In Tandem Intragenic Duplication of Doublesex and Mab-3-Related Transcription Factor 1 (DMRT1) in an SRY-Negative Boy with a 46,XX Disorder of Sex Development
Abstract
:1. Introduction
2. Case Report and Materials and Methods
2.1. Patient
2.2. Next-Generation Sequencing (NGS)
2.3. Whole Genome Sequencing (WGS)
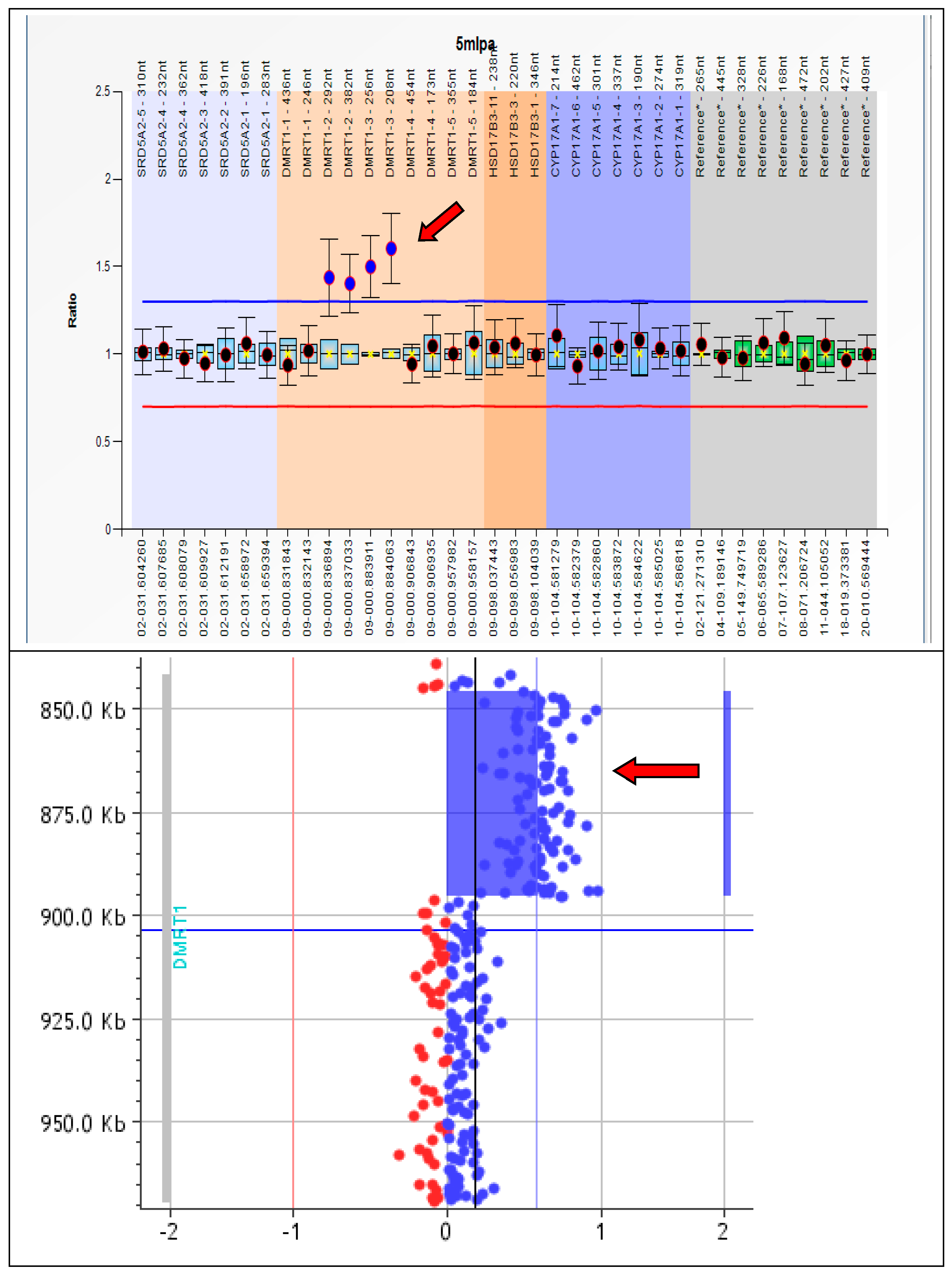
2.4. Multiplex Ligation-Dependent Probe Amplification (MLPA)
2.5. Comparative Genomic Hybridization Array (CGH-Array)
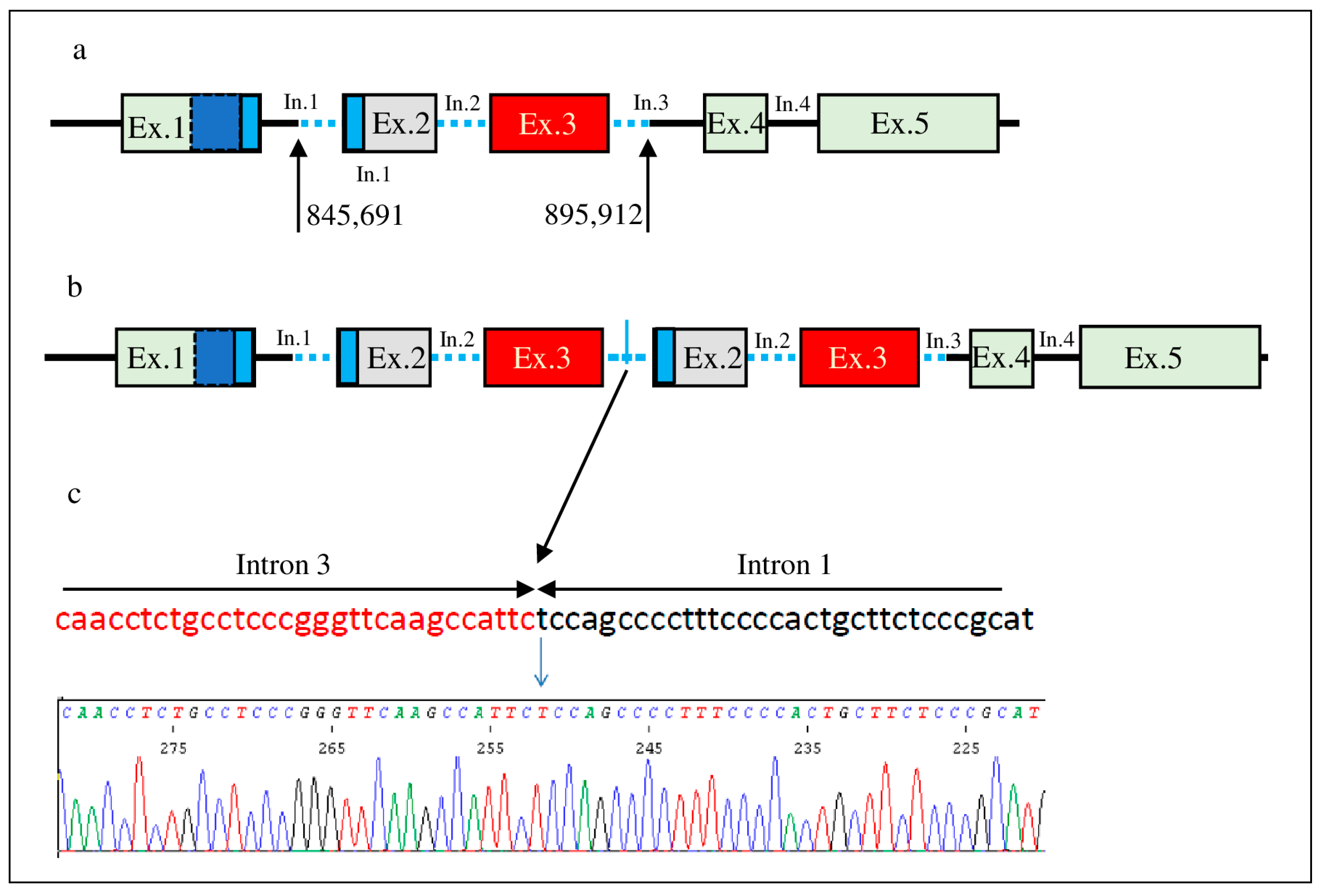
2.6. Amplification and Sequencing of the Junction Fragment
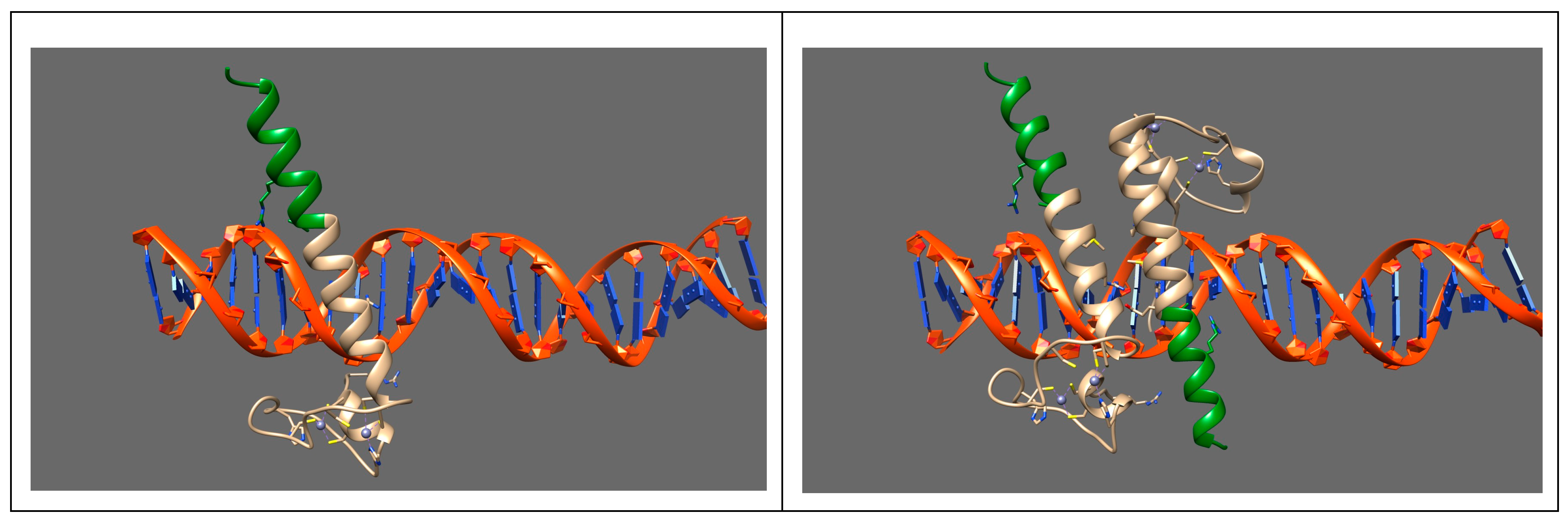
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Biason-Lauber, A. The battle of the sexes: Human sex development and its disorders. Results Probl. Cell Differ. 2016, 58, 337–382. [Google Scholar]
- Nagahama, Y.; Chakraborty, T.; Paul-Prasanth, B.; Ohta, K.; Nakamura, M. Sex determination, gonadal sex differentiation, and plasticity in vertebrate species. Physiol. Rev. 2021, 101, 1237–1308. [Google Scholar] [PubMed]
- Baetens, D.; Verdin, H.; De Baere, E.; Cools, M. Update on the genetics of differences of sex development (DSD). Best Pract. Res. Clin. Endocrinol. Metab. 2019, 33, 101271. [Google Scholar] [PubMed]
- Grinspon, R.P.; Rey, R.A. Molecular Characterization of XX Maleness. Int. J. Mol. Sci. 2019, 20, 6089. [Google Scholar]
- Svingen, T.; Koopman, P. Building the mammalian testis: Origins, differentiation, and assembly of the component cell populations. Genes Dev. 2013, 27, 2409–2426. [Google Scholar]
- Zarkower, D.; Murphy, M.W. DMRT1: An ancient sexual regulator required for human gonadogenesis. Sex. Dev. 2021, 16, 112–125. [Google Scholar]
- Erdman, S.E.; Burtis, K.C. The Drosophila doublesex proteins share a novel zinc finger related DNA binding domain. EMBO J. 1993, 12, 527–535. [Google Scholar] [CrossRef] [PubMed]
- Raymond, C.S.; Murphy, M.W.; O’Sullivan, M.G.; Bardwell, V.J.; Zarkower, D. Dmrt1, a gene related to worm and fly sexual regulators, is required for mammalian testis differentiation. Genes Dev. 2000, 14, 2587–2595. [Google Scholar] [PubMed]
- De Grandi, A.; Calvari, V.; Bertini, V.; Bulfone, A.; Peverali, G.; Camerino, G.; Borsani, G.; Guioli, S. The expression pattern of a mouse doublesex-related gene is consistent with a role in gonadal differentiation. Mech. Dev. 2000, 90, 323–326. [Google Scholar]
- Matson, C.K.; Zarkower, D. Sex and the singular DM domain: Insights into sexual regulation, evolution and plasticity. Nat. Rev. Genet. 2012, 13, 163–174. [Google Scholar]
- Zhao, L.; Svingen, T.; Ng, E.T.; Koopman, P. Female-to-male sex reversal in mice caused by transgenic overexpression of Dmrt1. Development 2015, 142, 1083–1088. [Google Scholar] [PubMed]
- Jiménez, R.; Burgos, M.; Barrionuevo, J.M. Sex maintenance in mammals. Genes 2021, 12, 999. [Google Scholar] [PubMed]
- Matson, C.K.; Murphy, M.W.; Sarver, A.L.; Griswold, M.D.; Bardwell, V.J.; Zarkower, D. DMRT1 prevents female reprogramming in postnatal mammalian testis. Nature 2011, 476, 101–104. [Google Scholar]
- Lindeman, R.E.; Gearhart, M.D.; Minkina, A.; Krentz, A.D.; Bardwell, V.J.; Zarkower, D. Sexual cell-fate reprogramming in the ovary by DMRT1. Curr. Biol. 2015, 25, 764–771. [Google Scholar] [PubMed]
- Goede, J.; Hack, W.W.M.; Sijstermans, K.; van der Voort-Doedens, L.M.; Van der Ploeg, T.; Meij-de Vries, A.; Delemarre-van de Waal, H.A. Normative values for testicular volume measured by ultrasonography in a normal population from infancy to adolescence. Horm. Res. Paediatr. 2011, 76, 56–64. [Google Scholar] [PubMed]
- Fanelli, F.; Baronio, F.; Ortolano, R.; Mezzullo, M.; Cassio, A.; Pagotto, U.; Balsamo, A. Normative basal values of hormones and proteins of gonadal and adrenal functions from birth to adulthood. Sex. Dev. 2018, 12, 50–94. [Google Scholar]
- Danecek, P.; Bonfield, J.K.; Liddle, J.; Marshall, J.; Ohan, V.; Pollard, M.O.; Whitwham, A.; Keane, T.; McCarthy, S.A.; Davies, R.M.; et al. Twelve years of SAMtools and BCFtools. Gigascience 2021, 10, giab008. [Google Scholar] [PubMed]
- McKenna, A.; Hanna, M.; Banks, E.; Sivachenko, A.; Cibulskis, K.; Kernytsky, A.; Garimella, K.; Altshuler, D.; Gabriel, S.; Daly, M.; et al. The Genome Analysis Toolkit: A MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010, 20, 1297–1303. [Google Scholar]
- Chen, X.; Schulz-Trieglaff, O.; Shaw, R.; Barnes, B.; Schlesinger, F.; Källberg, M.; Cox, A.J.; Kruglyak, S.; Saunders, C.T. Manta: Rapid detection of structural variants and indels for germline and cancer sequencing applications. Bioinformatics 2016, 32, 1220–1222. [Google Scholar]
- McLaren, W.; Gil, L.; Hunt, S.E.; Riat, H.S.; Ritchie, G.R.; Thormann, A.; Flicek, P.; Cunningham, F. The Ensembl Variant Effect Predictor. Genome Biol. 2016, 17, 122. [Google Scholar]
- Elzaiat, M.; Jouneau, L.; Thépot, D.; Klopp, C.; Allais-Bonnet, A.; Cabau, C.; André, M.; Chaffaux, S.; Cribiu, E.-P.; Pailhoux, E.; et al. High-throughput sequencing analyses of XX genital ridges lacking FOXL2 reveal DMRT1 up-regulation before SOX9 expression during the sex-reversal process in goats. Biol. Reprod. 2014, 91, 153. [Google Scholar] [CrossRef] [PubMed]
- Calvari, V.; Bertini, V.; De Grandi, A.; Peverali, G.; Zuffardi, O.; Ferguson-Smith, M.; Knudtzon, J.; Camerino, G.; Borsani, G.; Guioli, S. A new submicroscopic deletion that refines the 9p region for sex reversal. Genomics 2000, 65, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Quinonez, S.C.; Park, J.M.; Rabah, R.; Owens, K.M.; Yashar, B.M.; Glover, T.W.; Keegan, C.E. 9p partial monosomy and disorders of sex development: Review and postulation of a pathogenetic mechanism. Am. J. Med. Genet. A 2013, 161A, 1882–1896. [Google Scholar] [CrossRef]
- Fan, Y.; Zhang, X.; Wang, L.; Wang, R.; Huang, Z.; Sun, Y.; Yao, R.; Huang, X.; Ye, J.; Han, L.; et al. Diagnostic application of targeted next-generation sequencing of 80 genes associated with disorders of sexual development. Sci. Rep. 2017, 7, 44536. [Google Scholar] [CrossRef]
- Dewaele, A.; Dujardin, E.; André, M.; Albina, A.; Jammes, H.; Giton, F.; Sellem, E.; Jolivet, G.; Pailhoux, E.; Pannetier, M. Absence of Testicular Estrogen Leads to Defects in Spermatogenesis and Increased Semen Abnormalities in Male Rabbits. Genes 2022, 13, 2070. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.W.; Lee, J.K.; Rojo, S.; Gearhart, M.D.; Kurahashi, K.; Banerjee, S.; Loeuille, G.A.; Bashamboo, A.; McElreavey, K.; Zarkower, D.; et al. An ancient protein-DNA interaction underlying metazoan sex determination. Nat. Struct. Mol. Biol. 2015, 22, 442–451. [Google Scholar] [CrossRef]
| Custom NGS panel for DSDs (GRCh37/hg19) |
| AKR1C2 (NM_001354.4), AMH (NM_000479), AMHR2 (NM_020547.2), AR (NM_000044.2), ARX (NM_139058.2), ATRX (NM_000489.3), BMP15 (NM_005448.2), CBX2 (NM_005189.2), CYB5A (NM_001914.3), CYP11A1 (NM_000781.2), CYP11B1 (NM_000497.3), CYP17A1 (NM_000102), CYP19A1 (NM_031226.2), DHH (NM_021044.2), DHX37 (NM_032656.3), DMRT1 (NM_021951.2), GATA4 (NM_002052.3), HSD17B3 (NM_000197), HSD3B2 (NM_000198.3), LHCGR (NM_000233.3), MAMLD1 (NM_005491.3), MAP3K1 (NM_005921.1), NR0B1 (NM_000475), NR2F2(NM_021005), NR5A1 (NM_004959), POR (NM_000941.2), PPP1R12A (NM_002480.3), RSPO1 (NM_001038633.3), SOX9 (NM_000346), SRD5A2 (NM_000348), SRY (NM_003140), STAR (NM_000349), WNT4 (NM_030761.4), WT1 (NM_024426.4), ZFPM2 (NM_012082.3). |
| Custom CGH-array for DSDs (GRCh37/hg19) |
| AKR1C2, AKR1C4, AMH, AMHR2, AR, BMP15, CBX2, CTNNB1 β-catenin, CYP11A1, CYP11B1, CYP17A1, CY19A1, CYP21A2, DHH, DMRT1, DMRT2, GATA4, FGF9, FGFR2, FOXL2, HHAT, HSD17B3, HSD3B2, LHCGR, MAMLD1, MAP3K1, NR0B1 (DAX1), NR5A1 (SF1), PGD2, POR, RSPO1, SOX3, SOX9, SOX10, SRD5A2, SRY, STAR, WNT4, WT1, WWOX, ZFPM2 (FOG2). |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bertini, V.; Baldinotti, F.; Parma, P.; Tyutyusheva, N.; Sepich, M.; Bertolucci, G.; Rosano, C.; Caligo, M.A.; Peroni, D.; Valetto, A.; et al. In Tandem Intragenic Duplication of Doublesex and Mab-3-Related Transcription Factor 1 (DMRT1) in an SRY-Negative Boy with a 46,XX Disorder of Sex Development. Genes 2023, 14, 2067. https://doi.org/10.3390/genes14112067
Bertini V, Baldinotti F, Parma P, Tyutyusheva N, Sepich M, Bertolucci G, Rosano C, Caligo MA, Peroni D, Valetto A, et al. In Tandem Intragenic Duplication of Doublesex and Mab-3-Related Transcription Factor 1 (DMRT1) in an SRY-Negative Boy with a 46,XX Disorder of Sex Development. Genes. 2023; 14(11):2067. https://doi.org/10.3390/genes14112067
Chicago/Turabian StyleBertini, Veronica, Fulvia Baldinotti, Pietro Parma, Nina Tyutyusheva, Margherita Sepich, Giulia Bertolucci, Camillo Rosano, Maria Adelaide Caligo, Diego Peroni, Angelo Valetto, and et al. 2023. "In Tandem Intragenic Duplication of Doublesex and Mab-3-Related Transcription Factor 1 (DMRT1) in an SRY-Negative Boy with a 46,XX Disorder of Sex Development" Genes 14, no. 11: 2067. https://doi.org/10.3390/genes14112067







