A Simulated Shift Work Schedule Does Not Increase DNA Double-Strand Break Repair by NHEJ in the Drosophila Rr3 System
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. A Non-24 H Light:Dark Schedule Disrupts Activity Rhythms
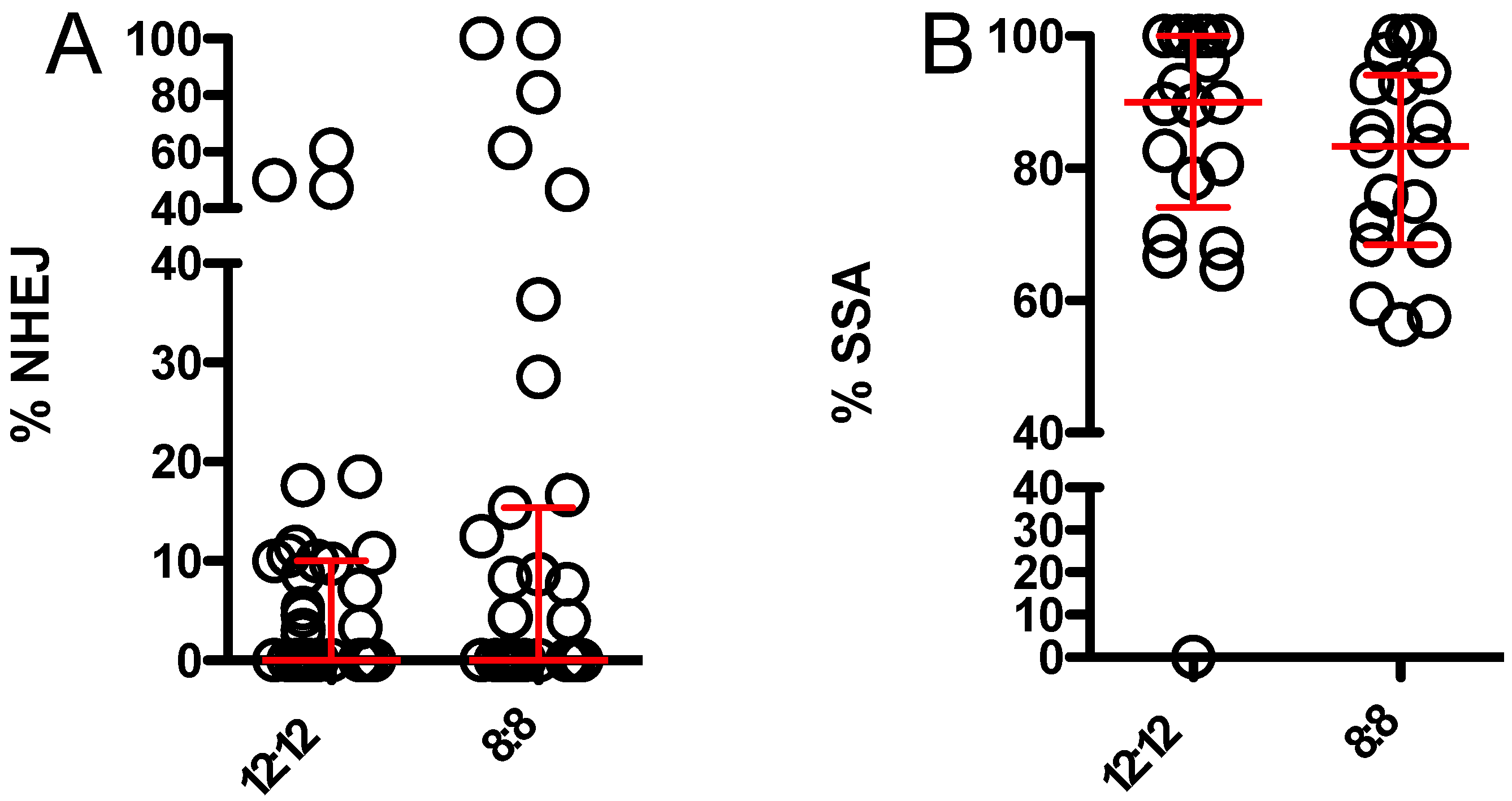
3.2. 8:8 L:D Does Not Alter Relative Usage of NHEJ and SSA When Endonuclease Is Supplied by Maternal Effect Only
3.3. 8:8 L:D Does Not Alter Relative Usage of NHEJ and SSA When Endonuclease Is Supplied by Maternal Effect and Zygotic Expression
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DSB | DNA double-strand break |
| HRR | homologous recombination repair |
| NHEJ | non-homologous end joining |
| Rr3 | Repair Reporter 3 |
| SSA | single strand annealing |
References
- Stevens, R.G.; Hansen, J.; Costa, G.; Haus, E.; Kauppinen, T.; Aronson, K.J.; Castano-Vinyals, G.; Davis, S.; Frings-Dresen, M.H.W.; Fritschi, L.; et al. Considerations of Circadian Impact for Defining “shift Work” in Cancer Studies: IARC Working Group Report. Occup. Environ. Med. 2011, 68, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Wegrzyn, L.R.; Tamimi, R.M.; Rosner, B.A.; Brown, S.B.; Stevens, R.G.; Eliassen, A.H.; Laden, F.; Willett, W.C.; Hankinson, S.E.; Schernhammer, E.S. Rotating Night-Shift Work and the Risk of Breast Cancer in the Nurses’ Health Studies. Am. J. Epidemiol. 2017, 186, 532–540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hansen, J. Night Shift Work and Risk of Breast Cancer. Curr. Environ. Health Rep. 2017, 4, 325–339. [Google Scholar] [CrossRef]
- Gehlert, S.; Clanton, M.; on behalf of the Shift Work and Breast Cancer Strategic Advisory. Group Shift Work and Breast Cancer. Int. J. Environ. Res. Public Health 2020, 17, 9544. [Google Scholar] [CrossRef]
- Wendeu-Foyet, M.G.; Bayon, V.; Cénée, S.; Trétarre, B.; Rébillard, X.; Cancel-Tassin, G.; Cussenot, O.; Lamy, P.-J.; Faraut, B.; Ben Khedher, S.; et al. Night Work and Prostate Cancer Risk: Results from the EPICAP Study. Occup. Environ. Med. 2018, 75, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Papantoniou, K.; Castaño-Vinyals, G.; Espinosa, A.; Turner, M.C.; Alonso-Aguado, M.H.; Martin, V.; Aragonés, N.; Pérez-Gómez, B.; Pozo, B.M.; Gómez-Acebo, I.; et al. Shift Work and Colorectal Cancer Risk in the MCC-Spain Case–Control Study. Scand. J. Work Environ. Health 2017, 43, 250–259. [Google Scholar] [CrossRef] [Green Version]
- Travis, R.C.; Balkwill, A.; Fensom, G.K.; Appleby, P.N.; Reeves, G.K.; Wang, X.-S.; Roddam, A.W.; Gathani, T.; Peto, R.; Green, J.; et al. Night Shift Work and Breast Cancer Incidence: Three Prospective Studies and Meta-Analysis of Published Studies. JNCI J. Natl. Cancer Inst. 2016, 108, djw169. [Google Scholar] [CrossRef]
- Barul, C.; Richard, H.; Parent, M.-E. Night-Shift Work and Risk of Prostate Cancer: Results from a Canadian Case-Control Study, the Prostate Cancer and Environment Study. Am. J. Epidemiol. 2019, 188, 1801–1811. [Google Scholar] [CrossRef]
- Boivin, D.B.; Boudreau, P. Impacts of Shift Work on Sleep and Circadian Rhythms. Pathol. Biol. 2014, 62, 292–301. [Google Scholar] [CrossRef]
- Nea, F.M.; Kearney, J.; Livingstone, M.B.E.; Pourshahidi, L.K.; Corish, C.A. Dietary and Lifestyle Habits and the Associated Health Risks in Shift Workers. Nutr. Res. Rev. 2015, 28, 143–166. [Google Scholar] [CrossRef] [Green Version]
- Reid, K.J.; Abbott, S.M. Jet Lag and Shift Work Disorder. Sleep Med. Clin. 2015, 10, 523–535. [Google Scholar] [CrossRef] [PubMed]
- Papagiannakopoulos, T.; Bauer, M.R.; Davidson, S.M.; Heimann, M.; Subbaraj, L.; Bhutkar, A.; Bartlebaugh, J.; Vander Heiden, M.G.; Jacks, T. Circadian Rhythm Disruption Promotes Lung Tumorigenesis. Cell Metab. 2016, 24, 324–331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wright, K.P.; Drake, A.L.; Frey, D.J.; Fleshner, M.; Desouza, C.A.; Gronfier, C.; Czeisler, C.A. Influence of Sleep Deprivation and Circadian Misalignment on Cortisol, Inflammatory Markers, and Cytokine Balance. Brain Behav. Immun. 2015, 47, 24–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leproult, R.; Holmbäck, U.; Van Cauter, E. Circadian Misalignment Augments Markers of Insulin Resistance and Inflammation, Independently of Sleep Loss. Diabetes 2014, 63, 1860–1869. [Google Scholar] [CrossRef] [Green Version]
- Chaix, A.; Zarrinpar, A.; Panda, S. The Circadian Coordination of Cell Biology. J. Cell Biol. 2016, 215, 15–25. [Google Scholar] [CrossRef]
- Uth, K.; Sleigh, R. Deregulation of the Circadian Clock Constitutes a Significant Factor in Tumorigenesis: A Clockwork Cancer. Part II. In Vivo Studies. Biotechnol. Biotechnol. Equip. 2014, 28, 379–386. [Google Scholar] [CrossRef]
- Brum, M.C.B.; Filho, F.F.D.; Schnorr, C.C.; Bottega, G.B.; Rodrigues, T.C. Shift Work and Its Association with Metabolic Disorders. Diabetol. Metab. Syndr. 2015, 7, 45. [Google Scholar] [CrossRef] [Green Version]
- Lee, G.-J.; Kim, K.; Kim, S.; Kim, J.-H.; Suh, C.; Son, B.-C.; Lee, C.-K.; Choi, J. Effects of Shift Work on Abdominal Obesity among 20–39-Year-Old Female Nurses: A 5-Year Retrospective Longitudinal Study. Ann. Occup. Environ. Med. 2016, 28, 69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Q.; Shi, J.; Duan, P.; Liu, B.; Li, T.; Wang, C.; Li, H.; Yang, T.; Gan, Y.; Wang, X.; et al. Is Shift Work Associated with a Higher Risk of Overweight or Obesity? A Systematic Review of Observational Studies with Meta-Analysis. Int. J. Epidemiol. 2018, 47, dyy079. [Google Scholar] [CrossRef] [Green Version]
- Sun, M.; Feng, W.; Wang, F.; Li, P.; Li, Z.; Li, M.; Tse, G.; Vlaanderen, J.; Vermeulen, R.; Tse, L.A. Meta-Analysis on Shift Work and Risks of Specific Obesity Types: Shift Work and Specific Obesity Types. Obes. Rev. 2018, 19, 28–40. [Google Scholar] [CrossRef]
- Lu, Y.-C.; Wang, C.-P.; Yu, T.-H.; Tsai, I.-T.; Hung, W.-C.; Lu, I.-C.; Hsu, C.-C.; Tang, W.-H.; Houng, J.-Y.; Chung, F.-M.; et al. Shift Work Is Associated with Metabolic Syndrome in Male Steel Workers-the Role of Resistin and WBC Count-Related Metabolic Derangements. Diabetol. Metab. Syndr. 2017, 9, 83. [Google Scholar] [CrossRef]
- Scheer, F.A.J.L.; Hilton, M.F.; Mantzoros, C.S.; Shea, S.A. Adverse Metabolic and Cardiovascular Consequences of Circadian Misalignment. Proc. Natl. Acad. Sci. USA 2009, 106, 4453–4458. [Google Scholar] [CrossRef] [Green Version]
- Salgado-Delgado, R.C.; Saderi, N.; Basualdo, M.d.C.; Guerrero-Vargas, N.N.; Escobar, C.; Buijs, R.M. Shift Work or Food Intake during the Rest Phase Promotes Metabolic Disruption and Desynchrony of Liver Genes in Male Rats. PLoS ONE 2013, 8, e60052. [Google Scholar] [CrossRef] [Green Version]
- Wefers, J.; van Moorsel, D.; Hansen, J.; Connell, N.J.; Havekes, B.; Hoeks, J.; van Marken Lichtenbelt, W.D.; Duez, H.; Phielix, E.; Kalsbeek, A.; et al. Circadian Misalignment Induces Fatty Acid Metabolism Gene Profiles and Compromises Insulin Sensitivity in Human Skeletal Muscle. Proc. Natl. Acad. Sci. USA 2018, 115, 7789–7794. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roskoden, F.C.; Krüger, J.; Vogt, L.J.; Gärtner, S.; Hannich, H.J.; Steveling, A.; Lerch, M.M.; Aghdassi, A.A. Physical Activity, Energy Expenditure, Nutritional Habits, Quality of Sleep and Stress Levels in Shift-Working Health Care Personnel. PLoS ONE 2017, 12, e0169983. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, Y.; Nakamura, K.; Sakurai, M.; Nagasawa, S.-Y.; Ishizaki, M.; Nakashima, M.; Kido, T.; Naruse, Y.; Nakagawa, H. The Effect of Age on the Relationships between Work-Related Factors and Heavy Drinking. J. Occup. Health 2014, 56, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Basu, A. DNA Damage, Mutagenesis and Cancer. Int. J. Mol. Sci. 2018, 19, 970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhatti, P.; Mirick, D.K.; Randolph, T.W.; Gong, J.; Buchanan, D.T.; Zhang, J.; Davis, S. Oxidative DNA Damage during Night Shift Work. Occup. Environ. Med. 2017, 74, 680–683. [Google Scholar] [CrossRef] [PubMed]
- Gery, S.; Komatsu, N.; Baldjyan, L.; Yu, A.; Koo, D.; Koeffler, H.P. The Circadian Gene Per1 Plays an Important Role in Cell Growth and DNA Damage Control in Human Cancer Cells. Mol. Cell 2006, 22, 375–382. [Google Scholar] [CrossRef]
- Ünsal-Kaçmaz, K.; Mullen, T.E.; Kaufmann, W.K.; Sancar, A. Coupling of Human Circadian and Cell Cycles by the Timeless Protein. Mol. Cell. Biol. 2005, 25, 3109–3116. [Google Scholar] [CrossRef] [Green Version]
- Terabayashi, T.; Hanada, K. Genome Instability Syndromes Caused by Impaired DNA Repair and Aberrant DNA Damage Responses. Cell Biol. Toxicol. 2018, 34, 337–350. [Google Scholar] [CrossRef] [PubMed]
- Scully, R.; Panday, A.; Elango, R.; Willis, N.A. DNA Double-Strand Break Repair-Pathway Choice in Somatic Mammalian Cells. Nat. Rev. Mol. Cell Biol. 2019, 20, 698–714. [Google Scholar] [CrossRef] [PubMed]
- Blasiak, J. Single-Strand Annealing in Cancer. Int. J. Mol. Sci. 2021, 22, 2167. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.H.Y.; Pannunzio, N.R.; Adachi, N.; Lieber, M.R. Non-Homologous DNA End Joining and Alternative Pathways to Double-Strand Break Repair. Nat. Rev. Mol. Cell Biol. 2017, 18, 495–506. [Google Scholar] [CrossRef] [PubMed]
- Aparicio, T.; Baer, R.; Gautier, J. DNA Double-Strand Break Repair Pathway Choice and Cancer. DNA Repair 2014, 19, 169–175. [Google Scholar] [CrossRef] [Green Version]
- Preston, C.R. Differential Usage of Alternative Pathways of Double-Strand Break Repair in Drosophila. Genetics 2005, 172, 1055–1068. [Google Scholar] [CrossRef] [Green Version]
- Rosato, E.; Kyriacou, C.P. Analysis of Locomotor Activity Rhythms in Drosophila. Nat. Protoc. 2006, 1, 559–568. [Google Scholar] [CrossRef]
- Cichewicz, K.; Hirsh, J. ShinyR-DAM: A Program Analyzing Drosophila Activity, Sleep and Circadian Rhythms. Commun. Biol. 2018, 1, 65. [Google Scholar] [CrossRef]
- Refinetti, R.; Cornélissen, G.; Halberg, F. Procedures for Numerical Analysis of Circadian Rhythms. Biol. Rhythm Res. 2007, 38, 275–325. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing Real-Time PCR Data by the Comparative CT Method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Allada, R.; Chung, B.Y. Circadian Organization of Behavior and Physiology in Drosophila. Annu. Rev. Physiol. 2010, 72, 605–624. [Google Scholar] [CrossRef] [Green Version]
- Franco, D.L.; Frenkel, L.; Ceriani, M.F. The Underlying Genetics of Drosophila Circadian Behaviors. Physiology 2018, 33, 50–62. [Google Scholar] [CrossRef]
- Zhao, J.; Warman, G.R.; Stanewsky, R.; Cheeseman, J.F. Development of the Molecular Circadian Clock and Its Light Sensitivity in Drosophila Melanogaster. J. Biol. Rhythm. 2019, 34, 272–282. [Google Scholar] [CrossRef]
- Plikus, M.V.; Vollmers, C.; de la Cruz, D.; Chaix, A.; Ramos, R.; Panda, S.; Chuong, C.-M. Local Circadian Clock Gates Cell Cycle Progression of Transient Amplifying Cells during Regenerative Hair Cycling. Proc. Natl. Acad. Sci. USA 2013, 110, E2106–E2115. [Google Scholar] [CrossRef] [Green Version]
- Shostak, A. Circadian Clock, Cell Division, and Cancer: From Molecules to Organism. Int. J. Mol. Sci. 2017, 18, 873. [Google Scholar] [CrossRef] [PubMed]
- Caron, P.; Pobega, E.; Polo, S.E. DNA Double-Strand Break Repair: All Roads Lead to HeterochROMAtin Marks. Front. Genet. 2021, 12, 730696. [Google Scholar] [CrossRef] [PubMed]
- Suh, J.; Jackson, F.R. Drosophila Ebony Activity Is Required in Glia for the Circadian Regulation of Locomotor Activity. Neuron 2007, 55, 435–447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beaver, L.M.; Gvakharia, B.O.; Vollintine, T.S.; Hege, D.M.; Stanewsky, R.; Giebultowicz, J.M. Loss of Circadian Clock Function Decreases Reproductive Fitness in Males of Drosophila Melanogaster. Proc. Natl. Acad. Sci. USA 2002, 99, 2134–2139. [Google Scholar] [CrossRef] [Green Version]
- Beaver, L.M.; Rush, B.L.; Gvakharia, B.O.; Giebultowicz, J.M. Noncircadian Regulation and Function of Clock Genes Period and Timeless in Oogenesis of Drosophila Melanogaster. J. Biol. Rhythm. 2003, 18, 463–472. [Google Scholar] [CrossRef] [PubMed]
- Kotwica, J.; Larson, M.K.; Bebas, P.; Giebultowicz, J.M. Developmental Profiles of PERIOD and DOUBLETIME in Drosophila Melanogaster Ovary. J. Insect Physiol. 2009, 55, 419–425. [Google Scholar] [CrossRef]
- Milev, N.B.; Reddy, A.B. Circadian Redox Oscillations and Metabolism. Trends Endocrinol. Metab. 2015, 26, 430–437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ditch, S.; Paull, T.T. The ATM Protein Kinase and Cellular Redox Signaling: Beyond the DNA Damage Response. Trends Biochem. Sci. 2012, 37, 15–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Genotype | |
|---|---|
| 1 | w; TM3 Sb P{UIE}72C/TM6 Ubx |
| 2 | w/Y; al wgSp−1 P{Rr3}48C L sp/CyO |
| 3 | w1118 |
| 4 | w/Y; al wgSp−1 P{Rr3}48C L sp/+; TM3 Sb P{UIE}72C/+ |
| 5 | w/Y; al wgSp−1 P{Rr3}48C L sp/+ |
| 6 | w1118/Y; al wgSp−1 P{Rr3}48C L sp/+ |
| 7 | w1118/Y; al wgSp−1 P{Rr3}48C L sp/+; TM3 Sb P{UIE}72C/+ |
| 8 | w/Y; al wgSp−1P{Rr3}48C L sp/+; TM3 Sb P{UIE}72C/+ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bergerson, L.; Fitzmaurice, C.; Knudtson, T.; McCormick, H.; Yu, A.M. A Simulated Shift Work Schedule Does Not Increase DNA Double-Strand Break Repair by NHEJ in the Drosophila Rr3 System. Genes 2022, 13, 150. https://doi.org/10.3390/genes13010150
Bergerson L, Fitzmaurice C, Knudtson T, McCormick H, Yu AM. A Simulated Shift Work Schedule Does Not Increase DNA Double-Strand Break Repair by NHEJ in the Drosophila Rr3 System. Genes. 2022; 13(1):150. https://doi.org/10.3390/genes13010150
Chicago/Turabian StyleBergerson, Lydia, Caleb Fitzmaurice, Tyler Knudtson, Halle McCormick, and Alder M. Yu. 2022. "A Simulated Shift Work Schedule Does Not Increase DNA Double-Strand Break Repair by NHEJ in the Drosophila Rr3 System" Genes 13, no. 1: 150. https://doi.org/10.3390/genes13010150
APA StyleBergerson, L., Fitzmaurice, C., Knudtson, T., McCormick, H., & Yu, A. M. (2022). A Simulated Shift Work Schedule Does Not Increase DNA Double-Strand Break Repair by NHEJ in the Drosophila Rr3 System. Genes, 13(1), 150. https://doi.org/10.3390/genes13010150






