Escherichia coli Increases its ATP Concentration in Weakly Acidic Environments Principally through the Glycolytic Pathway
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strains, Plasmids, Culture Media and Reagents
2.2. One-Step Inactivation of Chromosomal Genes in E. coli
2.3. P1 Transduction
2.4. Acid Tolerance Response (ATR) and Acid Resistance (AR) Test
2.5. ATP Content Measurement
2.6. RNA Extraction and RNA-seq Analysis
2.7. Gene Ontology Annotation and KEGG Pathway Analysis
2.8. Other Methods
2.9. Statistics
3. Results
3.1. Carbonyl Cyanide 3-Chlorophenylhydrazone (CCCP) and N,N′-Dicyclohexylcarbodiimide (DCCD) Affect the ATP Level and Acid Resistance under Different Conditions
3.2. Global Identification and Functional Inference of Acid-Regulated Genes, and Transcription Analysis of Known Glycolysis Genes
3.3. Characteristics of E. coli Knockout Mutants in Glycolysis and TCA Cycle Genes
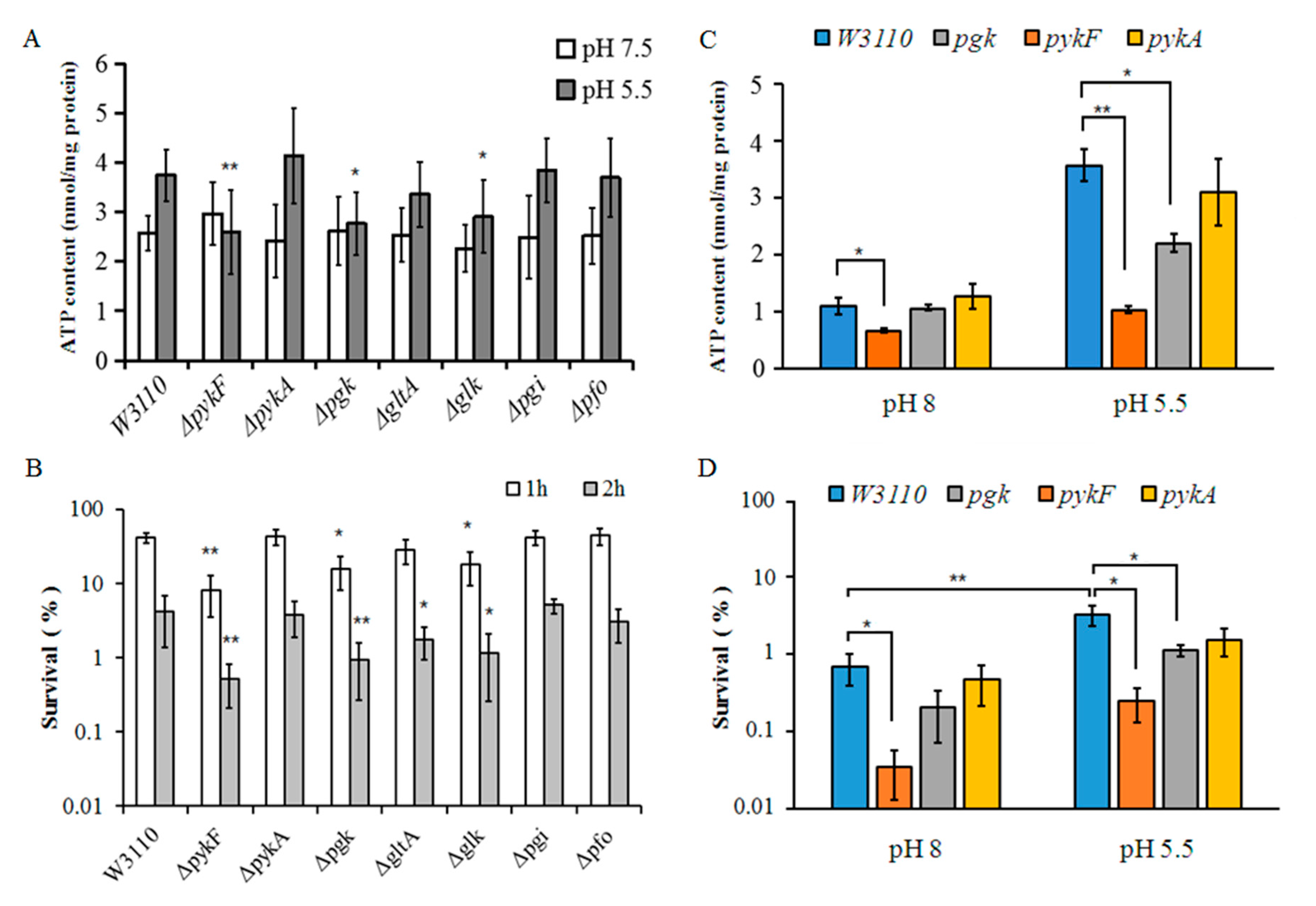
3.4. Comparisons of ATP Levels between Different Mutant Strains under Weakly Acidic Conditions
3.5. Comparisons of Acid Resistance between Different Mutant Strains
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lund, P.; Tramonti, A.; De Biase, D. Coping with low pH: Molecular strategies in neutralophilic bacteria. FEMS Microbiol. Rev. 2014, 38, 1091–1125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foster, J.W. Escherichia coli acid resistance: Tales of an amateur acidophile. Nat. Rev. Microbiol. 2004, 2, 898–907. [Google Scholar] [CrossRef] [PubMed]
- Hobbs, E.C.; Astarita, J.L.; Storz, G. Small RNAs and small proteins involved in resistance to cell envelope stress and acid shock in Escherichia coli: Analysis of a bar-coded mutant collection. J. Bacteriol. 2010, 192, 59–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leyer, G.J.; Wang, L.L.; Johnson, E.A. Acid adaptation of Escherichia coli O157:H7 increases survival in acidic foods. Appl. Environ. Microbiol. 1995, 61, 3752–3755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maurer, L.M.; Yohannes, E.; Bondurant, S.S.; Radmacher, M.; Slonczewski, J.L. pH regulates genes for flagellar motility, catabolism, and oxidative stress in Escherichia coli K-12. J. Bacteriol. 2005, 187, 304–319. [Google Scholar] [CrossRef] [Green Version]
- Seo, S.W.; Kim, D.; O′Brien, E.J.; Szubin, R.; Palsson, B.O. Decoding genome-wide GadEWX-transcriptional regulatory networks reveals multifaceted cellular responses to acid stress in Escherichia coli. Nat. Commun. 2015, 6, 7970. [Google Scholar] [CrossRef]
- Richard, H.; Foster, J.W. Escherichia coli glutamate- and arginine-dependent acid resistance systems increase internal pH and reverse transmembrane potential. J. Bacteriol. 2004, 186, 6032–6041. [Google Scholar] [CrossRef] [Green Version]
- De Biase, D.; Lund, P.A. The Escherichia coli Acid Stress Response and Its Significance for Pathogenesis. Adv. Appl. Microbiol. 2015, 92, 49–88. [Google Scholar]
- Castanie-Cornet, M.-P.; Penfound, T.A.; Smith, D.; Elliott, J.F.; Foster, J.W. Control of Acid Resistance in Escherichia coli. J. Bacteriol. 1999, 181, 3525. [Google Scholar] [CrossRef] [Green Version]
- Price, S.B.; Cheng, C.M.; Kaspar, C.W.; Wright, J.C.; DeGraves, F.J.; Penfound, T.A.; Castanie-Cornet, M.P.; Foster, J.W. Role of rpoS in acid resistance and fecal shedding of Escherichia coli O157:H7. Appl. Environ. Microbiol. 2000, 66, 632–637. [Google Scholar] [CrossRef] [Green Version]
- Castanie-Cornet, M.P.; Foster, J.W. Escherichia coli acid resistance: cAMP receptor protein and a 20 bp cis-acting sequence control pH and stationary phase expression of the gadA and gadBC glutamate decarboxylase genes. Microbiology 2001, 147, 709–715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hersh, B.M.; Farooq, F.T.; Barstad, D.N.; Blankenhorn, D.L.; Slonczewski, J.L. A glutamate-dependent acid resistance gene in Escherichia coli. J. Bacteriol. 1996, 178, 3978–3981. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masuda, N.; Church, G.M. Regulatory network of acid resistance genes in Escherichia coli. Mol. Microbiol. 2003, 48, 699–712. [Google Scholar] [CrossRef] [PubMed]
- Meng, S.Y.; Bennett, G.N. Nucleotide sequence of the Escherichia coli cad operon: A system for neutralization of low extracellular pH. J. Bacteriol. 1992, 174, 2659–2669. [Google Scholar] [CrossRef] [Green Version]
- Meng, S.Y.; Bennett, G.N. Regulation of the Escherichia coli cad operon: Location of a site required for acid induction. J. Bacteriol. 1992, 174, 2670–2678. [Google Scholar] [CrossRef] [Green Version]
- Gong, S.; Richard, H.; Foster, J.W. YjdE (AdiC) is the Arginine: Agmatine antiporter essential for arginine-dependent acid resistance in Escherichia coli. J. Bacteriol. 2003, 185, 4402–4409. [Google Scholar] [CrossRef] [Green Version]
- Vazquez-Juarez, R.C.; Kuriakose, J.A.; Rasko, D.A.; Ritchie, J.M.; Kendall, M.M.; Slater, T.M.; Sinha, M.; Luxon, B.A.; Popov, V.L.; Waldor, M.K.; et al. CadA negatively regulates Escherichia coli O157:H7 adherence and intestinal colonization. Infect. Immun. 2008, 76, 5072–5081. [Google Scholar] [CrossRef] [Green Version]
- Lu, P.; Ma, D.; Chen, Y.; Guo, Y.; Chen, G.-Q.; Deng, H.; Shi, Y. L-glutamine provides acid resistance for Escherichia coli through enzymatic release of ammonia. Cell Res. 2013, 23, 635–644. [Google Scholar] [CrossRef] [Green Version]
- Ma, D.; Lu, P.; Shi, Y. Substrate Selectivity of the Acid-activated Glutamate/γ-Aminobutyric acid (GABA) Antiporter GadC from Escherichia coli. J. Biol. Chem. 2013, 288, 15148–15153. [Google Scholar] [CrossRef] [Green Version]
- Tramonti, A.; De Canio, M.; Delany, I.; Scarlato, V.; De Biase, D. Mechanisms of transcription activation exerted by GadX and GadW at the gadA and gadBC gene promoters of the glutamate-Based acid resistance system in Escherichia coli. J. Bacteriol. 2006, 188, 8118. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Fukamachi, T.; Saito, H.; Kobayashi, H. Adenosine deamination increases the survival under acidic conditions in Escherichia coli. J. Appl. Microbiol. 2012, 112, 775–781. [Google Scholar] [CrossRef]
- Pennacchietti, E.; D′Alonzo, C.; Freddi, L.; Occhialini, A.; De Biase, D. The Glutaminase-Dependent Acid Resistance System: Qualitative and Quantitative Assays and Analysis of Its Distribution in Enteric Bacteria. Front. Microbiol. 2018, 9, 2869. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Y.; Fukamachi, T.; Saito, H.; Kobayashi, H. ATP requirement for acidic resistance in Escherichia coli. J. Bacteriol. 2011, 193, 3072–3077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sato, M.; Machida, K.; Arikado, E.; Saito, H.; Kakegawa, T.; Kobayashi, H. Expression of outer membrane proteins in Escherichia coli growing at acid pH. Appl. Environ. Microbiol. 2000, 66, 943–947. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Y.; Fukamachi, T.; Saito, H.; Kobayashi, H. Respiration and the F(1)Fo-ATPase enhance survival under acidic conditions in Escherichia coli. PLoS ONE 2012, 7, e52577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trchounian, A.; Trchounian, K. Fermentation Revisited: How Do Microorganisms Survive Under Energy-Limited Conditions? Trends Biochem. Sci. 2019, 44, 391–400. [Google Scholar] [CrossRef]
- Bardey, V.; Vallet, C.; Robas, N.; Charpentier, B.; Thouvenot, B.; Mougin, A.; Hajnsdorf, E.; Regnier, P.; Springer, M.; Branlant, C. Characterization of the molecular mechanisms involved in the differential production of erythrose-4-phosphate dehydrogenase, 3-phosphoglycerate kinase and class II fructose-1,6-bisphosphate aldolase in Escherichia coli. Mol. Microbiol. 2005, 57, 1265–1287. [Google Scholar] [CrossRef]
- Young, T.A.; Skordalakes, E.; Marqusee, S. Comparison of proteolytic susceptibility in phosphoglycerate kinases from yeast and E. coli: Modulation of conformational ensembles without altering structure or stability. J. Mol. Biol. 2007, 368, 1438–1447. [Google Scholar] [CrossRef]
- Cunningham, D.S.; Liu, Z.; Domagalski, N.; Koepsel, R.R.; Ataai, M.M.; Domach, M.M. Pyruvate kinase-deficient Escherichia coli exhibits increased plasmid copy number and cyclic AMP levels. J. Bacteriol. 2009, 191, 3041–3049. [Google Scholar] [CrossRef] [Green Version]
- Soellner, S.; Rahnert, M.; Siemann-Herzberg, M.; Takors, R.; Altenbuchner, J. Evolution of pyruvate kinase-deficient Escherichia coli mutants enables glycerol-based cell growth and succinate production. J. Appl. Microbiol. 2013, 115, 1368–1378. [Google Scholar] [CrossRef]
- Donovan, K.A.; Zhu, S.; Liuni, P.; Peng, F.; Kessans, S.A.; Wilson, D.J.; Dobson, R.C. Conformational Dynamics and Allostery in Pyruvate Kinase. J. Biol. Chem. 2016, 291, 9244–9256. [Google Scholar] [CrossRef] [Green Version]
- Zhao, C.; Lin, Z.; Dong, H.; Zhang, Y.; Li, Y. Reexamination of the Physiological Role of PykA in Escherichia coli Revealed that It Negatively Regulates the Intracellular ATP Levels under Anaerobic Conditions. Appl. Environ. Microbiol. 2017, 83, e00316–e00317. [Google Scholar] [CrossRef] [Green Version]
- Bhayana, V.; Duckworth, H.W. Amino acid sequence of Escherichia coli citrate synthase. Biochemistry 1984, 23, 2900–2905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Picossi, S.; Belitsky, B.R.; Sonenshein, A.L. Molecular mechanism of the regulation of Bacillus subtilis gltAB expression by GltC. J. Mol. Biol. 2007, 365, 1298–1313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jensen, K.F. The Escherichia coli K-12 “wild types” W3110 and MG1655 have an rph frameshift mutation that leads to pyrimidine starvation due to low pyrE expression levels. J. Bacteriol. 1993, 175, 3401–3407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Y.; Zhang, W.; Ma, J.; Pang, H.; Wang, H. Overproduction of alpha-Lipoic Acid by Gene Manipulated Escherichia coli. PLoS ONE 2017, 12, e0169369. [Google Scholar] [CrossRef]
- Datsenko, K.A.; Wanner, B.L. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 2000, 97, 6640–6645. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Sun, B.; Huang, H.; Jiang, Y.; Diao, L.; Chen, B.; Xu, C.; Wang, X.; Liu, J.; Jiang, W.; et al. High-efficiency scarless genetic modification in Escherichia coli by using lambda red recombination and I-SceI cleavage. Appl. Environ. Microbiol. 2014, 80, 3826–3834. [Google Scholar] [CrossRef] [Green Version]
- Zeph, L.R.; Onaga, M.A.; Stotzky, G. Transduction of Escherichia coli by bacteriophage P1 in soil. Appl. Environ. Microbiol. 1988, 54, 1731–1737. [Google Scholar] [CrossRef] [Green Version]
- Lin, J.; Lee, I.S.; Frey, J.; Slonczewski, J.L.; Foster, J.W. Comparative analysis of extreme acid survival in Salmonella typhimurium, Shigella flexneri, and Escherichia coli. J. Bacteriol. 1995, 177, 4097–4104. [Google Scholar] [CrossRef] [Green Version]
- Lasko, D.R.; Wang, D.I. In situ fermentation monitoring with recombinant firefly luciferase. Biotechnol. Bioeng. 1993, 42, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Maharjan, R.P.; Ferenci, T. Global metabolite analysis: The influence of extraction methodology on metabolome profiles of Escherichia coli. Anal. Biochem. 2003, 313, 145–154. [Google Scholar] [CrossRef]
- Mortazavi, A.; Williams, B.A.; McCue, K.; Schaeffer, L.; Wold, B. Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat. Methods 2008, 5, 621–628. [Google Scholar] [CrossRef] [PubMed]
- Huang da, W.; Sherman, B.T.; Lempicki, R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2009, 4, 44–57. [Google Scholar] [CrossRef]
- Ghoul, M.; Pommepuy, M.; Moillo-Batt, A.; Cormier, M. Effect of carbonyl cyanide m-chlorophenylhydrazone on Escherichia coli halotolerance. Appl. Environ. Microbiol. 1989, 55, 1040–1043. [Google Scholar] [CrossRef] [Green Version]
- Araki, M.; Hoshi, K.; Fujiwara, M.; Sasaki, Y.; Yonezawa, H.; Senpuku, H.; Iwamoto-Kihara, A.; Maeda, M. Complementation of the Fo c Subunit of Escherichia coli with That of Streptococcus mutans and Properties of the Hybrid FOF1 ATP Synthase. J. Bacteriol. 2013, 195, 4873. [Google Scholar] [CrossRef] [Green Version]
- Karapetyan, L.; Valle, A.; Bolivar, J.; Trchounian, A.; Trchounian, K. Evidence for Escherichia coli DcuD carrier dependent FOF1-ATPase activity during fermentation of glycerol. Sci. Rep. 2019, 9, 4279. [Google Scholar] [CrossRef] [Green Version]
- Stincone, A.; Daudi, N.; Rahman, A.S.; Antczak, P.; Henderson, I.; Cole, J.; Johnson, M.D.; Lund, P.; Falciani, F. A systems biology approach sheds new light on Escherichia coli acid resistance. Nucleic Acids Res. 2011, 39, 7512–7528. [Google Scholar] [CrossRef] [Green Version]
- Richards, G.R.; Patel, M.V.; Lloyd, C.R.; Vanderpool, C.K. Depletion of glycolytic intermediates plays a key role in glucose-phosphate stress in Escherichia coli. J. Bacteriol. 2013, 195, 4816–4825. [Google Scholar] [CrossRef] [Green Version]
- Russell, T.L.; Berardi, R.R.; Barnett, J.L.; Dermentzoglou, L.C.; Jarvenpaa, K.M.; Schmaltz, S.P.; Dressman, J.B. Upper gastrointestinal pH in seventy-nine healthy, elderly, North American men and women. Pharm. Res. 1993, 10, 187–196. [Google Scholar] [CrossRef] [Green Version]
- Ohwada, T.; Sagisaka, S.; Sato, T. An Exclusive Increase in the Concentration of ATP as a Result of Osmotic Stress in Escherichia coli B. Biosci. Biotechnol. Biochem. 1994, 58, 1512–1513. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, T.; Kobayashi, H. Regulation of the cytoplasmic pH by a proton-translocating ATPase in Streptococcus faecalis (faecium). A computer simulation. Eur. J. Biochem. 1989, 180, 467–471. [Google Scholar] [CrossRef] [PubMed]
- Krulwich, T.A.; Sachs, G.; Padan, E. Molecular aspects of bacterial pH sensing and homeostasis. Nat. Rev. Microbiol. 2011, 9, 330–343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mugikura, S.; Nishikawa, M.; Igarashi, K.; Kobayashi, H. Maintenance of a Neutral Cytoplasmic pH Is Not Obligatory for Growth of Escherichia coli and Streptococcus faecalis at an Alkaline pH. J. Biochem. 1990, 108, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Comb, D.G.; Roseman, S. Glucosamine 6-phosphate deaminase from Escherichia coli: Glucosamine 6-phosphate + H2O ⇄ Fructose 6-phosphate + NH3. Methods Enzymol. 1962, 5, 422–426. [Google Scholar]
- Lee, M.; Smith, G.M.; Eiteman, M.A.; Altman, E. Aerobic production of alanine by Escherichia coli aceF ldhA mutants expressing the Bacillus sphaericus alaD gene. Appl. Microbiol. Biotechnol. 2004, 65, 56–60. [Google Scholar] [CrossRef]
- De Mets, F.; Van Melderen, L.; Gottesman, S. Regulation of acetate metabolism and coordination with the TCA cycle via a processed small RNA. Proc. Natl. Acad. Sci. USA 2019, 116, 1043–1052. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Li, Y.; Cai, Z.; Jin, Y. Pyruvate-associated acid resistance in bacteria. Appl. Environ. Microbiol. 2014, 80, 4108–4113. [Google Scholar] [CrossRef] [Green Version]
- Diez-Gonzalez, F.; Russell, J.B. The ability of Escherichia coli O157:H7 to decrease its intracellular pH and resist the toxicity of acetic acid. Microbiology 1997, 143, 1175–1180. [Google Scholar] [CrossRef] [Green Version]
- Moreau, P.L.; Loiseau, L. Characterization of acetic acid-detoxifying Escherichia coli evolved under phosphate starvation conditions. Microb. Cell Fact. 2016, 15, 42. [Google Scholar] [CrossRef] [Green Version]
- Bae, Y.M.; Lee, S.Y. Effect of salt addition on acid resistance response of Escherichia coli O157:H7 against acetic acid. Food Microbiol. 2017, 65, 74–82. [Google Scholar] [CrossRef]
- Negrete, A.; Shiloach, J. Constitutive expression of the sRNA GadY decreases acetate production and improves E. coli growth. Microb. Cell Fact. 2015, 14, 148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noguchi, K.; Riggins, D.P.; Eldahan, K.C.; Kitko, R.D.; Slonczewski, J.L. Hydrogenase-3 contributes to anaerobic acid resistance of Escherichia coli. PLoS ONE 2010, 5, e10132. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Li, H.; Vuong, C.; Vadyvaloo, V.; Wang, J.; Yao, Y.; Otto, M.; Gao, Q. Role of the luxS quorum-sensing system in biofilm formation and virulence of Staphylococcus epidermidis. Infect. Immun. 2006, 74, 488–496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niba, E.T.; Naka, Y.; Nagase, M.; Mori, H.; Kitakawa, M. A genome-wide approach to identify the genes involved in biofilm formation in E. coli. DNA Res. 2007, 14, 237–246. [Google Scholar] [CrossRef]
- Tramonti, A.; Visca, P.; De Canio, M.; Falconi, M.; De Biase, D. Functional characterization and regulation of gadX, a gene encoding an AraC/XylS-like transcriptional activator of the Escherichia coli glutamic acid decarboxylase system. J. Bacteriol. 2002, 184, 2603–2613. [Google Scholar] [CrossRef] [Green Version]





| Strains or Plasmids | Genotype or Description | Source or Reference |
|---|---|---|
| Strains | ||
| W3110 | λ− F− derived from E. coli K-12 | [34] |
| BW25113 | lacIqrrnBT14 ΔlacZWJ16hsdR514 ΔaraBADAH33ΔrhaBADLD78 | [36] |
| SZ001 | BW25113 pgk::Cmr | This study |
| SZ002 | BW25113 pykA::Cmr | This study |
| SZ003 | BW25113 pykF::Kmr | This study |
| SZ004 | BW25113 pykA:Cmr pykF::Kmr | This study |
| SZ005 | BW25113 gltA::Cmr | This study |
| SZ006 | BW25113 pgi::Cmr | This study |
| SZ007 | BW25113 pfo::Cmr | This study |
| SZ008 | BW25113 glk::Cmr | This study |
| WB001 | W3110 pgk::Cmr | This study, W3110 × P1 (SZ001) |
| WB002 | W3110 pykA::Cmr | This study, W3110 × P1 (SZ002) |
| WB003 | W3110 pykF::Kmr | This study, W3110 × P1 (SZ003) |
| WB004 | W3110 gltA::Cmr | This study, W3110 × P1 (SZ004) |
| WB005 | W3110 pykA::Cmr pykF::Kmr | This study, W3110 × P1 (SZ005) |
| WB006 | W3110 pgi::Cmr | This study, W3110 × P1 (SZ006) |
| WB007 | W3110 pfo::Cmr | This study, W3110 × P1 (SZ007) |
| WB008 | W3110 glk::Cmr | This study, W3110 × P1 (SZ008) |
| Plasmids | ||
| pKD3 | bla, FRT, Cmr | [36] |
| pKD4 | bla, FRT, Kmr | [36] |
| pKD46 | bla, araC, gam-bet-exo | [36] |
| Primer Name | Sequence(5′→3′) |
|---|---|
| pykAP1 | ATTTCATTCGGATTTCATGTTCAAGCAACACCTGGTTGTTTCAGTCAACGGAGTATTACTGTGTAGGCTGGAGCTGCTTCG |
| pykAP2 | TGTTGAACTATCATTGAACTGTAGGCCGGATGTGGCGTTTTC GCCGCATCCGGCAACGTACCATATGAATATCCTCCTTAG |
| pykFP1 | GCAGTGCGCCCAGAAAGCAAGTTTCTCCCATCCTTCTCAACTTAAAGACTAAGACTGTCTGTGTAGGCTGGAGCTGCTTCG |
| pykFP2 | TTAAATAAAAAAAGCGCCCATCAGGGCGCTTCGATATACAAATTAATTCACAAAAGCAATACATATGAATATCCTCCTTAG |
| gltAP1 | TGCGAAGGCAAATTTAAGTTCCGGCAGTCTTACGCAATAAG GCGCTAAGGAGACCTTAATGTGTAGGCTGGAGCTGCTTCG |
| gltA P2 | AAAAATCAACCCGCCATATGAACGGCGGGTTAAAATATTTACAACTTAGCAATCAACCACATATGAATATCCTCCTTAG |
| pgk P1 | TTTCAGGTAAGACGCAAGCAGCGTCTGCAAAACTTTTAGAATCAACGAGAGGATTCACCTGTGTAGGCTGGAGCTGCTTCG |
| pgk P2 | AAAAATTGCGTGCTCTAAAAGCGCGCTGAAACAAGGGCAGGTTTCCCTGCCCTGTGATTTTCATATGAATATCCTCCTTAG |
| pgi p1 | GTGACTGGCGCTACAATCTTCCAAAGTCACAATTCTCAAAATCAGAAGAGTATTGCTATGTGTAGGCTGGAGCTGCTTCG |
| pgi p2 | TCAGGCATCGGTTGCCGGATGCGGCGTGAACGCCTTATCCGGCCTACATATCGACGATGACATATGAATATCCTCCTTAG |
| glk p1 | ATGACAAAGTATGCATTAGTCGGTGATGTGGGCGGCACCAACGCACGTCTTGCTCTGTGTGTAGGCTGGAGCTGCTTCG |
| glk p2 | TACAGAATGTGACCTAAGGTCTGGCGTAAATGTGCACCGGAACCGAGAAGGCCCGGCATATGAATATCCTCCTTAG |
| pfo p1 | TTTCGTGCGCCCCTCATTTGCGCAATGTAAGGGTGTCATATGATTACTATTGACGGTAATTGTGTAGGCTGGAGCTGCTTCG |
| pfo p2 | TTTAGAATTGGATAATCCTTATCCAGAGCATTTAATCGGTGTT GCTTTTCATATGAATATCCTCCTTAG |
| check for pgk KO | CCAATGAAGTCAACCTGTTGC |
| check for gltA KO | GATCCAGGTCACGATAACAAC |
| check for pykA KO | GAAGCAACGCTGGCAATTAC |
| check for pykF KO | CTGTAGCAATTGAGCGATG |
| check for pgi KO | CAGAGCGATACTTCGCTACTA |
| check for glk KO | CCGCAATACCCGTTAGCGTT |
| check for pfo KO | CTTCCTCTGATCTTCAAGCC |
| Km inbox | GTGAGATGACAGGAGATCCTG |
| Cm inbox | TCTTGCCCGCCTGATGAATGCTC |
| RT-glk F | CTGTATTGCCATCGCTTGCC |
| RT-glk R | TTACCTTCGACCGGTTCTGC |
| RT-pgi F | TTCATCGCTCCGGCTATCAC |
| RT-pgi R | CCAGGCTGAACGGAGTGATT |
| RT-pfkA F | TACATGGGTGCAATGCGTCT |
| RT-pfkA R | TAACGGCCCATCACTTCCAC |
| RT-fbaA F | ACTCCATCAACGCCGTACTG |
| RT-fbaA R | CAGTGGTCAGTGTGCAGGAT |
| RT-tpiA F | GCAAAACGTGGACCTGAACC |
| RT-tpiA R | ATGCACAGAACCGGAGTCAG |
| RT-gapA F | TTGACCTGACCGTTCGTCTG |
| RT-gapA R | GAAGTGCAAACTTCGCCGTT |
| RT-pgk F | CTCTCTGCTGCCGGTTGTTA |
| RT-pgk R | GCCTTTGTTGAAGCGAACGT |
| RT-gpmA F | ATCATCGCTGCACACGGTAA |
| RT-gpmA R | GCGATCTCGTCAGCATTACC |
| RT-eno F | TGGCGCGAAAACTGTGAAAG |
| RT-eno R | TGAACGCTTTGTTGCCTTCG |
| RT-pykA F | TCGCCTCTGACGTGGTAATG |
| RT-pykA F | GCATCACAGCGTCAGTACCA |
| RT-pykF F | GAACAGCCGTCTCGAGTTCA |
| RT-pykF R | TTAACAAGCTGCGGCACAAC |
| RT-pfo F | TTGCCACACATGCACTCTCT |
| RT-pfo R | CCTGCGGCATGAGATCAAGA |
| RT-gltA F | CGCATCCAATGGCAGTCATG |
| RT-gltA R | TTGCGCGGGTAAACAAATGG |
| RT-16S F | TACCGCATAACGTCGCAAGA |
| RT-16S R | AGTCTGGACCGTGTCTCAGT |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.; Chen, X.; Sun, W.; Nie, T.; Quanquin, N.; Sun, Y. Escherichia coli Increases its ATP Concentration in Weakly Acidic Environments Principally through the Glycolytic Pathway. Genes 2020, 11, 991. https://doi.org/10.3390/genes11090991
Zhang W, Chen X, Sun W, Nie T, Quanquin N, Sun Y. Escherichia coli Increases its ATP Concentration in Weakly Acidic Environments Principally through the Glycolytic Pathway. Genes. 2020; 11(9):991. https://doi.org/10.3390/genes11090991
Chicago/Turabian StyleZhang, Wenbin, Xin Chen, Wei Sun, Tao Nie, Natalie Quanquin, and Yirong Sun. 2020. "Escherichia coli Increases its ATP Concentration in Weakly Acidic Environments Principally through the Glycolytic Pathway" Genes 11, no. 9: 991. https://doi.org/10.3390/genes11090991
APA StyleZhang, W., Chen, X., Sun, W., Nie, T., Quanquin, N., & Sun, Y. (2020). Escherichia coli Increases its ATP Concentration in Weakly Acidic Environments Principally through the Glycolytic Pathway. Genes, 11(9), 991. https://doi.org/10.3390/genes11090991





