Genetic, Genomics, and Responses to Stresses in Cyanobacteria: Biotechnological Implications
Abstract
1. Introduction
2. Cyanobacteria Being Inevitably Exposed to Photo-Oxidative Stress Have Developed the Evolutionary-Conserved Glutathione System
3. Cyanobacteria Possess Widely Diverse Genomes That Contain a Wealth of Unknown Genes and Poorly Characterized Multigene Families
4. Interest and Current Limitations of Comparative Genomics
4.1. Danger of Genome Annotation Based Only on Sequence Comparison
4.2. Identification of Essential Genes at the Level of a Whole Genome for a Better Understanding of the Genotype-Phenotype Relationships
4.3. Importance of Deciphering the Selectivity/Redundancy of Multiple Gene Families
5. A Few Cyanobacteria Are Currently Amenable to Gene Manipulation, Leaving the Wide Biodiversity of Cyanobacteria Largely Unexplored
6. Properties of the Intensively Studied Unicellular Cyanobacteria Synechocystis PCC 6803, Synechococcus PCC 7942, and Synechococcus PCC 7002
6.1. Genome Organization in the Intensively Studied Unicellular Cyanobacteria Synechocystis PCC 6803, Synechococcus PCC 7942, and Synechococcus PCC 7002
6.2. Physiological Properties of Synechocystis PCC 6803, Synechococcus PCC 7942, and Synechococcus PCC 7002 and Biotechnological Implication
6.3. Comparison of the Stress-Responsive Glutathione and DNA Repair Systems of the Model Cyanobacteria Synechocystis PCC 6803, Synechococcus PCC 7942, and Synechococcus PCC 7002
6.4. Comparative Analysis of the Growth and Response to Stresses of the Three Model Cyanobacteria Synechocystis PCC 6803, Synechococcus PCC 7002, and Synechococcus PCC 7942
6.5. As Observed in Synechocystis PCC 6803, the Sub-Strains of a Single Cyanobacterium Cultivated in Various Laboratories Can Have Different Behaviors
7. Genetic Characteristics of the Model Cyanobacteria Synechocystis PCC 6803, Synechococcus PCC 7942, and Synechococcus PCC 7002
7.1. Synechocystis PCC 6803, Synechococcus PCC 7942, and Synechococcus PCC 7002 Are Naturally Competent for Genetic Transformation
7.2. Interest and Limitation of the Polyploidy of Synechocystis PCC 6803, Synechococcus PCC 7942, and Synechococcus PCC 7002
7.3. Utilization of Neutral Genome Sites for Gene Manipulation in Synechocystis PCC 6803, Synechococcus PCC 7942, and Synechococcus PCC 7002
| Neutral Site and Objective of the Gene Manipulation | References |
|---|---|
| psbA1: a silent gene | [235] |
| Photoproduction of extra bicarbonate transporters to increase biomass | [265] |
| Increase carbon import to improve growth | [266] |
| Photoproduction of isobutanol | [267] |
| psbA2 (slr1311): gene encoding the D1 protein of the PSII | [259] |
| Photoproduction of zeaxanthin | [268] |
| Analysis of a thioredoxin-interacting LuxR-like regulator | [269] |
| Photoproduction of beta-caryophyllene | [270] |
| Photoproduction of D1 protein of the PSII | [271] |
| Photoproduction of polyhydroxybutyrate (PHB) biodegradable bioplastics | [272] |
| Photoproduction of isoprene | [273,274,275,276,277] |
| Photoproduction of lipids | [278] |
| Photoproduction of aromatic amino-acids | [279] |
| Photoproduction of tryptophan | [280] |
| Analysis of endogenous flavodiiron proteins | [281,282] |
| cpcB: phycocyanin synthesis gene | |
| Photoproduction of isoprene | [283,284] |
| Photoproduction of geranyllinalool | [285] |
| glpK, the gene encoding the glycerol kinase enzyme | |
| Photoproduction of ipid | [286] |
| Combination psbA2 and cpcB | |
| Cloning of various genes for the photoproduction of isoprene | [287] |
| Photoproduction of β-phellandrene | [288,289,290,291,292,293] |
| slr0646 encoding the PBP5 dispensable penicillin binding protein | [294] |
| Photoproduction of the p-coumaric acid | [295] |
| ndhB (sll0223): encoding subunit 2 of the NAD(P)H-dehydrogenase | |
| Analysis of the circadian expression of the DnaK (heat-shock protein) encoding gene | [296] |
| Construction of bioluminescent reporter strains for metal detection | [297] |
| Analysis of the light regulation of the photosystem I genes | [298,299] |
| slr0168 | [220] |
| Analysis of tolerance to stresses | [300,301,302] |
| Analysis of endogenous and heterologous Fe- or Cu/Zn superoxide dismutase | [302] |
| Promoter analysis | [303] |
| Analysis of fatty-acids synthesis | [304] |
| Photoproduction of ethylene | [305,306] |
| Photoproduction of lactate | [307,308,309,310] |
| Photoproduction of 2,3-butanediol | [311] |
| Photoproduction of sucrose | [312] |
| Development of a marker-less gene replacement tool | [313] |
| Photoproduction of fatty alcohol | [314] |
| Photoproduction of isoprene | [275] |
| Photoproduction of ethanol | [315,316,317] |
| Photoproduction of glycerol | [318] |
| Photoproduction of n-butanol | [319] |
| Analysis of the regulation of Rubisco | [320] |
| Promoter analysis | [321] |
| Photoproduction of 1,2-propanediol | [322] |
| Analysis of alka(e)ne turnover | [323] |
| Photoproduction of mannitol | [324] |
| Photoproduction of bisabolene | [325] |
| slr0168 and slr1193 | |
| Photoproduction of ethanol | [326] |
| slr0168 and slr1556 | |
| Photoproduction of alkanes | [327] |
| ndhB and slr0168 | |
| Photoproduction of ethanol | [315] |
| psbA1 and slr0168 | |
| Analysis of promoters | [328] |
| psbA2 and slr0168 | |
| Photoproduction of fatty-acids | [329] |
| Analysis of the cyanobacterial iron superoxide dismutase SOD | [302] |
| Photoproduction of ethylene | [330] |
| phaCE genes operating in the synthesis of polyhydroxybutyrate (PHB) biodegradable bioplastics | [331] |
| Photoproduction of acetone | [332] |
| Intergenic region between slr2030 andslr2031 | |
| Analysis of glutathione synthesis | [333] |
| Analysis of heme oxygenase encoding genes | [334] |
| Photoproduction of poly-hydroxybutyrate (PHB) biodegradable bioplastics | [335] |
| Analysis of Flv3 flavodiiron protein | [336] |
| Photoproduction of pinene | [337] |
| Analysis of promoters and ribosome binding sites | [338] |
| Development of the CRISPR technologies for gene deletion or silencing | [339] |
| Photoproduction of ethylene | [340] |
| slr0846 and slr2030-slr2031 intergenic region | |
| Photoproduction of glutamate, linalool, and valencene | [341] |
| Intergenic regions between sll0821-slr0846 and slr2030-slr2031 | |
| Photoproduction of limonene | [342] |
| slr0168 and slr1704-sll1575 intergenic region | |
| Photoproduction of 3-hydroxypropionic acid | [343] |
| slr0168, psbA2, and slr2030-Slr2031 intergenic region | |
| Photoproduction of the manoyl oxide terpene | [344] |
| Photoproduction of ethanol and butanol | [345] |
| Intergenic regions between slr1495-sll1397, slr1362-sll1274, slr1828-sll1736, and slr1992-phaA2 | |
| Photoproduction of 3-hydroxybutyrate the precursor of the synthesis of PHB | [346] |
| Neutral Site and Objective of the Gene Manipulation | References |
|---|---|
| NSI | |
| Photoproduction of 1,2-propanediol | [347] |
| Photoproduction of glycerol | [348] |
| Photoproduction of lactate | [349] |
| Photoproduction of succinate | [350] |
| Photoproduction of ethylene | [351] |
| Photoproduction of ethanol | [352] |
| NSII | |
| Photoproduction of free fatty acids | [353,354,355] |
| Photoproduction of B12 vitamin | [356] |
| NSIII | |
| Photoproduction of isobutyraldehyde | [357] |
| Photoproduction of carboxysome proteins | [358] |
| NSI and NSII | |
| Analysis of the circadian rhythm | [359] |
| Photoproduction of isobutyraldehyde | [360] |
| Photoproduction of 1-butanol | [361,362] |
| Analysis of carboxysomes | [363,364] |
| Photoproduction of isopropanol | [364] |
| Photoproduction of isobutanol | [365] |
| Photoproduction of 3-hydroxypropionic acid | [366] |
| Photoproduction of 1,3-propanediol | [367] |
| Photoproduction of amorphadiene and squalene | [368] |
| Photoproduction of limonene | [369] |
| Photoproduction of acetone | [370] |
| Photoproduction of isoprene | [371] |
| Analysis of gene-expression control systems | [372] |
| Photoproduction of 2,3-butanediol | [373] |
| Photoproduction of farnesene | [374,375] |
| Photoproduction of lactate | [376] |
| NSI and NSIII | |
| Photoproduction of biomass and sucrose export | [377] |
| Photoproduction of a synthetic CO2-fixing photorespiratory bypass | [378] |
| Photoproduction of 2,3 butanediol | [379] |
| Photoproduction of amorphadiene or squalene | [380] |
| NSI, NSII, and NSIII | |
| Overproduction of transporters to facilitate sugar export | [254] |
| Promoter analysis | [381] |
| Photoproduction of polyketides | [382] |
| Analysis of the influence of pilus biogenesis on the natural transformation | [229] |
| psbA1 | |
| Photoproduction of ethylene | [383] |
| glgc | |
| Photoproduction of isobutanol | [366] |
| Intergenic region Synpcc7942_0893 and Synpcc7942_0894 | |
| Photoproduction of 2,3-butanediol | [384] |
| Neutral Cloning Sites | Objective of the Gene Manipulation and References |
|---|---|
| glpK (SYNPCC7002_A2842) encoding the glycerol kinase [385,386] | Removal of carboxysomes for containment of genetically modified strains [387] |
| acsA gene (SYNPCC7002_A1838) encoding an acetyl-CoA ligase and glpK | Analysis of an organic acid-based counter selection system [386] |
| acsA and glpK | Analysis of promoters and ribosome binding sites [388] |
| Integration between SYNPCC7002_A0935 and SYNPCC7002_A0936 | Photoproduction of bisabolene and limonene [388] |
| glpK and desB (SYNPCC7002_ A0159 encoding a ω3 acyl-lipid desaturase [389] and integration between SYNPCC7002_A0935 and SYNPCC7002_A0936 | Development of genetic tools [390] |
| glpK and integration between A0935-A0936 | Engineering a strain for melamine degradation [391] |
| Intergenic regions between SYNPCC7002_A0932 and SYNPCC7002_A0933, SYNPCC7002_A1202 and SYNPCC7002_A1203, SYNPCC7002_A1778 and SYNPCC7002_A1779 | Development of genetic tools [257] |
| SYNPCC7002_A1838, SYNPCC7002_A2542, and SYNPC- C7002_A2842 | Photoproduction of L-lysine [392] |
7.4. Development of Transformable Shuttle Vectors Based on the Endogenous Plasmids of Synechocystis PCC 6803, Synechococcus PCC 7942, and Synechococcus PCC 7002
7.5. The Chimeric Shuttle Vectors Based on an Endogenous Cyanobacterial Plasmid Tend to Have a Narrow-Host-Range of Replication
7.6. The Development of Autonomously Replicating Vectors Derived from the Broad-Host-Range Conjugative Plasmid RSF1010 Has Boosted the Genetics of Cyanobacteria
8. Interest and Limitation of the CRISPR/Cas Genome Editing Technology
9. Responses to Stresses: The Recent Progress in Omics Technics Are Limited by the Large Number of Genes of Still Unknown Function
10. Conclusions
- (i)
- verify the function of numerous genes that have been annotated merely by sequence analogy with those genes characterized only in intensively studied non-photosynthetic models (E. coli, yeast, etc.), which may have a different function in cyanobacteria;
- (ii)
- and analyze the specificity/redundancy of multiple gene families;
- (iii)
- characterize the function of the large number of as yet unknown genes and non-coding RNAs;
- (iv)
- identify the comprehensive set of genes that are essential to the growth of cells incubated in well-defined conditions.
Author Contributions
Funding
Conflicts of Interest
References
- Soo, R.M.; Hemp, J.; Hugenholtz, P. Evolution of photosynthesis and aerobic respiration in the cyanobacteria. Free Radic. Biol. Med. 2019, 140, 200–205. [Google Scholar] [CrossRef]
- Garcia-Pichel, F.; Zehr, J.P.; Bhattacharya, D.; Pakrasi, H.B. What’s in a name? The case of cyanobacteria. J. Phycol. 2020, 56, 1–5. [Google Scholar] [CrossRef]
- Hamilton, T.L.; Bryant, D.A.; Macalady, J.L. The role of biology in planetary evolution: Cyanobacterial primary production in low-oxygen Proterozoic oceans. Environ. Microbiol. 2016, 18, 325–340. [Google Scholar] [CrossRef]
- Ponce-Toledo, R.I.; Deschamps, P.; López-García, P.; Zivanovic, Y.; Benzerara, K.; Moreira, D. An Early-Branching Freshwater Cyanobacterium at the Origin of Plastids. Curr. Biol. 2017, 27, 386–391. [Google Scholar] [CrossRef]
- Ducat, D.C.; Way, J.C.; Silver, P.A. Engineering cyanobacteria to generate high-value products. Trends Biotechnol. 2011, 29, 95–103. [Google Scholar] [CrossRef]
- Veaudor, T.; Blanc-Garin, V.; Chenebault, C.; Diaz-Santos, E.; Sassi, J.F.; Cassier-Chauvat, C.; Chauvat, F. Recent advances in the photoautotrophic metabolism of cyanobacteria: Biotechnological implications. Life 2020, 10, 71. [Google Scholar] [CrossRef] [PubMed]
- Mills, L.A.; McCormick, A.J.; Lea-Smith, D.J. Current knowledge and recent advances in understanding metabolism of the model cyanobacterium Synechocystis sp. PCC 6803. Biosci. Rep. 2020, 40. [Google Scholar] [CrossRef]
- Garcia-Pichel, F.; Belnap, J.; Neuer, S.; Schanz, F. Estimates of global cyanobacterial biomass and its distribution. Arch. Hydrobiol. Suppl. Algol. Stud. 2009, 109, 213–227. [Google Scholar] [CrossRef]
- Chang, A.C.G.; Chen, T.; Li, N.; Duan, J. Perspectives on endosymbiosis in coralloid roots: Association of cycads and cyanobacteria. Front. Microbiol. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Cassier-Chauvat, C.; Chauvat, F. Cyanobacteria: Wonderful Microorganisms for Basic and Applied Research. eLS 2018, 1–11. [Google Scholar] [CrossRef]
- Cassier-Chauvat, C.; Chauvat, F. Cell division in cyanobacteria. In The Cell Biology of Cyanobacteria; Flores, E., Herrero, A., Eds.; Caister Academic Press: Poole, UK, 2014. [Google Scholar]
- Springstein, B.L.; Nürnberg, D.J.; Weiss, G.L.; Pilhofer, M.; Stucken, K. Structural determinants and their role in cyanobacterial morphogenesis. Life 2020, 10, 355. [Google Scholar] [CrossRef] [PubMed]
- Chauvat, F.; Corre, B.; Herdman, M.; Joset-Espardellier, F. Energetic and metabolic requirements for the germination of akinetes of the cyanobacterium Nostoc PCC 7524. Arch. Microbiol. 1982, 133, 44–49. [Google Scholar] [CrossRef]
- Herrero, A.; Stavans, J.; Flores, E. The multicellular nature of filamentous heterocyst-forming cyanobacteria. FEMS Microbiol. Rev. 2016, 40, 831–854. [Google Scholar] [CrossRef]
- Dittmann, E.; Gugger, M.; Sivonen, K.; Fewer, D.P. Natural Product Biosynthetic Diversity and Comparative Genomics of the Cyanobacteria. Trends Microbiol. 2015, 23, 642–652. [Google Scholar] [CrossRef]
- Cassier-Chauvat, C.; Dive, V.; Chauvat, F. Cyanobacteria: Photosynthetic factories combining biodiversity, radiation resistance, and genetics to facilitate drug discovery. Appl. Microbiol. Biotechnol. 2017, 101, 1359–1364. [Google Scholar] [CrossRef]
- Demay, J.; Bernard, C.; Reinhardt, A.; Marie, B. Natural products from cyanobacteria: Focus on beneficial activities. Mar. Drugs 2019, 17, 320. [Google Scholar] [CrossRef]
- Pereira, S.B.; Sousa, A.; Santos, M.; Araújo, M.; Serôdio, F.; Granja, P.; Tamagnini, P. Strategies to obtain designer polymers based on cyanobacterial extracellular polymeric substances (EPS). Int. J. Mol. Sci. 2019, 20, 5693. [Google Scholar] [CrossRef]
- Knoot, C.J.; Ungerer, J.; Wangikar, P.P.; Pakrasi, H.B. Cyanobacteria: Promising biocatalysts for sustainable chemical production. J. Biol. Chem. 2018, 293, 5044–5052. [Google Scholar] [CrossRef]
- Lin, P.C.; Pakrasi, H.B. Engineering cyanobacteria for production of terpenoids. Planta 2019, 249, 145–154. [Google Scholar] [CrossRef]
- Schopf, J.W. The paleobiological record of photosynthesis. Photosynth. Res. 2011, 107, 87–101. [Google Scholar] [CrossRef] [PubMed]
- Imlay, J.A. The molecular mechanisms and physiological consequences of oxidative stress: Lessons from a model bacterium. Nat. Rev. Microbiol. 2013, 11, 443–454. [Google Scholar] [CrossRef] [PubMed]
- Shimakawa, G.; Matsuda, Y.; Nakajima, K.; Tamoi, M.; Shigeoka, S.; Miyake, C. Diverse strategies of O2 usage for preventing photo-oxidative damage under CO2 limitation during algal photosynthesis. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Diaz, J.M.; Plummer, S. Production of extracellular reactive oxygen species by phytoplankton: Past and future directions. J. Plankton Res. 2018, 40, 655–666. [Google Scholar] [CrossRef] [PubMed]
- Narainsamy, K.; Marteyn, B.; Sakr, S.; Cassier-Chauvat, C.; Chauvat, F. Genomics of the Pleïotropic Glutathione System in Cyanobacteria. Adv. Bot. Res. 2013, 65, 157–188. [Google Scholar]
- Picciocchi, A.; Saguez, C.; Boussac, A.; Cassier-Chauvat, C.; Chauvat, F. CGFS-type monothiol glutaredoxins from the cyanobacterium Synechocystis PCC6803 and other evolutionary distant model organisms possess a glutathione-ligated [2Fe-2S] cluster. Biochemistry 2007, 46, 15018–15026. [Google Scholar] [CrossRef] [PubMed]
- Fahey, R.C. Glutathione analogs in prokaryotes. Biochim. Biophys. Acta Gen. Subj. 2013, 1830, 3182–3198. [Google Scholar] [CrossRef]
- Cassier-Chauvat, C.; Chauvat, F. Genomics of the Resistance to Metal and Oxidative Stresses in Cyanobacteria. Stress Environ. Regul. Gene Expr. Adapt. Bact. 2016, 2, 1154–1164. [Google Scholar]
- Kammerscheit, X.; Chauvat, F.; Cassier-Chauvat, C. From cyanobacteria to human, MAPEG-type glutathione-S-transferases operate in cell tolerance to heat, cold, and lipid peroxidation. Front. Microbiol. 2019, 10, 2248. [Google Scholar] [CrossRef]
- Berndt, C.; Lillig, C.H. Glutathione, Glutaredoxins, and Iron. Antioxid. Redox Signal. 2017, 27, 1235–1251. [Google Scholar] [CrossRef]
- Narainsamy, K.; Farci, S.; Braun, E.; Junot, C.; Cassier-Chauvat, C.; Chauvat, F. Oxidative-stress detoxification and signalling in cyanobacteria: The crucial glutathione synthesis pathway supports the production of ergothioneine and ophthalmate. Mol. Microbiol. 2016, 100, 15–24. [Google Scholar] [CrossRef]
- Kammerscheit, X.; Chauvat, F.; Cassier-Chauvat, C. First in vivo evidence that glutathione-s-transferase operates in photo-oxidative stress in cyanobacteria. Front. Microbiol. 2019, 10, 1899. [Google Scholar] [CrossRef]
- Noctor, G.; Mhamdi, A.; Chaouch, S.; Han, Y.; Neukermans, J.; Marquez-Garcia, B.; Queval, G.; Foyer, C.H. Glutathione in plants: An integrated overview. Plant Cell Environ. 2012, 35, 454–484. [Google Scholar] [CrossRef] [PubMed]
- Marteyn, B.; Sakr, S.; Farci, S.; Bedhomme, M.; Chardonnet, S.; Decottignies, P.; Lemaire, S.D.; Cassier-Chauvat, C.; Chauvat, F. The Synechocystis PCC6803 MerA-like enzyme operates in the reduction of both mercury and uranium under the control of the glutaredoxin 1 enzyme. J. Bacteriol. 2013, 195, 4138–4145. [Google Scholar] [CrossRef] [PubMed]
- Cassier-Chauvat, C.; Chauvat, F. Responses to oxidative and heavy metal stresses in cyanobacteria: Recent advances. Int. J. Mol. Sci. 2015, 16, 871. [Google Scholar] [CrossRef] [PubMed]
- Thorsen, M.; Jacobson, T.; Vooijs, R.; Navarrete, C.; Bliek, T.; Schat, H.; Tamás, M.J. Glutathione serves an extracellular defence function to decrease arsenite accumulation and toxicity in yeast. Mol. Microbiol. 2012, 84, 1177–1188. [Google Scholar] [CrossRef] [PubMed]
- Przybyla-Toscano, J.; Roland, M.; Gaymard, F.; Couturier, J.; Rouhier, N. Roles and maturation of iron–sulfur proteins in plastids. J. Biol. Inorg. Chem. 2018, 23, 545–566. [Google Scholar] [CrossRef]
- Daniel, T.; Faruq, H.M.; Laura Magdalena, J.; Manuela, G.; Christopher Horst, L. Role of GSH and Iron-Sulfur Glutaredoxins in Iron Metabolism-Review. Molecules 2020, 27, 3860. [Google Scholar] [CrossRef]
- Mailloux, R.J. Protein S-glutathionylation reactions as a global inhibitor of cell metabolism for the desensitization of hydrogen peroxide signals. Redox Biol. 2020, 32. [Google Scholar] [CrossRef]
- Chardonnet, S.; Sakr, S.; Cassier-Chauvat, C.; Le Maréchal, P.; Chauvat, F.; Lemaire, S.D.; Decottignies, P. First proteomic study of S-glutathionylation in cyanobacteria. J. Proteome Res. 2015, 14, 59–71. [Google Scholar] [CrossRef]
- Sakr, S.; Dutheil, J.; Saenkham, P.; Bottin, H.; Leplat, C.; Ortega-Ramos, M.; Aude, J.C.; Chapuis, V.; Guedeney, G.; Decottignies, P.; et al. The activity of the Synechocystis PCC6803 AbrB2 regulator of hydrogen production can be post-translationally controlled through glutathionylation. Int. J. Hydrog. Energy 2013, 38, 13547–13555. [Google Scholar] [CrossRef]
- Gallé, Á.; Czékus, Z.; Bela, K.; Horváth, E.; Ördög, A.; Csiszár, J.; Poór, P. Plant glutathione transferases and light. Front. Plant Sci. 2019, 9. [Google Scholar] [CrossRef] [PubMed]
- Sylvestre-Gonon, E.; Law, S.R.; Schwartz, M.; Robe, K.; Keech, O.; Didierjean, C.; Dubos, C.; Rouhier, N.; Hecker, A. Functional, structural and biochemical features of plant serinyl-glutathione transferases. Front. Plant Sci. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Couto, N.; Wood, J.; Barber, J. The role of glutathione reductase and related enzymes on cellular redox homoeostasis network. Free Radic. Biol. Med. 2016, 95, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Marteyn, B.; Domain, F.; Legrain, P.; Chauvat, F.; Cassier-Chauvat, C. The thioredoxin reductase-glutaredoxins-ferredoxin crossroad pathway for selenate tolerance in Synechocystis PCC6803. Mol. Microbiol. 2009, 71, 520–532. [Google Scholar] [CrossRef] [PubMed]
- Fleischmann, R.D.; Adams, M.D.; White, O.; Clayton, R.A.; Kirkness, E.F.; Kerlavage, A.R.; Bult, C.J.; Tomb, J.F.; Dougherty, B.A.; Merrick, J.M.; et al. Whole-genome random sequencing and assembly of Haemophilus influenzae Rd. Science 1995, 269, 496–512. [Google Scholar] [CrossRef]
- Fraser, C.M.; Gocayne, J.D.; White, O.; Adams, M.D.; Clayton, R.A.; Fleischmann, R.D.; Bult, C.J.; Kerlavage, A.R.; Sutton, G.; Kelley, J.M.; et al. The minimal gene complement of Mycoplasma genitalium. Science 1995, 270, 397–403. [Google Scholar] [CrossRef]
- Kaneko, T.; Sato, S.; Kotani, H.; Tanaka, A.; Asamizu, E.; Nakamura, Y.; Miyajima, N.; Hirosawa, M.; Sugiura, M.; Sasamoto, S.; et al. Sequence analysis of the genome of the unicellular cyanobacterium Synechocystis sp. strain PCC6803. II. Sequence determination of the entire genome and assignment of potential protein-coding regions. DNA Res. 1996, 3, 109–136. [Google Scholar] [CrossRef]
- Cao, H.; Shimura, Y.; Steffen, M.M.; Yang, Z.; Lu, J.; Joel, A.; Jenkins, L.; Kawachi, M.; Yin, Y.; Garcia-Pichel, F. The trait repertoire enabling cyanobacteria to bloom assessed through comparative genomic complexity and metatranscriptomics. MBio 2020, 11. [Google Scholar] [CrossRef]
- Fernandez, L.; Peura, S.; Eiler, A.; Linz, A.M.; McMahon, K.D.; Bertilsson, S. Diazotroph Genomes and Their Seasonal Dynamics in a Stratified Humic Bog Lake. Front. Microbiol. 2020, 11. [Google Scholar] [CrossRef]
- Grettenberger, C.L.; Sumner, D.Y.; Wall, K.; Brown, C.T.; Eisen, J.A.; Mackey, T.J.; Hawes, I.; Jospin, G.; Jungblut, A.D. A phylogenetically novel cyanobacterium most closely related to Gloeobacter. ISME J. 2020, 14, 2142–2152. [Google Scholar] [CrossRef]
- Salazar, V.W.; Tschoeke, D.A.; Swings, J.; Cosenza, C.A.; Mattoso, M.; Thompson, C.C.; Thompson, F.L. A new genomic taxonomy system for the Synechococcus collective. Environ. Microbiol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Tripp, H.J.; Bench, S.R.; Turk, K.A.; Foster, R.A.; Desany, B.A.; Niazi, F.; Affourtit, J.P.; Zehr, J.P. Metabolic streamlining in an open-ocean nitrogen-fixing cyanobacterium. Nature 2010, 464, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Dagan, T.; Roettger, M.; Stucken, K.; Landan, G.; Koch, R.; Major, P.; Gould, S.B.; Goremykin, V.V.; Rippka, R.; De Marsac, N.T.; et al. Genomes of stigonematalean cyanobacteria (subsection V) and the evolution of oxygenic photosynthesis from prokaryotes to plastids. Genome Biol. Evol. 2013, 5, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Larsson, J.; Nylander, J.A.A.; Bergman, B. Genome fluctuations in cyanobacteria reflect evolutionary, developmental and adaptive traits. BMC Evol. Biol. 2011, 11. [Google Scholar] [CrossRef] [PubMed]
- Cassier-Chauvat, C.; Poncelet, M.; Chauvat, F. Three insertion sequences from the cyanobacterium Synechocystis PCC6803 support the occurrence of horizontal DNA transfer among bacteria. Gene 1997, 195, 257–266. [Google Scholar] [CrossRef]
- Zhaxybayeva, O.; Gogarten, J.P.; Charlebois, R.L.; Doolittle, W.F.; Papke, R.T. Phylogenetic analyses of cyanobacterial genomes: Quantification of horizontal gene transfer events. Genome Res. 2006, 16, 1099–10108. [Google Scholar] [CrossRef]
- Cassier-Chauvat, C.; Veaudor, T.; Chauvat, F. Comparative genomics of DNA recombination and repair in cyanobacteria: Biotechnological implications. Front. Microbiol. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Godde, J.S.; Baichoo, S.; Mungloo-Dilmohamud, Z.; Jaufeerally-Fakim, Y. Comparison of genomic islands in cyanobacteria: Evidence of bacteriophage-mediated horizontal gene transfer from eukaryotes. Microbiol. Res. 2018, 211, 31–46. [Google Scholar] [CrossRef]
- Zeidner, G.; Bielawski, J.P.; Shmoish, M.; Scanlan, D.J.; Sabehi, G.; Béjà, O. Potential photosynthesis gene recombination between Prochlorococcus and Synechococcus via viral intermediates. Environ. Microbiol. 2005, 7, 1505–1513. [Google Scholar] [CrossRef]
- Scanlan, D.J.; Ostrowski, M.; Mazard, S.; Dufresne, A.; Garczarek, L.; Hess, W.R.; Post, A.F.; Hagemann, M.; Paulsen, I.; Partensky, F. Ecological Genomics of Marine Picocyanobacteria. Microbiol. Mol. Biol. Rev. 2009, 73, 249–299. [Google Scholar] [CrossRef]
- Nicholson, M.L.; Gaasenbeek, M.; Laudenbach, D.E. Two enzymes together capable of cysteine biosynthesis are encoded on a cyanobacterial plasmid. MGG Mol. Gen. Genet. 1995, 247, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Kimura, A.; Hamada, T.; Morita, E.H.; Hayashi, H. A high temperature-sensitive mutant of Synechococcus sp. PCC 7002 with modifications in the endogenous plasmid, pAQ1. Plant Cell Physiol. 2002, 43, 217–223. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nagarajan, S.; Sherman, D.M.; Shaw, I.; Sherman, L.A. Functions of the duplicated hik31 operons in central metabolism and responses to light, dark, and carbon sources in Synechocystis sp. strain PCC 6803. J. Bacteriol. 2012, 194, 448–459. [Google Scholar] [CrossRef] [PubMed]
- Swingley, W.D.; Chen, M.; Cheung, P.C.; Conrad, A.L.; Dejesa, L.C.; Hao, J.; Honchak, B.M.; Karbach, L.E.; Kurdoglu, A.; Lahiri, S.; et al. Niche adaptation and genome expansion in the chlorophyll d-producing cyanobacterium Acaryochloris marina. Proc. Natl. Acad. Sci. USA 2008, 105, 2005–2010. [Google Scholar] [CrossRef] [PubMed]
- Welsh, E.A.; Liberton, M.; Stöckel, J.; Loh, T.; Elvitigala, T.; Wang, C.; Wollam, A.; Fulton, R.S.; Clifton, S.W.; Jacobs, J.M.; et al. The genome of Cyanothece 51142, a unicellular diazotrophic cyanobacterium important in the marine nitrogen cycle. Proc. Natl. Acad. Sci. USA 2008, 105, 15094–15099. [Google Scholar] [CrossRef]
- Bandyopadhyay, A.; Elvitigala, T.; Welsh, E.; Stöckel, J.; Liberton, M.; Min, H.; Sherman, L.A.; Pakrasi, H.B. Novel metabolic attributes of the genus Cyanothece, comprising a group of unicellular nitrogen-fixing cyanobacteria. MBio 2011, 2. [Google Scholar] [CrossRef]
- Quinn, G.A.; Banat, A.M.; Abdelhameed, A.M.; Banat, I.M. Streptomyces from traditional medicine: Sources of new innovations in antibiotic discovery. J. Med. Microbiol. 2020, 69, 1040–1048. [Google Scholar] [CrossRef]
- Ahmed, M.N.; Reyna-González, E.; Schmid, B.; Wiebach, V.; Süssmuth, R.D.; Dittmann, E.; Fewer, D.P. Phylogenomic Analysis of the Microviridin Biosynthetic Pathway Coupled with Targeted Chemo-Enzymatic Synthesis Yields Potent Protease Inhibitors. ACS Chem. Biol. 2017, 12, 1538–1546. [Google Scholar] [CrossRef]
- Hoff, G.; Bertrand, C.; Piotrowski, E.; Thibessard, A.; Leblond, P. Genome plasticity is governed by double strand break DNA repair in Streptomyces. Sci. Rep. 2018, 8. [Google Scholar] [CrossRef]
- Labella, J.I.; Llop, A.; Contreras, A. The default cyanobacterial linked genome: An interactive platform based on cyanobacterial linkage networks to assist functional genomics. FEBS Lett. 2020, 594, 1661–1674. [Google Scholar] [CrossRef]
- Simm, S.; Keller, M.; Selymesi, M.; Schleiff, E. The composition of the global and feature specific cyanobacterial core-genomes. Front. Microbiol. 2015, 6. [Google Scholar] [CrossRef]
- Alvarenga, D.O.; Fiore, M.F.; Varani, A.M. A metagenomic approach to cyanobacterial genomics. Front. Microbiol. 2017. [Google Scholar] [CrossRef]
- Gonzalez-Esquer, C.R.; Smarda, J.; Rippka, R.; Axen, S.D.; Guglielmi, G.; Gugger, M.; Kerfeld, C.A. Cyanobacterial ultrastructure in light of genomic sequence data. Photosynth. Res. 2016, 192, 147–157. [Google Scholar] [CrossRef]
- Shih, P.M.; Wu, D.; Latifi, A.; Axen, S.D.; Fewer, D.P.; Talla, E.; Calteau, A.; Cai, F.; Tandeau de Marsac, N.; Rippka, R.; et al. Improving the coverage of the cyanobacterial phylum using diversity-driven genome sequencing. Proc. Natl. Acad. Sci. USA 2013, 110, 1053–1058. [Google Scholar] [CrossRef] [PubMed]
- Harel, A.; Karkar, S.; Cheng, S.; Falkowski, P.G.; Bhattacharya, D. Deciphering primordial cyanobacterial genome functions from protein network analysis. Curr. Biol. 2015, 25, 628–634. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Baracaldo, P.; Cardona, T. On the origin of oxygenic photosynthesis and Cyanobacteria. New Phytol. 2020, 225, 1440–1446. [Google Scholar] [CrossRef]
- Ponce-Toledo, R.I.; López-García, P.; Moreira, D. Horizontal and endosymbiotic gene transfer in early plastid evolution. New Phytol. 2019, 224, 618–624. [Google Scholar] [CrossRef] [PubMed]
- Kopf, M.; Klähn, S.; Pade, N.; Weingärtner, C.; Hagemann, M.; Voß, B.; Hess, W.R. Comparative genome analysis of the closely related Synechocystis strains PCC 6714 and PCC 6803. DNA Res. 2014, 21, 255–266. [Google Scholar] [CrossRef]
- Gale, G.A.R.; Osorio, A.A.S.; Mills, L.A.; Wang, B.; Lea-Smith, D.J.; McCormick, A.J. Emerging species and genome editing tools: Future prospects in cyanobacterial synthetic biology. Microorganisms 2019, 7, 409. [Google Scholar] [CrossRef]
- Janasch, M.; Asplund-Samuelsson, J.; Steuer, R.; Hudson, E.P. Kinetic modeling of the Calvin cycle identifies flux control and stable metabolomes in Synechocystis carbon fixation. J. Exp. Bot. 2019, 70, 973–983. [Google Scholar] [CrossRef]
- Lasry Testa, R.; Delpino, C.; Estrada, V.; Diaz, S.M. In silico strategies to couple production of bioethanol with growth in cyanobacteria. Biotechnol. Bioeng. 2019, 116, 2061–2073. [Google Scholar] [CrossRef]
- Khan, A.Z.; Bilal, M.; Mehmood, S.; Sharma, A.; Iqbal, H.M.N. State-of-the-art genetic modalities to engineer cyanobacteria for sustainable biosynthesis of biofuel and fine-chemicals to meet bio–economy challenges. Life 2019, 9, 54. [Google Scholar] [CrossRef] [PubMed]
- Santos-Merino, M.; Singh, A.K.; Ducat, D.C. New applications of synthetic biology tools for cyanobacterial metabolic engineering. Front. Bioeng. Biotechnol. 2019, 7. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Jiang, H.; Kalra, I.; Wang, X.; Cano, M.; Maness, P.C.; Yu, J.; Xiong, W. A generalized computational framework to streamline thermodynamics and kinetics analysis of metabolic pathways. Metab. Eng. 2020, 57, 140–150. [Google Scholar] [CrossRef]
- Hendry, J.I.; Bandyopadhyay, A.; Srinivasan, S.; Pakrasi, H.B.; Maranas, C.D. Metabolic model guided strain design of cyanobacteria. Curr. Opin. Biotechnol. 2020, 64, 17–23. [Google Scholar] [CrossRef]
- Mukherjee, B.; Madhu, S.; Wangikar, P.P. The role of systems biology in developing non-model cyanobacteria as hosts for chemical production. Curr. Opin. Biotechnol. 2020, 64, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Bender, R.A. The danger of annotation by analogy: Most “thii” genes play no role in thiamine biosynthesis. J. Bacteriol. 2011, 193, 4574–4575. [Google Scholar] [CrossRef]
- Fujisawa, T.; Narikawa, R.; Maeda, S.I.; Watanabe, S.; Kanesaki, Y.; Kobayashi, K.; Nomata, J.; Hanaoka, M.; Watanabe, M.; Ehira, S.; et al. CyanoBase: A large-scale update on its 20th anniversary. Nucleic Acids Res. 2017, 45, D551–D554. [Google Scholar] [CrossRef]
- Domain, F.; Houot, L.; Chauvat, F.; Cassier-Chauvat, C. Function and regulation of the cyanobacterial genes lexA, recA and ruvB: LexA is critical to the survival of cells facing inorganic carbon starvation. Mol. Microbiol. 2004, 53, 65–80. [Google Scholar] [CrossRef]
- Figge, R.M.; Cassier-Chauvat, C.; Chauvat, F.; Cerff, R. Characterization and analysis of an NAD(P)H dehydrogenase transcriptional regulator critical for the survival of cyanobacteria facing inorganic carbon starvation and osmotic stress. Mol. Microbiol. 2001, 39, 455–468. [Google Scholar] [CrossRef]
- Jiang, Y.L.; Wang, X.P.; Sun, H.; Han, S.J.; Li, W.F.; Cui, N.; Lin, G.M.; Zhang, J.Y.; Cheng, W.; Cao, D.D.; et al. Coordinating carbon and nitrogen metabolic signaling through the cyanobacterial global repressor NdhR. Proc. Natl. Acad. Sci. USA 2017, 115, 403–408. [Google Scholar] [CrossRef]
- Forchhammer, K.; Selim, K.A. Carbon/nitrogen homeostasis control in cyanobacteria. FEMS Microbiol. Rev. 2019, 44, 33–53. [Google Scholar] [CrossRef]
- Cotton, C.A.R.; Kabasakal, B.V.; Miah, N.A.; Murray, J.W. Structure of the dual-function fructose-1,6/sedoheptulose-1,7-bisphosphatase from Thermosynechococcus elongatus bound with sedoheptulose-7-phosphate. Acta Crystallogr. Sect. Struct. Biol. Commun. 2015, 71, 1341–1345. [Google Scholar] [CrossRef]
- Mohamed, H.E.; Vermaas, W.F.J. S110254 (CrtLdiox) is a bifunctional lycopene cyclase/dioxygenase in cyanobacteria producing myxoxanthophyll. J. Bacteriol. 2006, 188, 3337–3344. [Google Scholar] [CrossRef][Green Version]
- Yeremenko, N.; Kouřil, R.; Ihalainen, J.A.; D’Haene, S.; Van Oosterwijk, N.; Andrizhiyevskaya, E.G.; Keegstra, W.; Dekker, H.L.; Hagemann, M.; Boekema, E.J.; et al. Supramolecular organization and dual function of the IsiA chlorophyll-binding protein in cyanobacteria. Biochemistry 2004, 43, 10308–10313. [Google Scholar] [CrossRef] [PubMed]
- Burnat, M.; Picossi, S.; Valladares, A.; Herrero, A.; Flores, E. Catabolic pathway of arginine in Anabaena involves a novel bifunctional enzyme that produces proline from arginine. Mol. Microbiol. 2019, 111, 883–897. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Rhee, S. Structural and mutational analyses of the bifunctional arginine dihydrolase and ornithine cyclodeaminase AgrE from the cyanobacterium Anabaena. J. Biol. Chem. 2020, 295, 5751–5760. [Google Scholar] [CrossRef]
- Phogosee, S.; Hibino, T.; Kageyama, H.; Waditee-Sirisattha, R. Bifunctional alanine dehydrogenase from the halotolerant cyanobacterium Aphanothece halophytica: Characterization and molecular properties. Arch. Microbiol. 2018, 200, 719–727. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Noël, G.M.A.; Cumino, A.C.; De Los Angeles Kolman, M.; Salerno, G.L. First evidence of sucrose biosynthesis by single cyanobacterial bimodular proteins. FEBS Lett. 2013, 587, 1669–1674. [Google Scholar] [CrossRef]
- Jakopitsch, C.; Regelsberger, G.; Furtmüller, P.G.; Rüker, F.; Peschek, G.A.; Obinger, C. Catalase-peroxidase from Synechocystis is capable of chlorination and bromination reactions. Biochem. Biophys. Res. Commun. 2001, 287, 682–687. [Google Scholar] [CrossRef]
- Jakopitsch, C.; Auer, M.; Ivancich, A.; Rüker, F.; Furtmüller, P.G.; Obinger, C. Total conversion of bifunctional catalase-peroxidase (KatG) to monofunctional peroxidase by exchange of a conserved distal side tyrosine. J. Biol. Chem. 2003, 278, 20185–20191. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Laborde, S.M.; Frankel, L.K.; Bricker, T.M. Four Novel Genes Required for Optimal Photoautotrophic Growth of the Cyanobacterium Synechocystis sp. Strain PCC 6803 Identified by In Vitro Transposon Mutagenesis. J. Bacteriol. 2004, 186, 875–879. [Google Scholar] [CrossRef]
- Terauchi, K.; Sobue, R.; Furutani, Y.; Aoki, R.; Fujita, Y. Isolation of cyanobacterial mutants exhibiting growth defects under microoxic conditions by transposon tagging mutagenesis of Synechocystis sp. PCC 6803. J. Gen. Appl. Microbiol. 2017, 63, 131–138. [Google Scholar] [CrossRef]
- Katayama, M.; Tsinoremas, N.F.; Kondo, T.; Golden, S.S. cpmA, a gene involved in an output pathway of the cyanobacterial circadian system. J. Bacteriol. 1999, 181, 3516–3524. [Google Scholar] [CrossRef] [PubMed]
- Miyagishima, S.Y.; Wolk, P.P.; Osteryoung, K.W. Identification of cyanobacterial cell division genes by comparative and mutational analyses. Mol. Microbiol. 2005, 56, 126–143. [Google Scholar] [CrossRef] [PubMed]
- Watabe, K.; Mimuro, M.; Tsuchiya, T. Development of a high-frequency in vivo transposon mutagenesis system for Synechocystis sp. PCC 6803 and Synechococcus elongatus PCC 7942. Plant Cell Physiol. 2014, 55, 2017–2028. [Google Scholar] [CrossRef] [PubMed]
- Rubin, B.E.; Wetmore, K.M.; Price, M.N.; Diamond, S.; Shultzaberger, R.K.; Lowe, L.C.; Curtin, G.; Arkin, A.P.; Deutschbauer, A.; Golden, S.S. The essential gene set of a photosynthetic organism. Proc. Natl. Acad. Sci. USA 2015, 112. [Google Scholar] [CrossRef]
- Ozaki, H.; Ikeuchi, M.; Ogawa, T.; Fukuzawa, H.; Sonoike, K. Large-scale analysis of chlorophyll fluorescence kinetics in Synechocystis sp. PCC 6803: Identification of the factors involved in the modulation of photosystem stoichiometry. Plant Cell Physiol. 2007, 48, 451–458. [Google Scholar] [CrossRef]
- Kato, K.; Tanaka, R.; Sano, S.; Tanaka, A.; Hosaka, H. Identification of a gene essential for protoporphyrinogen IX oxidase activity in the cyanobacterium Synechocystis sp. PCC6803. Proc. Natl. Acad. Sci. USA 2010, 107, 16649–16654. [Google Scholar] [CrossRef]
- Zhang, S.; Frankel, L.K.; Bricker, T.M. The Sll0606 protein is required for photosystem II assembly/stability in the cyanobacterium Synechocystis sp. PCC 6803. J. Biol. Chem. 2010, 285, 32047–32054. [Google Scholar] [CrossRef]
- Shibata, M.; Ohkawa, H.; Kaneko, T.; Fukuzawa, H.; Tabata, S.; Kaplan, A.; Ogawa, T. Distinct constitutive and low-CO2-induced CO2 uptake systems in cyanobacteria: Genes involved and their phylogenetic relationship with homologous genes in other organisms. Proc. Natl. Acad. Sci. USA 2001, 98, 11789–11794. [Google Scholar] [CrossRef]
- Shibata, M.; Katoh, H.; Sonoda, M.; Ohkawa, H.; Shimoyama, M.; Fukuzawa, H.; Kaplan, A.; Ogawa, T. Genes essential to sodium-dependent bicarbonate transport in cyanobacteria: Function and phylogenetic analysis. J. Biol. Chem. 2002, 277, 18658–18664. [Google Scholar] [CrossRef]
- Tyo, K.E.J.; Espinoza, F.A.; Stephanopoulos, G.; Jin, Y.S. Identification of gene disruptions for increased poly-3-hydroxybutyrate accumulation in Synechocystis PCC 6803. Biotechnol. Prog. 2009, 25, 1236–1243. [Google Scholar] [CrossRef] [PubMed]
- Battchikova, N.; Wei, L.; Du, L.; Bersanini, L.; Aro, E.M.; Ma, W. Identification of novel Ssl0352 protein (NdhS), essential for efficient operation of cyclic electron transport around photosystem I, in NADPH:plastoquinone oxidoreductase (NDH-1) complexes of Synechocystis sp. PCC 6803. J. Biol. Chem. 2011, 286, 36992–37001. [Google Scholar] [CrossRef]
- Dai, H.; Zhang, L.; Zhang, J.; Mi, H.; Ogawa, T.; Ma, W. Identification of a cyanobacterial CRR6 protein, Slr1097, required for efficient assembly of NDH-1 complexes in Synechocystis sp. PCC 6803. Plant J. 2013, 75, 858–866. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Gao, F.; Zhao, J.; Ogawa, T.; Wang, Q.; Ma, W.W. NdhP Is an exclusive subunit of large complex of NADPH dehydrogenase essential to stabilize the complex in Synechocystis sp. Strain PCC 6803. J. Biol. Chem. 2014, 289, 26669–26676. [Google Scholar] [CrossRef]
- Zhao, J.; Rong, W.; Gao, F.; Ogawa, T.; Ma, W. Subunit Q is required to stabilize the large complex of NADPH dehydrogenase in Synechocystis sp. Strain PCC 6803. Plant Physiol. 2015, 168, 443–451. [Google Scholar] [CrossRef]
- Gao, F.; Zhao, J.; Wang, X.; Qin, S.; Wei, L.; Ma, W. NdhV is a subunit of NADPH dehydrogenase essential for cyclic electron transport in Synechocystis sp. strain PCC 6803. Plant Physiol. 2016, 170, 752–760. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Xu, M.; Wu, Y.; Lv, J.; Fu, P.; Mi, H. NdhM subunit is required for the stability and the function of NAD(P)H dehydrogenase complexes involved in CO2 uptake in Synechocystis sp. strain PCC 6803. J. Biol. Chem. 2016, 60, 1374–1385. [Google Scholar] [CrossRef]
- Wang, X.; Gao, F.; Zhang, J.; Zhao, J.; Ogawa, T.; Ma, W. A cytoplasmic protein Ssl3829 is important for NDH-1 hydrophilic arm assembly in Synechocystis sp. Strain PCC 6803. Plant Physiol. 2016, 171, 864–877. [Google Scholar] [CrossRef] [PubMed]
- Voshol, G.P.; Meyer, V.; van den Hondel, C.A.M.J.J. GTP-binding protein Era: A novel gene target for biofuel production. BMC Biotechnol. 2015, 15. [Google Scholar] [CrossRef]
- Cassier-Chauvat, C.; Chauvat, F. Function and Regulation of Ferredoxins in the Cyanobacterium, Synechocystis PCC6803: Recent Advances. Life 2014, 4, 666–680. [Google Scholar] [CrossRef]
- Lelong, C.; Boekema, E.J.; Kruip, J.; Bottin, H.; Rögner, M.; Sétif, P. Characterization of a redox active cross-linked complex between cyanobacterial photosystem I and soluble ferredoxin. EMBO J. 1996, 15, 2160–2168. [Google Scholar] [CrossRef]
- Poncelet, M.; Cassier-Chauvat, C.; Leschelle, X.; Bottin, H.; Chauvat, F. Targeted deletion and mutational analysis of the essential (2Fe-2S) plant-like ferredoxin in Synechocystis PCC6803 by plasmid shuffling. Mol. Microbiol. 1998, 28, 813–821. [Google Scholar] [CrossRef] [PubMed]
- Mazouni, K.; Domain, F.; Chauvat, F.; Cassier-Chauvat, C. Expression and regulation of the crucial plant-like ferredoxin of cyanobacteria. Mol. Microbiol. 2003, 49, 1019–1029. [Google Scholar] [CrossRef] [PubMed]
- Schorsch, M.; Kramer, M.; Goss, T.; Eisenhut, M.; Robinson, N.; Osman, D.; Wilde, A.; Sadaf, S.; Brückler, H.; Walder, L.; et al. A unique ferredoxin acts as a player in the low-iron response of photosynthetic organisms. Proc. Natl. Acad. Sci. USA 2018, 115, 12111–12120. [Google Scholar] [CrossRef]
- Mustila, H.; Allahverdiyeva, Y.; Isojärvi, J.; Aro, E.M.; Eisenhut, M. The bacterial-type [4Fe-4S] ferredoxin 7 has a regulatory function under photooxidative stress conditions in the cyanobacterium Synechocystis sp. PCC 6803. Biochim. Biophys. Acta Bioenerg. 2014, 1837, 1293–1304. [Google Scholar] [CrossRef] [PubMed]
- Hanke, G.T.; Satomi, Y.; Shinmura, K.; Takao, T.; Hase, T. A screen for potential ferredoxin electron transfer partners uncovers new, redox dependent interactions. Biochim. Biophys. Acta Proteins Proteom. 2011, 1814, 366–374. [Google Scholar] [CrossRef]
- Angeleri, M.; Zorina, A.; Aro, E.M.; Battchikova, N. Interplay of SpkG kinase and the Slr0151 protein in the phosphorylation of ferredoxin 5 in Synechocystis sp. strain PCC 6803. FEBS Lett. 2018, 592, 411–421. [Google Scholar] [CrossRef]
- Mondal, S.; Kumar, V.; Singh, S.P. Phylogenetic distribution and structural analyses of cyanobacterial glutaredoxins (Grxs). Comput. Biol. Chem. 2020, 84. [Google Scholar] [CrossRef]
- Zaffagnini, M.; Bedhomme, M.; Marchand, C.H.; Morisse, S.; Trost, P.; Lemaire, S.D. Redox regulation in photosynthetic organisms: Focus on glutathionylation. Antioxid. Redox Signal. 2012, 16, 567–586. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Riego, A.M.; López-Maury, L.; Florencio, F.J. Glutaredoxins are essential for stress adaptation in the cyanobacterium Synechocystis sp. PCC 6803. Front. Plant Sci. 2013. [Google Scholar] [CrossRef] [PubMed]
- López-Maury, L.; Sánchez-Riego, A.M.; Reyes, J.C.; Florencio, F.J. The glutathione/glutaredoxin system is essential for arsenate reduction in Synechocystis sp. strain PCC 6803. J. Bacteriol. 2009, 191, 3534–3543. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.G.; Chung, J.S.; Sutton, R.B.; Lee, J.S.; López-Maury, L.; Lee, S.Y.; Florencio, F.J.; Lin, T.; Zabet-Moghaddam, M.; Wood, M.J.; et al. Redox, mutagenic and structural studies of the glutaredoxin/arsenate reductase couple from the cyanobacterium Synechocystis sp. PCC 6803. Biochim. Biophys. Acta Proteins Proteom. 2012, 1824, 392–403. [Google Scholar] [CrossRef] [PubMed]
- Iwema, T.; Picciocchi, A.; Traore, D.A.K.; Ferrer, J.L.; Chauvat, F.; Jacquamet, L. Structural basis for delivery of the intact [Fe2S2] cluster by monothiol glutaredoxin. Biochemistry 2009, 48, 6041–6043. [Google Scholar] [CrossRef] [PubMed]
- Deponte, M. Glutathione catalysis and the reaction mechanisms of glutathione-dependent enzymes. Biochim. Biophys. Acta Gen. Subj. 2013, 1830, 3217–3266. [Google Scholar] [CrossRef]
- Nianiou-Obeidat, I.; Madesis, P.; Kissoudis, C.; Voulgari, G.; Chronopoulou, E.; Tsaftaris, A.; Labrou, N.E. Plant glutathione transferase-mediated stress tolerance: Functions and biotechnological applications. Plant Cell Rep. 2017, 36, 791–805. [Google Scholar] [CrossRef]
- Oakley, A. Glutathione transferases: A structural perspective. Drug Metab. Rev. 2011, 43, 138–151. [Google Scholar] [CrossRef]
- Wiktelius, E.; Stenberg, G. Novel class of glutathione transferases from cyanobacteria exhibit high catalytic activities towards naturally occurring isothiocyanates. Biochem. J. 2007, 406, 115–123. [Google Scholar] [CrossRef]
- Naciyar, M.S.; Karthick, L.; Prakasam, P.A.; Deviram, G.; Uma, L.; Prabaharan, D.; Saha, S.K. Diversity of glutathione S-transferases (GSTs) in cyanobacteria with reference to their structures, substrate recognition and catalytic functions. Microorganisms 2020, 8, 712. [Google Scholar] [CrossRef]
- Pandey, T.; Chhetri, G.; Chinta, R.; Kumar, B.; Singh, D.B.; Tripathi, T.; Singh, A.K. Functional classification and biochemical characterization of a novel rho class glutathione S-transferase in Synechocystis PCC 6803. FEBS Open Bio 2015, 5, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Pandey, T.; Singh, S.K.; Chhetri, G.; Tripathi, T.; Singh, A.K. Characterization of a highly pH stable Chi-class glutathione S-transferase from Synechocystis PCC 6803. PLoS ONE 2015, 10, e0126811. [Google Scholar] [CrossRef] [PubMed]
- Pandey, T.; Shukla, R.; Shukla, H.; Sonkar, A.; Tripathi, T.; Singh, A.K. A combined biochemical and computational studies of the rho-class glutathione s-transferase sll1545 of Synechocystis PCC 6803. Int. J. Biol. Macromol. 2017, 94, 378–385. [Google Scholar] [CrossRef]
- Kammerscheit, X.; Hecker, A.; Rouhier, N.; Chauvat, F.; Cassier-Chauvat, C. Methylglyoxal Detoxification Revisited: Role of Glutathione Transferase in Model Cyanobacterium Synechocystis sp. Strain PCC 6803. MBio 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Stanier, R.Y.; Kunisawa, R.; Mandel, M.; Cohen-Bazire, G. Purification and properties of unicellular blue-green algae (order Chroococcales). Bacteriol. Rev. 1971, 35, 171–205. [Google Scholar] [CrossRef] [PubMed]
- Rippka, R.; Deruelles, J.; Waterbury, J.B.; Herdman, M.; Stanier, R.Y. Generic assignments, strain histories and properties of pure cultures of cyano- bacteria. Journal of General Microbiology 11: 1-61. J. Gen. Microbiol. 1979, 111, 1–61. [Google Scholar] [CrossRef]
- Niu, T.C.; Lin, G.M.; Xie, L.R.; Wang, Z.Q.; Xing, W.Y.; Zhang, J.Y.; Zhang, C.C. Expanding the Potential of CRISPR-Cpf1-Based Genome Editing Technology in the Cyanobacterium Anabaena PCC 7120. ACS Synth. Biol. 2019, 8, 170–180. [Google Scholar] [CrossRef]
- Yu, J.; Liberton, M.; Cliften, P.F.; Head, R.D.; Jacobs, J.M.; Smith, R.D.; Koppenaal, D.W.; Brand, J.J.; Pakrasi, H.B. Synechococcus elongatus UTEX 2973, a fast growing cyanobacterial chassis for biosynthesis using light and CO2. Sci. Rep. 2015, 5. [Google Scholar] [CrossRef]
- Jaiswal, D.; Sengupta, A.; Sohoni, S.; Sengupta, S.; Phadnavis, A.G.; Pakrasi, H.B.; Wangikar, P.P. Genome Features and Biochemical Characteristics of a Robust, Fast Growing and Naturally Transformable Cyanobacterium Synechococcus elongatus PCC 11801 Isolated from India. Sci. Rep. 2018, 8. [Google Scholar] [CrossRef]
- Jaiswal, D.; Sengupta, A.; Sengupta, S.; Madhu, S.; Pakrasi, H.B.; Wangikar, P.P. A Novel Cyanobacterium Synechococcus elongatus PCC 11802 has Distinct Genomic and Metabolomic Characteristics Compared to its Neighbor PCC 11801. Sci. Rep. 2020, 10. [Google Scholar] [CrossRef]
- Włodarczyk, A.; Selão, T.T.; Norling, B.; Nixon, P.J. Newly discovered Synechococcus sp. PCC 11901 is a robust cyanobacterial strain for high biomass production. Commun. Biol. 2020, 3. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Hou, S.; Song, K.; Georg, J.; Klähn, S.; Lu, X.; Hess, W.R. The primary transcriptome of the fast-growing cyanobacterium Synechococcus elongatus UTEX 2973. Biotechnol. Biofuels 2018, 11. [Google Scholar] [CrossRef] [PubMed]
- Lou, W.; Tan, X.; Song, K.; Zhang, S.; Luan, G.; Li, C.; Lu, X. A specific single nucleotide polymorphism in the ATP synthase gene significantly improves environmental stress tolerance of Synechococcus elongatus PCC 7942. Appl. Environ. Microbiol. 2018, 84. [Google Scholar] [CrossRef] [PubMed]
- Bachin, D.; Nazarenko, L.V.; Mironov, K.S.; Pisareva, T.; Allakhverdiev, S.I.; Los, D.A. Mechanosensitive ion channel MscL controls ionic fluxes during cold and heat stress in Synechocystis. FEMS Microbiol. Lett. 2015, 362. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Sun, T.; Xu, C.; Chen, L.; Zhang, W. Development and optimization of genetic toolboxes for a fast-growing cyanobacterium Synechococcus elongatus UTEX 2973. Metab. Eng. 2018, 48, 163–174. [Google Scholar] [CrossRef]
- Ungerer, J.; Wendt, K.E.; Hendry, J.I.; Maranas, C.D.; Pakrasi, H.B. Comparative genomics reveals the molecular determinants of rapid growth of the cyanobacterium Synechococcus elongatus UTEX 2973. Proc. Natl. Acad. Sci. USA 2018, 115. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Qiao, C.; Luan, G.; Luo, Q.; Lu, X. Systematic Identification of Target Genes for Cellular Morphology Engineering in Synechococcus elongatus PCC7942. Front. Microbiol. 2020, 11. [Google Scholar] [CrossRef]
- Golden, S.S. Principles of rhythmicity emerging from cyanobacteria. Eur. J. Neurosci. 2020, 51, 13–18. [Google Scholar] [CrossRef]
- MacCready, J.S.; Basalla, J.L.; Vecchiarelli, A.G. Origin and Evolution of Carboxysome Positioning Systems in Cyanobacteria. Mol. Biol. Evol. 2020. [Google Scholar] [CrossRef]
- Labarre, J.; Chauvat, F.; Thuriaux, P. Insertional mutagenesis by random cloning of antibiotic resistance genes into the genome of the cyanobacterium Synechocystis strain PCC 6803. J. Bacteriol. 1989, 171, 3449–3457. [Google Scholar] [CrossRef]
- Zerulla, K.; Ludt, K.; Soppa, J. The ploidy level of Synechocystis sp. PCC 6803 is highly variable and is influenced by growth phase and by chemical and physical external parameters. Microbiology (UK) 2016, 162, 730–739. [Google Scholar] [CrossRef] [PubMed]
- Chauvat, F.; De Vries, L.; Van der Ende, A.; Van Arkel, G.A. A host-vector system for gene cloning in the cyanobacterium Synechocystis PCC 6803. MGG Mol. Gen. Genet. 1986, 204, 185–191. [Google Scholar] [CrossRef]
- Yang, X.; McFadden, B.A. A small plasmid, pCA2.4, from the cyanobacterium Synechocystis sp. strain PCC 6803 encodes a Rep protein and replicates by a rolling circle mechanism. J. Bacteriol. 1993, 175, 3981–3991. [Google Scholar] [CrossRef][Green Version]
- Yang, X.; McFadden, B.A. The complete DNA sequence and replication analysis of the plasmid pCB2.4 from the cyanobacterium Synechocystis PCC 6803. Plasmid 1994, 31, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; McFadden, B.A. Sequence analysis of plasmid pCC5.2 from cyanobacterium Synechocystis PCC 6803 that replicates by a rolling circle mechanism. Plasmid 1997, 37, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, T.; Nakamura, Y.; Sasamoto, S.; Watanabe, A.; Kohara, M.; Matsumoto, M.; Shimpo, S.; Yamada, M.; Tabata, S. Structural analysis of four large plasmids harboring in a unicellular cyanobacterium, Synechocystis sp. PCC 6803. DNA Res. 2003, 10, 221–228. [Google Scholar] [CrossRef]
- Berla, B.M.; Pakrasi, H.B. Upregulation of plasmid genes during stationary phase in Synechocystis sp. strain PCC 6803, a cyanobacterium. Appl. Environ. Microbiol. 2012, 18. [Google Scholar] [CrossRef]
- Castets, A.M.; Houmard, J.; Tandeau de Marsac, N. Is cell motility a plasmid-encoded function in the cyanobacterium Synechocystis 6803? FEMS Microbiol. Lett. 1986, 37, 277–281. [Google Scholar] [CrossRef]
- Kopfmann, S.; Hess, W.R. Toxin-antitoxin systems on the large defense plasmid pSYSA of Synechocystis sp. pCC 6803. J. Biol. Chem. 2013, 288, 7399–7409. [Google Scholar] [CrossRef]
- Kopfmann, S.; Roesch, S.K.; Hess, W.R. Type II toxin-antitoxin systems in the unicellular cyanobacterium Synechocystis sp. PCC 6803. Toxins 2016, 8, 228. [Google Scholar] [CrossRef]
- Paynter, J.J.; Andres-Enguix, I.; Fowler, P.W.; Tottey, S.; Cheng, W.; Enkvetchakul, D.; Bavro, V.N.; Kusakabe, Y.; Sansom, M.S.P.; Robinson, N.J.; et al. Functional complementation and genetic deletion studies of KirBac channels: Activatory mutations highlight gating-sensitive domains. J. Biol. Chem. 2010, 285, 40754–40761. [Google Scholar] [CrossRef] [PubMed]
- Giner-Lamia, J.; López-Maury, L.; Reyes, J.C.; Florencio, F.J. The CopRS two-component system is responsible for resistance to copper in the cyanobacterium Synechocystis sp. PCC 6803. Plant Physiol. 2012, 159, 1806–1818. [Google Scholar] [CrossRef] [PubMed]
- De Alvarenga, L.V.; Hess, W.R.; Hagemann, M. AcnSP—A Novel Small Protein Regulator of Aconitase Activity in the Cyanobacterium Synechocystis sp. PCC 6803. Front. Microbiol. 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Bird, A.J.; Turner-Cavet, J.S.; Lakey, J.H.; Robinson, N.J. A carboxyl-terminal Cys2/His2-type zinc-finger motif in DNA primase influences DNA content in Synechococcus PCC 7942. J. Biol. Chem. 1998, 273, 21246–21252. [Google Scholar] [CrossRef]
- Griese, M.; Lange, C.; Soppa, J. Ploidy in cyanobacteria. FEMS Microbiol. Lett. 2011, 323, 124–131. [Google Scholar] [CrossRef]
- Watanabe, S.; Ohbayashi, R.; Kanesaki, Y.; Saito, N.; Chibazakura, T.; Soga, T.; Yoshikawa, H. Intensive DNA replication and metabolism during the lag phase in Cyanobacteria. PLoS ONE 2015, 10, e0136800. [Google Scholar] [CrossRef]
- Van Den Hondel, C.A.M.J.J.; Verbeek, S.; van Der Ende, A.; Weisbeek, P.J.; Borrias, W.E.; van Arkel, G.A. Introduction of transposon Tn901 into a plasmid of Anacystis nidulans: Preparation for cloning in cyanobacteria. Proc. Natl. Acad. Sci. USA 1980, 77, 1570–1574. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Kay Holtman, C.; Magnuson, R.D.; Youderian, P.A.; Golden, S.S. The complete sequence and functional analysis of pANL, the large plasmid of the unicellular freshwater cyanobacterium Synechococcus elongatus PCC 7942. Plasmid 2008, 59, 176–192. [Google Scholar] [CrossRef]
- Roberts, T.M.; Koths, K.E. The blue-green alga Agmenellum quadruplicatum contains covalently closed DNA circles. Cell 1976, 9, 551–557. [Google Scholar] [CrossRef]
- Akiyama, H.; Kanai, S.; Hirano, M.; Miyasaka, H. Nucleotide sequence of plasmid pAQ1 of marine cyanobacterium Synechococcus sp. PCC7002. DNA Res. 1998, 5, 127–129. [Google Scholar] [CrossRef][Green Version]
- Kuhlemeier, C.J.; Borrias, W.E.; van den Hondel, C.A.M.J.J.; van Arkel, G.A. Vectors for cloning in cyanobacteria: Construction and characterization of two recombinant plasmids capable of transformation to Escherichia coli K12 and Anacystis nidulans R2. MGG Mol. Gen. Genet. 1981, 184, 249–254. [Google Scholar] [CrossRef]
- Chauvat, F.; Astier, C.; Vedel, F.; Joset-Espardellier, F. Transformation in the cyanobacterium Synechococcus R2: Improvement of efficiency; Role of the pUH24 plasmid. MGG Mol. Gen. Genet. 1983, 191, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, T.; Nakamura, Y.; Wolk, C.P.; Kuritz, T.; Sasamoto, S.; Watanabe, A.; Iriguchi, M.; Ishikawa, A.; Kawashima, K.; Kimura, T.; et al. Complete genomic sequence of the filamentous nitrogen-fixing cyanobacterium Anabaena sp. strain PCC 7120. DNA Res. 2001, 8, 205–213. [Google Scholar] [CrossRef]
- Ludwig, M.; Bryant, D.A. Acclimation of the global transcriptome of the cyanobacterium Synechococcus sp. strain PCC 7002 to nutrient limitations and different nitrogen sources. Front. Microbiol. 2012, 11. [Google Scholar] [CrossRef]
- Labarre, J.; Thuriaux, P.; Chauvat, F. Genetic analysis of amino acid transport in the facultatively heterotrophic cyanobacterium Synechocystis sp. strain 6803. J. Bacteriol. 1987, 169, 4668–4673. [Google Scholar] [CrossRef] [PubMed]
- Pérez, A.A.; Liu, Z.; Rodionov, D.A.; Li, Z.; Bryant, D.A. Complementation of cobalamin auxotrophy in Synechococcus sp. strain PCC 7002 and validation of a putative cobalamin riboswitch in vivo. J. Bacteriol. 2016, 198, 2743–2752. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, T.; Delgaizo, V.B.; Bryant, D.A. Growth on urea can trigger death and peroxidation of the cyanobacterium Synechococcus sp. strain PCC 7002. Appl. Environ. Microbiol. 1998, 64, 2361–2366. [Google Scholar] [CrossRef]
- Valladares, A.; Montesinos, M.L.; Herrero, A.; Flores, E. An ABC-type, high-affinity urea permease identified in cyanobacteria. Mol. Microbiol. 2002, 43, 703–715. [Google Scholar] [CrossRef]
- Veaudor, T.; Cassier-Chauvat, C.; Chauvat, F. Genomics of Urea Transport and Catabolism in Cyanobacteria: Biotechnological Implications. Front. Microbiol. 2019, 10. [Google Scholar] [CrossRef]
- Kirsch, F.; Klähn, S.; Hagemann, M. Salt-Regulated Accumulation of the Compatible Solutes Sucrose and Glucosylglycerol in Cyanobacteria and Its Biotechnological Potential. Front. Microbiol. 2019, 10. [Google Scholar] [CrossRef]
- Årstøl, E.; Hohmann-Marriott, M.F. Cyanobacterial siderophores—physiology, structure, biosynthesis, and applications. Mar. Drugs 2019, 17, 281. [Google Scholar] [CrossRef] [PubMed]
- Onizuka, T.; Endo, S.; Akiyama, H.; Kanai, S.; Hirano, M.; Yokota, A.; Tanaka, S.; Miyasaka, H. The rbcX gene product promotes the production and assembly of ribulose-1,5-bisphosphate carboxylase/oxygenase of Synechococcus sp. PCC7002 in Escherichia coli. Plant Cell Physiol. 2004, 45, 1390–1395. [Google Scholar] [CrossRef]
- Emlyn-Jones, D.; Woodger, F.J.; Price, G.D.; Whitney, S.M. RbcX can function as a Rubisco chaperonin, but is non-essential in Synechococcus PCC7942. Plant Cell Physiol. 2006, 47, 1630–1640. [Google Scholar] [CrossRef] [PubMed]
- Bracher, A.; Whitney, S.M.; Hartl, F.U.; Hayer-Hartl, M. Biogenesis and Metabolic Maintenance of Rubisco. Annu. Rev. Plant Biol. 2017, 68, 29–60. [Google Scholar] [CrossRef]
- Helman, Y.; Tchernov, D.; Reinhold, L.; Shibata, M.; Ogawa, T.; Schwarz, R.; Ohad, I.; Kaplan, A. Genes encoding A-type flavoproteins are essential for photoreduction of O2 in cyanobacteria. Curr. Biol. 2003, 13, 230–235. [Google Scholar] [CrossRef]
- Vicente, J.B.; Gomes, C.M.; Wasserfallen, A.; Teixeira, M. Module fusion in an A-type flavoprotein from the cyanobacterium Synechocystis condenses a multiple-component pathway in a single polypeptide chain. Biochem. Biophys. Res. Commun. 2002, 294, 82–87. [Google Scholar] [CrossRef]
- Allahverdiyeva, Y.; Mustila, H.; Ermakova, M.; Bersanini, L.; Richaud, P.; Ajlani, G.; Battchikova, N.; Cournac, L.; Aro, E.M. Flavodiiron proteins Flv1 and Flv3 enable cyanobacterial growth and photosynthesis under fluctuating light. Proc. Natl. Acad. Sci. USA 2013, 110, 4111–4116. [Google Scholar] [CrossRef]
- Zhang, P.; Allahverdiyeva, Y.; Eisenhut, M.; Aro, E.M. Flavodiiron proteins in oxygenic photosynthetic organisms: Photoprotection of photosystem II by FIv2 and FIv4 in Synechocystis sp. PCC 6803. PLoS ONE 2009, 4, e5331. [Google Scholar] [CrossRef] [PubMed]
- Shimakawa, G.; Shaku, K.; Nishi, A.; Hayashi, R.; Yamamoto, H.; Sakamoto, K.; Makino, A.; Miyake, C. FLAVODIIRON2 and FLAVODIIRON4 proteins mediate an oxygen-dependent alternative electron flow in Synechocystis sp. pcc 6803 under CO2-limited conditions. Plant Physiol. 2015, 167, 472–480. [Google Scholar] [CrossRef]
- Fujisawa, T.; Okamoto, S.; Katayama, T.; Nakao, M.; Yoshimura, H.; Kajiya-Kanegae, H.; Yamamoto, S.; Yano, C.; Yanaka, Y.; Maita, H.; et al. CyanoBase and RhizoBase: Databases of manually curated annotations for cyanobacterial and rhizobial genomes. Nucleic Acids Res. 2014, 42. [Google Scholar] [CrossRef]
- Jittawuttipoka, T.; Planchon, M.; Spalla, O.; Benzerara, K.; Guyot, F.; Cassier-Chauvat, C.; Chauvat, F. Multidisciplinary Evidences that Synechocystis PCC6803 Exopolysaccharides Operate in Cell Sedimentation and Protection against Salt and Metal Stresses. PLoS ONE 2013, 8, e55564. [Google Scholar] [CrossRef]
- Planchon, M.; Jittawuttipoka, T.; Cassier-Chauvat, C.; Guyot, F.; Gelabert, A.; Benedetti, M.F.; Chauvat, F.; Spalla, O. Exopolysaccharides protect Synechocystis against the deleterious effects of Titanium dioxide nanoparticles in natural and artificial waters. J. Colloid Interface Sci. 2013, 405, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Naveed, S.; Yu, Q.; Zhang, C.; Ge, Y. Extracellular polymeric substances alter cell surface properties, toxicity, and accumulation of arsenic in Synechocystis PCC6803. Environ. Pollut. 2020, 261. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.; Chai, H.; Yu, Z.; Wu, X.; Liu, Y.; Shen, L.; Li, J.; Ye, J.; Liu, D.; Ma, T.; et al. Behavior and mechanism of cesium biosorption from aqueous solution by living Synechococcus PCC7002. Microorganisms 2020, 8, 491. [Google Scholar] [CrossRef] [PubMed]
- Schatz, D.; Nagar, E.; Sendersky, E.; Parnasa, R.; Zilberman, S.; Carmeli, S.; Mastai, Y.; Shimoni, E.; Klein, E.; Yeger, O.; et al. Self-suppression of biofilm formation in the cyanobacterium Synechococcus elongatus. Environ. Microbiol. 2013, 15, 1786–1794. [Google Scholar] [CrossRef]
- Muzzopappa, F.; Kirilovsky, D. Changing Color for Photoprotection: The Orange Carotenoid Protein. Trends Plant Sci. 2020, 25, 92–104. [Google Scholar] [CrossRef]
- Kämäräinen, J.; Knoop, H.; Stanford, N.J.; Guerrero, F.; Akhtar, M.K.; Aro, E.M.; Steuer, R.; Jones, P.R. Physiological tolerance and stoichiometric potential of cyanobacteria for hydrocarbon fuel production. J. Biotechnol. 2012, 162, 67–74. [Google Scholar] [CrossRef]
- Gupta, A.; Bhagwat, S.G.; Sainis, J.K. Synechococcus elongatus PCC 7942 is more tolerant to chromate as compared to Synechocystis sp. PCC 6803. BioMetals 2013, 26, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Ballal, A. Unraveling the mechanism responsible for the contrasting tolerance of Synechocystis and Synechococcus to Cr(VI): Enzymatic and non-enzymatic antioxidants. Aquat. Toxicol. 2015, 164, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Kämäräinen, J.; Nylund, M.; Aro, E.M.; Kallio, P. Comparison of ethanol tolerance between potential cyanobacterial production hosts. J. Biotechnol. 2018, 283, 140–145. [Google Scholar] [CrossRef]
- Ikeuchi, M.; Tabata, S. Synechocystis sp. PCC 6803—A useful tool in the study of the genetics of cyanobacteria. Photosynth. Res. 2001, 70, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Tajima, N.; Sato, S.; Maruyama, F.; Kaneko, T.; Sasaki, N.V.; Kurokawa, K.; Ohta, H.; Kanesaki, Y.; Yoshikawa, H.; Tabata, S.; et al. Genomic structure of the cyanobacterium Synechocystis sp. PCC 6803 strain GT-S. DNA Res. 2011, 18, 393–399. [Google Scholar] [CrossRef]
- Morris, J.N.; Crawford, T.S.; Jeffs, A.; Stockwell, P.A.; Eaton-Rye, J.J.; Summerfield, T.C. Whole genome re-sequencing of two “wild-type” strains of the model cyanobacterium Synechocystis sp. PCC 6803. N. Z. J. Bot. 2014, 52, 36–47. [Google Scholar] [CrossRef]
- Trautmann, D.; Voß, B.; Wilde, A.; Al-Babili, S.; Hess, W.R. Microevolution in cyanobacteria: Re-sequencing a motile substrain of Synechocystis sp. PCC 6803. DNA Res. 2012, 19, 435–448. [Google Scholar] [CrossRef]
- Kanesaki, Y.; Shiwa, Y.; Tajima, N.; Suzuki, M.; Watanabe, S.; Sato, N.; Ikeuchi, M.; Yoshikawa, H. Identification of substrain-specific mutations by massively parallel whole-genome resequencing of Synechocystis sp. PCC 6803. DNA Res. 2012, 19, 67–79. [Google Scholar] [CrossRef] [PubMed]
- Tichý, M.; Bečková, M.; Kopečná, J.; Noda, J.; Sobotka, R.; Komenda, J. Strain of Synechocystis PCC 6803 with aberrant assembly of photosystem II contains tandem duplication of a large chromosomal region. Front. Plant Sci. 2016, 7. [Google Scholar] [CrossRef]
- Ding, Q.; Chen, G.; Wang, Y.; Wei, D. Identification of specific variations in a non-motile strain of cyanobacterium Synechocystis sp. PCC 6803 originated from ATCC 27184 by whole genome resequencing. Int. J. Mol. Sci. 2015, 16, 24081–24093. [Google Scholar] [CrossRef]
- Morris, J.N.; Eaton-Rye, J.J.; Summerfield, T.C. Phenotypic variation in wild-type substrains of the model cyanobacterium Synechocystis sp. PCC 6803. N Z. J. Bot. 2017, 25–35. [Google Scholar] [CrossRef]
- Williams, J.G.K. Construction of Specific Mutations in Photosystem II Photosynthetic Reaction Center by Genetic Engineering Methods in Synechocystis 6803. Methods Enzymol. 1988, 167, 766–778. [Google Scholar] [CrossRef]
- Zavřel, T.; Očenášová, P.; Červený, J. Phenotypic characterization of Synechocystis sp. PCC 6803 substrains reveals differences in sensitivity to abiotic stress. PLoS ONE 2017, 12, e0189130. [Google Scholar] [CrossRef]
- Shestakov, S.V.; Khyen, N.T. Evidence for genetic transformation in blue-green alga Anacystis nidulans. MGG Mol. Gen. Genet. 1970, 107. [Google Scholar] [CrossRef] [PubMed]
- Stevens, S.E.; Porter, R.D. Transformation in Agmenellum quadruplicatum. Proc. Natl. Acad. Sci. USA 1980, 77, 6052–6056. [Google Scholar] [CrossRef]
- Lambert, D.H.; Stevens, S.E. Photoheterotrophic growth of Agmenellum quadruplicatum PR-6. J. Bacteriol. 1986, 165. [Google Scholar] [CrossRef] [PubMed]
- Grigorieva, G.; Shestakov, S. Transformation in the cyanobacterium Synechocystis sp. 6803. FEMS Microbiol. Lett. 1982, 13, 367–370. [Google Scholar] [CrossRef]
- Orkwiszewski, K.G.; Kaney, A.R. Genetic transformation of the blue-green bacterium, Anacystis nidulans. Arch. Microbiol. 1974, 98, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Kufryk, G.I.; Sachet, M.; Schmetterer, G.; Vermaas, W.F.J. Transformation of the cyanobacterium Synechocystis sp. PCC 6803 as a tool for genetic mapping: Optimization of efficiency. FEMS Microbiol. Lett. 2002, 206, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Wendt, K.E.; Pakrasi, H.B. Genomics approaches to deciphering natural transformation in cyanobacteria. Front. Microbiol. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Taton, A.; Erikson, C.; Yang, Y.; Rubin, B.E.; Rifkin, S.A.; Golden, J.W.; Golden, S.S. The circadian clock and darkness control natural competence in cyanobacteria. Nat. Commun. 2020, 11. [Google Scholar] [CrossRef]
- Essich, E.; Stevens, E.; Porter, R.D. Chromosomal transformation in the cyanobacterium Agmenellum quadruplicatum. J. Bacteriol. 1990, 172, 1916–1922. [Google Scholar] [CrossRef]
- Williams, J.G.K.; Szalay, A.A. Stable integration of foreign DNA into the chromosome of the cyanobacterium Synechococcus R2. Gene 1983, 24, 37–51. [Google Scholar] [CrossRef]
- Buzby, J.S.; Porter, R.D.; Stevens, S.E. Expression of the Escherichia coli lacZ gene on a plasmid vector in a cyanobacterium. Science 1985, 230, 805–807. [Google Scholar] [CrossRef] [PubMed]
- Debus, R.J.; Barry, B.A.; Babcock, G.T.; McIntosh, L. Site-directed mutagenesis identifies a tyrosine radical involved in the photosynthetic oxygen-evolving system. Proc. Natl. Acad. Sci. USA 1988, 85. [Google Scholar] [CrossRef]
- De Vos, W.M.; Venema, G.; Canosi, U.; Trautner, T.A. Plasmid transformation in Bacillus subtilis: Fate of plasmid DNA. MGG Mol. Gen. Genet. 1981, 181, 424–433. [Google Scholar] [CrossRef] [PubMed]
- Jansson, C.; Debus, R.J.; Osiewacz, H.D.; Gurevitz, M.; McIntosh, L. Construction of an Obligate Photoheterotrophic Mutant of the Cyanobacterium Synechocystis 6803. Plant Physiol. 1987, 85, 1021–1025. [Google Scholar] [CrossRef]
- Pakrasi, H.B.; Williams, J.G.; Arntzen, C.J. Targeted mutagenesis of the psbE and psbF genes blocks photosynthetic electron transport: Evidence for a functional role of cytochrome b559 in photosystem II. EMBO J. 1988, 7, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Vermaas, W.F.J.; Ikeuchi, M.; Inoue, Y. Protein composition of the photosystem II core complex in genetically engineered mutants of the cyanobacterium Synechocystis sp. PCC 6803. Photosynth. Res. 1988, 17, 97–113. [Google Scholar] [CrossRef]
- Chitnis, P.R.; Reilly, P.A.; Miedel, M.C.; Nelson, N. Structure and targeted mutagenesis of the gene encoding 8-kDa subunit of photosystem I from the cyanobacterium Synechocystis sp. PCC 6803. J. Biol. Chem. 1989, 264, 18374–18380. [Google Scholar] [CrossRef]
- Chauvat, F.; Rouet, P.; Bottin, H.; Boussac, A. Mutagenesis by random cloning of an Escherichia coli kanamycin resistance gene into the genome of the cyanobacterium Synechocystis PCC 6803: Selection of mutants defective in photosynthesis. MGG Mol. Gen. Genet. 1989, 216, 51–59. [Google Scholar] [CrossRef]
- Muller, E.G.; Buchanan, B.B. Thioredoxin is essential for photosynthetic growth. The thioredoxin m gene of Anacystis nidulans. J. Biol. Chem. 1989, 264, 4008–4014. [Google Scholar] [CrossRef]
- Golden, S.S.; Brusslan, J.; Haselkorn, R. Expression of a family of psbA genes encoding a photosystem II polypeptide in the cyanobacterium Anacystis nidulans R2. EMBO J. 1986, 5, 2789–2798. [Google Scholar] [CrossRef] [PubMed]
- Golden, S.S.; Cho, D.S.; Nalty, M.S. Two functional psbD genes in the cyanobacterium Synechococcus sp. strain PCC 7942. J. Bacteriol. 1989, 171, 4707–4713. [Google Scholar] [CrossRef]
- Omata, T.; Carlson, T.J.; Ogawa, T.; Pierce, J. Sequencing and modification of the gene encoding the 42-kilodalton protein in the cytoplasmic membrane of Synechococcus PCC 7942. Plant Physiol. 1990, 93, 305–312. [Google Scholar] [CrossRef]
- Laudenbach, D.E.; Herbert, S.K.; McDowell, C.; Fork, D.C.; Grossman, A.R.; Straus, N.A. Cytochrome c-553 is not required for photosynthetic activity in the cyanobacterium Synechococcus. Plant Cell 1990, 2, 913–924. [Google Scholar] [CrossRef]
- Bustos, S.A.; Golden, S.S. Light-regulated expression of the psbD gene family in Synecbococcus sp. strain PCC 7942: Evidence for the role of duplicated psbD genes in cyanobacteria. MGG Mol. Gen. Genet. 1992. [Google Scholar] [CrossRef]
- Swanson, R.V.; Zhou, J.; Leary, J.A.; Williams, T.; De Lorimier, R.; Bryant, D.A.; Glazer, A.N. Characterization of phycocyanin produced by cpcE and cpcF mutants and identification of an intergenic suppressor of the defect in bilin attachment. J. Biol. Chem. 1992, 267, 16146–16154. [Google Scholar] [CrossRef]
- Zhou, J.; Gasparich, G.E.; Stirewalt, V.L.; De Lorimier, R.; Bryant, D.A. The cpcE and cpcF genes of Synechococcus sp. PCC 7002. Construction and phenotypic characterization of interposon mutants. J. Biol. Chem. 1992, 267, 16138–16145. [Google Scholar] [CrossRef]
- Schirmacher, A.M.; Hanamghar, S.S. Function and Benefits of Natural Competence in Cyanobacteria: From Ecology to Targeted Manipulation. Life 2020, 10, 249. [Google Scholar] [CrossRef] [PubMed]
- Armshaw, P.; Carey, D.; Sheahan, C.; Pembroke, J.T. Utilising the native plasmid, pCA2.4, from the cyanobacterium Synechocystis sp. strain PCC6803 as a cloning site for enhanced product production. Biotechnol. Biofuels 2015, 8. [Google Scholar] [CrossRef] [PubMed]
- Kopf, M.; Hess, W.R. Regulatory RNAs in photosynthetic cyanobacteria. FEMS Microbiol. Rev. 2015, 39, 301–315. [Google Scholar] [CrossRef] [PubMed]
- Ng, A.H.; Berla, B.M.; Pakrasi, H.B. Fine-tuning of photoautotrophic protein production by combining promoters and neutral sites in the cyanobacterium Synechocystis sp. strain PCC 6803. Appl. Environ. Microbiol. 2015, 81, 6857–6863. [Google Scholar] [CrossRef] [PubMed]
- Pinto, F.; Pacheco, C.C.; Oliveira, P.; Montagud, A.; Landels, A.; Couto, N.; Wright, P.C.; Urchueguía, J.F.; Tamagnini, P. Improving a Synechocystis-based photoautotrophic chassis through systematic genome mapping and validation of neutral sites. DNA Res. 2015, 22, 425–437. [Google Scholar] [CrossRef]
- Andersson, C.R.; Tsinoremas, N.F.; Shelton, J.; Lebedeva, N.V.; Yarrow, J.; Min, H.; Golden, S.S. Application of bioluminescence to the study of circadian rhythms in cyanobacteria. Methods Enzymol. 2000, 305. [Google Scholar] [CrossRef]
- Niederholtmeyer, H.; Wolfstädter, B.T.; Savage, D.F.; Silver, P.A.; Way, J.C. Engineering cyanobacteria to synthesize and export hydrophilic products. Appl. Environ. Microbiol. 2010, 76, 3462–3466. [Google Scholar] [CrossRef]
- Taton, A.; Unglaub, F.; Wright, N.E.; Zeng, W.Y.; Paz-Yepes, J.; Brahamsha, B.; Palenik, B.; Peterson, T.C.; Haerizadeh, F.; Golden, S.S.; et al. Broad-host-range vector system for synthetic biology and biotechnology in cyanobacteria. Nucleic Acids Res. 2014, 42. [Google Scholar] [CrossRef]
- Xu, Y.; Alvey, R.M.; Byrne, P.O.; Graham, J.E.; Shen, G.; Bryant, D.A. Expression of genes in cyanobacteria: Adaptation of endogenous plasmids as platforms for high-level gene expression in Synechococcus sp. PCC 7002. Methods Mol. Biol. 2011. [Google Scholar] [CrossRef]
- Ruffing, A.M.; Jensen, T.J.; Strickland, L.M. Genetic tools for advancement of Synechococcus sp. PCC 7002 as a cyanobacterial chassis. Microb. Cell Fact. 2016, 15. [Google Scholar] [CrossRef]
- Nozzi, N.E.; Case, A.E.; Carroll, A.L.; Atsumi, S. Systematic Approaches to Efficiently Produce 2,3-Butanediol in a Marine Cyanobacterium. ACS Synth. Biol. 2017, 6, 2136–2144. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, A.; Jansson, C. Influence of light on accumulation of photosynthesis-specific transcripts in the cyanobacterium Synechocystis 6803. Plant Mol. Biol. 1989, 13, 693–700. [Google Scholar] [CrossRef]
- Mohamed, A.; Eriksson, J.; Osiewacz, H.D.; Jansson, C. Differential expression of the psbA genes in the cyanobacterium Synechocystis 6803. MGG Mol. Gen. Genet. 1993, 238, 161–168. [Google Scholar] [CrossRef]
- Albers, S.C.; Gallegos, V.A.; Peebles, C.A.M. Engineering of genetic control tools in Synechocystis sp. PCC 6803 using rational design techniques. J. Biotechnol. 2015, 216, 36–46. [Google Scholar] [CrossRef]
- Lin, P.-C.; Saha, R.; Zhang, F.; Pakrasi, H.B. Metabolic engineering of the pentose phosphate pathway for enhanced limonene production in the cyanobacterium Synechocystis sp. PCC 6803. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Wang, Y.; Idoine, A.; Bhaya, D. Construction of a shuttle vector using an endogenous plasmid from the cyanobacterium Synechocystis sp. PCC6803. Front. Microbiol. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Pakrasi, H.B. Exploring native genetic elements as plug-in tools for synthetic biology in the cyanobacterium Synechocystis sp. PCC 6803. Microb. Cell Fact. 2018, 17. [Google Scholar] [CrossRef]
- Kamennaya, N.A.; Post, A.F. Characterization of cyanate metabolism in marine Synechococcus and Prochlorococcus spp. Appl. Environ. Microbiol. 2011, 29, 76–85. [Google Scholar] [CrossRef]
- Kamennaya, N.A.; Ahn, S.E.; Park, H.; Bartal, R.; Sasaki, K.A.; Holman, H.Y.; Jansson, C. Installing extra bicarbonate transporters in the cyanobacterium Synechocystis sp. PCC6803 enhances biomass production. Metab. Eng. 2015. [Google Scholar] [CrossRef] [PubMed]
- Varman, A.M.; Xiao, Y.; Pakrasi, H.B.; Tang, Y.J. Metabolic engineering of Synechocystis sp. Strain PCC 6803 for isobutanol production. Appl. Environ. Microbiol. 2013, 79, 908–914. [Google Scholar] [CrossRef] [PubMed]
- Lagarde, D.; Beuf, L.; Vermaas, W. Increased production of zeaxanthin and other pigments by application of genetic engineering techniques to Synechocystis sp. strain PCC 6803. Appl. Environ. Microbiol. 2000, 66, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Horiuchi, M.; Nakamura, K.; Kojima, K.; Nishiyama, Y.; Hatakeyama, W.; Hisabori, T.; Hihara, Y. The PedR transcriptional regulator interacts with thioredoxin to connect photosynthesis with gene expression in cyanobacteria. Biochem. J. 2010, 431, 135–140. [Google Scholar] [CrossRef]
- Reinsvold, R.E.; Jinkerson, R.E.; Radakovits, R.; Posewitz, M.C.; Basu, C. The production of the sesquiterpene β-caryophyllene in a transgenic strain of the cyanobacterium Synechocystis. J. Plant Physiol. 2011, 168, 848–852. [Google Scholar] [CrossRef]
- Nagarajan, A.; Winter, R.; Eaton-Rye, J.; Burnap, R. A synthetic DNA and fusion PCR approach to the ectopic expression of high levels of the D1 protein of photosystem II in Synechocystis sp. PCC 6803. J. Photochem. Photobiol. B Biol. 2011, 104, 212–219. [Google Scholar] [CrossRef]
- Khetkorn, W.; Incharoensakdi, A.; Lindblad, P.; Jantaro, S. Enhancement of poly-3-hydroxybutyrate production in Synechocystis sp. PCC 6803 by overexpression of its native biosynthetic genes. Bioresour. Technol. 2016, 214, 761–768. [Google Scholar] [CrossRef]
- Lindberg, P.; Park, S.; Melis, A. Engineering a platform for photosynthetic isoprene production in cyanobacteria, using Synechocystis as the model organism. Metab. Eng. 2010, 12, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Bentley, F.K.; Melis, A. Diffusion-based process for carbon dioxide uptake and isoprene emission in gaseous/aqueous two-phase photobioreactors by photosynthetic microorganisms. Biotechnol. Bioeng. 2012, 109. [Google Scholar] [CrossRef]
- Bentley, F.K.; Zurbriggen, A.; Melis, A. Heterologous expression of the mevalonic acid pathway in cyanobacteria enhances endogenous carbon partitioning to isoprene. Mol. Plant 2014, 7, 71–86. [Google Scholar] [CrossRef]
- Kudoh, K.; Hotta, S.; Sekine, M.; Fujii, R.; Uchida, A.; Kubota, G.; Kawano, Y.; Ihara, M. Overexpression of endogenous 1-deoxy-D-xylulose 5-phosphate synthase (DXS) in cyanobacterium Synechocystis sp. PCC6803 accelerates protein aggregation. J. Biosci. Bioeng. 2017, 123, 590–596. [Google Scholar] [CrossRef]
- Englund, E.; Shabestary, K.; Hudson, E.P.; Lindberg, P. Systematic overexpression study to find target enzymes enhancing production of terpenes in Synechocystis PCC 6803, using isoprene as a model compound. Metab. Eng. 2018, 49, 164–177. [Google Scholar] [CrossRef] [PubMed]
- Eungrasamee, K.; Miao, R.; Incharoensakdi, A.; Lindblad, P.; Jantaro, S. Improved lipid production via fatty acid biosynthesis and free fatty acid recycling in engineered Synechocystis sp. PCC 6803. Biotechnol. Biofuels 2019, 12. [Google Scholar] [CrossRef] [PubMed]
- Brey, L.F.; Włodarczyk, A.J.; Bang Thøfner, J.F.; Burow, M.; Crocoll, C.; Nielsen, I.; Zygadlo Nielsen, A.J.; Jensen, P.E. Metabolic engineering of Synechocystis sp. PCC 6803 for the production of aromatic amino acids and derived phenylpropanoids. Metab. Eng. 2020, 57, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, A.; Vue, J.; Morgan, J. Combining random mutagenesis and metabolic engineering for enhanced tryptophan production in Synechocystis sp. strain PCC 6803. Appl. Environ. Microbiol. 2020, 86. [Google Scholar] [CrossRef]
- Bersanini, L.; Battchikova, N.; Jokel, M.; Rehman, A.; Vass, I.; Allahverdiyeva, Y.; Aro, E.M. Flavodiiron protein Flv2/Flv4-related photoprotective mechanism dissipates excitation pressure of PSII in cooperation with phycobilisomes in cyanobacteria. Plant Physiol. 2014, 164, 805–818. [Google Scholar] [CrossRef]
- Mustila, H.; Paananen, P.; Battchikova, N.; Santana-Sánchez, A.; Muth-Pawlak, D.; Hagemann, M.; Aro, E.M.; Allahverdiyeva, Y. The flavodiiron protein Flv3 functions as a homo-oligomer during stress acclimation and is distinct from the Flv1/Flv3 hetero-oligomer specific to the O2 photoreduction pathway. Plant Cell Physiol. 2016, 57, 1468–1483. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chaves, J.E.; Romero, P.R.; Kirst, H.; Melis, A. Role of isopentenyl-diphosphate isomerase in heterologous cyanobacterial (Synechocystis) isoprene production. Photosynth. Res. 2016, 130, 517–527. [Google Scholar] [CrossRef] [PubMed]
- Chaves, J.E.; Rueda-Romero, P.; Kirst, H.; Melis, A. Engineering Isoprene Synthase Expression and Activity in Cyanobacteria. ACS Synth. Biol. 2017, 6, 2281–2292. [Google Scholar] [CrossRef]
- Formighieri, C.; Melis, A. Heterologous synthesis of geranyllinalool, a diterpenol plant product, in the cyanobacterium Synechocystis. Appl. Microbiol. Biotechnol. 2017, 101, 2791–2800. [Google Scholar] [CrossRef] [PubMed]
- Sivaramakrishnan, R.; Incharoensakdi, A. Enhancement of lipid production in Synechocystis sp. PCC 6803 overexpressing glycerol kinase under oxidative stress with glycerol supplementation. Bioresour. Technol. 2018, 267, 532–540. [Google Scholar] [CrossRef] [PubMed]
- Chaves, J.E.; Melis, A. Biotechnology of cyanobacterial isoprene production. Appl. Microbiol. Biotechnol. 2018, 102, 6452–6458. [Google Scholar] [CrossRef] [PubMed]
- Formighieri, C.; Melis, A. Regulation of β-phellandrene synthase gene expression, recombinant protein accumulation, and monoterpene hydrocarbons production in Synechocystis transformants. Planta 2014, 240, 309–324. [Google Scholar] [CrossRef]
- Formighieri, C.; Melis, A. A phycocyanin·phellandrene synthase fusion enhances recombinant protein expression and β-phellandrene (monoterpene) hydrocarbons production in Synechocystis (cyanobacteria). Metab. Eng. 2015, 32, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Formighieri, C.; Melis, A. Sustainable heterologous production of terpene hydrocarbons in cyanobacteria. Photosynth. Res. 2016, 130, 123–135. [Google Scholar] [CrossRef]
- Betterle, N.; Melis, A. Heterologous Leader Sequences in Fusion Constructs Enhance Expression of Geranyl Diphosphate Synthase and Yield of β-Phellandrene Production in Cyanobacteria (Synechocystis). ACS Synth. Biol. 2018, 7, 912–921. [Google Scholar] [CrossRef]
- Betterle, N.; Melis, A. Photosynthetic generation of heterologous terpenoids in cyanobacteria. Biotechnol. Bioeng. 2019, 116, 2041–2051. [Google Scholar] [CrossRef]
- Valsami, E.A.; Psychogyiou, M.E.; Pateraki, A.; Chrysoulaki, E.; Melis, A.; Ghanotakis, D.F. Fusion constructs enhance heterologous β-phellandrene production in Synechocystis sp. PCC 6803. J. Appl. Phycol. 2020. [Google Scholar] [CrossRef]
- Marbouty, M.; Mazouni, K.; Saguez, C.; Cassier-Chauvat, C.; Chauvat, F. Characterization of the Synechocystis strain PCC 6803 penicillin-binding proteins and cytokinetic proteins FtsQ and FtsW and their network of interactions with ZipN. J. Bacteriol. 2009, 191, 5123–5133. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Zhang, Y.; Cheng, D.; Daddy, S.; He, Q. Genetically engineering Synechocystis sp. Pasteur Culture Collection 6803 for the sustainable production of the plant secondary metabolite p-coumaric acid. Proc. Natl. Acad. Sci. USA 2014, 111, 9449–9454. [Google Scholar] [CrossRef]
- Aoki, S.; Kondo, T.; Ishiura, M. Circadian expression of the dnaK gene in the cyanobacterium Synechocystis sp. strain PCC 6803. J. Bacteriol. 1995, 177, 5606–5611. [Google Scholar] [CrossRef] [PubMed]
- Peca, L.; Kós, P.B.; Máté, Z.; Farsang, A.; Vass, I. Construction of bioluminescent cyanobacterial reporter strains for detection of nickel, cobalt and zinc. FEMS Microbiol. Lett. 2008, 1837, 1293–1304. [Google Scholar] [CrossRef] [PubMed]
- Muramatsu, M.; Hihara, Y. Transcriptional regulation of genes encoding subunits of photosystem I during acclimation to high-light conditions in Synechocystis sp. PCC 6803. Planta 2003, 216, 446–453. [Google Scholar] [CrossRef]
- Takahashi, T.; Nakai, N.; Muramatsu, M.; Hihara, Y. Role of multiple HLR1 sequences in the regulation of the dual promoters of the psaAB genes in Synechocystis sp. PCC 6803. J. Bacteriol. 2010, 192, 4031–4036. [Google Scholar] [CrossRef]
- Rutherford, J.C.; Cavet, J.S.; Robinson, N.J. Cobalt-dependent transcriptional switching by a dual-effector MerR-like protein regulates a cobalt-exporting variant CPx-type ATPase. J. Biol. Chem. 1999, 274, 25827–25832. [Google Scholar] [CrossRef]
- Calderon, R.H.; García-Cerdán, J.G.; Malnoë, A.; Cook, R.; Russell, J.J.; Gaw, C.; Dent, R.M.; De Vitry, C.; Niyogi, K.K. A conserved rubredoxin is necessary for photosystem II accumulation in diverse oxygenic photoautotrophs. J. Biol. Chem. 2013, 288, 26688–26696. [Google Scholar] [CrossRef]
- Ke, W.T.; Dai, G.Z.; Jiang, H.B.; Zhang, R.; Qiu, B.S. Essential roles of iron superoxide dismutase in photoautotrophic growth of Synechocystis sp. PCC 6803 and heterogeneous expression of marine Synechococcus sp. CC9311 copper/zinc superoxide dismutase within its sodB knockdown mutant. Microbiology (UK) 2014, 160, 228–241. [Google Scholar] [CrossRef] [PubMed]
- Kunert, A.; Hagemann, M.; Erdmann, N. Construction of promoter probe vectors for Synechocystis sp. PCC 6803 using the light-emitting reporter systems Gfp and LuxAB. J. Microbiol. Methods 2000, 41, 185–194. [Google Scholar] [CrossRef]
- Tan, X.; Yao, L.; Gao, Q.; Wang, W.; Qi, F.; Lu, X. Photosynthesis driven conversion of carbon dioxide to fatty alcohols and hydrocarbons in cyanobacteria. Metab. Eng. 2011, 13, 169–176. [Google Scholar] [CrossRef]
- Ungerer, J.; Tao, L.; Davis, M.; Ghirardi, M.; Maness, P.C.; Yu, J. Sustained photosynthetic conversion of CO2 to ethylene in recombinant cyanobacterium Synechocystis 6803. Energy Environ. Sci. 2012, 5, 8998–9006. [Google Scholar] [CrossRef]
- Veetil, V.P.; Angermayr, S.A.; Hellingwerf, K.J. Ethylene production with engineered Synechocystis sp PCC 6803 strains. Microb. Cell Fact. 2017, 16. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Angermayr, S.A.; Paszota, M.; Hellingwerf, K.J. Engineering a cyanobacterial cell factory for production of lactic acid. Appl. Environ. Microbiol. 2012, 78, 7098–7106. [Google Scholar] [CrossRef]
- Angermayr, S.A.; Van Der Woude, A.D.; Correddu, D.; Vreugdenhil, A.; Verrone, V.; Hellingwerf, K.J. Exploring metabolic engineering design principles for the photosynthetic production of lactic acid by Synechocystis sp. PCC6803. Biotechnol. Biofuels 2014, 7. [Google Scholar] [CrossRef]
- Van der Woude, A.D.; Angermayr, S.A.; Puthan Veetil, V.; Osnato, A.; Hellingwerf, K.J. Carbon sink removal: Increased photosynthetic production of lactic acid by Synechocystis sp. PCC6803 in a glycogen storage mutant. J. Biotechnol. 2014, 184, 100–102. [Google Scholar] [CrossRef] [PubMed]
- Hidese, R.; Matsuda, M.; Osanai, T.; Hasunuma, T.; Kondo, A. Malic Enzyme Facilitates d -Lactate Production through Increased Pyruvate Supply during Anoxic Dark Fermentation in Synechocystis sp. PCC 6803. ACS Synth. Biol. 2020, 9, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Savakis, P.E.; Angermayr, S.A.; Hellingwerf, K.J. Synthesis of 2,3-butanediol by Synechocystis sp. PCC6803 via heterologous expression of a catabolic pathway from lactic acid- and enterobacteria. Metab. Eng. 2013, 20, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Du, W.; Liang, F.; Duan, Y.; Tan, X.; Lu, X. Exploring the photosynthetic production capacity of sucrose by cyanobacteria. Metab. Eng. 2013, 19, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Viola, S.; Rühle, T.; Leister, D. A single vector-based strategy for marker-less gene replacement in Synechocystis sp. PCC 6803. Microb. Cell Fact. 2014, 13. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Qi, F.; Tan, X.; Lu, X. Improved production of fatty alcohols in cyanobacteria by metabolic engineering. Biotechnol. Biofuels 2014, 7. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, K.; Hirasawa, T.; Shimizu, H. Effect of malic enzyme on ethanol production by Synechocystis sp. PCC 6803. J. Biosci. Bioeng. 2015, 119, 82–84. [Google Scholar] [CrossRef]
- Namakoshi, K.; Nakajima, T.; Yoshikawa, K.; Toya, Y.; Shimizu, H. Combinatorial deletions of glgC and phaCE enhance ethanol production in Synechocystis sp. PCC 6803. J. Biotechnol. 2016, 239, 13–19. [Google Scholar] [CrossRef]
- Bartasun, P.; Prandi, N.; Storch, M.; Aknin, Y.; Bennett, M.; Palma, A.; Baldwin, G.; Sakuragi, Y.; Jones, P.R.; Rowland, J. The effect of modulating the quantity of enzymes in a model ethanol pathway on metabolic flux in Synechocystis sp. PCC 6803. PeerJ 2019, 7. [Google Scholar] [CrossRef] [PubMed]
- Savakis, P.; Tan, X.; Du, W.; Branco Dos Santos, F.; Lu, X.; Hellingwerf, K.J. Photosynthetic production of glycerol by a recombinant cyanobacterium. J. Biotechnol. 2015, 195, 46–51. [Google Scholar] [CrossRef]
- Anfelt, J.; Kaczmarzyk, D.; Shabestary, K.; Renberg, B.; Rockberg, J.; Nielsen, J.; Uhlén, M.; Hudson, E.P. Genetic and nutrient modulation of acetyl-CoA levels in Synechocystis for n-butanol production. Microb. Cell Fact. 2015, 14. [Google Scholar] [CrossRef]
- Hu, J.; Li, T.; Xu, W.; Zhan, J.; Chen, H.; He, C.; Wang, Q. Small antisense RNA RblR positively regulates RuBisCo in Synechocystis sp. PCC 6803. Front. Microbiol. 2017. [Google Scholar] [CrossRef][Green Version]
- Wang, B.; Eckert, C.; Maness, P.C.; Yu, J. A Genetic Toolbox for Modulating the Expression of Heterologous Genes in the Cyanobacterium Synechocystis sp. PCC 6803. ACS Synth. Biol. 2018, 7, 276–286. [Google Scholar] [CrossRef]
- David, C.; Schmid, A.; Adrian, L.; Wilde, A.; Bühler, K. Production of 1,2-propanediol in photoautotrophic Synechocystis is linked to glycogen turn-over. Biotechnol. Bioeng. 2018, 115, 300–311. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.; Wang, W.; Lu, X. High Light Induced Alka(e)ne Biodegradation for Lipid and Redox Homeostasis in Cyanobacteria. Front. Microbiol. 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Du, W.; Gallego, R.P.; Hellingwerf, K.J.; Van Der Woude, A.D.; Dos Santos, F.B. Using osmotic stress to stabilize mannitol production in Synechocystis sp. PCC6803. Biotechnol. Biofuels 2020, 13. [Google Scholar] [CrossRef] [PubMed]
- Sebesta, J.; Peebles, C.A. Improving heterologous protein expression in Synechocystis sp. PCC 6803 for alpha-bisabolene production. Metab. Eng. Commun. 2020, 10. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Zhao, H.; Li, Z.; Tan, X.; Lu, X. Photosynthetic production of ethanol from carbon dioxide in genetically engineered cyanobacteria. Energy Environ. Sci. 2012, 5, 9857–9865. [Google Scholar] [CrossRef]
- Wang, W.; Liu, X.; Lu, X. Engineering cyanobacteria to improve photosynthetic production of alka(e)nes. Biotechnol. Biofuels 2013, 6. [Google Scholar] [CrossRef]
- Immethun, C.M.; DeLorenzo, D.M.; Focht, C.M.; Gupta, D.; Johnson, C.B.; Moon, T.S. Physical, chemical, and metabolic state sensors expand the synthetic biology toolbox for Synechocystis sp. PCC 6803. Biotechnol. Bioeng. 2017, 114, 1561–1569. [Google Scholar] [CrossRef]
- Gao, Q.; Wang, W.; Zhao, H.; Lu, X. Effects of fatty acid activation on photosynthetic production of fatty acid-based biofuels in Synechocystis sp. PCC6803. Biotechnol. Biofuels 2012, 5. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.C.; Xiong, W.; Paddock, T.; Carrieri, D.; Chang, I.F.; Chiu, H.F.; Ungerer, J.; Hank Juo, S.H.; Maness, P.C.; Yu, J. Engineered xylose utilization enhances bio-products productivity in the cyanobacterium Synechocystis sp. PCC 6803. Metab. Eng. 2015, 30, 179–189. [Google Scholar] [CrossRef]
- Hein, S.; Tran, H.; Steinbüchel, A. Synechocystis sp. PCC6803 possesses a two-component polyhydroxyalkanoic acid synthase similar to that of anoxygenic purple sulfur bacteria. Arch. Microbiol. 1998, 170, 162–170. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, H.; Zhang, Y.; Li, Y.; Ma, Y. Designing and creating a modularized synthetic pathway in cyanobacterium Synechocystis enables production of acetone from carbon dioxide. Metab. Eng. 2012, 14, 394–400. [Google Scholar] [CrossRef]
- Cameron, J.C.; Pakrasi, H.B. Essential role of glutathione in acclimation to environmental and redox perturbations in the cyanobacterium Synechocystis sp. PCC 6803. Plant Physiol. 2010, 154, 1672–1685. [Google Scholar] [CrossRef]
- Aoki, R.; Goto, T.; Fujita, Y. A heme oxygenase isoform is essential for aerobic growth in the cyanobacterium Synechocystis sp. PCC 6803: Modes of differential operation of two isoforms/enzymes to adapt to low oxygen environments in cyanobacteria. Plant Cell Physiol. 2011, 52, 1744–1756. [Google Scholar] [CrossRef] [PubMed]
- Osanai, T.; Oikawa, A.; Numata, K.; Kuwahara, A.; Iijima, H.; Doi, Y.; Saito, K.; Hirai, M.Y. Pathway-level acceleration of glycogen catabolism by a response regulator in the cyanobacterium Synechocystis species PCC 6803. Plant Physiol. 2014, 164, 1831–1841. [Google Scholar] [CrossRef]
- Hasunuma, T.; Matsuda, M.; Senga, Y.; Aikawa, S.; Toyoshima, M.; Shimakawa, G.; Miyake, C.; Kondo, A. Overexpression of flv3 improves photosynthesis in the cyanobacterium Synechocystis sp. PCC6803 by enhancement of alternative electron flow. Biotechnol. Biofuels 2014, 7. [Google Scholar] [CrossRef]
- Tashiro, M.; Kiyota, H.; Kawai-Noma, S.; Saito, K.; Ikeuchi, M.; Iijima, Y.; Umeno, D. Bacterial Production of Pinene by a Laboratory-Evolved Pinene-Synthase. ACS Synth. Biol. 2016, 5. [Google Scholar] [CrossRef]
- Englund, E.; Liang, F.; Lindberg, P. Evaluation of promoters and ribosome binding sites for biotechnological applications in the unicellular cyanobacterium Synechocystis sp. PCC 6803. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef]
- Behler, J.; Vijay, D.; Hess, W.R.; Akhtar, M.K. CRISPR-Based Technologies for Metabolic Engineering in Cyanobacteria. Trends Biotechnol. 2018, 36, 996–1010. [Google Scholar] [CrossRef] [PubMed]
- Durall, C.; Lindberg, P.; Yu, J.; Lindblad, P. Increased ethylene production by overexpressing phosphoenolpyruvate carboxylase in the cyanobacterium Synechocystis PCC 6803. Biotechnol. Biofuels 2020, 13. [Google Scholar] [CrossRef] [PubMed]
- Matsudaira, A.; Hoshino, Y.; Uesaka, K.; Takatani, N.; Omata, T.; Usuda, Y. Production of glutamate and stereospecific flavors, (S)-linalool and (+)-valencene, by Synechocystis sp. PCC6803. J. Biosci. Bioeng. 2020, 130, 464–470. [Google Scholar] [CrossRef]
- Kiyota, H.; Okuda, Y.; Ito, M.; Hirai, M.Y.; Ikeuchi, M. Engineering of cyanobacteria for the photosynthetic production of limonene from CO2. J. Biotechnol. 2014, 185, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Sun, T.; Gao, X.; Shi, M.; Wu, L.; Chen, L.; Zhang, W. Biosynthesis of platform chemical 3-hydroxypropionic acid (3-HP) directly from CO2 in cyanobacterium Synechocystis sp. PCC 6803. Metab. Eng. 2016, 34, 60–70. [Google Scholar] [CrossRef]
- Englund, E.; Andersen-Ranberg, J.; Miao, R.; Hamberger, B.; Lindberg, P. Metabolic Engineering of Synechocystis sp. PCC 6803 for Production of the Plant Diterpenoid Manoyl Oxide. ACS Synth. Biol. 2015, 4, 1270–1278. [Google Scholar] [CrossRef]
- Shabestary, K.; Anfelt, J.; Ljungqvist, E.; Jahn, M.; Yao, L.; Hudson, E.P. Targeted Repression of Essential Genes to Arrest Growth and Increase Carbon Partitioning and Biofuel Titers in Cyanobacteria. ACS Synth. Biol. 2018, 7, 1669–1675. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Pugh, S.; Nielsen, D.R.; Zhang, W.; Meldrum, D.R. Engineering cyanobacteria for photosynthetic production of 3-hydroxybutyrate directly from CO2. Metab. Eng. 2013, 16, 68–77. [Google Scholar] [CrossRef]
- Li, H.; Liao, J.C. Engineering a cyanobacterium as the catalyst for the photosynthetic conversion of CO2 to 1,2-propanediol. Microb. Cell Fact. 2013, 12. [Google Scholar] [CrossRef]
- Wang, Y.; Tao, F.; Ni, J.; Li, C.; Xu, P. Production of C3 platform chemicals from CO2 by genetically engineered cyanobacteria. Green Chem. 2015, 17. [Google Scholar] [CrossRef]
- Li, C.; Tao, F.; Ni, J.; Wang, Y.; Yao, F.; Xu, P. Enhancing the light-driven production of d-lactate by engineering cyanobacterium using a combinational strategy. Sci. Rep. 2015, 5, 9777. [Google Scholar] [CrossRef]
- Lan, E.I.; Wei, C.T. Metabolic engineering of cyanobacteria for the photosynthetic production of succinate. Metab. Eng. 2016, 38, 483–493. [Google Scholar] [CrossRef]
- Carbonell, V.; Vuorio, E.; Aro, E.M.; Kallio, P. Enhanced stable production of ethylene in photosynthetic cyanobacterium Synechococcus elongatus PCC 7942. World J. Microbiol. Biotechnol. 2019, 35, 77. [Google Scholar] [CrossRef]
- Velmurugan, R.; Incharoensakdi, A. Heterologous Expression of Ethanol Synthesis Pathway in Glycogen Deficient Synechococcus elongatus PCC 7942 Resulted in Enhanced Production of Ethanol and Exopolysaccharides. Front Plant Sci 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Ruffing, A.M.; Jones, H.D.T. Physiological effects of free fatty acid production in genetically engineered Synechococcus elongatus PCC 7942. Biotechnol. Bioeng. 2012, 109, 2190–2199. [Google Scholar] [CrossRef]
- Ruffing, A.M. Borrowing genes from Chlamydomonas reinhardtii for free fatty acid production in engineered cyanobacteria. J. Appl. Phycol. 2013, 25, 1495–1507. [Google Scholar] [CrossRef]
- Ruffing, A.M. Improved free fatty acid production in cyanobacteria with Synechococcus sp. PCC 7002 as host. Front. Bioeng. Biotechnol. 2014, 2, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kachel, B.; Mack, M. Engineering of Synechococcus sp. strain PCC 7002 for the photoautotrophic production of light-sensitive riboflavin (vitamin B2). Metab. Eng. 2020, 62, 275–286. [Google Scholar] [CrossRef]
- Jazmin, L.J.; Xu, Y.; Cheah, Y.E.; Adebiyi, A.O.; Johnson, C.H.; Young, J.D. Isotopically nonstationary 13C flux analysis of cyanobacterial isobutyraldehyde production. Metab. Eng. 2017, 42, 9–18. [Google Scholar] [CrossRef]
- Sommer, M.; Sutter, M.; Gupta, S.; Kirst, H.; Turmo, A.; Lechno-Yossef, S.; Burton, R.L.; Saechao, C.; Sloan, N.B.; Cheng, X.; et al. Heterohexamers formed by CcmK3 and CcmK4 increase the complexity of beta carboxysome shells. Plant Physiol. 2019, 179, 156–167. [Google Scholar] [CrossRef]
- Clerico, E.M.; Cassone, V.M.; Golden, S.S. Stability and lability of circadian period of gene expression in the cyanobacterium Synechococcus elongatus. Microbiology 2009, 155, 635–641. [Google Scholar] [CrossRef][Green Version]
- Atsumi, S.; Higashide, W.; Liao, J.C. Direct photosynthetic recycling of carbon dioxide to isobutyraldehyde. Nat. Biotechnol. 2009, 27, 1177–1180. [Google Scholar] [CrossRef] [PubMed]
- Lan, E.I.; Liao, J.C. Metabolic engineering of cyanobacteria for 1-butanol production from carbon dioxide. Metab. Eng. 2011, 13, 353–363. [Google Scholar] [CrossRef]
- Lan, E.I.; Liao, J.C. ATP drives direct photosynthetic production of 1-butanol in cyanobacteria. Proc. Natl. Acad. Sci. USA 2012, 109, 6018–6023. [Google Scholar] [CrossRef] [PubMed]
- Cameron, J.C.; Wilson, S.C.; Bernstein, S.L.; Kerfeld, C.A. Biogenesis of a bacterial organelle: The carboxysome assembly pathway. Cell 2013, 155, 1131–1140. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.H.; Robinson-Mosher, A.; Savage, D.F.; Silver, P.A.; Polka, J.K. The Bacterial Carbon-Fixing Organelle Is Formed by Shell Envelopment of Preassembled Cargo. PLoS ONE 2013, 8, e76127. [Google Scholar] [CrossRef] [PubMed]
- Kusakabe, T.; Tatsuke, T.; Tsuruno, K.; Hirokawa, Y.; Atsumi, S.; Liao, J.C.; Hanai, T. Engineering a synthetic pathway in cyanobacteria for isopropanol production directly from carbon dioxide and light. Metab. Eng. 2013, 20, 101–108. [Google Scholar] [CrossRef]
- Li, X.; Shen, C.R.; Liao, J.C. Isobutanol production as an alternative metabolic sink to rescue the growth deficiency of the glycogen mutant of Synechococcus elongatus PCC 7942. Photosynth. Res. 2014, 120, 301–310. [Google Scholar] [CrossRef]
- Lan, E.I.; Chuang, D.S.; Shen, C.R.; Lee, A.M.; Ro, S.Y.; Liao, J.C. Metabolic engineering of cyanobacteria for photosynthetic 3-hydroxypropionic acid production from CO2 using Synechococcus elongatus PCC 7942. Metab. Eng. 2015, 31, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Hirokawa, Y.; Maki, Y.; Tatsuke, T.; Hanai, T. Cyanobacterial production of 1,3-propanediol directly from carbon dioxide using a synthetic metabolic pathway. Metab. Eng. 2016, 34, 97–103. [Google Scholar] [CrossRef]
- Wang, X.; Liu, W.; Xin, C.; Zheng, Y.; Cheng, Y.; Sun, S.; Li, R.; Zhu, X.-G.; Dai, S.Y.; Rentzepis, P.M.; et al. Enhanced limonene production in cyanobacteria reveals photosynthesis limitations. Proc. Natl. Acad. Sci. USA 2016, 113, 14225–14230. [Google Scholar] [CrossRef]
- Chwa, J.W.; Kim, W.J.; Sim, S.J.; Um, Y.; Woo, H.M. Engineering of a modular and synthetic phosphoketolase pathway for photosynthetic production of acetone from CO2 in Synechococcus elongatus PCC 7942 under light and aerobic condition. Plant Biotechnol. J. 2016, 14, 1768–1776. [Google Scholar] [CrossRef]
- Gao, X.; Gao, F.; Liu, D.; Zhang, H.; Nie, X.; Yang, C. Engineering the methylerythritol phosphate pathway in cyanobacteria for photosynthetic isoprene production from CO2. Energy Environ. Sci. 2016, 7, 71–86. [Google Scholar] [CrossRef]
- Taton, A.; Ma, A.T.; Ota, M.; Golden, S.S.; Golden, J.W. NOT Gate Genetic Circuits to Control Gene Expression in Cyanobacteria. ACS Synth. Biol. 2017, 6, 2175. [Google Scholar] [CrossRef]
- Kanno, M.; Atsumi, S. Engineering an Obligate photoautotrophic cyanobacterium to utilize glycerol for growth and chemical production. ACS Synth. Biol. 2017, 6, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Lee, J.; Lee, S.-M.; Um, Y.; Kim, Y.; Sim, S.J.; Choi, J.-I.; Woo, H.M. Direct Conversion of CO2 to α-Farnesene Using Metabolically Engineered Synechococcus elongatus PCC 7942. J. Agric. Food Chem. 2017, 60, 10424–10428. [Google Scholar] [CrossRef] [PubMed]
- Pattharaprachayakul, N.; Lee, H.J.; Incharoensakdi, A.; Woo, H.M. Evolutionary Engineering of Cyanobacteria to Enhance the Production of α-Farnesene from CO2. J. Agric. Food Chem. 2019, 67, 13658–13664. [Google Scholar] [CrossRef] [PubMed]
- Hirokawa, Y.; Goto, R.; Umetani, Y.; Hanai, T. Construction of a novel D-lactate producing pathway from dihydroxyacetone phosphate of the Calvin cycle in cyanobacterium, Synechococcus elongatus PCC 7942. J. Biosci. Bioeng. 2017, 124, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Ducat, D.C.; Avelar-Rivas, J.A.; Way, J.C.; Silvera, P.A. Rerouting carbon flux to enhance photosynthetic productivity. Appl. Environ. Microbiol. 2012, 78, 2660–2668. [Google Scholar] [CrossRef] [PubMed]
- Shih, P.M.; Zarzycki, J.; Niyogi, K.K.; Kerfeld, C.A. Introduction of a synthetic CO2-fixing photorespiratory bypass into a cyanobacterium. J. Biol. Chem. 2014, 289, 9493–9500. [Google Scholar] [CrossRef]
- Oliver, J.W.K.; Atsumi, S. A carbon sink pathway increases carbon productivity in cyanobacteria. Metab. Eng. 2015, 29, 106–112. [Google Scholar] [CrossRef]
- Choi, S.Y.; Sim, S.J.; Choi, J.I.; Woo, H.M. Identification of small droplets of photosynthetic squalene in engineered Synechococcus elongatus PCC 7942 using TEM and selective fluorescent Nile red analysis. Lett. Appl. Microbiol. 2018, 66, 523–529. [Google Scholar] [CrossRef]
- Kim, W.J.; Lee, S.M.; Um, Y.; Sim, S.J.; Woo, H.M. Development of synebrick vectors as a synthetic biology platform for gene expression in Synechococcus elongatus PCC 7942. Front. Plant Sci. 2017, 8. [Google Scholar] [CrossRef]
- Roulet, J.; Taton, A.; Golden, J.W.; Arabolaza, A.; Burkart, M.D.; Gramajo, H. Development of a cyanobacterial heterologous polyketide production platform. Metab. Eng. 2018, 49, 94–104. [Google Scholar] [CrossRef] [PubMed]
- Takahama, K.; Matsuoka, M.; Nagahama, K.; Ogawa, T. Construction and analysis of a recombinant cyanobacterium expressing a chromosomally inserted gene for an ethylene-forming enzyme at the psbAI locus. J. Biosci. Bioeng. 2003, 15, 302–305. [Google Scholar] [CrossRef]
- Oliver, J.W.K.; Machado, I.M.P.; Yoneda, H.; Atsumi, S. Cyanobacterial conversion of carbon dioxide to 2,3-butanediol. Proc. Natl. Acad. Sci. USA 2013, 110, 1249–1254. [Google Scholar] [CrossRef]
- Peterson, E.S.; McCue, L.A.; Schrimpe-Rutledge, A.C.; Jensen, J.L.; Walker, H.; Kobold, M.A.; Webb, S.R.; Payne, S.H.; Ansong, C.; Adkins, J.N.; et al. VESPA: Software to facilitate genomic annotation of prokaryotic organisms through integration of proteomic and transcriptomic data. BMC Genom. 2012, 13. [Google Scholar] [CrossRef]
- Begemann, M.B.; Zess, E.K.; Walters, E.M.; Schmitt, E.F.; Markley, A.L.; Pfleger, B.F. An Organic Acid Based Counter Selection System for Cyanobacteria. PLoS ONE 2013, 8, e76594. [Google Scholar] [CrossRef]
- Clark, R.L.; Gordon, G.C.; Bennett, N.R.; Lyu, H.; Root, T.W.; Pfleger, B.F. High-CO2 Requirement as a Mechanism for the Containment of Genetically Modified Cyanobacteria. ACS Synth. Biol. 2018, 7, 384–391. [Google Scholar] [CrossRef] [PubMed]
- Markley, A.L.; Begemann, M.B.; Clarke, R.E.; Gordon, G.C.; Pfleger, B.F. Synthetic Biology Toolbox for Controlling Gene Expression in the Cyanobacterium Synechococcus sp. strain PCC 7002. ACS Synth. Biol. 2015, 4, 595–603. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, T.; Shen, G.; Higashi, S.; Murata, N.; Bryant, D.A. Alteration of low-temperature susceptibility of the cyanobacterium Synechococcus sp. PCC 7002 by genetic manipulation of membrane lipid unsaturation. Arch. Microbiol. 1997, 169, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Vogel, A.I.M.; Lale, R.; Hohmann-Marriott, M.F. Streamlining recombination-mediated genetic engineering by validating three neutral integration sites in Synechococcus sp. PCC 7002. J. Biol. Eng. 2017, 11. [Google Scholar] [CrossRef]
- Selão, T.T.; Włodarczyk, A.; Nixon, P.J.; Norling, B. Growth and selection of the cyanobacterium Synechococcus sp. PCC 7002 using alternative nitrogen and phosphorus sources. Metab. Eng. 2019, 54, 2. [Google Scholar] [CrossRef]
- Korosh, T.C.; Markley, A.L.; Clark, R.L.; McGinley, L.L.; McMahon, K.D.; Pfleger, B.F. Engineering photosynthetic production of L-lysine. Metab. Eng. 2017, 44, 273–283. [Google Scholar] [CrossRef]
- Daniell, H.; Sarojini, G.; McFadden, B.A. Transformation of the cyanobacterium Anacystis nidulans 6301 with the Escherichia coli plasmid pBR322. Proc. Natl. Acad. Sci. USA 1986, 83, 2546–2550. [Google Scholar] [CrossRef]
- Koksharova, O.; Wolk, C. Genetic tools for cyanobacteria. Appl. Microbiol. Biotechnol. 2002, 58, 123–137. [Google Scholar] [CrossRef]
- Sherman, L.A.; Van de Putte, P. Construction of a hybrid plasmid capable of replication in the bacterium Escherichia coli and the cyanobacterium Anacystis nidulans. J. Bacteriol. 1982, 150, 410–413. [Google Scholar] [CrossRef]
- Golden, S.S.; Sherman, L.A. Optimal conditions for genetic transformation of the cyanobacterium Anacystis nidulans R2. J. Bacteriol. 1984, 158, 36–42. [Google Scholar] [CrossRef]
- Buzby, J.S.; Porter, R.D.; Stevens, S.E. Plasmid transformation in Agmenellum quadruplicatum PR-6: Construction of biphasic plasmids and characterization of their transformation properties. J. Bacteriol. 1983, 154, 1446–1450. [Google Scholar] [CrossRef] [PubMed]
- Scharnagl, M.; Richter, S.; Hagemann, M. The cyanobacterium Synechocystis sp. strain PCC 6803 expresses a DNA methyltransferase specific for the recognition sequence of the restriction endonuclease PvuI. J. Bacteriol. 1998, 180, 4116–4122. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Yu, J.; Zhang, W.; Meldrum, D.R. Premethylation of Foreign DNA Improves Integrative Transformation. Appl. Environ. Microbiol. 2015, 81, 8500–8506. [Google Scholar] [CrossRef] [PubMed]
- Hagemann, M.; Gartner, K.; Scharnagl, M.; Bolay, P.; Lott, S.C.; Fuss, J.; Huettel, B.; Reinhardt, R.; Klahn, S.; Hess, W.R. Identification of the DNA methyltransferases establishing the methylome of the cyanobacterium Synechocystis sp. PCC 6803. DNA Res. 2018, 25, 343–352. [Google Scholar] [CrossRef]
- Wu, D.; Wang, Y.; Xu, X. Effects of a Type I RM System on Gene Expression and Glycogen Catabolism in Synechocystis sp. PCC 6803. Front. Microbiol. 2020, 11, 1258. [Google Scholar] [CrossRef]
- Kuhlemeier, C.J.; Thomas, A.A.M.; van der Ende, A.; van Leen, R.W.; Borrias, W.E.; van den Hondel, C.A.M.J.J.; van Arkel, G.A. A host-vector system for gene cloning in the cyanobacterium Anacystis nidulans R2. Plasmid 1983, 10, 156–163. [Google Scholar] [CrossRef]
- Golden, S.S.; Sherman, L.A. A hybrid plasmid is a stable cloning vector for the cyanobacterium Anacystis nidulans R2. J. Bacteriol. 1983, 155, 966–972. [Google Scholar] [CrossRef] [PubMed]
- Gendel, S.; Straus, N.; Pulleyblank, D.; Williams, J. Shuttle cloning vectors for the cyanobacterium Anacystis nidulans. J. Bacteriol. 1983, 156, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Murphy, R.C.; Bryant, D.A.; Porter, R.D.; Tandeau De Marsac, N. Molecular cloning and characterization of the recA gene from the Cyanobacterium Synechococcus sp. strain PCC 7002. J. Bacteriol. 1987, 172, 967–976. [Google Scholar] [CrossRef] [PubMed]
- Kuhlemeier, C.J.; Logtenberg, T.; Stoorvogel, W.; van Heugten, H.A.; Borrias, W.E.; van Arkel, G.A. Cloning of nitrate reductase genes from the cyanobacterium Anacystis nidulans. J. Bacteriol. 1984, 159, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Gruber, M.Y.; Glick, B.R.; Thompson, J.E. Cloned manganese superoxide dismutase reduces oxidative stress in Escherichia coli and Anacystis nidulans. Proc. Natl. Acad. Sci. USA 1990, 87, 2608–2612. [Google Scholar] [CrossRef] [PubMed]
- Ferino, F.; Chauvat, F. A promoter-probe vector-host system for the cyanobacterium, Synechocystis PCC6803. Gene 1989, 84, 257–266. [Google Scholar] [CrossRef]
- Marraccini, P.; Bulteau, S.; Cassier-Chauvat, C.; Mermet-Bouvier, P.; Chauvat, F. A conjugative plasmid vector for promoter analysis in several cyanobacteria of the genera Synechococcus and Synechocystis. Plant Mol. Biol. 1993, 23, 905–909. [Google Scholar] [CrossRef]
- Chenebault, C.; Diaz-Santos, E.; Kammerscheit, X.; Görgen, S.; Ilioaia, C.; Streckaite, S.; Gall, A.; Robert, B.; Marcon, E.; Buisson, D.-A.; et al. A Genetic Toolbox for the New Model Cyanobacterium Cyanothece PCC 7425: A Case Study for the Photosynthetic Production of Limonene. Front. Microbiol. 2020, 11, 586–601. [Google Scholar] [CrossRef]
- Murphy, R.C.; Stevens, S.E. Cloning and expression of the cryIVD gene of Bacillus thuringiensis subsp. israelensis in the cyanobacterium Agmenellum quadruplicatum PR-6 and its resulting larvicidal activity. Appl. Environ. Microbiol. 1992, 58, 1650–1655. [Google Scholar] [CrossRef] [PubMed]
- Soltes-Rak, E.; Kushner, D.J.; Williams, D.D.; Coleman, J.R. Effect of promoter modification on mosquitocidal cryIVB gene expression in Synechococcus sp. strain PCC 7942. Appl. Environ. Microbiol. 1993, 59, 2404–2410. [Google Scholar] [CrossRef]
- Erbe, J.L.; Taylor, K.B.; Hall, L.M. Expression of mouse metallothionein in the cyanobacterium Synechococcus PCC7942. J. Ind. Microbiol. 1996, 17, 41–46. [Google Scholar] [CrossRef]
- Nomura, M.; Ishitani, M.; Takabe, T.; Rai, A.K.; Takabe, T. Synechococcus sp. PCC7942 transformed with Escherichia coli bet genes produces glycine betaine from choline and acquires resistance to salt stress. Plant Physiol. 1995, 107, 703–708. [Google Scholar] [CrossRef]
- Kaku, N.; Hibino, T.; Tanaka, Y.; Ishikawa, H.; Araki, E.; Takabe, T.; Takabe, T. Effects of overexpression of Escherichia coli katE and bet genes on the tolerance for salt stress in a freshwater cyanobacterium Synechococcus sp. PCC 7942. Plant Sci. 2000, 159, 281–288. [Google Scholar] [CrossRef]
- De Deng, M.; Coleman, J.R. Ethanol synthesis by genetic engineering in cyanobacteria. Appl. Environ. Microbiol. 1999, 65, 523–528. [Google Scholar] [CrossRef]
- Fukuda, H.; Sakai, M.; Nagahama, K.; Fujii, T.; Matsuoka, M.; Inoue, Y.; Ogawa, T. Heterologous expression of the gene for the ethylene-forming enzyme from Pseudomonas syringae in the cyanobacterium Synechococcus. Biotechnol. Lett. 1994, 16, 1–6. [Google Scholar] [CrossRef]
- Sakai, M.; Ogawa, T.; Matsuoka, M.; Fukuda, H. Photosynthetic conversion of carbon dioxide to ethylene by the recombinant cyanobacterium, Synechococcus sp. PCC 7942, which harbors a gene for the ethylene-forming enzyme of Pseudomonas syringae. J. Ferment. Bioeng. 1997, 4, 434–443. [Google Scholar] [CrossRef]
- Jindou, S.; Ito, Y.; Mito, N.; Uematsu, K.; Hosoda, A.; Tamura, H. Engineered platform for bioethylene production by a cyanobacterium expressing a chimeric complex of plant enzymes. ACS Synth. Biol. 2014, 3, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Iwaki, T.; Haranoh, K.; Inoue, N.; Kojima, K.; Satoh, R.; Nishino, T.; Wada, S.; Ihara, H.; Tsuyama, S.; Kobayashi, H.; et al. Expression of foreign type I ribulose-1,5-bisphosphate carboxylase/ oxygenase (EC 4.1.1.39) stimulates photosynthesis in cyanobacterium Synechococcus PCC7942 cells. Photosynth. Res. 2006, 88, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Taton, A.; Go, M.; London, R.E.; Pieper, L.M.; Golden, S.S.; Golden, J.W. Self-replicating shuttle vectors based on pANS, a small endogenous plasmid of the unicellular cyanobacterium Synechococcus elongatus PCC 7942. Microbiology (UK) 2016, 162, 2029–2041. [Google Scholar] [CrossRef]
- Scholz, P.; Haring, V.; Wittmann-Liebold, B.; Ashman, K.; Bagdasarian, M.; Scherzinger, E. Complete nucleotide sequence and gene organization of the broad-host-range plasmid RSF1010. Gene 1989, 75, 271–288. [Google Scholar] [CrossRef]
- Bagdasarian, M.; Lurz, R.; Rückert, B.; Franklin, F.C.H.; Bagdasarian, M.M.; Frey, J.; Timmis, K.N. Specific-purpose plasmid cloning vectors II. Broad host range, high copy number, RSF 1010-derived vectors, and a host-vector system for gene cloning in Pseudomonas. Gene 1981, 16, 237–247. [Google Scholar] [CrossRef]
- Kues, U.; Stahl, U. Replication of plasmids in gram-negative bacteria. Microbiol. Rev. 1989, 53, 491–516. [Google Scholar] [CrossRef] [PubMed]
- Meyer, R. Replication and conjugative mobilization of broad host-range IncQ plasmids. Plasmid 2009, 62, 57–70. [Google Scholar] [CrossRef] [PubMed]
- Kreps, S.; Ferino, F.; Mosrin, C.; Gerits, J.; Mergeay, M.; Thuriaux, P. Conjugative transfer and autonomous replication of a promiscuous IncQ plasmid in the cyanobacterium Synechocystis PCC 6803. MGG Mol. Gen. Genet. 1990, 221, 129–133. [Google Scholar] [CrossRef]
- Sode, K.; Tatara, M.; Takeyama, H.; Burgess, J.G.; Matsunaga, T. Conjugative gene transfer in marine cyanobacteria: Synechococcus sp., Synechocystis sp. and Pseudanabaena sp. Appl. Microbiol. Biotechnol. 1992, 37, 369–373. [Google Scholar] [CrossRef] [PubMed]
- Mermet-Bouvier, P.; Cassier-Chauvat, C.; Marraccini, P.; Chauvat, F. Transfer and replication of RSF1010-derived plasmids in several cyanobacteria of the genera Synechocystis and Synechococcus. Curr. Microbiol. 1993, 27, 323–327. [Google Scholar] [CrossRef]
- Mühlenhoff, U.; Chauvat, F. Gene transfer and manipulation in the thermophilic cyanobacterium Synechococcus elongatus. Mol. Gen. Genet. 1996, 252, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Brahamsha, B. A genetic manipulation system for oceanic cyanobacteria of the genus Synechococcus. Appl. Environ. Microbiol. 1996, 62, 1745–1751. [Google Scholar] [CrossRef]
- Guo, H.; Xu, X. Broad host range plasmid-based gene transfer system in the cyanobacterium Gloeobacter violaceus which lacks thylakoids. Prog. Nat. Sci. 2004, 14, 31–35. [Google Scholar] [CrossRef][Green Version]
- Araki, M.; Shimada, Y.; Mimuro, M.; Tsuchiya, T. Establishment of the reporter system for a thylakoid-lacking cyanobacterium, Gloeobacter violaceus PCC 7421. FEBS Open Bio 2013, 3, 11–15. [Google Scholar] [CrossRef]
- Tolonen, A.C.; Liszt, G.B.; Hess, W.R. Genetic manipulation of Prochlorococcus strain MIT9313: Green fluorescent protein expression from an RSF1010 plasmid and Tn5 transposition. Appl. Environ. Microbiol. 2006, 72, 7607–7613. [Google Scholar] [CrossRef]
- Huang, H.H.; Camsund, D.; Lindblad, P.; Heidorn, T. Design and characterization of molecular tools for a synthetic biology approach towards developing cyanobacterial biotechnology. Nucleic Acids Res. 2010, 38, 2577–2593. [Google Scholar] [CrossRef] [PubMed]
- Bishé, B.; Taton, A.; Golden, J.W. Modification of RSF1010-Based Broad-Host-Range Plasmids for Improved Conjugation and Cyanobacterial Bioprospecting. iScience 2019, 20, 216–228. [Google Scholar] [CrossRef] [PubMed]
- Taton, A.; Lis, E.; Adin, D.M.; Dong, G.; Cookson, S.; Kay, S.A.; Golden, S.S.; Golden, J.W. Gene transfer in Leptolyngbya sp. strain BL0902, a cyanobacterium suitable for production of biomass and bioproducts. PLoS ONE 2012, 7, e30901. [Google Scholar] [CrossRef]
- Mermet-Bouvier, P.; Chauvat, F. A conditional expression vector for the cyanobacteria Synechocystis sp. strains PCC6803 and PCC6714 or Synechococcus sp. strains PCC7942 and PCC6301. Curr. Microbiol. 1994, 28, 145–148. [Google Scholar] [CrossRef] [PubMed]
- Mazouni, K.; Bulteau, S.; Cassier-Chauvat, C.; Chauvat, F. Promoter element spacing controls basal expression and light inducibility of the cyanobacterial secA gene. Mol. Microbiol. 1998, 30, 1113–1122. [Google Scholar] [CrossRef]
- Dutheil, J.; Saenkham, P.; Sakr, S.; Leplat, C.; Ortega-Ramos, M.; Bottin, H.; Cournac, L.; Cassier-Chauvat, C.; Chauvat, F. The AbrB2 autorepressor, expressed from an atypical promoter, represses the hydrogenase operon to regulate hydrogen production in Synechocystis strain PCC6803. J. Bacteriol. 2012, 194, 5423–5433. [Google Scholar] [CrossRef]
- Mazouni, K.; Domain, F.; Cassier-Chauvat, C.; Chauvat, F. Molecular analysis of the key cytokinetic components of cyanobacteria: FtsZ, ZipN and MinCDE. Mol. Microbiol. 2004, 52, 1145–1158. [Google Scholar] [CrossRef]
- Marbouty, M.; Saguez, C.; Cassier-Chauvat, C.; Chauvat, F. Characterization of the FtsZ-interacting septal proteins SepF and Ftn6 in the spherical-celled cyanobacterium Synechocystis strain PCC 6803. J. Bacteriol. 2009, 191, 6178–6185. [Google Scholar] [CrossRef]
- Marbouty, M.; Saguez, C.; Cassier-Chauvat, C.; Chauvat, F. ZipN, an FtsA-like orchestrator of divisome assembly in the model cyanobacterium Synechocystis PCC6803. Mol. Microbiol. 2009, 74, 409–420. [Google Scholar] [CrossRef]
- Damrow, R.; Maldener, I.; Zilliges, Y. The multiple functions of common microbial carbon polymers, glycogen and PHB, during stress responses in the non-diazotrophic cyanobacterium Synechocystis sp. PCC 6803. Front. Microbiol. 2016, 7. [Google Scholar] [CrossRef]
- Ng, W.O.; Zentella, R.; Wang, Y.; Taylor, J.S.A.; Pakrasi, H.B. phrA, the major photoreactivating factor in the cyanobacterium Synechocystis sp. strain PCC 6803 codes for a cyclobutane-pyrimidine-dimer- specific DNA photolyase. Arch. Microbiol. 2000, 173, 412–416. [Google Scholar] [CrossRef]
- De Porcellinis, A.J.; Nørgaard, H.; Brey, L.M.F.; Erstad, S.M.; Jones, P.R.; Heazlewood, J.L.; Sakuragi, Y. Overexpression of bifunctional fructose-1,6-bisphosphatase/sedoheptulose-1,7-bisphosphatase leads to enhanced photosynthesis and global reprogramming of carbon metabolism in Synechococcus sp. PCC 7002. Metab. Eng. 2018, 47, 170–183. [Google Scholar] [CrossRef] [PubMed]
- Dienst, D.; Wichmann, J.; Mantovani, O.; Rodrigues, J.S.; Lindberg, P. High density cultivation for efficient sesquiterpenoid biosynthesis in Synechocystis sp. PCC 6803. Sci. Rep. 2020, 10, 5932. [Google Scholar] [CrossRef]
- Liang, F.; Englund, E.; Lindberg, P.; Lindblad, P. Engineered cyanobacteria with enhanced growth show increased ethanol production and higher biofuel to biomass ratio. Metab. Eng. 2018, 46, 51–59. [Google Scholar] [CrossRef]
- Guerrero, F.; Carbonell, V.; Cossu, M.; Correddu, D.; Jones, P.R. Ethylene Synthesis and Regulated Expression of Recombinant Protein in Synechocystis sp. PCC 6803. PLoS ONE 2012, 7, e50470. [Google Scholar] [CrossRef] [PubMed]
- Kuchmina, E.; Klähn, S.; Jakob, A.; Bigott, W.; Enke, H.; Dühring, U.; Wilde, A. Ethylene production in Synechocystis sp. PCC 6803 promotes phototactic movement. Microbiology (UK) 2017, 16, 1937–1947. [Google Scholar] [CrossRef] [PubMed]
- Thiel, K.; Mulaku, E.; Dandapani, H.; Nagy, C.; Aro, E.M.; Kallio, P. Translation efficiency of heterologous proteins is significantly affected by the genetic context of RBS sequences in engineered cyanobacterium Synechocystis sp. PCC 6803. Microb. Cell Fact. 2018, 17, 34. [Google Scholar] [CrossRef] [PubMed]
- Van der Woude, A.D.; Perez Gallego, R.; Vreugdenhil, A.; Puthan Veetil, V.; Chroumpi, T.; Hellingwerf, K.J. Genetic engineering of Synechocystis PCC6803 for the photoautotrophic production of the sweetener erythritol. Microb. Cell Fact. 2016, 15. [Google Scholar] [CrossRef]
- Ortega-Ramos, M.; Jittawuttipoka, T.; Saenkham, P.; Czarnecka-Kwasiborski, A.; Bottin, H.; Cassier-Chauvat, C.; Chauvat, F. Engineering Synechocystis PCC6803 for hydrogen production: Influence on the tolerance to oxidative and sugar stresses. PLoS ONE 2014, 9, e89372. [Google Scholar] [CrossRef]
- Veaudor, T.; Cassier-Chauvat, C.; Chauvat, F. Overproduction of the cyanobacterial hydrogenase and selection of a mutant thriving on urea, as a possible step towards the future production of hydrogen coupled with water treatment. PLoS ONE 2018, 13, e0198836. [Google Scholar] [CrossRef]
- Miao, R.; Liu, X.; Englund, E.; Lindberg, P.; Lindblad, P. Isobutanol production in Synechocystis PCC 6803 using heterologous and endogenous alcohol dehydrogenases. Metab. Eng. Commun. 2017, 5, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Miao, R.; Xie, H.; Ho, F.M.; Lindblad, P. Protein engineering of α-ketoisovalerate decarboxylase for improved isobutanol production in Synechocystis PCC 6803. Metab. Eng. 2018, 47, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Loeschcke, A.; Dienst, D.; Wewer, V.; Hage-Hülsmann, J.; Dietsch, M.; Kranz-Finger, S.; Hüren, V.; Metzger, S.; Urlacher, V.B.; Gigolashvili, T.; et al. The photosynthetic bacteria Rhodobacter capsulatus and Synechocystis sp. PCC 6803 as new hosts for cyclic plant triterpene biosynthesis. PLoS ONE 2017, 12, e189816. [Google Scholar] [CrossRef] [PubMed]
- Carroll, A.L.; Case, A.E.; Zhang, A.; Atsumi, S. Metabolic engineering tools in model cyanobacteria. Metab. Eng. 2018, 50, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Shabestary, K.; Björk, S.M.; Asplund-Samuelsson, J.; Joensson, H.N.; Jahn, M.; Hudson, E.P. Pooled CRISPRi screening of the cyanobacterium Synechocystis sp PCC 6803 for enhanced industrial phenotypes. Nat. Commun. 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Hihara, Y.; Kamei, A.; Kanehisa, M.; Kaplan, A.; Ikeuchi, M. DNA microarray analysis of cyanobacterial gene expression during acclimation to high light. Plant Cell 2001, 13, 793–806. [Google Scholar] [CrossRef] [PubMed]
- Hihara, Y.; Sonoike, K.; Kanehisa, M.; Ikeuchi, M. DNA microarray analysis of redox-responsive genes in the genome of the cyanobacterium Synechocystis sp. strain PCC 6803. J. Bacteriol. 2003, 185, 1719–1725. [Google Scholar] [CrossRef]
- Kanesaki, Y.; Suzuki, I.; Allakhverdiev, S.I.; Mikami, K.; Murata, N. Salt stress and hyperosmotic stress regulate the expression of different sets of genes in Synechocystis sp. PCC 6803. Biochem. Biophys. Res. Commun. 2002, 290, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Marin, K.; Suzuki, I.; Yamaguchi, K.; Ribbeck, K.; Yamamoto, H.; Kanesaki, Y.; Hagemann, M.; Murata, N. Identification of histidine kinases that act as sensors in the perception of salt stress in Synechocystis sp. PCC 6803. Proc. Natl. Acad. Sci. USA 2003, 100, 9061–9066. [Google Scholar] [CrossRef]
- Marin, K.; Kanesaki, Y.; Los, D.A.; Murata, N.; Suzuki, I.; Hagemann, M. Gene expression profiling reflects physiological processes in salt acclimation of Synechocystis sp. strain PCC 6803. Plant Physiol. 2004, 13, 3290–3300. [Google Scholar] [CrossRef]
- Shoumskaya, M.A.; Paithoonrangsarid, K.; Kanesaki, Y.; Los, D.A.; Zinchenko, V.V.; Tanticharoen, M.; Suzuki, I.; Murata, N. Identical Hik-Rre systems are involved in perception and transduction of salt signals and hyperosmotic signals but regulate the expression of individual genes to different extents in Synechocystis. J. Biol. Chem. 2005, 280, 21531–21538. [Google Scholar] [CrossRef]
- Mikami, K.; Kanesaki, Y.; Suzuki, I.; Murata, N. The histidine kinase Hik33 perceives osmotic stress and cold stress in Synechocystis sp. PCC 6803. Mol. Microbiol. 2002, 46, 905–915. [Google Scholar] [CrossRef]
- Ohta, H.; Shibata, Y.; Haseyama, Y.; Yoshino, Y.; Suzuki, T.; Kagasawa, T.; Kamei, A.; Ikeuchi, M.; Enami, I. Identification of genes expressed in response to acid stress in Synechocystis sp. PCC 6803 using DNA microarrays. Photosynth. Res. 2005, 84, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Uchiyama, J.; Asakura, R.; Kimura, M.; Moriyama, A.; Tahara, H.; Kobayashi, Y.; Kubo, Y.; Yoshihara, T.; Ohta, H. Slr0967 and Sll0939 induced by the SphR response regulator in Synechocystis sp. PCC 6803 are essential for growth under acid stress conditions. Biochim. Biophys. Acta Bioenerg. 2012, 1817, 1270–1276. [Google Scholar] [CrossRef]
- Suzuki, I.; Kanesaki, Y.; Hayashi, H.; Hall, J.J.; Simon, W.J.; Slabas, A.R.; Murata, N. The histidine kinase Hik34 is involved in thermotolerance by regulating the expression of heat shock genes in Synechocystis. Plant Physiol. 2005, 138, 1409–1421. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, I.; Simon, W.J.; Slabas, A.R. The heat shock response of Synechocystis sp. PCC 6803 analysed by transcriptomics and proteomics. J. Exp. Bot. 2006, 57, 1573–1578. [Google Scholar] [CrossRef] [PubMed]
- Rowland, J.G.; Pang, X.; Suzuki, I.; Murata, N.; Simon, W.J.; Slabas, A.R. Identification of components associated with thermal acclimation of photosystem II in Synechocystis sp. PCC6803. PLoS ONE 2010, 5, e10511. [Google Scholar] [CrossRef] [PubMed]
- Paithoonrangsarid, K.; Shoumskaya, M.A.; Kanesaki, Y.; Satoh, S.; Tabata, S.; Los, D.A.; Zinchenko, V.V.; Hayashi, H.; Tanticharoen, M.; Suzuki, I.; et al. Five histidine kinases perceive osmotic stress and regulate distinct sets of genes in Synechocystis. J. Biol. Chem. 2004, 279, 53078–53086. [Google Scholar] [CrossRef] [PubMed]
- Shapiguzov, A.; Lyukevich, A.A.; Allakhverdiev, S.I.; Sergeyenko, T.V.; Suzuki, I.; Murata, N.; Los, D.A. Osmotic shrinkage of cells of Synechocystis sp. PCC 6803 by water efflux via aquaporins regulates osmostress-inducible gene expression. Microbiology 2005, 151, 447–455. [Google Scholar] [CrossRef]
- Kobayashi, M.; Ishizuka, T.; Katayama, M.; Kanehisa, M.; Bhattacharyya-Pakrasi, M.; Pakrasi, H.B.; Ikeuchi, M. Response to oxidative stress involves a novel peroxiredoxin gene in the unicellular cyanobacterium Synechocystis sp. PCC 6803. Plant Cell Physiol. 2004, 45, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Houot, L.; Floutier, M.; Marteyn, B.; Michaut, M.; Picciocchi, A.; Legrain, P.; Aude, J.C.; Cassier-Chauvat, C.; Chauvat, F. Cadmium triggers an integrated reprogramming of the metabolism of Synechocystis PCC6803, under the control of the Slr1738 regulator. BMC Genom. 2007, 8. [Google Scholar] [CrossRef] [PubMed]
- Kanesaki, Y.; Yamamoto, H.; Paithoonrangsarid, K.; Shoumskaya, M.; Suzuki, I.; Hayashi, H.; Murata, N. Histidine kinases play important roles in the perception and signal transduction of hydrogen peroxide in the cyanobacterium, Synechocystis sp. PCC 6803. Plant J. 2007, 49, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Pendse, N.D.; Phillips, K.N.; Cotner, J.B.; Khodursky, A. Gene expression patterns of sulfur starvation in Synechocystis sp. PCC 6803. BMC Genom. 2008, 9. [Google Scholar] [CrossRef]
- Leplat, C.; Champeimont, R.; Saenkham, P.; Cassier-Chauvat, C.; Jean-Christophe, A.; Chauvat, F. Genome-wide transcriptome analysis of hydrogen production in the cyanobacterium Synechocystis: Towards the identification of new players. Int. J. Hydrog. Energy 2013, 38, 1866–1872. [Google Scholar] [CrossRef]
- Mitschke, J.; Georg, J.; Scholz, I.; Sharma, C.M.; Dienst, D.; Bantscheff, J.; Voß, B.; Steglich, C.; Wilde, A.; Vogel, J.; et al. An experimentally anchored map of transcriptional start sites in the model cyanobacterium Synechocystis sp. PCC6803. Proc. Natl. Acad. Sci. USA 2011, 108, 2124–2129. [Google Scholar] [CrossRef]
- Muro-Pastor, A.M.; Hess, W.R. Regulatory RNA at the crossroads of carbon and nitrogen metabolism in photosynthetic cyanobacteria. Biochim. Biophys. Acta Gene Regul. Mech. 2020, 1863, 194477. [Google Scholar] [CrossRef]
- Baumgartner, D.; Kopf, M.; Klähn, S.; Steglich, C.; Hess, W.R. Small proteins in cyanobacteria provide a paradigm for the functional analysis of the bacterial micro-proteome. BMC Microbiol. 2016, 16. [Google Scholar] [CrossRef]
- Huang, S.; Chen, L.; Te, R.; Qiao, J.; Wang, J.; Zhang, W. Complementary iTRAQ proteomics and RNA-seq transcriptomics reveal multiple levels of regulation in response to nitrogen starvation in Synechocystis sp. PCC 6803. Mol. Biosyst. 2013, 9, 2565–2574. [Google Scholar] [CrossRef]
- Pei, G.; Sun, T.; Chen, S.; Chen, L.; Zhang, W. Systematic and functional identification of small non-coding RNAs associated with exogenous biofuel stress in cyanobacterium Synechocystis sp. PCC 6803. Biotechnol. Biofuels 2017, 10. [Google Scholar] [CrossRef]
- Hernández-Prieto, M.A.; Semeniuk, T.A.; Futschik, M.E. Toward a systems-level understanding of gene regulatory, protein interaction, and metabolic networks in cyanobacteria. Front. Genet. 2014, 5. [Google Scholar] [CrossRef]
- Nakao, M.; Okamoto, S.; Kohara, M.; Fujishiro, T.; Fujisawa, T.; Sato, S.; Tabata, S.; Kaneko, T.; Nakamura, Y. CyanoBase: The cyanobacteria genome database update 2010. Nucleic Acids Res. 2009, 38, D379–D381. [Google Scholar] [CrossRef]
- Zhang, Y.; Fonslow, B.R.; Shan, B.; Baek, M.C.; Yates, J.R. Protein analysis by shotgun/bottom-up proteomics. Chem. Rev. 2013, 113, 2343–2394. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Wang, J.; Ge, H.; Fang, L.; Zhang, Y.; Huang, X.; Wang, Y. Toward the complete proteome of Synechocystis sp. PCC 6803. Photosynth. Res. 2015, 126, 203–219. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Shen, C.; Liao, L.; Huang, X.; Liu, K.; Wang, W.; Guo, L.; Jin, W.; Huang, F.; Xu, W.; et al. Functional proteomic discovery of Slr0110 as a central regulator of carbohydrate metabolism in Synechocystis species PCC6803. Mol. Cell. Proteom. 2014, 13, 204–219. [Google Scholar] [CrossRef]
- Song, Z.; Chen, L.; Wang, J.; Lu, Y.; Jiang, W.; Zhang, W. A transcriptional regulator Sll0794 regulates tolerance to biofuel ethanol in photosynthetic Synechocystis sp. PCC 6803. Mol. Cell. Proteom. 2014, 13, 3519–3532. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Fang, L.; Huang, X.; Ge, H.; Wang, J.; Wang, X.; Zhang, Y.; Sui, N.; Xu, W.; Wang, Y. Systematic identification of light-regulated cold-responsive proteome in a model cyanobacterium. J. Proteom. 2018, 179, 100–109. [Google Scholar] [CrossRef]
- Angeleri, M.; Muth-Pawlak, D.; Wilde, A.; Aro, E.M.; Battchikova, N. Global proteome response of Synechocystis 6803 to extreme copper environments applied to control the activity of the inducible petJ promoter. J. Appl. Microbiol. 2019, 126, 826–841. [Google Scholar] [CrossRef]
- Battchikova, N.; Vainonen, J.P.; Vorontsova, N.; Kerãnen, M.; Carmel, D.; Aro, E.M. Dynamic changes in the proteome of Synechocystis 6803 in response to CO2 limitation revealed by quantitative proteomics. J. Proteome Res. 2010, 9, 5896–5912. [Google Scholar] [CrossRef]
- Miranda, H.; Cheregi, O.; Netotea, S.; Hvidsten, T.R.; Moritz, T.; Funk, C. Co-expression analysis, proteomic and metabolomic study on the impact of a Deg/HtrA protease triple mutant in Synechocystis sp. PCC 6803 exposed to temperature and high light stress. J. Proteom. 2013, 78, 294–311. [Google Scholar] [CrossRef]
- Slabas, A.R.; Suzuki, I.; Murata, N.; Simon, W.J.; Hall, J.J. Proteomic analysis of the heat shock response in Synechocystis PCC6803 and a thermally tolerant knockout strain lacking the histidine kinase 34 gene. Proteomics 2006, 6, 845–864. [Google Scholar] [CrossRef] [PubMed]
- Rowland, J.G.; Simon, W.J.; Prakash, J.S.S.; Slabas, A.R. Proteomics reveals a role for the RNA helicase crhR in the modulation of multiple metabolic pathways during cold acclimation of Synechocystis sp. PCC6803. J. Proteome Res. 2011, 10, 3674–3689. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.F.; Yang, H.M.; Cui, S.X.; Hu, J.; Wang, J.; Kuang, T.Y.; Norling, B.; Huang, F. Proteomic analysis of plasma membranes of cyanobacterium Synechocystis sp. strain PCC 6803 in response to high pH stress. J. Proteome Res. 2009, 8, 2892–2902. [Google Scholar] [CrossRef] [PubMed]
- Kurian, D.; Phadwal, K.; Mäenpää, P. Proteomic characterization of acid stress response in Synechocystis sp. PCC 6803. Proteomics 2006, 6, 3614–3624. [Google Scholar] [CrossRef]
- Ren, Q.; Shi, M.; Chen, L.; Wang, J.; Zhang, W. Integrated proteomic and metabolomic characterization of a novel two-component response regulator Slr1909 involved in acid tolerance in Synechocystis sp. PCC 6803. J. Proteom. 2014, 109, 76–89. [Google Scholar] [CrossRef] [PubMed]
- Wegener, K.M.; Singh, A.K.; Jacobs, J.M.; Elvitigala, T.; Welsh, E.A.; Keren, N.; Gritsenko, M.A.; Ghosh, B.K.; Camp, D.G.; Smith, R.D.; et al. Global proteomics reveal an atypical strategy for carbon/nitrogen assimilation by a cyanobacterium under diverse environmental perturbations. Mol. Cell. Proteom. 2010, 12, 2678–2689. [Google Scholar] [CrossRef]
- Fuszard, M.A.; Ow, S.Y.; Gan, C.S.; Noirel, J.; Ternan, N.G.; McMullan, G.; Biggs, C.A.; Reardon, K.F.; Wright, P.C. The quantitative proteomic response of Synechocystis sp. PCC6803 to phosphate acclimation. Aquat. Biosyst. 2013, 9. [Google Scholar] [CrossRef]
- Chen, L.; Zhu, Y.; Song, Z.; Wang, J.; Zhang, W. An orphan response regulator Sll0649 involved in cadmium tolerance and metal homeostasis in photosynthetic Synechocystis sp. PCC 6803. J. Proteom. 2014, 103, 87–102. [Google Scholar] [CrossRef]
- Mehta, A.; López-Maury, L.; Florencio, F.J. Proteomic pattern alterations of the cyanobacterium Synechocystis sp. PCC 6803 in response to cadmium, nickel and cobalt. J. Proteom. 2014, 102, 98–112. [Google Scholar] [CrossRef]
- Fulda, S.; Huang, F.; Nilsson, F.; Hagemann, M.; Norling, B. Proteomics of Synechocystis sp. strain PCC 6803: Identification of periplasmic proteins in cells grown at low and high salt concentrations. Eur. J. Biochem. 2000, 267, 5900–5907. [Google Scholar] [CrossRef]
- Fulda, S.; Mikkat, S.; Huang, F.; Huckauf, J.; Marin, K.; Norling, B.; Hagemann, M. Proteome analysis of salt stress response in the cyanobacterium Synechocystis sp. strain PCC 6803. Proteomics 2006, 6, 2733–2745. [Google Scholar] [CrossRef]
- Huang, F.; Fulda, S.; Hagemann, M.; Norling, B. Proteomic screening of salt-stress-induced changes in plasma membranes of Synechocystis sp. strain PCC 6803. Proteomics 2006, 6, 910–920. [Google Scholar] [CrossRef]
- Pandhal, J.; Ow, S.Y.; Wright, P.C.; Biggs, C.A. Comparative proteomics study of salt tolerance between a nonsequenced extremely halotolerant cyanobacterium and its mildly halotolerant relative using in vivo metabolic labeling and in vitro isobaric labeling. J. Proteome Res. 2009, 8, 818–828. [Google Scholar] [CrossRef]
- Li, T.; Yang, H.M.; Cui, S.X.; Suzuki, I.; Zhang, L.F.; Li, L.; Bo, T.T.; Wang, J.; Murata, N.; Huang, F. Proteomic study of the impact of Hik33 mutation in Synechocystis sp. PCC 6803 under normal and salt stress conditions. J. Proteome Res. 2012, 11, 502–514. [Google Scholar] [CrossRef]
- Qiao, J.; Huang, S.; Te, R.; Wang, J.; Chen, L.; Zhang, W. Integrated proteomic and transcriptomic analysis reveals novel genes and regulatory mechanisms involved in salt stress responses in Synechocystis sp. PCC 6803. Appl. Microbiol. Biotechnol. 2013, 97, 8253–8264. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Xiong, W.; Li, X.B.; Gao, C.F.; Zhang, Y.L.; Li, H.; Wu, Q.Y. Identification of the proteomic changes in Synechocystis sp. PCC 6803 following prolonged UV-B irradiation. J. Exp. Bot. 2009, 60, 1141–1154. [Google Scholar] [CrossRef]
- Liu, J.; Chen, L.; Wang, J.; Qiao, J.; Zhang, W. Proteomic analysis reveals resistance mechanism against biofuel hexane in Synechocystis sp. PCC 6803. Biotechnol. Biofuels 2012, 5. [Google Scholar] [CrossRef] [PubMed]
- Qiao, J.; Wang, J.; Chen, L.; Tian, X.; Huang, S.; Ren, X.; Zhang, W. Quantitative iTRAQ LC-MS/MS proteomics reveals metabolic responses to biofuel ethanol in cyanobacterial Synechocystis sp. PCC 6803. J. Proteome Res. 2012, 11, 5286–5300. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Chen, L.; Wang, J.; Qiao, J.; Zhang, W. Quantitative proteomics reveals dynamic responses of Synechocystis sp. PCC 6803 to next-generation biofuel butanol. J. Proteom. 2013, 78, 326–345. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wu, L.; Wang, J.; Zhang, W. Butanol tolerance regulated by a two-component response regulator Slr1037 in photosynthetic Synechocystis sp. PCC 6803. Biotechnol. Biofuels 2014, 7. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, Y.; Chen, L.; Zhang, W. Proteomic and metabolomic analyses reveal metabolic responses to 3-hydroxypropionic acid synthesized internally in cyanobacterium Synechocystis sp. PCC 6803. Biotechnol. Biofuels 2016, 9. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Yang, M.; Lin, X.; Liu, X.; Huang, H.; Ge, F. Malonylome Analysis Reveals the Involvement of Lysine Malonylation in Metabolism and Photosynthesis in Cyanobacteria. J. Proteome Res. 2017, 15, 2030–2043. [Google Scholar] [CrossRef]
- Lin, X.; Yang, M.; Liu, X.; Cheng, Z.; Ge, F. Characterization of Lysine Monomethylome and Methyltransferase in Model Cyanobacterium Synechocystis sp. PCC 6803. Genom. Proteom. Bioinform. 2020, 18, 289–304. [Google Scholar] [CrossRef] [PubMed]
- Mikkat, S.; Fulda, S.; Hagemann, M. A 2D gel electrophoresis-based snapshot of the phosphoproteome in the cyanobacterium Synechocystis sp. strain PCC 6803. Microbiology (UK) 2014, 160, 296–306. [Google Scholar] [CrossRef] [PubMed]
- Spät, P.; Macek, B.; Forchhammer, K. Phosphoproteome of the cyanobacterium Synechocystis sp. PCC 6803 and its dynamics during nitrogen starvation. Front. Microbiol. 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- Toyoshima, M.; Tokumaru, Y.; Matsuda, F.; Shimizu, H. Assessment of protein content and phosphorylation level in Synechocystis sp. PCC 6803 under various growth conditions using quantitative phosphoproteomic analysis. Molecules 2020, 20, 3582. [Google Scholar] [CrossRef]
- Krall, L.; Huege, J.; Catchpole, G.; Steinhauser, D.; Willmitzer, L. Assessment of sampling strategies for gas chromatography-mass spectrometry (GC-MS) based metabolomics of cyanobacteria. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2009, 877, 2952–2960. [Google Scholar] [CrossRef]
- Narainsamy, K.; Cassier-Chauvat, C.; Junot, C.; Chauvat, F. High performance analysis of the cyanobacterial metabolism via liquid chromatography coupled to a LTQ-Orbitrap mass spectrometer: Evidence that glucose reprograms the whole carbon metabolism and triggers oxidative stress. Metabolomics 2013, 9, 21–32. [Google Scholar] [CrossRef]
- Achyuthan, K.E.; Harper, J.C.; Manginell, R.P.; Moorman, M.W. Volatile metabolites emission by in vivo microalgae—An overlooked opportunity? Metabolites 2017, 7, 39. [Google Scholar] [CrossRef]
- Majchrzak, T.; Wojnowski, W.; Rutkowska, M.; Wasik, A. Real-Time Volatilomics: A Novel Approach for Analyzing Biological Samples. Trends Plant Sci. 2020, 25, 302–312. [Google Scholar] [CrossRef]
- Yoshikawa, K.; Hirasawa, T.; Ogawa, K.; Hidaka, Y.; Nakajima, T.; Furusawa, C.; Shimizu, H. Integrated transcriptomic and metabolomic analysis of the central metabolism of Synechocystis sp. PCC 6803 under different trophic conditions. Biotechnol. J. 2013, 8, 571–580. [Google Scholar] [CrossRef]
- Nakajima, T.; Kajihata, S.; Yoshikawa, K.; Matsuda, F.; Furusawa, C.; Hirasawa, T.; Shimizu, H. Integrated metabolic flux and omics analysis of Synechocystis sp. PCC 6803 under mixotrophic and photoheterotrophic conditions. Plant Cell Physiol. 2014, 55, 1605–1612. [Google Scholar] [CrossRef] [PubMed]
- Cheregi, O.; Miranda, H.; Gröbner, G.; Funk, C. Inactivation of the Deg protease family in the cyanobacterium Synechocystis sp. PCC 6803 has impact on the outer cell layers. J. Photochem. Photobiol. B Biol. 2015, 152, 383–394. [Google Scholar] [CrossRef] [PubMed]
- Klähn, S.; Orf, I.; Schwarz, D.; Matthiessen, J.K.F.; Kopka, J.; Hess, W.R.; Hagemann, M. Integrated transcriptomic and metabolomic characterization of the low-carbon response using an ndhR mutant of Synechocystis sp. PCC 6803. Plant Physiol. 2015, 169, 1540–1556. [Google Scholar] [CrossRef]
- Jablonsky, J.; Papacek, S.; Hagemann, M. Different strategies of metabolic regulation in cyanobacteria: From transcriptional to biochemical control. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Saha, R.; Liu, D.; Hoynes-O’Connor, A.; Liberton, M.; Yu, J.; Bhattacharyya-Pakrasi, M.; Balassy, A.; Zhang, F.; Moon, T.S.; Maranas, C.D.; et al. Diurnal regulation of cellular processes in the cyanobacterium Synechocystis sp. strain PCC 6803: Insights from transcriptomic, fluxomic, and physiological analyses. MBio 2016, 7, e00464-16. [Google Scholar] [CrossRef] [PubMed]
- Tibiletti, T.; Hernández-Prieto, M.A.; Matthijs, H.C.P.; Niyogi, K.K.; Funk, C. Deletion of the gene family of small chlorophyll-binding proteins (ScpABCDE) offsets C/N homeostasis in Synechocystis PCC 6803. Biochim. Biophys. Acta Bioenerg. 2016, 1857, 396–407. [Google Scholar] [CrossRef] [PubMed]
- Hasunuma, T.; Matsuda, M.; Kondo, A. Improved sugar-free succinate production by Synechocystis sp. PCC 6803 following identification of the limiting steps in glycogen catabolism. Metab. Eng. Commun. 2016, 3, 130–141. [Google Scholar] [CrossRef]
- Zhu, H.; Ren, X.; Wang, J.; Song, Z.; Shi, M.; Qiao, J.; Tian, X.; Liu, J.; Chen, L.; Zhang, W. Integrated OMICS guided engineering of biofuel butanol-tolerance in photosynthetic Synechocystis sp. PCC 6803. Biotechnol. Biofuels 2013, 6. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.X.; Li, H.C.; Wei, Y.P.; Chu, W.Y.; Chong, Y.L.; Long, X.H.; Liu, Z.P.; Qin, S.; Shao, H.B. Signal transduction pathways in Synechocystis sp. PCC 6803 and biotechnological implications under abiotic stress. Crit. Rev. Biotechnol. 2015, 35, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Gärtner, K.; Klähn, S.; Watanabe, S.; Mikkat, S.; Scholz, I.; Hess, W.R.; Hagemann, M. Cytosine N4-methylation via M.Ssp6803II is involved in the regulation of transcription, fine-tuning of DNA replication and DNA repair in the cyanobacterium Synechocystis sp. PCC 6803. Front. Microbiol. 2019, 10. [Google Scholar] [CrossRef]
- Xu, W.; Wang, Y. Post-translational Modifications of Serine/Threonine and Histidine Kinases and Their Roles in Signal Transductions in Synechocystis Sp. PCC 6803. Appl. Biochem. Biotechnol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Broddrick, J.T.; Rubin, B.E.; Welkie, D.G.; Du, N.; Mih, N.; Diamond, S.; Lee, J.J.; Golden, S.S.; Palsson, B.O. Unique attributes of cyanobacterial metabolism revealed by improved genome-scale metabolic modeling and essential gene analysis. Proc. Natl. Acad. Sci. USA 2016, 113, E8344–E8353. [Google Scholar] [CrossRef]
- Broddrick, J.T.; Welkie, D.G.; Jallet, D.; Golden, S.S.; Peers, G.; Palsson, B.O. Predicting the metabolic capabilities of Synechococcus elongatus PCC 7942 adapted to different light regimes. Metab. Eng. 2019, 52, 42–56. [Google Scholar] [CrossRef] [PubMed]
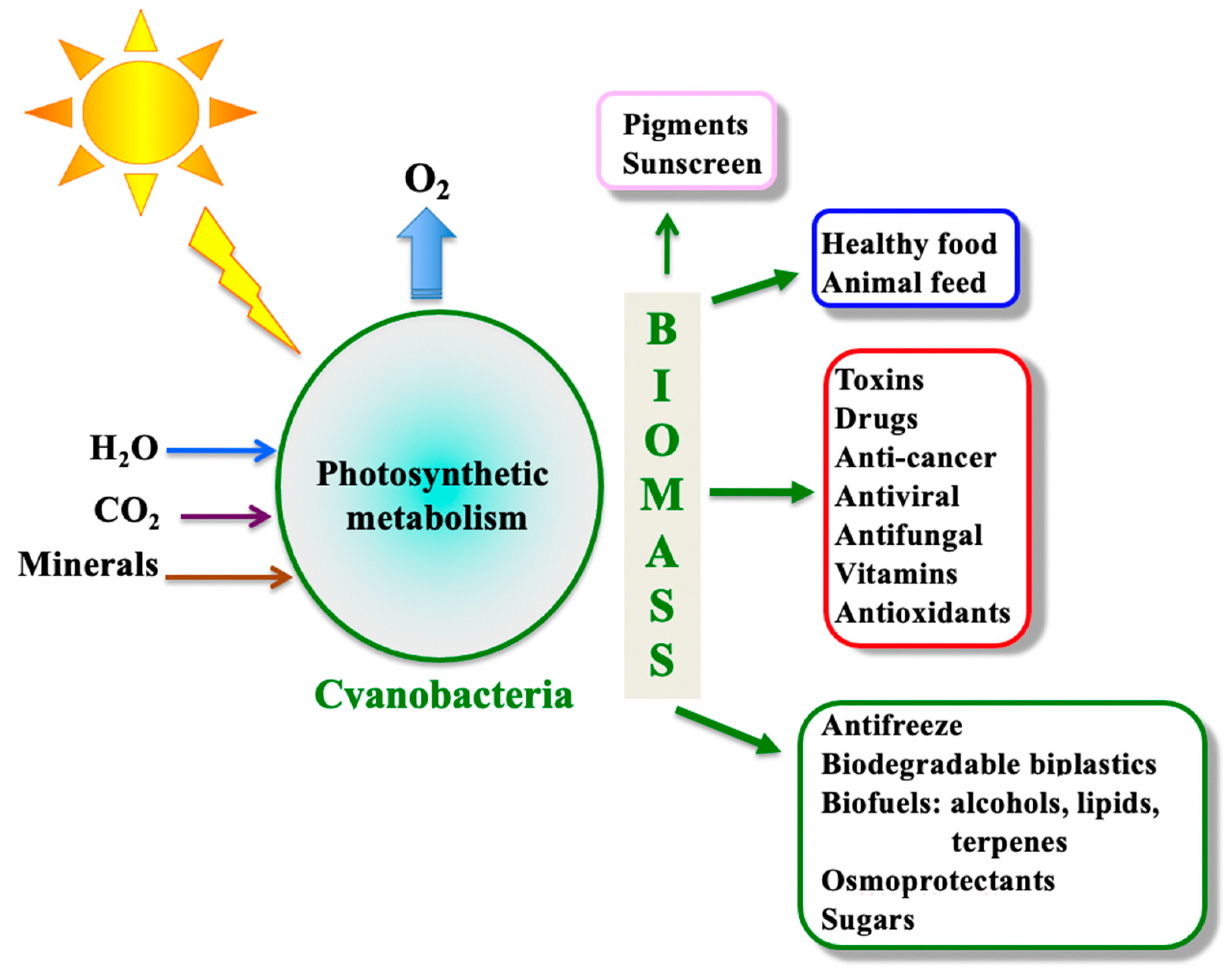
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cassier-Chauvat, C.; Blanc-Garin, V.; Chauvat, F. Genetic, Genomics, and Responses to Stresses in Cyanobacteria: Biotechnological Implications. Genes 2021, 12, 500. https://doi.org/10.3390/genes12040500
Cassier-Chauvat C, Blanc-Garin V, Chauvat F. Genetic, Genomics, and Responses to Stresses in Cyanobacteria: Biotechnological Implications. Genes. 2021; 12(4):500. https://doi.org/10.3390/genes12040500
Chicago/Turabian StyleCassier-Chauvat, Corinne, Victoire Blanc-Garin, and Franck Chauvat. 2021. "Genetic, Genomics, and Responses to Stresses in Cyanobacteria: Biotechnological Implications" Genes 12, no. 4: 500. https://doi.org/10.3390/genes12040500
APA StyleCassier-Chauvat, C., Blanc-Garin, V., & Chauvat, F. (2021). Genetic, Genomics, and Responses to Stresses in Cyanobacteria: Biotechnological Implications. Genes, 12(4), 500. https://doi.org/10.3390/genes12040500






