Association of Early Childhood Caries with Bitter Taste Receptors: A Meta-Analysis of Genome-Wide Association Studies and Transcriptome-Wide Association Study
Abstract
:1. Introduction
2. Materials and Methods
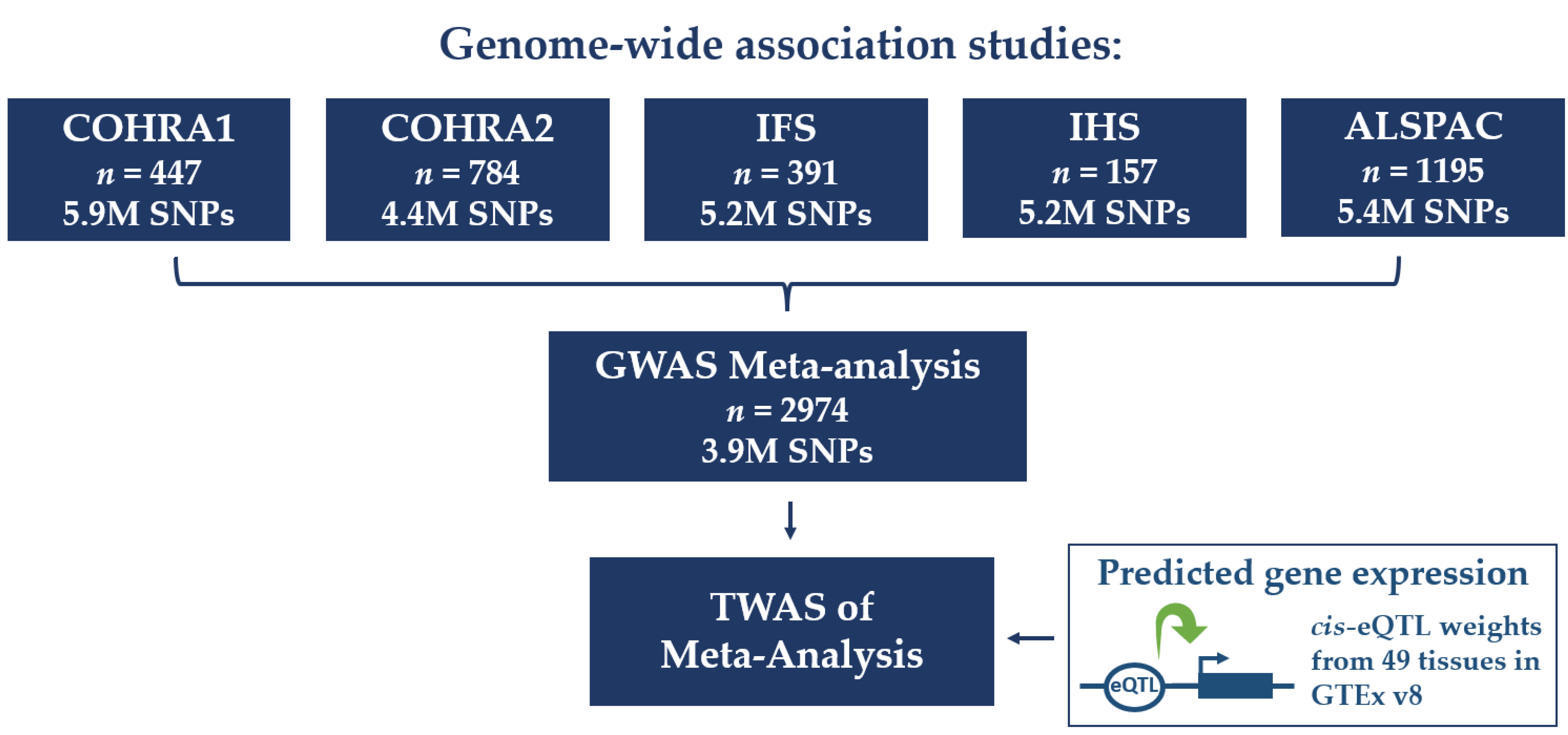
2.1. Participant Cohorts
2.2. Caries Phenotypes
2.3. Genome-Wide Association Studies (GWASs) and Meta-Analysis
2.4. Transcriptome-Wide Association Study (TWAS)
3. Results
3.1. GWASs and GWAS Meta-Analysis
3.2. TWAS
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wen, P.; Chen, M.; Zhong, Y.; Dong, Q.; Wong, H. Global Burden and Inequality of Dental Caries, 1990 to 2019. J. Dent. Res. 2022, 101, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Pakkhesal, M.; Riyahi, E.; Alhosseini, A.N.; Amdjadi, P.; Behnampour, N. Impact of dental caries on oral health related quality of life among preschool children: Perceptions of parents. BMC Oral Health 2021, 21, 68. [Google Scholar] [CrossRef] [PubMed]
- Sheiham, A.; James, W.P.T. Diet and Dental Caries: The Pivotal Role of Free Sugars Reemphasized. J. Dent. Res. 2015, 94, 1341–1347. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Shaffer, J.R.; Weyant, R.J.; Cuenco, K.T.; DeSensi, R.S.; Crout, R.; McNeil, D.W.; Marazita, M.L. Genes and Their Effects on Dental Caries May Differ between Primary and Permanent Dentitions. Caries Res. 2010, 44, 277–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haworth, S.; Shungin, D.; Van Der Tas, J.T.; Vucic, S.; Medina-Gomez, C.; Yakimov, V.; Feenstra, B.; Shaffer, J.R.; Lee, M.K.; Standl, M.; et al. Consortium-based genome-wide meta-analysis for childhood dental caries traits. Hum. Mol. Genet. 2018, 27, 3113–3127. [Google Scholar] [CrossRef] [Green Version]
- Shungin, D.; Haworth, S.; Divaris, K.; Agler, C.; Kamatani, Y.; Lee, M.K.; Grinde, K.; Hindy, G.; Alaraudanjoki, V.; Pesonen, P.; et al. Genome-wide analysis of dental caries and periodontitis combining clinical and self-reported data. Nat. Commun. 2019, 10, 2773. [Google Scholar] [CrossRef] [Green Version]
- Ballantine, J.L.; Carlson, J.; Zandoná, A.G.F.; Agler, C.; Zeldin, L.P.; Rozier, R.G.; Roberts, M.W.; Basta, P.V.; Luo, J.; Antonio-Obese, M.E.; et al. Exploring the genomic basis of early childhood caries: A pilot study. Int. J. Paediatr. Dent. 2017, 28, 217–225. [Google Scholar] [CrossRef] [Green Version]
- Shaffer, J.R.; Wang, X.; Feingold, E.; Lee, M.; Begum, F.; Weeks, D.E.; Cuenco, K.T.; Barmada, M.M.; Wendell, S.K.; Crosslin, D.R.; et al. Genome-wide Association Scan for Childhood Caries Implicates Novel Genes. J. Dent. Res. 2011, 90, 1457–1462. [Google Scholar] [CrossRef] [Green Version]
- Yang, F.; Zeng, X.; Ning, K.; Liu, K.-L.; Lo, C.-C.; Wang, W.; Chen, J.; Wang, D.; Huang, R.; Chang, X.; et al. Saliva microbiomes distinguish caries-active from healthy human populations. ISME J. 2012, 6, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Zeng, Z.; Feingold, E.; Wang, X.; Weeks, D.; Lee, M.; Cuenco, K.; Broffitt, B.; Weyant, R.; Crout, R.; McNeil, D.; et al. Genome-Wide Association Study of Primary Dentition Pit-and-Fissure and Smooth Surface Caries. Caries Res. 2014, 48, 330–338. [Google Scholar] [CrossRef]
- Alotaibi, R.N.; Howe, B.J.; Chernus, J.M.; Mukhopadhyay, N.; Sanchez, C.; Deleyiannis, F.W.; Neiswanger, K.; Padilla, C.; Poletta, F.A.; Orioli, I.M.; et al. Genome-Wide Association Study (GWAS) of dental caries in diverse populations. BMC Oral Health 2021, 21, 377. [Google Scholar] [CrossRef]
- Orlova, E.; Carlson, J.C.; Lee, M.K.; Feingold, E.; McNeil, D.W.; Crout, R.J.; Weyant, R.; Marazita, M.L.; Shaffer, J.R. Pilot GWAS of caries in African-Americans shows genetic heterogeneity. BMC Oral Health 2019, 19, 215. [Google Scholar] [CrossRef]
- Wang, X.; Shaffer, J.R.; Zeng, Z.; Begum, F.; Vieira, A.R.; Noel, J.; Anjomshoaa, I.; Cuenco, K.T.; Lee, M.K.; Beck, J.; et al. Genome-wide association Scan of dental caries in the permanent dentition. BMC Oral Health 2012, 12, 57. [Google Scholar] [CrossRef] [Green Version]
- Halldorsdottir, T.; Piechaczek, C.; Soares de Matos, A.P.; Czamara, D.; Pehl, V.; Wagenbuechler, P.; Feldmann, L.; Quickenstedt-Reinhardt, P.; Allgaier, A.K.; Freisleder, F.J.; et al. Polygenic Risk: Predicting Depression Outcomes in Clinical and Epidemiological Cohorts of Youths. Am. J. Psychiatry 2019, 176, 615–625. [Google Scholar] [CrossRef]
- Polk, D.E.; Weyant, R.J.; Crout, R.J.; McNeil, D.W.; Tarter, R.E.; Thomas, J.G.; Marazita, M.L. Study protocol of the Center for Oral Health Research in Appalachia (COHRA) etiology study. BMC Oral Health 2008, 8, 18. [Google Scholar] [CrossRef] [Green Version]
- Neiswanger, K.; McNeil, D.W.; Foxman, B.; Govil, M.; Cooper, M.E.; Weyant, R.J.; Shaffer, J.R.; Crout, R.J.; Simhan, H.N.; Beach, S.R.; et al. Oral Health in a Sample of Pregnant Women from Northern Appalachia (2011–2015). Int. J. Dent. 2015, 2015, 469376. [Google Scholar] [CrossRef] [Green Version]
- Hamilton, C.M.; Strader, L.C.; Pratt, J.G.; Maiese, D.; Hendershot, T.; Kwok, R.K.; Hammond, J.A.; Huggins, W.; Jackman, D.; Pan, H.; et al. The PhenX Toolkit: Get the most from your measures. Am. J. Epidemiol. 2011, 174, 253–260. [Google Scholar] [CrossRef]
- Warren, J.J.; Levy, S.M.; Ms, B.B.; Kanellis, M.J. Longitudinal Study of Non-cavitated Carious Lesion Progression in the Primary Dentition. J. Public Health Dent. 2006, 66, 83–87. [Google Scholar] [CrossRef]
- Marshall, T.A.; Levy, S.M.; Broffitt, B.; Warren, J.J.; Eichenberger-Gilmore, J.M.; Burns, T.L.; Stumbo, P.J. Dental caries and beverage consumption in young children. Pediatrics 2003, 112 Pt 1, e184–e191. [Google Scholar] [CrossRef] [Green Version]
- Levy, S.M.; Kiritsy, M.C.; Slager, S.L.; Warren, J.J. Patterns of dietary fluoride supplement use during infancy. J. Public Health Dent. 1998, 58, 228–233. [Google Scholar] [CrossRef]
- Slayton, R.L.; Cooper, M.; Marazita, M. Tuftelin, Mutans Streptococci, and Dental Caries Susceptibility. J. Dent. Res. 2005, 84, 711–714. [Google Scholar] [CrossRef] [PubMed]
- Pitts, N.B.; Fyffe, H.E. The effect of varying diagnostic thresholds upon clinical caries data for a low prevalence group. J. Dent. Res. 1988, 67, 592–596. [Google Scholar] [CrossRef] [PubMed]
- Boyd, A.; Golding, J.; Macleod, J.; Lawlor, D.A.; Fraser, A.; Henderson, J.; Molloy, L.; Ness, A.; Ring, S.; Davey Smith, G. Cohort Profile: The ‘Children of the 90s’—the indexoffspring of the Avon Longitudinal Study of Parents and Children. Int. J. Epidemiol. 2013, 42, 111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fraser, A.; Macdonald-Wallis, C.; Tilling, K.; Boyd, A.; Golding, J.; Davey Smith, G.; Henderson, J.; Macleod, J.; Molloy, L.; Ness, A.; et al. Cohort Profile: The Avon Longitudinal Study of Parents and Children: ALSPAC mothers cohort. Int. J. Epidemiol. 2013, 42, 97–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, H.M.; Sul, J.H.; Service, S.K.; Zaitlen, N.A.; Kong, S.-Y.; Freimer, N.B.; Sabatti, C.; Eskin, E. Variance component model to account for sample structure in genome-wide association studies. Nat. Genet. 2010, 42, 348–354. [Google Scholar] [CrossRef] [Green Version]
- Purcell, S.; Neale, B.; Todd-Brown, K.; Thomas, L.; Ferreira, M.A.R.; Bender, D.; Maller, J.; Sklar, P.; de Bakker, P.I.W.; Daly, M.J.; et al. PLINK: A Tool Set for Whole-Genome Association and Population-Based Linkage Analyses. Am. J. Hum. Genet. 2007, 81, 559–575. [Google Scholar] [CrossRef] [Green Version]
- Willer, C.; Li, Y.; Abecasis, G.R. METAL: Fast and efficient meta-analysis of genomewide association scans. Bioinformatics 2010, 26, 2190–2191. [Google Scholar] [CrossRef] [Green Version]
- Pruim, R.J.; Welch, R.P.; Sanna, S.; Teslovich, T.M.; Chines, P.S.; Gliedt, T.P.; Boehnke, M.; Abecasis, G.; Willer, C.J. LocusZoom: Regional visualization of genome-wide association scan results. Bioinformatics 2010, 26, 2336–2337. [Google Scholar] [CrossRef] [Green Version]
- Barbeira, A.N.; Dickinson, S.P.; Bonazzola, R.; Zheng, J.; Wheeler, H.E.; Torres, J.M.; Torstenson, E.S.; Shah, K.P.; Garcia, T.; Edwards, T.L.; et al. Exploring the phenotypic consequences of tissue specific gene expression variation inferred from GWAS summary statistics. Nat. Commun. 2018, 9, 1825. [Google Scholar] [CrossRef]
- Barbeira, A.N.; Pividori, M.; Zheng, J.; Wheeler, H.E.; Nicolae, D.L.; Im, H.K. Integrating predicted transcriptome from multiple tissues improves association detection. PLOS Genet. 2019, 15, e1007889. [Google Scholar] [CrossRef]
- Wainberg, M.; Sinnott-Armstrong, N.; Mancuso, N.; Barbeira, A.N.; Knowles, D.A.; Golan, D.; Ermel, R.; Ruusalepp, A.; Quertermous, T.; Hao, K.; et al. Opportunities and challenges for transcriptome-wide association studies. Nat. Genet. 2019, 51, 592–599. [Google Scholar] [CrossRef]
- Kamila, T.; Agnieszka, K. An update on extra-oral bitter taste receptors. J. Transl. Med. 2021, 19, 440. [Google Scholar]
- Pirastu, N.; Kooyman, M.; Traglia, M.; Robino, A.; Willems, S.M.; Pistis, G.; D’Adamo, P.; Amin, N.; D’Eustacchio, A.; Navarini, L.; et al. Association Analysis of Bitter Receptor Genes in Five Isolated Populations Identifies a Significant Correlation between TAS2R43 Variants and Coffee Liking. PLoS ONE 2014, 9, e92065. [Google Scholar] [CrossRef] [Green Version]
- Erciyas, K.; Pehlivan, S.; Sever, T.; Orbak, R. Genetic variation of myeloperoxidase gene contributes to aggressive periodontitis: A preliminary association study in Turkish population. Dis. Markers 2010, 28, 95–99. [Google Scholar] [CrossRef]
- Medapati, M.R.; Singh, N.; Bhagirath, A.Y.; Duan, K.; Triggs-Raine, B.; Batista, E.L.; Chelikani, P. Bitter taste receptor T2R14 detects quorum sensing molecules from cariogenic Streptococcus mutans and mediates innate immune responses in gingival epithelial cells. FASEB J. 2021, 35, e21375. [Google Scholar] [CrossRef]
- Chang, Y.-Y.; Yu, L.C.-H.; Yu, I.-S.; Jhuang, Y.-L.; Huang, W.-J.; Yang, C.-Y.; Jeng, Y.-M. Deletion of cadherin-17 enhances intestinal permeability and susceptibility to intestinal tumour formation. J. Pathol. 2018, 246, 289–299. [Google Scholar] [CrossRef]
- Yue, F.; Cheng, Y.; Breschi, A.; Vierstra, J.; Wu, W.; Ryba, T.; Sandstrom, R.; Samantha, K.; Davis, C.; Pope, B.D.; et al. A comparative encyclopedia of DNA elements in the mouse genome. Nature 2014, 515, 355–364. [Google Scholar] [CrossRef] [Green Version]
- Chung, C.S.; Witkop, C.J.; Henry, J.L. A Genetic Study of Dental Caries with Special Reference to PTC Taste Sensitivity. Am. J. Hum. Genet. 1964, 16, 245. [Google Scholar]
- Wendell, S.; Wang, X.; Brown, M.; Cooper, M.E.; DeSensi, R.S.; Weyant, R.J.; Crout, R.; McNeil, D.W.; Marazita, M.L. Taste genes associated with dental caries. J. Dent. Res. 2010, 89, 1198–1202. [Google Scholar] [CrossRef]
- Dong, H.; Liu, J.; Zhu, J.; Zhou, Z.; Tizzano, M.; Peng, X.; Zhou, X.; Xu, X.; Zheng, X. Oral Microbiota-Host Interaction Mediated by Taste Receptors. Front. Cell. Infect. Microbiol. 2022, 12, 802504. [Google Scholar] [CrossRef]
- Medapati, M.R.; Bhagirath, A.Y.; Singh, N.; Schroth, R.J.; Bhullar, R.P.; Duan, K.; Chelikani, P. Bitter Taste Receptor T2R14 Modulates Gram-Positive Bacterial Internalization and Survival in Gingival Epithelial Cells. Int. J. Mol. Sci. 2021, 22, 9920. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.J.; Xiong, G.; Kofonow, J.M.; Chen, B.; Lysenko, A.; Jiang, P.; Abraham, V.; Doghramji, L.; Adappa, N.D.; Palmer, J.N.; et al. T2R38 taste receptor polymorphisms underlie susceptibility to upper respiratory infection. J. Clin. Investig. 2012, 122, 4145–4159. [Google Scholar] [CrossRef] [PubMed]
- Gil, S.; Coldwell, S.; Drury, J.L.; Arroyo, F.; Phi, T.; Saadat, S.; Kwong, D.; Chung, W.O. Genotype-specific regulation of oral innate immunity by T2R38 taste receptor. Mol. Immunol. 2015, 68 Pt C, 663–670. [Google Scholar] [CrossRef] [Green Version]
- Lee, R.J.; Kofonow, J.M.; Rosen, P.L.; Siebert, A.P.; Chen, B.; Doghramji, L.; Xiong, G.; Adappa, N.D.; Palmer, J.N.; Kennedy, D.W.; et al. Bitter and sweet taste receptors regulate human upper respiratory innate immunity. J. Clin. Investig. 2014, 124, 1393–1405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diószegi, J.; Llanaj, E.; Ádány, R. Genetic Background of Taste Perception, Taste Preferences, and Its Nutritional Implications: A Systematic Review. Front. Genet. 2019, 10, 1272. [Google Scholar] [CrossRef] [Green Version]
- Hertel, S.; Mühlig, L.; Hannig, C.; Hummel, T. Taste perception in children with different caries activity. Eur. Arch. Paediatr. Dent. 2022, 23, 929–934. [Google Scholar] [CrossRef]
- Jurczak, A.; Jamka-Kasprzyk, M.; Bębenek, Z.; Staszczyk, M.; Jagielski, P.; Kościelniak, D.; Gregorczyk-Maga, I.; Kołodziej, I.; Kępisty, M.; Kukurba-Setkowicz, M.; et al. Differences in Sweet Taste Perception and Its Association with the Streptococcus mutans Cariogenic Profile in Preschool Children with Caries. Nutrients 2020, 12, 2592. [Google Scholar] [CrossRef]
- Burnham, L.; Matlak, S.; Makrigiorgos, G.; Braun, N.; Knapp, B.P.; Merewood, A. Breastfeeding and Coffee Consumption in Children Younger than 2 Years in Boston, Massachusetts, USA. J. Hum. Lact. 2015, 31, 267–272. [Google Scholar] [CrossRef]
- Flores, G.; Lin, H. Factors predicting severe childhood obesity in kindergarteners. Int. J. Obes. 2013, 37, 31–39. [Google Scholar] [CrossRef] [Green Version]
- Majewski, R.F. Dental caries in adolescents associated with caffeinated carbonated beverages. Pediatr. Dent. 2001, 23, 198–203. [Google Scholar]
- Godavarthy, D.; Naik, R.; Gali, P.K.; Mujib, B.A.; Baddam, V.R.R. Can coffee combat caries? An in vitro study. J. Oral Maxillofac. Pathol. 2020, 24, 64–67. [Google Scholar] [CrossRef]
- Bischoff, S.C.; Barbara, G.; Buurman, W.; Ockhuizen, T.; Schulzke, J.-D.; Serino, M.; Tilg, H.; Watson, A.; Wells, J.M. Intestinal permeability—A new target for disease prevention and therapy. BMC Gastroenterol. 2014, 14, 189. [Google Scholar] [CrossRef] [Green Version]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; et al. Chronic inflammation in the etiology of disease across the life span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef]
- Grundberg, E.; Small, K.S.; Hedman, Å.K.; Nica, A.C.; Buil, A.; Keildson, S.; Bell, J.T.; Yang, T.P.; Meduri, E.; Barrett, A.; et al. Mapping cis- and trans-regulatory effects across multiple tissues in twins. Nat. Genet. 2012, 44, 1084–1089. [Google Scholar] [CrossRef] [Green Version]
- National Institute of Dental and Craniofacial Research. Dental Caries (Tooth Decay) in Children Ages 2 to 11 Year. Available online: https://www.nidcr.nih.gov/research/data-statistics/dental-caries/children (accessed on 12 December 2022).
- Ricciuto, L.; Fulgoni, V.L.; Gaine, P.C.; Scott, O.M.; DiFrancesco, L. Trends in Added Sugars Intake and Sources Among US Children, Adolescents, and Teens Using NHANES 2001–2018. J. Nutr. 2022, 152, 568–578. [Google Scholar] [CrossRef]
- Powell, E.S.; Smith-Taillie, L.P.; Popkin, B.M. Added Sugars Intake Across the Distribution of US Children and Adult Consumers: 1977–2012. J. Acad. Nutr. Diet. 2016, 116, 1543–1550. [Google Scholar] [CrossRef] [Green Version]
- Shaffer, J.R.; Wang, X.; McNeil, D.W.; Weyant, R.J.; Crout, R.; Marazita, M.L. Genetic Susceptibility to Dental Caries Differs between the Sexes: A Family-Based Study. Caries Res. 2015, 49, 133–140. [Google Scholar] [CrossRef] [Green Version]
- Slayton, R.L.; Warren, J.J.; Kanellis, M.J.; Levy, S.M.; Islam, M. Prevalence of enamel hypoplasia and isolated opacities in the primary dentition. Pediatr. Dent. 2001, 23, 32–36. [Google Scholar]
- Dudding, T.; Thomas, S.J.; Duncan, K.; Lawlor, D.A.; Timpson, N.J. Re-Examining the Association between Vitamin D and Childhood Caries. PLoS ONE 2015, 10, e0143769. [Google Scholar] [CrossRef] [Green Version]
- Durbin, R.M.; Altshuler, D.L.; Durbin, R.M.; Abecasis, G.R.; Bentley, D.R.; Clark, A.G.; Chakravarti, A. A map of human genome variation from population-scale sequencing. Nature 2010, 467, 1061–1073. [Google Scholar]
- Delaneau, O.; Marchini, J.; Zagury, J.-F. A linear complexity phasing method for thousands of genomes. Nat. Methods 2011, 9, 179–181. [Google Scholar] [CrossRef] [PubMed]
- Howie, B.N.; Donnelly, P.; Marchini, J. A Flexible and Accurate Genotype Imputation Method for the Next Generation of Genome-Wide Association Studies. PLOS Genet. 2009, 5, e1000529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das, S.; Forer, L.; Schönherr, S.; Sidore, C.; Locke, A.E.; Kwong, A.; Vrieze, S.I.; Chew, E.Y.; Levy, S.; McGue, M.; et al. Next-generation genotype imputation service and methods. Nat. Genet. 2016, 48, 1284–1287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCarthy, S.; Das, S.; Kretzschmar, W.; Delaneau, O.; Wood, A.R.; Teumer, A. A reference panel of 64,976 haplotypes for genotype imputation. Nat. Genet. 2016, 48, 1279–1283. [Google Scholar] [PubMed] [Green Version]
- Watanabe, K.; Taskesen, E.; van Bochoven, A.; Posthuma, D. Functional mapping and annotation of genetic associations with FUMA. Nat. Commun. 2017, 8, 1826. [Google Scholar] [CrossRef] [PubMed]
- Munz, M.; Richter, G.M.; Loos, B.G.; Jepsen, S.; Divaris, K.; Offenbacher, S.; Teumer, A.; Holtfreter, B.; Kocher, T.; Bruckmann, C.; et al. Meta-analysis of genome-wide association studies of aggressive and chronic periodontitis identifies two novel risk loci. Eur. J. Hum. Genet. 2019, 27, 102–113. [Google Scholar] [CrossRef] [Green Version]
- Matalova, E.; Lesot, H.; Svandova, E.; Berghe, T.V.; Sharpe, P.T.; Healy, C.; Vandenabeele, P.; Tucker, A.S. Caspase-7 participates in differentiation of cells forming dental hard tissues. Dev. Growth Differ. 2013, 55, 615–621. [Google Scholar] [CrossRef]
- Xie, M.; Kobayashi, I.; Kiyoshima, T.; Nagata, K.; Ookuma, Y.; Fujiwara, H.; Sakai, H. In situ expression of ribosomal protein L21 in developing tooth germ of the mouse lower first molar. J. Mol. Histol. 2009, 40, 361–367. [Google Scholar] [CrossRef]
- Zhou, C.; Zang, D.; Jin, Y.; Wu, H.; Liu, Z.; Du, J.; Zhang, J. Mutation in ribosomal protein L21 underlies hereditary hypotrichosis simplex. Hum. Mutat. 2011, 32, 710–714. [Google Scholar] [CrossRef]
- Liu, Q.; Guan, X.M.; Martin, W.J.; McDonald, T.P.; Clements, M.K.; Jiang, Q.; Zeng, Z.; Jacobson, M.; Williams, D.L.; Yu, H.; et al. Identification and characterization of novel mammalian neuropeptide FF-like peptides that attenuate morphine-induced antinociception. J. Biol. Chem. 2001, 276, 36961–36969. [Google Scholar] [CrossRef] [Green Version]
- Peluso, G.; Tian, E.; Abusleme, L.; Munemasa, T.; Mukaibo, T.; Hagen, K.G.T. Loss of the disease-associated glycosyltransferase Galnt3 alters Muc10 glycosylation and the composition of the oral microbiome. J. Biol. Chem. 2020, 295, 1411–1425. [Google Scholar] [CrossRef]
- Statello, L.; Guo, C.J.; Chen, L.L.; Huarte, M. Gene regulation by long non-coding RNAs and its biological functions. Nat. Rev. Mol. Cell Biol. 2020, 22, 96–118. [Google Scholar] [CrossRef]
- Shaffer, J.R.; Feingold, E.; Wang, X.; Lee, M.; Tcuenco, K.; Weeks, D.E.; Weyant, R.J.; Crout, R.; McNeil, D.W.; Marazita, M.L. GWAS of Dental Caries Patterns in the Permanent Dentition. J. Dent. Res. 2013, 92, 38–44. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Jia, P.; Cuenco, K.T.; Zeng, Z.; Feingold, E.; Marazita, M.L.; Wang, L.; Zhao, Z. Association Signals Unveiled by a Comprehensive Gene Set Enrichment Analysis of Dental Caries Genome-Wide Association Studies. PLoS ONE 2013, 8, e72653. [Google Scholar] [CrossRef] [Green Version]
- Ozturk, A.; Famili, P.; Vieira, A. The Antimicrobial Peptide DEFB1 Is Associated with Caries. J. Dent. Res. 2010, 89, 631–636. [Google Scholar] [CrossRef]
- Faheem, S.; Maqsood, S.; Hasan, A.; Imtiaz, F.; Shaikh, F.; Farooqui, W.A. Associations of early childhood caries with salivary β defensin-3 and childhood anemia: A case–control study. BMC Oral Health 2021, 21, 445. [Google Scholar] [CrossRef]
- Jurczak, A.; Kościelniak, D.; Papież, M.; Vyhouskaya, P.; Krzyściak, W. A study on β-defensin-2 and histatin-5 as a diagnostic marker of early childhood caries progression. Biol. Res. 2015, 48, 61. [Google Scholar] [CrossRef] [Green Version]
- De Lima, J.M.; Morand, G.B.; Macedo, C.C.S.; Diesel, L.; Hier, M.P.; Mlynarek, A.; Kowalski, L.P.; Maschietto, M.; Alaoui-Jamali, M.A.; da Silva, S.D. NDRG1 deficiency is associated with regional metastasis in oral cancer by inducing epithelial-mesenchymal transition. Carcinogenesis 2020, 41, 769–777. [Google Scholar] [CrossRef]
- Grant, S.F.A.; Thorleifsson, G.; Reynisdottir, I.; Benediktsson, R.; Manolescu, A.; Sainz, J.; Helgason, A.; Stefansson, H.; Emilsson, V.; Helgadottir, A.; et al. Variant of transcription factor 7-like 2 (TCF7L2) gene confers risk of type 2 diabetes. Nat. Genet. 2006, 38, 320–323. [Google Scholar] [CrossRef]
- Kotoh, J.; Sasaki, D.; Matsumoto, K.; Maeda, A. Plekhs1 and Prdx3 are candidate genesresponsible for mild hyperglycemia associated with obesity in a new animal model ofF344-fa-nidd6 rat. J. Vet. Med. Sci. 2016, 78, 1683. [Google Scholar] [CrossRef] [Green Version]
- Dauwerse, J.G.; Dixon, J.; Seland, S.; Ruivenkamp, C.A.; Van Haeringen, A.; Hoefsloot, L.H.; Peters, D.J.; Boers, A.C.D.; Daumer-Haas, C.; Maiwald, R.; et al. Mutations in genes encoding subunits of RNA polymerases I and III cause Treacher Collins syndrome. Nat Genet. 2011, 43, 20–22. [Google Scholar] [CrossRef] [PubMed]
- Marneros, A.G.; Beck, A.E.; Turner, E.H.; McMillin, M.J.; Edwards, M.J.; Field, M.; Sobreira, N.L.D.M.; Perez, A.B.A.; Fortes, J.A.; Lampe, A.K.; et al. Mutations in KCTD1 Cause Scalp-Ear-Nipple Syndrome. Am. J. Hum. Genet. 2013, 92, 621–626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liao, C.; Zhou, Y.; Li, M.; Xia, Y.; Peng, W. LINC00968 promotes osteogenic differentiation in vitro and bone formation in vivo via regulation of miR-3658/RUNX2. Differentiation 2020, 116, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Cheng, B.; Ling, J.; Chen, X.; Liu, J. The role of Fos protein in modulation of dental pain in central nerve system. Hua Xi Kou Qiang Yi Xue Za Zhi 2001, 19, 253–255. [Google Scholar] [PubMed]
- Astrom, A.; Voz, M.; Kas, K.; Roijer, E.; Wedell, B.; Mandahl, N.; de Ven, W.V.; Mark, J.; Stenman, G. Conserved mechanism of PLAG1 activation in salivary gland tumors with and without chromosome 8q12 abnormalities: Identification of SII as a new fusion partner gene. Cancer Res. 1999, 59, 918–923. [Google Scholar]
- Baus-Domínguez, M.; Gómez-Díaz, R.; Torres-Lagares, D.; Corcuera-Flores, J.R.; Ruiz-Villandiego, J.C.; Machuca-Portillo, G.; Gutiérrez-Pérez, J.L.; Serrera-Figallo, M.A. Differential Expression of Inflammation-Related Genes in Down Syndrome Patients with or without Periodontal Disease. Mediat. Inflamm. 2019, 2019, 4567106. [Google Scholar] [CrossRef]
- Rhodin, K.; Divaris, K.; North, K.E.; Barros, S.P.; Moss, K.; Beck, J.D.; Offenbacher, S. Chronic periodontitis genome-wide association studies: Gene-centric and gene set enrichment analyses. J. Dent. Res. 2014, 93, 882–890. [Google Scholar] [CrossRef] [Green Version]
- Ning, W.; Ma, Y.; Li, S.; Wang, X.; Pan, H.; Wei, C.; Zhang, S.; Bai, D.; Liu, X.; Deng, Y.; et al. Shared Molecular Mechanisms between Atherosclerosis and Periodontitis by Analyzing the Transcriptomic Alterations of Peripheral Blood Monocytes. Comput. Math. Methods Med. 2021, 2021, 1498431. [Google Scholar] [CrossRef]
- Streelman, J.T.; Bloomquist, R.; Fowler, T.E. Developmental Plasticity of Patterned and Regenerating Oral Organs. Curr. Top. Dev. Biol. 2015, 115, 321–333. [Google Scholar]
- Luo, H.; Wang, C.; Liu, M.; Yin, B.; Peng, A.; Huang, D.; Ye, L. Inhibition of SOX9 Promotes Inflammatory and Immune Responses of Dental Pulp. J. Endod. 2018, 44, 792–799. [Google Scholar] [CrossRef]
- Ainetdin, A.; Nieminen, P.; Rice, D.; Sanz-Navarro, M. Expression of the Rnf43 and Znrf3 Genes during Murine Teeth Development. Master’s Thesis, University of Helsinki, Helsinki, Finland, 2019. [Google Scholar]
- Magacz, M.; Kędziora, K.; Sapa, J.; Krzyściak, W. The Significance of Lactoperoxidase System in Oral Health: Application and Efficacy in Oral Hygiene Products. Int. J. Mol. Sci. 2019, 20, 1443. [Google Scholar] [CrossRef] [Green Version]
- Ramos-Lopez, O.; Project, M.; Riezu-Boj, J.I.; Milagro, F.I.; Zulet, M.A.; Santos, J.L.; Martinez, J.A. Associations between olfactory pathway gene methylation marks, obesity features and dietary intakes. Genes Nutr. 2019, 14, 11. [Google Scholar] [CrossRef] [Green Version]
- Russell, S.L.; Gold, M.J.; Hartmann, M.; Willing, B.P.; Thorson, L.; Wlodarska, M.; Gill, N.; Blanchet, M.-R.; Mohn, W.W.; McNagny, K.M.; et al. Early life antibiotic-driven changes in microbiota enhance susceptibility to allergic asthma. EMBO Rep. 2012, 13, 440–447. [Google Scholar] [CrossRef]
- Fatemifar, G.; Hoggart, C.J.; Paternoster, L.; Kemp, J.P.; Prokopenko, I.; Horikoshi, M.; Wright, V.J.; Tobias, J.H.; Richmond, S.; Zhurov, A.I.; et al. Genome-wide association study of primary tooth eruption identifies pleiotropic loci associated with height and craniofacial distances. Hum. Mol. Genet. 2013, 22, 3807–3817. [Google Scholar] [CrossRef] [Green Version]
- Kantaputra, P.N.; Wangtiraumnuay, N.; Ngamphiw, C.; Olsen, B.; Intachai, W.; Tucker, A.S.; Tongsima, S. Cryptophthalmos, dental anomalies, oral vestibule defect, and a novel FREM2 mutation. J. Hum. Genet. 2021, 67, 115–118. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.K.; Shaffer, J.R.; Leslie, E.J.; Orlova, E.; Carlson, J.C.; Feingold, E.; Marazita, M.L.; Weinberg, S.M. Genome-wide association study of facial morphology reveals novel associations with FREM1 and PARK2. PLoS ONE 2017, 12, e0176566. [Google Scholar] [CrossRef] [Green Version]
- Koromila, T.; Georgoulias, P.; Dailiana, Z.; Ntzani, E.E.; Samara, S.; Chassanidis, C.; Aleporou-Marinou, V.; Kollia, P. CER1 gene variations associated with bone mineral density, bone markers, and early menopause in postmenopausal women. Hum. Genom. 2013, 7, 21. [Google Scholar] [CrossRef]
- Mellas, R.E.; Kim, H.; Osinski, J.; Sadibasic, S.; Gronostajski, R.M.; Cho, M.; Baker, O.J. NFIB regulates embryonic development of submandibular glands. J. Dent. Res. 2015, 94, 312–319. [Google Scholar] [CrossRef] [Green Version]
- Mahtout, H.; Curt, S.; Chandad, F.; Rouabhia, M.; Grenier, D. Effect of periodontopathogen lipopolysaccharides and proinflammatory cytokines on CD46, CD55, and CD59 gene/protein expression by oral epithelial cells. FEMS Immunol. Med. Microbiol. 2011, 62, 295–303. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, S.; Fukuda, T.; Nagayasu, S.; Nakanishi, J.; Yoshida, K.; Hirata-Tsuchiya, S.; Nakao, Y.; Sano, T.; Yamashita, A.; Yamada, S.; et al. Dental pulp cell-derived powerful inducer of TNF-α comprises PKR containing stress granule rich microvesicles. Sci. Rep. 2019, 9, 3825. [Google Scholar] [CrossRef] [Green Version]
- Teng, M.-S.; Wu, S.; Er, L.-K.; Hsu, L.-A.; Chou, H.-H.; Ko, Y.-L. LIPC variants as genetic determinants of adiposity status, visceral adiposity indicators, and triglyceride-glucose (TyG) index-related parameters mediated by serum triglyceride levels. Diabetol. Metab. Syndr. 2018, 10, 791. [Google Scholar] [CrossRef] [PubMed]
- Vieira, A.R.; McHenry, T.G.; Daack-Hirsch, S.; Murray, J.C.; Marazita, M.L. Candidate Gene/Loci Studies in Cleft Lip/Palate and Dental Anomalies Finds Novel Susceptibility Genes for Clefts. Genet. Med. 2008, 10, 668–674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ikeda, A.; Shahid, S.; Blumberg, B.R.; Suzuki, M.; Bartlett, J.D. ADAM10 is Expressed by Ameloblasts, Cleaves the RELT TNF Receptor Extracellular Domain and Facilitates Enamel Development. Sci. Rep. 2019, 9, 14086. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Natour, B.; Rankin, R.; McKenna, R.; McMillan, H.; Zhang, S.; About, I.; Khan, A.A.; Galicia, J.C.; Lundy, F.T.; El-Karim, I.A. Identification and validation of novel biomarkers and therapeutics for pulpitis using connectivity mapping. Int. Endod. J. 2021, 54, 1571–1580. [Google Scholar] [CrossRef] [PubMed]
- Krivanek, J.; Soldatov, R.A.; Kastriti, M.E.; Chontorotzea, T.; Herdina, A.N.; Petersen, J.; Szarowska, B.; Landova, M.; Matejova, V.K.; Holla, L.I.; et al. Dental cell type atlas reveals stem and differentiated cell types in mouse and human teeth. Nat. Commun. 2020, 11, 4816. [Google Scholar] [CrossRef]
- Sayed, I.M.; Chakraborty, A.; El-Hafeez, A.; Ali, A.; Sharma, A.; Sahan, A.Z.; Huang, W.J.M.; Sahoo, D.; Ghosh, P.; Hazra, T.K.; et al. The DNA Glycosylase NEIL2 Suppresses Fusobacterium-Infection-Induced Inflammation and DNA Damage in Colonic Epithelial Cells. Cells 2020, 9, 1980. [Google Scholar] [CrossRef]
- Lakshmi, K.R.; Benarji, K.A.; Nelakurthi, H.; Haritha, P.; Amrutha, R. Cathepsins in oral diseases. J. Dr. NTR Univ. Health Sci. 2019, 8, 153. [Google Scholar] [CrossRef]
- Yang, R.; Huang, H.; Han, C.; Cui, S.; Zhou, Y. Serine Metabolism Controls Dental Pulp Stem Cell Aging by Regulating the DNA Methylation of p16. J. Dent. Res. 2021, 100, 90–97. [Google Scholar] [CrossRef]
- Cho, S.-G.; Lee, J.-W.; Heo, J.S.; Kim, S.-Y. Gene Expression Change in Human Dental Pulp Cells Exposed to a Low-Level Toxic Concentration of Triethylene Glycol Dimethacrylate: An RNA-seq Analysis. Basic Clin. Pharmacol. Toxicol. 2014, 115, 282–290. [Google Scholar] [CrossRef]
- Pemberton, T.; Li, F.-Y.; Oka, S.; Mendoza-Fandino, G.A.; Hsu, Y.-H.; Bringas, P.; Chai, Y.; Snead, M.L.; Mehrian-Shai, R.; Patel, P.I. Identification of novel genes expressed during mouse tooth development by microarray gene expression analysis. Dev. Dyn. 2007, 236, 2245–2257. [Google Scholar] [CrossRef] [Green Version]
- Salvi, A.; Giacopuzzi, E.; Bardellini, E.; Amadori, F.; Ferrari, L.; De Petro, G.; Borsani, G.; Majorana, A. Mutation analysis by direct and whole exome sequencing in familial and sporadic tooth agenesis. Int. J. Mol. Med. 2016, 38, 1338–1348. [Google Scholar] [CrossRef] [Green Version]
- Bauer, F.; Elbers, C.C.; Adan, R.A.; Loos, R.J.; Onland-Moret, N.C.; Grobbee, D.E.; van Vliet-Ostaptchouk, J.V.; Wijmenga, C.; van der Schouw, Y.T. Obesity genes identified in genome-wide association studies are associated with adiposity measures and potentially with nutrient-specific food preference. Am. J. Clin. Nutr. 2009, 90, 951–959. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Tatakis, D.N. Human gingiva transcriptome during wound healing. J. Clin. Periodontol. 2017, 44, 394–402. [Google Scholar] [CrossRef]
- Li, L.; Lin, M.; Wang, Y.; Cserjesi, P.; Chen, Z.; Chen, Y. BmprIa is required in mesenchymal tissue and has limited redundant function with BmprIb in tooth and palate development. Dev. Biol. 2011, 349, 451–461. [Google Scholar] [CrossRef] [Green Version]
- Uchibe, K.; Shimizu, H.; Yokoyama, S.; Kuboki, T.; Asahara, H. Identification of novel transcription-regulating genes expressed during murine molar development. Dev. Dyn. 2012, 241, 1217–1226. [Google Scholar] [CrossRef]
- Liu, Y.; Dong, N.; Miao, J.; Li, C.; Wang, X.; Ruan, J. Lin28 promotes dental pulp cell proliferation via upregulation of cyclin-dependent proteins and interaction with let-7a/IGF2BP2 pathways. Biomed. Pharmacother. 2019, 113, 108742. [Google Scholar] [CrossRef]
- Alaraudanjoki, V.K.; Koivisto, S.; Pesonen, P.; Männikkö, M.; Leinonen, J.; Tjäderhane, L.; Laitala, M.-L.; Lussi, A.; Anttonen, V.A.-M. Genome-Wide Association Study of Erosive Tooth Wear in a Finnish Cohort. Caries Res. 2019, 53, 49–59. [Google Scholar] [CrossRef]
- Liu, M.; Zhao, S.; Lin, Q.; Wang, X.P. YAP regulates the expression of Hoxa1 and Hoxc13 in mouse and human oral and skin epithelial tissues. Mol. Cell. Biol. 2015, 35, 1449–1461. [Google Scholar] [CrossRef] [Green Version]
- Gong, A.-X.; Zhang, J.-H.; Li, J.; Wu, J.; Wang, L.; Miao, D.-S. Comparison of gene expression profiles between dental pulp and periodontal ligament tissues in humans. Int. J. Mol. Med. 2017, 40, 647–660. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Ning, T.; Song, C.; Luo, X.; Xu, S.; Zhang, X.; Deng, Z.; Ma, D.; Wu, B. Priming integrin α5 promotes human dental pulp stem cells odontogenic differentiation due to extracellular matrix deposition and amplified extracellular matrix-receptor activity. J. Cell. Physiol. 2019, 234, 12897–12909. [Google Scholar] [CrossRef]
- Yang, H.Y.T.; Iadarola, M.J. Activation of spinal neuropeptide FF and the neuropeptide FF receptor 2 during inflammatory hyperalgesia in rats. Neuroscience 2003, 118, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Lee, J.; Kim, S.O.; Song, J.S.; Lee, J.H.; Lee, S.I.; Jung, H.S.; Choi, B. Comparative Gene-Expression Analysis of the Dental Follicle and Periodontal Ligament in Humans. PLoS ONE 2013, 8, 84201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, K.E.; Kang, C.-M.; Jeon, M.; Kim, S.-O.; Lee, J.-H.; Choi, H.-J. General gene expression patterns and stemness of the gingiva and dental pulp. J. Dent. Sci. 2022, 17, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.S.; Cheng, P.; Favis, R.; Wickenden, A.; Romano, G.; Wang, H. SCN9A Variants May be Implicated in Neuropathic Pain Associated With Diabetic Peripheral Neuropathy and Pain Severity. Clin. J. Pain 2015, 31, 976–982. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herrman, E.; Lyashenko, C.; Ortiz, S.; Raslan, K.; Bona, S.; Choi, D.; Maier, T.; Forsyth, A.; Machida, C. SCN9A Genetic Polymorphisms and Dental Pain Sensitivity in Autistic Children. In Proceedings of the IADR/AADR/CADR General Session, Vancouver, BC, Canada, 17–19 June 2019; p. 1. [Google Scholar]
- Moortgat, S.; Berland, S.; Aukrust, I.; Maystadt, I.; Baker, L.; Benoit, V.; Caro-Llopis, A.; Cooper, N.S.; Debray, F.-G.; Faivre, L.; et al. HUWE1 variants cause dominant X-linked intellectual disability: A clinical study of 21 patients. Eur. J. Hum. Genet. 2018, 26, 64–74. [Google Scholar] [CrossRef] [Green Version]
- Loenarz, C.; Ge, W.; Coleman, M.L.; Rose, N.R.; Cooper, C.D.; Klose, R.J.; Ratcliffe, P.J.; Schofield, C.J. PHF8, a gene associated with cleft lip/palate and mental retardation, encodes for an Nε-dimethyl lysine demethylase. Hum. Mol. Genet. 2010, 19, 217–222. [Google Scholar] [CrossRef] [Green Version]
- Chaussain-Miller, C.; Fioretti, F.; Goldberg, M.; Menashi, S. The Role of Matrix Metalloproteinases (MMPs) in Human Caries. J. Dent. Res. 2006, 85, 22–32. [Google Scholar] [CrossRef]
- Sahlberg, C.; Reponen, P.; Tryggvason, K.; Thesleff, I. Timp-1, -2 and -3 show coexpression with gelatinases A and B during mouse tooth morphogenesis. Eur. J. Oral Sci. 1999, 107, 121–130. [Google Scholar] [CrossRef]
- DenBesten, P.K.; Machule, D.; Gallagher, R.; Marshall, G.W.; Mathews, C.; Filvaroff, E. The effect of TGF-β 2 on dentin apposition and hardness in transgenic mice. Adv. Dent. Res. 2001, 15, 39–41. [Google Scholar] [CrossRef]
- Wang, Q.; Jia, P.; Cuenco, K.T.; Feingold, E.; Marazita, M.L.; Wang, L.; Zhao, Z. Multi-Dimensional Prioritization of Dental Caries Candidate Genes and Its Enriched Dense Network Modules. PLoS ONE 2013, 8, 76666. [Google Scholar] [CrossRef] [Green Version]
- Xiao, X.; Xin, C.; Zhang, Y.; Yan, J.; Chen, Z.; Xu, H.; Liang, M.; Wu, B.; Fang, F.; Qiu, W. Characterization of Odontogenic Differentiation from Human Dental Pulp Stem Cells Using TMT-Based Proteomic Analysis. BioMed Res. Int. 2020, 2020, 3871496. [Google Scholar] [CrossRef]
- Jeong, S.-J.; Jeong, M.-J. Increase of Grb2 and Ras Proteins and Expression of Growth Factors in LPS Stimulated Odontoblast-like Dental Pulp Cells. Appl. Microsc. 2013, 43, 27–33. [Google Scholar] [CrossRef] [Green Version]
- Sharir, A.; Marangoni, P.; Zilionis, R.; Wan, M.; Wald, T.; Hu, J.; Kawaguchi, K.; Castillo-Azofeifa, D.; Epstein, L.; Harrington, K.; et al. A large pool of actively cycling progenitors orchestrates self-renewal and injury repair of an ectodermal appendage. Nature 2019, 21, 1102–1112. [Google Scholar] [CrossRef]
- Fujihara, C.; Yamada, S.; Ozaki, N.; Takeshita, N.; Kawaki, H.; Takano-Yamamoto, T.; Murakami, S. Role of Mechanical Stress-induced Glutamate Signaling-associated Molecules in Cytodifferentiation of Periodontal Ligament Cells. J. Biol. Chem. 2010, 285, 28286–28297. [Google Scholar] [CrossRef] [Green Version]
- Sanders, A.E.; Sofer, T.; Wong, Q.; Kerr, K.F.; Agler, C.; Shaffer, J.R.; Beck, J.D.; Offenbacher, S.; Salazar, C.R.; North, K.E.; et al. Chronic Periodontitis Genome-wide Association Study in the Hispanic Community Health Study/Study of Latinos. J. Dent. Res. 2017, 96, 64. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Tong, X.; Zhu, J.; Tian, L.; Jie, Z.; Zou, Y.; Lin, X.; Liang, H.; Li, W.; Ju, Y.; et al. Metagenome-genome-wide association studies reveal human genetic impact on the oral microbiome. Cell Discov. 2021, 7, 117. [Google Scholar] [CrossRef]


| Cohort | Affected | Unaffected | Total | Age Mean (SD) | Female (%) | Location |
|---|---|---|---|---|---|---|
| COHRA1 | 176 (39.5%) | 269 (60.5%) | 447 | 3.88(1.37) | 215 (48.1%) | PA, WV |
| COHRA2 | 156 (20%) | 623 (80%) | 784 | 3.80(1.52) | 373 (47.9%) | PA, WV |
| Iowa Fluoride (IFS) | 137 (35%) | 254 (65%) | 391 | 5.12 (0.37) | 202 (51.7%) | IA |
| IOWA Head Start (IHS) | 54 (34.4%) | 103 (65.6%) | 157 | 3.96(0.78) | 76 (48.4%) | IA |
| ALSPAC | 320 (26.8%) | 875 (73.2%) | 1195 | 4.83(0.77) | 535 (44.8%) | SW England |
| SNP | Chr:Pos | Effect Allele/ Non-Effect Allele | MAF | Z score | Direction † | p | Select Gene(s) ‡ |
|---|---|---|---|---|---|---|---|
| rs76823412 | 8:134690570 | T/C | 0.47 | 4.806 | +++++ | 1.54 × 10−6 | ST3GAL1 * |
| rs74470773 | 3:33958083 | T/C | 0.47 | −4.589 | ----- | 4.46 × 10−6 | PDCD6IP * |
| rs1044956 | 7:24854765 | A/G | 0.49 | 4.551 | +++++ | 5.35 × 10−6 | OSBPL3 *, NPVF |
| rs9889096 | 16:13575143 | T/G | 0.50 | −4.541 | --+-- | 5.59 × 10−6 | SHISA9 * |
| rs563135 | 10:115067899 | A/C | 0.51 | −4.532 | ----- | 5.86 × 10−6 | TCF7L2 *, CASP7 |
| rs7325099 | 13:28104496 | A/C | 0.50 | 4.485 | ++?++ | 7.28 × 10−6 | LNX2 *, POLR1D, RPL21 |
| rs8091366 | 18:24715618 | A/G | 0.52 | 4.476 | +++++ | 7.60 × 10−6 | CHST9 *, KCTD1 |
| Gene | Locus | TWAS p-Value | Gene Start Chr:Pos * | N Tissues † | Z Score (±SD) |
|---|---|---|---|---|---|
| LINC02905 (C8Orf49) ‡ | chr8p23.1 | 1.82 × 10−8 | 8:11618765 | 9 (3) | −0.35 (1.33) |
| CDH17 | chr8q22.1 | 3.32 × 10−8 | 8:95139394 | 23 (5) | 1.74 (3.00) |
| TAS2R43 | chr12p13.2 | 8.78 × 10−8 | 12:11243886 | 27 (3) | 1.80 (1.89) |
| SMIM10L1 | chr12p13.2 | 1.70 × 10−7 | 12:11323780 | 46 (3) | −0.35 (1.62) |
| TAS2R14 | chr12p13.2 | 1.06 × 10−6 | 12:11090853 | 48 (4) | 0.47 (1.27) |
| NRAD1 (LINC00284) ‡ | chr13q14.11 | 1.69 × 10−6 | 13:44596471 | 30 (5) | 0.13 (1.07) |
| TAS2R31 | chr12p13.2 | 1.04 × 10−5 | 12:11182986 | 41 (4) | −0.25 (1.49) |
| LACC1 ‡ | chr13q14.11 | 3.98 × 10−5 | 13:44453420 | 48 (4) | 0.10 (1.16) |
| IGSF5 ‡ | chr21q22.2 | 7.22 × 10−5 | 21:41117334 | 27 (11) | 0.63 (1.40) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orlova, E.; Dudding, T.; Chernus, J.M.; Alotaibi, R.N.; Haworth, S.; Crout, R.J.; Lee, M.K.; Mukhopadhyay, N.; Feingold, E.; Levy, S.M.; et al. Association of Early Childhood Caries with Bitter Taste Receptors: A Meta-Analysis of Genome-Wide Association Studies and Transcriptome-Wide Association Study. Genes 2023, 14, 59. https://doi.org/10.3390/genes14010059
Orlova E, Dudding T, Chernus JM, Alotaibi RN, Haworth S, Crout RJ, Lee MK, Mukhopadhyay N, Feingold E, Levy SM, et al. Association of Early Childhood Caries with Bitter Taste Receptors: A Meta-Analysis of Genome-Wide Association Studies and Transcriptome-Wide Association Study. Genes. 2023; 14(1):59. https://doi.org/10.3390/genes14010059
Chicago/Turabian StyleOrlova, Ekaterina, Tom Dudding, Jonathan M. Chernus, Rasha N. Alotaibi, Simon Haworth, Richard J. Crout, Myoung Keun Lee, Nandita Mukhopadhyay, Eleanor Feingold, Steven M. Levy, and et al. 2023. "Association of Early Childhood Caries with Bitter Taste Receptors: A Meta-Analysis of Genome-Wide Association Studies and Transcriptome-Wide Association Study" Genes 14, no. 1: 59. https://doi.org/10.3390/genes14010059
APA StyleOrlova, E., Dudding, T., Chernus, J. M., Alotaibi, R. N., Haworth, S., Crout, R. J., Lee, M. K., Mukhopadhyay, N., Feingold, E., Levy, S. M., McNeil, D. W., Foxman, B., Weyant, R. J., Timpson, N. J., Marazita, M. L., & Shaffer, J. R. (2023). Association of Early Childhood Caries with Bitter Taste Receptors: A Meta-Analysis of Genome-Wide Association Studies and Transcriptome-Wide Association Study. Genes, 14(1), 59. https://doi.org/10.3390/genes14010059







