Higher Mast Cell Accumulation in Human Adipose Tissues Defines Clinically Favorable Obesity Sub-Phenotypes
Abstract
1. Introduction
2. Materials and Methods
2.1. Human Cohorts
2.2. RNA Extraction and Quantification
2.3. Histology and Immunostaining
2.4. Statistical Analyses
3. Results
3.1. Assessing AT-MC Histologically and by Gene Expression
3.2. Cross-Sectional Analyses
3.3. In Vitro Analyses
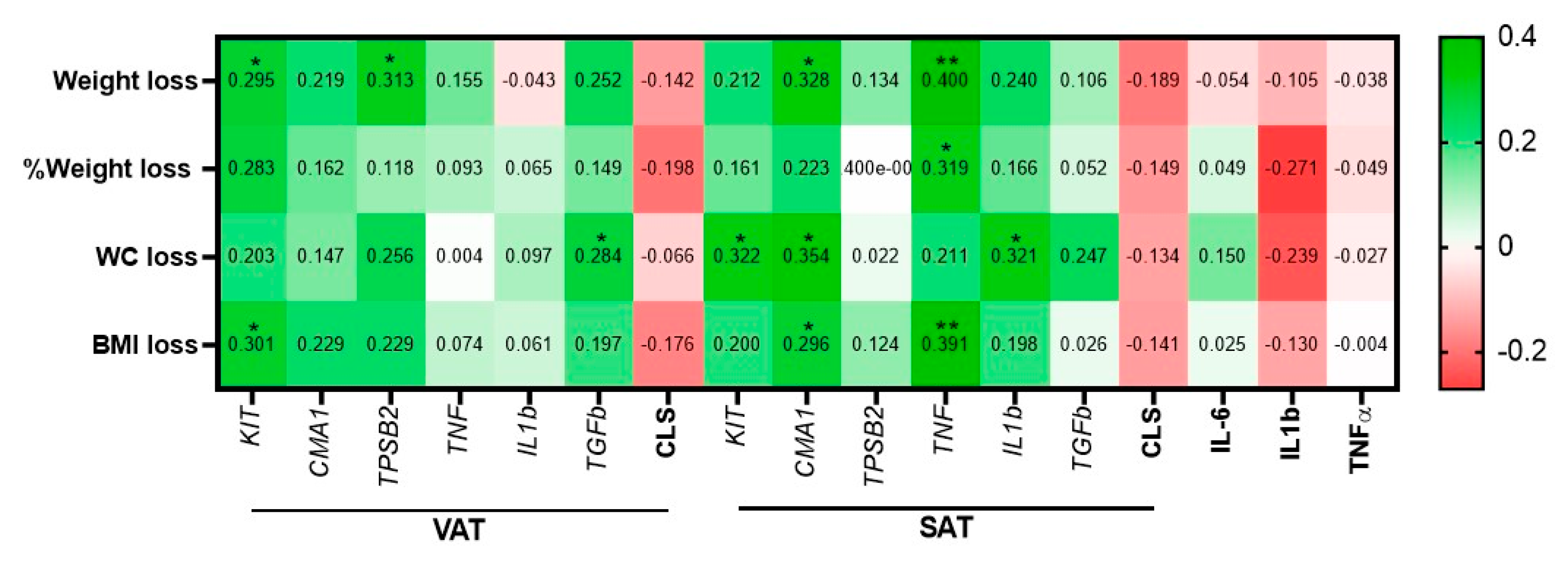
3.4. Prospective Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Weisberg, S.P.; McCann, D.; Desai, M.; Rosenbaum, M.; Leibel, R.L.; Ferrante, A.W., Jr. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Investig. 2003, 112, 1796–1808. [Google Scholar] [CrossRef] [PubMed]
- Mraz, M.; Haluzik, M. The role of adipose tissue immune cells in obesity and low-grade inflammation. J. Endocrinol. 2014, 222, R113–R127. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, J.; Evers, N.; Awazawa, M.; Nicholls, H.T.; Brönneke, H.S.; Dietrich, A.; Mauer, J.; Blüher, M.; Brüning, J.C. Obesogenic memory can confer long-term increases in adipose tissue but not liver inflammation and insulin resistance after weight loss. Mol. Metab. 2016, 5, 328–339. [Google Scholar] [CrossRef] [PubMed]
- Arivazhagan, L.; Ruiz, H.H.; Wilson, R.A.; Manigrasso, M.B.; Gugger, P.F.; Fisher, E.A.; Moore, K.J.; Ramasamy, R.; Schmidt, A.M. An eclectic cast of cellular actors orchestrates innate immune responses in the mechanisms driving obesity and metabolic perturbation. Circ. Res. 2020, 126, 1565–1589. [Google Scholar] [CrossRef] [PubMed]
- Weinstock, A.; Moura Silva, H.; Moore, K.J.; Schmidt, A.M.; Fisher, E.A. Leukocyte heterogeneity in adipose tissue, including in obesity. Circ. Res. 2020, 126, 1590–1612. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Divoux, A.; Sun, J.; Zhang, J.; Clément, K.; Glickman, J.N.; Sukhova, G.K.; Wolters, P.J.; Du, J.; Gorgun, C.Z.; et al. Genetic deficiency and pharmacological stabilization of mast cells reduce diet-induced obesity and diabetes in mice. Nat. Med. 2009, 15, 940–945. [Google Scholar] [CrossRef] [PubMed]
- Altintas, M.M.; Azad, A.; Nayer, B.; Contreras, G.; Zaias, J.; Faul, C.; Reiser, J.; Nayer, A. Mast cells, macrophages, and crown-like structures distinguish subcutaneous from visceral fat in mice. J. Lipid Res. 2011, 52, 480–488. [Google Scholar] [CrossRef]
- Tanaka, A.; Nomura, Y.; Matsuda, A.; Ohmori, K.; Matsuda, H. Mast cells function as an alternative modulator of adipogenesis through 15-deoxy-delta-12, 14-prostaglandin J2. Am. J. Physiol. Cell Physiol. 2011, 301, C1360–C1367. [Google Scholar] [CrossRef]
- Gutierrez, D.A.; Muralidhar, S.; Feyerabend, T.B.; Herzig, S.; Rodewald, H.R. Hematopoietic kit deficiency, rather than lack of mast cells, protects mice from obesity and insulin resistance. Cell Metab. 2015, 21, 678–691. [Google Scholar] [CrossRef]
- Chmelař, J.; Chatzigeorgiou, A.; Chung, K.J.; Prucnal, M.; Voehringer, D.; Roers, A.; Chavakis, T. No role for mast cells in obesity-related metabolic dysregulation. Front. Immunol. 2016, 7, 524. [Google Scholar] [CrossRef]
- Divoux, A.; Moutel, S.; Poitou, C.; Lacasa, D.; Veyrie, N.; Aissat, A.; Arock, M.; Guerre-Millo, M.; Clement, K. Mast cells in human adipose tissue: Link with morbid obesity, inflammatory status, and diabetes. J. Clin. Endocrinol. Metab. 2012, 97, E1677–E1685. [Google Scholar] [CrossRef] [PubMed]
- Einwallner, E.; Kiefer, F.W.; Di Caro, G.; Orthofer, M.; Witzeneder, N.; Hörmann, G.; Itariu, B.; Zeyda, M.; Penninger, J.M.; Stulnig, T.M.; et al. Mast cells are not associated with systemic insulin resistance. Eur. J. Clin. Investig. 2016, 46, 911–919. [Google Scholar] [CrossRef] [PubMed]
- Haim, Y.; Blüher, M.; Slutsky, N.; Goldstein, N.; Klöting, N.; Harman-Boehm, I.; Kirshtein, B.; Ginsberg, D.; Gericke, M.; Guiu Jurado, E.; et al. Elevated autophagy gene expression in adipose tissue of obese humans: A potential non-cell-cycle-dependent function of E2F1. Autophagy 2015, 11, 2074–2088. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, H.; Pecht, T.; Shaco-Levy, R.; Harman-Boehm, I.; Kirshtein, B.; Kuperman, Y.; Chen, A.; Blüher, M.; Shai, I.; Rudich, A. Adipose tissue foam cells are present in human obesity. J. Clin. Endocrinol. Metab. 2013, 98, 1173–1181. [Google Scholar] [CrossRef]
- Wallace, T.M.; Levy, J.C.; Matthews, D.R. Use and abuse of HOMA modeling. Diabetes Care 2004, 27, 1487–1495. [Google Scholar] [CrossRef]
- Keller, M.; Hopp, L.; Liu, X.; Wohland, T.; Rohde, K.; Cancello, R.; Klös, M.; Bacos, K.; Kern, M.; Eichelmann, F.; et al. Genome-wide DNA promoter methylation and transcriptome analysis in human adipose tissue unravels novel candidate genes for obesity. Mol. Metab. 2017, 6, 86–100. [Google Scholar] [CrossRef]
- Neville, M.J.; Collins, J.M.; Gloyn, A.L.; McCarthy, M.I.; Karpe, F. Comprehensive human adipose tissue mRNA and microRNA endogenous control selection for quantitative real-time-PCR normalization. Obesity 2011, 19, 888–892. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)). Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Saito, H.; Matsumoto, K.; Okumura, S.; Kashiwakura, J.I.; Oboki, K.; Yokoi, H.; Kambe, N.; Ohta, K.; Okayama, Y. Gene expression profiling of human mast cell subtypes: An in silico study. Allergol. Int. 2006, 55, 173–179. [Google Scholar] [CrossRef]
- Piche, M.E.; Tchernof, A.; Despres, J.P. Obesity Phenotypes, Diabetes, and Cardiovascular Diseases. Circ. Res. 2020, 126, 1477–1500. [Google Scholar] [CrossRef]
- Kovanen, P.T. Mast Cells as Potential Accelerators of Human Atherosclerosis-From Early to Late Lesions. Int. J. Mol. Sci. 2019, 20, 4479. [Google Scholar] [CrossRef] [PubMed]
- Fenger, R.V.; Linneberg, A.; Vidal, C.; Vizcaino, L.; Husemoen, L.L.; Aadahl, M.; Gonzalez-Quintela, A. Determinants of serum tryptase in a general population: The relationship of serum tryptase to obesity and asthma. Int. Arch. Allergy. Immunol. 2012, 157, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Amiot, L.; Vu, N.; Drenou, B.; Scrofani, M.; Chalin, A.; Devisme, C.; Samson, M. The anti-fibrotic role of mast cells in the liver is mediated by HLA-G and interaction with hepatic stellate cells. Cytokine 2019, 117, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Bradding, P.; Pejler, G. The controversial role of mast cells in fibrosis. Immunol. Rev. 2018, 282, 198–231. [Google Scholar] [CrossRef]
- Sun, K.; Tordjman, J.; Clement, K.; Scherer, P.E. Fibrosis and adipose tissue dysfunction. Cell Metab. 2013, 18, 470–477. [Google Scholar] [CrossRef]
- Crewe, C.; An, Y.A.; Scherer, P.E. The ominous triad of adipose tissue dysfunction: Inflammation, fibrosis, and impaired angiogenesis. J. Clin. Investig. 2017, 127, 74–82. [Google Scholar] [CrossRef]
- Bel Lassen, P.; Charlotte, F.; Liu, Y.; Bedossa, P.; Le Naour, G.; Tordjman, J.; Poitou, C.; Bouillot, J.L.; Genser, L.; Zucker, J.D.; et al. The FAT Score, a Fibrosis Score of Adipose Tissue: Predicting Weight-Loss Outcome After Gastric Bypass. J. Clin. Endocrinol. Metab. 2017, 102, 2443–2453. [Google Scholar] [CrossRef]
- Muir, L.A.; Neeley, C.K.; Meyer, K.A.; Baker, N.A.; Brosius, A.M.; Washabaugh, A.R.; Varban, O.A.; Finks, J.F.; Zamarron, B.F.; Flesher, C.G.; et al. Adipose tissue fibrosis, hypertrophy, and hyperplasia: Correlations with diabetes in human obesity. Obesity 2016, 24, 597–605. [Google Scholar] [CrossRef]
- Divoux, A.; Tordjman, J.; Lacasa, D.; Veyrie, N.; Hugol, D.; Aissat, A.; Basdevant, A.; Guerre-Millo, M.; Poitou, C.; Zucker, J.D.; et al. Fibrosis in human adipose tissue: Composition, distribution, and link with lipid metabolism and fat mass loss. Diabetes 2010, 59, 2817–2825. [Google Scholar] [CrossRef]
- Smith, U.; Kahn, B.B. Adipose tissue regulates insulin sensitivity: Role of adipogenesis, de novo lipogenesis and novel lipids. J. Intern. Med. 2016, 280, 465–475. [Google Scholar] [CrossRef]
- Finlin, B.S.; Zhu, B.; Confides, A.L.; Westgate, P.M.; Harfmann, B.D.; Dupont-Versteegden, E.E.; Kern, P.A. Mast Cells Promote Seasonal White Adipose Beiging in Humans. Diabetes 2017, 66, 1237–1246. [Google Scholar] [CrossRef] [PubMed]
- Finlin, B.S.; Confides, A.L.; Zhu, B.; Boulanger, M.C.; Memetimin, H.; Taylor, K.W.; Johnson, Z.R.; Westgate, P.M.; Dupont-Versteegden, E.E.; Kern, P.A. Adipose Tissue Mast Cells Promote Human Adipose Beiging in Response to Cold. Sci. Rep. 2019, 9, 8658. [Google Scholar] [CrossRef] [PubMed]
- Norrby, K. Mast cells and angiogenesis. APMIS 2002, 110, 355–371. [Google Scholar] [CrossRef]
- Ribatti, D.; Crivellato, E. Mast cells, angiogenesis, and tumour growth. Biochim. Biophys. Acta 2012, 1822, 2–8. [Google Scholar] [CrossRef] [PubMed]
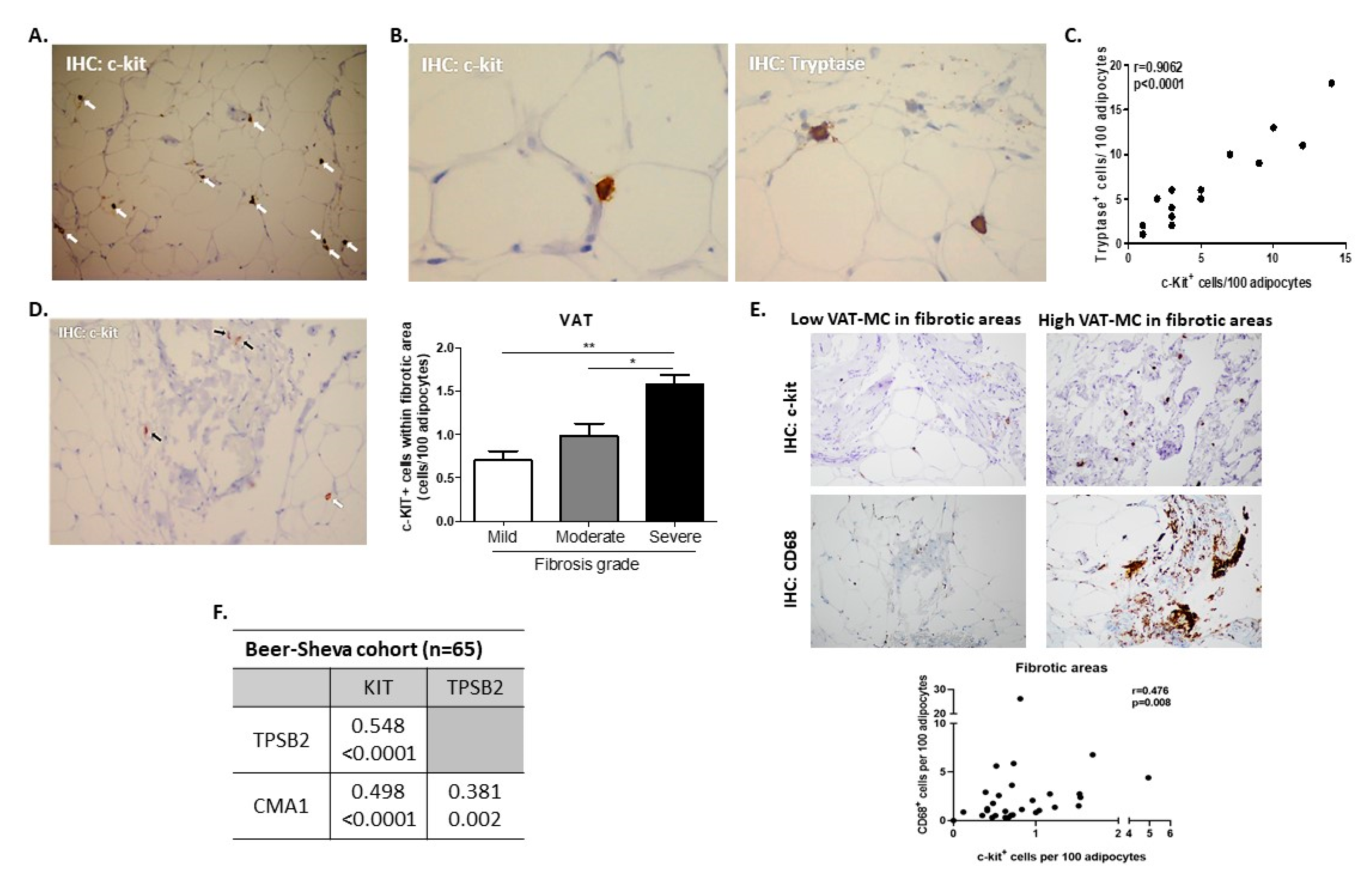
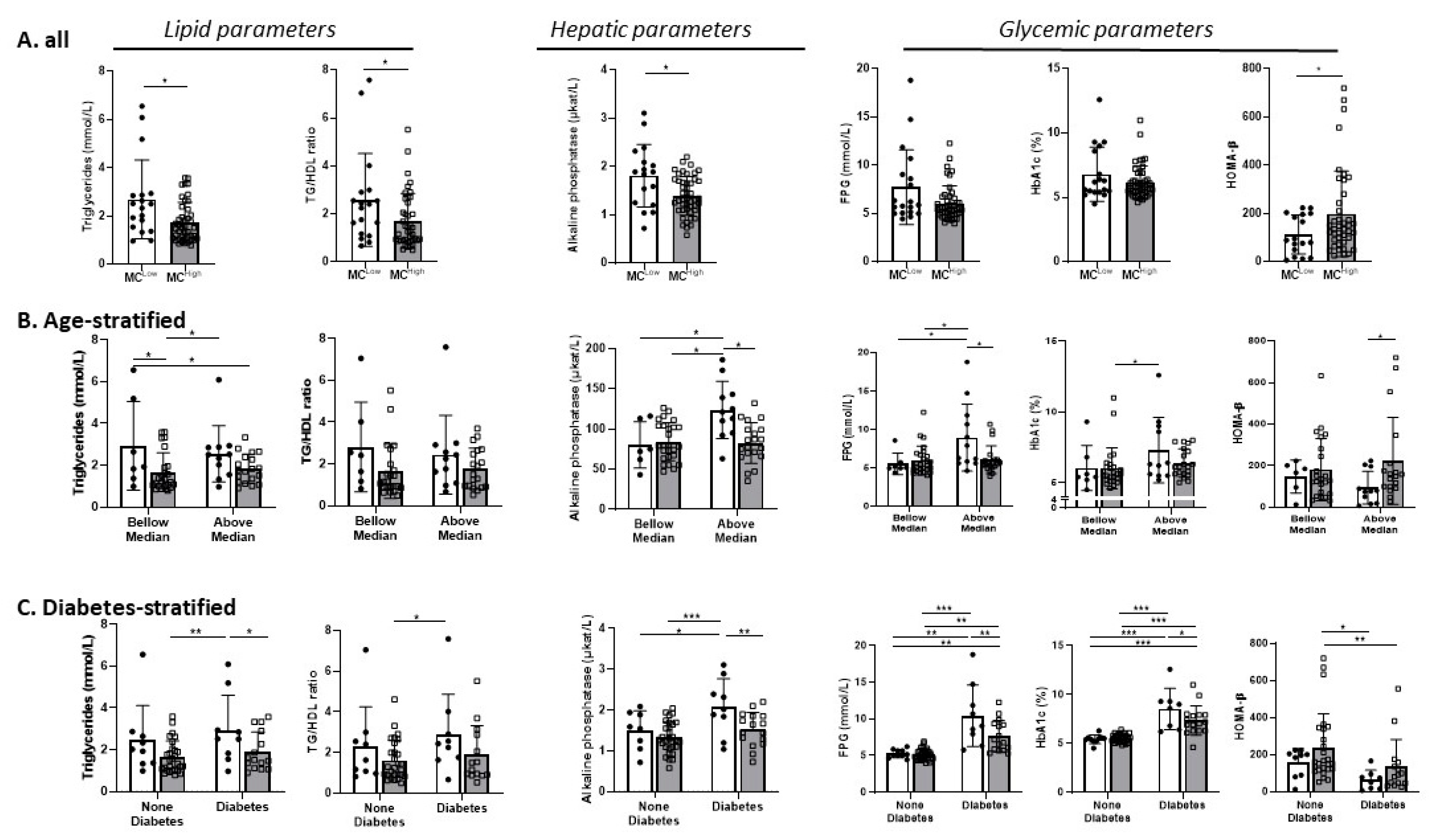
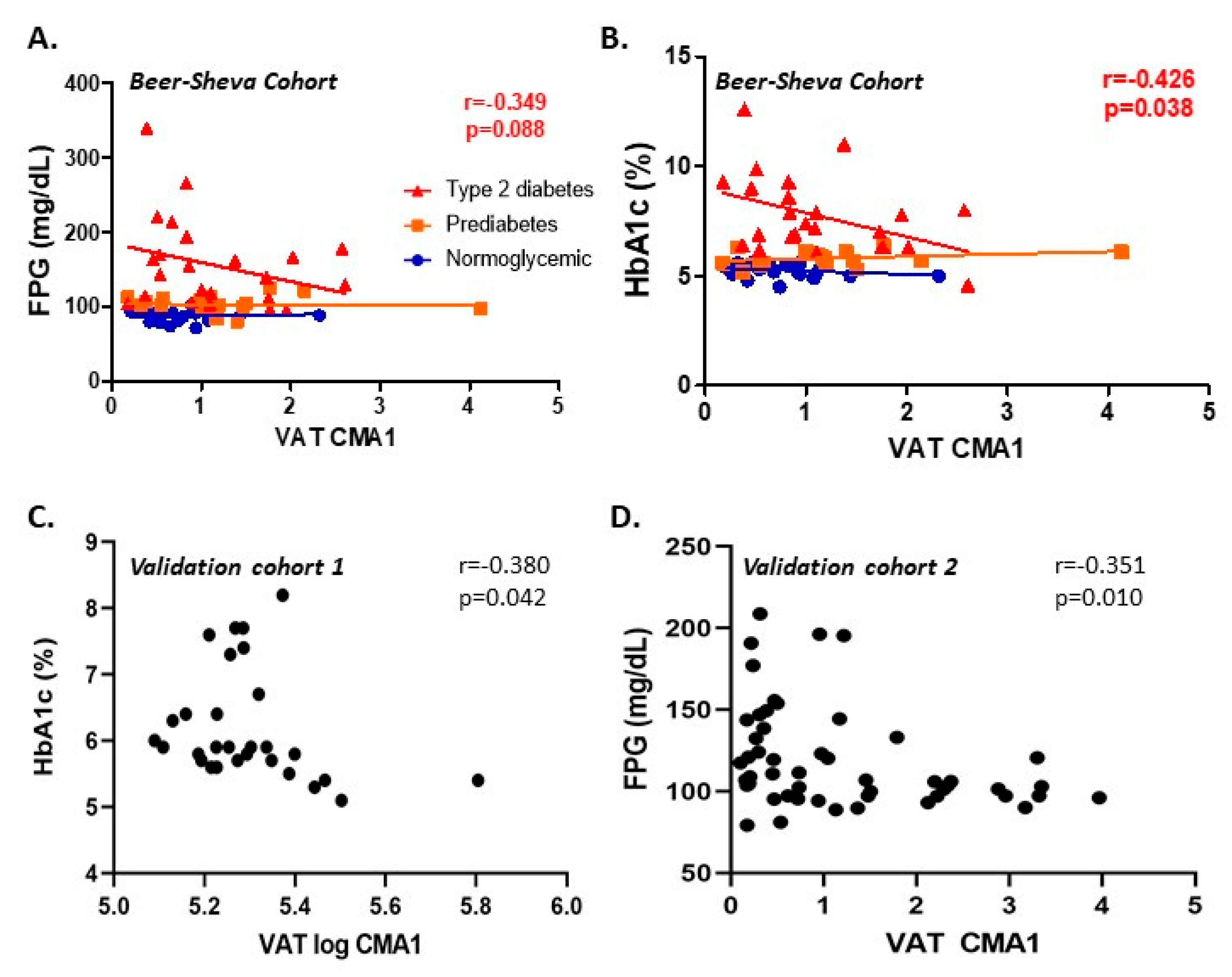
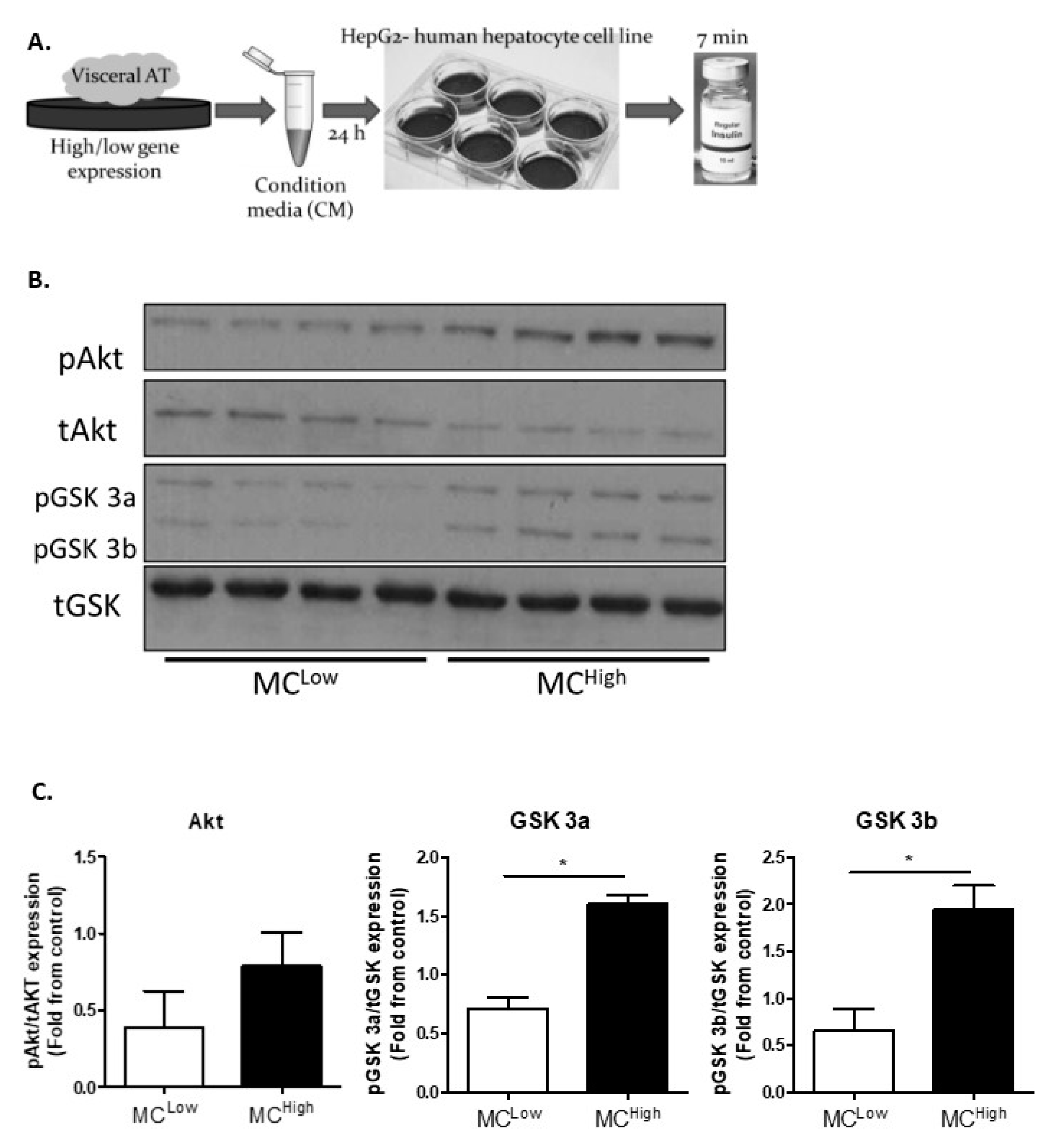

| Beer-Sheva (Main Cohort) | Leipzig-1 (Validation Cohort 1) | Leipzig 2 (Validation Cohort 2) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Normal Values/Units | Total | VAT-Low | VAT-High | Total | VAT-Low | VAT-High | Total | VAT-Low | VAT-High | |
| N | 65 | 19 | 46 | 32 | 7 | 25 | 56 | 18 | 38 | |
| Age | (year) | 46.7 ± 14.2 | 50.8 ± 13.7 | 45.0 ± 14.3 | 47.7 ± 15.3 | 44.1 ± 8.5 | 48.9 ± 11.7 | 43.9 ± 10.3 | 45.4 ± 6.9 | 43.2 ± 11.6 |
| Sex | M/F | 25/40 | 8/11 | 17/29 | 9/23 | 1/6 | 8/17 | 13/43 | 3/15 | 10/28 |
| Weight | (kg) | 112.5 ± 23.6 | 111.7 ± 22.6 | 112.8 ± 24.2 | 134.9 ± 32.6 | 120.4 ± 24.3 | 139.0 ± 33.8 | 144.2 ± 30.3 | 132.2 ± 24.8 | 149.9 ± 31.2 * |
| BMI | <25 (kg/m2) | 40.7 ± 6.0 | 40.2 ± 5.8 | 40.9 ± 6.2 | 47.9 ± 11.2 | 44.1 ± 8.5 | 48.9 ± 11.7 | 50.5 ± 8.6 | 47.9 ± 7.8 | 51.7 ± 8.8 |
| FPG | 3.9–5.4 mmol/L | 6.5 ± 2.7 | 7.7 ± 3.9 | 6.0 ± 1.8 | 6.7 ± 2.2 | 6.8 ± 1.8 | 6.7 ± 2.3 | 6.6 ± 1.7 | 7.0 ± 1.8 | 6.4 ± 1.6 |
| Insulin | <174 pmol/L | 105.1 ± 72.5 | 89.9 ± 49.8 | 111.4 ± 79.8 | 135.1 ± 158.9 | 74.3 ± 49.5 | 147.2 ± 171.1 | 206.1 ± 163.4 | 211.6 ± 215.2 | 203.5 ± 135.1 |
| HbA1c | <39 mmol/mol | 45.9 ± 16.7 | 49.2 ± 22.6 | 43.9 ± 13.9 | 44.3 ± 9.1 | 47.7 ± 10.7 | 43.2 ± 8.5 | 46.4 ± 11.8 | 46.9 ± 9.6 | 46.2 ± 12.9 |
| <5.7% | 6.3 ± 1.6 | 6.8 ± 2.1 | 6.2 ± 1.3 | 6.2 ± 0.8 | 6.5 ± 1.0 | 6.1 ± 0.8 | 6.4 ± 1.1 | 6.4 ± 0.9 | 6.4 ± 1.2 | |
| HOMA-IR | <2.5 | 5.2 ± 3.8 | 5.3 ± 3.8 | 5.1 ± 3.9 | 6.7 ± 11.4 | 3.0 ± 1.9 | 7.4 ± 12.3 | 8.9 ± 7.4 | 9.0 ± 9.3 | 8.8 ± 6.5 |
| HOMA-β % | 173.4 ± 159.6 | 112.4 ± 80.6 | 198.6 ± 177.4 * | 130.6 ± 92.3 | 82.6 ± 66.7 | 140.3 ± 95.1 | 219.3 ± 166.8 | 210.6 ± 208.1 | 223.6 ± 145.9 | |
| Total cholesterol | <5.2 mmol/L | 4.8 ± 1.1 | 5.1 ± 1.3 | 4.6 ± 1.0 | 4.7 ± 1.1 | 5.2 ± 1.2 | 4.4 ± 1.0 | 5.3 ± 1.1 | 5.2 ± 1.0 | 5.3 ± 1.2 |
| LDL-c | <1.8 mmol/L | 2.8 ± 0.9 | 2.7 ± 0.9 | 2.8 ± 0.9 | 3.2 ± 1.3 | 3.3 ± 0.9 | 3.2 ± 1.5 | 3.3 ± 1.0 | 3.1 ± 0.5 | 3.4 ± 1.2 |
| Triglycerides | <1.7 mmol/L | 2.0 ± 1.2 | 2.7 ± 1.6 | 1.7 ± 0.8 * | 1.9 ± 1.0 | 1.7 ± 0.8 | 1.9 ± 1.2 | 2.0 ± 1.0 | 1.9 ± 0.7 | 2.1 ± 1.1 |
| HDL: male | >1.03 mmol/L | 1.0 ± 0.2 | 1.0 ± 0.2 | 1.0 ± 0.2 | 1.1 ± 0.2 | 1.2 ± 0.2 | 1.1 ± 0.2 | 1.1 ± 0.3 | 1.0 ± 0.1 | 1.1 ± 0.4 |
| female | >1.29 mmol/L | 1.2 ± 0.4 | 1.2 ± 0.4 | 1.2 ± 0.4 | 1.2 ± 0.3 | 1.2 ± 0.4 | 1.2 ± 0.3 | |||
| TG/HDL ratio: male | 2.6 ± 1.5 | 3.1 ± 1.8 | 2.3 ± 1.4 | 1.8 ± 1.2 | 1.3 ± 0.6 | 2.0 ± 1.3 | 2.6 ± 1.9 | 2.0 ± 0.4 | 2.7 ± 2.1 | |
| female | 1.6 ± 1.3 | 2.2 ± 2.1 | 1.4 ± 0.8 * | 1.7 ± 1.1 | 1.6 ± 0.9 | 1.7 ± 1.2 | ||||
| CRP | <47.6 nmol/L | 15.7 ± 25.2 | 10.5 ± 9.7 | 17.9 ± 29.2 | 17.6 ± 19.1 | 10.1 ± 8.6 | 19.4 ± 20.6 | 59.4 ± 65.3 | 46.5 ± 63.8 | 65.4 ± 65.6 |
| AST | <0.68 µkat/L | 0.5 ± 0.4 | 0.6 ± 0.4 | 0.5 ± 0.4 | 0.7 ± 0.7 | 0.5 ± 0.1 | 0.8 ± 0.8 | 0.6 ± 0.3 | 0.6 ± 0.3 | 0.6 ± 0.3 |
| ALT | <0.68 µkat/L | 0.6 ± 0.4 | 0.7 ± 0.4 | 0.5 ± 0.4 | 0.7 ± 0.5 | 0.5 ± 0.1 | 0.8 ± 0.6 | 0.7 ± 0.4 | 0.7 ± 0.5 | 0.6 ± 0.4 |
| AP | <2 µkat/L | 1.5 ± 0.5 | 1.8 ± 0.6 | 1.4 ± 0.4 * | ||||||
| Diastolic BP | <85 mmHg | 81.0 ± 17.2 | 88.2 ± 27.7 | 78.4 ± 10.6 | ||||||
| Systolic BP | <130 mmHg | 138.5 ± 16.1 | 139.1 ± 19.7 | 138.3 ± 14.8 | ||||||
| Visceral Adipocyte area | (µm2) | 4213.6 ± 1323.8 | 4455.8 ± 1138.7 | 4097.8 ± 1412.8 | ||||||
| Subcutaneous Adipocyte area | (µm2) | 5720.9 ± 1284.1 † | 5771.7 ± 1337.8 | 5696.7 ± 1290.7 | ||||||
| SAT-KIT | 1.0 ± 0.7 | 0.8 ± 0.3 | 1.1 ± 0.8 | 5.9 ± 0.3 | 5.9 ± 0.2 | 5.9 ± 0.4 | 1.2 ± 0.7 | 1.0 ± 0.5 | 1.3 ± 0.8 | |
| SAT-TPSB2 | 1.2 ± 0.8 | 1.23 ± 0.8 | 1.2 ± 0.8 | 6.3 ± 0.5 | 6.1 ± 0.3 | 6.2 ± 0.5 | 1.2 ± 0.9 | 0.8 ± 0.5 | 1.3 ± 0.9 * | |
| SAT-CMA1 | 2.1 ± 1.0 | 1.6 ± 0.8 | 2.3 ± 1.1 | 5.3 ± 0.1 | 5.2 ± 0.1 | 5.3 ± 0.5 | 1.3 ± 0.8 | 1.1 ± 1.1 | 1.3 ± 0.6 | |
| VAT fibrosis grade | 1.8 ± 0.7 | 1.8 ± 0.6 | 1.8 ± 0.7 | |||||||
| KIT | TPSB2 | CMA1 | COLA3A1 | COLA6A1 | |
|---|---|---|---|---|---|
| COLA1A1 | 0.342 | n.s. | 0.236 | 0.895 | 0.305 |
| 0.007 | 0.065 | <0.001 | 0.015 | ||
| COLA3A1 | 0.333 | n.s. | 0.275 | 0.303 | |
| 0.009 | 0.032 | 0.017 | |||
| COLA6A1 | n.s. | 0.435 | 0.26 | ||
| <0.001 | 0.038 |
| Characteristics | Baseline (n = 56) | After 12 Months (n = 56) | p-Value |
|---|---|---|---|
| Weight (kg) | 144.2 ± 30.3 | 105.6 ± 23.8 | <0.0001 |
| BMI (kg/m2) | 50.5 ± 8.6 | 37.1 ± 8.0 | <0.0001 |
| Body fat (%) | 45.1 ± 7.6 | 35.5 ± 8.2 | <0.0001 |
| FPG (mmol/L) | 6.6 ± 1.7 | 5.6 ± 1.5 | <0.0001 |
| HbA1c (%) | 6.4 ± 1.1 | 5.4 ± 0.8 | <0.0001 |
| Insulin (pmol/L) | 206.1 ± 163.4 | 88.8 ± 102.4 | <0.0001 |
| HOMA-IR | 8.9 ± 7.4 | 3.8 ± 5.3 | <0.0001 |
| Total cholesterol (mmol/L) | 5.3 ± 1.1 | 4.9 ± 0.9 | 0.067 |
| LDL-c (mmol/L) | 3.3 ± 1.0 | 3.1 ± 1.0 | ns |
| HDL (mmol/L) | 1.2 ± 0.3 | 1.4 ± 0.4 | <0.0001 |
| Triglycerides (mmol/L) | 2.0 ± 1.0 | 1.3 ± 0.6 | <0.0001 |
| CRP (nmol/L) | 59.4 ± 65.0 | 32.9 ± 42.8 | 0.024 |
| Interleukin 6 (pg/mL) | 5.5 ± 3.4 | 2.6 ± 2.0 | <0.0001 |
| Interleukin 1 beta (pg/mL) | 9.6 ± 6.7 | 9.1 ± 8.0 | ns |
| Tumor necrosis factor alpha (pg/mL) | 8.3 ± 3.1 | 8.0 ± 3.7 | ns |
| VAT | |||
| CLS (per 100 adipocytes) | 9.0 ± 2.9 | 7.7 ± 3.3 | <0.0001 |
| TNF (AU) | 2.3 ± 1.9 | 1.7 ± 1.9 | ns |
| IL1b (AU) | 1.9 ± 1.2 | 1.2 ± 0.9 | 0.003 |
| TGFb (AU) | 1.5 ± 1.4 | 1.4 ± 1.1 | ns |
| KIT (AU) | 1.1 ± 0.7 | 1.1 ± 0.7 | ns |
| TPSB2 (AU) | 1.0 ± 1.0 | 1.1 ± 1.0 | ns |
| CMA1 (AU) | 1.2 ± 1.1 | 0.8 ± 1.0 | 0.044 |
| SAT | |||
| CLS (per 100 adipocytes) | 4.3 ± 2.1 | 3.6 ± 2.3 | 0.005 |
| TNF (AU) | 1.9 ± 1.8 | 1.5 ± 1.2 | ns |
| IL1b (AU) | 1.9 ± 1.8 | 1.3 ± 0.9 | 0.035 |
| TGFb (AU) | 1.5 ± 0.9 | 1.2 ± 0.7 | 0.024 |
| KIT (AU) | 1.3 ± 0.7 | 1.1 ± 0.8 | ns |
| TPSB2 (AU) | 1.2 ± 0.9 | 1.1 ± 0.7 | ns |
| CMA1 (AU) | 1.3 ± 0.8 | 1.4 ± 1.0 | 0.146 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goldstein, N.; Kezerle, Y.; Gepner, Y.; Haim, Y.; Pecht, T.; Gazit, R.; Polischuk, V.; Liberty, I.F.; Kirshtein, B.; Shaco-Levy, R.; et al. Higher Mast Cell Accumulation in Human Adipose Tissues Defines Clinically Favorable Obesity Sub-Phenotypes. Cells 2020, 9, 1508. https://doi.org/10.3390/cells9061508
Goldstein N, Kezerle Y, Gepner Y, Haim Y, Pecht T, Gazit R, Polischuk V, Liberty IF, Kirshtein B, Shaco-Levy R, et al. Higher Mast Cell Accumulation in Human Adipose Tissues Defines Clinically Favorable Obesity Sub-Phenotypes. Cells. 2020; 9(6):1508. https://doi.org/10.3390/cells9061508
Chicago/Turabian StyleGoldstein, Nir, Yarden Kezerle, Yftach Gepner, Yulia Haim, Tal Pecht, Roi Gazit, Vera Polischuk, Idit F. Liberty, Boris Kirshtein, Ruthy Shaco-Levy, and et al. 2020. "Higher Mast Cell Accumulation in Human Adipose Tissues Defines Clinically Favorable Obesity Sub-Phenotypes" Cells 9, no. 6: 1508. https://doi.org/10.3390/cells9061508
APA StyleGoldstein, N., Kezerle, Y., Gepner, Y., Haim, Y., Pecht, T., Gazit, R., Polischuk, V., Liberty, I. F., Kirshtein, B., Shaco-Levy, R., Blüher, M., & Rudich, A. (2020). Higher Mast Cell Accumulation in Human Adipose Tissues Defines Clinically Favorable Obesity Sub-Phenotypes. Cells, 9(6), 1508. https://doi.org/10.3390/cells9061508






