Pre-Administration of PLX-R18 Cells Protects Mice from Radiation-Induced Hematopoietic Failure and Lethality
Abstract
:1. Introduction
2. Materials and Methods
2.1. PLX-R18 Preparation and Administration
2.2. Mice
2.3. Safety Study Design
2.4. Total Body Irradiation (TBI)
2.5. Survival Study in TBI Model Using Cobalt-60 γ Radiation
2.6. Hematopoietic Recovery Study in TBI Model Using Cobalt-60 γ Radiation
2.6.1. CBC and Differential Analyses
2.6.2. Blood Collection and Tissue Collection
2.6.3. Sternal Histopathology
2.6.4. Hematopoietic Progenitor Clonogenic Assay (CFU Assay)
2.6.5. ELISA to Quantify Radiation Injury and Endothelial Biomarkers from Serum
2.6.6. Luminex Assay to Study Cytokines
2.6.7. Total RNA Extraction from Spleen Tissue and Autoimmune Pathway Analysis
2.6.8. Western Blot Analysis from Spleen Tissue
3. Results
3.1. PLX-R18 Administered Twice Four Days Apart Found to Be Safe
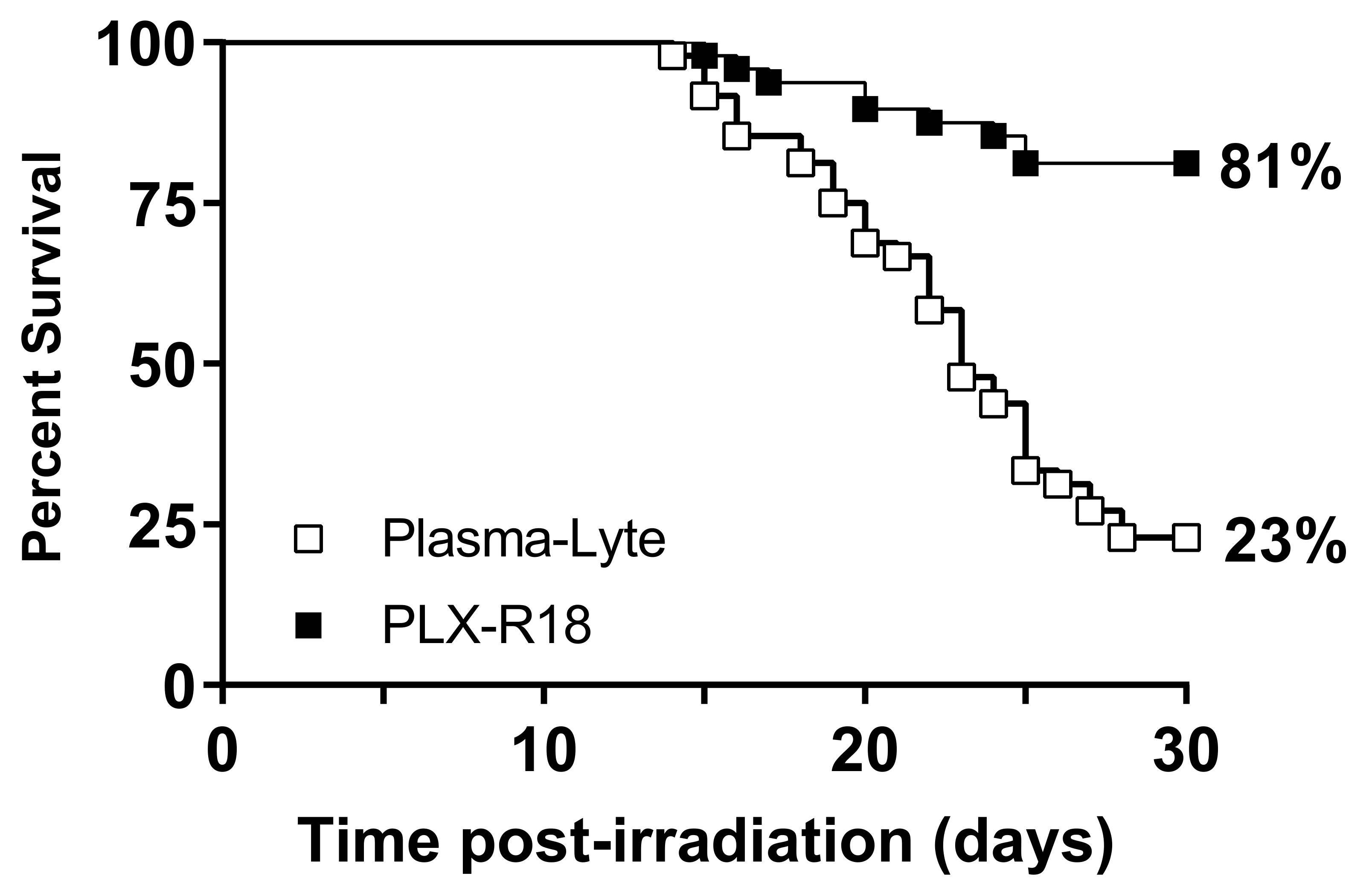
3.2. PLX-R18 Protects Mice from Lethal Dose of Irradiation
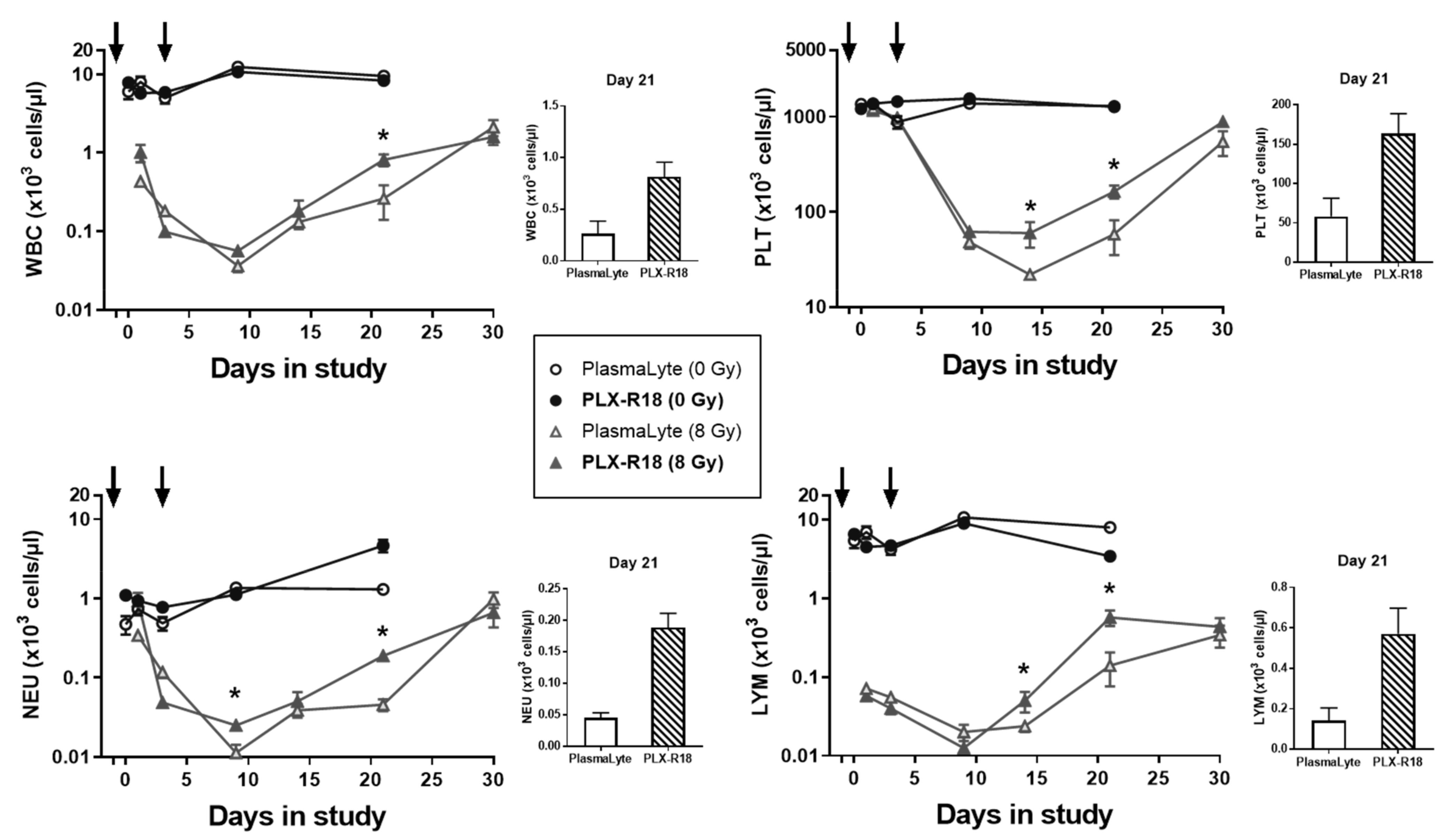
3.3. PLX-R18 Administration Accelerated Hematological Recovery in Irradiated Mice
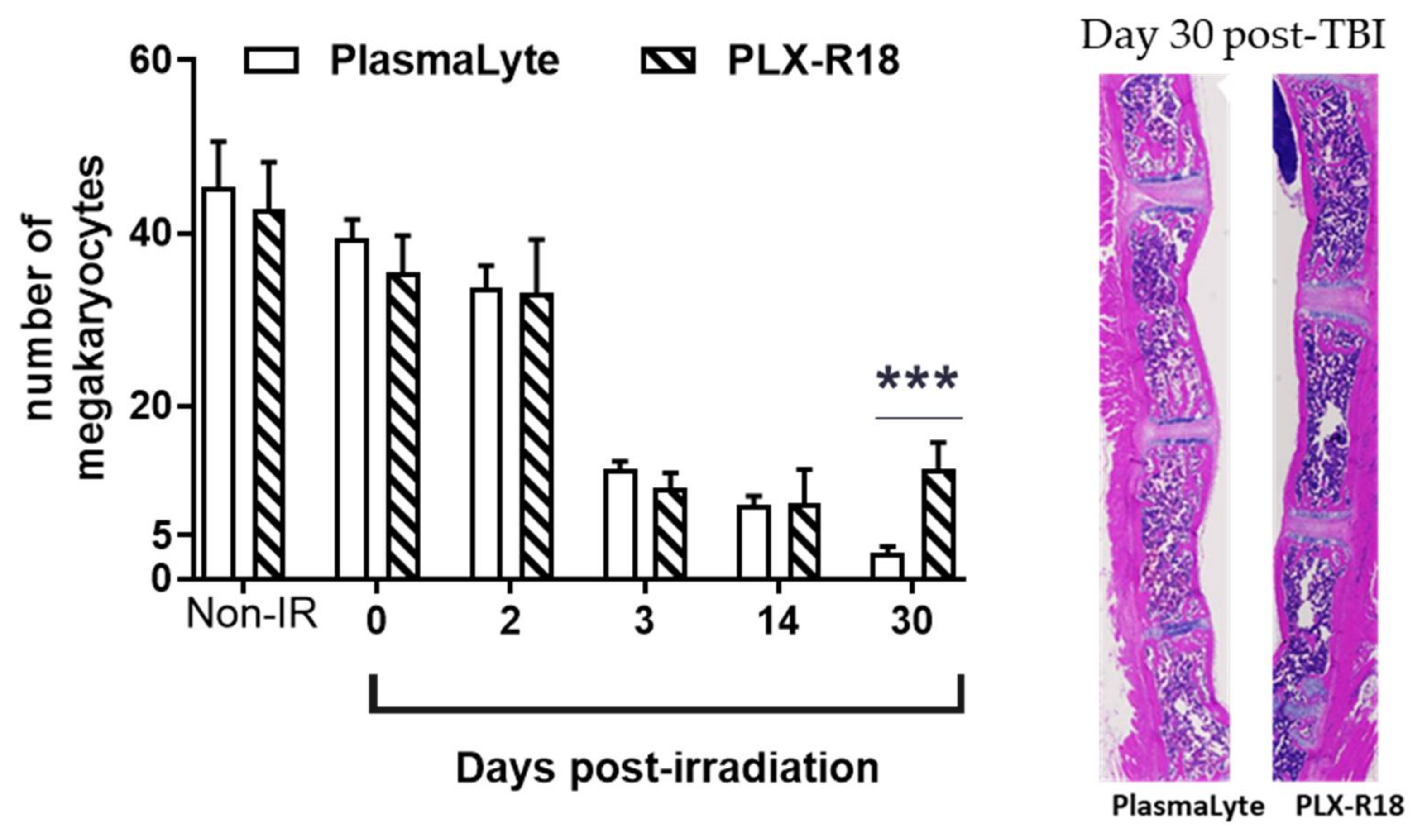
3.4. PLX-R18 Mitigates Radiation-Induced Bone Marrow Damage
3.5. PLX-R18 Ameliorates Radiation-Induced Inflammation and Vascular Endothelial Damage
3.6. PLX-R18 Treatment Modulated Levels of Various Inflammatory Chemokines, and Growth Factors
3.7. PLX-R18 Abrogated Expression of Genes Involved in Autoimmune Pathways
3.8. PLX-R18 Modulated Phosphorylation of AKT
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singh, V.K.; Seed, T.M. Radiation countermeasures for hematopoietic acute radiation syndrome: Growth factors, cytokines and beyond. Int. J. Radiat. Biol. 2021, 97, 1526–1547. [Google Scholar] [CrossRef] [PubMed]
- Waselenko, J.K.; MacVittie, T.J.; Blakely, W.F.; Pesik, N.; Wiley, A.L.; Dickerson, W.E.; Tsu, H.; Confer, D.L.; Coleman, C.N.; Seed, T.; et al. Medical Management of the Acute Radiation Syndrome: Recommendations of the Strategic National Stockpile Radiation Working Group. Ann. Intern. Med. 2004, 140, 1037–1051. [Google Scholar] [CrossRef] [PubMed]
- Walker, R.I.; Cerveny, T.J.; Alt, L.A. Medical Consequences of Nuclear Warfare; Armed Forces Radiobiology Research Institute: Bethesda, MD, USA, 1989. [Google Scholar]
- Amgen Inc. Neupogen (Filgrastim) Injection for Subcutaneous or Intravenous Use; Amgen Inc.: Thousand Oaks, CA, USA, 2015. [Google Scholar]
- Amgen Inc. NEULASTA® (Pegfilgrastim) Injection, for Subcutaneous Use; Amgen Inc.: Thousand Oaks, CA, USA, 2015. [Google Scholar]
- Sanofi-Aventis US LLC. LEUKINE® (Sargramostim) for Injection, for Subcutaneous or Intravenous Use; Sanofi-Aventis US LLC.: Bridgewater, NJ, USA, 2018. [Google Scholar]
- Amgen Inc. NPLATE® (Romiplostim) for Injection, for Subcutaneous Use; Amgen Inc.: Thousand Oaks, CA, USA, 2021. [Google Scholar]
- Hérodin, F.; Mayol, J.F.; Mourcin, F.; Drouet, M. Which place for stem cell therapy in the treatment of acute radiation syndrome? Folia Histochem. Cytobiol. 2005, 43, 223–227. [Google Scholar]
- Fukumoto, R. Mesenchymal stem cell therapy for acute radiation syndrome. Mil. Med. Res. 2016, 3, 17. [Google Scholar] [CrossRef]
- Diaz, M.F.; Horton, P.L.D.; Dumbali, S.P.; Kumar, A.; Livingston, M.; Skibber, M.A.; Mohammadalipour, A.; Gill, B.S.; Zhang, S.; Cox, C.S.; et al. Bone marrow stromal cell therapy improves survival after radiation injury but does not restore endogenous hematopoiesis. Sci. Rep. 2020, 10, 22211. [Google Scholar] [CrossRef] [PubMed]
- Qian, L.; Cen, J. Hematopoietic Stem Cells and Mesenchymal Stromal Cells in Acute Radiation Syndrome. Oxidat. Med. Cell. Longev. 2020, 2020, 8340756. [Google Scholar] [CrossRef]
- Eaton, E.B.; Varney, T.R. Mesenchymal stem cell therapy for acute radiation syndrome: Innovative medical approaches in military medicine. Mil. Med. Res. 2015, 2, 2. [Google Scholar] [CrossRef]
- Hu, K.X.; Sun, Q.Y.; Guo, M.; Ai, H.S. The radiation protection and therapy effects of mesenchymal stem cells in mice with acute radiation injury. Br. J. Radiol. 2010, 83, 52–58. [Google Scholar] [CrossRef]
- Shim, S.; Lee, S.B.; Lee J-g Jang, W.-S.; Lee, S.-J.; Park, S.; Lee, S.-S. Mitigating effects of hUCB-MSCs on the hematopoietic syndrome resulting from total body irradiation. Exp. Hematol. 2013, 41, 346–353.e2. [Google Scholar] [CrossRef]
- Bandekar, M.; Maurya, D.K.; Sharma, D.; Checker, R.; Gota, V.; Mishra, N.; Sandur, S.K. Xenogeneic transplantation of human WJ-MSCs rescues mice from acute radiation syndrome via Nrf-2-dependent regeneration of damaged tissues. Am. J. Transplant. 2020, 20, 2044–2057. [Google Scholar] [CrossRef]
- Lange, C.; Brunswig-Spickenheier, B.; Cappallo-Obermann, H.; Eggert, K.; Gehling, U.M.; Rudolph, C.; Schlegelberger, B.; Cornils, K.; Zustin, J.; Spiess, A.-N.; et al. Radiation Rescue: Mesenchymal Stromal Cells Protect from Lethal Irradiation. PLoS ONE 2011, 6, e14486. [Google Scholar] [CrossRef] [PubMed]
- Sher, N.; Prezma, T.; Fuchs-Telem, D.; Shraga-Heled, N.; Authier, S.; Chang, P.; Ascah, A.; Bakke, J.; Gahagen, J.; Ofir, R. Intramuscular Administration of Placenta-Derived Stromal Cells Enhances Survival of Rhesus Macaque Monkeys Exposed to Total Body Irradiation. Blood 2017, 130 (Suppl. S1), 3784. [Google Scholar]
- Ofir, R.; Pinzur, L.; Levent, A.; Aberman, Z.; Gorodetsky, R.; Volk, H.D. Mechanism of Action of PLX-R18, a Placental-Derived Cellular Therapy for the Treatment of Radiation-Induced Bone Marrow Failure: Poster Abstract. Blood 2015, 126, 2417. [Google Scholar] [CrossRef]
- Gaberman, E.; Pinzur, L.; Levdansky, L.; Tsirlin, M.; Netzer, N.; Aberman, Z.; Gorodetsky, R. Mitigation of Lethal Radiation Syndrome in Mice by Intramuscular Injection of 3D Cultured Adherent Human Placental Stromal Cells. PLoS ONE 2013, 8, e66549. [Google Scholar] [CrossRef]
- Pinzur, L.; Akyuez, L.; Levdansky, L.; Blumenfeld, M.; Volinsky, E.; Aberman, Z.; Reinke, P.; Ofir, R.; Volk, H.-D.; Gorodetsky, R. Rescue from lethal acute radiation syndrome (ARS) with severe weight loss by secretome of intramuscularly injected human placental stromal cells. J. Cachexia Sarcopenia Muscle 2018, 9, 1079–1092. [Google Scholar] [CrossRef] [PubMed]
- Sher, N.; Ofir, R. Placenta-Derived Adherent Stromal Cell Therapy for Hematopoietic Disorders: A Case Study of PLX-R18. Cell Transpl. 2018, 27, 140–150. [Google Scholar] [CrossRef] [PubMed]
- Pluristem Therapeutics Inc. Analyst and Investor Call—HCT Phase I Topline Results Powerpoint Presentation; Pluristem Therapeutics Inc.: Haifa, Israel, 2021; press release. [Google Scholar]
- Lazarus, H.; Escobar, C.; Metheny, L.; McGuirk, J.P.; Zuckerman, T.; Litzow, M.; Rowley, S.D.; Ofir, R.; Shani, L.; Rowe, J.M. Safety and Demonstrated Efficacy of Placenta-Derived Cell Therapy PLX-R18 in Subjects with Incomplete Hematopoietic Recovery Following Hematopoietic Cell Transplantation: A Phase I International Multi-Center Study. Blood 2020, 136, 24–25. [Google Scholar] [CrossRef]
- Corre, I.; Pineau, D.; Hermouet, S. Interleukin-8: An autocrine/paracrine growth factor for human hematopoietic progenitors acting in synergy with colony stimulating factor-1 to promote monocyte-macrophage growth and differentiation. Exp. Hematol. 1999, 27, 28–36. [Google Scholar] [CrossRef]
- Roberts, A.W. G-CSF: A key regulator of neutrophil production, but that’s not all! Growth Factors 2005, 23, 33–41. [Google Scholar] [CrossRef]
- Deshmane, S.L.; Kremlev, S.; Amini, S.; Sawaya, B.E. Monocyte chemoattractant protein-1 (MCP-1): An overview. J. Interferon Cytokine Res. 2009, 29, 313–326. [Google Scholar] [CrossRef]
- Weissenbach, M.; Clahsen, T.; Weber, C.; Spitzer, D.; Wirth, D.; Vestweber, D.; Heinrich, P.C.; Schaper, F. Interleukin-6 is a direct mediator of T cell migration. Eur. J. Immunol. 2004, 34, 2895–3906. [Google Scholar] [CrossRef] [PubMed]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Krause, D.S.; Deans, R.J.; Keating, A.; Prockop, D.J.; Horwitz, E.M. Minimal criteria for defining multipotent mesenchymal stromal cells. Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Papait, A.; Vertua, E.; Magatti, M.; Ceccariglia, S.; De Munari, S.; Silini, A.R.; Sheleg, M.; Ofir, R.; Parolini, O. Mesenchymal Stromal Cells from Fetal and Maternal Placenta Possess Key Similarities and Differences: Potential Implications for Their Applications in Regenerative Medicine. Cells 2020, 9, 127. [Google Scholar] [CrossRef]
- Ghosh, S.P.; Perkins, M.W.; Hieber, K.; Kulkarni, S.; Kao, T.-C.; Reddy, E.P.; Reddy, M.V.R.; Maniar, M.; Seed, T.; Kumar, K.S. Radiation protection by a new chemical entity, Ex-Rad: Efficacy and mechanisms. Radiat. Res. 2009, 171, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, S.; Ghosh, S.P.; Satyamitra, M.; Mog, S.; Hieber, K.; Romanyukha, L.; Gambles, K.; Toles, R.; Kao, T.-C.; Hauer-Jensen, M.; et al. Gamma-tocotrienol protects hematopoietic stem and progenitor cells in mice after total-body irradiation. Radiat. Res. 2010, 173, 738–747. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.P.; Kulkarni, S.; Hieber, K.; Toles, R.; Romanyukha, L.; Kao, T.-C.; Hauer-Jensen, M.; Kumar, K.S. Gamma-tocotrienol, a tocol antioxidant as a potent radioprotector. Int. J. Radiat. Biol. 2009, 85, 598–606. [Google Scholar] [CrossRef]
- Kumar, V.P.; Biswas, S.; Sharma, N.K.; Stone, S.; Fam, C.M.; Cox, G.N.; Ghosh, S.P. PEGylated IL-11 (BBT-059): A Novel Radiation Countermeasure for Hematopoietic Acute Radiation Syndrome. Health Phys. 2018, 115, 65–76. [Google Scholar] [CrossRef]
- Kulkarni, S.S.; Cary, L.H.; Gambles, K.; Hauer-Jensen, M.; Kumar, K.S.; Ghosh, S.P. Gamma-tocotrienol, a radiation prophylaxis agent, induces high levels of granulocyte colony-stimulating factor. Int. Immunopharmacol. 2012, 14, 495–503. [Google Scholar] [CrossRef]
- Cheema, A.K.; Byrum, S.D.; Sharma, N.K.; Altadill, T.; Kumar, V.P.; Biswas, S.; Balgley, B.M.; Hauer-Jensen, M.; Tackett, A.J.; Ghosh, S.P. Proteomic Changes in Mouse Spleen after Radiation-Induced Injury and its Modulation by Gamma-Tocotrienol. Radiat. Res. 2018, 190, 449–463. [Google Scholar] [CrossRef]
- Berbee, M.; Fu, Q.; Boerma, M.; Wang, J.; Kumar, K.S.; Hauer-Jensen, M. Gamma-Tocotrienol ameliorates intestinal radiation injury and reduces vascular oxidative stress after total-body irradiation by an HMG-CoA reductase-dependent mechanism. Radiat. Res. 2009, 171, 596–605. [Google Scholar] [CrossRef]
- Ossetrova, N.I.; Condliffe, D.P.; Ney, P.H.; Krasnopolsky, K.; Hieber, K.P.; Rahman, A.; Sandgren, D.J. Early-response biomarkers for assessment of radiation exposure in a mouse total-body irradiation model. Health Phys. 2014, 106, 772–786. [Google Scholar] [CrossRef] [PubMed]
- Biju, P.G.; Garg, S.; Wang, W.; Choudhry, M.A.; Kovacs, E.J.; Fink, L.M.; Hauer-Jensen, M. Procalcitonin as a predictive biomarker for total body irradiation-induced bacterial load and lethality in mice. Shock 2012, 38, 170–176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tessner, T.G.; Muhale, F.; Riehl, T.E.; Anant, S.; Stenson, W.F. Prostaglandin E2 reduces radiation-induced epithelial apoptosis through a mechanism involving AKT activation and bax translocation. J. Clin. Investig. 2004, 114, 1676–1685. [Google Scholar] [CrossRef] [PubMed]
- Park, H.S.; You, G.E.; Yang, K.H.; Kim, J.Y.; An, S.; Song, J.-Y.; Lee, S.-J.; Lim, Y.-K.; Nam, S.Y. Role of AKT and ERK pathways in controlling sensitivity to ionizing radiation and adaptive response induced by low-dose radiation in human immune cells. Eur. J. Cell. Biol. 2015, 94, 653–660. [Google Scholar] [CrossRef]
- Buzhor, E.; Leshansky, L.; Blumenthal, J.; Barash, H.; Warshawsky, D.; Mazor, Y.; Shtrichman, R. Cell-based therapy approaches: The hope for incurable diseases. Regen Med. 2014, 9, 649–672. [Google Scholar] [CrossRef]
- Singh, V.K.; Christensen, J.; O Fatanmi, O.; Gille, D.; Ducey, E.J.; Wise, S.Y.; Karsunky, H.; Sedello, A.K. Myeloid progenitors: A radiation countermeasure that is effective when initiated days after irradiation. Radiat. Res. 2012, 177, 781–791. [Google Scholar] [CrossRef]
- Kumar, V.P.; Holmes-Hampton, G.P.; Biswas, S.; Stone, S.; Sharma, N.K.; Hritzo, B.; Guilfoyle, M.; Eichenbaum, G.; Guha, C.; Ghosh, S.P. Mitigation of total body irradiation-induced mortality and hematopoietic injury of mice by a thrombopoietin mimetic (JNJ-26366821). Sci. Rep. 2022, 12, 3485. [Google Scholar] [CrossRef]
- Bhavsar, I.; Miller, C.S.; Al-Sabbagh, M. Macrophage Inflammatory Protein-1 Alpha (MIP-1 alpha)/CCL3: As a Biomarker. Gen. Methods Biomark. Res. Appl. 2015, 223–249. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumar, V.P.; Biswas, S.; Holmes-Hampton, G.P.; Sheleg, M.; Stone, S.; Legesse, B.; Ofir, R.; Ghosh, S.P. Pre-Administration of PLX-R18 Cells Protects Mice from Radiation-Induced Hematopoietic Failure and Lethality. Genes 2022, 13, 1756. https://doi.org/10.3390/genes13101756
Kumar VP, Biswas S, Holmes-Hampton GP, Sheleg M, Stone S, Legesse B, Ofir R, Ghosh SP. Pre-Administration of PLX-R18 Cells Protects Mice from Radiation-Induced Hematopoietic Failure and Lethality. Genes. 2022; 13(10):1756. https://doi.org/10.3390/genes13101756
Chicago/Turabian StyleKumar, Vidya P., Shukla Biswas, Gregory P. Holmes-Hampton, Michal Sheleg, Sasha Stone, Betre Legesse, Racheli Ofir, and Sanchita P. Ghosh. 2022. "Pre-Administration of PLX-R18 Cells Protects Mice from Radiation-Induced Hematopoietic Failure and Lethality" Genes 13, no. 10: 1756. https://doi.org/10.3390/genes13101756
APA StyleKumar, V. P., Biswas, S., Holmes-Hampton, G. P., Sheleg, M., Stone, S., Legesse, B., Ofir, R., & Ghosh, S. P. (2022). Pre-Administration of PLX-R18 Cells Protects Mice from Radiation-Induced Hematopoietic Failure and Lethality. Genes, 13(10), 1756. https://doi.org/10.3390/genes13101756