γδ T Cells: The Ideal Tool for Cancer Immunotherapy
Abstract
:1. Introduction
1.1. Human Adult γδ T Cells Subsets
1.2. The Hybrid αβ/γδ T Cells
1.3. γδ T Cells: An Appealing Source for Adoptive Cell Immunotherapy
2. Expansion Strategies
2.1. Ex Vivo Expansion of Vδ2 γδ T Cells
2.2. Ex Vivo Expansion of Vδ1 γδ T Cells
2.3. Ex Vivo Expansion Using mAbs
2.4. γδ T Cell Modulation with Different Substances
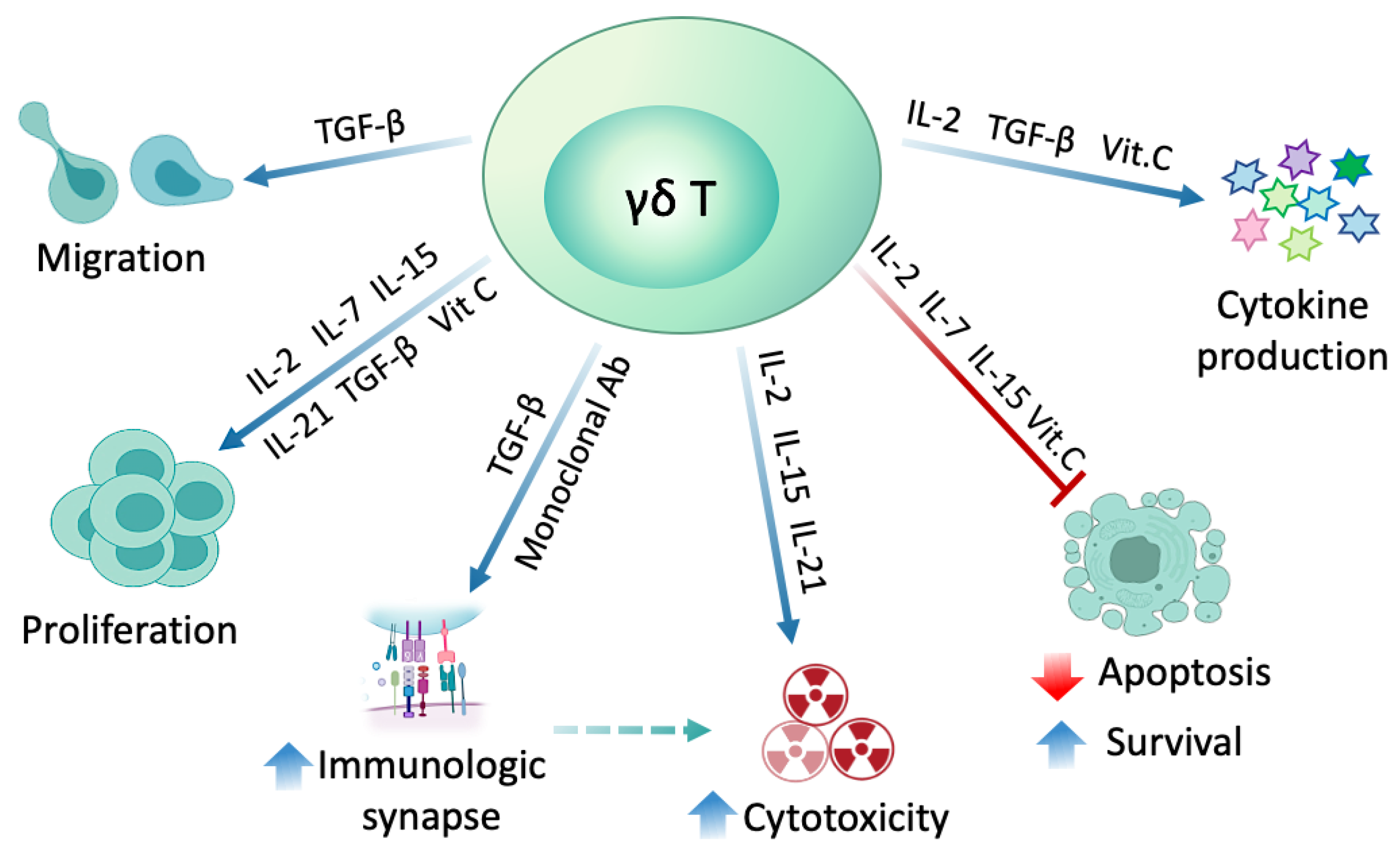
2.4.1. Interleukins
2.4.2. Transforming Growth Factor-β (TGF-β)
2.4.3. Vitamin C
2.4.4. Monoclonal Antibodies
2.5. In Vivo (Systemic) Expansion of γδ T Cells
3. Toward Engineering γδ T Cells: Transduction Strategies
4. Preclinical and Clinical Experience: The Lesson Learned
5. Challenges
6. Conclusions and Future Perspective
Funding
Acknowledgments
Conflicts of Interest
References
- Melandri, D.; Zlatareva, I.; Chaleil, R.A.G.; Dart, R.J.; Chancellor, A.; Nussbaumer, O.; Polyakova, O.; Roberts, N.A.; Wesch, D.; Kabelitz, D.; et al. The γδTCR combines innate immunity with adaptive immunity by utilizing spatially distinct regions for agonist selection and antigen responsiveness. Nat. Immunol. 2018, 19, 1352–1365. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-L.; Ding, Y.-P.; Tanaka, Y.; Shen, L.-W.; Wei, C.-H.; Minato, N.; Zhang, W. γδ T Cells and Their Potential for Immunotherapy. Int. J. Biol. Sci. 2014, 10, 119–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halary, F.; Pitard, V.; Dlubek, D.; Krzysiek, R.; De La Salle, H.; Merville, P.; Dromer, C.; Emilie, D.; Moreau, J.-F.; Déchanet-Merville, J. Shared reactivity of Vδ2neg γδ T cells against cytomegalovirus-infected cells and tumor intestinal epithelial cells. J. Exp. Med. 2005, 201, 1567–1578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mordmüller, B.; Surat, G.; Lagler, H.; Chakravarty, S.; Ishizuka, A.; Lalremruata, A.; Gmeiner, M.; Campo, J.J.; Esen, M.; Ruben, A.J.; et al. Sterile protection against human malaria by chemoattenuated PfSPZ vaccine. Nature 2017, 542, 445–449. [Google Scholar] [CrossRef]
- Couzi, L.; Pitard, V.; Sicard, X.; Garrigue, I.; Hawchar, O.; Merville, P.; Moreau, J.-F.; Déchanet-Merville, J. Antibody-dependent anti-cytomegalovirus activity of human γδ T cells expressing CD16 (FcγRIIIa). Blood 2012, 119, 1418–1427. Available online: http://www.ncbi.nlm.nih.gov/pubmed/22180442 (accessed on 11 March 2020). [CrossRef]
- Ramstead, A.G.; Jutila, M.A. Complex Role of γδ T-Cell-Derived Cytokines and Growth Factors in Cancer. J. Interf. Cytokine Res. 2012, 32, 563–569. [Google Scholar] [CrossRef] [Green Version]
- Brandes, M.; Willimann, K.; Moser, B. Professional Antigen-Presentation Function by Human γδ T Cells. Science 2005, 309, 264–268. Available online: http://science.sciencemag.org/content/309/5732/264.abstract (accessed on 17 May 2020). [CrossRef]
- Airoldi, I.; Bertaina, A.; Prigione, I.; Zorzoli, A.; Pagliara, D.; Cocco, C.; Meazza, R.; LoIacono, F.; Lucarelli, B.; Bernardo, M.E.; et al. γδ T-cell reconstitution after HLA-haploidentical hematopoietic transplantation depleted of TCR-αβ+/CD19+ lymphocytes. Blood 2015, 125, 2349–2358. [Google Scholar] [CrossRef]
- Vantourout, P.; Hayday, A. Six-of-the-best: Unique contributions of gammadelta T cells to immunology. Nat. Rev. Immunol. 2013, 13, 88–100. [Google Scholar] [CrossRef] [Green Version]
- Gentles, A.J.; Newman, A.M.; Liu, C.L.; Bratman, S.V.; Feng, W.; Kim, N.; Nair, V.S.; Xu, Y.; Khuong, A.; Hoang, C.D.; et al. The prognostic landscape of genes and infiltrating immune cells across human cancers. Nat. Med. 2015, 21, 938–945. [Google Scholar] [CrossRef]
- Vermijlen, D.; Gatti, D.; Kouzeli, A.; Rus, T.; Eberl, M. γδ T cell responses: How many ligands will it take till we know? Semin. Cell Dev. Biol. 2018, 84, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Willcox, B.E.; Willcox, C.R. γδ TCR ligands: The quest to solve a 500-million-year-old mystery. Nat. Immunol. 2019, 20, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Legut, M.; Cole, D.K.; Sewell, A.K. The promise of γδ T cells and the γδ T cell receptor for cancer immunotherapy. Cell. Mol. Immunol. 2015, 12, 656–668. [Google Scholar] [CrossRef] [PubMed]
- Minculescu, L.; Sengeløv, H. The Role of Gamma Delta T Cells in Haematopoietic Stem Cell Transplantation. Scand. J. Immunol. 2015, 81, 459–468. Available online: http://www.ncbi.nlm.nih.gov/pubmed/25753378 (accessed on 5 March 2020). [CrossRef] [PubMed]
- Rui, L.; Johnson, R.; Yu, G.; Mckenna, D.; Hubel, A. Preservation of cell-based immunotherapies for clinical trials. Cytotherapy 2019, 21, 943–957. Available online: http://www.ncbi.nlm.nih.gov/pubmed/31416704 (accessed on 22 February 2020).
- Kabelitz, D.; Marischen, L.; Oberg, H.-H.; Holtmeier, W.; Wesch, D. Epithelial Defence by γδ T Cells. Int. Arch. Allergy Immunol. 2005, 137, 73–81. Available online: https://www.karger.com/Article/FullText/85107 (accessed on 12 May 2020). [CrossRef]
- Sharma, A.; Zumwalde, N.A.; Gumperz, J. Expansion and Adoptive Transfer of Human Vδ2+ T cells to Assess Antitumor Effects in Vivo. In Advanced Structural Safety Studies; Humana Press Inc.: New York, NY, USA, 2019; pp. 57–72. Available online: http://www.ncbi.nlm.nih.gov/pubmed/30465195 (accessed on 23 February 2020).
- Pauza, C.D.; Liou, M.-L.; Lahusen, T.; Xiao, L.; Lapidus, R.G.; Cairo, C.; Li, H. Gamma Delta T Cell Therapy for Cancer: It Is Good to be Local. Front. Immunol. 2018, 9, 1305. [Google Scholar] [CrossRef]
- Davey, M.S.; Willcox, C.R.; Hunter, S.; Kasatskaya, S.A.; Remmerswaal, E.B.M.; Salim, M.; Mohammed, F.; Bemelman, F.J.; Chudakov, D.M.; Oo, Y.H.; et al. The human Vδ2+ T-cell compartment comprises distinct innate-like Vγ9+ and adaptive Vγ9-subsets. Nat. Commun. 2018, 9, 1760. [Google Scholar] [CrossRef]
- Papadopoulou, M.; Tieppo, P.; McGovern, N.; Gosselin, F.; Chan, J.K.Y.; Goetgeluk, G.; Dauby, N.; Cogan, A.; Donner, C.; Ginhoux, F.; et al. TCR Sequencing Reveals the Distinct Development of Fetal and Adult Human Vγ9Vδ2 T Cells. J. Immunol. 2019, 203, 1468–1479. [Google Scholar] [CrossRef] [Green Version]
- Fichtner, A.S.; Bubke, A.; Rampoldi, F.; Wilharm, A.; Tan, L.; Steinbrück, L.; Schultze-Florey, C.; Von Kaisenberg, C.; Prinz, I.; Herrmann, T.; et al. TCR repertoire analysis reveals phosphoantigen-induced polyclonal proliferation of Vγ9Vδ2 T cells in neonates and adults. J. Leukoc. Biol. 2020, 107. [Google Scholar] [CrossRef]
- Ravens, S.; Schultze-Florey, C.; Raha, S.; Sandrock, I.; Drenker, M.; Oberdörfer, L.; Reinhardt, A.; Ravens, I.; Beck, M.; Geffers, R.; et al. Human γδ T cells are quickly reconstituted after stem-cell transplantation and show adaptive clonal expansion in response to viral infection. Nat. Immunol. 2017, 18, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Davey, M.S.; Willcox, C.R.; Joyce, S.P.; Ladell, K.; Kasatskaya, S.A.; McLaren, J.E.; Hunter, S.; Salim, M.; Mohammed, F.; Price, D.A.; et al. Clonal selection in the human Vδ1 T cell repertoire indicates γδ TCR-dependent adaptive immune surveillance. Nat. Commun. 2017, 8, 14760. [Google Scholar] [CrossRef] [PubMed]
- Tieppo, P.; Papadopoulou, M.; Gatti, D.; McGovern, N.; Chan, J.K.; Gosselin, F.; Goetgeluk, G.; Weening, K.; Ma, L.; Dauby, N.; et al. The human fetal thymus generates invariant effector γδ T cells. J. Exp. Med. 2020, 217, e20190580. [Google Scholar] [CrossRef] [PubMed]
- Ghadially, H.; Brown, L.; Lloyd, C.; Lewis, L.; Lewis, A.; Dillon, J.; Sainson, R.; Jovanovic, J.; Tigue, N.; Bannister, D.; et al. MHC class I chain-related protein A and B (MICA and MICB) are predominantly expressed intracellularly in tumour and normal tissue. Br. J. Cancer 2017, 116, 1208–1217. [Google Scholar] [CrossRef] [Green Version]
- Petrasca, A.; Melo, A.; Breen, E.P.; Doherty, D.G. Human Vδ3 + γδ T cells induce maturation and IgM secretion by B cells. Immunol. Lett. 2018, 196, 126–134. Available online: http://www.ncbi.nlm.nih.gov/pubmed/29438730 (accessed on 3 March 2020). [CrossRef]
- Zheng, H.; Wang, X.; Ma, Y.; Xu, B.; Chen, S.; Yang, L.; Wu, X.; Przybylski, G.K.; Huang, S.; Ye, T.; et al. The TCR γδ Repertoire and Relative Gene Expression Characteristics of T-ALL Cases with Biclonal Malignant Vδ1 and Vδ2 T Cells. DNA Cell Biol. 2014, 33, 49–56. [Google Scholar] [CrossRef] [Green Version]
- Adams, E.J.; Gu, S.; Luoma, A.M. Human gamma delta T cells: Evolution and ligand recognition. Cell. Immunol. 2015, 296, 31–40. [Google Scholar] [CrossRef] [Green Version]
- Christopoulos, P.; Bukatz, D.; Kock, S.; Malkovsky, M.; Finke, J.; Fisch, P. Improved analysis of TCRγδ variable region expression in humans. J. Immunol. Methods 2016, 434, 66–72. Available online: http://www.ncbi.nlm.nih.gov/pubmed/27109705 (accessed on 3 March 2020). [CrossRef]
- Bowen, S.; Sun, P.; Livak, F.; Sharrow, S.; Hodes, R.J. A novel T cell subset with trans-rearranged Vγ-Cβ TCRs shows Vβ expression is dispensable for lineage choice and MHC restriction. J. Immunol. 2013, 192, 169–177. [Google Scholar] [CrossRef]
- Hochstenbach, F.; Brenner, M.B. T-cell receptor δ-chain can substitute for α to form a βδ heterodimer. Nature 1989, 340, 562–565. [Google Scholar] [CrossRef]
- Ishida, I.; Verbeek, S.; Bonneville, M.; Itohara, S.; Berns, A.; Tonegawa, S. T-cell receptor γδ and γ transgenic mice suggest a role of a γ gene silencer in the generation of αβ T cells. Proc. Natl. Acad. Sci. USA 1990, 87, 3067–3071. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Livak, F.; Petrie, H.T.; Crispe, I.N.; Schatz, D.G. In-frame TCR delta gene rearrangements play a critical role in the alpha beta/gamma delta T cell lineage decision. Immunity 1995, 2, 617–627. [Google Scholar] [CrossRef] [Green Version]
- Bosco, N.; Engdahl, C.; Bénard, A.; Rolink, J.; Ceredig, R.; Rolink, A.G. TCR-β chains derived from peripheral γδ T cells can take part in αβ T-cell development. Eur. J. Immunol. 2008, 38, 3520–3529. Available online: http://www.ncbi.nlm.nih.gov/pubmed/18991270 (accessed on 9 April 2020). [CrossRef] [PubMed]
- Pellicci, D.G.; Uldrich, A.P.; Le Nours, J.; Ross, F.; Chabrol, E.; Eckle, S.B.G.; De Boer, R.; Lim, R.T.; McPherson, K.; Besra, G.; et al. The molecular bases of δ/αβ T cell–mediated antigen recognition. J. Exp. Med. 2014, 211, 2599–2615. Available online: http://www.ncbi.nlm.nih.gov/pubmed/25452463 (accessed on 9 April 2020). [CrossRef] [PubMed]
- Ziegler, H.; Welker, C.; Sterk, M.; Haarer, J.; Rammensee, H.G.; Handgretinger, R.; Handgretinger, R.; Schilbach, K. Human peripheral CD4+ Vδ1+ γδT cells can develop into αβT cells. Front Immunol. 2014, 5, 645. [Google Scholar] [CrossRef] [PubMed]
- Bertaina, A.; Zorzoli, A.; Petretto, A.; Barbarito, G.; Inglese, E.; Merli, P.; Lavarello, C.; Brescia, L.P.; de Angelis, B.; Tripodi, G.; et al. Zoledronic acid boosts gammadelta T-cell activity in children receiving alphabeta(+) T and CD19(+) cell-depleted grafts from an HLA-haplo-identical donor. Oncoimmunology 2017, 6, e1216291. [Google Scholar] [CrossRef] [Green Version]
- Babbe, H.; Chester, N.; Leder, P.; Reizis, B. The Bloom’s syndrome helicase is critical for development and function of the alphabeta T-cell lineage. Mol. Cell Biol. 2007, 27, 1947–1959. Available online: http://www.ncbi.nlm.nih.gov/pubmed/17210642 (accessed on 10 April 2020). [CrossRef] [Green Version]
- Edwards, S.C.; Sutton, C.E.; Ladell, K.; Grant, E.J.; McLaren, J.E.; Roche, F.; Dash, P.; Apiwattanakul, N.; Awad, W.; Miners, K.L.; et al. A population of proinflammatory T cells coexpresses αβ and γδ T cell receptors in mice and humans. J. Exp. Med. 2020, 217, e20190834. [Google Scholar] [CrossRef] [Green Version]
- Di Lorenzo, B.; Simões, A.E.; Caiado, F.; Tieppo, P.; Correia, D.V.; Carvalho, T.; Da Silva, M.G.; Déchanet-Merville, J.; Schumacher, T.N.; Prinz, I.; et al. Broad Cytotoxic Targeting of Acute Myeloid Leukemia by Polyclonal Delta One T Cells. Cancer Immunol. Res. 2019, 7, 552–558. [Google Scholar] [CrossRef] [Green Version]
- Nussbaumer, O.; Koslowski, M. The emerging role of γδ T cells in cancer immunotherapy. Immuno-Oncol. Technol. 2019, 1, 3–10. [Google Scholar] [CrossRef] [Green Version]
- Capsomidis, A.; Benthall, G.; Van Acker, H.H.; Fisher, J.; Kramer, A.M.; Abeln, Z.; Majani, Y.; Gileadi, T.; Wallace, R.; Gustafsson, K.; et al. Chimeric Antigen Receptor-Engineered Human Gamma Delta T Cells: Enhanced Cytotoxicity with Retention of Cross Presentation. Mol. Ther. 2017, 26, 354–365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fisher, J.; Anderson, J. Engineering Approaches in Human Gamma Delta T Cells for Cancer Immunotherapy. Front. Immunol. 2018, 9, 1409. [Google Scholar] [CrossRef] [PubMed]
- Silva-Santos, B.; Serre, K.; Norell, H.; Norell, H. γδ T cells in cancer. Nat. Rev. Immunol. 2015, 15, 683–691. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Chen, C.; Li, Z.; Zhu, S.; Tay, J.C.; Zhang, X.; Zha, S.; Zeng, J.; Tan, W.K.; Liu, X.; et al. Large-scale expansion of Vγ9Vδ2 T cells with engineered K562 feeder cells in G-Rex vessels and their use as chimeric antigen receptor–modified effector cells. Cytotherapy 2018, 20, 420–435. Available online: http://www.ncbi.nlm.nih.gov/pubmed/29402645 (accessed on 20 February 2020). [CrossRef]
- Fisher, J.P.; Heuijerjans, J.; Yan, M.; Gustafsson, K.; Anderson, J. γδ T cells for cancer immunotherapy. OncoImmunology 2014, 3, e27572. Available online: http://www.ncbi.nlm.nih.gov/pubmed/24734216 (accessed on 23 February 2020). [CrossRef] [Green Version]
- Baker, F.L.; Bigley, A.B.; Agha, N.H.; Pedlar, C.R.; O’Connor, D.P.; Bond, R.A.; Bollard, C.M.; Katsanis, E.; Simpson, R.J. Systemic β-Adrenergic Receptor Activation Augments the ex vivo Expansion and Anti-Tumor Activity of Vγ9Vδ2 T-Cells. Front. Immunol. 2020, 10, 3082. [Google Scholar] [CrossRef] [Green Version]
- Vermijlen, D.; Ellis, P.; Langford, C.; Klein, A.; Engel, R.; Willimann, K.; Jomaa, H.; Hayday, A.; Eberl, M. Distinct Cytokine-Driven Responses of Activated Blood γδ T Cells: Insights into Unconventional T Cell Pleiotropy1. J. Immunol. 2007, 178, 4304–4314. [Google Scholar] [CrossRef]
- Wesch, D.; Glatzel, A.; Kabelitz, D. Differentiation of Resting Human Peripheral Blood γδ T Cells toward Th1- or Th2-Phenotype. Cell. Immunol. 2001, 212, 110–117. [Google Scholar] [CrossRef]
- Berglund, S.; Gaballa, A.; Sawaisorn, P.; Sundberg, B.; Uhlin, M. Expansion of Gammadelta T Cells from Cord Blood: A Therapeutical Possibility. Stem Cells Int. 2018, 2018, 8529104. [Google Scholar] [CrossRef] [Green Version]
- Eberl, M.; Engel, R.; Beck, E.; Jomaa, H. Differentiation of human γδ T cells towards distinct memory phenotypes. Cell. Immunol. 2002, 218, 1–6. Available online: https://www.sciencedirect.com/science/article/pii/S0008874902005191?via%3Dihub (accessed on 19 February 2020). [CrossRef]
- Caccamo, N.; Meraviglia, S.; Ferlazzo, V.; Angelini, D.F.; Borsellino, G.; Poccia, F.; Battistini, L.; Dieli, F.; Salerno, A. Differential requirements for antigen or homeostatic cytokines for proliferation and differentiation of human Vγ9Vδ2 naive, memory and effector T cell subsets. Eur. J. Immunol. 2005, 35, 1764–1772. [Google Scholar] [CrossRef] [PubMed]
- Fisher, J.P.; Yan, M.; Heuijerjans, J.; Carter, L.; Abolhassani, A.; Frosch, J.; Wallace, R.; Flutter, B.; Capsomidis, A.; Hubank, M.; et al. Neuroblastoma killing properties of Vδ2 and Vδ2-negative γδT cells following expansion by artificial antigen-presenting cells. Clin. Cancer Res. 2014, 20, 5720–5732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Chai, W.; Yang, W.; Han, W.; Mou, W.; Xi, Y.; Chen, X.; Wang, H.; Wang, W.; Qin, H.; et al. The increased IL-17-producing γδT cells promote tumor cell proliferation and migration in neuroblastoma. Clin. Immunol. 2020, 211, 108343. Available online: http://www.ncbi.nlm.nih.gov/pubmed/31931123 (accessed on 10 April 2020). [CrossRef] [PubMed]
- Beucke, N.; Wesch, D.; Oberg, H.; Peters, C.; Bochem, J.; Weide, B.; Garbe, C.; Pawelec, G.; Sebens, S.; Röcken, C.; et al. Pitfalls in the characterization of circulating and tissue-resident human γδ T cells. J. Leukoc. Biol. 2020, 107. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1002/JLB.5MA1219-296R (accessed on 9 April 2020). [CrossRef] [PubMed]
- Schilbach, K.; Krickeberg, N.; Kaißer, C.; Mingram, S.; Kind, J.; Siegers, G.M.; Hashimoto, H. Suppressive activity of Vδ2+ γδ T cells on αβ T cells is licensed by TCR signaling and correlates with signal strength. Cancer Immunol. Immunother. 2020, 69, 593–610. Available online: http://www.ncbi.nlm.nih.gov/pubmed/31982940 (accessed on 9 April 2020). [CrossRef] [Green Version]
- Wu, K.; Zhao, H.; Xiu, Y.; Li, Z.; Zhao, J.; Xie, S.; Zeng, H.; Xu, B.; Yu, L.; Xu, B. IL-21-mediated expansion of Vγ9Vδ2 T cells is limited by the Tim-3 pathway. Int. Immunopharmacol. 2019, 69, 136–142. Available online: http://www.ncbi.nlm.nih.gov/pubmed/30708194 (accessed on 23 February 2020). [CrossRef]
- Wang, R.; Wen, Q.; He, W.; Yang, J.; Zhou, C.-Y.; Xiong, W.; Ma, L. Optimized protocols for γδ T cell expansion and lentiviral transduction. Mol. Med. Rep. 2019, 19, 1471–1480. Available online: http://www.ncbi.nlm.nih.gov/pubmed/30628681 (accessed on 5 March 2020). [CrossRef] [Green Version]
- Fisher, J.; Sharma, R.; Don, D.W.; Barisa, M.; Hurtado, M.O.; Abramowski, P.; Porter, L.; Day, W.; Borea, R.; Inglott, S.; et al. Engineering γ∞T cells limits tonic signaling associated with chimeric antigen receptors. Sci. Signal. 2019, 12, eaax1872. [Google Scholar] [CrossRef]
- Harrer, D.C.; Simon, B.; Fujii, A.S.-I.; Shimizu, K.; Uslu, U.; Schuler, G.; Gerer, K.F.; Hoyer, S.; Dörrie, J.; Schaft, N. RNA-transfection of γ/δ T cells with a chimeric antigen receptor or an α/β T-cell receptor: A safer alternative to genetically engineered α/β T cells for the immunotherapy of melanoma. BMC Cancer 2017, 17, 551. Available online: http://bmccancer.biomedcentral.com/articles/10.1186/s12885-017-3539-3 (accessed on 4 March 2020). [CrossRef]
- Rischer, M.; Pscherer, S.; Duwe, S.; Vormoor, J.; Jürgens, H.; Rossig, C. Human gammadelta T cells as mediators of chimaeric-receptor redirected anti-tumour immunity. Br. J. Haematol. 2004, 126, 583–592. Available online: http://www.ncbi.nlm.nih.gov/pubmed/15287953 (accessed on 4 March 2020). [CrossRef]
- Lamb, L.S.; Bowersock, J.; Dasgupta, A.; Gillespie, G.Y.; Su, Y.; Johnson, A.; Spencer, H.T. Engineered Drug Resistant γδ T Cells Kill Glioblastoma Cell Lines during a Chemotherapy Challenge: A Strategy for Combining Chemo- and Immunotherapy. PLoS ONE 2013, 8, e51805. Available online: http://www.ncbi.nlm.nih.gov/pubmed/23326319 (accessed on 4 March 2020). [CrossRef] [PubMed]
- Shimizu, K.; Shinga, J.; Yamasaki, S.; Kawamura, M.; Dörrie, J.; Schaft, N.; Sato, Y.; Iyoda, T.; Fujii, A.S.-I. Transfer of mRNA Encoding Invariant NKT Cell Receptors Imparts Glycolipid Specific Responses to T Cells and γδT Cells. PLoS ONE 2015, 10, e0131477. Available online: https://dx.plos.org/10.1371/journal.pone.0131477 (accessed on 4 March 2020). [CrossRef] [PubMed] [Green Version]
- Aft, R.; Naughton, M.; Trinkaus, K.; Watson, M.; Ylagan, L.; Mac Gregor, M.C.; Zhai, J.; Kuo, S.; Shannon, W.; Diemer, K.; et al. Effect of zoledronic acid on disseminated tumour cells in women with locally advanced breast cancer: An open label, randomised, phase 2 trial. Lancet Oncol. 2010, 11, 421–428. [Google Scholar] [CrossRef] [Green Version]
- Wada, I.; Matsushita, H.; Noji, S.; Mori, K.; Yamashita, H.; Nomura, S.; Shimizu, N.; Seto, Y.; Kakimi, K. Intraperitoneal injection of in vitro expanded Vγ9Vδ2 T cells together with zoledronate for the treatment of malignant ascites due to gastric cancer. Cancer Med. 2014, 3, 362–375. Available online: http://doi.wiley.com/10.1002/cam4.196 (accessed on 24 February 2020). [CrossRef]
- Dieli, F.; Vermijlen, D.; Fulfaro, F.; Caccamo, N.; Meraviglia, S.; Cicero, G.; Roberts, A.; Buccheri, S.; D’Asaro, M.; Gebbia, N.; et al. Targeting human γδ T cells with zoledronate and interleukin-2 for immunotherapy of hormone-refractory prostate cancer. Cancer Res. 2007, 67, 7450–7457. [Google Scholar] [CrossRef] [Green Version]
- Nakajima, J.; Murakawa, T.; Fukami, T.; Goto, S.; Kaneko, T.; Yoshida, Y.; Takamoto, S.; Kakimi, K. A phase I study of adoptive immunotherapy for recurrent non-small-cell lung cancer patients with autologous γδ T cells☆☆☆. Eur. J. Cardio-Thorac. Surg. 2010, 37, 1191–1197. Available online: http://www.ncbi.nlm.nih.gov/pubmed/20137969 (accessed on 9 April 2020). [CrossRef] [Green Version]
- Sakamoto, M.; Nakajima, J.; Murakawa, T.; Fukami, T.; Yoshida, Y.; Murayama, T.; Takamoto, S.; Matsushita, H.; Kakimi, K. Adoptive immunotherapy for advanced non-small celllung cancer using zoledronate-expanded γδT cells: APhase i clinical study. J. Immunother. 2011, 34, 202–211. Available online: http://www.ncbi.nlm.nih.gov/pubmed/21304399 (accessed on 9 April 2020). [CrossRef]
- Noguchi, A.; Kaneko, T.; Kamigaki, T.; Fujimoto, K.; Ozawa, M.; Saito, M.; Ariyoshi, N.; Goto, S. Zoledronate-activated Vγ9γδ T cell-based immunotherapy is feasible and restores the impairment of γδ T cells in patients with solid tumors. Cytotherapy 2011, 13, 92–97. Available online: https://www.tandfonline.com/action/journalInformation?journalCode=icyt20 (accessed on 9 April 2020). [CrossRef]
- Lang, J.M.; Kaikobad, M.R.; Wallace, M.; Staab, M.J.; Horvath, R.L.; Wilding, G.; Liu, G.; Eickhoff, J.C.; McNeel, U.G.; Malkovsky, M. Pilot trial of interleukin-2 and zoledronic acid to augment γδ T cells as treatment for patients with refractory renal cell carcinoma. Cancer Immunol. Immunother. 2011, 60, 1447–1460. [Google Scholar] [CrossRef] [Green Version]
- Wilhelm, M.; Smetak, M.; Schaefer-Eckart, K.; Kimmel, B.; Birkmann, J.; Einsele, H.; Kunzmann, V. Successful adoptive transfer and in vivo expansion of haploidentical γδ T cells. J. Transl. Med. 2014, 12, 45. Available online: http://translational-medicine.biomedcentral.com/articles/10.1186/1479-5876-12-45 (accessed on 24 March 2020). [CrossRef]
- Izumi, T.; Kondo, M.; Takahashi, T.; Fujieda, N.; Kondô, A.; Tamura, N.; Murakawa, T.; Nakajima, J.; Matsushita, H.; Kakimi, K. Ex vivo characterization of γδ T-cell repertoire in patients after adoptive transfer of Vγ9Vδ2 T cells expressing the interleukin-2 receptor β-chain and the common γ-chain. Cytotherapy 2013, 15, 481–491. Available online: http://www.ncbi.nlm.nih.gov/pubmed/23391461 (accessed on 9 April 2020). [CrossRef] [PubMed]
- Kunzmann, V.; Smetak, M.; Kimmel, B.; Weigang-Koehler, K.; Goebeler, M.; Birkmann, J.; Becker, J.; Schmidt-Wolf, I.G.H.; Einsele, H.; Wilhelm, M. Tumor-promoting versus tumor-antagonizing roles of γδ T cells in cancer immunotherapy: Results from a prospective phase I/II trial. J. Immunother. 2012, 35, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, M.; Kunzmann, V.; Eckstein, S.; Reimer, P.; Weissinger, F.; Ruediger, T.; Tony, H.-P. γδ T cells for immune therapy of patients with lymphoid malignancies. Blood 2003, 102, 200–206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobayashi, H.; Tanaka, Y.; Yagi, J.; Osaka, Y.; Nakazawa, H.; Uchiyama, T.; Minato, N.; Toma, H. Safety profile and anti-tumor effects of adoptive immunotherapy using gamma-delta T cells against advanced renal cell carcinoma: A pilot study. Cancer Immunol. Immunother. 2006, 56, 469–476. [Google Scholar] [CrossRef]
- Kobayashi, H.; Tanaka, Y.; Yagi, J.; Minato, N.; Tanabe, K. Phase I/II study of adoptive transfer of γδ T cells in combination with zoledronic acid and IL-2 to patients with advanced renal cell carcinoma. Cancer Immunol. Immunother. 2011, 60, 1075–1084. [Google Scholar] [CrossRef]
- Bennouna, J.; Bompas, E.; Neidhardt, E.M.; Rolland, F.; Philip, I.; Galéa, C.; Salot, S.; Saiagh, S.; Audrain, M.; Rimbert, M.; et al. Phase-I study of Innacell γδ™, an autologous cell-therapy product highly enriched in γ9δ2 T lymphocytes, in combination with IL-2, in patients with metastatic renal cell carcinoma. Cancer Immunol. Immunother. 2008, 57, 1599–1609. [Google Scholar] [CrossRef]
- Tanaka, Y.; Morita, C.; Tanaka, Y.; Nieves, E.; Brenner, M.B.; Bloom, B.R. Natural and synthetic non-peptide antigens recognized by human γδ T cells. Nature 1995, 375, 155–158. [Google Scholar] [CrossRef]
- Bürk, M.R.; Mori, L.; De Libero, G. Human Vγ9-Vδ2 cells are stimulated in a crossreactive fashion by a variety of phosphorylated metabolites. Eur. J. Immunol. 1995, 25, 2052–2058. [Google Scholar] [CrossRef]
- Hintz, M.; Reichenberg, A.; Altincicek, B.; Bahr, U.; Gschwind, R.M.; Kollas, A.-K.; Beck, E.; Wiesner, J.; Eberl, M.; Jomaa, H. Identification of (E)-4-hydroxy-3-methyl-but-2-enyl pyrophosphate as a major activator for human γδ T cells inEscherichia coli. FEBS Lett. 2001, 509, 317–322. [Google Scholar] [CrossRef] [Green Version]
- Wiemer, D.F.; Wiemer, A.J. Opportunities and challenges in development of phosphoantigens as Vγ9Vδ2 T cell agonists. Biochem. Pharmacol. 2014, 89, 301–312. [Google Scholar] [CrossRef]
- Gober, H.-J.; Kistowska, M.; Angman, L.; Jenö, P.; Mori, L.; De Libero, G. Human T Cell Receptor γδ Cells Recognize Endogenous Mevalonate Metabolites in Tumor Cells. J. Exp. Med. 2003, 197, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Gu, S.; Nawrocka, W.; Adams, E.J. Sensing of Pyrophosphate Metabolites by Vγ9Vδ2 T Cells. Front. Immunol. 2015, 5, 688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kistowska, M.; Rossy, E.; Sansano, S.; Gober, H.-J.; Landmann, R.; Mori, L.; De Libero, G. Dysregulation of the host mevalonate pathway during early bacterial infection activates human TCR γδ cells. Eur. J. Immunol. 2008, 38, 2200–2209. [Google Scholar] [CrossRef] [PubMed]
- Soares, A.P.; Espírito-Santo, R.; Line, S.R.P.; Pinto, M.D.G.F.; Santos, P.D.M.; Toralles, M.B.P.; Santo, A.D.E. Bisphosphonates: Pharmacokinetics, bioavailability, mechanisms of action, clinical applications in children, and effects on tooth development. Environ. Toxicol. Pharmacol. 2016, 42, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Thompson, K.; Roelofs, A.J.; Jauhiainen, M.; Mönkkönen, H.; Mönkkönen, J.; Rogers, M.J. Activation of γδ T Cells by Bisphosphonates. Adv. Exp. Med. Biol. 2010, 658, 11–20. [Google Scholar] [PubMed]
- Green, J.R.; Müller, K.; Jaeggi, K.A. Preclinical pharmacology of CGP 42′446, a new, potent, heterocyclic bisphosphonate compound. J. Bone Miner. Res. 1994, 9, 745–751. [Google Scholar] [CrossRef]
- Lin, J.-F.; Lin, Y.-C.; Lin, Y.-H.; Tsai, T.-F.; Chou, K.-Y.; Chen, H.-E.; Hwang, T.I.-S. Zoledronic Acid Induces Autophagic Cell Death in Human Prostate Cancer Cells. J. Urol. 2011, 185, 1490–1496. Available online: https://www.sciencedirect.com/science/article/pii/S0022534710051554 (accessed on 25 February 2020). [CrossRef]
- Morita, C.; Beckman, E.M.; Bukowski, J.F.; Tanaka, Y.; Band, H.; Bloom, B.R.; Golan, D.E.; Brenner, M.B. Direct presentation of nonpeptide prenyl pyrophosphate antigens to human γδ T cells. Immunity 1995, 3, 495–507. [Google Scholar] [CrossRef] [Green Version]
- Henneman, L.; Van Cruchten, A.G.; Kulik, W.; Waterham, H.R. Inhibition of the isoprenoid biosynthesis pathway; detection of intermediates by UPLC–MS/MS. Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2011, 1811, 227–233. [Google Scholar] [CrossRef]
- Vavassori, S.; Kumar, A.; Wan, G.S.; Ramanjaneyulu, G.S.; Cavallari, M.; El Daker, S.; Beddoe, T.; Theodossis, A.; Williams, N.K.; Gostick, E.; et al. Butyrophilin 3A1 binds phosphorylated antigens and stimulates human γδ T cells. Nat. Immunol. 2013, 14, 908–916. [Google Scholar] [CrossRef]
- Sandstrom, A.; Peigné, C.-M.; Léger, A.; Crooks, J.E.; Konczak, F.; Gesnel, M.-C.; Breathnach, R.; Bonneville, M.; Scotet, E.; Adams, E.J. The intracellular B30.2 domain of Butyrophilin 3A1 binds phosphoantigens to mediate activation of human Vγ9Vδ2 T cells. Immunity 2014, 40, 490–500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blazquez, J.-L.; Benyamine, A.; Pasero, C.; Olive, D. New Insights Into the Regulation of γδ T Cells by BTN3A and Other BTN/BTNL in Tumor Immunity. Front. Immunol. 2018, 9, 1601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palakodeti, A.; Sandstrom, A.; Sundaresan, L.; Harly, C.; Nedellec, S.; Olive, D.; Scotet, E.; Bonneville, M.; Adams, E.J. The molecular basis for modulation of human Vγ9Vδ2 T cell responses by CD277/butyrophilin-3 (BTN3A)-specific antibodies. J. Biol. Chem. 2012, 287, 32780–32790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rigau, M.; Ostrouska, S.; Fulford, T.S.; Johnson, D.N.; Woods, K.; Ruan, Z.; McWilliam, H.E.G.; Hudson, C.; Tutuka, C.; Wheatley, A.K.; et al. Butyrophilin 2A1 is essential for phosphoantigen reactivity by gd T cells. Science 2020, 367, eaay5516. [Google Scholar] [CrossRef] [PubMed]
- Hodgins, N.O.; Wang, J.T.W.; Al-Jamal, K.T. Nano-technology based carriers for nitrogen-containing bisphosphonates delivery as sensitisers of γδ T cells for anticancer immunotherapy. Adv. Drug Deliv. Rev. 2017, 114, 143–160. Available online: http://www.ncbi.nlm.nih.gov/pubmed/28694026 (accessed on 25 March 2020). [CrossRef] [Green Version]
- Riviere, I.; Brose, K.; Mulligan, R.C. Effects of retroviral vector design on expression of human adenosine deaminase in murine bone marrow transplant recipients engrafted with genetically modified cells. Proc. Natl. Acad. Sci. USA 1995, 92, 6733–6737. Available online: https://www.pnas.org/content/92/15/6733 (accessed on 4 March 2020). [CrossRef] [Green Version]
- Hoeres, T.; Smetak, M.; Pretscher, D.; Wilhelm, M. Improving the Efficiency of Vγ9Vδ2 T-Cell Immunotherapy in Cancer. Front. Immunol. 2018, 9, 800. [Google Scholar] [CrossRef]
- Nada, M.H.; Wang, H.; Workalemahu, G.; Tanaka, Y.; Morita, C. Enhancing adoptive cancer immunotherapy with Vγ2Vδ2 T cells through pulse zoledronate stimulation. J. Immunother. Cancer 2017, 5, 9. [Google Scholar] [CrossRef] [Green Version]
- Siegers, G.M.; Ribot, E.J.; Keating, A.; Foster, P.J. Extensive expansion of primary human gamma delta T cells generates cytotoxic effector memory cells that can be labeled with Feraheme for cellular MRI. Cancer Immunol. Immunother. 2012, 62, 571–583. [Google Scholar] [CrossRef]
- Siegers, G.M.; Dhamko, H.; Wang, X.-H.; Mathieson, A.M.; Kosaka, Y.; Felizardo, T.C.; A Medin, J.; Tohda, S.; Schueler, J.; Fisch, P.; et al. Human Vδ1 γδ T cells expanded from peripheral blood exhibit specific cytotoxicity against B-cell chronic lymphocytic leukemia-derived cells. Cytotherapy 2011, 13, 753–764. [Google Scholar] [CrossRef]
- Almeida, A.; Correia, D.; Fernandes-Platzgummer, A.; Silva, M.R.; Anjos, D.R.; Silva-Santos, B. Delta One T cells for immunotherapy of chronic lymphocytic leukemia: Clinical-grade expansion/ differentiation and preclinical proof-of-concept. Clin. Cancer Res. 2016, 22, 5795–5804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, S.; Borowska, M.T.; Boughter, C.T.; Adams, E.J. Butyrophilin3A proteins and Vγ9Vδ2 T cell activation. Semin. Cell Dev. Biol. 2018, 84, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Harly, C.; Guillaume, Y.; Nedellec, S.; Peigné, C.M.; Mönkkönen, H.; Mönkkönen, J.; Li, J.; Kuball, J.; Adams, E.J.; Netzer, S.; et al. Key implication of CD277/butyrophilin-3 (BTN3A) in cellular stress sensing by a major human γδ T-cell subset. Blood 2012, 120, 2269–2279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopez, R.D.; Xu, S.; Guo, B.; Negrin, R.S.; Waller, E.K. CD2-mediated IL-12-dependent signals render human gamma delta-T cells resistant to mitogen-induced apoptosis, permitting the large-scale ex vivo expansion of functionally distinct lymphocytes: Implications for the development of adoptive immunotherapy strategies. Blood 2000, 96, 3827–3837. Available online: http://www.ncbi.nlm.nih.gov/pubmed/11090067 (accessed on 24 March 2020).
- Dunne, M.R.; Mangan, B.A.; Madrigal-Estebas, L.; Doherty, D.G. Preferential Th1 Cytokine Profile of Phosphoantigen-Stimulated Human Vγ9Vδ2 T Cells. Mediat. Inflamm. 2011, 2010, 704941. [Google Scholar] [CrossRef] [Green Version]
- Capietto, A.-H.; Martinet, L.; Cendron, D.; Fruchon, S.; Pont, F.; Fournié, J.-J. Phosphoantigens Overcome Human TCRVγ9+ γδ Cell Immunosuppression by TGF-β: Relevance for Cancer Immunotherapy. J. Immunol. 2010, 184, 6680–6687. [Google Scholar] [CrossRef] [Green Version]
- Viey, E.; Lucas, C.; Romagné, F.; Escudier, B.; Chouaib, S.; Caignard, A. Chemokine Receptors Expression and Migration Potential of Tumor-infiltrating and Peripheral-expanded Vγ9Vδ2 T Cells From Renal Cell Carcinoma Patients. J. Immunother. 2008, 31, 313–323. [Google Scholar] [CrossRef]
- Peters, C.; Meyer, A.; Kouakanou, L.; Feder, J.; Schricker, T.; Lettau, M.; Janssen, O.; Wesch, D.; Kabelitz, D. TGF-β enhances the cytotoxic activity of Vδ2 T cells. OncoImmunology 2018, 8, e1522471. Available online: https://www.tandfonline.com/doi/full/10.1080/2162402X.2018.1522471 (accessed on 27 February 2020). [CrossRef] [Green Version]
- Alexander, A.A.Z.; Maniar, A.; Cummings, J.S.; Hebbeler, A.M.; Schulze, D.H.; Gastman, B.R.; Pauza, C.D.; Strome, S.E.; Chapoval, A.I. Isopentenyl pyrophosphate-activated CD56+ γδ T lymphocytes display potent antitumor activity toward human squamous cell carcinoma. Clin. Cancer Res. 2008, 14, 4232–4240. [Google Scholar] [CrossRef] [Green Version]
- Van Acker, H.H.; Anguille, S.; Willemen, Y.; Bergh, J.M.V.D.; Berneman, Z.N.; Lion, E.; Smits, E.; Van Tendeloo, V. Interleukin-15 enhances the proliferation, stimulatory phenotype, and antitumor effector functions of human gamma delta T cells. J. Hematol. Oncol. 2016, 9, 101–113. [Google Scholar] [CrossRef] [Green Version]
- Thedrez, A.; Harly, C.; Morice, A.; Salot, S.; Bonneville, M.; Scotet, E. IL-21-Mediated Potentiation of Antitumor Cytolytic and Proinflammatory Responses of Human Vγ9Vδ2 T Cells for Adoptive Immunotherapy. J. Immunol. 2009, 182, 3423–3431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, K.; Feng, J.; Xiu, Y.; Li, Z.; Lin, Z.; Zhao, H.; Zeng, H.; Xia, W.; Yu, L.; Xu, B. Vδ2 T cell subsets, defined by PD-1 and TIM-3 expression, present varied cytokine responses in acute myeloid leukemia patients. Int. Immunopharmacol. 2020, 80, 106122. Available online: http://www.ncbi.nlm.nih.gov/pubmed/31955066 (accessed on 11 March 2020). [CrossRef] [PubMed]
- Martin, C.E.; Spasova, D.S.; Frimpong-Boateng, K.; Kim, H.-O.; Lee, M.; Kim, K.S.; Surh, C.D. Interleukin-7 Availability Is Maintained by a Hematopoietic Cytokine Sink Comprising Innate Lymphoid Cells and T Cells Article Interleukin-7 Availability Is Maintained by a Hematopoietic Cytokine Sink Comprising Innate Lymphoid Cells and T Cells. Immunity 2017, 47, 171–182. Available online: http://dx.doi.org/10.1016/j.immuni.2017.07.005http://dx.doi.org/10.1016/j.immuni.2017.07.005 (accessed on 6 May 2020). [CrossRef] [PubMed] [Green Version]
- Baccala, R.; Witherden, D.; Gonzalez-Quintial, R.; Dummer, W.; Surh, C.D.; Havran, W.L.; Theofilopoulos, A.N. γδ T Cell Homeostasis Is Controlled by IL-7 and IL-15 Together with Subset-Specific Factors. J. Immunol. 2005, 174, 4606–4612. [Google Scholar] [CrossRef]
- Sumaria, N.; Roediger, B.; Gascoigne, N.R.J.; Qin, J.; Pinto, R.; Cavanagh, L.L.; Shklovskaya, E.; Groth, B.F.D.S.; Triccas, J.A.; Weninger, W. Cutaneous immunosurveillance by self-renewing dermal γδ T cells. J. Exp. Med. 2011, 208, 505–518. [Google Scholar] [CrossRef] [Green Version]
- Bekiaris, V.; Sedy, J.; Macauley, M.G.; Rhode-Kurnow, A.; Ware, C.F. The inhibitory receptor BTLA controls γδ T cell homeostasis and inflammatory responses. Immunity 2013, 39, 1082–1094. [Google Scholar] [CrossRef] [Green Version]
- Michel, M.-L.; Pang, D.J.; Haque, S.F.Y.; Potocnik, A.; Pennington, D.J.; Hayday, A. Interleukin 7 (IL-7) selectively promotes mouse and human IL-17-producing γδ cells. Proc. Natl. Acad. Sci. USA 2012, 109, 17549–17554. Available online: https://www.pnas.org/cgi/doi/10.1073/pnas.1204327109 (accessed on 6 May 2020). [CrossRef] [Green Version]
- Chen, H.; Eling, N.; Martinez-Jimenez, C.P.; O’Brien, L.M.; Carbonaro, V.; Marioni, J.C.; Odom, D.T.; de la Roche, M. IL -7-dependent compositional changes within the γδ T cell pool in lymph nodes during ageing lead to an unbalanced anti-tumour response. EMBO Rep. 2019, 20, e47379. [Google Scholar] [CrossRef]
- Hou, L.; Jie, Z.; Desai, M.; Liang, Y.; Soong, L.; Wang, T.; Sun, J. Early IL-17 production by intrahepatic T cells is important for adaptive immune responses in viral hepatitis. J. Immunol. 2012, 190, 621–629. [Google Scholar] [CrossRef] [Green Version]
- Wu, D.; Wu, P.; Wu, X.; Ye, J.; Wang, Z.; Zhao, S.; Ni, C.; Hu, G.; Xu, J.; Han, Y.; et al. Ex vivo expanded human circulating Vδ1 γδT cells exhibit favorable therapeutic potential for colon cancer. OncoImmunology 2015, 4, e992749. [Google Scholar] [CrossRef] [Green Version]
- Kouakanou, L.; Xu, Y.; Peters, C.; He, J.; Wu, Y.; Yin, Z.; Kabelitz, D. Vitamin C promotes the proliferation and effector functions of human γδ T cells. Cell. Mol. Immunol. 2019, 17, 462–473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Capietto, A.-H.; Martinet, L.; Fournié, J.-J. Stimulated γδ T Cells Increase the In Vivo Efficacy of Trastuzumab in HER-2+ Breast Cancer. J. Immunol. 2011, 187, 1031–1038. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zoine, J.T.; Knight, K.A.; Fleischer, L.C.; Sutton, K.S.; Goldsmith, K.C.; Doering, C.B.; Spencer, H.T. Ex vivo expanded patient-derived γδ T-cell immunotherapy enhances neuroblastoma tumor regression in a murine model. OncoImmunology 2019, 8, 1593804–1593813. Available online: http://www.ncbi.nlm.nih.gov/pubmed/31413905 (accessed on 12 March 2020). [CrossRef] [PubMed] [Green Version]
- Laurent, G.; De Micheaux, S.L.; Solal-Celigny, P.; Soubeyran, P.; Delwail, V.; Ghesquières, H.; Thieblemont, C.; Jourdan, E.; Beautier, L.; Audibert, F.; et al. Phase I/II Study of IPH1101, γσ T Cell Agonist, Combined with Rituximab, in Low Grade Follicular Lymphoma Patients. Blood 2009, 114, 1649. [Google Scholar] [CrossRef]
- Oberg, H.H.; Peipp, M.; Kellner, C.; Sebens, S.; Krause, S.; Petrick, D.; Adam-Klages, S.; Röcken, C.; Becker, T.; Vogel, I.; et al. Novel bispecific antibodies increase gd t-cell cytotoxicity against pancreatic cancer cells. Cancer Res. 2014, 74, 1349–1360. [Google Scholar] [CrossRef] [Green Version]
- Oberg, H.-H.; Kellner, C.; Gonnermann, D.; Sebens, S.; Bauerschlag, D.; Gramatzki, M.; Kabelitz, D.; Peipp, M.; Wesch, D. Tribody [(HER2)2xCD16] Is More Effective Than Trastuzumab in Enhancing γδ T Cell and Natural Killer Cell Cytotoxicity Against HER2-Expressing Cancer Cells. Front. Immunol. 2018, 9, 814. [Google Scholar] [CrossRef]
- Hahn, A.M.; Winkler, T.H. Resolving the mystery—How TCR transgenic mouse models shed light on the elusive case of gamma delta T cells. J. Leukoc. Biol. 2020, 107. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1002/JLB.1MR0120-237R (accessed on 18 February 2020). [CrossRef]
- Simmons, A.; Alberola-Ila, J. Retroviral Transduction of T Cells and T Cell Precursors. In T-Cell Development: Methods and Protocols; Humana Press: New York, NY, USA, 2016; pp. 99–108. [Google Scholar]
- Fisher, J.; Abramowski, P.; Don, N.D.W.; Flutter, B.; Capsomidis, A.; Cheung, G.W.-K.; Gustafsson, K.; Anderson, J. Avoidance of On-Target Off-Tumor Activation Using a Co-stimulation-Only Chimeric Antigen Receptor. Mol. Ther. 2017, 25, 1234–1247. [Google Scholar] [CrossRef] [Green Version]
- Biasco, L.; Rothe, M.; Schott, J.W.; Schambach, A. Integrating Vectors for Gene Therapy and Clonal Tracking of Engineered Hematopoiesis. Hematol. Clin. N. Am. 2017, 31, 737–752. [Google Scholar] [CrossRef]
- Bosticardo, M.; Ghosh, A.; Du, Y.; A Jenkins, N.; Copeland, N.G.; Candotti, F. Self-inactivating Retroviral Vector-mediated Gene Transfer Induces Oncogene Activation and Immortalization of Primary Murine Bone Marrow Cells. Mol. Ther. 2009, 17, 1910–1918. [Google Scholar] [CrossRef]
- Eyquem, J.; Mansilla-Soto, J.; Giavridis, T.; Van Der Stegen, S.J.C.; Hamieh, M.; Cunanan, K.M.; Odak, A.; Gönen, M.; Sadelain, M. Targeting a CAR to the TRAC locus with CRISPR/Cas9 enhances tumour rejection. Nature 2017, 543, 113–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- MacLeod, D.T.; Antony, J.; Martin, A.J.; Moser, R.J.; Hekele, A.; Wetzel, K.J.; Brown, A.E.; Triggiano, M.A.; Hux, J.A.; Pham, C.D.; et al. Integration of a CD19 CAR into the TCR Alpha Chain Locus Streamlines Production of Allogeneic Gene-Edited CAR T Cells. Mol. Ther. 2017, 25, 949–961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ivics, Z.; Hackett, P.B.; Plasterk, R.H.; Izsvák, Z. Molecular Reconstruction of Sleeping Beauty, a Tc1-like Transposon from Fish, and Its Transposition in Human Cells. Cell 1997, 91, 501–510. [Google Scholar] [CrossRef] [Green Version]
- Aronovich, E.L.; McIvor, R.S.; Hackett, P.B. The Sleeping Beauty transposon system: A non-viral vector for gene therapy. Hum. Mol. Genet. 2011, 20, R14–R20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levine, B.L.; Miskin, J.; Wonnacott, K.; Keir, C. Global Manufacturing of CAR T Cell Therapy. Mol. Ther.-Methods Clin. Dev. 2016, 4, 92–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deniger, D.C.; Switzer, K.; Mi, T.; Maiti, S.; Hurton, L.; Singh, H.; Huls, H.; Olivares, S.; Lee, D.-J.; E Champlin, R.; et al. Bispecific T-cells Expressing Polyclonal Repertoire of Endogenous γδ T-cell Receptors and Introduced CD19-specific Chimeric Antigen Receptor. Mol. Ther. 2013, 21, 638–647. [Google Scholar] [CrossRef] [Green Version]
- Beatty, G.L.; Haas, A.R.; Maus, M.V.; Torigian, E.A.; Soulen, M.C.; Plesa, G.; Chew, A.; Zhao, Y.; Levine, B.L.; Albelda, S.M.; et al. Mesothelin-specific Chimeric Antigen Receptor mRNA-Engineered T cells Induce Anti-Tumor Activity in Solid Malignancies. Cancer Immunol. Res. 2013, 2, 112–120. [Google Scholar] [CrossRef] [Green Version]
- Lamb, L.S.; Lopez, R.D. γδ T cells: A new frontier for immunotherapy? Biol. Blood Marrow Transplant. 2005, 11, 161–168. Available online: http://www.ncbi.nlm.nih.gov/pubmed/15744234 (accessed on 5 March 2020). [CrossRef] [Green Version]
- Liu, Z.; Guo, B.L.; Gehrs, B.C.; Nan, L.; Lopez, R.D. Ex vivo expanded human Vγ9Vδ2+ γδ-T cells mediate innate antitumor activity against human prostate cancer cells in vitro. J. Urol. 2005, 173, 1552–1556. [Google Scholar] [CrossRef]
- To, W.C.; Wood, B.G.; Krauss, J.; Strome, M.; Esclamado, R.M.; Lavertu, P.; Dasko, D.; Kim, J.A.; Plautz, G.E.; Leff, B.E.; et al. Systemic Adoptive T-Cell Immunotherapy in Recurrent and Metastatic Carcinoma of the Head and Neck. Arch. Otolaryngol.-Head Neck Surg. 2000, 126, 1225. [Google Scholar] [CrossRef] [Green Version]
- Meraviglia, S.; Eberl, M.; Vermijlen, D.; Todaro, M.; Buccheri, S.; Cicero, G.; La Mendola, C.; Guggino, G.; D’Asaro, M.; Orlando, V.; et al. In vivo manipulation of Vγ9Vδ2 T cells with zoledronate and low-dose interleukin-2 for immunotherapy of advanced breast cancer patients. Clin. Exp. Immunol. 2010, 161, 290–297. Available online: http://www.ncbi.nlm.nih.gov/pubmed/20491785 (accessed on 24 March 2020). [CrossRef] [PubMed]
- Otto, M.; Barfield, R.; Martin, W.J.; Iyengar, R.; Leung, W.; Leimig, T.; Chaleff, S.; Gillies, S.; Handgretinger, R. Combination Immunotherapy with Clinical-Scale Enriched Human T cells, hu14.18 Antibody, and the Immunocytokine Fc-IL7 in Disseminated Neuroblastoma. Clin. Cancer Res. 2005, 11, 8486–8491. Available online: http://www.ncbi.nlm.nih.gov/pubmed/16322312 (accessed on 24 March 2020). [CrossRef] [PubMed] [Green Version]
- Kang, N.; Zhou, J.; Zhang, T.; Wang, L.; Lu, F.; Cui, Y.; Cui, L.; He, W. Adoptive immunotherapy of lung cancer with immobilized anti-TCRγδ antibody-expanded human γδ T Cells in peripheral blood. Cancer Biol. Ther. 2009, 8, 1540–1549. Available online: http://www.ncbi.nlm.nih.gov/pubmed/19471115 (accessed on 24 March 2020). [CrossRef] [PubMed] [Green Version]
- Kakimi, K.; Matsushita, H.; Murakawa, T.; Nakajima, J. γδ T cell therapy for the treatment of non-small cell lung cancer. Transl. Lung Cancer Res. 2014, 3, 23–33. [Google Scholar]
- Buccheri, S.; Guggino, G.; Caccamo, N.; Li Donni, P.; Dieli, F. Efficacy and safety of γδT cell-based tumor immunotherapy: A meta-analysis. J. Biol. Regul. Homeost. Agents 2014, 28, 81–90. [Google Scholar]
- Fournié, J.-J.; Sicard, H.; Poupot, M.; Bezombes, C.; Blanc, A.; Romagné, F.; Ysebaert, L.; Laurent, G. What lessons can be learned from γδ T cell-based cancer immunotherapy trials? Cell. Mol. Immunol. 2012, 10, 35–41. [Google Scholar] [CrossRef] [Green Version]
- Gnant, M.; Mlineritsch, B.; Stoeger, H.; Luschin-Ebengreuth, G.; Heck, D.; Menzel, C.; Jakesz, R.; Seifert, M.; Hubalek, M.; Pristauz, G.; et al. Adjuvant endocrine therapy plus zoledronic acid in premenopausal women with early-stage breast cancer: 62-month follow-up from the ABCSG-12 randomised trial. Lancet Oncol. 2011, 12, 631–641. [Google Scholar] [CrossRef]
- Zumwalde, N.A.; Sharma, A.; Xu, X.; Ma, S.; Schneider, C.L.; Romero-Masters, J.C.; Hudson, A.W.; Gendron-Fitzpatrick, A.; Kenney, S.C.; Gumperz, J. Adoptively transferred Vγ9Vδ2 T cells show potent antitumor effects in a preclinical B cell lymphomagenesis model. JCI Insight 2017, 2, e93179. [Google Scholar] [CrossRef] [Green Version]
- De Witte, M.A.; Sarhan, D.; Davis, Z.; Felices, M.; Vallera, D.A.; Hinderlie, P.; Curtsinger, J.; Cooley, S.; Wagner, J.; Kuball, J.; et al. Early Reconstitution of NK and γδ T Cells and Its Implication for the Design of Post-Transplant Immunotherapy. Biol. Blood Marrow Transplant. 2018, 24, 1152–1162. [Google Scholar] [CrossRef] [Green Version]
- Basingab, F.S.; Ahmadi, M.; Morgan, D.J. IFNg-Dependent Interactions between ICAM-1 and LFA-1 counteract prostaglandin E2-mediated inhibition of antitumor CTL responses. Cancer Immunol. Res. 2016, 4, 400–411. Available online: http://www.ncbi.nlm.nih.gov/pubmed/26928462 (accessed on 24 March 2020). [CrossRef] [Green Version]
- Eil, R.; Vodnala, S.K.; Clever, D.; Klebanoff, C.A.; Sukumar, M.; Pan, J.H.; Palmer, D.C.; Gros, A.; Yamamoto, T.N.; Patel, S.J.; et al. Ionic immune suppression within the tumour microenvironment limits T cell effector function. Nature 2016, 537, 539–543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alnaggar, M.; Xu, Y.; Li, J.; He, J.; Chen, J.; Li, M.; Wu, Q.; Lin, L.; Liang, Y.; Wang, X.; et al. Allogenic Vγ9Vδ2 T cell as new potential immunotherapy drug for solid tumor: A case study for cholangiocarcinoma. J. Immunother. Cancer 2019, 7, 36–37. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Niu, C.; Cui, J. Gamma-delta (γδ) T cells: Friend or foe in cancer development? J. Transl. Med. 2018, 16, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lawand, M.; Déchanet-Merville, J.; Dieu-Nosjean, M.C. Key features of gamma-delta T-cell subsets in human diseases and their immunotherapeutic implications. Front. Immunol. 2017, 8, 761. Available online: http://www.ncbi.nlm.nih.gov/pubmed/28713381 (accessed on 5 March 2020). [CrossRef] [PubMed] [Green Version]
- Chitadze, G.; Oberg, H.-H.; Wesch, D.; Kabelitz, D. The Ambiguous Role of γδ T Lymphocytes in Antitumor Immunity. Trends Immunol. 2017, 38, 668–678. [Google Scholar] [CrossRef]
- Janssen, A.; Hidalgo, J.V.; Beringer, D.X.; Van Dooremalen, S.; Fernando, F.; Van Diest, E.; Terrizzi, A.R.; Bronsert, P.; Kock, S.; Schmitt-Graeff, A.; et al. γδ T-cell Receptors Derived from Breast Cancer-Infiltrating T Lymphocytes Mediate Antitumor Reactivity. Cancer Immunol. Res. 2020, 8, 530–543. [Google Scholar] [CrossRef]
- Nicol, A.J.; Tokuyama, H.; Mattarollo, S.; Hagi, T.; Suzuki, K.; Yokokawa, K.; Nieda, M. Clinical evaluation of autologous gamma delta T cell-based immunotherapy for metastatic solid tumours. Br. J. Cancer 2011, 105, 778–786. [Google Scholar] [CrossRef] [Green Version]
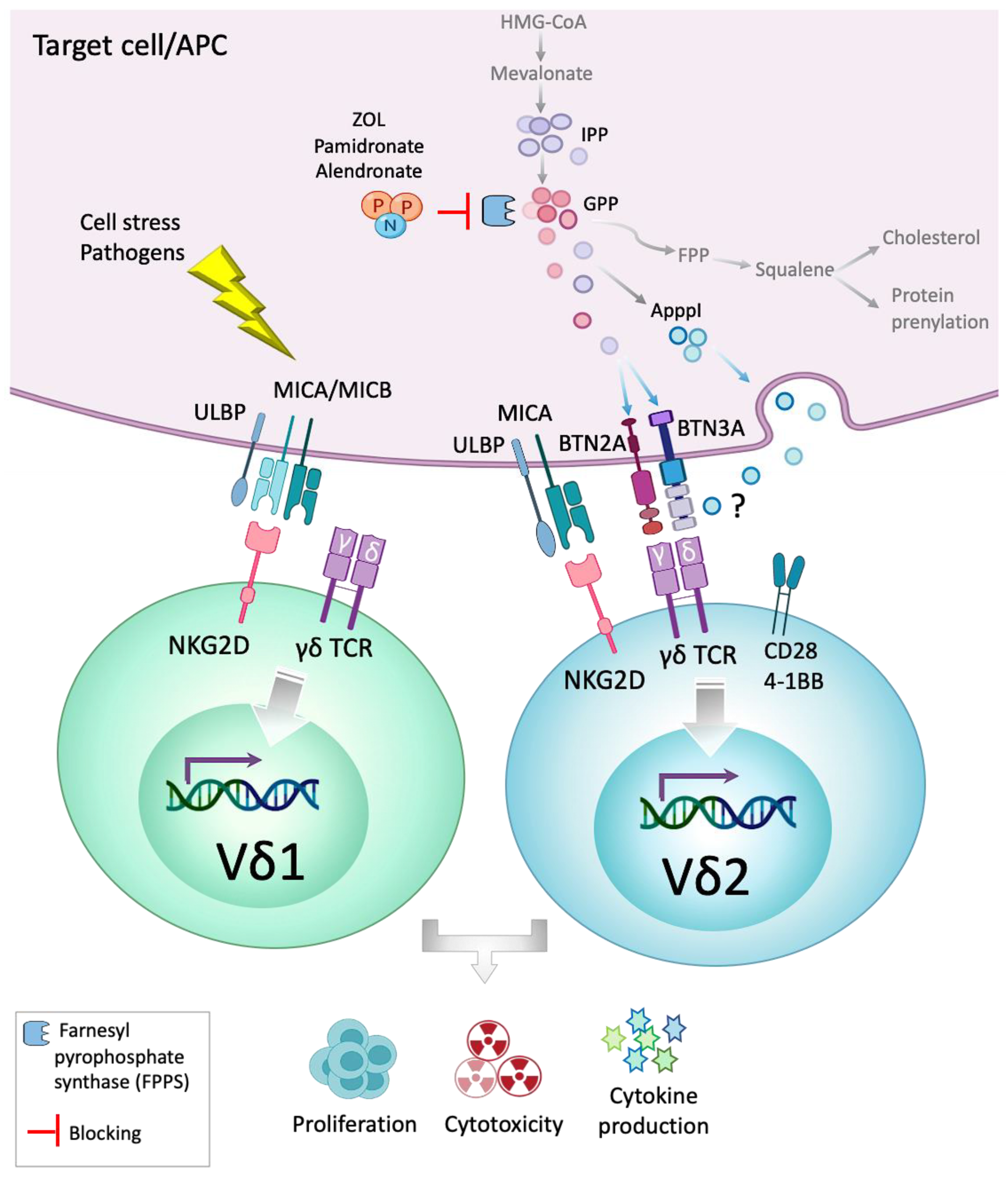
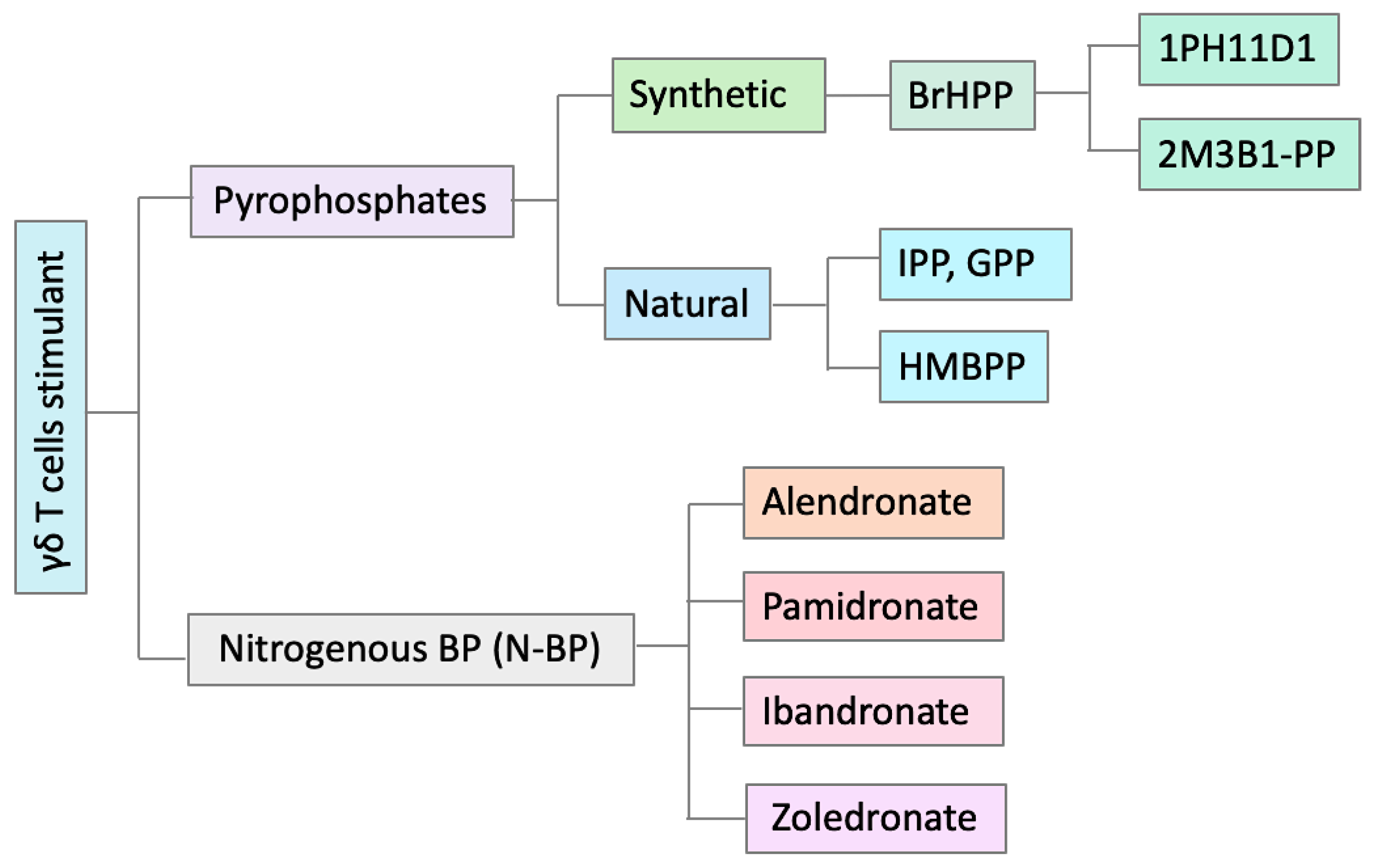
| pAg or BP (conc.) | Additional Stimuli | Cytokine (conc.) | Transduction | Subset | Target | Citation | ||
|---|---|---|---|---|---|---|---|---|
| ZOL (5 uM) | IL-2 (100 IU/mL) | - | - | - | Baker FL. 2020 [47] | |||
| Synthetic HMBPP (0.1–1.0 nM) | IL-2, IL-4, IL-7, IL-15, IL-21, IFNα/β etc. | - | Vγ9Vδ2 | - | Vermijlen D. 2007 [48] | |||
| IPP (2 ug/mL) | Irradiated lymphoma cells | IL-12/IL-4 or IL-4/IL-12 | - | - | - | Wesch D. 2001 [49] | ||
| ZOL (5 uM) | IL-2 (200 IU/mL) | - | - | Cholangiocarci-noma | Berglund S. 2018 [50] * | |||
| HMBPP (0.1–10 ng/mL) | IL-2, IL-7, Il-15, IL-21 | - | - | - | Eberl M. 2002 [51] | |||
| IPP (variable) | IL-2, IL-7, IL-15 | - | Vγ9Vδ2 | - | Caccamo N. 2005 [52] | |||
| IPP (50 uM) | aAPC, anti-γδ T mAbs | IL-2, IL-21 | - | Polyclonal | Neuroblastoma | Fisher J. 2014 [53] | ||
| Pamidronate (10 μg/mL) | IL-2, IL-23, IL-1β, IL-6 | - | - | - | Zhang H. 2020 [54] | |||
| ZOL (5 μM), PMA/Ionomycin (750 ng/mL) | - | - | Vδ2, Vδ1 | - | Beucke N. 2019 [55] | |||
| ZOL, IPP (20 μg/mL) | Anti-γδ TCR mAb | IL-2, IL-15 | - | Vδ2 | - | Schilbach K. 2020 [56] | ||
| HMBPP (20 ng/mL) | Feeder cells | IL-2, IL-21 | Retroviral | Vγ9Vδ2 | - | Wu K. 2019 [57] | ||
| IPP (2–5 ug/mL) | IL-2 (100–1000 U/mL) | Lentiviral | - | - | Wang RN. 2019 [58] | |||
| ZOL (5 uM) | IL-2 (100–200 IU/mL) | Retroviral | - | - | Fisher J. 2019 [59] | |||
| ZOL (40 ug/mL), Con-A (1 mg/mL) | IL-2, IL-4 | Retroviral | Vδ2, Vδ1 | - | Capsomidis A. 2017 [42] | |||
| ZOL (5 uM), OKT3 | IL-2 (1000 IU/mL) | RNA electroporation | - | Melanoma | Harrer DC. 2017 [60] | |||
| ZOL (1 ug/mL) | Irradiated feeder cells | IL-2 (100 IU/mL), IL-15 (10 ng/mL) | Retroviral | - | - | Rischer M. 2004 [61] | ||
| ZOL (1 uM) | IL-2 (50 U/mL) | Lentiviral | - | Glioblastoma | Lamb LS. 2013 [62] | |||
| ZOL (5 μM) | IL-2 (300 IU/mL) | RNA electroporation | Vγ9Vδ2 | - | Shimizu K. 2015 [63] | |||
| ZOL (5 uM) | Engineered K562 feeder cells | IL-2 (300 IU/mL) | RNA electroporation | Vγ9Vδ2 | - | Xiao L. 2018 [45] | ||
| pAg or BP (conc.) | Treatment Strategy | Cytokine | Subset | Target | Citation |
|---|---|---|---|---|---|
| ZOL (0.05 mg/kg,1–3 doses) | IV infusion, then in vitro expansion | - | Vδ2, Vδ1 | Leukemia | Bertaina A. 2017 [37] |
| ZOL (4 mg starting dose) | IV infusion + chemotherapy | - | - | Breast cancer | Aft R. 2010 [64] |
| ZOL (5 uM) | Ex vivo expansion and IP injection | IL-2 (1000 IU/mL) | Vγ9Vδ2 | Gastric cancer | Wada I. 2014 [65] |
| ZOL (4 mg, every 21 days) | IV infusion + Ca and vit. D supplement | IL-2 (0.6 × 106 IU), SQ | - | Prostate cancer | Dieli F. 2007 [66] |
| ZOL | Ex vivo expansion and adoptive transfer | IL-2 (1000 IU/mL) | - | Non-small cell lung cancer | Nakajima J. 2010 [67], Sakamoto M. 2011 [68] |
| ZOL (5 uM) | Ex vivo expansion and adoptive transfer | IL-2 (1000 IU/mL) | Vγ9Vδ2 | Solid tumors | Noguchi A. 2011 [69] |
| ZOL (4 mg starting dose) | IV infusion | IL-2 (7 × 106U/m2), SQ | Vγ9Vδ2 | Renal carcinoma | Lang JM. 2011 [70] |
| ZOL (4 mg starting dose) | IV infusion post-CD4/CD8 depleted leukapheresis product infusion | IL-2 (1 × 106 U/m2), SQ | - | Hematological malignancies | Wilhelm M. 2014 [71] |
| ZOL (5 uM) | Ex vivo expansion and adoptive transfer | IL-2 (1000 IU/mL) | Vγ9Vδ2 | Colorectal cancer | Izumi T. 2013 [72] |
| ZOL (4 mg starting dose) | IV infusion | IL-2 (2 × 106 IU/m2) | - | Renal cell carcinoma, melanoma, acute myeloid leukemia | Kunzmann V. 2012 [73] |
| Pamidronate (90 mg starting dose) | IV infusion | IL-2 (3 × 106 IU/m2) | - | Non-Hodgkin lymphoma or multiple myeloma | Wilhelm M. 2003 [74] |
| 2M3B1-PP (100 uM) | Ex vivo expansion and adoptive transfer | IL-2 (100 IU/mL) | - | Renal carcinoma | Kobayashi H. 2007 [75] |
| 2M3B1-PP (100 uM) + ZOL (4 mg) | Ex vivo expansion and adoptive transfer, + ZOL IV infusion | IL-2 (100 IU/mL), IL-2 (1.4 × 106 IU) | Renal carcinoma | Kobayashi H. 2011 [76] | |
| BrHPP (IPH1101, Phosphostim) (3 uM) | Ex vivo expansion and adoptive transfer | IL-2 (20–60 ng/mL), (2 × 106 IU/m2), SQ | Vγ9Vδ2 | Metastatic renal cell carcinoma | Bennouna J. 2008 [77] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yazdanifar, M.; Barbarito, G.; Bertaina, A.; Airoldi, I. γδ T Cells: The Ideal Tool for Cancer Immunotherapy. Cells 2020, 9, 1305. https://doi.org/10.3390/cells9051305
Yazdanifar M, Barbarito G, Bertaina A, Airoldi I. γδ T Cells: The Ideal Tool for Cancer Immunotherapy. Cells. 2020; 9(5):1305. https://doi.org/10.3390/cells9051305
Chicago/Turabian StyleYazdanifar, Mahboubeh, Giulia Barbarito, Alice Bertaina, and Irma Airoldi. 2020. "γδ T Cells: The Ideal Tool for Cancer Immunotherapy" Cells 9, no. 5: 1305. https://doi.org/10.3390/cells9051305
APA StyleYazdanifar, M., Barbarito, G., Bertaina, A., & Airoldi, I. (2020). γδ T Cells: The Ideal Tool for Cancer Immunotherapy. Cells, 9(5), 1305. https://doi.org/10.3390/cells9051305





