Extreme Low Cytosolic pH Is a Signal for Cell Survival in Acid Stressed Yeast
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strains and Growth Conditions
2.2. Growth and Recovery Determination
2.3. Spot Assays
2.4. Cell Viability
2.5. Cytosolic pH (pHc) Measurements
2.6. Protein Analysis
3. Results
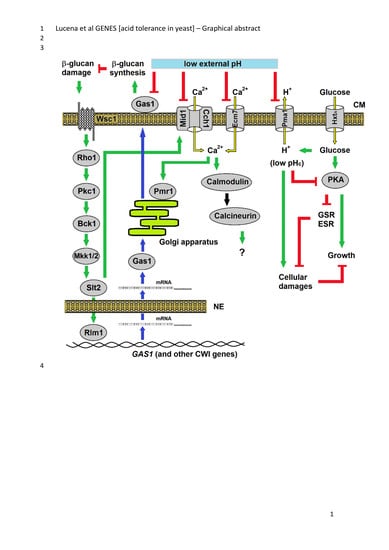
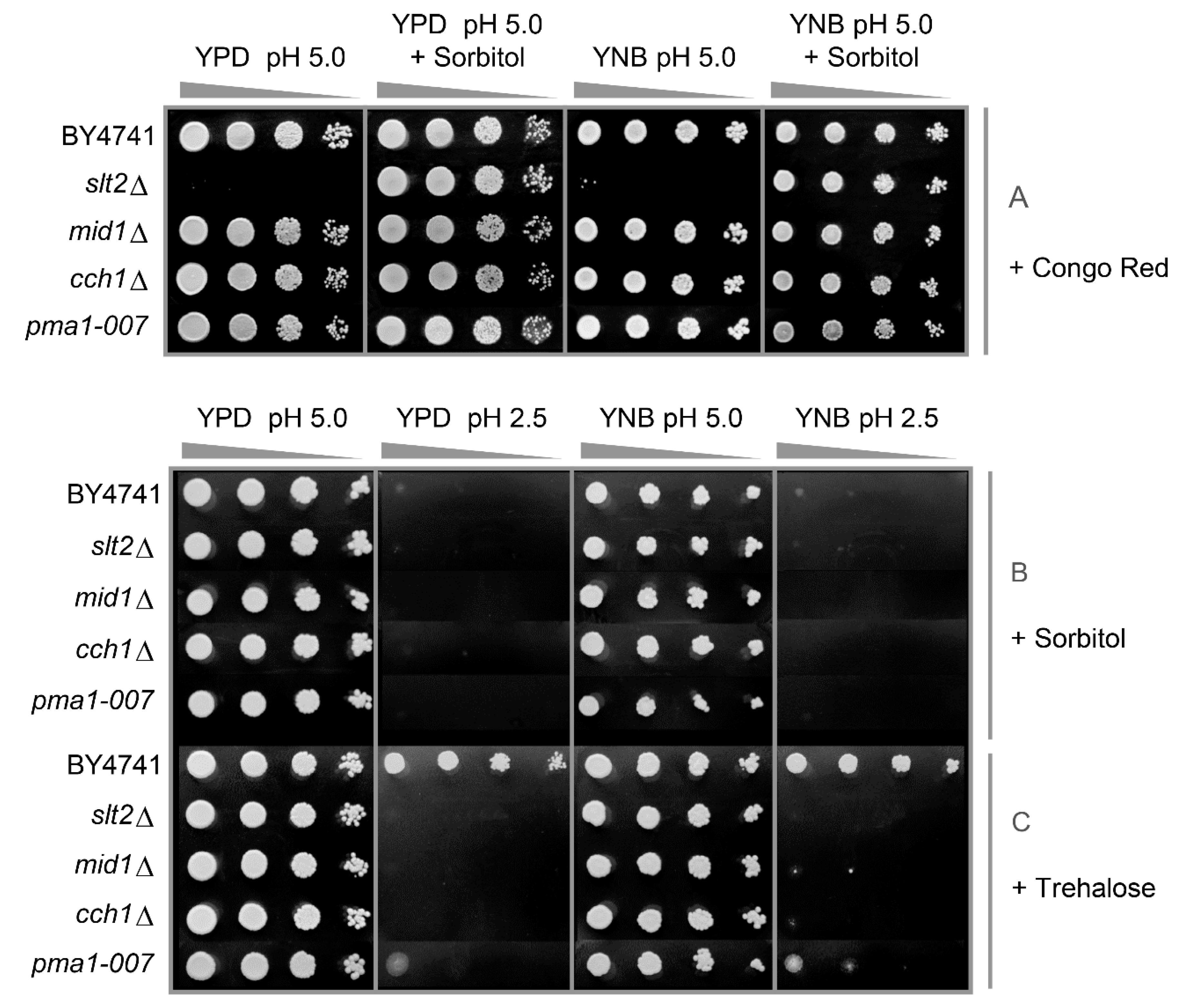
3.1. Components of the PKC, Ca2+ Signalling Pathway and Cell Wall Biosynthesis Are Required for Low-pH Stress Tolerance
3.2. Low pHex and High Osmolarity Exert Synergistic Stresses
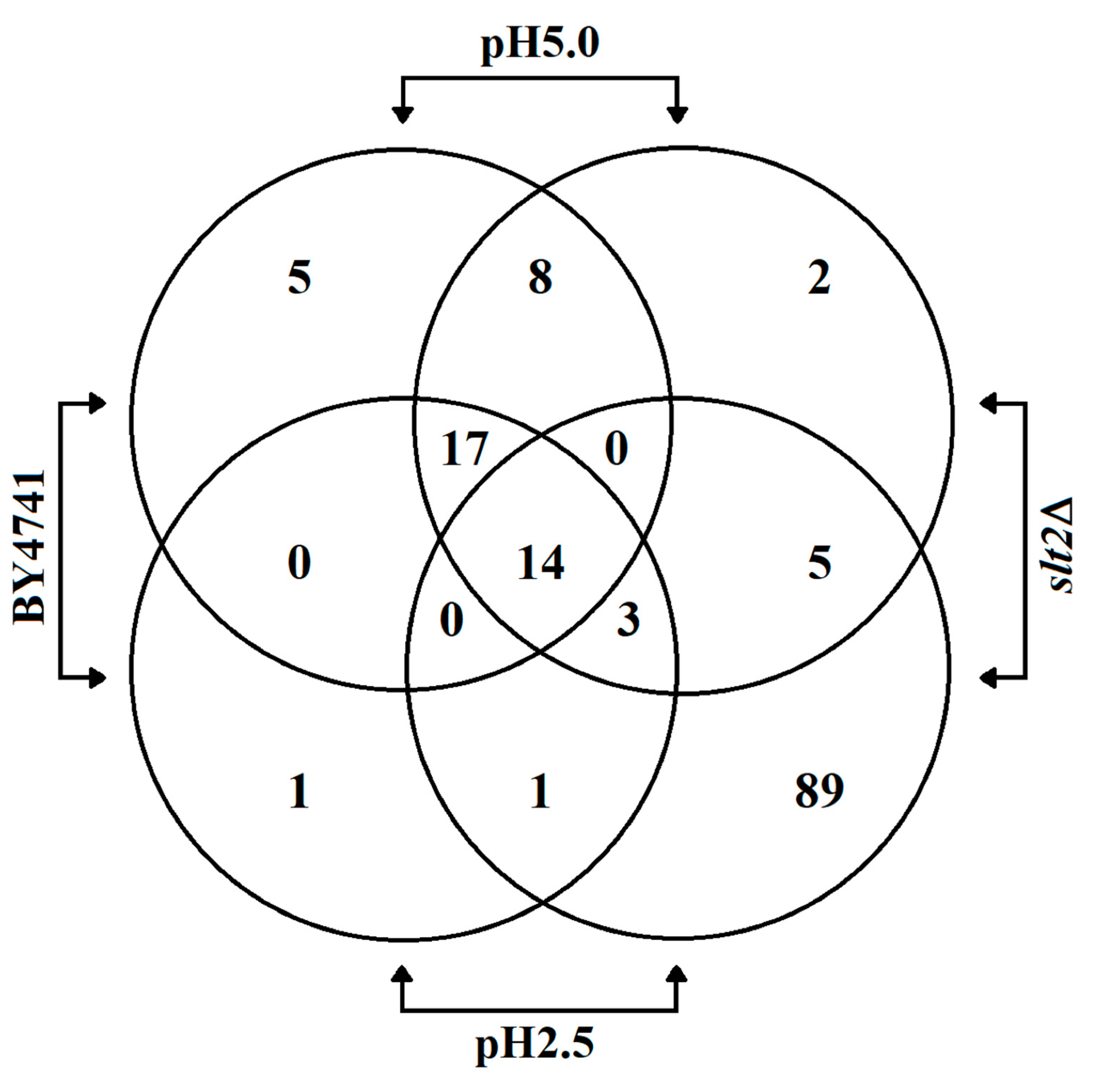
3.3. Low pHex Induces Cell Lysis in CWI Deficient Cells
3.4. Effects of Low pHex on Intracellular Acidification
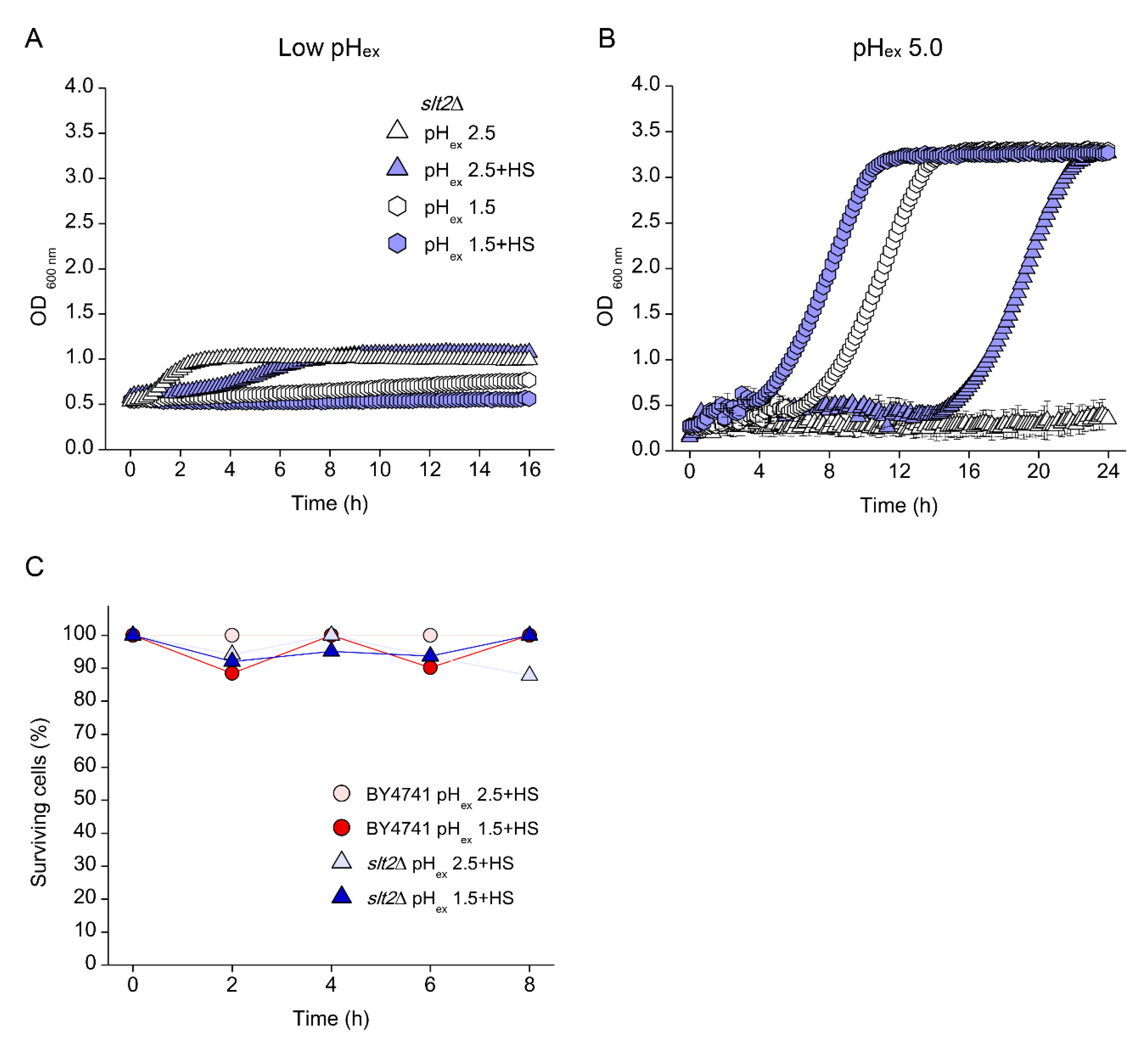
3.5. Low pHc Protects Cells from Loss of Viability at Extreme Low pHex
3.6. Absence of Growth Protects Cells from Low pHex Lethality
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Melo, H.F.; Bonini, B.M.; Thevelein, J.; Simões, D.A.; de Morais, M.A., Jr. Physiological and molecular analysis of the stress response of Saccharomyces cerevisiae imposed by strong inorganic acid with implication to industrial fermentations. J. Appl. Microbiol. 2010, 109, 116–127. [Google Scholar] [PubMed]
- Lucena, R.M.; Elsztein, C.; de Barros Pita, W.; de Souza, R.B.; Paiva, S.L., Jr.; de Morais, M.A., Jr. Transcriptomic response of Saccharomyces cerevisiae for its adaptation to sulphuric acid-induced stress. Antonie Van Leeuwenhoek 2015, 108, 1147–1160. [Google Scholar] [CrossRef]
- Ullah, A.; Orij, R.; Brul, S.; Smits, G.J. Quantitative analysis of the modes of growth inhibition by weak organic acids in Saccharomyces cerevisiae. Appl. Environ. Microbiol. 2012, 78, 8377–8387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beasley, D.E.; Koltz, A.M.; Lambert, J.E.; Fierer, N.; Dunn, R.R. The Evolution of Stomach Acidity and Its Relevance to the Human Microbiome. PLoS ONE 2015, 10, e0134116. [Google Scholar] [CrossRef] [PubMed]
- Orij, R.; Postmus, J.; Ter Beek, A.; Brul, S.; Smits, G.J. In vivo measurement of cytosolic and mitochondrial pH using a pH-sensitive GFP derivative in Saccharomyces cerevisiae reveals a relation between intracellular pH and growth. Microbiology 2009, 155, 268–278. [Google Scholar] [CrossRef] [Green Version]
- Orij, R.; Brul, S.; Smits, G.J. Intracellular pH is a tightly controlled signal in yeast. Biochim. Biophys. Acta 2011, 1810, 933–944. [Google Scholar] [CrossRef]
- Horák, J. Yeast nutrient transporters. Biochim. Biophys. Acta 1997, 1331, 41–79. [Google Scholar] [CrossRef]
- Morsomme, P.; Slayman, C.W.; Goffeau, A. Mutagenic study of the structure, function and biogenesis of the yeast plasma membrane H(+)-ATPase. Biochim. Biophys. Acta 2000, 1469, 133–157. [Google Scholar] [CrossRef]
- Dechant, R.; Binda, M.; Lee, S.S.; Pelet, S.; Winderickx, J.; Peter, M. Cytosolic pH is a second messenger for glucose and regulates the PKA pathway through V-ATPase. EMBO J. 2010, 29, 2515–2526. [Google Scholar] [CrossRef] [Green Version]
- Ullah, A.; Lopes, M.I.; Brul, S.; Smits, G.J. Intracellular pH homeostasis in Candida glabrata in infection-associated conditions. Microbiology 2013, 59, 803–813. [Google Scholar] [CrossRef] [Green Version]
- Denker, S.P.; Huang, D.C.; Orlowski, J.; Furthmayr, H.; Barber, D.L. Direct binding of the Na-H exchanger NHE1 to ERM proteins regulates the cortical cytoskeleton and cell shape independently of H(+) translocation. Mol. Cell 2000, 6, 1425–1436. [Google Scholar] [CrossRef]
- Orij, R.; Urbanus, M.L.; Vizeacoumar, F.J.; Giaever, G.; Boone, C.; Nislow, C.; Brul, S.; Smits, G.J. Genome-wide analysis of intracellular pH reveals quantitative control of cell division rate by pH(c) in Saccharomyces cerevisiae. Genome Biol. 2012, 13, R80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boussouf, A.; Gaillard, S. Intracellular pH changes during oligodendrocyte differentiation in primary culture. J. Neurosci. Res. 2000, 59, 731–739. [Google Scholar] [CrossRef]
- Putney, L.K.; Barber, D.L. Na-H exchange-dependent increase in intracellular pH times G2/M entry and transition. J. Biol. Chem. 2003, 278, 44645–44649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lagadic-Gossmann, D.; Huc, L.; Lecureur, V. Alterations of intracellular pH homeostasis in apoptosis: origins and roles. Cell Death Differ. 2004, 11, 953–961. [Google Scholar] [CrossRef] [Green Version]
- Damaghi, M.; Wojtkowiak, J.W.; Gillies, R.J. pH sensing and regulation in cancer. Front. Physiol. 2013, 4, 370. [Google Scholar] [CrossRef] [Green Version]
- Webb, B.A.; Chimenti, M.; Jacobson, M.P.; Barber, D.L. Dysregulated pH: a perfect storm for cancer progression. Nat. Rev. Cancer 2011, 11, 671–677. [Google Scholar] [CrossRef]
- Piper, P.; Calderon, C.O.; Hatzixanthis, K.; Mollapour, M. Weak acid adaptation: the stress response that confers yeasts with resistance to organic acid food preservatives. Microbiology 2001, 147, 2635–2642. [Google Scholar] [CrossRef] [Green Version]
- Hatzixanthis, K.; Mollapour, M.; Seymour, I.; Bauer, B.E.; Krapf, G.; Schüller, C.; Kuchler, K.; Piper, P.W. Moderately lipophilic carboxylate compounds are the selective inducers of the Saccharomyces cerevisiae Pdr12p ATP-binding cassette transporter. Yeast 2003, 20, 575–585. [Google Scholar] [CrossRef] [Green Version]
- Kapteyn, J.C.; ter Riet, B.; Vink, E.; Blad, S.; de Nobel, H.; van den Ende, H.; Klis, F.M. Low external pH induces HOG1-dependent changes in the organization of the Saccharomyces cerevisiae cell wall. Mol. Microbiol. 2001, 39, 469–479. [Google Scholar] [CrossRef] [Green Version]
- Ullah, A.; Chandrasekaran, G.; Brul, S.; Smits, G.J. Yeast adaptation to weak acids prevents futile energy expenditure. Front. Microbiol. 2013, 4, 142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, A.K.; Gelling, C.; Rogers, P.L.; Dawes, I.W.; Rosche, B. Response of Saccharomyces cerevisiae to stress-free acidification. J. Microbiol. 2009, 47, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Claret, S.; Gatti, X.; Doignon, F.; Thoraval, D.; Crouzet, M. The Rgd1p Rho GTPase activating protein and the Mid2p cell wall sensor are required at low pH for protein kinase C pathway activation and cell survival in Saccharomyces cerevisiae. Eukaryot. Cell 2005, 4, 1375–1386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmidt, M.; Schaumberg, J.Z.; Steen, C.M.; Boyer, M.P. Boric Acid Disturbs Cell Wall Synthesis in Saccharomyces cerevisiae. Int. J. Microbiol. 2010, 930465. [Google Scholar] [CrossRef] [Green Version]
- Lucena, R.M.; Elsztein, C.; Simões, D.A.; de Morais, M.A., Jr. Participation of CWI, HOG and Calcineurin pathways in the tolerance of Saccharomyces cerevisiae to low pH by inorganic acid. J. Appl. Microbiol. 2012, 113, 629–640. [Google Scholar] [CrossRef]
- Brandão, R.L.; Rosa, J.C.C.; Nicoli, J.R.; Almeida, M.V.S.; Carmo, A.P.; Queiros, H.T.; Castro, I.M. Investigating acid stress response in different Saccharomyces strains. J. Mycol. 2014, 178274. [Google Scholar] [CrossRef] [Green Version]
- Lucena, R.M.; Elsztein, C.; de Souza, R.B.; de Barros Pita, W.; Paiva, S.S., Jr.; de Morais, M.A., Jr. Genetic Interaction between HOG1 and SLT2 Genes in Signalling the Cellular Stress Caused by Sulphuric Acid in Saccharomyces cerevisiae. J. Mol. Microbiol. Biotechnol. 2015, 25, 423–427. [Google Scholar] [CrossRef]
- Gietz, R.D.; Woods, R.A. Transformation of yeast by lithium acetate/single-stranded carrier DNA/polyethylene glycol method. Methods Enzymol. 2002, 350, 87–96. [Google Scholar]
- Porat, Z.; Wender, N.; Erez, O.; Kahana, C. Mechanism of polyamine tolerance in yeast: novel regulators and insights. Cell Mol. Life Sci. 2005, 62, 3106–3116. [Google Scholar] [CrossRef]
- Young, B.P.; Shin, J.J.; Orij, R.; Chao, J.T.; Li, S.C.; Guan, X.L.; Khong, A.; Jan, E.; Wenk, M.R.; Prinz, W.A.; et al. Phosphatidic acid is a pH biosensor that links membrane biogenesis to metabolism. Science 2010, 329, 1085–1088. [Google Scholar] [CrossRef]
- Groppi, S.; Belotti, F.; Brandão, R.L.; Martegani, E.; Tisi, R. Glucose-induced calcium influx in budding yeast involves a novel calcium transport system and can activate calcineurin. Cell Calcium 2011, 49, 376–386. [Google Scholar] [CrossRef] [PubMed]
- Levin, D.E.; Bartlett-Heubusch, E. Mutants in the S. cerevisiae PKC1 gene display a cell cyclespecific osmotic stability defect. J. Cell Biol. 1992, 116, 1221–1229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hounsa, C.G.; Brandt, E.V.; Thevelein, J.; Hohmann, S.; Prior, B.A. Role of trehalose in survival of Saccharomyces cerevisiae under osmotic stress. Microbiology 1998, 144, 671–680. [Google Scholar] [CrossRef] [PubMed]
- Herdeiro, R.S.; Pereira, M.D.; Panek, A.D.; Eleutherio, E.C. Trehalose protects Saccharomyces cerevisiae from lipid peroxidation during oxidative stress. Biochim. Biophys. Acta 2006, 1760, 340–346. [Google Scholar] [CrossRef]
- Bandara, A.; Fraser, S.; Chambers, P.J.; Stanley, G.A. Trehalose promotes the survival of Saccharomyces cerevisiae during lethal ethanol stress, but does not influence growth under sublethal ethanol stress. FEMS Yeast Res. 2009, 9, 1208–1216. [Google Scholar] [CrossRef]
- Branco, P.; Francisco, D.; Chambon, C.; Hébraud, M.; Arneborg, N.; Almeida, M.G.; Caldeira, J.; Albergaria, H. Identification of novel GAPDH-derived antimicrobial peptides secreted by Saccharomyces cerevisiae and involved in wine microbial interactions. Appl. Microbiol. Biotechnol. 2014, 98, 843–853. [Google Scholar] [CrossRef] [Green Version]
- Miesenböck, G.; de Angelis, D.A.; and Rothman, J.E. Visualizing secretion and synaptic transmission with pH-sensitive green fluorescent proteins. Nature 1998, 394, 192–195. [Google Scholar] [CrossRef]
- Zakrzewska, A.; van Eikenhorst, G.; Burggraaff, J.E.; Vis, D.J.; Hoefsloot, H.; Delneri, D.; Oliver, S.G.; Brul, S.; Smits, G.J. Genome-wide analysis of yeast stress survival and tolerance acquisition to analyze the central trade-off between growth rate and cellular robustness. Mol. Biol. Cell 2011, 22, 4435–4446. [Google Scholar] [CrossRef]
- Bush, K.; Bradford, P.A. β-Lactams and β-Lactamase Inhibitors: An Overview. Cold Spring Harb. Perspect. Med. 2016, 6, a025247. [Google Scholar] [CrossRef]
- Mouyna, I.; Fontaine, T.; Vai, M.; Monod, M.; Fonzi, W.A.; Diaquin, M.; Popolo, L.; Hartland, R.P.; Latgé, J.P. Glycosylphosphatidylinositol-anchored glucanosyltransferases play an active role in the biosynthesis of the fungal cell wall. J. Biol. Chem. 2000, 275, 14882–14889. [Google Scholar] [CrossRef] [Green Version]
- Klis, F.M.; Mol, P.; Hellingwerf, K.; Brul, S. Dynamics of cell wall structure in Saccharomyces cerevisiae. FEMS Microbiol. Rev. 2002, 26, 239–256. [Google Scholar] [CrossRef] [PubMed]
- Ragni, E.; Fontaine, T.; Gissi, C.; Latgè, J.P.; Popolo, L. The Gas family of proteins of Saccharomyces cerevisiae: characterization and evolutionary analysis. Yeast 2007, 24, 297–308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazáň, M.; Ragni, E.; Popolo, L.; Farkaš, V. Catalytic properties of the Gas family β-(1,3)-glucanosyltransferases active in fungal cell-wall biogenesis as determined by a novel fluorescent assay. Biochem. J. 2011, 438, 275–282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elsztein, C.; de Lucena, R.M.; de Morais, M.A., Jr. The resistance of the yeast Saccharomyces cerevisiae to the biocide polyhexamethylene biguanide: involvement of cell wall integrity pathway and emerging role for YAP1. BMC Mol. Biol. 2011, 12, 38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Queiroz, M.G.; Elsztein, C.; de Morais, M.A., Jr. The effects of the Ncw2 protein of Saccharomyces cerevisiae on the positioning of chitin in response to cell wall damage. Antonie Van Leeuwenhoek 2020, 113, 265–277. [Google Scholar] [CrossRef] [PubMed]
- Schiavone, M.; Formosa-Dague, C.; Elsztein, C.; Teste, M.A.; Martin-Yken, H.; de Morais, M.A., Jr.; Dague, E.; François, J.M. Evidence for a Role for the Plasma Membrane in the Nanomechanical Properties of the Cell Wall as Revealed by an Atomic Force Microscopy Study of the Response of Saccharomyces cerevisiae to Ethanol Stress. Appl. Environ. Microbiol. 2016, 82, 4789–4801. [Google Scholar] [CrossRef] [Green Version]
- Udom, N.; Chansongkrow, P.; Charoensawan, V.; Auesukaree, C. Coordination of the Cell Wall Integrity and High-Osmolarity Glycerol Pathways in Response to Ethanol Stress in Saccharomyces cerevisiae. Appl. Environm. Microbiol. 2019, 85, e00551-19. [Google Scholar] [CrossRef] [Green Version]
- Sanz, A.B.; García, R.; Rodríguez-Peña, J.M.; Arroyo, J. The CWI Pathway: Regulation of the Transcriptional Adaptive Response to Cell Wall Stress in Yeast. J. Fungi 2018, 4, 1. [Google Scholar] [CrossRef] [Green Version]
- Verna, J.; Lodder, A.; Lee, K.; Vagts, A.; Ballester, R. A family of genes required for maintenance of cell wall integrity and for the stress response in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 2007, 94, 13804–13809. [Google Scholar] [CrossRef] [Green Version]
- Futagami, T.; Nakao, S.; Kido, Y.; Oka, T.; Kajiwara, Y.; Takashita, H.; Omori, T.; Furukawa, K.; Goto, M. Putative stress sensors WscA and WscB are involved in hypo-osmotic and acidic pH stress tolerance in Aspergillus nidulans. Eukaryot. Cell 2011, 10, 1504–1515. [Google Scholar] [CrossRef] [Green Version]
- Dupres, V.; Alsteens, D.; Wilk, S.; Hansen, B.; Heinisch, J.J.; Dufrêne, Y.F. The yeast Wsc1 cell surface sensor behaves like a nanospring in vivo. Nat. Chem. Biol. 2009, 5, 857–862. [Google Scholar] [CrossRef] [PubMed]
- Cyert, M.S. Calcineurin signaling in Saccharomyces cerevisiae: how yeast go crazy in response to stress. Biochem. Biophys. Res. Commun. 2003, 311, 1143–1150. [Google Scholar] [CrossRef]
- Cunningham, K.W. Acidic calcium stores of Saccharomyces cerevisiae. Cell Calcium 2011, 50, 129–138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonilla, M.; Cunningham, K.W. Mitogen-activated protein kinase stimulation of Ca(2+) signaling is required for survival of endoplasmic reticulum stress in yeast. Mol. Biol. Cell. 2003, 14, 4296–4305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martínez-Muñoz, G.A.; Kane, P.J. Vacuolar and plasma membrane proton pumps collaborate to achieve cytosolic pH homeostasis in yeast. Biol. Chem. 2008, 283, 20309–20319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Kok, S.; Yilmaz, D.; Daran, J.M.; Pronk, J.T.; van Maris, A.J. In vivo analysis of Saccharomyces cerevisiae plasma membrane ATPase Pma1p isoforms with increased in vitro H+/ATP stoichiometry. Antonie Van Leeuwenhoek 2012, 102, 401–406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ambesi, A.; Miranda, M.; Petrov, V.V.; Slayman, C.W. Biogenesis and function of the yeast plasma membrane H(+)-ATPase. J. Exp. Biol. 2000, 203, 155–160. [Google Scholar]
- Peña, A.; Sánchez, N.S.; Calahorra, M. Estimation of the electric plasma membrane potential difference in yeast with fluorescent dyes: comparative study of methods. J. Bioenerg. Biomembr. 2010, 42, 419–432. [Google Scholar] [CrossRef]
- Estruch, F. Stress-controlled transcription factors, stress-induced genes and stress tolerance in budding yeast. FEMS Microbiol. Rev. 2000, 24, 469–486. [Google Scholar] [CrossRef]
- Berry, D.B.; Guan, Q.; Hose, J.; Haroon, S.; Gebbia, M.; Heisler, L.E.; Nislow, C.; Giaever, G.; Gasch, A.P. Multiple means to the same end: the genetic basis of acquired stress resistance in yeast. PLoS Genet. 2011, 7, e1002353. [Google Scholar] [CrossRef] [Green Version]
- Mira, N.P.; Palma, M.; Guerreiro, J.F.; Sá-Correia, I. Genome-wide identification of Saccharomyces cerevisiae genes required for tolerance to acetic acid. Microb. Cell Fact. 2010, 9, 79. [Google Scholar] [CrossRef] [PubMed] [Green Version]






| Strain | Biological Function of the Mutated Gene According to Saccharomyces Genome Database |
|---|---|
| wsc1Δ | Sensor-transducer of PKC1 MAPKKK pathway involved in maintenance of cell wall integrity (CWI) |
| wsc2Δ | Sensor-transducer of PKC1 MAPKKK pathway involved in maintenance of CWI |
| wsc3Δ | Sensor-transducer of PKC1 MAPKKK pathway involved in maintenance of CWI |
| mid2Δ | O-glycosylated sensor plasma membrane protein acting for CWI signalling |
| mtl1Δ | Putative plasma membrane sensor involved in CWI signalling and stress response |
| rom1Δ | Guanine nucleotide exchange factor (GEF) for Rho1p in the activation of PKC signalling cascade |
| rom2Δ | GEF protein with overlapping function to Rom1p |
| bck1Δ | Mitogen Activating Protein kinase kinase kinase (MAPKKK), first of the PKC signalling cascade |
| mkk1Δ | Target of Bck1p, MAPKK second kinase of the PKC signalling cascade |
| mkk2Δ | MAPKK protein with overlapping function to Mkk1p |
| slt2Δ | Target of Mkk1/2, MAPK third kinase of the PKC signalling cascade |
| rlm1Δ | Target of Slt2p, MADS-box transcription factor involved in the expression of CWI genes. |
| swi4Δ | DNA binding component of the SBF complex (Swi4p-Swi6p) that regulates G1/S checkpoint genes |
| swi6Δ | Transcription cofactor of SBF complex (Swi4p-Swi6p) |
| fks1Δ | Catalytic subunit of 1,3-β-D-glucan synthase involved in CWI mechanism |
| fks2Δ | Catalytic subunit of 1,3-β-D-glucan synthase involved in spore wall biosynthesis. |
| kdx1Δ | Protein kinase implicated in Slt2p signalling pathway of CWI mechanism and mating |
| gas1Δ | Glycosylphosphatidylinositol (GPI)-anchored β-1,3-glucanosyltransferase required for CW assembly |
| gas2Δ | GPI-anchored β-1,3-glucanosyltransferase involved in spore wall assembly |
| gas3Δ | Putative 1,3-β-glucanosyltransferase, member of GAS family |
| gas4Δ | 1,3-β-glucanosyltransferase involved with Gas2p in spore wall assembly |
| gas5Δ | 1,3-β-glucanosyltransferase; has similarity to Gas1p; localises to the cell wall. |
| mnn1Δ | α-1,3-mannosyltransferase of the Golgi complex required for N-mannosylation of secreted proteins |
| chs1Δ | Chitin synthase I requires that catalysis the transfer of N-acetylglucosamine (GlcNAc) to chitin. |
| sed1Δ | Major stress-induced structural GPI-cell wall glycoprotein associated with translating ribosomes |
| pir3Δ | O-glycosylated covalently-bound cell wall protein required for cell wall stability |
| hsp12Δ | Plasma membrane protein involved in maintaining membrane organisation during stress conditions |
| skn7Δ | Nuclear response regulator and transcription factor that interacts with the Tup1-Cyc8 complex |
| pma1-007 | Major plasma membrane H+-ATPase pump |
| mid1Δ | Subunit of the Voltage-gated high-affinity calcium Mid1/Cch1 channel |
| cch1Δ | Subunit of the Voltage-gated high-affinity calcium Mid1/Cch1 channel |
| cna1Δ | Catalytic subunit of the Ca2+/calmodulin-regulated protein phosphatase calcineurin A complex |
| cna2Δ | Catalytic subunit of the Ca2+/calmodulin-regulated protein phosphatase calcineurin A complex |
| cnb1Δ | Calcineurin B; regulatory subunit of calcineurin A complex |
| crz1Δ | Transcription factor regulated by Ca2+/calmodulin in response to stress condition |
| cmk1Δ | Calmodulin-dependent protein kinase acting on stress response |
| cmk2Δ | Calmodulin-dependent protein kinase with overlapping function to Cmk1 |
| rcn1Δ | Protein involved in calcineurin regulation during calcium signalling |
| rcn2Δ | Protein of unknown function, paralogous to Rcn1p |
| slm1Δ | Phosphoinositide PI4,5P(2) binding protein that acts on cytoskeleton organisation during stress |
| slm2Δ | Phosphoinositide PI4,5P(2) binding protein that forms a complex with Slm1p |
| hph1Δ | Calcineurin substrate tail-anchored ER membrane protein of unknown function |
| hph2Δ | Tail-anchored ER membrane protein of unknown function involved in growth in osmotic and CW stress |
| pmr1Δ | High affinity Ca2+/Mn2+ P-type ATPase involved in Ca2+-dependent protein sorting in the Golgi complex |
| pmc1Δ | Vacuolar Ca2+ ATPase involved in depleting cytosolic Ca2+ and preventing calcineurin activation |
| vcx1Δ | Vacuolar membrane antiporter with Ca2+/H+ and K+/H+ exchange activity for cell ion homeostasis |
| ecm7Δ | Putative integral membrane protein with a role in calcium uptake |
| yvc1Δ | Vacuolar cation channel that mediates vacuolar Ca2+ release in response to hyperosmotic shock |
| Strains | pHex 2.5 | pHex 1.5 | ||||
|---|---|---|---|---|---|---|
| −HS | +HS | −HS | +HS | |||
| BY4741 | 6.96 ± 0.00 | 6.40 ± 0.00 | 6.68 ± 0.01 | 6.10 ± 0.01 | ||
| slt2Δ | 7.00 ± 0.02 | 6.45 ± 0.02 | 6.70 ± 0.01 | 6.01 ± 0.01 | ||
| pma1-007 | 6.83 ± 0.01 | 6.33 ± 0.02 | 6.49 ± 0.01 | 5.90 ± 0.01 | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lucena, R.M.; Dolz-Edo, L.; Brul, S.; de Morais, M.A., Jr.; Smits, G. Extreme Low Cytosolic pH Is a Signal for Cell Survival in Acid Stressed Yeast. Genes 2020, 11, 656. https://doi.org/10.3390/genes11060656
Lucena RM, Dolz-Edo L, Brul S, de Morais MA Jr., Smits G. Extreme Low Cytosolic pH Is a Signal for Cell Survival in Acid Stressed Yeast. Genes. 2020; 11(6):656. https://doi.org/10.3390/genes11060656
Chicago/Turabian StyleLucena, Rodrigo Mendonça, Laura Dolz-Edo, Stanley Brul, Marcos Antonio de Morais, Jr., and Gertien Smits. 2020. "Extreme Low Cytosolic pH Is a Signal for Cell Survival in Acid Stressed Yeast" Genes 11, no. 6: 656. https://doi.org/10.3390/genes11060656
APA StyleLucena, R. M., Dolz-Edo, L., Brul, S., de Morais, M. A., Jr., & Smits, G. (2020). Extreme Low Cytosolic pH Is a Signal for Cell Survival in Acid Stressed Yeast. Genes, 11(6), 656. https://doi.org/10.3390/genes11060656