DNA Satellites Are Transcribed as Part of the Non-Coding Genome in Eukaryotes and Bacteria
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Caenorhabditis Elegans
3.2. Bacillus coagulans
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thakur, J.; Packiaraj, J.; Henikoff, S. Sequence, Chromatin and Evolution of Satellite DNA. Int. J. Mol. Sci. 2021, 22, 4309. [Google Scholar] [CrossRef] [PubMed]
- Perea-Resa, C.; Blower, M.D. Centromere biology: Transcription goes on stage. Mol. Cell. Biol. 2018, 38, e00263-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, X.; Eickbush, D.G.; Speece, I.; Larracuente, A.M. Heterochromatin-dependent transcription of satellite DNAs in the Drosophila melanogaster female germline. eLife 2021, 10, e62375. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, G.C.S. Satellite DNA transcripts have diverse biological roles in Drosophila. Heredity 2015, 115, 1–2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shatskikh, A.S.; Kotov, A.A.; Adashev, V.E.; Bazylev, S.S.; Olenina, L.V. Functional Significance of Satellite DNAs: Insights from Drosophila. Front. Cell Dev. Biol. 2020, 8, 312. [Google Scholar] [CrossRef] [PubMed]
- Bury, L.; Moodie, B.; Ly, J.; McKay, L.S.; Miga, K.H.H.; Cheeseman, I.M. Alpha-satellite RNA transcripts are repressed by centromere–nucleolus associations. eLife 2020, 9, e59770. [Google Scholar] [CrossRef] [PubMed]
- Schoeftner, S.; Blasco, M.A. Chromatin regulation and non-coding RNAs at mammalian telomeres. Semin. Cell Dev. Biol. 2010, 21, 186–193. [Google Scholar] [CrossRef]
- Chen, L.; Yang, L. ALUternative Regulation for Gene Expression. Trends Cell Biol. 2017, 27, 480–490. [Google Scholar] [CrossRef] [Green Version]
- Kaletsky, R.; Yao, V.; Williams, A.; Runnels, A.M.; Tadych, A.; Zhou, S.; Troyanskaya, O.G.; Murphy, C.T. Transcriptome analysis of adult Caenorhabditis elegans cells reveals tissue specific gene and isoform expression. PLoS Genet. 2018, 14, e1007559. [Google Scholar] [CrossRef] [Green Version]
- Miki, T.S.; Carl, S.H.; Großhans, H. Two distinct transcription termination modes dictated by promoters. Genes Dev. 2017, 31, 1870–1879. [Google Scholar] [CrossRef] [Green Version]
- Qin, J.; Wang, X.; Wang, L.; Zhu, B.; Zhang, X.; Yao, Q.; Xu, P. Comparative transcriptome analysis reveals different molecular mechanisms of Bacillus coagulans 2-6 response to sodium lactate and calcium lactate during lactic acid production. PLoS ONE 2015, 10, e0124316. [Google Scholar] [CrossRef] [Green Version]
- Yoshimura, J.; Ichikawa, K.; Shoura, M.J.; Artiles, K.L.; Gabdan, I.; Wahba, L.; Smith, C.L.; Edgley, M.L.; Rougvie, A.E.; Fire, A.Z.; et al. Recompleting the Caenorhabditis elegans genome. Genome Res. 2019, 29, 1009–1022. [Google Scholar] [CrossRef] [Green Version]
- Subirana, J.A.; Albà, M.M.; Messeguer, X. High evolutionary turnover of tandem repeat families in Caenorhabditis. BMC Evol. Biol. 2015, 15, 218. [Google Scholar] [CrossRef] [Green Version]
- NCBI SRA Web Site. Available online: https://www.ncbi.nlm.nih.gov/sra/ (accessed on 10 October 2021).
- Subirana, J.A.; Messeguer, X. Unique features of tandem repeats in bacteria. J. Bacteriol. 2020, 202, e00229-20. [Google Scholar] [CrossRef]
- Kalvari, I.; Nawrocki, E.P.; Ontiveros-Palacios, N.; Argasinska, J.; Lamkiewicz, K.; Marz, M.; Griffiths-Jones, S.; Toffano-Nioche, C.; Gautheret, D.; Weinberg, Z.; et al. Rfam 14: Expanded coverage of metagenomic, viral and microRNA families. Nucleic Acids Res. 2021, 49, D192–D200. [Google Scholar] [CrossRef]
- Subirana, J.A.; Messeguer, X. Tandem Repeats in Bacillus: Unique Features and Taxonomic Distribution. Int. J. Mol. Sci. 2021, 22, 5373. [Google Scholar] [CrossRef] [PubMed]
- RNAfold WebServer. Available online: http://rna.tbi.univie.ac.at//cgi-bin/RNAWebSuite/RNAfold.cgi (accessed on 10 October 2021).
- Khan, M.R.; Wellinger, R.J.; Laurent, B. Exploring the Alternative Splicing of Long Noncoding RNAs. Trends Genet. 2021, 37, 695–698. [Google Scholar] [CrossRef] [PubMed]
- Britton, C.; Laing, R.; Devaney, E. Small RNAs in parasitic nematodes—Forms and functions. Parasitology 2020, 147, 855–864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ambros, V.; Ruvkun, G. Recent Molecular Genetic Explorations of Caenorhabditis elegans MicroRNAs. Genetics 2018, 209, 651–673. [Google Scholar]
- Ruby, J.G.; Jan, C.; Player, C.; Axtell, M.J.; Lee, W.; Nusbaum, C.; Ge, H.; Bartel, D.P. Large-Scale Sequencing Reveals 21U-RNAs and Additional MicroRNAs and Endogenous siRNAs in C. elegans. Cell 2006, 127, 1193–1207. [Google Scholar] [CrossRef] [Green Version]
- Almeida, M.V.; Andrade-Navarro, M.A.; Ketting, R.F. Function and Evolution of Nematode RNAi Pathways. Non-Coding RNA 2019, 5, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cevec, M.; Thibaudeau, C.; Plavec, J. NMR structure of the let-7 miRNA interacting with the site LCS1 of lin-41 mRNA from Caenorhabditis elegans. Nucleic Acids Res. 2010, 38, 7814–7821. [Google Scholar] [CrossRef] [PubMed]
- Olina, A.V.; Kulbachinskiy, A.V.; Aravin, A.A.; Esyunina, D.M. Argonaute Proteins and Mechanisms of RNA Interference in Eukaryotes and Prokaryotes. Biochemistry 2018, 83, 483–497. [Google Scholar] [CrossRef] [PubMed]
- Hansen, T.B.; Jensen, T.I.; Clausen, B.H.; Bramsen, J.B.; Finsen, B.; Damgaard, C.K.; Kjems, J. Natural RNA circles function as efficient microRNA sponges. Nature 2013, 495, 384–388. [Google Scholar] [CrossRef] [PubMed]
- Chekulaeva, M.; Rajewsky, N. Roles of Long Noncoding RNAs and Circular RNAs in Translation. Cold Spring Harb. Perspect. Biol. 2019, 11, a032680. [Google Scholar] [CrossRef]
- Thakur, J.; Henikoff, S. Architectural RNA in chromatin organization. Biochem. Soc. Trans. 2020, 48, 1967–1978. [Google Scholar] [CrossRef]
- Amaral, P.P.; Dinger, M.E.; Mercer, T.R.; Mattick, J.S. The Eukaryotic Genome as an RNA Machine. Science 2008, 319, 1787–1789. [Google Scholar] [CrossRef]
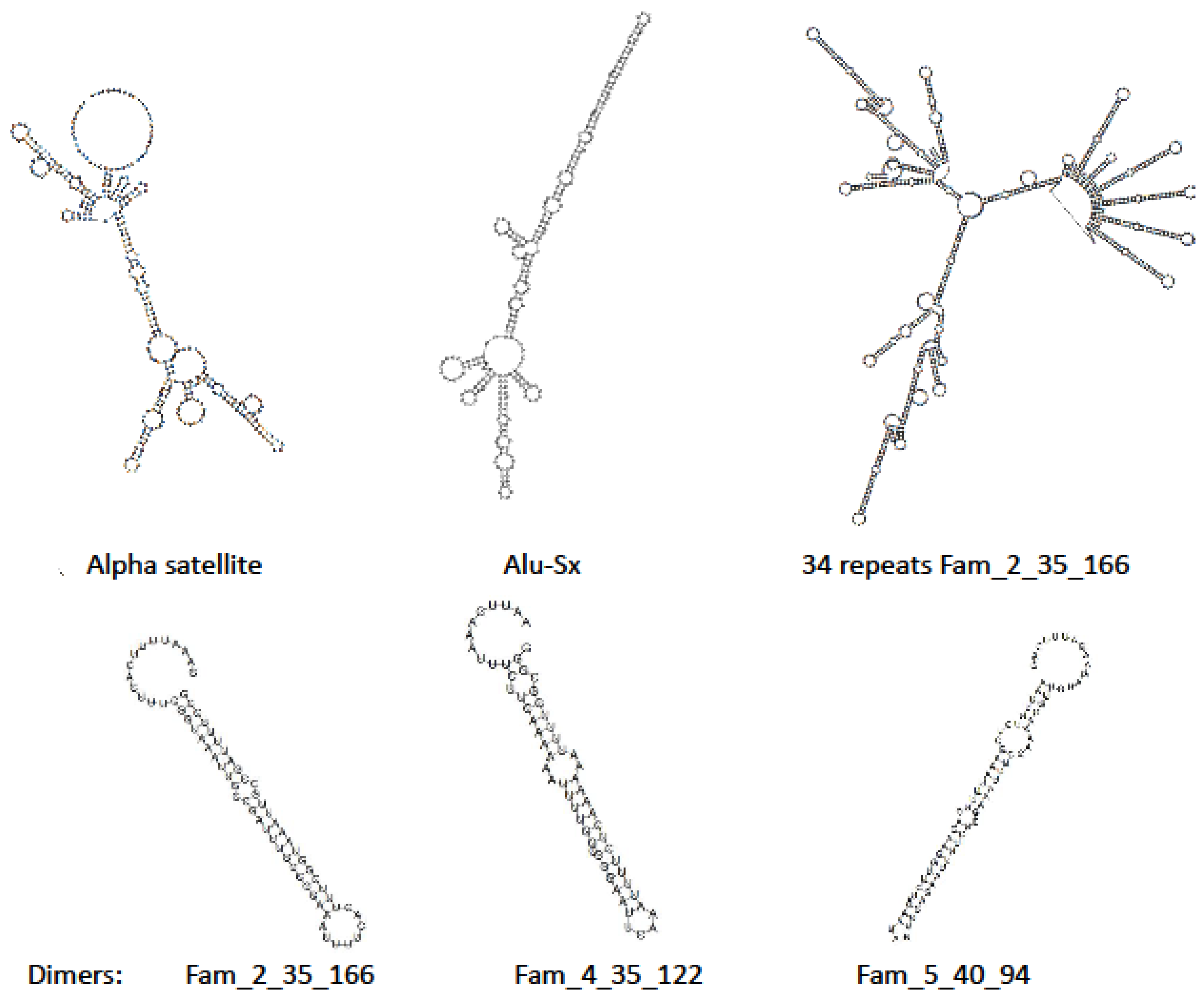
| Experiment | Average Spot Length | Bases (Gb) | Library Name | Satellite Family | Number of Hits | |
|---|---|---|---|---|---|---|
| 85% | 95% | |||||
| SRX4314529 | 139 | 34.44 | hypodermis_1 | 2_35_166 | 494 | 44 |
| SRX4314521 | 85 | 33.14 | hypodermis_7 | 500 | 157 | |
| SRX4314518 | 115 | 22.00 | intestine_2 | 500 | 107 | |
| SRX4314515 | 117 | 24.28 | intestine_3 | 500 | 134 | |
| SRX4314514 | 103 | 31.28 | neurons_1 | 500 | 233 | |
| SRX4314512 | 113 | 28.26 | neurons_3 | 500 | 402 | |
| SRX4314519 | 115 | 37.93 | neurons_4 | 500 | 315 | |
| SRX4314505 | 117 | 22.97 | muscle_6 | 495 | 130 | |
| SRX4314522 | 112 | 25.57 | muscle_1 | 494 | 85 | |
| Average values | ||||||
| 24.3 | muscle | 494 | 107 | |||
| 33.2 | neurons | 499 | 317 | |||
| 23.1 | intestine | 500 | 120 | |||
| 33.7 | hypodermis | 497 | 101 | |||
| Comparison of satellite families | ||||||
| SRX4314512 | 113 | 28.26 | neurons_3 | 1_12_169 | 500 | 364 |
| SRX4314512 | 113 | 28.26 | neurons_3 | 2_35_166 | 500 | 402 |
| SRX4314512 | 113 | 28.26 | neurons_3 | 4_35_122 | 500 | 73 |
| SRX4314512 | 113 | 28.26 | neurons_3 | 5_40_94 | 317 | 10 |
| SRX4314512 | 113 | 28.26 | neurons_3 | 9_20_48 | 500 | 441 |
| SRX4314512 | 113 | 28.26 | neurons_3 | 10_25_41 | 500 | 143 |
| SRX4314512 | 113 | 28.26 | neurons_3 | 11_45_30 | 289 | 8 |
| SRX4314512 | 113 | 28.26 | neurons_3 | 12_20_29 | 74 | 3 |
| SRX4314512 | 113 | 28.26 | neurons_3 | 13_31_27 | 324 | 49 |
| SRX4314512 | 113 | 28.26 | neurons_3 | 14_43_26 | 500 | 0 |
| SRX4314512 | 113 | 28.26 | neurons_3 | 15_26_22 | 500 | 174 |
| SRX4314512 | 113 | 28.26 | neurons_3 | 22_59_13 | 194 | 3 |
| SRX4314512 | 113 | 28.26 | neurons_3 | 24_32_11 | 500 | 330 |
| SRX4314512 | 113 | 28.26 | neurons_3 | Transfer RNA | 500 | 330 |
| Results obtained by Miki et al. [10] | ||||||
| SRX3104615 | 51 | 4.5 | Whole worms | 2_35_166 | 500 | 138 |
| SRX2737099 | 100 | 3.8 | Whole body | 2_35_166 | 496 | 119 |
| Conditions | SRX Code | Number of Hits in Each Repeat Family | |||
|---|---|---|---|---|---|
| 1_52_139 | 2_52_35 | 8_52_18 | 360_52_1 | ||
| No stress | 700697 | 500 | 482 | 399 | 341 |
| Ca lactate | 700698 | 500 | 142 | 203 | 290 |
| Na lactate | 700710 | 500 | 500 | 498 | 499 |
| Number of satellites | 9 | 4 | 1 | 1 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Subirana, J.A.; Messeguer, X. DNA Satellites Are Transcribed as Part of the Non-Coding Genome in Eukaryotes and Bacteria. Genes 2021, 12, 1651. https://doi.org/10.3390/genes12111651
Subirana JA, Messeguer X. DNA Satellites Are Transcribed as Part of the Non-Coding Genome in Eukaryotes and Bacteria. Genes. 2021; 12(11):1651. https://doi.org/10.3390/genes12111651
Chicago/Turabian StyleSubirana, Juan A., and Xavier Messeguer. 2021. "DNA Satellites Are Transcribed as Part of the Non-Coding Genome in Eukaryotes and Bacteria" Genes 12, no. 11: 1651. https://doi.org/10.3390/genes12111651
APA StyleSubirana, J. A., & Messeguer, X. (2021). DNA Satellites Are Transcribed as Part of the Non-Coding Genome in Eukaryotes and Bacteria. Genes, 12(11), 1651. https://doi.org/10.3390/genes12111651





