Subfunctionalization of Parental Polyamine Oxidase (PAO) Genes in the Allopolyploid Tobacco Nicotiana tabacum (L.)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials, Growth Condition, and Stress Treatments
2.2. RNA Extraction and Quantitative Real-Time Polymerase Chain Reation (qRT-PCR) Analysis
2.3. Statistical Analysis
2.4. Sequence Analysis and Bionformatic Tools
3. Results
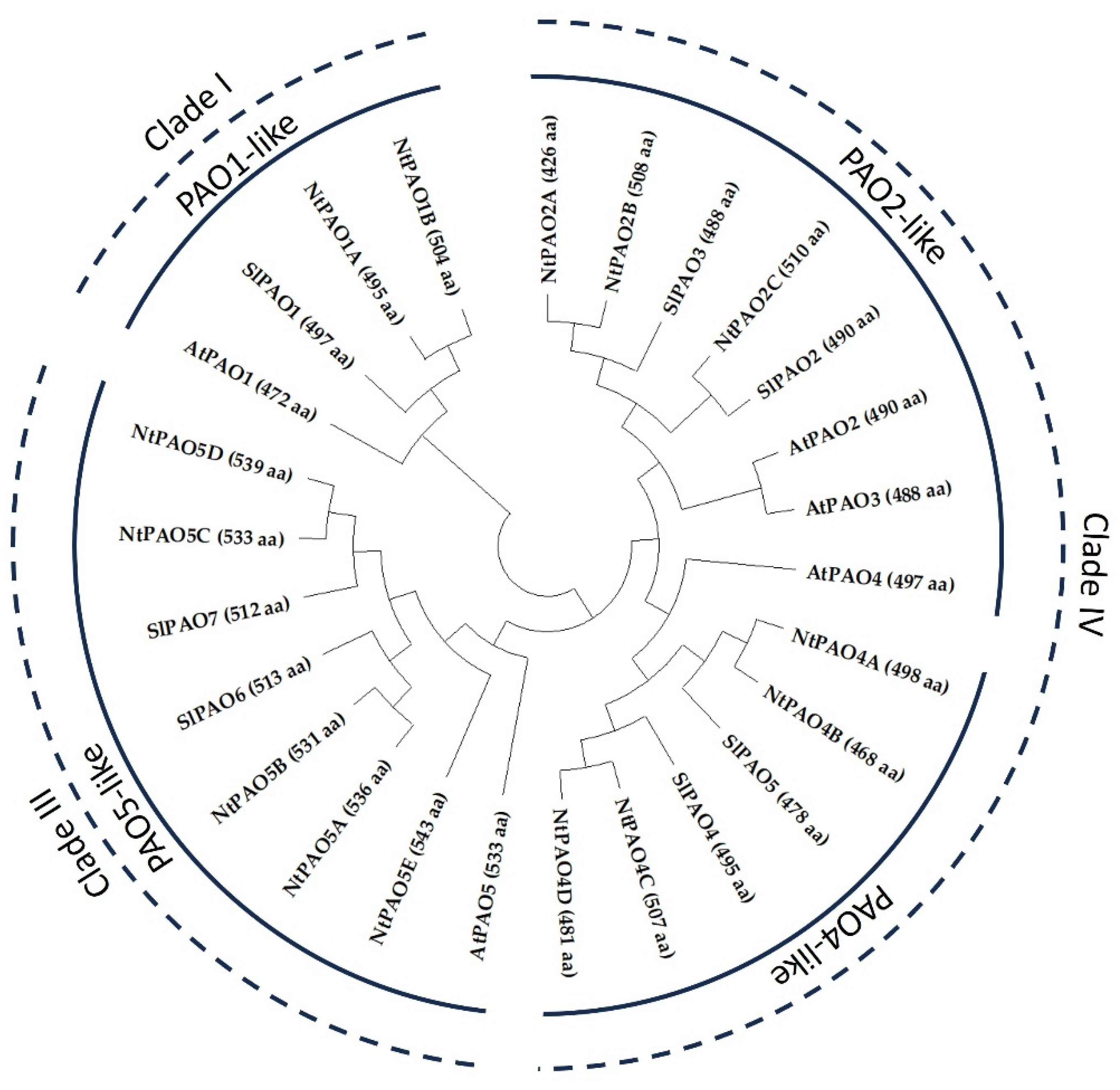
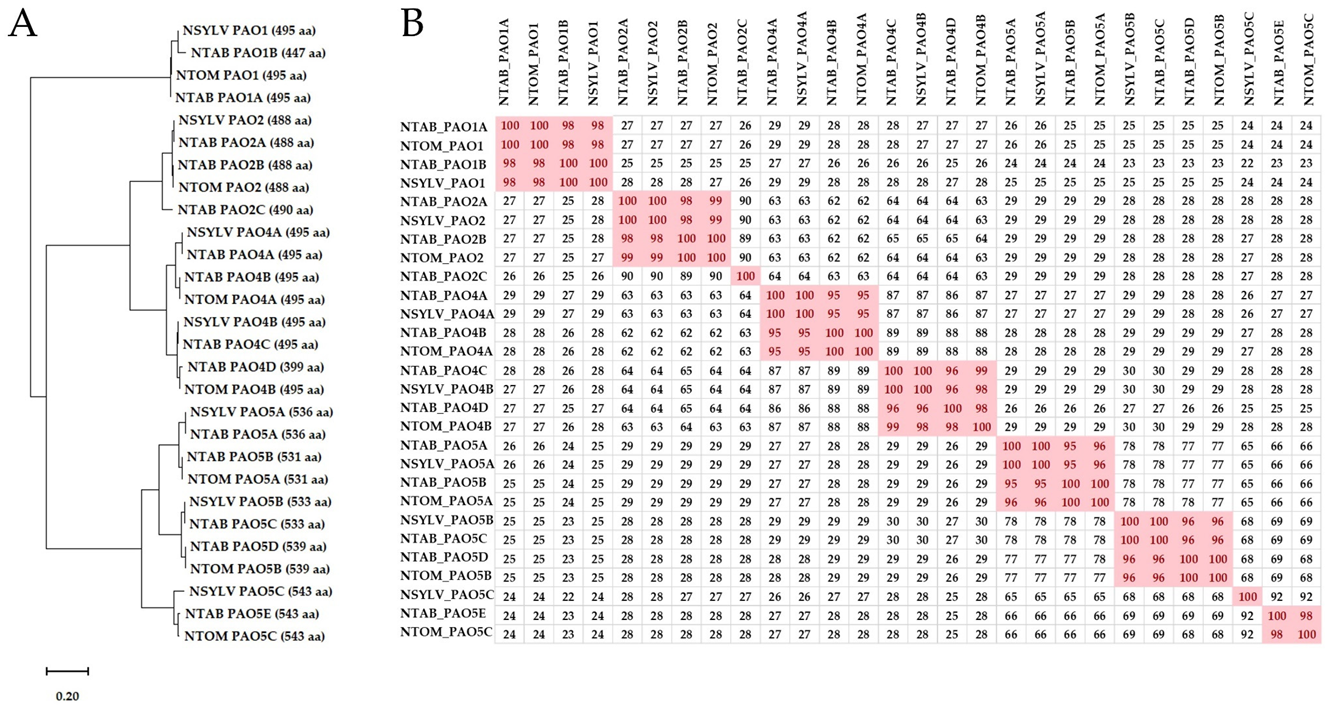
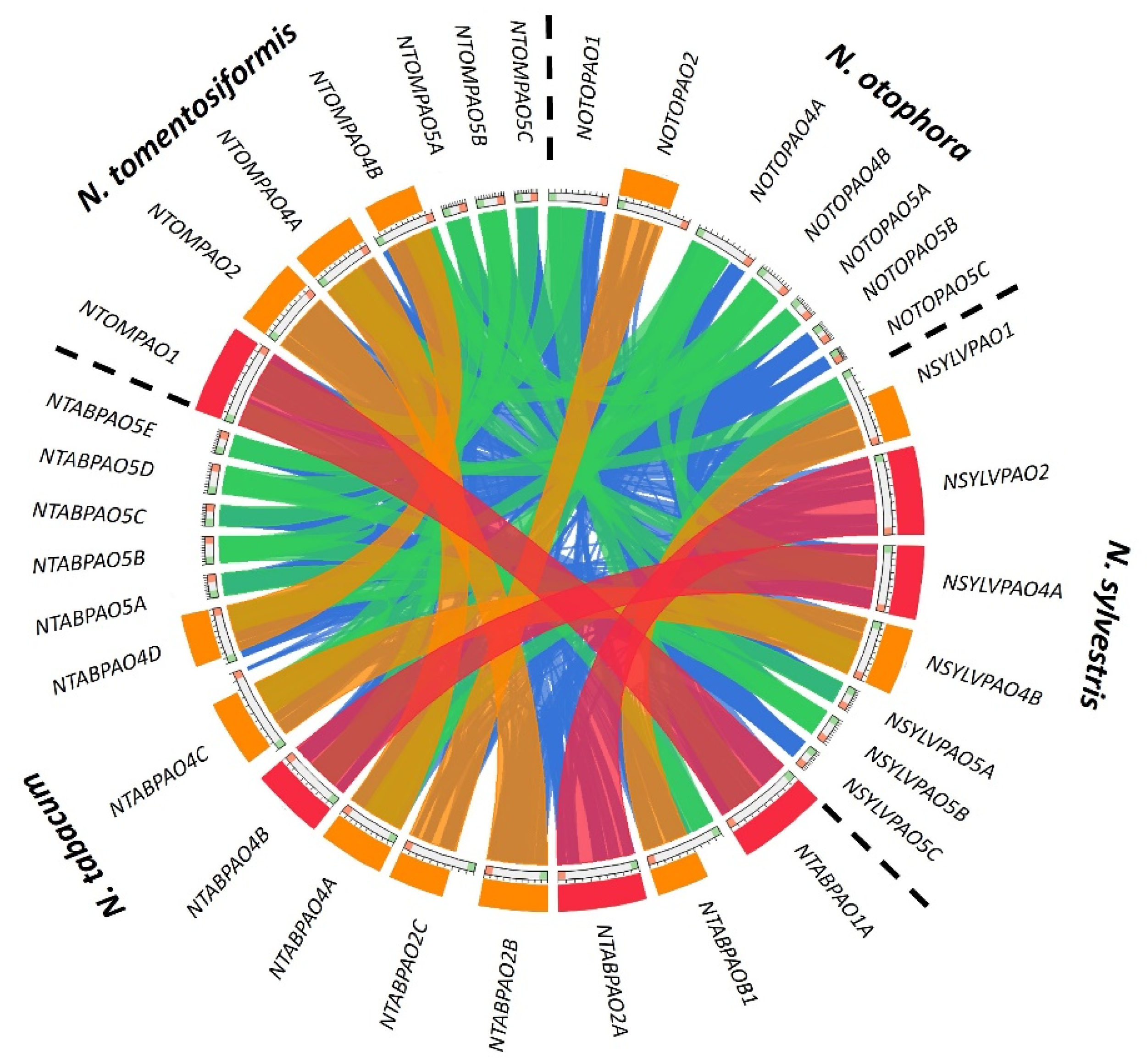
3.1. The Nicotiana tabacum (L.) Genome Contains Fourteen PAO-Coding Genes
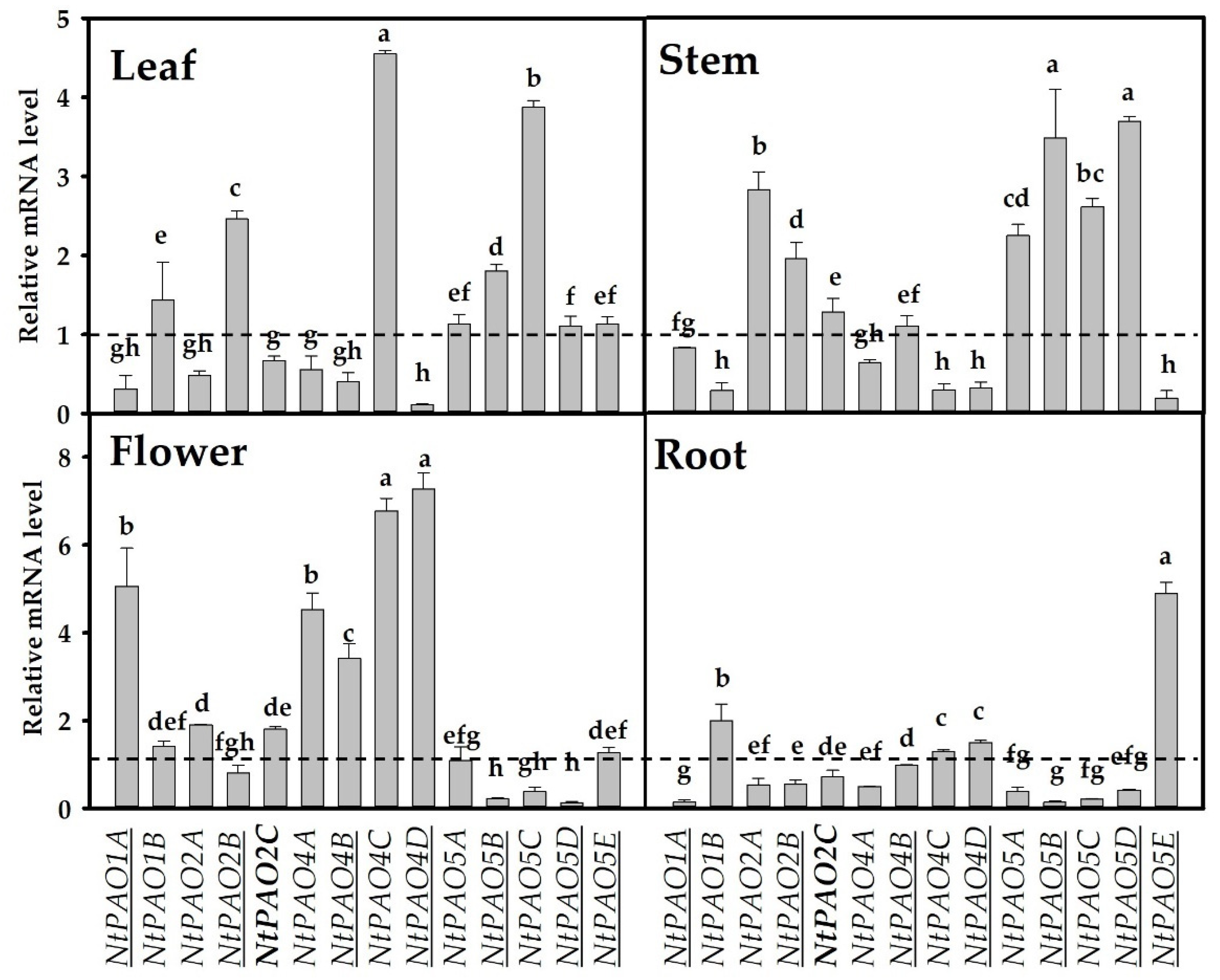
3.2. Expression Analysis Indicates the Subfunctionalization of Parental PAO-Coding Genes in the Hybrid Tobacco
3.3. Organ-Specific Expression of 14 NtPAOs
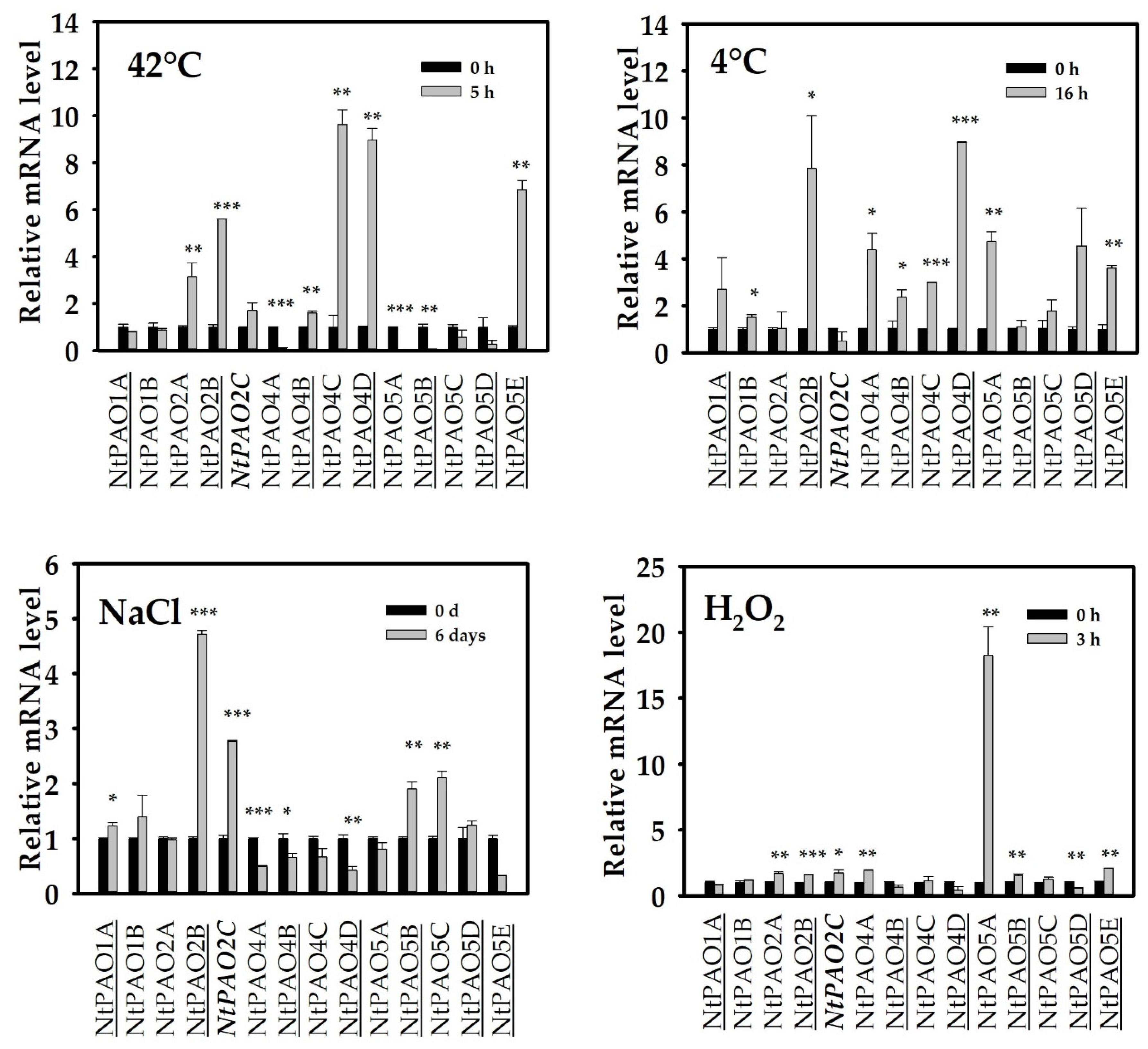
3.4. Expression Changes of Tobacco PAOs in Response to Abiotic Stress Treatments
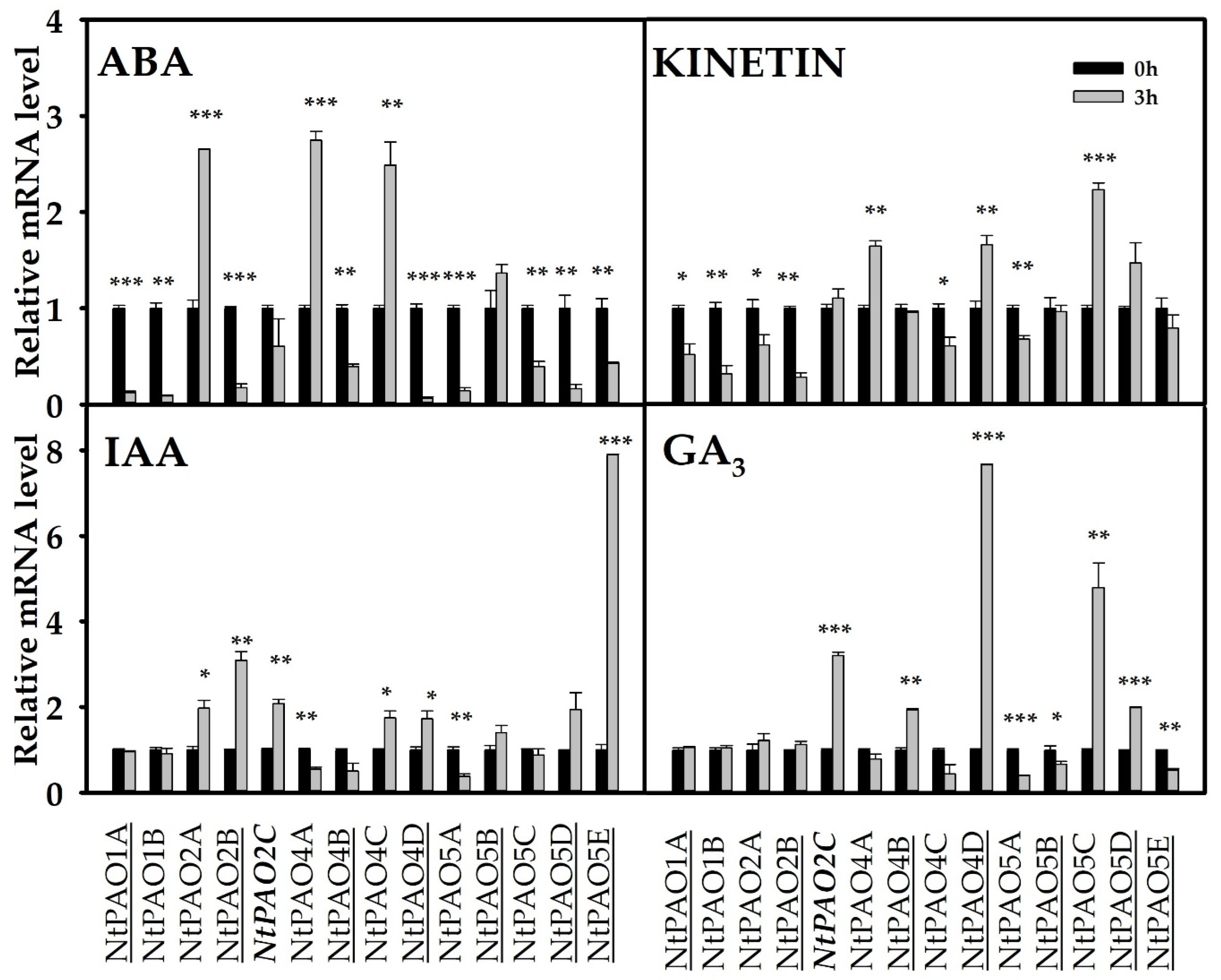
3.5. Expression Responses of Tobacco PAO-Coding Genes in Response to Phytohormones
4. Discussion
4.1. The PAO Genes of the Allotetraploid Hybrid N. tabacum Do Not Exactly Match the Parental Sequences
4.2. The Expression Patterns of PAO-Coding Genes in Tobacco Exhibit Diversification in Comparison to Other Dicotyledonous Species
4.3. The Expression Pattern of Homeologous PAO Genes Exhibit Signs of Subfunctionalization
5. Conclusions and Outlook
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tiburcio, A.F.; Altabella, T.; Bitrián, M.; Alcázar, R. The Roles of Polyamines during the Lifespan of Plants: From Development to Stress. Planta 2014, 240, 1–18. [Google Scholar] [CrossRef]
- Benkő, P.; Jee, S.; Kaszler, N.; Fehér, A.; Gémes, K. Polyamines Treatment during Pollen Germination and Pollen Tube Elongation in Tobacco Modulate Reactive Oxygen Species and Nitric Oxide Homeostasis. J. Plant Physiol. 2020, 244, 153085. [Google Scholar] [CrossRef]
- Kaszler, N.; Benkő, P.; Bernula, D.; Szepesi, Á.; Fehér, A.; Gémes, K.; Benk, P.; Bernula, D.; Szepesi, Á.; Fehér, A.; et al. Polyamine Metabolism Is Involved in the Direct Regeneration of Shoots from Arabidopsis Lateral Root Primordia. Plants 2021, 10, 305. [Google Scholar] [CrossRef] [PubMed]
- Fortes, A.M.; Agudelo-Romero, P. Polyamine Metabolism in Climacteric and Non-Climacteric Fruit Ripening; Humana Press Inc.: Totowa, NJ, USA, 2018; pp. 433–447. [Google Scholar]
- Cai, G.; Sobieszczuk-Nowicka, E.; Aloisi, I.; Fattorini, L.; Serafini-Fracassini, D.; Del Duca, S. Polyamines Are Common Players in Different Facets of Plant Programmed Cell Death. Amino Acids 2015, 47, 27–44. [Google Scholar] [CrossRef]
- Groppa, M.D.; Benavides, M.P. Polyamines and Abiotic Stress: Recent Advances. Amino Acids 2008, 34, 35–45. [Google Scholar] [CrossRef]
- Moschou, P.N.; Paschalidis, K.A.; Roubelakis-Angelakis, K.A. Plant Polyamine Catabolism. Plant Signal. Behav. 2008, 3, 1061–1066. [Google Scholar] [CrossRef]
- Gémes, K.; Kim, Y.J.; Park, K.Y.; Moschou, P.N.; Andronis, E.; Valassaki, C.; Roussis, A.; Roubelakis-Angelakis, K.A. An NADPH-Oxidase/Polyamine Oxidase Feedback Loop Controls Oxidative Burst under Salinity. Plant Physiol. 2016, 172, 1418–1431. [Google Scholar] [CrossRef] [PubMed]
- Gémes, K.; Mellidou, Ι.; Karamanoli, K.; Beris, D.; Park, K.Y.; Matsi, T.; Haralampidis, K.; Constantinidou, H.I.; Roubelakis-Angelakis, K.A. Deregulation of Apoplastic Polyamine Oxidase Affects Development and Salt Response of Tobacco Plants. J. Plant Physiol. 2017, 211, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ebeed, H.T.; Hassan, N.M.; Aljarani, A.M. Exogenous Applications of Polyamines Modulate Drought Responses in Wheat through Osmolytes Accumulation, Increasing Free Polyamine Levels and Regulation of Polyamine Biosynthetic Genes. Plant Physiol. Biochem. 2017, 118, 438–448. [Google Scholar] [CrossRef]
- Takahashi, T. Plant Polyamines. Plants 2020, 9, 511. [Google Scholar] [CrossRef]
- Smith, T.A. Polyamines. Annu. Rev. Plant Physiol. 1985, 36, 117–143. [Google Scholar] [CrossRef]
- Kusano, T.; Berberich, T.; Tateda, C.; Takahashi, Y. Polyamines: Essential Factors for Growth and Survival. Planta 2008, 228, 367–381. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; Cong, R.; Sagor, G.H.M.M.H.M.M.; Niitsu, M.; Berberich, T.; Kusano, T. Characterization of Five Polyamine Oxidase Isoforms in Arabidopsis Thaliana. Plant Cell Rep. 2010, 29, 955–965. [Google Scholar] [CrossRef] [PubMed]
- Sagor, G.H.M.; Inoue, M.; Kusano, T.; Berberich, T. Expression Profile of Seven Polyamine Oxidase Genes in Rice (Oryza sativa) in Response to Abiotic Stresses, Phytohormones and Polyamines. Physiol. Mol. Biol. Plants 2021, 27, 1353–1359. [Google Scholar] [CrossRef]
- Fujita, M.; Shinozaki, K. Polyamine Transport Systems in Plants. In Polyamines: A Universal Molecular Nexus for Growth, Survival, and Specialized Metabolism; Springer: Tokyo, Japan, 2015; pp. 179–185. ISBN 9784431552123. [Google Scholar]
- Liu, T.; Kim, D.W.; Niitsu, M.; Berberich, T.; Kusano, T. Oryza Sativa Polyamine Oxidase 1 Back-Converts Tetraamines, Spermine and Thermospermine, to Spermidine. Plant Cell Rep. 2014, 33, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Bagni, N.; Tassoni, A. Biosynthesis, Oxidation and Conjugation of Aliphatic Polyamines in Higher Plants. Amino Acids 2001, 20, 301–317. [Google Scholar] [CrossRef]
- Cona, A.; Rea, G.; Angelini, R.; Federico, R.; Tavladoraki, P. Functions of Amine Oxidases in Plant Development and Defence. Trends Plant Sci. 2006, 11, 80–88. [Google Scholar] [CrossRef]
- Benkő, P.; Gémes, K.; Fehér, A. Polyamine Oxidase-Generated Reactive Oxygen Species in Plant Development and Adaptation: The Polyamine Oxidase—NADPH Oxidase Nexus. Antioxidants 2022, 11, 2488. [Google Scholar] [CrossRef]
- Hao, Y.; Huang, B.; Jia, D.; Mann, T.; Jiang, X.; Qiu, Y.; Niitsu, M.; Berberich, T.; Kusano, T.; Liu, T. Identification of Seven Polyamine Oxidase Genes in Tomato (Solanum lycopersicum L.) and Their Expression Profiles under Physiological and Various Stress Conditions. J. Plant Physiol. 2018, 228, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Paschalidis, K.; Feng, J.-C.C.; Song, J.; Liu, J.-H.H. Polyamine Catabolism in Plants: A Universal Process with Diverse Functions. Front. Plant Sci. 2019, 10, 561. [Google Scholar] [CrossRef]
- Toumi, I.; Pagoulatou, M.G.; Margaritopoulou, T.; Milioni, D.; Roubelakis-Angelakis, K.A. Genetically Modified Heat Shock Protein90s and Polyamine Oxidases in Arabidopsis Reveal Their Interaction under Heat Stress Affecting Polyamine Acetylation, Oxidation and Homeostasis of Reactive Oxygen Species. Plants 2019, 8, 323. [Google Scholar] [CrossRef]
- Mellidou, I.; Karamanoli, K.; Beris, D.; Haralampidis, K.; Constantinidou, H.-I.A.; Roubelakis-Angelakis, K.A. Underexpression of Apoplastic Polyamine Oxidase Improves Thermotolerance in Nicotiana Tabacum. J. Plant Physiol. 2017, 218, 171–174. [Google Scholar] [CrossRef]
- Konstantinos, P.A.; Imene, T.; Panagiotis, M.N.; Roubelakis-Angelakis, K.A. ABA-Dependent Amine Oxidases-Derived H2O2 Affects Stomata Conductance. Plant Signal. Behav. 2010, 5, 1153–1156. [Google Scholar] [CrossRef]
- Bordenave, C.D.; Granados Mendoza, C.; Jiménez Bremont, J.F.; Gárriz, A.; Rodríguez, A.A. Defining Novel Plant Polyamine Oxidase Subfamilies through Molecular Modeling and Sequence Analysis. BMC Evol. Biol. 2019, 19, 28. [Google Scholar] [CrossRef]
- Kim, D.W.; Watanabe, K.; Murayama, C.; Izawa, S.; Niitsu, M.; Michael, A.J.; Berberich, T.; Kusano, T. Polyamine Oxidase5 Regulates Arabidopsis Growth through Thermospermine Oxidase Activity. Plant Physiol. 2014, 165, 1575–1590. [Google Scholar] [CrossRef] [PubMed]
- Ono, Y.; Kim, D.W.; Watanabe, K.; Sasaki, A.; Niitsu, M.; Berberich, T.; Kusano, T.; Takahashi, Y. Constitutively and Highly Expressed Oryza Sativa Polyamine Oxidases Localize in Peroxisomes and Catalyze Polyamine Back Conversion. Amino Acids 2012, 42, 867–876. [Google Scholar] [CrossRef]
- Edwards, K.D.; Fernandez-Pozo, N.; Drake-Stowe, K.; Humphry, M.; Evans, A.D.; Bombarely, A.; Allen, F.; Hurst, R.; White, B.; Kernodle, S.P.; et al. A Reference Genome for Nicotiana Tabacum Enables Map-Based Cloning of Homeologous Loci Implicated in Nitrogen Utilization Efficiency. BMC Genom. 2017, 18, 448. [Google Scholar] [CrossRef] [PubMed]
- Magadum, S.; Banerjee, U.; Murugan, P.; Gangapur, D.; Ravikesavan, R. Gene Duplication as a Major Force in Evolution. J. Genet. 2013, 92, 155–161. [Google Scholar] [CrossRef]
- Clarkson, J.J.; Dodsworth, S.; Chase, M.W. Time-Calibrated Phylogenetic Trees Establish a Lag between Polyploidisation and Diversification in Nicotiana (Solanaceae). Plant. Syst. Evol. 2017, 303, 1001–1012. [Google Scholar] [CrossRef]
- Bombarely, A.; Edwards, K.D.; Sanchez-Tamburrino, J.; Mueller, L.A. Deciphering the Complex Leaf Transcriptome of the Allotetraploid Species Nicotiana Tabacum: A Phylogenomic Perspective. BMC Genom. 2012, 13, 406. [Google Scholar] [CrossRef] [PubMed]
- Murashige, T.; Skoog, F. A Revised Medium for Rapid Growth and Bio Assays with Tobacco Tissue Cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Ye, J.; Coulouris, G.; Zaretskaya, I.; Cutcutache, I.; Rozen, S.; Madden, T.L. Primer-BLAST: A Tool to Design Target-Specific Primers for Polymerase Chain Reaction. BMC Bioinform. 2012, 13, 134. [Google Scholar] [CrossRef]
- Sierro, N.; Battey, J.N.D.; Ouadi, S.; Bakaher, N.; Bovet, L.; Willig, A.; Goepfert, S.; Peitsch, M.C.; Ivanov, N.V. The Tobacco Genome Sequence and Its Comparison with Those of Tomato and Potato. Nat. Commun. 2014, 5, 3833. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.; Zaretskaya, I.; Raytselis, Y.; Merezhuk, Y.; McGinnis, S.; Madden, T.L. NCBI BLAST: A Better Web Interface. Nucleic Acids Res. 2008, 36, W5–W9. [Google Scholar] [CrossRef] [PubMed]
- Bailey, T.L.; Johnson, J.; Grant, C.E.; Noble, W.S. The MEME Suite. Nucleic Acids Res. 2015, 43, W39–W49. [Google Scholar] [CrossRef]
- Darzentas, N. Circoletto: Visualizing Sequence Similarity with Circos. Bioinformatics 2010, 26, 2620–2621. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Larkin, M.A.; Blackshields, G.; Brown, N.P.; Chenna, R.; McGettigan, P.A.; McWilliam, H.; Valentin, F.; Wallace, I.M.; Wilm, A.; Lopez, R.; et al. Clustal W and Clustal X Version 2.0. Bioinformatics 2007, 23, 2947–2948. [Google Scholar] [CrossRef]
- Saitou, N.; Nei, M. The Neighbor-Joining Method: A New Method for Reconstructing Phylogenetic Trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [CrossRef]
- Zuckerland, E.; Pauling, L. Evolutionary Divergence and Convergence in Proteins. In Evolving Genes and Proteins; Bryson, V., Vogel, H.J., Eds.; Academic Press: New York, NY, USA, 1965; pp. 97–166. [Google Scholar]
- Horton, P.; Park, K.-J.; Obayashi, T.; Fujita, N.; Harada, H.; Adams-Collier, C.J.; Nakai, K. WoLF PSORT: Protein Localization Predictor. Nucleic Acids Res. 2007, 35, W585–W587. [Google Scholar] [CrossRef]
- Sierro, N.; Battey, J.N.; Ouadi, S.; Bovet, L.; Goepfert, S.; Bakaher, N.; Peitsch, M.C.; Ivanov, N.V. Reference Genomes and Transcriptomes of Nicotiana Sylvestris and Nicotiana Tomentosiformis. Genome Biol. 2013, 14, R60. [Google Scholar] [CrossRef] [PubMed]
- Kenton, A.; Parokonny, A.S.; Gleba, Y.Y.; Bennett, M.D. Characterization of the Nicotiana tabacum L. Genome by Molecular Cytogenetics. Mol. Gen. Genet. 1993, 240, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Riechers, D.E.; Timko, M.P. Structure and Expression of the Gene Family Encoding Putrescine N-Methyltransferase in Nicotiana Tabacum: New Clues to the Evolutionary Origin of Cultivated Tobacco. Plant. Mol. Biol. 1999, 41, 387–401. [Google Scholar] [CrossRef] [PubMed]
- Ren, N.; Timko, M.P. AFLP Analysis of Genetic Polymorphism and Evolutionary Relationships among Cultivated and Wild Nicotiana Species. Genome 2001, 44, 559–571. [Google Scholar] [CrossRef]
- Li, M.; Lu, J.; Tao, M.; Li, M.; Yang, H.; Xia, E.; Chen, Q.; Wan, X. Genome-Wide Identification of Seven Polyamine Oxidase Genes in Camellia sinensis (L.) and Their Expression Patterns Under Various Abiotic Stresses. Front. Plant Sci. 2020, 11, 544933. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liang, L.; Xiao, J.; Xie, Y.; Zhu, L.; Xue, X.; Xu, L.; Zhou, P.; Ran, J.; Huang, Z.; et al. Genome-Wide Identification of Polyamine Oxidase (PAO) Family Genes: Roles of CaPAO2 and CaPAO4 in the Cold Tolerance of Pepper (Capsicum annuum L.). Int. J. Mol. Sci. 2022, 23, 9999. [Google Scholar] [CrossRef]
- Shoji, T.; Moriyama, K.; Sierro, N.; Ouadi, S.; Ivanov, N.V.; Hashimoto, T.; Saito, K. Natural and Induced Variations in Transcriptional Regulator Genes Result in Low-Nicotine Phenotypes in Tobacco. Plant J. 2022, 111, 1768–1779. [Google Scholar] [CrossRef]
- Dewey, R.E.; Xie, J. Molecular Genetics of Alkaloid Biosynthesis in Nicotiana Tabacum. Phytochemistry 2013, 94, 10–27. [Google Scholar] [CrossRef]
- Nölke, G.; Chudobova, I.; Houdelet, M.; Volke, D.; Lusso, M.; Frederick, J.; Kudithipudi, C.; Shen, Y.; Warek, U.; Strickland, J.A.; et al. Impact of Nicotine Pathway Downregulation on Polyamine Biosynthesis and Leaf Ripening in Tobacco. Plant Direct 2021, 5, e00329. [Google Scholar] [CrossRef]
- Fincato, P.; Moschou, P.N.; Ahou, A.; Angelini, R.; Roubelakis-Angelakis, K.A.; Federico, R.; Tavladoraki, P. The Members of Arabidopsis Thaliana PAO Gene Family Exhibit Distinct Tissue- and Organ-Specific Expression Pattern during Seedling Growth and Flower Development. Amino Acids 2012, 42, 831–841. [Google Scholar] [CrossRef]
- Samanta, I.; Roy, P.C.; Das, E.; Mishra, S.; Chowdhary, G. Plant Peroxisomal Polyamine Oxidase: A Ubiquitous Enzyme Involved in Abiotic Stress Tolerance. Plants 2023, 12, 652. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Liu, J.-H. CsPAO4 of Citrus Sinensis Functions in Polyamine Terminal Catabolism and Inhibits Plant Growth under Salt Stress. Sci. Rep. 2016, 6, 31384. [Google Scholar] [CrossRef] [PubMed]
- Zarza, X.; Atanasov, K.E.; Marco, F.; Arbona, V.; Carrasco, P.; Kopka, J.; Fotopoulos, V.; Munnik, T.; Gómez-Cadenas, A.; Tiburcio, A.F.; et al. Polyamine Oxidase 5 Loss-of-Function Mutations in Arabidopsis Thaliana Trigger Metabolic and Transcriptional Reprogramming and Promote Salt Stress Tolerance. Plant Cell Environ. 2017, 40, 527–542. [Google Scholar] [CrossRef]
- Sagor, G.H.M.; Zhang, S.; Kojima, S.; Simm, S.; Berberich, T.; Kusano, T. Reducing Cytoplasmic Polyamine Oxidase Activity in Arabidopsis Increases Salt and Drought Tolerance by Reducing Reactive Oxygen Species Production and Increasing Defense Gene Expression. Front. Plant Sci. 2016, 7, 214. [Google Scholar] [CrossRef] [PubMed]
| Gene Accession a | Gene Accession b | Scaffold Accession (Gene Position) | mRNA Accession | mRNA Size (bp) | Protein Accession | Protein Size (aa) | Gene Annotation | Gene Name |
|---|---|---|---|---|---|---|---|---|
| LOC107832568 | Nitab4.5_0000707g0200.1 | NW_015864215.1 (20974..27671) | NM_001326282.2 | 1888 | NP_001313211.1 | 495 | polyamine oxidase 1 | NtPAO1A |
| LOC107788770 | Nitab4.5_0008441g0030.1 | NW_015916267.1 (46836..53239) | XM_016610479.1 | 1801 | XP_016465965.1 | 447 | polyamine oxidase 1-like | NtPAO1B |
| LOC107762338 | Nitab4.5_0002015g0070.1 | NW_015876609.1 (111786..118428, | XM_016580681.1 | 2235 | XP_016436167.1 | 488 | probable polyamine oxidase 2 | NtPAO2A |
| LOC107775961 | Nitab4.5_0004862g0070.1 | NW_015901231.1 (12262..17390) | XM_016595768.1 | 2295 | XP_016451254.1 | 488 | probable polyamine oxidase 2 | NtPAO2B |
| LOC107799822 | Nitab4.5_0007665g0030.1 | NW_015931733.1 (28305..34373, | XM_016622969.1 | 2300 | XP_016478455.1 | 490 | probable polyamine oxidase 2 | NtPAO2C |
| LOC107800087 | Nitab4.5_0001374g0220.1 | NW_015932137.1 (603..6066, | XM_016623232.1 | 2199 | XP_016478718.1 | 495 | probable polyamine oxidase 4 | NtPAO4A |
| LOC107775720 | Nitab4.5_0000091g0280.1 | NW_015900952.1 (12745..17683 | XM_016595473.1 | 1492 | XP_016450959.1 | 416 | probable polyamine oxidase 4 | NtPAO4B |
| LOC107761719 | Nitab4.5_0003412g0030.1 | NW_015874368.1 (104479..111727 | XM_016579980.1 | 2802 | XP_016435466.1 | 495 | probable polyamine oxidase 4 | NtPAO4_C |
| LOC107812697 | Nitab4.5_0000483g0140.1 | NW_015954488.1 (6193..10774) | XM_016637848.1 | 2094 | XP_016493334.1 | 399 | probable polyamine oxidase 4 | NtPAO4D |
| LOC107813795 | Nitab4.5_0004978g0010.1 | NW_015789106.1 (85803..87734) | XM_016639103.1 | 1932 | XP_016494589.1 | 536 | probable polyamine oxidase 5 | NtPAO5A |
| LOC107765565 | Nitab4.5_0016456g0010.1 | NW_015794575.1 (4084..5978, | XM_016584233.1 | 1895 | XP_016439719.1 | 531 | probable polyamine oxidase 5 | NtPAO5B |
| LOC107791914 | Nitab4.5_0003310g0050.1 | NW_015920426.1 (26034..28297, | XM_016614062.1 | 2264 | XP_016469548.1 | 533 | probable polyamine oxidase 5 | NtPAO5C |
| LOC107767845 | Nitab4.5_0000095g0080.1 | NW_015887926.1 (167021..169486 | XM_016586944.1 | 2466 | XP_016442430.1 | 539 | probable polyamine oxidase 5 | NtPAO5D |
| LOC107778196 | Nitab4.5_0008523g0010.1 | NW_015903755.1 (63036..64820) | XM_016598410.1 | 1785 | XP_016453896.1 | 543 | probable polyamine oxidase 5 | NtPAO5E |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benkő, P.; Kaszler, N.; Gémes, K.; Fehér, A. Subfunctionalization of Parental Polyamine Oxidase (PAO) Genes in the Allopolyploid Tobacco Nicotiana tabacum (L.). Genes 2023, 14, 2025. https://doi.org/10.3390/genes14112025
Benkő P, Kaszler N, Gémes K, Fehér A. Subfunctionalization of Parental Polyamine Oxidase (PAO) Genes in the Allopolyploid Tobacco Nicotiana tabacum (L.). Genes. 2023; 14(11):2025. https://doi.org/10.3390/genes14112025
Chicago/Turabian StyleBenkő, Péter, Nikolett Kaszler, Katalin Gémes, and Attila Fehér. 2023. "Subfunctionalization of Parental Polyamine Oxidase (PAO) Genes in the Allopolyploid Tobacco Nicotiana tabacum (L.)" Genes 14, no. 11: 2025. https://doi.org/10.3390/genes14112025






