The Role of miRNA-221 and miRNA-34a in Non-Melanoma Skin Cancer of the Head and Neck Region
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples Collection of NMSC Patients
2.2. RNA Isolation and Extraction
2.3. cDNA Synthesis and Quantitative Real Time RT-PCR
2.4. Statistical Analyses
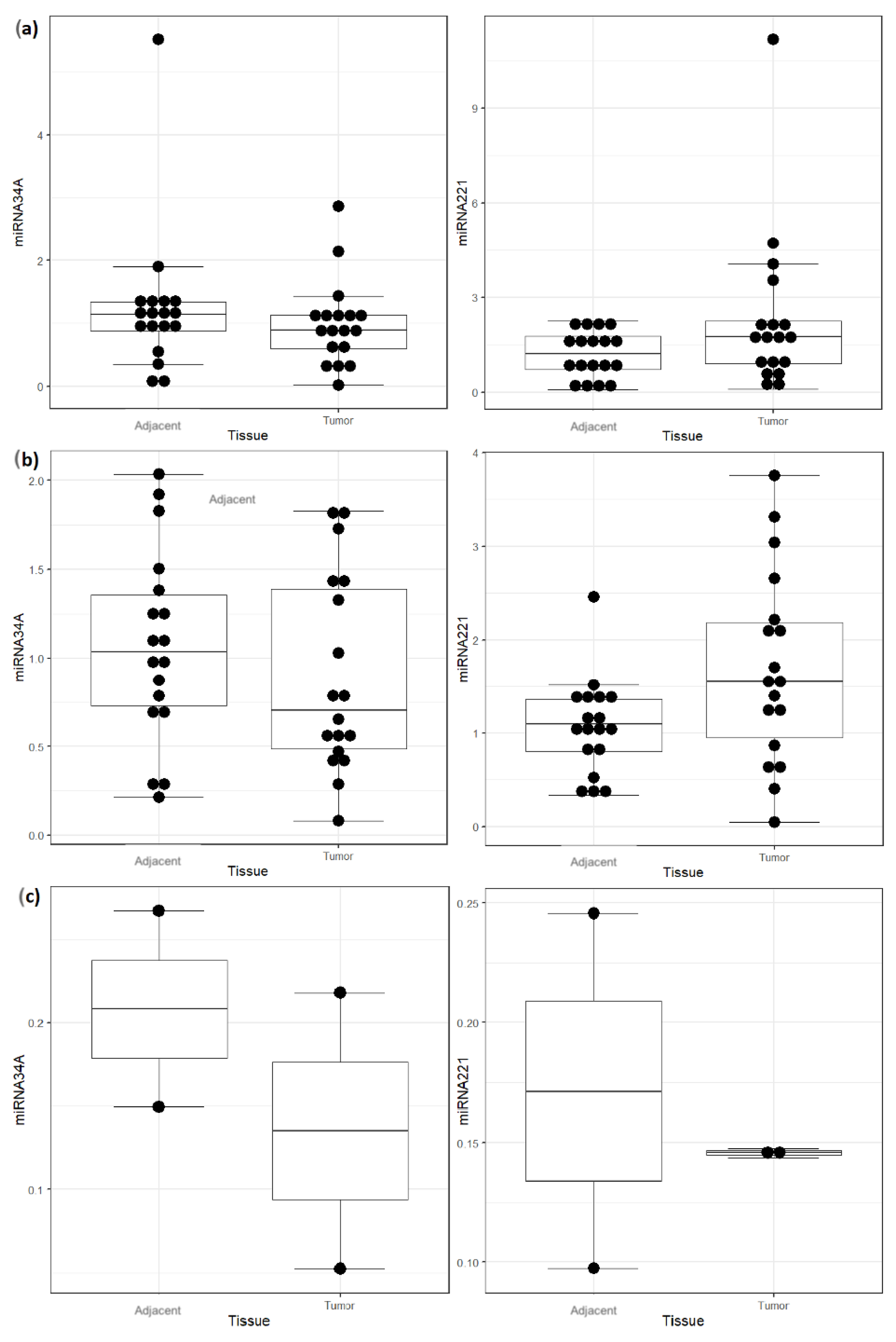
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, C.; Athar, M. Ionizing Radiation Exposure and Basal Cell Carcinoma Pathogenesis. Radiat. Res. 2016, 185, 217–228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamas, T.; Baciut, M.; Nutu, A.; Bran, S.; Armencea, G.; Stoia, S.; Manea, A.; Crisan, L.; Opris, H.; Onisor, F.; et al. Is miRNA Regulation the Key to Controlling Non-Melanoma Skin Cancer Evolution? Genes 2021, 12, 1929. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, M.P.; Sinha, R.; Mukhtar, M.S.; Athar, M. Epigenetic regulation in the pathogenesis of non-melanoma skin cancer. Semin. Cancer Biol. 2020, in press. [CrossRef] [PubMed]
- Moodycliffe, A.M.; Nghiem, D.; Clydesdale, G.; Ullrich, S.E. Immune suppression and skin cancer development: Regulation by NKT cells. Nat. Immunol. 2000, 1, 521–525. [Google Scholar] [CrossRef] [PubMed]
- Di Meglio, P.; Perera, G.K.; Nestle, F.O. The multitasking organ: Recent insights into skin immune function. Immunity 2011, 23, 857–869. [Google Scholar] [CrossRef] [Green Version]
- Gordon, R. Skin Cancer: An Overview of Epidemiology and Risk Factors. Semin. Oncol. Nurs. 2013, 29, 160–169. [Google Scholar] [CrossRef]
- D’Errico, M.; Calcagnile, A.; Canzona, F.; Didona, B.; Posteraro, P.; Cavalieri, R.; Corona, R.; Vorechovsky, I.; Nardo, T.; Stefanini, M.; et al. UV mutation signature in tumor suppressor genes involved in skin carcinogenesis in xeroderma pigmentosum patients. Oncogene 2000, 19, 463–467. [Google Scholar] [CrossRef] [Green Version]
- Agrawal, R.; Woodfolk, J.A. Skin barrier defects in atopic dermatitis. Curr. Allergy Asthma Rep. 2014, 14, 433. [Google Scholar] [CrossRef] [Green Version]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Glass, A.G.; Hoover, R.N. The emerging epidemic of melanoma and squamous cell skin cancer. JAMA 1989, 262, 2097–2100. [Google Scholar] [CrossRef]
- Dubas, L.E.; Ingraffea, A. Nonmelanoma skin cancer. Facial. Plast. Surg. Clin. N. Am. 2013, 21, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Parekh, V.; Seykora, J.T. Cutaneous Squamous Cell Carcinoma. Clin. Lab. Med. 2017, 37, 503–525. [Google Scholar] [CrossRef] [PubMed]
- Green, A.C.; Olsen, C.M. Cutaneous squamous cell carcinoma: An epidemiological review. Br. J. Dermatol. 2017, 177, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Bernardo, B.C.; Ooi, J.Y.; Lin, R.C.; McMullen, J.R. miRNA therapeutics: A new class of drugs with potential therapeutic applications in the heart. Future Med. Chem. 2015, 7, 1771–1792. [Google Scholar] [CrossRef]
- Di Leva, G.; Garofalo, M.; Croce, C.M. MicroRNAs in cancer. Annual review of pathology. Annu. Rev. 2014, 9, 287–314. [Google Scholar] [CrossRef] [Green Version]
- Sand, M.; Gambichler, T.; Sand, D.; Skrygan, M.; Altmeyer, P.; Bechara, F.G. MicroRNAs and the skin: Tiny players in the body’s largest organ. J. Dermatol. Sci. 2009, 53, 169–175. [Google Scholar] [CrossRef]
- Sand, M.; Skrygan, M.; Sand, D.; Georgas, D.; Hahn, S.A.; Gambichler, T.; Altmeyer, P.; Bechara, F.G. Expression of microRNAs in basal cell carcinoma. Br. J. Dermatol. 2012, 167, 847–855. [Google Scholar] [CrossRef]
- Sand, M.; Sand, D.; Altmeyer, P.; Bechara, F.G. MicroRNA in non-melanoma skin cancer. Cancer Biomark. 2012, 11, 253–257. [Google Scholar] [CrossRef]
- Han, C.; Seebacher, N.A.; Hornicek, F.J.; Kan, Q.; Duan, Z. Regulation of microRNAs function by circular RNAs in human cancer. Oncotarget 2017, 8, 64622–64637. [Google Scholar] [CrossRef] [Green Version]
- Tian, J.; Shen, R.; Yan, Y.; Deng, L. miRNA-186 promotes tumor growth in cutaneous squamous cell carcinoma by inhibiting apoptotic protease activating factor-1. Exp. Ther. Med. 2018, 16, 4010–4018. [Google Scholar]
- Self-Fordham, J.B.; Naqvi, A.R.; Uttamani, J.R.; Kulkarni, V.; Nares, S. MicroRNA: Dynamic Regulators of Macrophage Polarization and Plasticity. Front. Immunol. 2017, 8, 1062. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wightman, B.; Ha, I.; Ruvkun, G. Posttranscriptional regulation of the heterochronic gene lin-14 by lin-4 mediates temporal pattern formation in C. elegans. Cell 1993, 75, 855–862. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.C.; Feinbaum, R.L.; Ambros, V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell 1993, 75, 843–854. [Google Scholar] [CrossRef] [PubMed]
- Ha, M.; Kim, V.N. Regulation of microRNA biogenesis. Nat. Rev. Mol. Cell Biol. 2014, 15, 509–524. [Google Scholar] [CrossRef]
- Broughton, J.P.; Lovci, M.T.; Huang, J.L.; Yeo, G.W.; Pasquinelli, A.E. Pairing beyond the Seed Supports MicroRNA Targeting Specificity. Mol. Cell 2016, 64, 320–333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vasudevan, S. Posttranscriptional upregulation by microRNAs. Wiley Interdiscip. Rev. RNA 2012, 3, 311–330. [Google Scholar] [CrossRef]
- Makarova, J.A.; Shkurnikov, M.U.; Wicklein, D.; Lange, T.; Samatov, T.R.; Turchinovich, A.A.; Tonevitsky, A.G. Intracellular and extracellular microRNA: An update on localization and biological role. Prog. Histochem. Cytochem. 2016, 51, 33–49. [Google Scholar] [CrossRef]
- O’Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front. Endocrinol. 2018, 3, 402. [Google Scholar] [CrossRef] [Green Version]
- Creemers, E.E.; Tijsen, A.J.; Pinto, Y.M. Circulating microRNAs: Novel biomarkers and extracellular communicators in cardiovascular disease? Circ. Res. 2012, 110, 483–495. [Google Scholar] [CrossRef] [Green Version]
- Nikolouzakis, T.K.; Falzone, L.; Lasithiotakis, K.; Krüger-Krasagakis, S.; Kalogeraki, A.; Sifaki, M.; Spandidos, D.A.; Chrysos, E.; Tsatsakis, A.; Tsiaoussis, J. Current and Future Trends in Molecular Biomarkers for Diagnostic, Prognostic, and Predictive Purposes in Non-Melanoma Skin Cancer. J. Clin. Med. 2020, 9, 2868. [Google Scholar] [CrossRef]
- Gong, Z.H.; Zhou, F.; Shi, C.; Xiang, T.; Zhou, C.K.; Wang, Q.Q.; Jiang, Y.S.; Gao, S.F. miRNA-221 promotes cutaneous squamous cell carcinoma progression by targeting PTEN. Cell Mol. Biol. Lett. 2019, 24, 9. [Google Scholar] [CrossRef] [PubMed]
- Mari, E.; Zicari, A.; Fico, F.; Massimi, I.; Martina, L.; Mardente, S. Action of HMGB1 on miRNA-221/222 cluster in neuroblastoma cell lines. Oncol. Lett. 2016, 12, 2133–2138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, M.; Liu, J.; Wang, L.; Wu, H.; Zhou, C.; Zhu, H.; Xu, N.; Xie, Y. Association of serum microRNA expression in hepatocellular carcinomas treated with transarterial chemoembolization and patient survival. PLoS ONE 2014, 9, e109347. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Peskoe, S.B.; Ribas, J.; Rafiqi, F.; Kudrolli, T.; Meeker, A.K.; De Marzo, A.M.; Platz, E.A.; Lupold, S.E. Investigation of miRNA-21, miRNA-141, and miRNA-221 expression levels in prostate adenocarcinoma for associated risk of recurrence after radical prostatectomy. Prostate 2014, 74, 1655–1662. [Google Scholar] [CrossRef] [Green Version]
- Tao, K.; Yang, J.; Guo, Z.; Hu, Y.; Sheng, H.; Gao, H.; Yu, H. Prognostic value of miRNA-221-3p, miRNA-342-3p and miRNA-491-5p expression in colon cancer. Am. J. Transl. Res. 2014, 6, 391–401. [Google Scholar]
- Yamashita, R.; Sato, M.; Kakumu, T.; Hase, T.; Yogo, N.; Maruyama, E.; Sekido, Y.; Kondo, M.; Hasegawa, Y. Growth inhibitory effects of miRNA-221 and miRNA-222 in non-small cell lung cancer cells. Cancer Med. 2015, 4, 551–564. [Google Scholar] [CrossRef]
- Li, W.J.; Wang, Y.; Liu, R.; Kasinski, A.L.; Shen, H.; Slack, F.J.; Tang, D.G. MicroRNA-34a: Potent Tumor Suppressor, Cancer Stem Cell Inhibitor, and Potential Anticancer Therapeutic. Front. Cell Dev. Biol. 2021, 9, 640587. [Google Scholar] [CrossRef]
- Duan, K.; Ge, Y.C.; Zhang, X.P.; Wu, S.Y.; Feng, J.S.; Chen, S.L.; Zhang, L.I.; Yuan, Z.H.; Fu, C.H. miRNA-34a inhibits cell proliferation in prostate cancer by downregulation of SIRT1 expression. Oncol. Lett. 2015, 10, 3223–3227. [Google Scholar] [CrossRef] [Green Version]
- Si, W.; Li, Y.; Shao, H.; Hu, R.; Wang, W.; Zhang, K.; Yang, Q. MiRNA-34a Inhibits Breast Cancer Proliferation and Progression by Targeting Wnt1 in Wnt/β-Catenin Signaling Pathway. Am. J. Med. Sci. 2016, 352, 191–199. [Google Scholar] [CrossRef]
- Shi, H.; Zhou, S.; Liu, J.; Zhu, J.; Xue, J.; Gu, L.; Chen, Y. miRNA-34a inhibits the in vitro cell proliferation and migration in human esophageal cancer. Pathol. Res. Pract. 2016, 212, 444–449. [Google Scholar] [CrossRef]
- Zhang, D.G.; Zheng, J.N.; Pei, D.S. P53/microRNA-34-induced metabolic regulation: New opportunities in anticancer therapy. Mol. Cancer 2014, 13, 115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, J.H.; Roh, K.S.; Suh, S.S.; Lee, S.; Sung, S.W.; Park, J.K.; Byun, J.H.; Kang, J.H. The expression of microRNA-34a is inversely correlated with c-MET and CDK6 and has a prognostic significance in lung adenocarcinoma patients. Tumour Biol. J. Int. Soc. Oncodevelopmental Biol. Med. 2015, 36, 9327–9337. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.; Ma, L.; Wu, Z.; Zheng, G.; Li, J. Expression of miRNA-34a in basal cell carcinoma patients and its relationship with prognosis. J. Buon. 2019, 24, 1283–1288. [Google Scholar] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Li, P.; He, Q.Y.; Luo, C.Q.; Qian, L.Y. Circulating miRNA-221 expression level and prognosis of cutaneous malignant melanoma. Med. Sci. Monit. 2014, 20, 2472–2477. [Google Scholar] [PubMed] [Green Version]
- Li, J.; Yen, C.; Liaw, D.; Podsypanina, K.; Bose, S.; Wang, S.I.; Puc, J.; Miliaresis, C.; Rodgers, L.; McCombie, R.; et al. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science 1991, 275, 1943–1947. [Google Scholar] [CrossRef] [PubMed]
- Teng, D.H.; Hu, R.; Lin, H.; Davis, T.; Iliev, D.; Frye, C.; Swedlund, B.; Hansen, K.L.; Vinson, V.L.; Gumpper, K.L.; et al. MMAC1/PTEN mutations in primary tumor specimens and tumor cell lines. Cancer Res. 1997, 57, 5221–5225. [Google Scholar] [PubMed]
- Zhang, H.; Xu, H.L.; Wang, Y.C.; Lu, Z.Y.; Yu, X.F.; Sui, D.Y. 20(S)-Protopanaxadiol-Induced Apoptosis in MCF-7 Breast Cancer Cell Line through the Inhibition of PI3K/AKT/mTOR Signaling Pathway. Int. J. Mol. Sci. 2018, 19, 1053. [Google Scholar] [CrossRef] [Green Version]
- Mardente, S.; Mari, E.; Massimi, I.; Fico, F.; Faggioni, A.; Pulcinelli, F.; Antonaci, A.; Zicari, A. HMGB1-Induced Cross Talk between PTEN and miRs 221/222 in Thyroid Cancer. BioMed Res. Int. 2015, 51, 2027. [Google Scholar] [CrossRef] [Green Version]
- Kanemaru, H.; Fukushima, S.; Yamashita, J.; Honda, N.; Oyama, R.; Kakimoto, A.; Masuguchi, S.; Ishihara, T.; Inoue, Y.; Jinnin, M.; et al. The circulating microRNA-221 level in patients with malignant melanoma as a new tumor marker. J. Dermatol. Sci. 2011, 61, 187–193. [Google Scholar] [CrossRef]
- Garofoli, M.; Volpicella, M.; Guida, M.; Porcelli, L.; Azzariti, A. The Role of Non-Coding RNAs as Prognostic Factor, Predictor of Drug Response or Resistance and Pharmacological Targets, in the Cutaneous Squamous Cell Carcinoma. Cancers 2020, 12, 2552. [Google Scholar] [CrossRef]
- Skourti, E.; Logotheti, S.; Kontos, C.K.; Pavlopoulou, A.; Dimoragka, P.T.; Trougakos, I.P.; Gorgoulis, V.; Scorilas, A.; Michalopoulos, I.; Zoumpourlis, V. Progression of mouse skin carcinogenesis is associated with the orchestrated deregulation of miRNA-200 family members, miRNA-205 and their common targets. Mol. Carcinog. 2016, 55, 1229–1242. [Google Scholar] [CrossRef]
- Lefort, K.; Brooks, Y.; Ostano, P.; Cario-André, M.; Calpini, V.; Guinea-Viniegra, J.; Albinger-Hegyi, A.; Hoetzenecker, W.; Kolfschoten, I.; Wagner, E.F.; et al. A miRNA-34a-SIRT6 axis in the squamous cell differentiation network. EMBO J. 2013, 32, 2248–2263. [Google Scholar] [CrossRef] [Green Version]
- Lange, A.M.; Lo, H.W. Inhibiting TRK Proteins in Clinical Cancer Therapy. Cancers 2018, 10, 105. [Google Scholar] [CrossRef] [Green Version]
- Gal-Yam, E.N.; Saito, Y.; Egger, G.; Jones, P.A. Cancer epigenetics: Modifications, screening, and therapy. Annu. Rev. Med. 2008, 59, 267–280. [Google Scholar] [PubMed]
- Lodygin, D.; Tarasov, V.; Epanchintsev, A.; Berking, C.; Knyazeva, T.; Körner, H.; Knyazev, P.; Diebold, J.; Hermeking, H. Inactivation of miRNA-34a by aberrant CpG methylation in multiple types of cancer. Cell Cycle 2008, 7, 2591–2600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vousden, K.H.; Lu, X. Live or let die: The cell’s response to p53. Nat. Rev. Cancer 2002, 2, 594–604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rivlin, N.; Brosh, R.; Oren, M.; Rotter, V. Mutations in the p53 Tumor Suppressor Gene: Important Milestones at the Various Steps of Tumorigenesis. Genes Cancer 2011, 2, 466–474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodust, P.M.; Stockfleth, E.; Ulrich, C.; Leverkus, M.; Eberle, J. UV-induced squamous cell carcinoma—A role for antiapoptotic signalling pathways. Br. J. Dermatol. 2009, 161 (Suppl. S3), 107–115. [Google Scholar] [CrossRef]
- Tamas, T.; Dinu, C.; Lenghel, M.; Băciuț, G.; Bran, S.; Stoia, S.; Băciuț, M. The role of ultrasonography in head and neck Non-Melanoma Skin Cancer approach: An update with a review of the literature. Med. Ultrason. 2021, 23, 83–88. [Google Scholar] [CrossRef]
- Neagu, M.; Constantin, C.; Cretoiu, S.M.; Zurac, S. miRNAs in the Diagnosis and Prognosis of Skin Cancer. Front. Cell Dev. Biol. 2020, 8, 71. [Google Scholar] [CrossRef] [Green Version]
| Assay Name | Assay ID |
|---|---|
| hsa-miRNA-34-5p | 000426 |
| hsa-miRNA-221-3p | 000524 |
| U6 snRNA | 001973 |
| RNU48 | 001006 |
| Histopathology: | BCC (n = 18) | BSC (n = 2) | SCC (n = 18) | p-Value |
|---|---|---|---|---|
| Age, median (IQR) | 70.5 (62–78) | 76 (74.5–77.5) | 76.5 (65.25–84.5) | 0.454 |
| Sex (F), n (%) | 10 (55.56) | 1 (50) | 7 (38.89) | 0.747 |
| Breslow, median (IQR) | 5.45 (3.67–9.38) | 4.1 (3.55–4.65) | 7 (5–12) | 0.439 {0.659/0.747/0.156} |
| Clark, n (%) | 0.267 | |||
| II: | 1 (5.56) | 0 (0) | 1 (5.88) | |
| III: | 1 (5.56) | 0 (0) | 0 (0) | |
| IV: | 7 (38.89) | 1 (50) | 2 (11.76) | |
| V: | 9 (50) | 1 (50) | 14 (82.35) | |
| Clark IV/V, n (%) | 16 (88.89) | 2 (100) | 16 (88.89) | |
| T3/4, n (%) | 4 (22.22) | 0 (0) | 12 (66.67) | 0.011 |
| V1, n (%) | 1 (5.56) | 1 (50) | 2 (11.11) | 0.285 |
| L1, n (%) | 1 (5.56) | 0 (0) | 5 (27.78) | 0.239 |
| R1, n (%) | 4 (22.22) | 1 (50) | 3 (16.67) | 0.523 |
| Pn1, n (%) | 4 (22.22) | 0 (0) | 8 (44.44) | 0.292 |
| Tumor Type and miRNA | Normal (n = 18) | Tumoral (n = 18) | Difference (95% CI) | p-Value |
|---|---|---|---|---|
| Basocellular carcinoma (BCC) | ||||
| miRNA-34A, median (IQR) | 1.14 (0.88–1.34) | 0.89 (0.6–1.13) | 0.24 (−0.2–0.53) | 0.279 |
| miRNA-221, median (IQR) | 1.21 (0.72–1.77) | 1.75 (0.91–2.25) | 0.54 (−1.37–0.2) | 0.161 |
| Squamous carcinoma (SCC) | ||||
| miRNA-34A, median (IQR) | 1.03 (0.73–1.36) | 0.7 (0.49–1.39) | 0.33 (−0.25–0.57) | 0.308 |
| miRNA-221, median (IQR) | 1.09 (0.8–1.36) | 1.55 (0.95–2.19) | 0.46 (−1.12–−0.03) | 0.04 |
| Basosquamous carcinoma (BSC) | ||||
| miRNA-34A, median (IQR) | 0.21 (0.18–0.24) | 0.14 (0.09–0.18) | 0.07 (−0.07–0.21) | 1 |
| miRNA-221, median (IQR) | 0.17 (0.13–0.21) | 0.15 (0.14–0.15) | 0.03 (−0.05–0.1) | 1 |
| R1: | R1 (n = 8) | R0 (n = 30) | Difference (95% CI) | p |
|---|---|---|---|---|
| miRNA-34A tumor tissue, median (IQR) | 1.05 (0.8–1.59) | 0.66 (0.42–1.13) | 0.39 (−0.08–0.98) | 0.14 [n1 = 8, n2 = 30] |
| miRNA-221 tumor tissue, median (IQR) | 3.64 (1.78–4.22) | 1.46 (0.67–1.95) | 2.18 (0.36–3.16) | 0.019 [n1 = 8, n2 = 30] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tamas, T.; Raduly, L.; Berindan-Neagoe, I.; Dinu, C.; Botan, E.; Bumbu, B.; Tamas, A.; Stoia, S.; Leucuta, D.C.; Bran, S.; et al. The Role of miRNA-221 and miRNA-34a in Non-Melanoma Skin Cancer of the Head and Neck Region. Genes 2023, 14, 503. https://doi.org/10.3390/genes14020503
Tamas T, Raduly L, Berindan-Neagoe I, Dinu C, Botan E, Bumbu B, Tamas A, Stoia S, Leucuta DC, Bran S, et al. The Role of miRNA-221 and miRNA-34a in Non-Melanoma Skin Cancer of the Head and Neck Region. Genes. 2023; 14(2):503. https://doi.org/10.3390/genes14020503
Chicago/Turabian StyleTamas, Tiberiu, Lajos Raduly, Ioana Berindan-Neagoe, Cristian Dinu, Emil Botan, Bogdan Bumbu, Adela Tamas, Sebastian Stoia, Daniel Corneliu Leucuta, Simion Bran, and et al. 2023. "The Role of miRNA-221 and miRNA-34a in Non-Melanoma Skin Cancer of the Head and Neck Region" Genes 14, no. 2: 503. https://doi.org/10.3390/genes14020503





