Analysis of CACNA1C and KCNH2 Risk Variants on Cardiac Autonomic Function in Patients with Schizophrenia
Abstract
1. Introduction
2. Participants and Methods
2.1. Participants
2.2. Assessment of Autonomic Function
2.3. Marker Selection, Genetic Analyses, and Identification of Subgroups
2.4. Statistical Analysis
3. Results
Associations of CACNA1C and KCNH2 Risk Variants with Parameters of Cardiac Autonomic Dysfunction
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hjorthøj, C.; Stürup, A.E.; McGrath, J.J.; Nordentoft, M. Years of potential life lost and life expectancy in schizophrenia: A systematic review and meta-analysis. Lancet Psychiatry 2017, 4, 295–301. [Google Scholar] [CrossRef]
- Laursen, T.M.; Nordentoft, M.; Mortensen, P.B. Excess early mortality in schizophrenia. Annu. Rev. Clin. Psychol. 2014, 10, 425–448. [Google Scholar] [CrossRef] [PubMed]
- Walker, E.R.; McGee, R.E.; Druss, B.G. Mortality in Mental Disorders and Global Disease Burden Implications: A Systematic Review and Meta-analysis. JAMA Psychiatry 2015, 72, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Westman, J.; Eriksson, S.V.; Gissler, M.; Hällgren, J.; Prieto, M.L.; Bobo, W.V.; Frye, M.A.; Erlinge, D.; Alfredsson, L.; Ösby, U. Increased cardiovascular mortality in people with schizophrenia: A 24-year national register study. Epidemiol. Psychiatr. Sci. 2018, 27, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Sweeting, J.; Duflou, J.; Semsarian, C. Postmortem analysis of cardiovascular deaths in schizophrenia: A 10-year review. Schizophr. Res. 2013, 150, 398–403. [Google Scholar] [CrossRef]
- So, H.C.; Chau, K.L.; Ao, F.K.; Mo, C.H.; Sham, P.C. Exploring shared genetic bases and causal relationships of schizophrenia and bipolar disorder with 28 cardiovascular and metabolic traits. Psychol. Med. 2019, 49, 1286–1298. [Google Scholar] [CrossRef]
- Shen, M.J.; Zipes, D.P. Role of the Autonomic Nervous System in Modulating Cardiac Arrhythmias. Circ. Res. 2014, 114, 1004–1021. [Google Scholar] [CrossRef]
- Bär, K.J.; Letzsch, A.; Jochum, T.; Wagner, G.; Greiner, W.; Sauer, H. Loss of efferent vagal activity in acute schizophrenia. J. Psychiatr. Res. 2005, 39, 519–527. [Google Scholar] [CrossRef]
- Bär, K.-J.; Berger, S.; Metzner, M.; Boettger, M.K.; Schulz, S.; Ramachandraiah, C.T.; Terhaar, J.; Voss, A.; Yeragani, V.K.; Sauer, H. Autonomic Dysfunction in Unaffected First-Degree Relatives of Patients Suffering From Schizophrenia. Schizophr. Bull. 2009, 36, 1050–1058. [Google Scholar] [CrossRef]
- Castro, M.N.; Vigo, D.E.; Chu, E.M.; Fahrer, R.D.; de Achaval, D.; Costanzo, E.Y.; Leiguarda, R.C.; Nogués, M.; Cardinali, D.P.; Guinjoan, S.M. Heart rate variability response to mental arithmetic stress is abnormal in first-degree relatives of individuals with schizophrenia. Schizophr. Res. 2009, 109, 134–140. [Google Scholar] [CrossRef]
- Bär. Cardiac Autonomic Dysfunction in Patients with Schizophrenia and Their Healthy Relatives—A Small Review. Front. Neurol. 2015, 6, 139. [Google Scholar] [CrossRef]
- Bär, K.J.; Koschke, M.; Boettger, M.K.; Berger, S.; Kabisch, A.; Sauer, H.; Voss, A.; Yeragani, V.K. Acute psychosis leads to increased QT variability in patients suffering from schizophrenia. Schizophr. Res. 2007, 95, 115–123. [Google Scholar] [CrossRef]
- Imbrici, P.; Conte Camerino, D.; Tricarico, D. Major channels involved in neuropsychiatric disorders and therapeutic perspectives [Review]. Front. Genet. 2013, 4, 76. [Google Scholar] [CrossRef]
- Mäki-Marttunen, T.; Lines, G.T.; Edwards, A.G.; Tveito, A.; Dale, A.M.; Einevoll, G.T.; Andreassen, O.A. Pleiotropic effects of schizophrenia-associated genetic variants in neuron firing and cardiac pacemaking revealed by computational modeling. Transl. Psychiatry 2017, 7, 5. [Google Scholar] [CrossRef]
- Bernardi, J.; Aromolaran, K.A.; Aromolaran, A.S. Neurological Disorders and Risk of Arrhythmia. Int. J. Mol. Sci. 2020, 22, 188. [Google Scholar] [CrossRef]
- Carlo, N.; Timothy, K.W.; Bloise, R.; Priori, S.G. CACNA1C-Related Disorders; Adam, M.P., Ardinger, H.H., Pagon, R.A., Eds.; (2006 Feb 15 [Updated 2021 Feb 11]); GeneReviews® [Internet] Seattle (WA): Seattle, WA, USA; University of Washington: Seattle, WA, USA, 2021. [Google Scholar]
- Lubeiro, A.; Fatjó-Vilas, M.; Guardiola, M.; Almodóvar, C.; Gomez-Pilar, J.; Cea-Cañas, B.; Poza, J.; Palomino, A.; Gómez-García, M.; Zugasti, J.; et al. Analysis of KCNH2 and CACNA1C schizophrenia risk genes on EEG functional network modulation during an auditory odd-ball task. Eur. Arch. Psychiatry Clin. Neurosci. 2020, 270, 433–442. [Google Scholar] [CrossRef]
- Striessnig, J.; Pinggera, A.; Kaur, G.; Bock, G.; Tuluc, P. L-type Ca(2+) channels in heart and brain. Wiley Interdiscip. Rev. Membr. Transp. Signal. 2014, 3, 15–38. [Google Scholar] [CrossRef]
- Yazawa, M.; Hsueh, B.; Jia, X.; Pasca, A.M.; Bernstein, J.A.; Hallmayer, J.; Dolmetsch, R.E. Using induced pluripotent stem cells to investigate cardiac phenotypes in Timothy syndrome. Nature 2011, 471, 230–234. [Google Scholar] [CrossRef]
- Boczek, N.J.; Best, J.M.; Tester, D.J.; Giudicessi, J.R.; Middha, S.; Evans, J.M.; Kamp, T.J.; Ackerman, M.J. Exome sequencing and systems biology converge to identify novel mutations in the L-type calcium channel, CACNA1C, linked to autosomal dominant long QT syndrome. Circ. Cardiovasc. Genet. 2013, 6, 279–289. [Google Scholar] [CrossRef]
- Antzelevitch, C.; Brugada, P.; Borggrefe, M.; Brugada, J.; Brugada, R.; Corrado, D.; Gussak, I.; LeMarec, H.; Nademanee, K.; Riera, A.R.P.; et al. Brugada syndrome: Report of the second consensus conference: Endorsed by the Heart Rhythm Society and the European Heart Rhythm Association. Circulation 2005, 111, 659–670. [Google Scholar] [CrossRef]
- Ripke, S.; Neale, B.M.; Corvin, A.; Walters, J.T.R.; Farh, K.-H.; Holmans, P.A.; O’Donovan, M.C. Biological Insights From 108 Schizophrenia-Associated Genetic Loci. Nature 2014, 511, 421–427. [Google Scholar] [CrossRef]
- Yoshimizu, T.; Pan, J.Q.; Mungenast, A.E.; Madison, J.M.; Su, S.; Ketterman, J.; Ongur, D.; McPhie, D.; Cohen, B.; Perlis, R.; et al. Functional implications of a psychiatric risk variant within CACNA1C in induced human neurons. Mol. Psychiatry 2015, 20, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Purcell, S.M.; Moran, J.L.; Fromer, M.; Ruderfer, D.; Solovieff, N.; Roussos, P.; O’dushlaine, C.; Chambert, K.; Bergen, S.E.; Kähler, A.; et al. A polygenic burden of rare disruptive mutations in schizophrenia. Nature 2014, 506, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Lintunen, J.; Lähteenvuo, M.; Tiihonen, J.; Tanskanen, A.; Taipale, H. Adenosine modulators and calcium channel blockers as add-on treatment for schizophrenia. NJP Schizophr. 2021, 7, 1. [Google Scholar] [CrossRef] [PubMed]
- Van Dyke, P.; Thomas, K.L. Concomitant calcium channel blocker and antipsychotic therapy in patients with schizophrenia: Efficacy analysis of the CATIE-Sz phase 1 data. Ann. Clin. Psychiatry 2018, 30, 6–16. [Google Scholar]
- Huffaker, S.J.; Chen, J.; Nicodemus, K.K.; Sambataro, F.; Yang, F.; Mattay, V.; Lipska, B.K.; Hyde, T.M.; Song, J.; Rujescu, D.; et al. A novel, primate-specific, brain isoform of KCNH2 impacts cortical physiology, cognition, neuronal repolarization and risk for schizophrenia. Nat. Med. 2009, 15, 509–518. [Google Scholar] [CrossRef]
- Carr, G.V.; Chen, J.; Yang, F.; Ren, M.; Yuan, P.; Tian, Q.; Bebensee, A.; Zhang, G.Y.; Du, J.; Glineburg, P.; et al. KCNH2-3.1 expression impairs cognition and alters neuronal function in a model of molecular pathology associated with schizophrenia. Mol. Psychiatry 2016, 21, 1517–1526. [Google Scholar] [CrossRef]
- Kay, S.R.; Fiszbein, A.; Opler, L.A. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr. Bull. 1987, 13, 261–276. [Google Scholar] [CrossRef]
- Lippman, N.; Stein, K.M.; Lerman, B.B. Comparison of methods for removal of ectopy in measurement of heart rate variability. Am. J. Physiol. 1994, 267, H411–H418. [Google Scholar] [CrossRef]
- Fortin, J.; Haitchi, G.; Bojic, A.; Habenbacher, W.; Grüllenberger, R.; Heller, A.; Pacher, R.; Wach, P.; Skrabal, F. Validation and verification of the task force® monitor. Results Clin. Stud. FDA 2001, 510, 1–7. [Google Scholar]
- Malik, M.C.J.; Bigger, J. Heart Rate Variability. Circulation 1996, 93, 1043–1065. [Google Scholar] [CrossRef]
- Montano, N.; Porta, A.; Cogliati, C.; Costantino, G.; Tobaldini, E.; Casali, K.R.; Iellamo, F. Heart rate variability explored in the frequency domain: A tool to investigate the link between heart and behavior. Neurosci. Biobehav. Rev. 2009, 33, 71–80. [Google Scholar] [CrossRef]
- Furlan, R.; Porta, A.; Costa, F.; Tank, J.; Baker, L.; Schiavi, R.; Robertson, D.; Malliani, A.; Mosqueda-Garcia, R. Oscillatory patterns in sympathetic neural discharge and cardiovascular variables during orthostatic stimulus. Circulation 2000, 101, 886–892. [Google Scholar] [CrossRef]
- Billman, G.E. The LF/HF ratio does not accurately measure cardiac sympatho-vagal balance. Front. Physiol. 2013, 4, 26. [Google Scholar] [CrossRef]
- Pagani, M.; Lucini, D.; Porta, A. Sympathovagal balance from heart rate variability: Time for a second round? Exp. Physiol. 2012, 97, 1141–1142. [Google Scholar] [CrossRef]
- Rajendra Acharya, U.; Paul Joseph, K.; Kannathal, N.; Lim, C.M.; Suri, J.S. Heart rate variability: A review. Med. Biol. Eng. Comput. 2006, 44, 1031–1051. [Google Scholar] [CrossRef]
- Reyes del Paso, G.A.; Langewitz, W.; Mulder, L.J.; van Roon, A.; Duschek, S. The utility of low frequency heart rate variability as an index of sympathetic cardiac tone: A review with emphasis on a reanalysis of previous studies. Psychophysiology 2013, 50, 477–487. [Google Scholar] [CrossRef]
- Bär, K.J.; Boettger, M.K.; Koschke, M.; Schulz, S.; Chokka, P.; Yeragani, V.K.; Voss, A. Non-linear complexity measures of heart rate variability in acute schizophrenia. Clin. Neurophysiol. 2007, 118, 2009–2015. [Google Scholar] [CrossRef]
- Baumert, M.; Baier, V.; Haueisen, J.; Wessel, N.; Meyerfeldt, U.; Schirdewan, A.; Voss, A. Forecasting of life threatening arrhythmias using the compression entropy of heart rate. Methods Inf. Med. 2004, 43, 202–206. [Google Scholar] [CrossRef]
- Berger, R.D.; Kasper, E.K.; Baughman, K.L.; Marban, E.; Calkins, H.; Tomaselli, G.F. Beat-to-beat QT interval variability: Novel evidence for repolarization lability in ischemic and nonischemic dilated cardiomyopathy. Circulation 1997, 96, 1557–1565. [Google Scholar] [CrossRef]
- Pardiñas, A.F.; Holmans, P.; Pocklington, A.J.; Escott-Price, V.; Ripke, S.; Carrera, N.; Legge, S.E.; Bishop, S.; Cameron, D.; Hamshere, M.L.; et al. Common schizophrenia alleles are enriched in mutation-intolerant genes and in regions under strong background selection. Nat. Genet. 2018, 50, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Eppinga, R.N.; Hagemeijer, Y.; Burgess, S.; Hinds, D.A.; Stefansson, K.; Gudbjartsson, D.F.; Van Veldhuisen, D.J.; Munroe, P.B.; Verweij, N.; van der Harst, P. Identification of genomic loci associated with resting heart rate and shared genetic predictors with all-cause mortality. Nat. Genet. 2016, 48, 1557–1563. [Google Scholar] [CrossRef] [PubMed]
- Ramírez, J.; Duijvenboden, S.V.; Ntalla, I.; Mifsud, B.; Warren, H.R.; Tzanis, E.; Orini, M.; Tinker, A.; Lambiase, P.D.; Munroe, P.B. Thirty loci identified for heart rate response to exercise and recovery implicate autonomic nervous system. Nat. Commun. 2018, 9, 1947. [Google Scholar] [CrossRef] [PubMed]
- Arking, D.E.; Pulit, S.L.; Crotti, L.; van der Harst, P.; Munroe, P.B.; Koopmann, T.T.; Sotoodehnia, N.; Rossin, E.J.; Morley, M. Genetic association study of QT interval highlights role for calcium signaling pathways in myocardial repolarization. Nat. Genet. 2014, 46, 826–836. [Google Scholar] [CrossRef] [PubMed]
- Newton-Cheh, C.; Eijgelsheim, M.; Rice, K.M.; de Bakker, P.I.; Yin, X.; Estrada, K.; Bis, J.C.; Marciante, K.; Rivadeneira, F.; Noseworthy, P.A.; et al. Common variants at ten loci influence QT interval duration in the QTGEN Study. Nat. Genet. 2009, 41, 399–406. [Google Scholar] [CrossRef]
- Braganholi, D.F.; Cicarelli, R.M. Analysis of SNP (single nucleotide polymorphism) multiplex markers related to sudden cardiac death in Brazilian families. Genet. Mol. Res. 2015, 14, 14348. [Google Scholar] [CrossRef]
- Wang, Q.S.; Wang, X.F.; Chen, X.D.; Yu, J.F.; Wang, J.; Sun, J.; Lu, S.-B.; Shen, M.-Y.; Lu, M.; Li, Y.-G.; et al. Genetic polymorphism of KCNH2 confers predisposition of acquired atrial fibrillation in Chinese. J. Cardiovasc. Electrophysiol. 2009, 20, 1158–1162. [Google Scholar] [CrossRef]
- Ripke, S.; O’dushlaine, C.; Chambert, K.; Moran, J.L.; Kähler, A.K.; Akterin, S.; Bergen, S.E.; Collins, A.L.; Crowley, J.J.; Fromer, M.; et al. Genome-wide association analysis identifies 13 new risk loci for schizophrenia. Nat. Genet. 2013, 45, 1150–1159. [Google Scholar] [CrossRef]
- Jia PHan, G.; Zhao, J.; Lu, P.; Zhao, P. SZGR 2.0: A one-stop shop of schizophrenia candidate genes. Nucleic Acids Res. 2017, 45, D915–D924. [Google Scholar] [CrossRef]
- Machiela, M.J.; Chanock, S.J. LDlink: A web-based application for exploring population-specific haplotype structure and linking correlated alleles of possible functional variants. Bioinformatics 2015, 31, 3555–3557. [Google Scholar] [CrossRef]
- Betzenhauser, M.J.; Pitt, G.S.; Antzelevitch, C. Calcium Channel Mutations in Cardiac Arrhythmia Syndromes. Curr. Mol. Pharm. 2015, 8, 133–142. [Google Scholar] [CrossRef]
- Zhu, D.; Yin, J.; Liang, C.; Luo, X.; Lv, D.; Dai, Z.; Xiong, S.; Fu, J.; Li, Y.; Lin, J.; et al. CACNA1C (rs1006737) may be a susceptibility gene for schizophrenia: An updated meta-analysis. Brain Behav. 2019, 9, e01292. [Google Scholar] [CrossRef]
- Porter, B.; van Duijvenboden, S.; Bishop, M.J.; Orini, M.; Claridge, S.; Gould, J.; Sieniewicz, B.; Sidhu, B.; Razavi, R.; Rinaldi, C.; et al. Beat-to-Beat Variability of Ventricular Action Potential Duration Oscillates at Low Frequency During Sympathetic Provocation in Humans. Front. Physiol. 2018, 9, 147. [Google Scholar] [CrossRef]
- Moon, A.L.; Haan, N.; Wilkinson, L.S.; Thomas, K.L.; Hall, J. CACNA1C: Association With Psychiatric Disorders, Behavior, and Neurogenesis. Schizophr. Bull. 2018, 44, 958–965. [Google Scholar] [CrossRef]
- Fujii, K.; Ozeki, Y.; Okayasu, H.; Takano, Y.; Shinozaki, T.; Hori, H.; Orui, M.; Horie, M.; Kunugi, H.; Shimoda, K. QT is longer in drug-free patients with schizophrenia compared with age-matched healthy subjects. PLoS ONE 2014, 9, e98555. [Google Scholar] [CrossRef]
- Yu, H.; Yan, H.; Wang, L.; Li, J.; Tan, L.; Deng, W.; Chen, Q.; Yang, G.; Zhang, F.; Lu, T.; et al. Five novel loci associated with antipsychotic treatment response in patients with schizophrenia: A genome-wide association study. Lancet Psychiatry 2018, 5, 327–338. [Google Scholar] [CrossRef]
- Apud, J.A.; Zhang, F.; Decot, H.; Bigos, K.L.; Weinberger, D.R. Genetic variation in KCNH2 associated with expression in the brain of a unique hERG isoform modulates treatment response in patients with schizophrenia. Am. J. Psychiatry 2012, 169, 725–734. [Google Scholar] [CrossRef]
- Hashimoto, R.; Ohi, K.; Yasuda, Y.; Fukumoto, M.; Yamamori, H.; Kamino, K.; Morihara, T.; Iwase, M.; Kazui, H.; Takeda, M. The KCNH2 gene is associated with neurocognition and the risk of schizophrenia. World J. Biol. Psychiatry 2013, 14, 114–120. [Google Scholar] [CrossRef]
- Baumert, M.; Schlaich, M.P.; Nalivaiko, E.; Lambert, E.; Sari, C.I.; Kaye, D.M.; Elser, M.D.; Sanders, P.; Lambert, G. Relation between QT interval variability and cardiac sympathetic activity in hypertension. Am. J. Physiol. Heart Circ. Physiol. 2011, 300, H1412–H1417. [Google Scholar] [CrossRef]
- Kusuki, H.; Tsuchiya, Y.; Mizutani, Y.; Nishio, M.; Oikawa, S.; Nagata, R.; Kiriyanagi, Y.; Horio, K.; Kojima, A.; Uchida, H.; et al. QT Variability Index is Correlated with Autonomic Nerve Activity in Healthy Children. Pediatr. Cardiol. 2020, 41, 1432–1437. [Google Scholar] [CrossRef]
- Hillebrand, S.; Gast, K.B.; de Mutsert, R.; Swenne, C.A.; Jukema, J.W.; Middeldorp, S.; Rosendaal, F.R.; Dekkers, O.M. Heart rate variability and first cardiovascular event in populations without known cardiovascular disease: Meta-analysis and dose-response meta-regression. Europace 2013, 15, 742–749. [Google Scholar] [CrossRef] [PubMed]
- Jensen, M.T.; Marott, J.L.; Lange, P.; Vestbo, J.; Schnohr, P.; Nielsen, O.W.; Jensen, J.S.; Jensen, G.B. Resting heart rate is a predictor of mortality in COPD. Eur. Respir J. 2013, 42, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Thayer, J.F.; Yamamoto, S.S.; Brosschot, J.F. The relationship of autonomic imbalance, heart rate variability and cardiovascular disease risk factors. Int. J. Cardiol. 2010, 141, 122–131. [Google Scholar] [CrossRef]
- Hancox, J.C.; McPate, M.J.; El Harchi, A.; Zhang, Y.H. The hERG potassium channel and hERG screening for drug-induced torsades de pointes. Pharmacol. Ther. 2008, 119, 118–132. [Google Scholar] [CrossRef] [PubMed]
- Perrin, M.J.; Subbiah, R.N.; Vandenberg, J.I.; Hill, A.P. Human ether-a-go-go related gene (hERG) K+ channels: Function and dysfunction. Prog. Biophys. Mol. Biol. 2008, 98, 137–148. [Google Scholar] [CrossRef]
- Atalar, F.; Acuner, T.T.; Cine, N.; Oncu, F.; Yesilbursa, D.; Ozbek, U.; Turkcan, S. Two four-marker haplotypes on 7q36.1 region indicate that the potassium channel gene HERG1 (KCNH2, Kv11.1) is related to schizophrenia: A case control study. Behav. Brain Funct. 2010, 6, 27. [Google Scholar] [CrossRef]
- Heide, J.; Zhang, F.; Bigos, K.L.; Mann, S.A.; Carr, V.J.; Shannon Weickert, C.; Green, M.J.; Weinberger, D.R.; Vandenberg, J.I. Differential Response to Risperidone in Schizophrenia Patients by KCNH2 Genotype and Drug Metabolizer Status. Am. J. Psychiatry 2016, 173, 53–59. [Google Scholar] [CrossRef]
- Heide, J.; Vandenberg, J.I.; Shannon Weickert, C. Expression of KCNH2-3.1 mRNA is increased in small neurons in the dorsolateral prefrontal cortex in patients with schizophrenia. Eur. J. Psychiatry 2015, 29, 85–103. [Google Scholar] [CrossRef][Green Version]
- Ren, M.; Hu, Z.; Chen, Q.; Jaffe, A.; Li, Y.; Sadashivaiah, V.; Zhu, S.; Rajpurohit, N.; Shin, J.H.; Xia, W.; et al. KCNH2-3.1 mediates aberrant complement activation and impaired hippocampal-medial prefrontal circuitry associated with working memory deficits. Mol. Psychiatry 2020, 25, 206–229. [Google Scholar] [CrossRef]
- Refisch, A.; Chung, H.Y.; Komatsuzaki, S.; Schumann, A.; Mühleisen, T.W.; Nöthen, M.M.; Hübner, C.A.; Bär, K.J. A common variation in HCN1 is associated with heart rate variability in schizophrenia. Schizophr. Res. 2021, 229, 73–79. [Google Scholar] [CrossRef]
- Refisch, A.; Komatsuzaki, S.; Ungelenk, M.; Chung, H.Y.; Schumann, A.; Schilling, S.S.; Jantzen, W.; Schröder, S.; Mühleisen, T.W.; Nöthen, M.M.; et al. Associations of common genetic risk variants of the muscarinic acetylcholine receptor M2 with cardiac autonomic dysfunction in patients with schizophrenia. World J. Biol. Psychiatry 2022. Available online: https://pubmed.ncbi.nlm.nih.gov/35172679/ (accessed on 4 February 2022). [CrossRef]
- Greenwood, T.A.; Lazzeroni, L.C.; Maihofer, A.X.; Swerdlow, N.R.; Calkins, M.E.; Freedman, R.; Green, M.F.; Light, G.A.; Nievergelt, C.M.; Nuechterlein, K.H.; et al. Genome-wide Association of Endophenotypes for Schizophrenia from the Consortium on the Genetics of Schizophrenia (COGS) Study. JAMA Psychiatry 2019, 76, 1274. [Google Scholar] [CrossRef]
- Stogios, N.; Gdanski, A.; Gerretsen, P.; Chintoh, A.F.; Graff-Guerrero, A.; Rajji, T.K.; Remington, G.; Hahn, M.K.; Agarwal, S.M. Autonomic nervous system dysfunction in schizophrenia: Impact on cognitive and metabolic health. Npj Schizophr. 2021, 7, 22. [Google Scholar] [CrossRef]
| SNP | HGVS Nomenclature | Annotation | Trait | Allele E/O | p Value | Source |
|---|---|---|---|---|---|---|
| rs11763131 | NC_000007.14:g.150971094G>A | upstream | schizophrenia | A/G | 0.07 | [27] |
| rs3807373 | NC_000007.14:g.150971633G>A | upstream | schizophrenia | A/G | 0.013 | |
| rs3807374 | NC_000007.14:g.150971258T>C | upstream | schizophrenia | G/T | 0.017 | |
| rs3800779 | NC_000007.14:g.150974126C>A | upstream | schizophrenia | C/T | 0.0054 | |
| rs748693 | NC_000007.14:g.150974349A>G | upstream | schizophrenia | G/A | 0.027 | |
| rs1036145 | NC_000007.14:g.150977342C>G | upstream | schizophrenia | T/C | 0.015 | |
| rs2968864 | NC_000007.14:g.150925074T>C | downstream | QT interval | C/T | 8 × 10−16 | [46] |
| rs4725982 | NC_000007.14:g.150940775C>G | downstream | QT interval sudden cardiac death | T/C | 5 × 10−16 | [46,47] |
| rs2072413 | NC_000007.14:g.150950881C>T | intronic | QT interval | T/C | 1 × 10−49 | [45] |
| rs1805120 | NC_000007.14:g.150952443G>A NM_000238.4:c.1539C>T NP_000229.1:p.Phe513= | synonymous variant | acquired atrial fibrillation | A/G | 0.021 | [48] |
| SNP | HGVS nomenclature | Annotation | Trait | Allele E/O | p value | Source |
| rs1006737 | NC_000012.12:g.2236129G>A | intronic | schizophrenia | A/G | 1.09 × 10−16 | [49] |
| rs4765905 | NC_000012.12:g.2240418G>A | intronic | schizophrenia | C/G | 1.08 × 10−16 | Psychiatric Genomics Consortium (PGC) [50] |
| rs2007044 | NC_000012.12:g.2235794A>G | intronic | schizophrenia | G/A | 2.63 × 10−17 | [22,42] |
| rs2239063 | NC_000012.12:g.2402665A>C | intronic | schizophrenia | C/A | 5.39 × 10−9 | PGC [50] |
| rs2283274 | NC_000012.12:g.2075300G>C | intronic | resting heart rate | C/G | 7.21 × 10−20 | [43,44] |
| Diagnostic Group | p | ||
|---|---|---|---|
| Healthy Controls | Schizophrenia Patients | ||
| Soziodemographic Data | |||
| N | 144 | 77 | NA |
| age (y) | 29.9 ± 6.9 (19 to 44 y) | 33.1 ± 11.5 (19 to 49 y) | 0.009 |
| gender (f/m) | 73/71 | 45/32 | n.s. |
| smoker status (y/n) | 30/114 | 44/33 | <0.001 |
| cig. per day | 1.3 ± 3.3 | 7.3 ± 9.5 | <0.001 |
| cups of coffee a day | 1.4 ± 1.4 | 2.4 ± 2.3 | 0.025 |
| BMI (m/kg2) | 23.1 ± 3.4 | 23.0 ± 8.0 | n.s. |
| hours of sport per week | 1.9 ± 2.0 | 0.9 ± 1.4 | n.s. |
| Psychopathology | |||
| PANSS gen | NA | 41.9 ± 11.4 | NA |
| PANSS pos | NA | 22.2 ± 6.3 | NA |
| CACNA1C rs2283274 G > C | ||||||
|---|---|---|---|---|---|---|
| Healthy Controls | Schizophrenia Patients | |||||
| MAF | C = 0.16 (45/288) | C = 0.22 (34/154) | ||||
| GG | 0.71 (103/144) | 0.58 (45/77) | ||||
| CG | 0.26 (37/144) | 0.39 (30/77) | ||||
| CC | 0.03 (4/144) | 0.03 (2/77) | ||||
| χ2 | 0.0011 | 0.014 | ||||
| CAF | GG | CG/CC | p | GG | CG/CC | p |
| N | 103 | 41 | n.s. | 45 | 32 | 0.011 |
| mHR | 65.4 ± 11.8 | 64.5 ± 10.6 | n.s. | 77.5 ± 12.5 | 78.7 ± 9.8 | n.s. |
| LF/HF | 2.1 ± 2.1 | 2.6 ± 4.4 | n.s. | 3.5 ± 2.9 | 2.6 ± 2.3 | n.s. |
| RMSSD | 66.5 ± 41.2 | 67.7 ± 52.4 | n.s. | 34.0 ± 30.9 | 37.1 ± 32.3 | n.s. |
| Hc | 0.83 ± 0.06 | 0.81 ± 0.07 | n.s. | 0.77 ± 0.11 | 0.78 ± 0.09 | n.s. |
| QTc | 0.413 ± 0.04 | 0.404 ± 0.04 | n.s. | 0.441 ± 0.05 | 0.466 ± 0.05 | 0.042 |
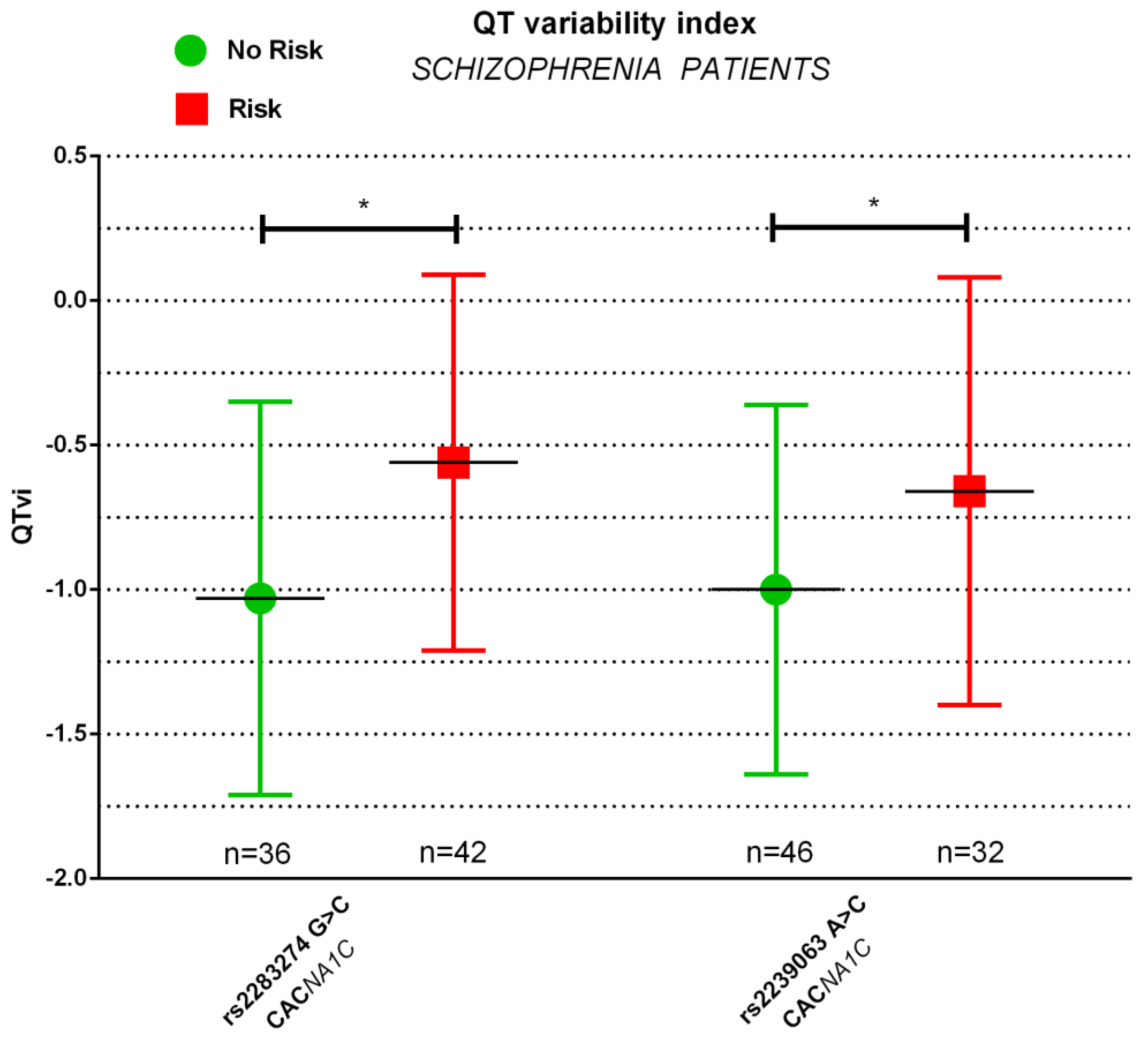
| QTvi | −1.35 ± 0.46 | −1.33 ± 0.46 | n.s. | −1.03 ± 0.68 | −0.56 ± 0.65 | 0.003 |
| CACNA1C rs2007044 A > G | ||||||
| Healthy Controls | Schizophrenia Patients | |||||
| MAF | G = 0.38 (110/288) | G = 0.34 (52/154) | ||||
| AA | 0.40 (58/144) | 0.45 (35/77) | ||||
| AG | 0.43 (62/144) | 0.42 (32/77) | ||||
| GG | 0.17 (24/144) | 0.13 (10/77) | ||||
| χ2 | 0.0085 | 0.0041 | ||||
| CAF | AA | AG/GG | p | AA | AG/GG | p |
| N | 58 | 86 | n.s. | 35 | 42 | n.s. |
| mHR | 65.9 ± 13.7 | 64.7 ± 9.7 | n.s. | 77.8 ± 10.8 | 78.2 ± 11.9 | n.s. |
| LF/HF | 2.8 ± 4.2 | 1.9 ± 1.6 | n.s. | 3.2 ± 2.9 | 3.1 ± 2.5 | n.s. |
| RMSSD | 61.7 ± 38.5 | 70.3 ± 48.0 | n.s. | 32.0 ± 30.5 | 38.0 ± 32.1 | n.s. |
| Hc | 0.82 ± 0.07 | 0.82 ± 0.06 | n.s. | 0.76 ± 0.12 | 0.79 ± 0.09 | n.s. |
| QTc | 0.412 ± 0.05 | 0.409 ± 0.04 | n.s. | 0.449 ± 0.05 | 0.453 ± 0.05 | n.s. |
| QTvi | −1.37 ± 0.45 | −1.33 ± 0.47 | n.s. | −0.86 ± 0.57 | −0.81 ± 0.80 | n.s. |
| CACNA1C rs1006737 G > A | ||||||
| Healthy Controls | Schizophrenia Patients | |||||
| MAF | A = 0.34 (99/288) | A = 0.29 (44/154) | ||||
| GG | 0.46 (66/144) | 0.53 (41/77) | ||||
| AG | 0.40 (57/144) | 0.36 (28/77) | ||||
| AA | 0.14 (21/144) | 0.10 (8/77) | ||||
| χ2 | 0.0118 | 0.0106 | ||||
| CAF | GG | AG/AA | p | GG | AG/AA | p |
| N | 66 | 78 | n.s. | 41 | 36 | n.s. |
| mHR | 65.2 ± 13.2 | 65.2 ± 9.8 | n.s. | 77.8 ± 11.7 | 78.2 ± 11.2 | n.s. |
| LF/HF | 2.8 ± 4.0 | 1.8 ± 1.6 | n.s. | 3.0 ± 2.8 | 3.2 ± 2.6 | n.s. |
| RMSSD | 61.9 ± 37.5 | 71.0 ± 49.5 | n.s. | 35.3 ± 36.3 | 35.3 ± 25.0 | n.s. |
| Hc | 0.82 ± 0.07 | 0.82 ± 0.06 | n.s. | 0.76 ± 0.11 | 0.79 ± 0.08 | n.s. |
| QTc | 0.408 ± 0.05 | 0.412 ± 0.04 | n.s. | 0.451 ± 0.05 | 0.452 ± 0.05 | n.s. |
| QTvi | −1.38 ± 0.44 | −1.31 ± 0.48 | n.s. | −0.87 ± 0.61 | −0.80 ± 0.81 | n.s. |
| CACNA1C rs4765905 G > C | ||||||
| Healthy Controls | Schizophrenia Patients | |||||
| MAF | C = 0.33 (96/288) | C = 0.29 (44/154) | ||||
| GG | 0.47 (68/144) | 0.53 (41/77) | ||||
| CG | 0.39 (56/144) | 0.36 (28/77) | ||||
| CC | 0.14 (20/144) | 0.11 (8/77) | ||||
| χ2 | 0.0155 | 0.0158 | ||||
| CAF | GG | CG/CC | p | GG | CG/CC | p |
| N | 68 | 76 | n.s. | 41 | 36 | n.s. |
| mHR | 65.1 ± 13.1 | 65.3 ± 9.9 | n.s. | 77.8 ± 11.7 | 78.2 ± 11.2 | n.s. |
| LF/HF | 2.8 ± 3.9 | 1.7 ± 1.5 | n.s. | 3.0 ± 2.8 | 3.2 ± 2.6 | n.s. |
| RMSSD | 61.1 ± 37.3 | 72.0 ± 49.7 | n.s. | 35.3 ± 36.3 | 35.3 ± 25.0 | n.s. |
| Hc | 0.82 ± 0.07 | 0.82 ± 0.06 | n.s. | 0.76 ± 0.11 | 0.79 ± 0.08 | n.s. |
| QTc | 0.408 ± 0.05 | 0.412 ± 0.04 | n.s. | 0.451 ± 0.05 | 0.452 ± 0.05 | n.s. |
| QTvi | −1.38 ± 0.43 | −1.31 ± 0.48 | n.s. | −0.87 ± 0.61 | −0.80 ± 0.81 | n.s. |
| CACNA1C rs2239063 A > C | ||||||
| Healthy Controls | Schizophrenia Patients | |||||
| MAF | C = 0.34 (99/288) | C = 0.29 (44/154) | ||||
| AA | 0.44 (64/144) | 0.52 (40/77) | ||||
| AC | 0.42 (61/144) | 0.39 (30/77) | ||||
| CC | 0.14 (19/144) | 0.09 (7/77) | ||||
| χ2 | 0.0059 | 0.0019 | ||||
| CAF | AA | AC/CC | p | AA | AC/CC | p |
| N | 64 | 80 | n.s. | 40 | 37 | 0.050 |
| mHR | 65.0 ± 9.3 | 65.3 ± 13.0 | n.s. | 79.0 ± 11.1 | 77.0 ± 11.8 | n.s. |
| LF/HF | 2.2 ± 2.4 | 2.3 ± 3.4 | n.s. | 3.6 ± 3.1 | 2.6 ± 2.1 | n.s. |
| RMSSD | 67.3 ± 41.7 | 66.4 ± 46.8 | n.s. | 37.1 ± 36.2 | 33.3 ± 25.4 | n.s. |
| Hc | 0.82 ± 0.07 | 0.82 ± 0.06 | n.s. | 0.79 ± 0.08 | 0.76 ± 0.12 | n.s. |
| QTc | 0.411 ± 0.04 | 0.409 ± 0.05 | n.s. | 0.447 ± 0.04 | 0.456 ± 0.06 | n.s. |
| QTvi | −1.35 ± 0.48 | −1.34 ± 0.45 | n.s. | −1.00 ± 0.64 | −0.66 ± 0.74 | 0.037 |
| KCNH2 rs2968864 T > C | ||||||
|---|---|---|---|---|---|---|
| Healthy Controls | Schizophrenia Patients | |||||
| MAF | C = 0.28 (80/288) | C = 0.27 (41/154) | ||||
| TT | 0.48 (69/144) | 0.51 (39/77) | ||||
| CT | 0.47 (68/144) | 0.45 (35/77) | ||||
| CC | 0.05 (6/144) | 0.04 (3/77) | ||||
| χ2 | 0.0235 | 0.0241 | ||||
| CAF | TT | CT/CC | p | TT | CT/CC | p |
| N | 70 | 74 | n.s. | 39 | 38 | n.s. |
| mHR | 64.1 ± 9.4 | 66.1 ± 13.1 | n.s. | 78.9 ± 11.6 | 77.2 ± 11.3 | n.s. |
| LF/HF | 1.8 ± 1.4 | 2.7 ± 3.8 | n.s. | 3.4 ± 3.1 | 2.8 ± 2.1 | n.s. |
| RMSSD | 66.5 ± 41.8 | 67.1 ± 47.2 | n.s. | 35.5 ± 32.7 | 35.1 ± 30.2 | n.s. |
| Hc | 0.82 ± 0.06 | 0.82 ± 0.07 | n.s. | 0.77 ± 0.12 | 0.78 ± 0.07 | n.s. |
| QTc | 0.404 ± 0.04 | 0.416 ± 0.05 | n.s. | 0.463 ± 0.05 | 0.439 ± 0.05 | n.s. |
| QTvi | −1.40 ± 0.42 | −1.29 ± 0.49 | n.s. | −0.67 ± 0.76 | −1.01 ± 0.61 | n.s. |
| KCNH2 rs4725982 C > T | ||||||
| Healthy Controls | Schizophrenia Patients | |||||
| MAF | T = 0.21 (60/288) | T = 0.18 (28/154) | ||||
| CC | 0.63 (90/144) | 0.68 (52/77) | ||||
| CT | 0.33 (48/144) | 0.29 (22/77) | ||||
| TT | 0.04 (6/144) | 0.03 (3/77) | ||||
| χ2 | 0.0002 | 0.0000 | ||||
| CAF | CC | CT/TT | p | CC | CT/TT | p |
| N | 90 | 54 | n.s. | 52 | 25 | n.s. |
| mHR | 66.0 ± 12.8 | 63.7 ± 8.7 | n.s. | 76.3 ± 11.1 | 81.6 ± 11.2 | n.s. |
| LF/HF | 2.3 ± 3.3 | 2.2 ± 2.2 | n.s. | 2.9 ± 2.5 | 3.6 ± 3.1 | n.s. |
| RMSSD | 67.9 ± 48.3 | 65.0 ± 37.6 | n.s. | 39.0 ± 35.1 | 27.5 ± 19.7 | n.s. |
| Hc | 0.82 ± 0.07 | 0.82 ± 0.07 | n.s. | 0.79 ± 0.09 | 0.75 ± 0.12 | n.s. |
| QTc | 0.413 ± 0.05 | 0.405 ± 0.04 | n.s. | 0.447 ± 0.05 | 0.461 ± 0.06 | n.s. |
| QTvi | −1.32 ± 0.48 | −1.38 ± 0.43 | n.s. | −0.87 ± 0.72 | −0.76 ± 0.68 | n.s. |
| KCNH2 1,805,120 G > A | ||||||
| Healthy Controls | Schizophrenia Patients | |||||
| MAF | A = 0.28 (80/288) | A = 0.26 (40/154) | ||||
| GG | 0.54 (78/144) | 0.52 (40/77) | ||||
| AG | 0.36 (52/144) | 0.44 (34/77) | ||||
| AA | 0.10 (14/144) | 0.04 (3/77) | ||||
| χ2 | 0.0115 | 0.0206 | ||||
| CAF | GG | AG/AA | p | GG | AG/AA | p |
| N | 78 | 66 | n.s. | 40 | 37 | n.s. |
| mHR | 64.6 ± 9.5 | 65.9 ± 13.3 | n.s. | 78.9 ± 11.6 | 77.0 ± 11.4 | n.s. |
| LF/HF | 2.0 ± 1.8 | 2.6 ± 4.0 | n.s. | 3.4 ± 3.1 | 2.9 ± 2.1 | n.s. |
| RMSSD | 66.2 ± 43.5 | 67.4 ± 46.1 | n.s. | 35.5 ± 32.7 | 32.9 ± 26.4 | n.s. |
| Hc | 0.82 ± 0.07 | 0.83 ± 0.06 | n.s. | 0.77 ± 0.12 | 0.78 ± 0.07 | n.s. |
| QTc | 0.405 ± 0.04 | 0.417 ± 0.05 | n.s. | 0.463 ± 0.05 | 0.439 ± 0.05 | n.s. |
| QTvi | −1.36 ± 0.47 | −1.31 ± 0.47 | n.s. | −0.67 ± 0.76 | −1.04 ± 0.61 | n.s. |
| KCNH2 rs2072413 C > T | ||||||
| Healthy Controls | Schizophrenia Patients | |||||
| MAF | T = 0.31 (90/288) | T = 0.27 (41/154) | ||||
| CC | 0.47 (67/144) | 0.52 (40/77) | ||||
| CT | 0.44 (64/144) | 0.44 (33/77) | ||||
| TT | 0.09 (13/144) | 0.04 (4/77) | ||||
| χ2 | 0.0008 | 0.0206 | ||||
| CAF | CC | CT/TT | p | CC | CT/TT | p |
| N | 67 | 77 | n.s. | 40 | 37 | n.s. |
| mHR | 65.2 ± 9.6 | 65.1 ± 12.9 | n.s. | 78.5 ± 11.6 | 77.4 ± 11.3 | n.s. |
| LF/HF | 1.8 ± 1.4 | 2.6 ± 3.8 | n.s. | 3.3 ± 3.2 | 2.9 ± 2.1 | n.s. |
| RMSSD | 64.6 ± 42.2 | 68.8 ± 46.6 | n.s. | 38.0 ± 35.6 | 32.4 ± 26.1 | n.s. |
| Hc | 0.81 ± 0.07 | 0.83 ± 0.07 | n.s. | 0.77 ± 0.12 | 0.78 ± 0.07 | n.s. |
| QTc | 0.408 ± 0.04 | 0.412 ± 0.05 | n.s. | 0.459 ± 0.05 | 0.443 ± 0.05 | n.s. |
| QTvi | −1.35 ± 0.46 | −1.34 ± 0.46 | n.s. | −0.72 ± 0.74 | −0.96 ± 0.66 | n.s. |
| KCNH2 rs11763131 G > A | ||||||
| Healthy Controls | Schizophrenia Patients | |||||
| MAF | A = 0.25 (71/288) | A = 0.34 (52/154) | ||||
| GG | 0.58 (84/144) | 0.44 (34/77) | ||||
| AG | 0.34 (49/144) | 0.44 (34/77) | ||||
| AA | 0.08 (11/144) | 0.12 (9/77) | ||||
| χ2 | 0.0087 | 0.0004 | ||||
| CAF | GG | AG/AA | p | GG | AG/AA | p |
| N | 84 | 60 | n.s. | 34 | 43 | 0.010 |
| mHR | 65.7 ± 12.5 | 64.5 ± 9.8 | n.s. | 74.9 ± 9.5 | 80.5 ± 12.2 | 0.033 |
| LF/HF | 2.6 ± 3.6 | 1.7 ± 1.7 | n.s. | 2.7 ± 2.2 | 3.4 ± 3.0 | n.s. |
| RMSSD | 63.0 ± 37.0 | 72.2 ± 53.1 | n.s. | 36.1 ± 29.5 | 34.7 ± 33.0 | n.s. |
| Hc | 0.82 ± 0.06 | 0.82 ± 0.07 | n.s. | 0.78 ± 0.08 | 0.77 ± 0.12 | n.s. |
| QTc | 0.414 ± 0.05 | 0.404 ± 0.04 | n.s. | 0.449 ± 0.05 | 0.453 ± 0.05 | n.s. |
| QTvi | −1.30 ± 0.50 | −1.41 ± 0.39 | n.s. | −1.07 ± 0.55 | −0.65 ± 0.76 | 0.008 |
| KCNH2 rs3807374 T > G | ||||||
| Healthy Controls | Schizophrenia Patients | |||||
| MAF | G = 0.26 (75/288) | G = 0.34 (53/154) | ||||
| TT | 0.58 (84/144) | 0.44 (34/77) | ||||
| GT | 0.31 (43/144) | 0.43 (33/77) | ||||
| GG | 0.11 (16/144) | 0.13 (10/77) | ||||
| χ2 | 0.0417 | 0.0024 | ||||
| CAF | TT | GT/GG | p | TT | GT/GG | p |
| N | 85 | 59 | n.s. | 34 | 43 | n.s. |
| mHR | 65.7 ± 12.4 | 64.1 ± 9.8 | n.s. | 75.9 ± 10.5 | 79.7 ± 11.9 | n.s. |
| LF/HF | 2.7 ± 3.6 | 1.6 ± 1.4 | n.s. | 2.7 ± 2.1 | 3.4 ± 3.0 | n.s. |
| RMSSD | 64.5 ± 39.4 | 70.5 ± 51.2 | n.s. | 35.9 ± 29.7 | 34.8 ± 32.9 | n.s. |
| Hc | 0.82 ± 0.06 | 0.82 ± 0.07 | n.s. | 0.78 ± 0.07 | 0.77 ± 0.12 | n.s. |
| QTc | 0.414 ± 0.05 | 0.403 ± 0.04 | n.s. | 0.452 ± 0.05 | 0.451 ± 0.05 | n.s. |
| QTvi | −1.32 ± 0.48 | −1.40 ± 0.39 | n.s. | −1.02 ± 0.62 | −0.69 ± 0.74 | n.s. |
| KCNH2 rs3807373 G > A | ||||||
| Healthy Controls | Schizophrenia Patients | |||||
| MAF | A = 0.25 (71/288) | A = 0.33 (51/154) | ||||
| GG | 0.58 (84/144) | 0.45 (35/77) | ||||
| AG | 0.34 (49/144) | 0.43 (33/77) | ||||
| AA | 0.08 (11/144) | 0.12 (9/77) | ||||
| χ2 | 0.0087 | 0.0012 | ||||
| CAF | GG | AG/AA | p | GG | AG/AA | p |
| N | 84 | 60 | n.s. | 35 | 42 | 0.024 |
| mHR | 65.7 ± 12.5 | 64.5 ± 9.8 | n.s. | 74.9 ± 9.4 | 80.6 ± 12.3 | 0.028 |
| LF/HF | 2.6 ± 3.6 | 1.7 ± 1.7 | n.s. | 2.7 ± 2.1 | 3.5 ± 3.1 | n.s. |
| RMSSD | 63.0 ± 37.0 | 72.2 ± 53.1 | n.s. | 36.1 ± 29.1 | 34.6 ± 33.4 | n.s. |
| Hc | 0.82 ± 0.06 | 0.82 ± 0.07 | n.s. | 0.78 ± 0.08 | 0.77 ± 0.12 | n.s. |
| QTc | 0.414 ± 0.05 | 0.404 ± 0.04 | n.s. | 0.448 ± 0.05 | 0.454 ± 0.05 | n.s. |
| QTvi | −1.30 ± 0.50 | −1.41 ± 0.39 | n.s. | −1.05 ± 0.56 | −0.66 ± 0.77 | 0.013 |
| KCNH2 rs3800779 T > C | ||||||
| Healthy Controls | Schizophrenia Patients | |||||
| MAF | C = 0.30 (85/288) | C = 0.25 (39/154) | ||||
| TT | 0.50 (72/144) | 0.42 (32/77) | ||||
| CT | 0.41 (59/144) | 0.40 (31/77) | ||||
| CC | 0.09 (13/144) | 0.18 (14/77) | ||||
| χ2 | 0.0002 | 0.0228 | ||||
| CAF | TT | CT/CC | p | TT | CT/CC | p |
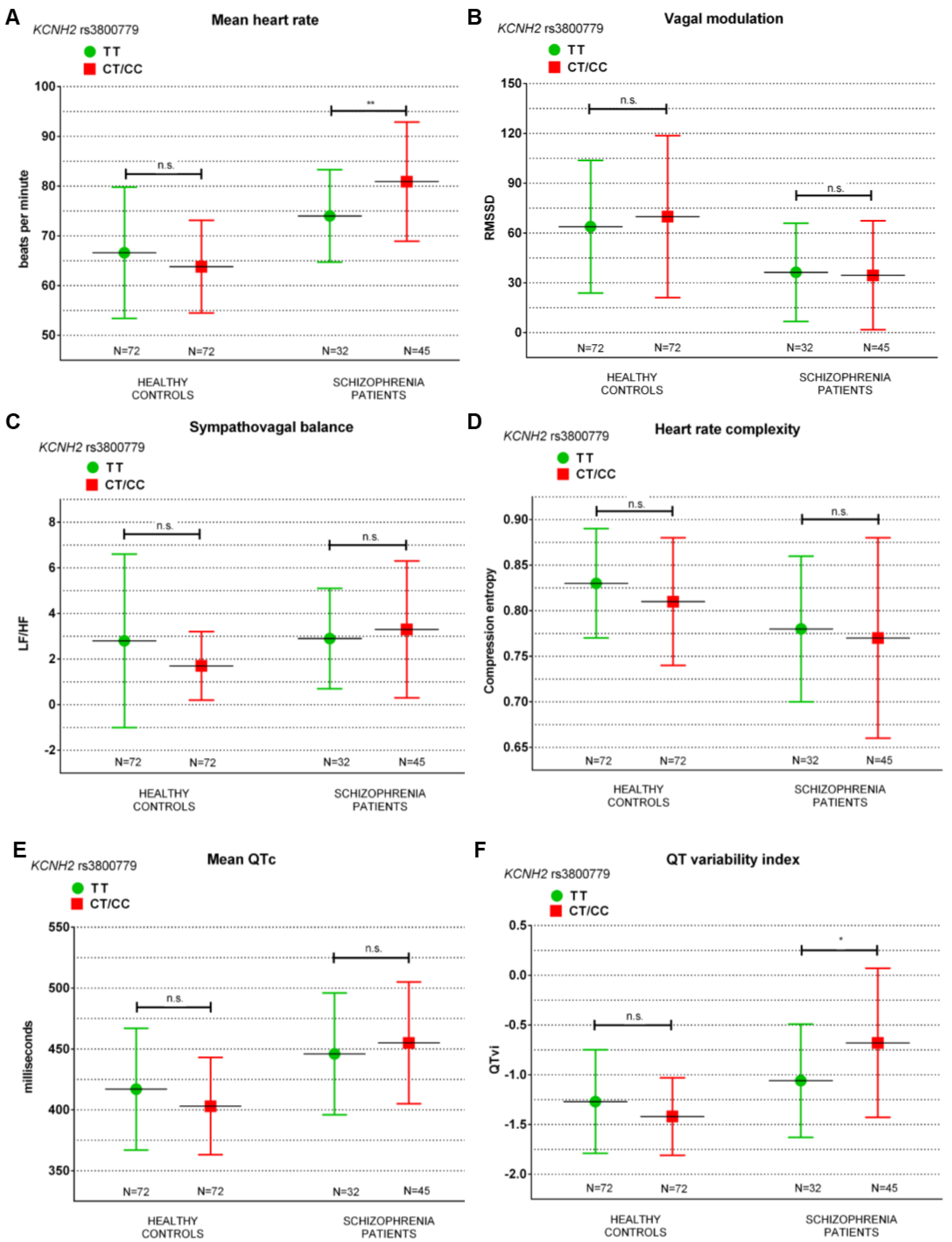
| N | 72 | 72 | n.s. | 32 | 45 | 0.018 |
| mHR | 66.6 ± 13.2 | 63.8 ± 9.3 | n.s. | 74.0 ± 9.3 | 80.9 ± 12.0 | 0.008 |
| LF/HF | 2.8 ± 3.8 | 1.7 ± 1.5 | n.s. | 2.9 ± 2.2 | 3.3 ± 3.0 | n.s. |
| RMSSD | 63.8 ± 39.9 | 69.9 ± 48.8 | n.s. | 36.3 ± 29.6 | 34.5 ± 32.8 | n.s. |
| Hc | 0.83 ± 0.06 | 0.81 ± 0.07 | n.s. | 0.78 ± 0.08 | 0.77 ± 0.11 | n.s. |
| QTc | 0.417 ± 0.05 | 0.403 ± 0.04 | n.s. | 0.446 ± 0.05 | 0.455 ± 0.05 | n.s. |
| QTvi | −1.27 ± 0.52 | −1.42 ± 0.39 | n.s. | −1.06 ± 0.57 | −0.68 ± 0.75 | 0.019 |
| KCNH2 rs748693 A > G | ||||||
| Healthy Controls | Schizophrenia Patients | |||||
| MAF | G = 0.31 (88/288) | G = 0.40 (62/154) | ||||
| AA | 0.49 (70/144) | 0.38 (29/77) | ||||
| AG | 0.42 (60/144) | 0.44 (34/77) | ||||
| GG | 0.09 (14/144) | 0.18 (14/77) | ||||
| χ2 | 0.0000 | 0.0069 | ||||
| CAF | AA | AG/GG | p | AA | AG/GG | p |
| N | 70 | 74 | n.s. | 29 | 48 | 0.025 |
| mHR | 66.5 ± 13.3 | 63.9 ± 9.2 | n.s. | 74.7 ± 9.3 | 80.0 ± 12.1 | 0.049 |
| LF/HF | 2.8 ± 3.8 | 1.8 ± 1.6 | n.s. | 3.1 ± 2.2 | 3.1 ± 2.9 | n.s. |
| RMSSD | 64.5 ± 40.2 | 69.0 ± 48.4 | n.s. | 34.4 ± 29.5 | 35.8 ± 32.6 | n.s. |
| Hc | 0.83 ± 0.06 | 0.81 ± 0.07 | n.s. | 0.77 ± 0.08 | 0.78 ± 0.11 | n.s. |
| QTc | 0.417 ± 0.05 | 0.404 ± 0.04 | n.s. | 0.450 ± 0.05 | 0.452 ± 0.05 | n.s. |
| QTvi | −1.26 ± 0.52 | −1.42 ± 0.38 | n.s. | −1.05 ± 0.57 | −0.71 ± 0.75 | 0.042 |
| KCNH2 rs1036145 C > T | ||||||
| Healthy Controls | Schizophrenia Patients | |||||
| MAF | T = 0.25 (73/288) | T = 0.39 (60/154) | ||||
| CC | 0.51 (74/144) | 0.39 (30/77) | ||||
| CT | 0.40 (57/144) | 0.44 (34/77) | ||||
| TT | 0.09 (13/144) | 0.17 (13/77) | ||||
| χ2 | 0.0008 | 0.0057 | ||||
| CAF | CC | CT/TT | p | CC | CT/TT | p |
| N | 74 | 70 | n.s. | 30 | 47 | 0.043 |
| mHR | 66.5 ± 13.3 | 63.9 ± 9.2 | n.s. | 75.0 ± 9.9 | 79.9 ± 11.9 | n.s. |
| LF/HF | 2.8 ± 3.8 | 1.8 ± 1.6 | n.s. | 3.2 ± 2.8 | 3.0 ± 2.7 | n.s. |
| RMSSD | 64.5 ± 40.2 | 69.0 ± 48.4 | n.s. | 35.8 ± 31.1 | 35.0 ± 31.7 | n.s. |
| Hc | 0.83 ± 0.06 | 0.81 ± 0.07 | n.s. | 0.78 ± 0.08 | 0.77 ± 0.11 | n.s. |
| QTc | 0.417 ± 0.05 | 0.404 ± 0.04 | n.s. | 0.450 ± 0.05 | 0.452 ± 0.05 | n.s. |
| QTvi | −1.26 ± 0.52 | −1.42 ± 0.38 | n.s. | −1.04 ± 0.54 | −0.71 ± 0.77 | 0.042 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Refisch, A.; Komatsuzaki, S.; Ungelenk, M.; Schumann, A.; Chung, H.-Y.; Schilling, S.S.; Jantzen, W.; Schröder, S.; Nöthen, M.M.; Mühleisen, T.W.; et al. Analysis of CACNA1C and KCNH2 Risk Variants on Cardiac Autonomic Function in Patients with Schizophrenia. Genes 2022, 13, 2132. https://doi.org/10.3390/genes13112132
Refisch A, Komatsuzaki S, Ungelenk M, Schumann A, Chung H-Y, Schilling SS, Jantzen W, Schröder S, Nöthen MM, Mühleisen TW, et al. Analysis of CACNA1C and KCNH2 Risk Variants on Cardiac Autonomic Function in Patients with Schizophrenia. Genes. 2022; 13(11):2132. https://doi.org/10.3390/genes13112132
Chicago/Turabian StyleRefisch, Alexander, Shoko Komatsuzaki, Martin Ungelenk, Andy Schumann, Ha-Yeun Chung, Susann S. Schilling, Wibke Jantzen, Sabine Schröder, Markus M. Nöthen, Thomas W. Mühleisen, and et al. 2022. "Analysis of CACNA1C and KCNH2 Risk Variants on Cardiac Autonomic Function in Patients with Schizophrenia" Genes 13, no. 11: 2132. https://doi.org/10.3390/genes13112132
APA StyleRefisch, A., Komatsuzaki, S., Ungelenk, M., Schumann, A., Chung, H.-Y., Schilling, S. S., Jantzen, W., Schröder, S., Nöthen, M. M., Mühleisen, T. W., Hübner, C. A., & Bär, K.-J. (2022). Analysis of CACNA1C and KCNH2 Risk Variants on Cardiac Autonomic Function in Patients with Schizophrenia. Genes, 13(11), 2132. https://doi.org/10.3390/genes13112132






