRef]
- Shen, T.; Shi, J.; Wang, N.; Yu, X.; Zhang, C.; Li, J.; Wei, L.; Ma, C.; Zhao, X.; Lian, M.; et al. 15-Lipoxygenase and 15-hydroxyeicosatetraenoic acid regulate intravascular thrombosis in pulmonary hypertension. Am. J. Physiol. Lung Cell Mol. Physiol. 2015, 309, L449–L462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ke, Y.; Karki, P.; Kim, J.; Son, S.; Berdyshev, E.; Bochkov, V.N.; Birukova, A.A.; Birukov, K.G. Elevated truncated oxidized phospholipids as a factor exacerbating ALI in the aging lungs. FASEB J. 2019, 33, 3887–3900. [Google Scholar] [CrossRef] [PubMed]
- Leitinger, N.; Tyner, T.R.; Oslund, L.; Rizza, C.; Subbanagounder, G.; Lee, H.; Shih, P.T.; Mackman, N.; Tigyi, G.; Territo, M.C.; et al. Structurally similar oxidized phospholipids differentially regulate endothelial binding of monocytes and neutrophils. Proc. Natl. Acad. Sci. USA 1999, 96, 12010–12015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shih, P.T.; Elices, M.J.; Fang, Z.T.; Ugarova, T.P.; Strahl, D.; Territo, M.C.; Frank, J.S.; Kovach, N.L.; Cabanas, C.; Berliner, J.A.; et al. Minimally modified low-density lipoprotein induces monocyte adhesion to endothelial connecting segment-1 by activating beta1 integrin. J. Clin. Invest. 1999, 103, 613–625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huber, J.; Furnkranz, A.; Bochkov, V.N.; Patricia, M.K.; Lee, H.; Hedrick, C.C.; Berliner, J.A.; Binder, B.R.; Leitinger, N. Specific monocyte adhesion to endothelial cells induced by oxidized phospholipids involves activation of cPLA2 and lipoxygenase. J. Lipid Res. 2006, 47, 1054–1062. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.A.; Territo, M.C.; Wayner, E.; Carlos, T.M.; Parhami, F.; Smith, C.W.; Haberland, M.E.; Fogelman, A.M.; Berliner, J.A. Partial characterization of leukocyte binding molecules on endothelial cells induced by minimally oxidized LDL. Arterioscler. Thromb. 1994, 14, 427–433. [Google Scholar] [CrossRef] [Green Version]
- Bekkering, S.; Quintin, J.; Joosten, L.A.; Van der Meer, J.W.; Netea, M.G.; Riksen, N.P. Oxidized low-density lipoprotein induces long-term proinflammatory cytokine production and foam cell formation via epigenetic reprogramming of monocytes. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 1731–1738. [Google Scholar] [CrossRef]
- Sohrabi, Y.; Lagache, S.M.M.; Schnack, L.; Godfrey, R.; Kahles, F.; Bruemmer, D.; Waltenberger, J.; Findeisen, H.M. mTOR-Dependent Oxidative Stress Regulates oxLDL-Induced Trained Innate Immunity in Human Monocytes. Front. Immunol. 2018, 9, 3155. [Google Scholar] [CrossRef] [Green Version]
- Furnkranz, A.; Schober, A.; Bochkov, V.N.; Bashtrykov, P.; Kronke, G.; Kadl, A.; Binder, B.R.; Weber, C.; Leitinger, N. Oxidized phospholipids trigger atherogenic inflammation in murine arteries. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 633–638. [Google Scholar] [CrossRef] [Green Version]
- Subbanagounder, G.; Wong, J.W.; Lee, H.; Faull, K.F.; Miller, E.; Witztum, J.L.; Berliner, J.A. Epoxyisoprostane and epoxycyclopentenone phospholipids regulate monocyte chemotactic protein-1 and interleukin-8 synthesis. Formation of these oxidized phospholipids in response to interleukin-1beta. J. Biol Chem. 2002, 277, 7271–7281. [Google Scholar] [CrossRef] [Green Version]
- Yeh, M.; Leitinger, N.; De Martin, R.; Onai, N.; Matsushima, K.; Vora, D.K.; Berliner, J.A.; Reddy, S.T. Increased transcription of IL-8 in endothelial cells is differentially regulated by TNF-alpha and oxidized phospholipids. Arterioscler. Thromb. Vasc. Biol. 2001, 21, 1585–1591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeh, M.; Gharavi, N.M.; Choi, J.; Hsieh, X.; Reed, E.; Mouillesseaux, K.P.; Cole, A.L.; Reddy, S.T.; Berliner, J.A. Oxidized phospholipids increase interleukin 8 (IL-8) synthesis by activation of the c-src/signal transducers and activators of transcription (STAT)3 pathway. J. Biol. Chem. 2004, 279, 30175–30181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeh, M.; Cole, A.L.; Choi, J.; Liu, Y.; Tulchinsky, D.; Qiao, J.H.; Fishbein, M.C.; Dooley, A.N.; Hovnanian, T.; Mouilleseaux, K.; et al. Role for sterol regulatory element-binding protein in activation of endothelial cells by phospholipid oxidation products. Circ. Res. 2004, 95, 780–788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gargalovic, P.S.; Gharavi, N.M.; Clark, M.J.; Pagnon, J.; Yang, W.P.; He, A.; Truong, A.; Baruch-Oren, T.; Berliner, J.A.; Kirchgessner, T.G.; et al. The unfolded protein response is an important regulator of inflammatory genes in endothelial cells. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 2490–2496. [Google Scholar] [CrossRef] [Green Version]
- Oskolkova, O.V.; Afonyushkin, T.; Leitner, A.; Von Schlieffen, E.; Gargalovic, P.S.; Lusis, A.J.; Binder, B.R.; Bochkov, V.N. ATF4-dependent transcription is a key mechanism in VEGF up-regulation by oxidized phospholipids: Critical role of oxidized sn-2 residues in activation of unfolded protein response. Blood 2008, 112, 330–339. [Google Scholar] [CrossRef]
- Yeon, S.H.; Yang, G.; Lee, H.E.; Lee, J.Y. Oxidized phosphatidylcholine induces the activation of NLRP3 inflammasome in macrophages. J. Leukoc. Biol. 2017, 101, 205–215. [Google Scholar] [CrossRef]
- Zanoni, I.; Tan, Y.; Di Gioia, M.; Broggi, A.; Ruan, J.; Shi, J.; Donado, C.A.; Shao, F.; Wu, H.; Springstead, J.R.; et al. An endogenous caspase-11 ligand elicits interleukin-1 release from living dendritic cells. Science 2016, 352, 1232–1236. [Google Scholar] [CrossRef] [Green Version]
- Miller, Y.I.; Choi, S.H.; Wiesner, P.; Fang, L.; Harkewicz, R.; Hartvigsen, K.; Boullier, A.; Gonen, A.; Diehl, C.J.; Que, X.; et al. Oxidation-specific epitopes are danger-associated molecular patterns recognized by pattern recognition receptors of innate immunity. Circ. Res. 2011, 108, 235–248. [Google Scholar] [CrossRef] [Green Version]
- Imai, Y.; Kuba, K.; Neely, G.G.; Yaghubian-Malhami, R.; Perkmann, T.; Van Loo, G.; Ermolaeva, M.; Veldhuizen, R.; Leung, Y.H.; Wang, H.; et al. Identification of oxidative stress and Toll-like receptor 4 signaling as a key pathway of acute lung injury. Cell 2008, 133, 235–249. [Google Scholar] [CrossRef]
- Shirey, K.A.; Lai, W.; Scott, A.J.; Lipsky, M.; Mistry, P.; Pletneva, L.M.; Karp, C.L.; McAlees, J.; Gioannini, T.L.; Weiss, J.; et al. The TLR4 antagonist Eritoran protects mice from lethal influenza infection. Nature 2013, 497, 498–502. [Google Scholar] [CrossRef] [Green Version]
- Walton, K.A.; Hsieh, X.; Gharavi, N.; Wang, S.; Wang, G.; Yeh, M.; Cole, A.L.; Berliner, J.A. Receptors involved in the oxidized 1-palmitoyl-2-arachidonoyl-sn-glycero-3-phosphorylcholine-mediated synthesis of interleukin-8. A role for Toll-like receptor 4 and a glycosylphosphatidylinositol-anchored protein. J. Biol. Chem. 2003, 278, 29661–29666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morales-Nebreda, L.; Mutlu, G.M.; Scott Budinger, G.R.; Radigan, K.A. Loss of TLR4 does not prevent influenza A-induced mortality. Am. J. Respir. Crit. Care Med. 2014, 189, 1280–1281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakai, T.; Imai, J.; Takagaki, H.; Ui, M.; Hatta, S. Cytoplasmic OH scavenger TA293 attenuates cellular senescence and fibrosis by activating macrophages through oxidized phospholipids/TLR4. Life Sci. 2019, 221, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Venosa, A.; Smith, L.C.; Murray, A.; Banota, T.; Gow, A.J.; Laskin, J.D.; Laskin, D.L. Regulation of Macrophage Foam Cell Formation During Nitrogen Mustard (NM)-Induced Pulmonary Fibrosis by Lung Lipids. Toxicol. Sci. 2019, 172, 344–358. [Google Scholar] [CrossRef]
- Kadl, A.; Sharma, P.R.; Chen, W.; Agrawal, R.; Meher, A.K.; Rudraiah, S.; Grubbs, N.; Sharma, R.; Leitinger, N. Oxidized phospholipid-induced inflammation is mediated by Toll-like receptor 2. Free Radic. Biol. Med. 2011, 51, 1903–1909. [Google Scholar] [CrossRef] [Green Version]
- Gillotte-Taylor, K.; Boullier, A.; Witztum, J.L.; Steinberg, D.; Quehenberger, O. Scavenger receptor class B type I as a receptor for oxidized low density lipoprotein. J. Lipid Res. 2001, 42, 1474–1482. [Google Scholar]
- Podrez, E.A.; Poliakov, E.; Shen, Z.; Zhang, R.; Deng, Y.; Sun, M.; Finton, P.J.; Shan, L.; Gugiu, B.; Fox, P.L.; et al. Identification of a novel family of oxidized phospholipids that serve as ligands for the macrophage scavenger receptor CD36. J. Biol Chem 2002, 277, 38503–38516. [Google Scholar] [CrossRef] [Green Version]
- Ishii, H.; Tezuka, T.; Ishikawa, H.; Takada, K.; Oida, K.; Horie, S. Oxidized phospholipids in oxidized low-density lipoprotein down-regulate thrombomodulin transcription in vascular endothelial cells through a decrease in the binding of RARbeta-RXRalpha heterodimers and Sp1 and Sp3 to their binding sequences in the TM promoter. Blood 2003, 101, 4765–4774. [Google Scholar] [CrossRef] [Green Version]
- Walton, K.A.; Cole, A.L.; Yeh, M.; Subbanagounder, G.; Krutzik, S.R.; Modlin, R.L.; Lucas, R.M.; Nakai, J.; Smart, E.J.; Vora, D.K.; et al. Specific phospholipid oxidation products inhibit ligand activation of toll-like receptors 4 and 2. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 1197–1203. [Google Scholar] [CrossRef] [Green Version]
- Erridge, C.; Kennedy, S.; Spickett, C.M.; Webb, D.J. Oxidized phospholipid inhibition of toll-like receptor (TLR) signaling is restricted to TLR2 and TLR4: Roles for CD14, LPS-binding protein, and MD2 as targets for specificity of inhibition. J. Biol. Chem. 2008, 283, 24748–24759. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.J.; Choi, N.Y.; Koo, J.E.; Kim, S.Y.; Joung, S.M.; Jeong, E.; Lee, J.Y. Suppression of Toll-like receptor 4 activation by endogenous oxidized phosphatidylcholine, KOdiA-PC by inhibiting LPS binding to MD2. Inflamm. Res. 2013, 62, 571–580. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.A.; Kim, S.M.; Son, Y.H.; Lee, C.W.; Chung, S.W.; Eo, S.K.; Rhim, B.Y.; Kim, K. Peptidoglycan enhances secretion of monocyte chemoattractants via multiple signaling pathways. Biochem. Biophys. Res. Commun. 2011, 408, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Tseng, P.O.; Wang, D.; Zhang, H.; Hunter, K.; Hertzberg, J.; Stenmark, K.R.; Tan, W. Stiffening-induced high pulsatility flow activates endothelial inflammation via a TLR2/NF-kappaB pathway. PLoS ONE 2014, 9, e102195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walton, K.A.; Gugiu, B.G.; Thomas, M.; Basseri, R.J.; Eliav, D.R.; Salomon, R.G.; Berliner, J.A. A role for neutral sphingomyelinase activation in the inhibition of LPS action by phospholipid oxidation products. J. Lipid Res. 2006, 47, 1967–1974. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mendel, I.; Shoham, A.; Propheta-Meiran, O.; Ishai, E.; Halperin, G.; Feige, E.; Breitbart, E. A Lecinoxoid, an oxidized phospholipid small molecule, constrains CNS autoimmune disease. J. Neuroimmunol. 2010, 226, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Mendel, I.; Yacov, N.; Shoham, A.; Ishai, E.; Breitbart, E. Treatment with Oxidized Phospholipids Directly Inhibits Nonalcoholic Steatohepatitis and Liver Fibrosis Without Affecting Steatosis. Dig. Dis. Sci. 2016, 61, 2545–2553. [Google Scholar] [CrossRef] [Green Version]
- Colombo, S.; Martin-Sierra, C.; Melo, T.; Laranjeira, P.; Paiva, A.; Domingues, P.; Domingues, M.R. Modulation of the inflammatory response of immune cells in human peripheral blood by oxidized arachidonoyl aminophospholipids. Arch. Biochem. Biophys. 2018, 660, 64–71. [Google Scholar] [CrossRef]
- Rossi, A.; Kapahi, P.; Natoli, G.; Takahashi, T.; Chen, Y.; Karin, M.; Santoro, M.G. Anti-inflammatory cyclopentenone prostaglandins are direct inhibitors of IkappaB kinase. Nature 2000, 403, 103–108. [Google Scholar] [CrossRef]
- Maciel, E.; Neves, B.M.; Martins, J.; Colombo, S.; Cruz, M.T.; Domingues, P.; Domingues, M.R.M. Oxidized phosphatidylserine mitigates LPS-triggered macrophage inflammatory status through modulation of JNK and NF-kB signaling cascades. Cell Signal. 2019, 61, 30–38. [Google Scholar] [CrossRef]
- Ghersa, P.; Hooft van Huijsduijnen, R.; Whelan, J.; Cambet, Y.; Pescini, R.; DeLamarter, J.F. Inhibition of E-selectin gene transcription through a cAMP-dependent protein kinase pathway. J. Biol. Chem. 1994, 269, 29129–29137. [Google Scholar]
- Rahman, A.; Anwar, K.N.; Minhajuddin, M.; Bijli, K.M.; Javaid, K.; True, A.L.; Malik, A.B. cAMP targeting of p38 MAP kinase inhibits thrombin-induced NF-kappaB activation and ICAM-1 expression in endothelial cells. Am. J. Physiol Lung Cell Mol. Physiol. 2004, 287, L1017–L1024. [Google Scholar] [CrossRef] [PubMed]
- Cole, A.L.; Subbanagounder, G.; Mukhopadhyay, S.; Berliner, J.A.; Vora, D.K. Oxidized phospholipid-induced endothelial cell/monocyte interaction is mediated by a cAMP-dependent R-Ras/PI3-kinase pathway. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 1384–1390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kronke, G.; Bochkov, V.N.; Huber, J.; Gruber, F.; Bluml, S.; Furnkranz, A.; Kadl, A.; Binder, B.R.; Leitinger, N. Oxidized phospholipids induce expression of human heme oxygenase-1 involving activation of cAMP-responsive element-binding protein. J. Biol. Chem. 2003, 278, 51006–51014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishikawa, K.; Navab, M.; Leitinger, N.; Fogelman, A.M.; Lusis, A.J. Induction of heme oxygenase-1 inhibits the monocyte transmigration induced by mildly oxidized LDL. J. Clin. Invest. 1997, 100, 1209–1216. [Google Scholar] [CrossRef] [PubMed]
- Otterbein, L.E.; Bach, F.H.; Alam, J.; Soares, M.; Tao Lu, H.; Wysk, M.; Davis, R.J.; Flavell, R.A.; Choi, A.M. Carbon monoxide has anti-inflammatory effects involving the mitogen-activated protein kinase pathway. Nat. Med. 2000, 6, 422–428. [Google Scholar] [CrossRef]
- Otterbein, L.E.; Zuckerbraun, B.S.; Haga, M.; Liu, F.; Song, R.; Usheva, A.; Stachulak, C.; Bodyak, N.; Smith, R.N.; Csizmadia, E.; et al. Carbon monoxide suppresses arteriosclerotic lesions associated with chronic graft rejection and with balloon injury. Nat. Med. 2003, 9, 183–190. [Google Scholar] [CrossRef]
- Pontsler, A.V.; St Hilaire, A.; Marathe, G.K.; Zimmerman, G.A.; McIntyre, T.M. Cyclooxygenase-2 is induced in monocytes by peroxisome proliferator activated receptor gamma and oxidized alkyl phospholipids from oxidized low density lipoprotein. J. Biol. Chem. 2002, 277, 13029–13036. [Google Scholar] [CrossRef] [Green Version]
- Itoh, K.; Mochizuki, M.; Ishii, Y.; Ishii, T.; Shibata, T.; Kawamoto, Y.; Kelly, V.; Sekizawa, K.; Uchida, K.; Yamamoto, M. Transcription factor Nrf2 regulates inflammation by mediating the effect of 15-deoxy-Delta(12,14)-prostaglandin j(2). Mol. Cell Biol. 2004, 24, 36–45. [Google Scholar] [CrossRef] [Green Version]
- Bretscher, P.; Egger, J.; Shamshiev, A.; Trotzmuller, M.; Kofeler, H.; Carreira, E.M.; Kopf, M.; Freigang, S. Phospholipid oxidation generates potent anti-inflammatory lipid mediators that mimic structurally related pro-resolving eicosanoids by activating Nrf2. EMBO Mol. Med. 2015, 7, 593–607. [Google Scholar] [CrossRef]
- Jyrkkanen, H.K.; Kansanen, E.; Inkala, M.; Kivela, A.M.; Hurttila, H.; Heinonen, S.E.; Goldsteins, G.; Jauhiainen, S.; Tiainen, S.; Makkonen, H.; et al. Nrf2 regulates antioxidant gene expression evoked by oxidized phospholipids in endothelial cells and murine arteries in vivo. Circ. Res. 2008, 103, e1–e9. [Google Scholar] [CrossRef] [Green Version]
- Serhan, C.N.; Chiang, N. Endogenous pro-resolving and anti-inflammatory lipid mediators: A new pharmacologic genus. Br. J. Pharmacol. 2008, 153, S200–S215. [Google Scholar] [CrossRef] [PubMed]
- Oskolkova, O.; Sarich, N.; Tian, Y.; Gawlak, G.; Meng, F.; Bochkov, V.N.; Berdyshev, E.; Birukova, A.A.; Birukov, K.G. Incorporation of iloprost in phospholipase-resistant phospholipid scaffold enhances its barrier protective effects on pulmonary endothelium. Sci. Rep. 2018, 8, 879. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, J.; Guo, S.; Xue, X.; Chen, Q.; Ge, J.; Zhuo, Y.; Zhong, H.; Chen, B.; Zhao, M.; Han, W.; et al. Identification of a novel series of anti-inflammatory and anti-oxidative phospholipid oxidation products containing the cyclopentenone moiety in vitro and in vivo: Implication in atherosclerosis. J. Biol. Chem. 2017, 292, 5378–5391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cherepanova, O.A.; Srikakulapu, P.; Greene, E.S.; Chaklader, M.; Haskins, R.M.; McCanna, M.E.; Bandyopadhyay, S.; Ban, B.; Leitinger, N.; McNamara, C.A.; et al. Novel Autoimmune IgM Antibody Attenuates Atherosclerosis in IgM Deficient Low-Fat Diet-Fed, but Not Western Diet-Fed Apoe(-/-) Mice. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 206–219. [Google Scholar] [CrossRef] [PubMed]
- Birukov, K.G.; Zebda, N.; Birukova, A.A. Barrier enhancing signals in pulmonary edema. Compr. Physiol. 2013, 3, 429–484. [Google Scholar] [CrossRef]
- Patterson, C.E.; Lum, H. Update on pulmonary edema: The role and regulation of endothelial barrier function. Endothelium 2001, 8, 75–105. [Google Scholar]
- Kasa, A.; Csortos, C.; Verin, A.D. Cytoskeletal mechanisms regulating vascular endothelial barrier function in response to acute lung injury. Tissue Barriers 2015, 3, e974448. [Google Scholar] [CrossRef] [Green Version]
- Sukriti, S.; Tauseef, M.; Yazbeck, P.; Mehta, D. Mechanisms regulating endothelial permeability. Pulm. Circ. 2014, 4, 535–551. [Google Scholar] [CrossRef] [Green Version]
- Birukova, A.A.; Malyukova, I.; Mikaelyan, A.; Fu, P.; Birukov, K.G. Tiam1 and betaPIX mediate Rac-dependent endothelial barrier protective response to oxidized phospholipids. J. Cell Physiol. 2007, 211, 608–617. [Google Scholar] [CrossRef]
- Birukova, A.A.; Alekseeva, E.; Cokic, I.; Turner, C.E.; Birukov, K.G. Cross talk between paxillin and Rac is critical for mediation of barrier-protective effects by oxidized phospholipids. Am. J. Physiol. Lung Cell Mol. Physiol 2008, 295, L593–L602. [Google Scholar] [CrossRef] [Green Version]
- Birukova, A.A.; Chatchavalvanich, S.; Oskolkova, O.; Bochkov, V.N.; Birukov, K.G. Signaling pathways involved in OxPAPC-induced pulmonary endothelial barrier protection. Microvasc. Res. 2007, 73, 173–181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Birukova, A.A.; Fu, P.; Wu, T.; Dubrovskyi, O.; Sarich, N.; Poroyko, V.; Birukov, K.G. Afadin controls p120-catenin-ZO-1 interactions leading to endothelial barrier enhancement by oxidized phospholipids. J. Cell Physiol. 2012, 227, 1883–1890. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Birukova, A.A.; Zebda, N.; Fu, P.; Poroyko, V.; Cokic, I.; Birukov, K.G. Association between adherens junctions and tight junctions via Rap1 promotes barrier protective effects of oxidized phospholipids. J. Cell Physiol. 2011, 226, 2052–2062. [Google Scholar] [CrossRef] [Green Version]
- Tian, Y.; Tian, X.; Gawlak, G.; Sarich, N.; Sacks, D.B.; Birukova, A.A.; Birukov, K.G. Role of IQGAP1 in endothelial barrier enhancement caused by OxPAPC. Am. J. Physiol. Lung Cell Mol. Physiol. 2016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Birukova, A.A.; Zebda, N.; Cokic, I.; Fu, P.; Wu, T.; Dubrovskyi, O.; Birukov, K.G. p190RhoGAP mediates protective effects of oxidized phospholipids in the models of ventilator-induced lung injury. Exp. Cell Res. 2011, 317, 859–872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Birukov, K.G.; Leitinger, N.; Bochkov, V.N.; Garcia, J.G. Signal transduction pathways activated in human pulmonary endothelial cells by OxPAPC, a bioactive component of oxidized lipoproteins. Microvasc. Res. 2004, 67, 18–28. [Google Scholar] [CrossRef]
- Singleton, P.A.; Chatchavalvanich, S.; Fu, P.; Xing, J.; Birukova, A.A.; Fortune, J.A.; Klibanov, A.M.; Garcia, J.G.; Birukov, K.G. Akt-mediated transactivation of the S1P1 receptor in caveolin-enriched microdomains regulates endothelial barrier enhancement by oxidized phospholipids. Circ. Res. 2009, 104, 978–986. [Google Scholar] [CrossRef] [Green Version]
- Birukova, A.A.; Singleton, P.A.; Gawlak, G.; Tian, X.; Mirzapoiazova, T.; Mambetsariev, B.; Dubrovskyi, O.; Oskolkova, O.V.; Bochkov, V.N.; Birukov, K.G. GRP78 is a novel receptor initiating a vascular barrier protective response to oxidized phospholipids. Mol. Biol. Cell 2014, 25, 2006–2016. [Google Scholar] [CrossRef] [Green Version]
- Peng, X.; Hassoun, P.M.; Sammani, S.; McVerry, B.J.; Burne, M.J.; Rabb, H.; Pearse, D.; Tuder, R.M.; Garcia, J.G. Protective effects of sphingosine 1-phosphate in murine endotoxin-induced inflammatory lung injury. Am. J. Respir. Crit. Care Med. 2004, 169, 1245–1251. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Chiang, E.T.; Simmons, J.T.; Garcia, J.G.; Dudek, S.M. FTY720-induced human pulmonary endothelial barrier enhancement is mediated by c-Abl. Eur. Respir. J. 2011, 38, 78–88. [Google Scholar] [CrossRef] [Green Version]
- Oskolkova, O.; Gawlak, G.; Tian, Y.; Ke, Y.; Sarich, N.; Son, S.; Andreasson, K.; Bochkov, V.N.; Birukova, A.A.; Birukov, K.G. Prostaglandin E receptor-4 receptor mediates endothelial barrier-enhancing and anti-inflammatory effects of oxidized phospholipids. FASEB J. 2017, 31, 4187–4202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Angelini, D.J.; Hyun, S.W.; Grigoryev, D.N.; Garg, P.; Gong, P.; Singh, I.S.; Passaniti, A.; Hasday, J.D.; Goldblum, S.E. TNF-alpha increases tyrosine phosphorylation of vascular endothelial cadherin and opens the paracellular pathway through fyn activation in human lung endothelia. Am. J. Physiol. Lung Cell Mol. Physiol. 2006, 291, L1232–L1245. [Google Scholar] [CrossRef] [PubMed]
- Shasby, D.M.; Ries, D.R.; Shasby, S.S.; Winter, M.C. Histamine stimulates phosphorylation of adherens junction proteins and alters their link to vimentin. Am. J. Physiol. Lung Cell Mol. Physiol. 2002, 282, L1330–L1338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Birukova, A.A.; Lee, S.; Starosta, V.; Wu, T.; Ho, T.; Kim, J.; Berliner, J.A.; Birukov, K.G. A role for VEGFR2 activation in endothelial responses caused by barrier disruptive OxPAPC concentrations. PLoS ONE 2012, 7, e30957. [Google Scholar] [CrossRef] [Green Version]
- Trpkovic, A.; Resanovic, I.; Stanimirovic, J.; Radak, D.; Mousa, S.A.; Cenic-Milosevic, D.; Jevremovic, D.; Isenovic, E.R. Oxidized low-density lipoprotein as a biomarker of cardiovascular diseases. Crit. Rev. Clin. Lab. Sci. 2015, 52, 70–85. [Google Scholar] [CrossRef]
- Karki, P.; Meliton, A.; Shah, A.; Tian, Y.; Ohmura, T.; Sarich, N.; Birukova, A.A.; Birukov, K.G. Role of truncated oxidized phospholipids in acute endothelial barrier dysfunction caused by particulate matter. PLoS ONE 2018, 13, e0206251. [Google Scholar] [CrossRef]
- Liu, J.; Li, W.; Chen, R.; McIntyre, T.M. Circulating biologically active oxidized phospholipids show on-going and increased oxidative stress in older male mice. Redox. Biol. 2013, 1, 110–114. [Google Scholar] [CrossRef] [Green Version]
- Frey, B.; Haupt, R.; Alms, S.; Holzmann, G.; Konig, T.; Kern, H.; Kox, W.; Rustow, B.; Schlame, M. Increase in fragmented phosphatidylcholine in blood plasma by oxidative stress. J. Lipid Res. 2000, 41, 1145–1153. [Google Scholar]
- Hong, J.H.; Kang, J.W.; Kim, D.K.; Baik, S.H.; Kim, K.H.; Shanta, S.R.; Jung, J.H.; Mook-Jung, I.; Kim, K.P. Global changes of phospholipids identified by MALDI imaging mass spectrometry in a mouse model of Alzheimer’s disease. J. Lipid Res. 2016, 57, 36–45. [Google Scholar] [CrossRef] [Green Version]
- Kawanishi, N.; Kato, Y.; Yokozeki, K.; Sawada, S.; Sakurai, R.; Fujiwara, Y.; Shinkai, S.; Goda, N.; Suzuki, K. Effects of aging on serum levels of lipid molecular species as determined by lipidomics analysis in Japanese men and women. Lipids Health Dis. 2018, 17, 135. [Google Scholar] [CrossRef] [Green Version]
- Gaudin, M.; Panchal, M.; Auzeil, N.; Duyckaerts, C.; Brunelle, A.; Laprevote, O.; Touboul, D. Choline-containing phospholipids in microdissected human Alzheimer’s disease brain senile plaque versus neuropil. Bioanalysis 2012, 4, 2153–5159. [Google Scholar] [CrossRef]
- Whiley, L.; Sen, A.; Heaton, J.; Proitsi, P.; Garcia-Gomez, D.; Leung, R.; Smith, N.; Thambisetty, M.; Kloszewska, I.; Mecocci, P.; et al. Evidence of altered phosphatidylcholine metabolism in Alzheimer’s disease. Neurobiol. Aging 2014, 35, 271–278. [Google Scholar] [CrossRef]
- Nomura, K.; Imai, H.; Koumura, T.; Kobayashi, T.; Nakagawa, Y. Mitochondrial phospholipid hydroperoxide glutathione peroxidase inhibits the release of cytochrome c from mitochondria by suppressing the peroxidation of cardiolipin in hypoglycaemia-induced apoptosis. Biochem. J. 2000, 351, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Paradies, G.; Petrosillo, G.; Paradies, V.; Ruggiero, F.M. Oxidative stress, mitochondrial bioenergetics, and cardiolipin in aging. Free Radic. Biol. Med. 2010, 48, 1286–1295. [Google Scholar] [CrossRef] [PubMed]
- Tyurina, Y.Y.; Tyurin, V.A.; Kaynar, A.M.; Kapralova, V.I.; Wasserloos, K.; Li, J.; Mosher, M.; Wright, L.; Wipf, P.; Watkins, S.; et al. Oxidative lipidomics of hyperoxic acute lung injury: Mass spectrometric characterization of cardiolipin and phosphatidylserine peroxidation. Am. J. Physiol. Lung Cell Mol. Physiol. 2010, 299, L73–L85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petrosillo, G.; Matera, M.; Casanova, G.; Ruggiero, F.M.; Paradies, G. Mitochondrial dysfunction in rat brain with aging Involvement of complex I, reactive oxygen species and cardiolipin. Neurochem. Int. 2008, 53, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Petrosillo, G.; Matera, M.; Moro, N.; Ruggiero, F.M.; Paradies, G. Mitochondrial complex I dysfunction in rat heart with aging: Critical role of reactive oxygen species and cardiolipin. Free Radic. Biol. Med. 2009, 46, 88–94. [Google Scholar] [CrossRef]
- Eum, J.Y.; Lee, J.C.; Yi, S.S.; Kim, I.Y.; Seong, J.K.; Moon, M.H. Aging-related lipidomic changes in mouse serum, kidney, and heart by nanoflow ultrahigh-performance liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2020. [Google Scholar] [CrossRef]
- Ni, Z.; Sousa, B.C.; Colombo, S.; Afonso, C.B.; Melo, T.; Pitt, A.R.; Spickett, C.M.; Domingues, P.; Domingues, M.R.; Fedorova, M.; et al. Evaluation of air oxidized PAPC: A multi laboratory study by LC-MS/MS. Free Radic. Biol. Med. 2019, 144, 156–166. [Google Scholar] [CrossRef]
- Colombo, S.; Melo, T.; Martinez-Lopez, M.; Carrasco, M.J.; Domingues, M.R.; Perez-Sala, D.; Domingues, P. Phospholipidome of endothelial cells shows a different adaptation response upon oxidative, glycative and lipoxidative stress. Sci. Rep. 2018, 8, 12365. [Google Scholar] [CrossRef] [Green Version]
- Carter, C.L.; Jones, J.W.; Farese, A.M.; MacVittie, T.J.; Kane, M.A. Lipidomic dysregulation within the lung parenchyma following whole-thorax lung irradiation: Markers of injury, inflammation and fibrosis detected by MALDI-MSI. Sci. Rep. 2017, 7, 10343. [Google Scholar] [CrossRef] [PubMed]
- Kagan, V.E.; Tyurina, Y.Y.; Sun, W.Y.; Vlasova, L.L.; Dar, H.; Tyurin, V.A.; Amoscato, A.A.; Mallampalli, R.; Van der Wel, P.C.A.; He, R.R.; et al. Redox phospholipidomics of enzymatically generated oxygenated phospholipids as specific signals of programmed cell death. Free Radic. Biol. Med. 2020, 147, 231–241. [Google Scholar] [CrossRef] [PubMed]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karki, P.; Birukov, K.G. Oxidized Phospholipids in Healthy and Diseased Lung Endothelium. Cells 2020, 9, 981. https://doi.org/10.3390/cells9040981
Karki P, Birukov KG. Oxidized Phospholipids in Healthy and Diseased Lung Endothelium. Cells. 2020; 9(4):981. https://doi.org/10.3390/cells9040981
Chicago/Turabian StyleKarki, Pratap, and Konstantin G. Birukov. 2020. "Oxidized Phospholipids in Healthy and Diseased Lung Endothelium" Cells 9, no. 4: 981. https://doi.org/10.3390/cells9040981
APA StyleKarki, P., & Birukov, K. G. (2020). Oxidized Phospholipids in Healthy and Diseased Lung Endothelium. Cells, 9(4), 981. https://doi.org/10.3390/cells9040981





