RNA-Sequencing Reveals the Involvement of Sesquiterpene Biosynthesis Genes and Transcription Factors during an Early Response to Mechanical Wounding of Aquilaria sinensis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and RNA Extraction
2.2. Transcriptome Profiling of the Wounded and Healthy Xylem Tissues from A. sinensis
2.3. Correlation Networks
2.4. qRT-PCR Analysis
3. Results
3.1. Global Analysis of Transcriptome of A. sinensis
3.2. Functional Enrichment Analysis of DEGs
3.3. DEGs Involved in Hormone Signal Transduction
3.4. Potential Genes Involved in 2-(2-Phenylethyl)chromone Biosynthesis
3.5. DEGs Involved in Sesquiterpene Biosynthesis
3.6. Transcription Factors Mediated Regulatory Networks Involved in Sesquiterpene Biosynthesis
3.7. RNA-Seq Verification by qRT-PCR
4. Discussion
4.1. A. sinensis Transcriptome Sequencing
4.2. Jasmonic Acid and Salicylic Acid Have Potential Regulatory Roles in Agarwood Formation
4.3. Key Genes Associated with 2-(2-Phenylethyl)chromone Biosynthesis in A. sinensis
4.4. Key Genes Associated with Sesquiterpene Biosynthesis in A. sinensis
4.5. A Transcriptomic Network Underlying the Regulation of Sesquiterpene Biosynthesis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Edreva, A.; Velikova, V.; Tsonev, T.; Dagnon, S.; Gürel, A.; Aktaş, L.; Gesheva, E. Stress-protective role of secondary metabolites: Diversity of functions and mechanisms. Gen. Appl. Plant Physiol. 2008, 34, 67–78. [Google Scholar]
- Davies, K.M.; Deroles, S.C. Prospects for the use of plant cell cultures in food biotechnology. Curr. Opin. Biotechnol. 2014, 26, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, R.; Jong, P.L.; Kamziah, A.K. Fungal inoculation induces agarwood in young Aquilaria malaccensis trees in the nursery. J. Forestry Res. 2014, 25, 201–204. [Google Scholar] [CrossRef]
- Wang, S.; Yu, Z.; Wang, C.; Wu, C.; Guo, P.; Wei, J. Chemical Constituents and Pharmacological Activity of Agarwood and Aquilaria Plants. Molecules 2018, 23, 342. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.Q.; Wei, J.H.; Yang, J.S.; Zhang, Z.; Yang, Y.; Gao, Z.H.; Sui, C.; Gong, B. Chemical constituents of agarwood originating from the endemic genus Aquilaria plants. Chem. Biodivers. 2012, 9, 236–250. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, H.; Yang, Y.; Zhang, Z.; Wei, J.; Meng, H.; Chen, W.; Feng, J.; Gan, B.; Chen, X.; et al. Whole-tree Agarwood-Inducing Technique: An Efficient Novel Technique for Producing High-Quality Agarwood in Cultivated Aquilaria sinensis Trees. Molecules 2013, 18, 3086–3106. [Google Scholar] [CrossRef]
- Peng, C.S.; Osman, M.F.; Bahari, N.; Zakaria, R.; Rahim, K.A. Agarwood inducement technology: A method for producing oil grade agarwood in cultivated Aquilaria malaccensis Lamk. J. Agrobiotechnol. 2015, 6, 1–16. [Google Scholar]
- Ye, W.; Wu, H.; He, X.; Wang, L.; Zhang, W.; Li, H.; Fan, Y.; Tan, G.; Liu, T.; Gao, X. Transcriptome Sequencing of Chemically Induced Aquilaria sinensis to Identify Genes Related to Agarwood Formation. PLoS ONE 2016, 11, e0155505. [Google Scholar] [CrossRef]
- Chhipa, H.; Chowdhary, K.; Kaushik, N. Artificial production of agarwood oil in Aquilaria sp. by fungi: A review. Phytochem. Rev. 2017, 16, 835–860. [Google Scholar] [CrossRef]
- Liu, J.; Xu, Y.; Zhang, Z.; Wei, J. Hydrogen peroxide promotes programmed cell death and salicylic acid accumulation during the induced production of sesquiterpenes in cultured cell suspensions of Aquilaria sinensis. Funct. Plant Biol. 2015, 42, 337–346. [Google Scholar] [CrossRef]
- Liu, P.; Zhang, Y.; Yang, Y.; Lv, F.; Wei, J. Programmed cell death might involve in progress of wounding induced agarwood formation in stems of Aquilaria sinensis. Microsc. Res. Tech. 2022, 85, 2904–2912. [Google Scholar] [CrossRef]
- Liu, P.; Zhang, X.; Yang, Y.; Sui, C.; Xu, Y.; Wei, J. Interxylary phloem and xylem rays are the structural foundation of agarwood resin formation in the stems of Aquilaria sinensis. Trees 2019, 33, 533–542. [Google Scholar] [CrossRef]
- Liu, J.; Li, T.; Chen, T.; Gao, J.; Zhang, X.; Jiang, C.; Yang, J.; Zhou, J.; Wang, T.; Chi, X. Integrating Multiple Omics Identifies Phaeoacremonium rubrigenum Acting as Aquilaria sinensis Marker Fungus to Promote Agarwood Sesquiterpene Accumulation by Inducing Plant Host Phosphorylation. Microbiol. Spectr. 2022, 10, e02722-21. [Google Scholar] [CrossRef] [PubMed]
- Celedon, J.M.; Bohlmann, J. An extended model of heartwood secondary metabolism informed by functional genomics. Tree Physiol. 2017, 38, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Bergström, B. Chemical and structural changes during heartwood formation in Pinus sylvestris. Forestry 2003, 76, 45–53. [Google Scholar] [CrossRef]
- Lim, K.-J.; Paasela, T.; Harju, A.; Venäläinen, M.; Paulin, L.; Auvinen, P.; Kärkkäinen, K.; Teeri, T.H. Developmental Changes in Scots Pine Transcriptome during Heartwood Formation. Plant Physiol. 2016, 172, 1403–1417. [Google Scholar] [CrossRef]
- Chen, S.Y.; Yen, P.; Chang, T.; Chang, S.; Huang, S.; Yeh, T. Distribution of living ray parenchyma cells and major bioactive compounds during the heartwood formation of Taiwania cryptomerioides Hayata. J. Wood Chem. Technol. 2018, 38, 84–95. [Google Scholar] [CrossRef]
- Wang, X.; Gao, B.; Liu, X.; Dong, X.; Zhang, Z.; Fan, H.; Zhang, L.; Wang, J.; Shi, S.; Tu, P. Salinity stress induces the production of 2-(2-phenylethyl)chromones and regulates novel classes of responsive genes involved in signal transduction in Aquilaria sinensis calli. BMC Plant Biol. 2016, 16, 119. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, Z.; Wang, M.; Wei, J.; Chen, H.; Gao, Z.; Sui, C.; Luo, H.; Zhang, X.; Yang, Y. Identification of genes related to agarwood formation: Transcriptome analysis of healthy and wounded tissues of Aquilaria sinensis. BMC Genom. 2013, 14, 227. [Google Scholar] [CrossRef]
- Xu, Y.; Sun, P.; Tang, X.; Gao, Z.; Zhang, Z.; Wei, J. Genome-wide analysis of WRKY transcription factors in Aquilaria sinensis (Lour.) Gilg. Sci. Rep. 2020, 10, 3018. [Google Scholar] [CrossRef]
- Li, R.; Zhu, J.; Guo, D.; Li, H.; Wang, Y.; Ding, X.; Mei, W.; Chen, Z.; Dai, H.; Peng, S. Genome-wide identification and expression analysis of terpene synthase gene family in Aquilaria sinensis. Plant Physiol. Biochem. 2021, 164, 185–194. [Google Scholar] [CrossRef]
- Xiao, M.; Feng, Y.; Sun, P.; Xu, Y.; Rong, M.; Liu, Y.; Jiang, J.; Yu, C.; Gao, Z.; Wei, J. Genome-wide investigation and expression analysis of the AP2/ERF family for selection of agarwood-related genes in Aquilaria sinensis (Lour.) Gilg. Genome 2022, 65, 443–457. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Liao, Y.; Lv, F.; Zhang, Z.; Sun, P.; Gao, Z.; Hu, K.; Sui, C.; Jin, Y.; Wei, J. Transcription Factor AsMYC2 Controls the Jasmonate-Responsive Expression of ASS1 Regulating Sesquiterpene Biosynthesis in Aquilaria sinensis (Lour.) Gilg. Plant Cell Physiol. 2017, 58, 1924–1933. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, R.; Jong, P.L.; Nurul Irdayu, I. Succession patterns of fungi associated to wound-induced agarwood in wild Aquilaria malaccensis revealed from quantitative PCR assay. World J. Microbiol. Biotechnol. 2014, 30, 2427–2436. [Google Scholar] [CrossRef]
- Zhang, P.; Li, X.; Cui, Z.; Xu, D. Morphological, physiological, biochemical and molecular analyses reveal wounding-induced agarwood formation mechanism in two types of Aquilaria sinensis (Lour.) Spreng. Ind. Crops Prod. 2022, 178, 114603. [Google Scholar] [CrossRef]
- Ding, X.; Mei, W.; Lin, Q.; Wang, H.; Wang, J.; Peng, S.; Li, H.; Zhu, J.; Li, W.; Wang, P.; et al. Genome sequence of the agarwood tree Aquilaria sinensis (Lour.) Spreng: The first chromosome-level draft genome in the Thymelaeceae family. GigaScience 2020, 9, giaa013. [Google Scholar] [CrossRef]
- Kim, D.; Paggi, J.M.; Park, C.; Bennett, C.; Salzberg, S.L. Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat. Biotechnol. 2019, 37, 907–915. [Google Scholar] [CrossRef]
- Liao, Y.; Smyth, G.K.; Shi, W. featureCounts: An efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 2013, 30, 923–930. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B (Methodological) 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Yu, G.; Wang, L.; Han, Y.; He, Q. clusterProfiler: An R Package for Comparing Biological Themes Among Gene Clusters. OMICS J. Integr. Biol. 2012, 16, 284–287. [Google Scholar] [CrossRef] [PubMed]
- Shannon, P.T.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Wang, X. Application of the Gini Correlation Coefficient to Infer Regulatory Relationships in Transcriptome Analysis. Plant Physiol. 2012, 160, 192–203. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Božunović, J.; Skorić, M.; Matekalo, D.; Živković, S.; Dragićević, M.; Aničić, N.; Filipović, B.; Banjanac, T.; Šiler, B.; Mišić, D. Secoiridoids Metabolism Response to Wounding in Common Centaury (Centaurium erythraea Rafn) Leaves. Plants 2019, 8, 589. [Google Scholar] [CrossRef]
- Wu, Z.; Liu, W.; Li, J.; Yu, L.; Lin, L. Dynamic analysis of gene expression and determination of chemicals in agarwood in Aquilaria sinensis. J. Forestry Res. 2020, 31, 1833–1841. [Google Scholar] [CrossRef]
- Kang, J.-N.; Lee, W.-H.; Won, S.Y.; Chang, S.; Hong, J.-P.; Oh, T.-J.; Lee, S.M.; Kang, S.-H. Systemic Expression of Genes Involved in the Plant Defense Response Induced by Wounding in Senna tora. Int. J. Mol. Sci. 2021, 22, 10073. [Google Scholar] [CrossRef] [PubMed]
- Dixon, R.A.; Paiva, N.L. Stress-Induced Phenylpropanoid Metabolism. Plant Cell 1995, 7, 1085–1097. [Google Scholar] [CrossRef]
- Petrussa, E.; Braidot, E.; Zancani, M.; Peresson, C.; Bertolini, A.; Patui, S.; Vianello, A. Plant Flavonoids—Biosynthesis, Transport and Involvement in Stress Responses. Int. J. Mol. Sci. 2013, 14, 14950–14973. [Google Scholar] [CrossRef] [PubMed]
- Chapman, J.M.; Muhlemann, J.K.; Gayomba, S.R.; Muday, G.K. RBOH-Dependent ROS Synthesis and ROS Scavenging by Plant Specialized Metabolites To Modulate Plant Development and Stress Responses. Chem. Res. Toxicol. 2019, 32, 370–396. [Google Scholar] [CrossRef] [PubMed]
- Isah, T. Stress and defense responses in plant secondary metabolites production. Biol. Res. 2019, 52, 39. [Google Scholar] [CrossRef] [PubMed]
- Zhan, X.; Chen, Z.; Chen, R.; Shen, C. Environmental and Genetic Factors Involved in Plant Protection-Associated Secondary Metabolite Biosynthesis Pathways. Front. Plant Sci. 2022, 13, 877304. [Google Scholar] [CrossRef] [PubMed]
- Bilgin, D.D.; Zavala, J.A.; Zhu, J.; Clough, S.J.; Ort, D.R.; Delucia, E.H. Biotic stress globally downregulates photosynthesis genes. Plant, Cell Environ. 2010, 33, 1597–1613. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-M.; Huang, G.-Q.; Li, Y.; Zheng, Y.; Li, X.-B. Cotton photosynthesis-related PSAK1 protein is involved in plant response to aphid attack. Mol. Biol. Rep. 2014, 41, 3191–3200. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, Z.; Fang, S.; Liu, Y.; Shang, X. Metabolome and Transcriptome Analyses Unravel the Molecular Regulatory Mechanisms Involved in Photosynthesis of Cyclocarya paliurus under Salt Stress. Int. J. Mol. Sci. 2022, 23, 1161. [Google Scholar] [CrossRef]
- Santner, A.; Calderon-Villalobos, L.I.A.; Estelle, M. Plant hormones are versatile chemical regulators of plant growth. Nat. Chem. Biol. 2009, 5, 301–307. [Google Scholar] [CrossRef]
- Peleg, Z.; Blumwald, E. Hormone balance and abiotic stress tolerance in crop plants. Curr. Opin. Plant Biol. 2011, 14, 290–295. [Google Scholar] [CrossRef]
- Fahad, S.; Hussain, S.; Bano, A.; Saud, S.; Hassan, S.; Shan, D.; Khan, F.A.; Khan, F.; Chen, Y.; Wu, C.; et al. Potential role of phytohormones and plant growth-promoting rhizobacteria in abiotic stresses: Consequences for changing environment. Environ. Sci. Pollut. Res. 2015, 22, 4907–4921. [Google Scholar] [CrossRef]
- Huang, J.; Reichelt, M.; Chowdhury, S.; Hammerbacher, A.; Hartmann, H. Increasing carbon availability stimulates growth and secondary metabolites via modulation of phytohormones in winter wheat. J. Exp. Bot. 2017, 68, 1251–1263. [Google Scholar] [CrossRef]
- Lv, F.; Li, S.; Feng, J.; Liu, P.; Gao, Z.; Yang, Y.; Xu, Y.; Wei, J. Hydrogen peroxide burst triggers accumulation of jasmonates and salicylic acid inducing sesquiterpene biosynthesis in wounded Aquilaria sinesis. J. Plant Physiol. 2019, 234-235, 167–175. [Google Scholar] [CrossRef]
- Sun, P.-W.; Xu, Y.-H.; Yu, C.-C.; Lv, F.-F.; Tang, X.-L.; Gao, Z.-H.; Zhang, Z.; Wang, H.; Liu, Y.; Wei, J.-H. WRKY44 represses expression of the wound-induced sesquiterpene biosynthetic gene ASS1 in Aquilaria sinensis. J. Exp. Bot. 2019, 71, 1128–1138. [Google Scholar] [CrossRef]
- Chini, A.; Fonseca, S.; Fernández, G.; Adie, B.; Chico, J.M.; Lorenzo, O.; García-Casado, G.; López-Vidriero, I.; Lozano, F.M.; Ponce, M.R.; et al. The JAZ family of repressors is the missing link in jasmonate signalling. Nature 2007, 448, 666–671. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Wei, J.; Xu, Y.; Zhang, Z. Cloning, expression and characterization of COI1 gene (AsCOI1) from Aquilaria sinensis (Lour.) Gilg. Acta Pharm. Sin. B 2015, 5, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Bari, R.; Jones, J.D.G. Role of plant hormones in plant defence responses. Plant Mol. Biol. 2009, 69, 473–488. [Google Scholar] [CrossRef]
- Verma, V.; Ravindran, P.; Kumar, P.P. Plant hormone-mediated regulation of stress responses. BMC Plant Biol. 2016, 16, 86. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Duan, G.; Li, C.; Liu, L.; Han, G.; Zhang, Y.; Wang, C. The Crosstalks Between Jasmonic Acid and Other Plant Hormone Signaling Highlight the Involvement of Jasmonic Acid as a Core Component in Plant Response to Biotic and Abiotic Stresses. Front. Plant Sci. 2019, 10, 1349. [Google Scholar] [CrossRef]
- Liu, J.; Yang, J.; Jiang, C.; Zhou, J.; Zhao, Y.; Huang, L. Volatile organic compound and endogenous phytohormone characteristics during callus browning in Aquilaria sinensis. Ind. Crops Prod. 2021, 168, 113605. [Google Scholar] [CrossRef]
- Bisht, R.; Bhattacharyya, A.; Shrivastava, A.; Saxena, P. An Overview of the Medicinally Important Plant Type III PKS Derived Polyketides. Front. Plant Sci. 2021, 12, 2155. [Google Scholar] [CrossRef]
- Liao, G.; Dong, W.-H.; Yang, J.-L.; Li, W.; Wang, J.; Mei, W.-L.; Dai, H.-F. Monitoring the Chemical Profile in Agarwood Formation within One Year and Speculating on the Biosynthesis of 2-(2-Phenylethyl)Chromones. Molecules 2018, 23, 1261. [Google Scholar] [CrossRef]
- Wang, X.-H.; Gao, B.-W.; Nakashima, Y.; Mori, T.; Zhang, Z.-X.; Kodama, T.; Lee, Y.-E.; Zhang, Z.-K.; Wong, C.-P.; Liu, Q.-Q.; et al. Identification of a diarylpentanoid-producing polyketide synthase revealing an unusual biosynthetic pathway of 2-(2-phenylethyl)chromones in agarwood. Nat. Commun. 2022, 13, 348. [Google Scholar] [CrossRef]
- Vranová, E.; Coman, D.; Gruissem, W. Network Analysis of the MVA and MEP Pathways for Isoprenoid Synthesis. Annu. Rev. Plant Biol. 2013, 64, 665–700. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Concepción, M. Early Steps in Isoprenoid Biosynthesis: Multilevel Regulation of the Supply of Common Precursors in Plant Cells. Phytochem. Rev. 2006, 5, 1–15. [Google Scholar] [CrossRef]
- Bick, J.A.; Lange, B.M. Metabolic cross talk between cytosolic and plastidial pathways of isoprenoid biosynthesis: Unidirectional transport of intermediates across the chloroplast envelope membrane. Arch. Biochem. Biophys. 2003, 415, 146–154. [Google Scholar] [CrossRef]
- Bartram, S.; Jux, A.; Gleixner, G.; Boland, W. Dynamic pathway allocation in early terpenoid biosynthesis of stress-induced lima bean leaves. Phytochemistry 2006, 67, 1661–1672. [Google Scholar] [CrossRef]
- Mendoza-Poudereux, I.; Kutzner, E.; Huber, C.; Segura, J.; Eisenreich, W.; Arrillaga, I. Metabolic cross-talk between pathways of terpenoid backbone biosynthesis in spike lavender. Plant Physiol. Biochem. 2015, 95, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Wei, Q.; Liu, Y.; Wei, X.; Chen, X.; Yin, X.; Xie, T. Transcriptome sequencing and functional characterization of new sesquiterpene synthases from Curcuma wenyujin. Arch. Biochem. Biophys. 2021, 709, 108986. [Google Scholar] [CrossRef]
- Meng, F.; Chu, T.; Tang, Q.; Chen, W. A tetraploidization event shaped the Aquilaria sinensis genome and contributed to the ability of sesquiterpenes synthesis. BMC Genom. 2021, 22, 647. [Google Scholar] [CrossRef]
- Meraj, T.A.; Fu, J.; Raza, M.A.; Zhu, C.; Shen, Q.; Xu, D.; Wang, Q. Transcriptional Factors Regulate Plant Stress Responses Through Mediating Secondary Metabolism. Genes 2020, 11, 346. [Google Scholar] [CrossRef]
- Zhang, F.; Fu, X.; Lv, Z.; Lu, X.; Shen, Q.; Zhang, L.; Zhu, M.; Wang, G.; Sun, X.; Liao, Z.; et al. A Basic Leucine Zipper Transcription Factor, AabZIP1, Connects Abscisic Acid Signaling with Artemisinin Biosynthesis in Artemisia annua. Mol. Plant 2015, 8, 163–175. [Google Scholar] [CrossRef]
- Shen, S.-l.; Yin, X.-r.; Zhang, B.; Xie, X.-l.; Jiang, Q.; Grierson, D.; Chen, K.-s. CitAP2.10 activation of the terpene synthase CsTPS1 is associated with the synthesis of (+)-valencene in ‘Newhall’ orange. J. Exp. Bot. 2016, 67, 4105–4115. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.-H.; Wang, J.-W.; Wang, S.; Wang, J.-Y.; Chen, X.-Y. Characterization of GaWRKY1, a Cotton Transcription Factor That Regulates the Sesquiterpene Synthase Gene (+)-δ-Cadinene Synthase-A. Plant Physiol. 2004, 135, 507–515. [Google Scholar] [CrossRef]
- Hong, G.-J.; Xue, X.-Y.; Mao, Y.-B.; Wang, L.-J.; Chen, X.-Y. Arabidopsis MYC2 Interacts with DELLA Proteins in Regulating Sesquiterpene Synthase Gene Expression. Plant Cell 2012, 24, 2635–2648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van der Fits, L.; Memelink, J. ORCA3, a Jasmonate-Responsive Transcriptional Regulator of Plant Primary and Secondary Metabolism. Science 2000, 289, 295–297. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.-X.; Li, J.-X.; Yang, C.-Q.; Hu, W.-L.; Wang, L.-J.; Chen, X.-Y. The Jasmonate-Responsive AP2/ERF Transcription Factors AaERF1 and AaERF2 Positively Regulate Artemisinin Biosynthesis in Artemisia annua L. Mol. Plant 2012, 5, 353–365. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Gou, J.; Chen, F.; Li, C.; Zhang, Y. Comparative Transcriptome Analysis Identifies Putative Genes Involved in the Biosynthesis of Xanthanolides in Xanthium strumarium L. Front. Plant Sci. 2016, 7, 1317. [Google Scholar] [CrossRef]
- Xu, J.; Wu, S.-R.; Xu, Y.-H.; Ge, Z.-Y.; Sui, C.; Wei, J.-H. Overexpression of BcbZIP134 negatively regulates the biosynthesis of saikosaponins. Plant Cell Tiss. Org. Cult. 2019, 137, 297–308. [Google Scholar] [CrossRef]
- Yang, Z.; Xie, C.; Huang, Y.; An, W.; Liu, S.; Huang, S.; Zheng, X. Metabolism and transcriptome profiling provides insight into the genes and transcription factors involved in monoterpene biosynthesis of borneol chemotype of Cinnamomum camphora induced by mechanical damage. PeerJ 2021, 9, e11465. [Google Scholar] [CrossRef] [PubMed]
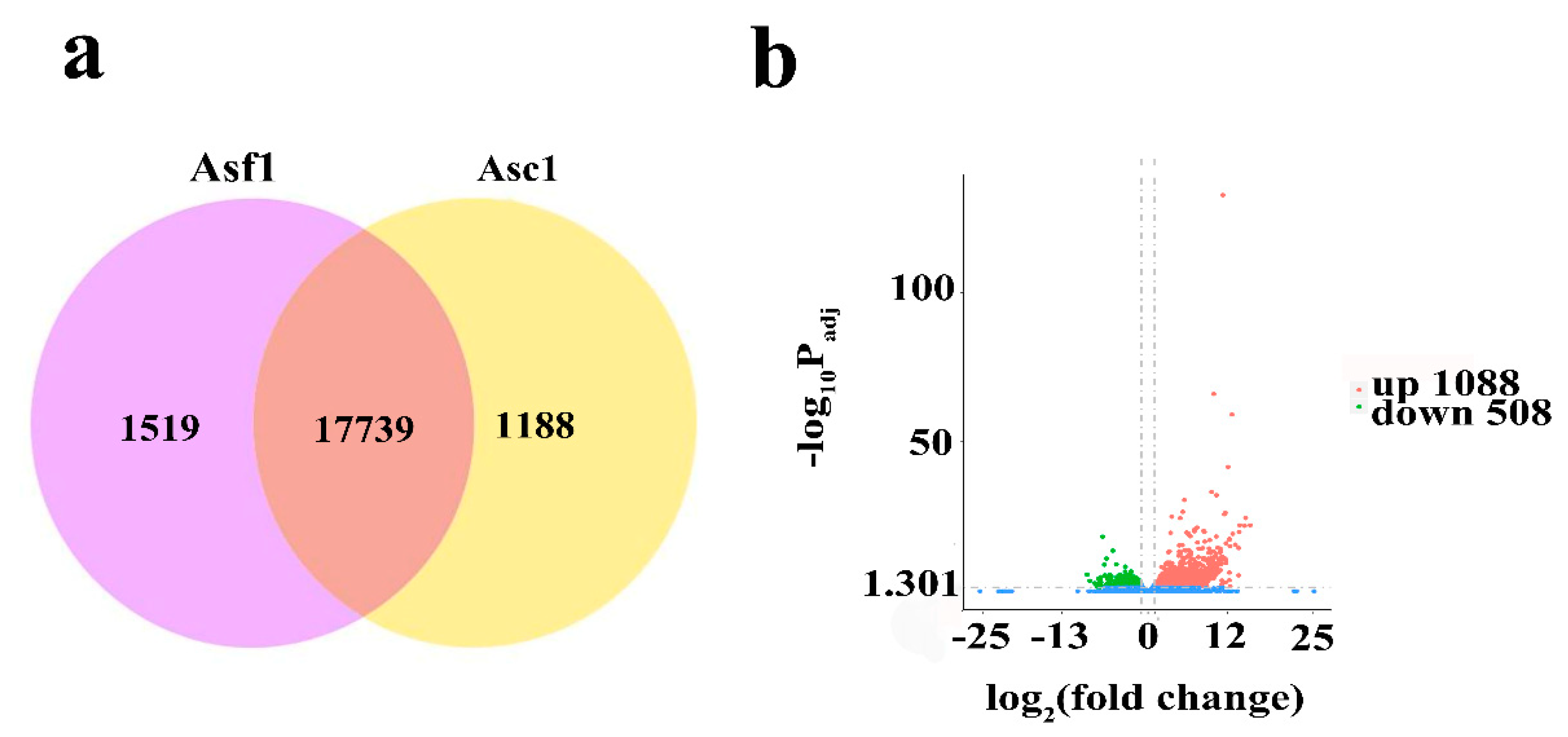
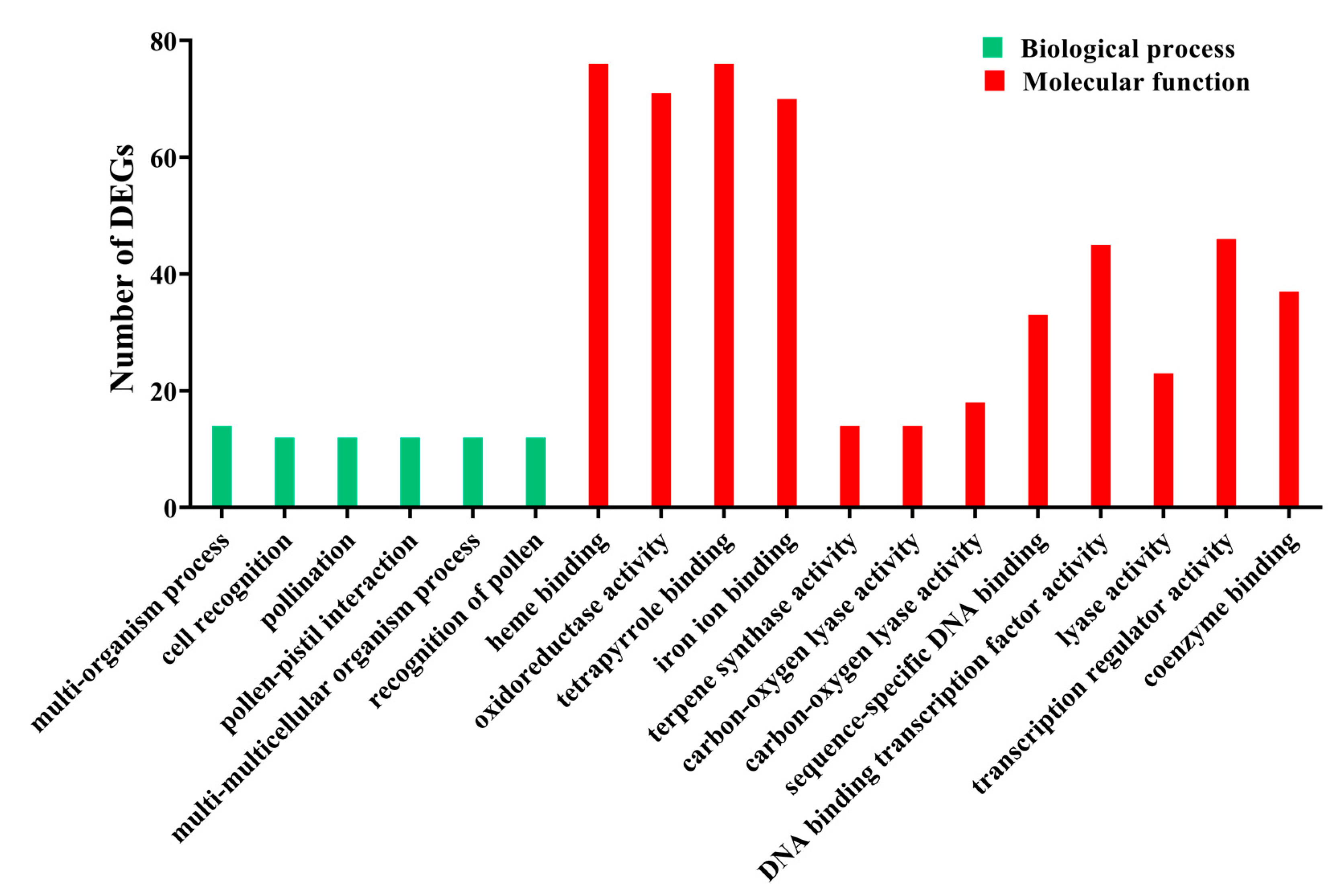
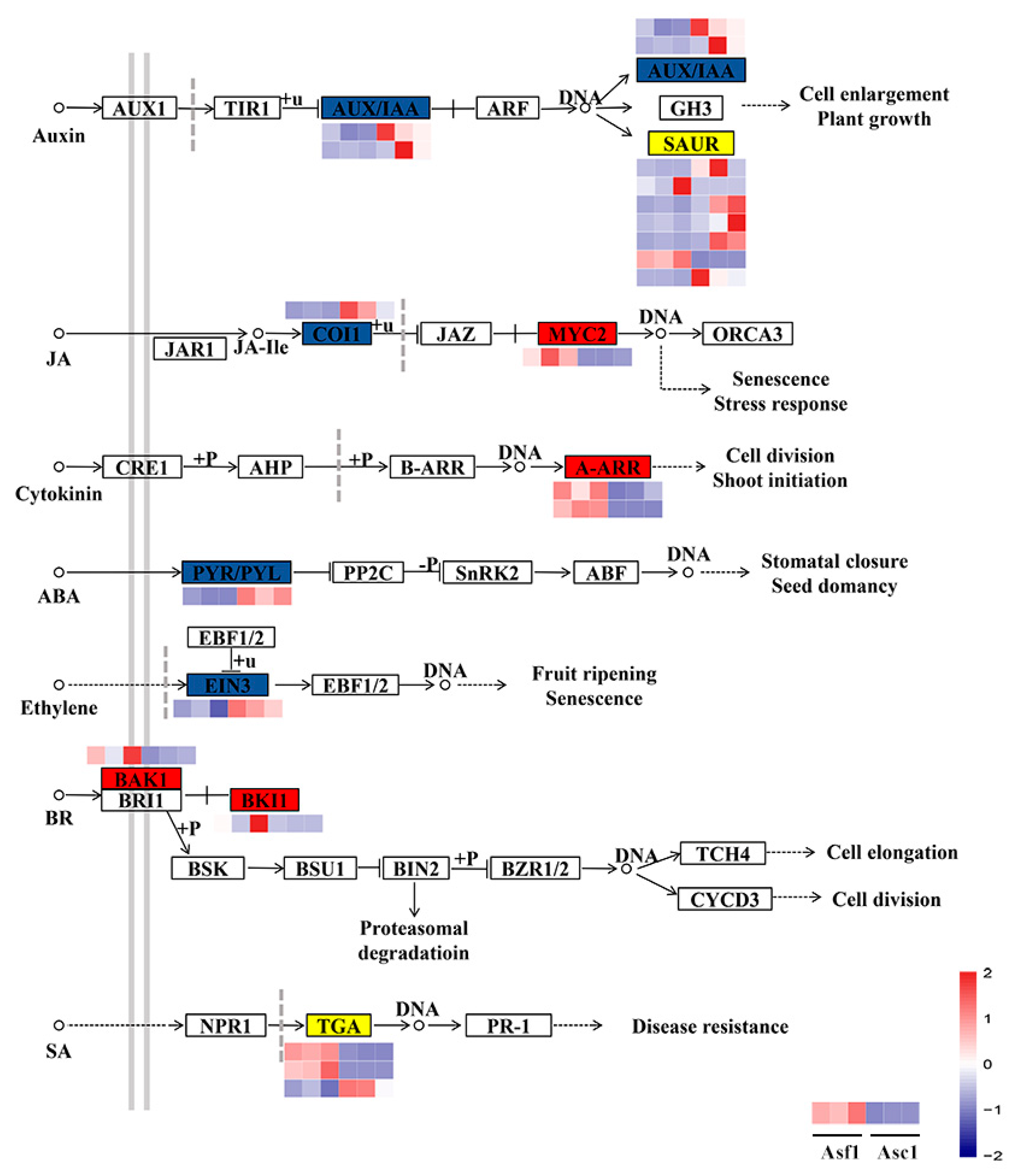

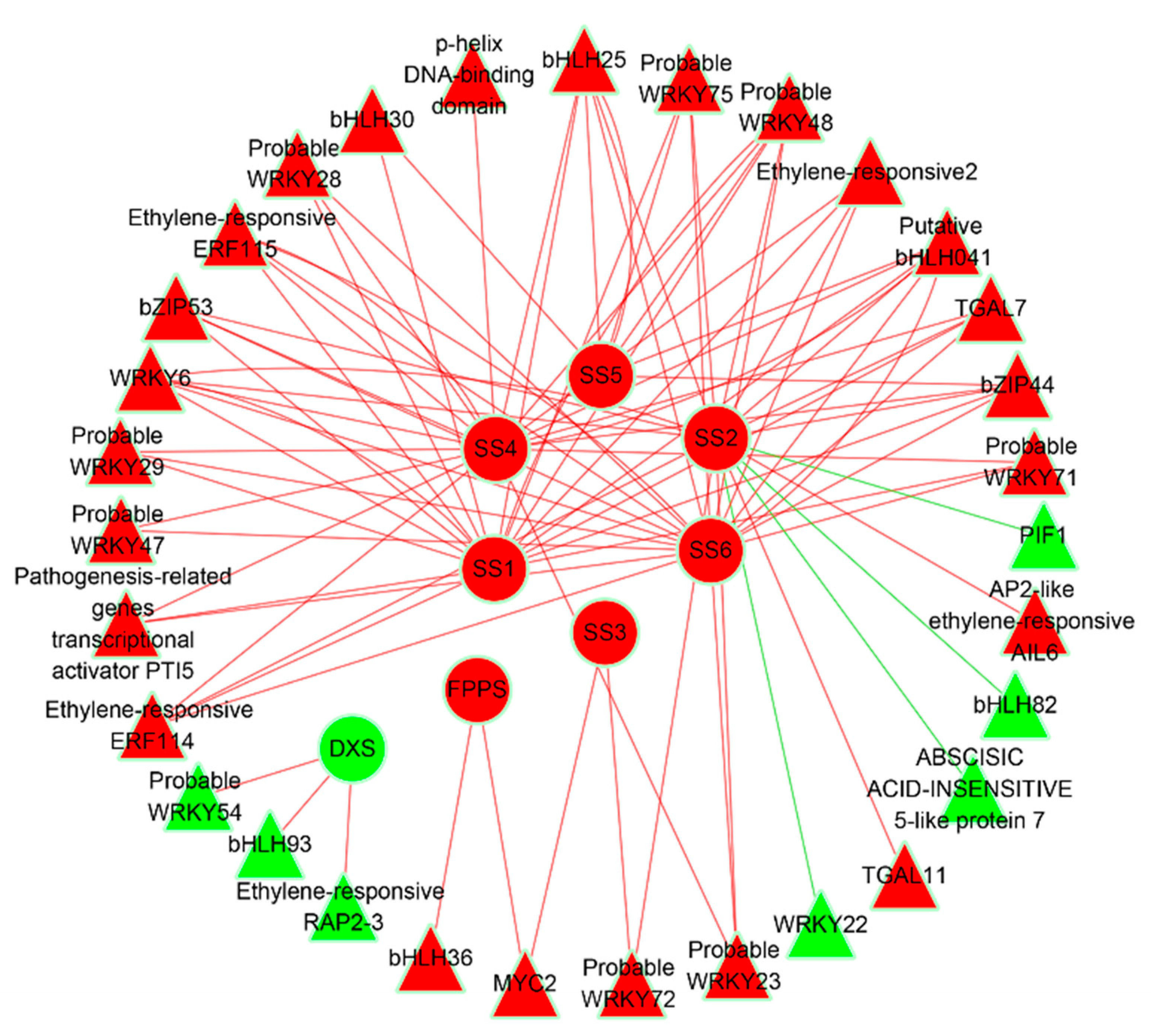
| Sample | Raw Reads | Clean Reads | Mapped to Genome | Q20 (%) | Q30 (%) | GC (%) |
|---|---|---|---|---|---|---|
| Asc11 | 48,158,840 | 45,845,016 | 41,524,528 (90.58%) | 98.33 | 94.85 | 46.96 |
| Asc12 | 49,229,480 | 48,487,386 | 44,432,205 (91.64%) | 97.59 | 93.02 | 46.49 |
| Asc13 | 55,055,938 | 52,975,166 | 48,697,477 (91.93%) | 98.20 | 94.51 | 46.32 |
| Asf11 | 46,747,414 | 43,317,726 | 38,986,805 (90.00%) | 98.34 | 94.86 | 46.17 |
| Asf12 | 50,747,240 | 47,754,238 | 43,633,605 (91.37%) | 98.10 | 94.27 | 46.50 |
| Asf13 | 45,935,934 | 44,470,980 | 36,366,124(81.77%) | 97.70 | 93.60 | 47.84 |
| KEGG_ID | Pathway Name | Number | Up | Down |
|---|---|---|---|---|
| pop00941 | Flavonoid biosynthesis | 14 | 14 | 0 |
| pop00940 | Phenylpropanoid biosynthesis | 24 | 24 | 0 |
| pop00480 | Glutathione metabolism | 12 | 12 | 0 |
| pop00909 | Sesquiterpenoid and triterpenoid biosynthesis | 6 | 6 | 0 |
| pop00400 | Phenylalanine, tyrosine and tryptophan biosynthesis | 8 | 8 | 0 |
| pop00360 | Phenylalanine metabolism | 7 | 6 | 1 |
| pop00195 | Photosynthesis | 9 | 0 | 9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, J.; Du, R.; Wang, Y.; Chen, J. RNA-Sequencing Reveals the Involvement of Sesquiterpene Biosynthesis Genes and Transcription Factors during an Early Response to Mechanical Wounding of Aquilaria sinensis. Genes 2023, 14, 464. https://doi.org/10.3390/genes14020464
Xu J, Du R, Wang Y, Chen J. RNA-Sequencing Reveals the Involvement of Sesquiterpene Biosynthesis Genes and Transcription Factors during an Early Response to Mechanical Wounding of Aquilaria sinensis. Genes. 2023; 14(2):464. https://doi.org/10.3390/genes14020464
Chicago/Turabian StyleXu, Jieru, Ruyue Du, Yue Wang, and Jinhui Chen. 2023. "RNA-Sequencing Reveals the Involvement of Sesquiterpene Biosynthesis Genes and Transcription Factors during an Early Response to Mechanical Wounding of Aquilaria sinensis" Genes 14, no. 2: 464. https://doi.org/10.3390/genes14020464
APA StyleXu, J., Du, R., Wang, Y., & Chen, J. (2023). RNA-Sequencing Reveals the Involvement of Sesquiterpene Biosynthesis Genes and Transcription Factors during an Early Response to Mechanical Wounding of Aquilaria sinensis. Genes, 14(2), 464. https://doi.org/10.3390/genes14020464






