The Influence of Methyl Jasmonate on Expression Patterns of Rosmarinic Acid Biosynthesis Genes, and Phenolic Compounds in Different Species of Salvia subg. Perovskia Kar L.
Abstract
:1. Introduction
2. Result and Discussion
2.1. RA Changes at Different MeJA Concentrations
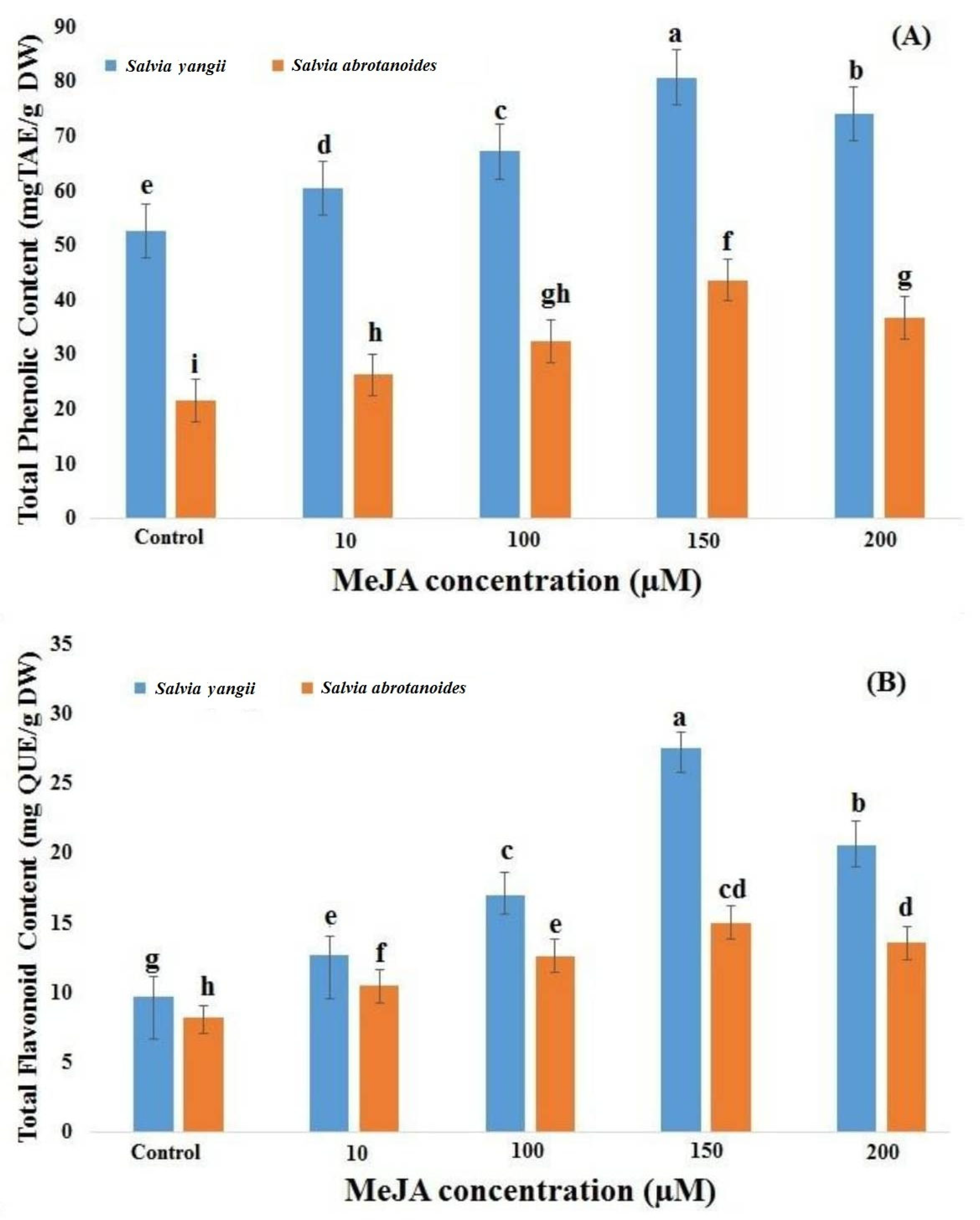
2.2. TPC and TFC as a Function of MeJA Concentration
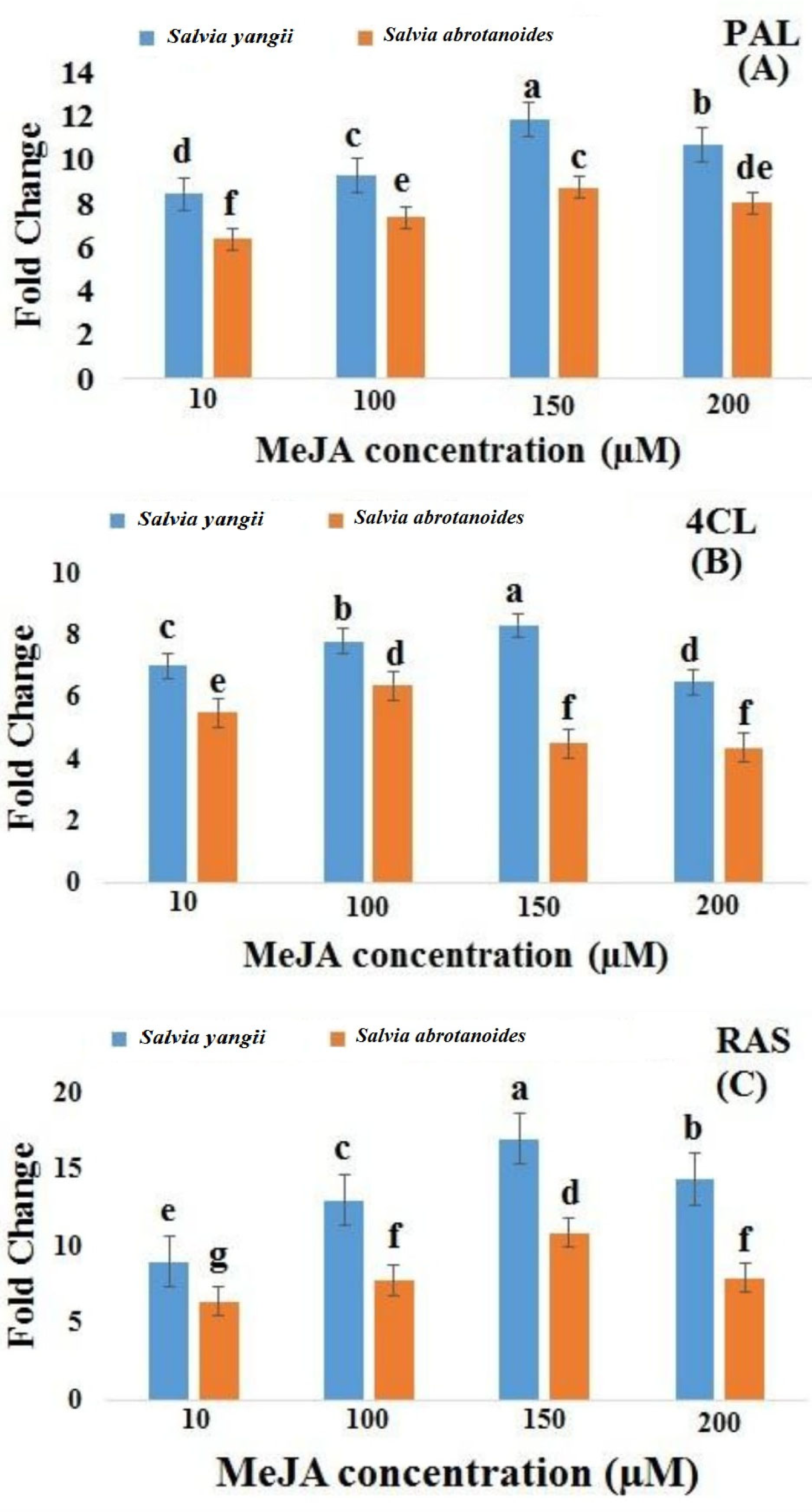
2.3. MeJA’s Effects on the Expression of the PAL, 4CL, and RAS Genes
3. Materials and Methods
3.1. Plant Materials and Environmental Factors
3.2. MeJA Treatments
3.3. Real-Time PCR Analysis
3.4. HPLC Analysis
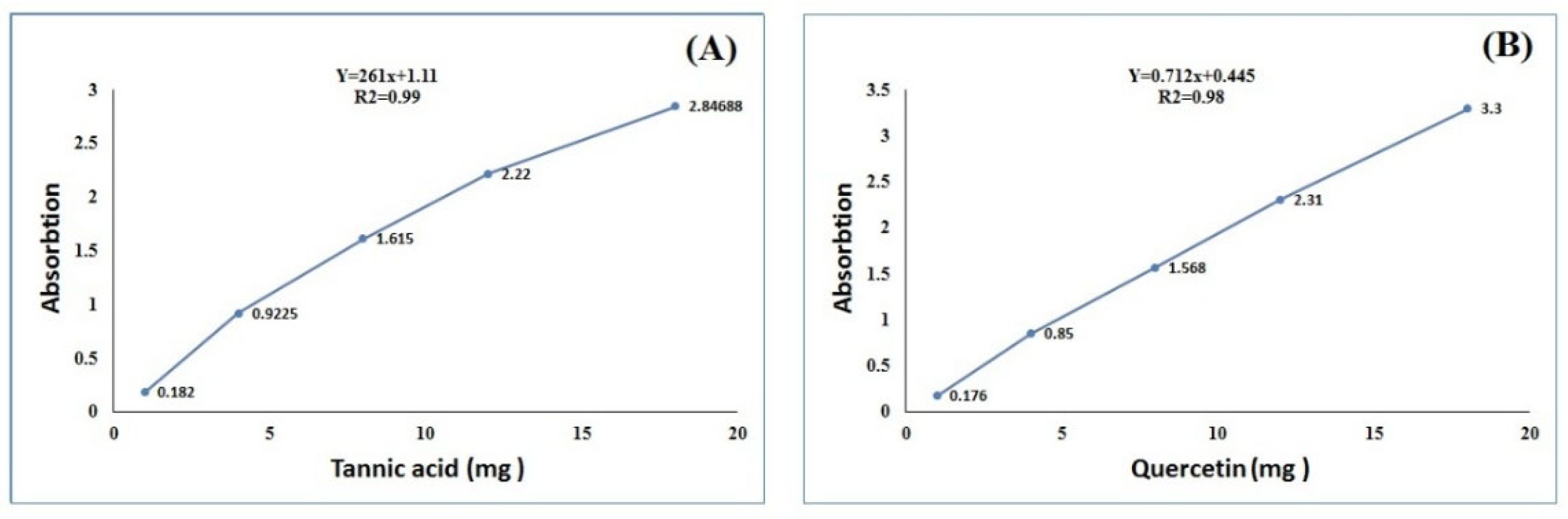
3.5. Total Phenolic and Flavonoid Contents Analysis
3.6. Data Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yilmaz, A.; Ciftci, V. Genetic relationships and diversity analysis in Turkish laurel (Laurus nobilis L.) germplasm using ISSR and SCoT markers. Mol. Biol. Rep. 2021, 48, 4537–4547. [Google Scholar] [CrossRef]
- Yilmaz, A.; Guler, E.; Soydemir, H.E.; Demirel, S.; Mollahaliloglu, S.; Karadeniz, T.; Ciftci, V. Miracle. plant: Aronia (Aronia melanocarpa). MAS J. Appl. Sci. 2021, 6, 83–94. [Google Scholar]
- Yilmaz, A.; Karik, Ü. AMF and PGPR enhance yield and secondary metabolite profile of basil (Ocimum basilicum L.). Ind. Crops Prod. 2022, 176, 114327. [Google Scholar] [CrossRef]
- Mátis, A.; Malkócs, T.; Kuhn, T.; Laczkó, L.; Moysiyenko, I.; Szabó, A.; Bădărău, A.; Sramkó, G. Hiding in plain sight: Integrative analyses uncover a cryptic Salvia species in Europe. Taxon 2023, 72, 78–97. [Google Scholar] [CrossRef]
- Drew, B.T.; González-Gallegos, J.G.; Xiang, C.L.; Kriebel, R.; Drummond, C.P.; Walked, J.B.; Sytsma, K.J. Salvia united: The greatest good for the greatest number. Taxon 2017, 66, 133–145. [Google Scholar] [CrossRef] [Green Version]
- Bielecka, M.; Pencakowski, B.; Stafiniak, M.; Jakubowski, K.; Rahimmalek, M.; Gharibi, S.; Matkowski, A.; Slusarczyk, S. Metabolomics and DNA-based authentication of two traditional Asian medicinal and aromatic species of Salvia subg. Perovskia. Cells 2021, 10, 112. [Google Scholar] [CrossRef]
- Mohammadhosseini, M.; Venditti, A.; Akbarzadeh, A. The genus Perovskia Kar.: Ethnobotany, chemotaxonomy and phytochemistry: A review. Toxin Rev. 2021, 40, 484–505. [Google Scholar] [CrossRef]
- Ghaffari, Z.; Rahimmalek, M.; Sabzalian, M.R. Variations in essential oil composition and antioxidant activity in Perovskia abrotanoides Kar. collected from different regions in Iran. Chem. Biodivers. 2018, 15, e1700565. [Google Scholar] [CrossRef]
- Afshari, M.; Rahimmalek, M. Variation in essential oil composition, anatomical, and antioxidant characteristics of Achillea filipendulina Lam. as affected by different phenological stages. J. Essent. Oil Res. 2021, 33, 283–298. [Google Scholar] [CrossRef]
- Orhan, E.I.; Senol, S.F.; Ercetin, T.; Kahraman, A.; Celep, F.; Akaydin, G.; Sener, B.; Dogan, M. Assessment of anticholinesterase and antioxidant properties of selected sage (Salvia) species with their total phenol and flavonoid contents. Ind. Crops Prod. 2013, 41, 21–30. [Google Scholar] [CrossRef]
- Flora of Pakistan. Available online: http://www.efloras.org/flora_page.aspx?flora_id=5 (accessed on 17 January 2020).
- Rechinger, K.H. Flora Iranica; Akademische Druck- und Verlagsanstalt: Graz, Austria, 1982. [Google Scholar]
- Mozaffarian, V.A. Dictionary of Iranian Plant Names; Farhang Moaser: Tehran, Iran, 1996. [Google Scholar]
- Flora of China. Available online: http://www.efloras.org/flora_page.aspx?flora_id=2 (accessed on 17 January 2020).
- Perveen, S.; Malik, A.; Noor, A.T.; Tareen, R.B. Pervosides A and B, new isoferulyl glucosides from Perovskia atriplicifolia. J. Asian Nat. Prod. Res. 2008, 10, 1105–1108. [Google Scholar] [CrossRef]
- Tareen, R.B.; Bibi, T.; Khan, M.A.; Ahmad, M.; Zafar, M. Indigenous knowledge of folk medicine by the women of Kalat and Khuzdar regions of Balochistan, Pakistan. Pak. J. Bot. 2010, 42, 1465–1485. [Google Scholar]
- Baquar, S.R. Medicinal and Poisonous Plants of Pakistan, 1st ed.; Printas Press: Karachi, Pakistan, 1989. [Google Scholar]
- Cardilea, V.; Russob, A.; Formisanoc, C.; Rigano, D.; Senatore, F.; Arnold, N.A.; Piozzi, F. Essential oils of Salvia bracteata and Salvia rubifolia from Lebanon: Chemical composition, antimicrobial activity and inhibitory effect on human melanoma cells. J. Ethnol. Pharmacol. 2009, 126, 265–272. [Google Scholar] [CrossRef]
- Eisenman, S.W.; Zaurov, D.E.; Struwe, L. Medicinal Plants of Central Asia: Uzbekistan and Kyrgyzstan; Springer: Berlin/Heidelberg, Germany, 2012; ISBN 978-1-46143-912-7. [Google Scholar]
- Gao, L.; Zhou, J.; Zhu, L.Y.; Zhang, J.R.; Jing, Y.X.; Zhao, J.W.; Huang, X.Z.; Li, G.P.; Jiang, Z.Y.; Xue, D.Y. Four New Diterpene Glucosides from Perovskia atriplicifolia. Chem. Biodivers. 2017, 14, 1–7. [Google Scholar] [CrossRef]
- Jiang, Z.Y.; Yu, Y.J.; Huang, C.G.; Huang, X.Z.; Hu, Q.F.; Yang, G.Y.; Wang, H.B.; Zhang, X.Y.; Li, G.P. Icetexane diterpenoids from Perovskia atriplicifolia. Planta Med. 2015, 81, 241–246. [Google Scholar] [CrossRef] [Green Version]
- Kumar, P.G.; Gupta, S.; Murugan, P.M.; Bala Singh, S. Ethnobotanical studies of Nubra valley—A cold arid zone of Himalaya. Ethnobot. Leafl. 2009, 13, 9. [Google Scholar]
- Moallem, S.A.; Niapour, M. Study of embryotoxicity of Perovskia abrotanoides, an adulterant in folk-medicine, during organogenesis in mice. J. Ethnopharmacol. 2008, 117, 108–114. [Google Scholar] [CrossRef]
- Ballabh, B.; Chaurasia, O.P.; Ahmed, Z.; Singh, S.B. Traditional medicinal plants of cold desert Ladakh-Used against kidney and urinary disorders. J. Ethnopharmacol. 2008, 118, 331–339. [Google Scholar] [CrossRef]
- Hosseinzadeh, H.; Amel, S. Antinociceptive Effect of the Aerial Parts of Perovskia abrotanoides Extracts in Mice. Iran. Red Crescent Med. J. 2009, 4, 15–17. [Google Scholar]
- Sairafianpour, M.; Christensen, J.; Staerk, D.; Budnik, B.; Kharazmi, A.; Bagherzadeh, K.; Jaroszewski, J.W. Leishmanicidal, antiplasmodial, and cytotoxic activity of novel diterpenoid 1,2-quinones from Perovskia abrotanoides: New source of tanshinones. J. Nat. Prod. 2001, 64, 1398–1403. [Google Scholar] [CrossRef]
- Jaafari, M.R.; Hooshmand, S.; Samiei, A.; Hossainzadeh, H. Evaluation of leishmanicidal effect of Perovskia abrotanoides Karel.Root extract by in vitro leishmanicidal assay using promastigotes of Leishmania major. Pharmacologyonline 2007, 1, 299–303. [Google Scholar]
- Petersen, M.; Simmonds, M.S.J. Molecules of interest: Rosmarinic acid. Phytochemistry 2003, 62, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Hamaguchi, T.; Ono, K.; Murase, A.; Yamada, M. Phenolic compounds prevent Alzheimer’s pathology through different effects on the amyloid-β aggregation pathway. Am. J. Pathol. 2009, 175, 2557–2565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazzanti, G.; Battinelli, L.; Pompeo, C.; Serrilli, A.M.; Rossi, R.; Sauzullo, I.; Megoni, F.; Vullo, V. Inhibitory activity of Melissa officinalis L. extracts on Herpes simplex virus type 2 replication. Nat. Prod. Res. 2008, 23, 1433–1440. [Google Scholar] [CrossRef] [PubMed]
- Al-Dhabi, N.A.; Arasu, M.V.; Park, C.H.; Park, S.U. Recent studies on rosmarinic acid and its biological and pharmacological activities. EXCLI J. 2014, 13, 1192–1195. [Google Scholar]
- Taha, R.A.; Hassan, M.M.; Ibrahim, E.A.; Abo-Bakr, N.H.; Shaaban, E.A. Carbon nanotubes impact on date palm in vitro cultures. Plant Cell Tissue Organ Cult. 2016, 127, 525–534. [Google Scholar] [CrossRef]
- Petersen, M. Rosmarinic acid: New aspects. Phytochem. Rev. 2013, 12, 207–227. [Google Scholar] [CrossRef]
- Petersen, M.; Abdullah, Y.; Benner, J.; Eberle, D.; Gehlen, K.; Hücherig, S.; Janiak, V.; Kim, K.H.; Sander, M.; Weitzel, C.; et al. Evolution of rosmarinic acid biosynthesis. Phytochemistry 2009, 70, 1663–1679. [Google Scholar] [CrossRef]
- Weitzel, C.; Petersen, M. Cloning and characterization of rosmarinic acid synthase from Melissa officinalis L. Phytochemistry 2011, 72, 572–578. [Google Scholar] [CrossRef]
- Deng, C.; Wang, Y.; Huang, F.; Lu, S.; Zhao, L.; Ma, X.; Kai, G. SmMYB2 promotes salvianolic acid biosynthesis in the medicinal herb Salvia miltiorrhiza. J. Integr. Plant. Biol. 2020, 62, 1688–1702. [Google Scholar] [CrossRef]
- Zhang, S.; Li, H.; Liang, X.; Yan, Y.; Xia, P.; Jia, Y.; Liang, Z. Enhanced production of phenolic acids in Salvia miltiorrhiza hairy root cultures by combing the RNAi-mediated silencing of chalcone synthase gene with salicylic acid treatment. Biochem. Eng. J. 2015, 103, 185–192. [Google Scholar] [CrossRef]
- Fu, R.; Shi, M.; Deng, C.; Zhang, Y.; Zhang, X.; Wang, Y.; Kai, G. Improved phenolic acid content and bioactivities of Salvia miltiorrhiza hairy roots by genetic manipulation of RAS and CYP98A14. Food Chem. 2020, 331, 127365. [Google Scholar] [CrossRef]
- Guerriero, G.; Berni, R.; Muñoz-Sanchez, J.A.; Apone, F.; Abdel-Salam, E.M.; Qahtan, A.A.; Alatar, A.A.; Cantini, C.; Cai, G.; Hausman, J.-F.; et al. Production of plant secondary metabolites: Examples, tips and suggestions for biotechnologists. Genes 2018, 9, 309. [Google Scholar] [CrossRef] [Green Version]
- Naik, P.M.; Al–Khayri, J.M. Abiotic and Biotic Elicitors—Role in Secondary Metabolites Production through In Vitro Culture of Medicinal Plants. In Abiotic and Biotic Stress in Plants—Recent Advances and Future Perspectives; Shanker, A.K., Shanker, C., Eds.; InTech: Rijeka, Croatia, 2016; pp. 247–278. [Google Scholar]
- Gonçalves, S.; Romano, A. Production of Plant Secondary Metabolites by Using Biotechnological Tools. In Secondary Metabolites: Sources and Applications; Vijayakumar, R., Raja, S.S.S., Eds.; InTech: Rijeka, Croatia, 2018. [Google Scholar]
- Sircar, D.; Cardoso, H.G.; Mukherjee, C.; Mitra, A.; Arnholdt-Schmitt, B. Alternative oxidase (AOX) and phenolic metabolism in methyl jasmonate-treated hairy root cultures of Daucus carota L. J. Plant Physiol. 2012, 169, 657–663. [Google Scholar] [CrossRef]
- Narayani, M.; Srivastava, S. Elicitation: A stimulation of stress in in vitro plant cell/tissue cultures for enhancement of secondary metabolite production. Phytochem. Rev. 2017, 16, 1227–1252. [Google Scholar] [CrossRef]
- Gai, Q.; Jiao, J.; Wang, X.; Zang, Y.; Niu, L.; Fu, Y. Elicitation of Isatis tinctoria L. hairy root cultures by salicylic acid and methyl jasmonate for the enhanced production of pharmacologically active alkaloids and flavonoids. Plant Cell Tissue Organ Cult. 2019, 137, 77–86. [Google Scholar] [CrossRef]
- Xing, B.; Yang, D.; Liu, L.; Han, R.; Sun, Y.; Liang, Z. Phenolic acid production is more effectively enhanced than tanshinone production by methyl jasmonate in Salvia miltiorrhiza hairy roots. Plant Cell Tissue Organ Cult. 2018, 134, 119–129. [Google Scholar] [CrossRef]
- Fatemi, F.; Abdollahi, M.R.; Mirzaie-asl, A.; Dastan, D.; Garagounis, C.; Papadopoulou, K. Identification and expression profiling of rosmarinic acid biosynthetic genes from Satureja khuzistanica under carbon nanotubes and methyl jasmonate elicitation. Plant Cell Tissue Organ Cult. 2019, 136, 561–573. [Google Scholar] [CrossRef]
- Kianersi, F.; Abdollahi, M.R.; Mirzaie-asl, A.; Dastan, D.; Rasheed, F. Identification and tissue-specific expression of rutin biosynthetic pathway genes in Capparis spinosa elicited with salicylic acid and methyl jasmonate. Sci. Rep. 2020, 10, 8884. [Google Scholar] [CrossRef]
- Kianersi, F.; Abdollahi, M.R.; Mirzaie-asl, A.; Dastan, D.; Rasheed, F. Biosynthesis of rutin changes in Capparis spinosa due to altered expression of its pathway genes under elicitors’ supplementation. Plant Cell Tissue Organ Cult. 2020, 141, 619–631. [Google Scholar] [CrossRef]
- Kianersi, F.; PourAboughadareh, A.; Majdi, M.; Poczai, P. Effect of Methyl Jasmonate on Thymol, Carvacrol, Phytochemical Accumulation, and Expression of Key Genes Involved in Thymol/Carvacrol Biosynthetic Pathway in Some Iranian Thyme Species. Int. J. Mol. Sci. 2021, 22, 11124. [Google Scholar] [CrossRef] [PubMed]
- Kianersi, F.; Amin Azarm, D.; Pour-Aboughadareh, A.; Poczai, P. Change in Secondary Metabolites and Expression Pattern of Key Rosmarinic Acid Related Genes in Iranian Lemon Balm (Melissa officinalis L.) Ecotypes Using Methyl Jasmonate Treatments. Molecules 2022, 27, 1715. [Google Scholar] [CrossRef] [PubMed]
- Abdollahi, M.R.; Kianersi, F.; Moosavi, S.S.; Dastan, D.; Asadi, S. Identification and Expression Analysis of Two Genes Involved in the Biosynthesis of t-Anethole in Fennel (Foeniculum vulgare Mill.) and Their Up-Regulation in Leaves in Response to Methyl Jasmonate Treatments. J. Plant Growth Regul. 2022, 42, 759–770. [Google Scholar] [CrossRef]
- Kianersi, F.; Amin Azarm, D.; Fatemi, F.; Pour-Aboughadareh, A.; Poczai, P. Methyl Jasmonate Induces Genes Involved in Linalool Accumulation and Increases the Content of Phenolics in Two Iranian Coriander (Coriandrum sativum L.) Ecotypes. Genes 2022, 13, 1717. [Google Scholar] [CrossRef]
- Zhang, S.; Yan, Y.; Wang, B.; Liang, Z.; Liu, Y.; Liu, F.; Qi, Z. Selective responses of enzymes in the two parallel pathways of rosmarinic acid biosynthetic pathway to elicitors in Salvia miltiorrhiza hairy root cultures. J. Biosci. Bioeng. 2014, 117, 645–651. [Google Scholar] [CrossRef]
- Yousefian, S.; Lohrasebi, T.; Farhadpour, M.; Haghbeen, K. Effect of methyl jasmonate on phenolic acids accumulation and the expression profile of their biosynthesis-related genes in Mentha spicata hairy root cultures. Plant Cell Tissue Organ Cult. 2020, 142, 285–297. [Google Scholar] [CrossRef]
- Kim, Y.B.; Kim, J.K.; Uddin, M.R.; Xu, H.; Park, W.T.; Tuan, P.A.; Li, X.; Chung, E.S.; Lee, J.-H.; Park, S.U. Metabolomics analysis and biosynthesis of rosmarinic acid in Agastache rugosa Kuntze treated with methyl jasmonate. PLoS ONE 2013, 8, e64199. [Google Scholar] [CrossRef] [Green Version]
- Stafiniak, M.; Ślusarczyk, S.; Pencakowski, B.; Matkowski, A.; Rahimmalek, M.; Bielecka, M. Seasonal Variations of Rosmarinic Acid and Its Glucoside and Expression of Genes Related to Their Biosynthesis in Two Medicinal and Aromatic Species of Salvia subg. Perovskia. Biology 2021, 10, 458. [Google Scholar] [CrossRef]
- Fatemi, F.; Abdollahi, M.R.; Mirzaie-Asl, A.; Dastan, D.; Papadopoulou, K. Phytochemical, antioxidant, enzyme activity and antifungal properties of Satureja khuzistanica in vitro and in vivo explants stimulated by some chemical elicitors. Pharm. Biol. 2020, 58, 286–296. [Google Scholar] [CrossRef] [Green Version]
- Ruan, J.; Zhou, Y.; Zhou, M.; Yan, J.; Khurshid, M.; Weng, W.; Cheng, J.; Zhang, K. Jasmonic acid signaling pathway in plants. Int. J. Mol. Sci. 2019, 20, 2479. [Google Scholar] [CrossRef] [Green Version]
- Xiao, Y.; Gao, S.; Di, P.; Chen, J.; Chen, W.; Zhang, L. Methyl jasmonate dramatically enhances the accumulation of phenolic acids in Salvia miltiorrhiza hairy root cultures. Physiol. Plant. 2009, 137, 1–9. [Google Scholar] [CrossRef]
- Park, W.T.; Arasu, M.V.; Al-Dhabi, N.A.; Yeo, S.K.; Jeon, J.; Park, J.S.; Lee, S.Y.; Park, S.U. Yeast extract and silver nitrate induce the expression of phenylpropanoid biosynthetic genes and induce the accumulation of rosmarinic acid in Agastache rugosa cell culture. Molecules 2016, 21, 426. [Google Scholar] [CrossRef]
- Kintzios, S.; Makri, O.; Panagiotopoulos, E.; Scapeti, M. In vitro rosmarinic acid accumulation in sweet basil (Ocimum basilicum L.). Biotechnol. Lett. 2003, 25, 405–408. [Google Scholar] [CrossRef]
- Mizukami, H.; Tabira, Y.; Ellis, B.E. Methyl jasmonate-induced rosmarinic acid biosynthesis in Lithospermum erythrorhizon cell suspension cultures. Plant. Cell. Rep. 1993, 12, 706–709. [Google Scholar] [CrossRef]
- Guan, Y.; Hu, W.; Jiang, A.; Xu, Y.; Sa, R.; Feng, K.; Zhao, M.; Yu, J.; Ji, Y.; Hou, M.; et al. Effect of Methyl Jasmonate on Phenolic Accumulation in Wounded Broccoli. Molecules 2019, 24, 3537. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, R.; Ma, G.; Zhang, L.; Hirai, M.; Yahata, M.; Yamawaki, K.; Shimada, T.; Fujii, H.; Endo, T.; Kato, M. Effects of Salicylic Acid and Methyl Jasmonate Treatments on Flavonoid and Carotenoid Accumulation in the Juice Sacs of Satsuma Mandarin In Vitro. Appl. Sci. 2020, 10, 8916. [Google Scholar] [CrossRef]
- Salami, M.; Rahimmalek, M.; Ehtemam, M.H. Inhibitory effect of different fennel (Foeniculum vulgare) samples and their phenolic compounds on formation of advanced glycation products and comparison of antimicrobial and antioxidant activities. Food Chem. 2016, 213, 196–205. [Google Scholar] [CrossRef]
- Roby, M.H.H.; Sarhana, M.A.; Selima, K.A.; Khalela, K.I. Antioxidant and Antimicrobial Activities of Essential Oil and Extracts of Fennel (Foeniculum vulgare L.) and Chamomile (Matricaria chamomilla L.). Ind. Crops Prod. 2013, 44, 437–445. [Google Scholar] [CrossRef]
- Kim, H.J.; Chen, F.; Wang, X.; Choi, J.H. Effect of methyl jasmonate on phenolics, isothiocyanate, and metabolic enzymes in radish sprout (Raphanus sativus L.). J. Agric. Food. Chem. 2006, 54, 7263–7269. [Google Scholar] [CrossRef]
- Kim, H.J.; Chen, F.; Wang, X.; Rajapakse, N.C. Effect of methyl jasmonate on secondary metabolites of sweet basil (Ocimum basilicum L.). J. Agric. Food. Chem. 2006, 54, 2327–2332. [Google Scholar] [CrossRef]
- Kim, H.J.; Park, K.J.; Lim, J.H. Metabolomic analysis of phenolic compounds in buckwheat (Fagopyrum esculentum M.) sprouts treated with methyl jasmonate. J. Agric. Food. Chem. 2011, 59, 5707–5713. [Google Scholar] [CrossRef] [PubMed]
- Awasthi, P.; Mahajan, V.; Jamwal, V.L.; Chouhan, R.; Kapoor, N.; Bedi, Y.S.; Gandhi, S.G. Characterization of the gene encoding 4-coumarate: CoA ligase in Coleus forskohlii. J. Plant. Biochem. Biotechnol. 2019, 28, 203–210. [Google Scholar] [CrossRef]
- Deng, Y.; Li, C.; Li, H.; Lu, S.; Iriti, M. Identification and characterization of flavonoid biosynthetic enzyme genes in Salvia miltiorrhiza (Lamiaceae). Molecules 2018, 23, 1467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, C.H.; Yeo, H.J.; Park, Y.E.; Chun, S.W.; Chung, Y.S.; Lee, S.Y.; Park, S.U. Influence of Chitosan, Salicylic Acid and Jasmonic Acid on Phenylpropanoid Accumulation in Germinated Buckwheat (Fagopyrum esculentum Moench). Foods 2019, 8, 153. [Google Scholar] [CrossRef] [Green Version]
- Jaafar, H.Z.; Ibrahim, M.H.; Mohamad-Fakri, N.F. Impact of soil field water capacity on secondary metabolites, phenylalanine ammonia-lyase (PAL), maliondialdehyde (MDA) and photosynthetic responses of Malaysian Kacip Fatimah (Labisia pumila Benth). Molecules 2012, 17, 7305–7322. [Google Scholar] [CrossRef] [Green Version]
- Zhou, P.; Li, Q.; Liu, G.; Xu, N.; Yang, Y.; Zeng, W.; Chen, A.; Wang, S. Integrated analysis of transcriptomic and metabolomic data reveals critical metabolic pathways involved in polyphenol biosynthesis in Nicotiana tabacum under chilling stress. Funct. Plant Biol. 2018, 46, 30–43. [Google Scholar] [CrossRef]
- Gharibi, S.; Sayed Tabatabaei, B.E.; Saeidi, G.; Talebi, M.; Matkowski, A. The effect of drought stress on polyphenolic compounds and expression of flavonoid biosynthesis related genes in Achillea pachycephala Rech.f. Phytochemistry 2019, 162, 90–98. [Google Scholar] [CrossRef]
- Majdi, M.; Abdollahi, M.R.; Maroufi, A. Parthenolide accumulation and expression of genes related to parthenolide biosynthesis affected by exogenous application of methyl jasmonate and salicylic acid in Tanacetum parthenium. Plant. Cell. Rep. 2015, 34, 1909–1918. [Google Scholar] [CrossRef]
- Elyasi, R.; Majdi, M.; Bahramnejad, B.; Mirzaghaderi, G. Spatial modulation and abiotic elicitors responses of the biosynthesis related genes of mono/triterpenes in black cumin (Nigella sativa). Ind. Crops Prod. 2016, 79, 240–247. [Google Scholar] [CrossRef]
- Brouki Milan, E.; Mandoulakani, B.A.; Kheradmand, F. The effect of methyl jasmonate on the expression of phenylalanine ammonia lyase and eugenol-o-methyl transferase genes in basil. Philipp. Agric. Sci. 2017, 100, 163–167. [Google Scholar]
- Chezem, W.R.; Clay, N.K. Regulation of plant secondary metabolism and associated specialized cell development by MYBs and bHLHs. Phytochemistry 2016, 131, 26–43. [Google Scholar] [CrossRef] [Green Version]
- Açıkgoz, M. Establishment of cell suspension cultures of Ocimum basilicum L. and enhanced production of pharmaceutical active ingredients. Ind. Crops Prod. 2020, 148, e112278. [Google Scholar] [CrossRef]
- Li, X.; Kim, J.K.; Park, S.U. Molecular cloning and characterization of rosmarinic acid biosynthetic genes and rosmarinic acid accumulation in Ocimum basilicum L. Saudi. J. Biol. Sci. 2017, 26, 469–472. [Google Scholar]
- Thiyagarajan, K.; Vitali, F.; Tolaini, V.; Galeffi, P.; Cantale, C.; Vikram, P.; Singh, S.; De Rossi, P.; Nobili, C.; Procacci, S.; et al. Genomic characterization of phenylalanine ammonia lyase gene in buckwheat. PLoS ONE 2016, 11, 151187. [Google Scholar] [CrossRef] [Green Version]
- Hao, X.; Pu, Z.; Cao, G.; You, D.; Zhou, Y.; Deng, C.; Shi, M.; Nile, S.H.; Wang, Y.; Zhou, W.; et al. Tanshinone and salvianolic acid biosynthesis are regulated bySmMYB98 in Salvia miltiorrhiza hairy roots. J. Adv. Res. 2020, 23, 1–12. [Google Scholar] [CrossRef]
- Huang, J.; Gu, M.; Lai, Z.; Fan, B.; Shi, K.; Zhou, Y.H.; Yu, J.Q.; Chen, Z. Functional analysis of the Arabidopsis PAL gene family in plant growth, development, and response to environmental stress. Plant Physiol. 2010, 153, 1526–1538. [Google Scholar] [CrossRef] [Green Version]
- Cocetta, G.; Rossoni, M.; Gardana, C.; Mignani, I.; Ferrante, A.; Spinardi, A. Methyl jasmonate affects phenolic metabolism and gene expression in blueberry (Vaccinium corymbosum). Physiol. Plant. 2015, 153, 269–283. [Google Scholar] [CrossRef]
- Biswas, T. Elicitor induced increased rosmarinic acid content of in vitro root cultures of Ocimum basilicum L. (Sweet Basil). Plant Sci. Today 2020, 7, 157–163. [Google Scholar] [CrossRef]
- Sarabandi, M.; Farokhzad, A.; Mandoulakani, B.A.; Ghasemzadeh, R. Biochemical and gene expression responses of two Iranian grape cultivars to foliar application of methyl jasmonate under boron toxicity conditions. Sci. Hort. 2019, 249, 355–363. [Google Scholar] [CrossRef]
- Belhadj, A.; Saigne, C.; Telef, N.; Cluzet, S.; Bouscaut, J.; Corio-Costet, M.F.; Mérillon, J.M. Methyl jasmonate induces defense responses in grapevine and triggers protection against Erysiphe necator. J. Agric. Food Chem. 2006, 54, 9119–9125. [Google Scholar] [CrossRef]
- Farooq, M.A.; Gill, R.A.; Islam, F.; Ali, B.; Liu, H.; Xu, J.; He, S.; Zhou, W. Methyl jasmonate regulates antioxidant defense and suppresses arsenic uptake in Brassica napus L. Front. Plant Sci. 2016, 7, 468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔct method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Skendi, A.; Irakli, M.; Chatzopoulou, P. Analysis of phenolic compounds in Greek plants of Lamiaceae family by HPLC. J. Appl. Res. Med. Aromat. Plants 2017, 6, 62–69. [Google Scholar] [CrossRef]
- Zhang, D.Y.; Yao, X.H.; Duan, M.H.; Wei, F.Y.; Wu, G.H.; Li, L. Variation of essential oil content and antioxidant activity of Lonicera species in different sites of China. Ind. Crops Prod. 2015, 77, 772–779. [Google Scholar] [CrossRef]


| Real-Time Primers | Sequences (5′ to 3′) |
|---|---|
| PAL F PAL R | ACATCCTGGCCGTCCTATC GTCCTGCTCGTGCAGCTT |
| 4CL F 4CL R | GCGATCTTGATCATGCAGAA AAGGTCATATTTGCCCACCA |
| RAS F RAS R | TCGATTTCTTGGAGCTGCAG GCACCCAACTAATCACCCAAAG |
| Actin Actin | ACCTCAAAATAGCATGGGGAAGT GGCCGTTCTCTCACTTTATGCTA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kianersi, F.; Amin Azarm, D.; Fatemi, F.; Jamshidi, B.; Pour-Aboughadareh, A.; Janda, T. The Influence of Methyl Jasmonate on Expression Patterns of Rosmarinic Acid Biosynthesis Genes, and Phenolic Compounds in Different Species of Salvia subg. Perovskia Kar L. Genes 2023, 14, 871. https://doi.org/10.3390/genes14040871
Kianersi F, Amin Azarm D, Fatemi F, Jamshidi B, Pour-Aboughadareh A, Janda T. The Influence of Methyl Jasmonate on Expression Patterns of Rosmarinic Acid Biosynthesis Genes, and Phenolic Compounds in Different Species of Salvia subg. Perovskia Kar L. Genes. 2023; 14(4):871. https://doi.org/10.3390/genes14040871
Chicago/Turabian StyleKianersi, Farzad, Davood Amin Azarm, Farzaneh Fatemi, Bita Jamshidi, Alireza Pour-Aboughadareh, and Tibor Janda. 2023. "The Influence of Methyl Jasmonate on Expression Patterns of Rosmarinic Acid Biosynthesis Genes, and Phenolic Compounds in Different Species of Salvia subg. Perovskia Kar L." Genes 14, no. 4: 871. https://doi.org/10.3390/genes14040871








