The Complete Chloroplast Genomes of Blepharoglossum elegans and B. grossum and Comparative Analysis with Related Species (Orchidaceae, Malaxideae)
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling and DNA Extraction
2.2. DNA Library Preparation and Sequencing
2.3. Chloroplast Genome Assembly and Annotations
2.4. Genome Comparison and Phylogenetic Identification
3. Results and Discussion
3.1. Chloroplast Genome Features
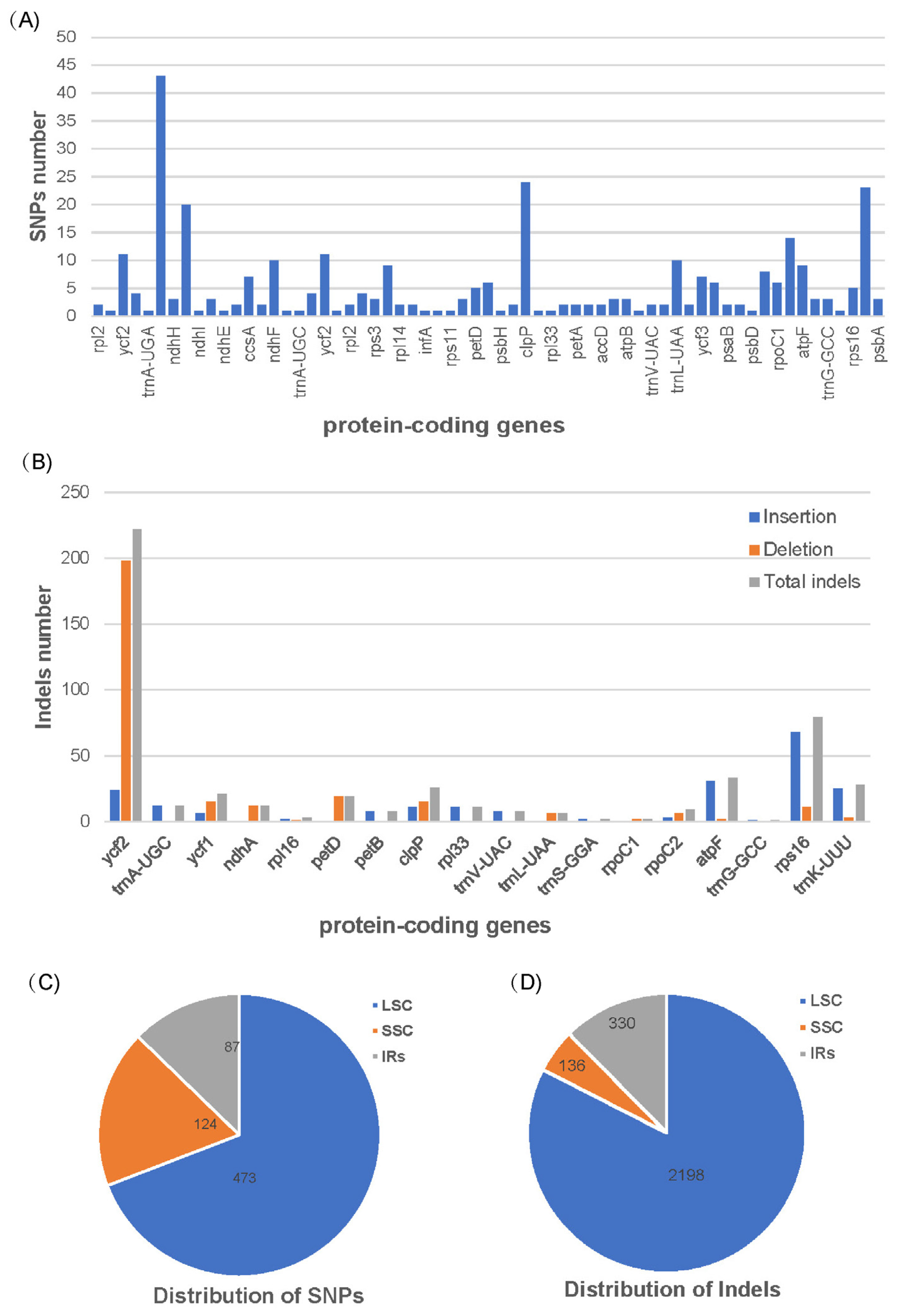
3.2. SNPs and Indels Detection between B. grossum and B. elegans
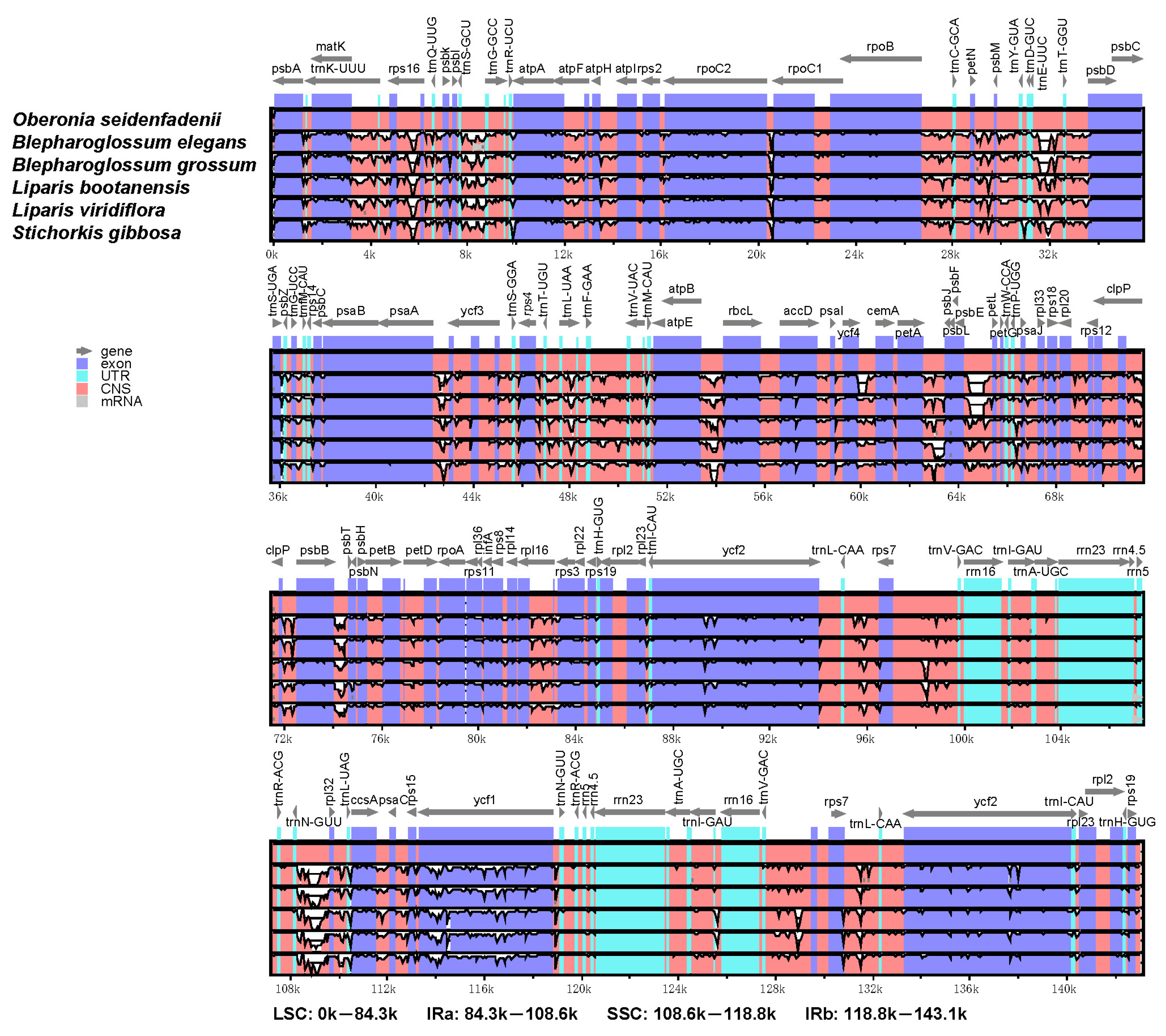
3.3. Details of the IR Contraction and Expansion
3.4. Comparative Analysis of the Chloroplast Genomes
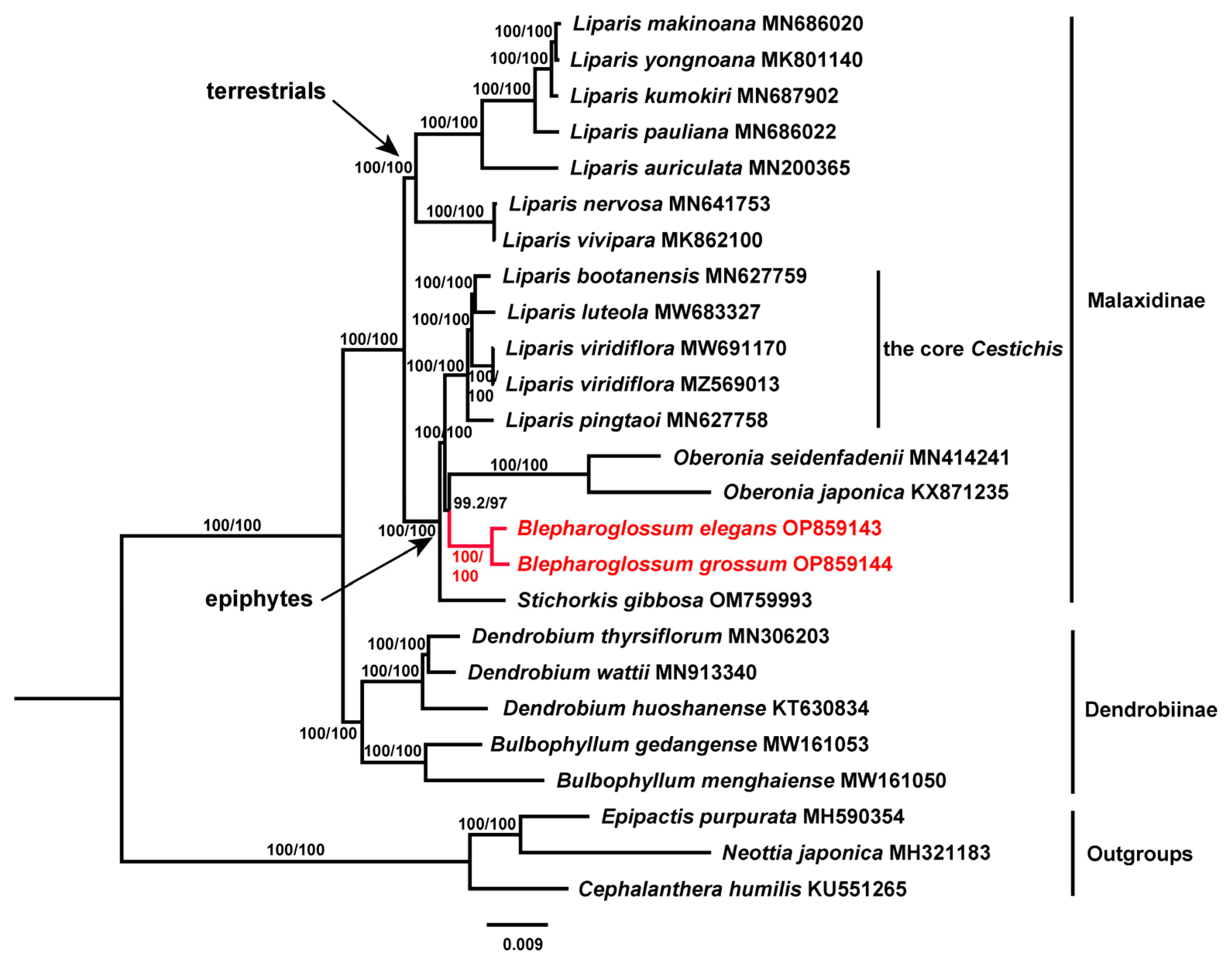
3.5. Phylogenetic Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schlechter, R. Die Orchidaceen von Deutsch-Neu-Guinea. Repertorium specierum novarum regni vegetabilis; Paul Parey: Berlin, Germany, 1914; pp. i–lxvi, 181–220. [Google Scholar]
- Jones, D.L.; Clements, M.A. Miscellaneous nomenclature notes and changes in Australian, New Guinea and New Zealand Orchidaceae. Orchiadian 2005, 15, 22–42. [Google Scholar]
- Li, L.; Chung, S.W.; Li, B.; Zeng, S.J.; Yan, H.F.; Li, S.J. New insight into the molecular phylogeny of the genus Liparis s.l. (Orchidaceae: Malaxideae) with a new generic segregate: Blepharoglossum. Plant Syst. Evol. 2020, 306, 54. [Google Scholar] [CrossRef]
- Ormerod, P.; Juswara, L. Notes on some Malesian Orchidaceae III. Harv. Pap. Bot. 2021, 26, 197–201. [Google Scholar] [CrossRef]
- Olmstead, R.; Palmer, J. Chloroplast DNA systematics: A review of methods and data analysis. Am. J. Bot. 1994, 81, 1205–1224. [Google Scholar] [CrossRef]
- Yang, J.B.; Tang, M.; Li, H.T.; Zhang, Z.R.; Li, D.Z. Complete chloroplast genome of the genus Cymbidium: Lights into the species identification, phylogenetic implications and population genetic analyses. BMC Evol. Biol. 2013, 13, 84. [Google Scholar] [CrossRef]
- Vieira, L.d.N.; Faoro, H.; Rogalski, M.; Fraga, H.; Cardoso, R.L.A.; Souza, E.M.; Pedrosa, F.O.; Nodari, R.; Guerra, M.P. The complete chloroplast genome sequence of Podocarpus lambertii: Genome structure, evolutionary aspects, gene content and SSR detection. PLoS ONE 2014, 9, e90618. [Google Scholar] [CrossRef]
- Daniell, H.; Lin, C.S.; Yu, M.; Chang, W.J. Chloroplast genomes: Diversity, evolution, and applications in genetic engineering. Genome Biol. 2016, 17, 134. [Google Scholar] [CrossRef]
- Viljoen, E.; Odeny, D.A.; Coetzee, M.P.A.; Berger, D.K.; Rees, D.J.G. Application of chloroplast phylogenomics to resolve species relationships within the plant genus Amaranthus. J. Mol. Evol. 2018, 86, 216–239. [Google Scholar] [CrossRef]
- Liu, D.K.; Tu, X.D.; Zhao, Z.; Zeng, M.Y.; Zhang, S.; Ma, L.; Zhang, G.Q.; Wang, M.M.; Liu, Z.J.; Lan, S.R.; et al. Plastid phylogenomic data yield new and robust insights into the phylogeny of Cleisostoma–Gastrochilus clades (Orchidaceae, Aeridinae). Mol. Phylogenet. Evol. 2020, 145, 106729. [Google Scholar] [CrossRef]
- Han, S.Y.; Wang, R.B.; Hong, X.; Wu, C.L.; Zhang, S.J.; Kan, X.Z. Plastomes of Bletilla (Orchidaceae) and phylogenetic implications. Int. J. Mol. Sci. 2022, 23, 10151. [Google Scholar] [CrossRef]
- Han, Y.W.; Duan, D.; Ma, X.F.; Jia, Y.; Liu, Z.L.; Zhao, G.F.; Li, Z.H. Efficient identification of the forest tree species in Aceraceae using DNA barcodes. Front. Plant Sci. 2016, 7, 1707. [Google Scholar] [CrossRef] [PubMed]
- Song, F.; Li, T.; Burgess, K.S.; Feng, Y.; Ge, X.J. Complete plastome sequencing resolves taxonomic relationships among species of Calligonum L. (Polygonaceae) in China. BMC Plant Biol. 2020, 20, 261. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.X.; Chen, Y.S.; Shi, C.M.; Huang, Z.B.; Zhang, Y.; Li, S.K.; Li, Y.; Ye, J.; Yu, C.; Li, Z.; et al. SOAPnuke: A MapReduce acceleration-supported software for integrated quality control and preprocessing of high-throughput sequencing data. GigaScience 2017, 7, gix120. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.J.; Yu, W.B.; Yang, J.B.; Song, Y.; de Pamphilis, C.W.; Yi, T.S.; Li, D.Z. GetOrganelle: A fast and versatile toolkit for accurate de novo assembly of organelle genomes. Genome Biol. 2020, 21, 241. [Google Scholar] [CrossRef]
- Qu, X.J.; Moore, M.J.; Li, D.Z.; Yi, T.S. PGA: A software package for rapid, accurate, and flexible batch annotation of plastomes. Plant Methods 2019, 15, 50. [Google Scholar] [CrossRef]
- Kearse, M.D.; Moir, R.; Wilson, A.; Stones-Havas, S.; Cheung, M.; Sturrock, S.; Buxton, S.; Cooper, A.; Markowitz, S.; Duran, C.; et al. Geneious Basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 2012, 28, 1647–1649. [Google Scholar] [CrossRef]
- Greiner, S.; Lehwark, P.; Bock, R. OrganellarGenomeDRAW (OGDRAW) version 1.3.1: Expanded toolkit for the graphical visualization of organellar genomes. Nucleic Acids Res. 2019, 47, W59–W64. [Google Scholar] [CrossRef]
- Amiryousefi, A.; Hyvönen, J.; Poczai, P. IRscope: An online program to visualize the junction sites of chloroplast genomes. Bioinformatics 2018, 34, 3030–3031. [Google Scholar] [CrossRef]
- Frazer, K.A.; Pachter, L.; Poliakov, A.; Rubin, E.M.; Dubchak, I. VISTA: Computational tools for comparative genomics. Nucleic Acids Res. 2004, 32, W273–W279. [Google Scholar] [CrossRef]
- van den Berg, C.; Goldman, D.H.; Freudenstein, J.V.; Pridgeon, A.M.; Cameron, K.M.; Chase, M.W. An overview of the phylogenetic relationships within Epidendroideae inferred from multiple DNA regions and recircumscription of Epidendreae and Arethuseae (Orchidaceae). Am. J. Bot. 2005, 92, 613–624. [Google Scholar] [CrossRef]
- Nguyen, L.T.; Schmidt, H.A.; von Haeseler, A.; Minh, B.Q. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum likelihood phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Guindon, S.; Dufayard, J.F.; Lefort, V.; Anisimova, M.; Hordijk, W.; Gascuel, O. New algorithms and methods to estimate maximum-likelihood phylogenies: Assessing the performance of PhyML 3. 0. Syst. Biol. 2010, 59, 307–321. [Google Scholar] [CrossRef] [PubMed]
- Minh, B.Q.; Nguyen, M.A.; von Haeseler, A. Ultrafast approximation for phylogenetic bootstrap. Mol. Biol. Evol. 2013, 30, 1188–1195. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.T.; Kim, J.S.; Moore, M.J.; Neubig, K.M.; Williams, N.H.; Whitten, W.M.; Kim, J.H. Seven new complete plastome sequences reveal rampant independent loss of the ndh gene family across Orchids and associated instability of the inverted repeat/small single-copy region boundaries. PLoS ONE 2015, 10, e0142215. [Google Scholar] [CrossRef]
- Niu, Z.T.; Xue, Q.Y.; Zhu, S.Y.; Sun, J.; Liu, W.; Ding, X.Y. The complete plastome sequences of four Orchid species: Insights into the evolution of the Orchidaceae and the utility of plastomic mutational hotspots. Front. Plant Sci. 2017, 8, 715. [Google Scholar] [CrossRef] [PubMed]
- Nanjala, C.; Wanga, V.O.; Odago, W.; Mutinda, E.S.; Waswa, E.N.; Oulo, M.A.; Mkala, E.M.; Kuja, J.; Yang, J.X.; Dong, X.; et al. Plastome structure of 8 Calanthe s.l. species (Orchidaceae): Comparative genomics, phylogenetic analysis. BMC Plant Biol. 2022, 22, 387. [Google Scholar] [CrossRef]
- Asaf, S.; Khan, A.L.; Khan, M.A.; Waqas, M.; Kang, S.M.; Yun, B.W.; Lee, I.J. Chloroplast genomes of Arabidopsis halleri ssp. gemmifera and Arabidopsis lyrata ssp. petraea: Structures and comparative analysis. Sci. Rep. 2017, 7, 7556. [Google Scholar] [CrossRef]
- Albalat, R.; Cañestro, C. Evolution by gene loss. Nat. Rev. Genet. 2016, 17, 379–391. [Google Scholar] [CrossRef]
- Han, C.; Ding, R.; Zong, X.; Zhang, L.; Chen, X.; Qu, B. Structural characterization of Platanthera ussuriensis chloroplast genome and comparative analyses with other species of Orchidaceae. BMC Genom. 2022, 23, 84. [Google Scholar] [CrossRef]
- Cameron, K.M. Leave it to the leaves: A molecular phylogenetic study of malaxideae (Epidendroideae, Orchidaceae). AM. J. Bot. 2005, 92, 1025–1032. [Google Scholar] [CrossRef]
- Tang, G.D.; Zhang, G.Q.; Hong, W.J.; Liu, Z.J.; Zhuang, X.Y. Phylogenetic analysis of Malaxideae (Orchidaceae: Epidendroideae): Two new species based on the combined nrDNA ITS and chloroplast matK sequences. Guihaia 2015, 35, 447–463. [Google Scholar]
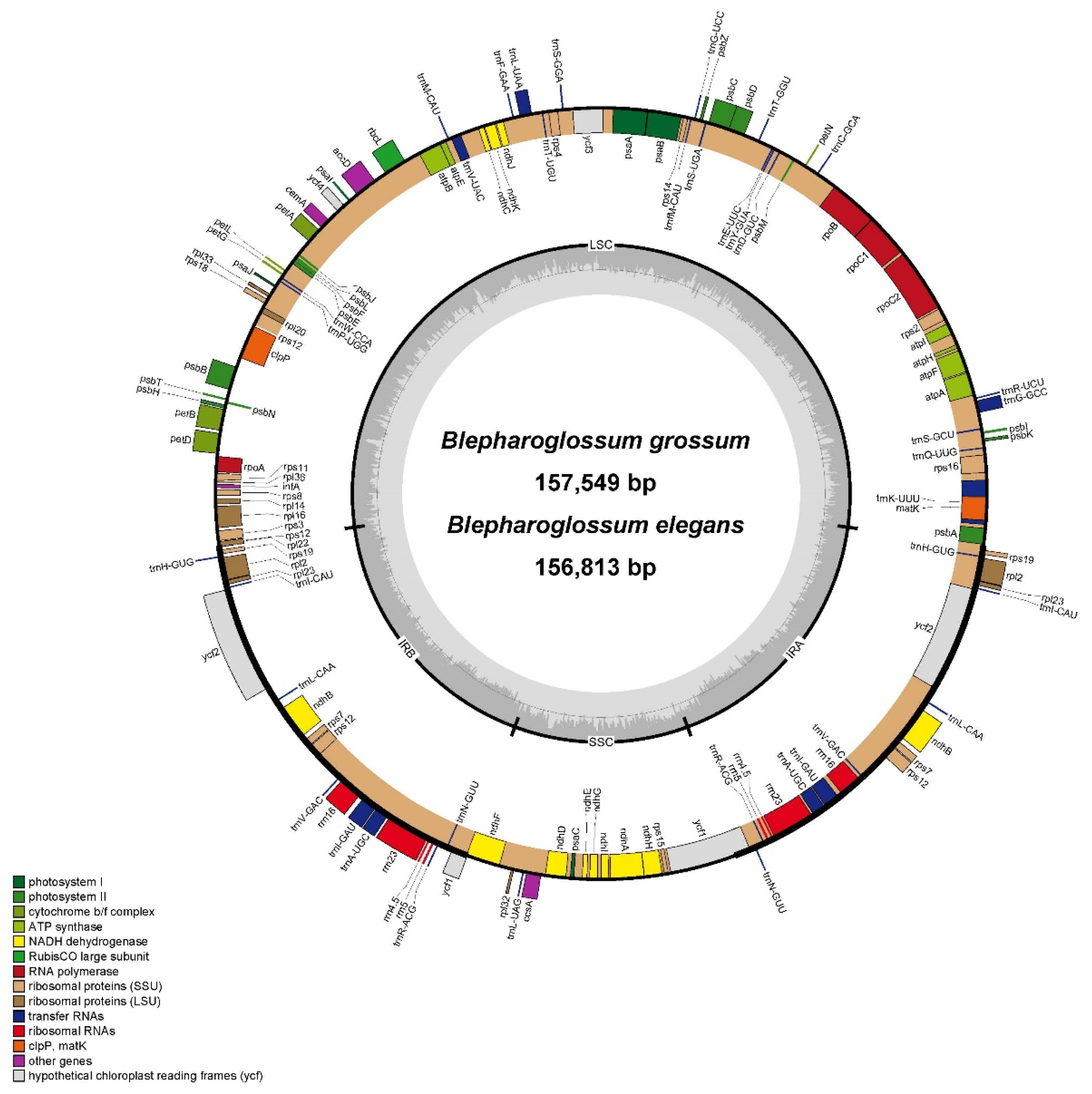

| Species | B. elegans | B. grossum | L. bootanensis | L. viridiflora | S. gibbosa | O. seidenfadenii | |
|---|---|---|---|---|---|---|---|
| GenBank ID | data | OP859143 | OP859144 | MN627759 | MW691170 | OM759993 | MN414241 |
| Length (bp) | Total | 156,813 | 157,549 | 158,325 | 158,258 | 158,056 | 143,062 |
| LSC | 84,984 | 85,588 | 86,584 | 86,668 | 86,280 | 84,282 | |
| SSC | 17,731 | 17,765 | 17,775 | 17,806 | 17,764 | 10,224 | |
| IR | 27,049 | 27,098 | 26,983 | 26,892 | 27,006 | 24,278 | |
| Gene number | Total | 133 | 133 | 133 | 133 | 133 | 120 |
| CDS | 87 | 87 | 83 | 83 | 86 | 74 | |
| tRNA | 38 | 38 | 38 | 38 | 38 | 38 | |
| rRNA | 8 | 8 | 8 | 8 | 8 | 8 | |
| GC content (%) | Total | 36.8 | 36.8 | 36.9 | 36.8 | 36.9 | 37.1 |
| LSC | 34.3 | 34.4 | 34.5 | 34.3 | 34.4 | 34.4 | |
| SSC | 29.6 | 29.8 | 30.0 | 29.8 | 29.7 | 27.9 | |
| IR | 43.0 | 43.1 | 43.1 | 43.1 | 43.1 | 43.7 |
| Gene Group | Gene Name |
|---|---|
| photosystem I | psaA, psaB, psaC, psaI, psaJ |
| photosystem II | psbA, psbB, psbC, psbD, psbE, psbF, psbH, psbI, psbJ, psbK, psbL, psbM, psbN, psbT, psbZ |
| NADH dehydrogenase | ndhA*, ndhB*(2), ndhC, ndhD, ndhE, ndhF, ndhG, ndhH, ndhI, ndhJ, ndhK |
| Cytochrome b/f | petA, petB*, petD*, petG, petL, petN |
| ATP synthase | atpA, atpB, atpE, atpF*, atpH, atpI |
| rubisco | rbcL |
| Proteins of large ribosomal subunit | rpl14, rpl16*, rpl2*(2), rpl20, rpl22, rpl23(2), rpl32, rpl33, rpl36 |
| Proteins of small ribosomal subunit | rps11, rps12**(2), rps14, rps15, rps16*, rps18, rps19(2), rps2, rps3, rps4, rps7(2), rps8 |
| RNA polymerase | rpoA, rpoB, rpoC1*, rpoC2 |
| Ribosomal RNAs | rrn16(2), rrn23(2), rrn4.5(2), rrn5(2) |
| Transfer RNAs | trnA-UGC*(2), trnC-GCA, trnD-GUC, trnE-UUC, trnF-GAA, trnG-GCC*, trnG-UCC*, trnH-GUG(2), trnI-CAU(2), trnI-GAU*(2), trnK-UUU*, trnL-CAA(2), trnL-UAA*, trnL-UAG, trnM-CAU, trnN-GUU(2), trnP-UGG, trnQ-UUG, trnR-ACG(2), trnR-UCU, trnS-GCU, trnS-GGA, trnS-UGA, trnT-GGU, trnT-UGU, trnV-GAC(2), trnV-UAC*, trnW-CCA, trnY-GUA, trnfM-CAU |
| Maturase | matK |
| Protease | clpP** |
| Envelope membrane protein | cemA |
| Acetyl-CoA carboxylase | accD |
| c-type cytochrome synthesis gene | ccsA |
| Translation initiation factor | infA |
| Conserved hypothetical chloroplast ORF | ycf1(2), ycf2(2), ycf3**, ycf4 |
| SNPs | Indels | ||
|---|---|---|---|
| Insertions | Deletions | ||
| CDS | 321 | 212 | 290 |
| IGS | 364 | 752 | 1410 |
| Total | 684 | 964 | 1700 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, W.; Wu, K.; Fang, L.; Zeng, S.; Li, L. The Complete Chloroplast Genomes of Blepharoglossum elegans and B. grossum and Comparative Analysis with Related Species (Orchidaceae, Malaxideae). Genes 2023, 14, 1069. https://doi.org/10.3390/genes14051069
Yang W, Wu K, Fang L, Zeng S, Li L. The Complete Chloroplast Genomes of Blepharoglossum elegans and B. grossum and Comparative Analysis with Related Species (Orchidaceae, Malaxideae). Genes. 2023; 14(5):1069. https://doi.org/10.3390/genes14051069
Chicago/Turabian StyleYang, Wenting, Kunlin Wu, Lin Fang, Songjun Zeng, and Lin Li. 2023. "The Complete Chloroplast Genomes of Blepharoglossum elegans and B. grossum and Comparative Analysis with Related Species (Orchidaceae, Malaxideae)" Genes 14, no. 5: 1069. https://doi.org/10.3390/genes14051069
APA StyleYang, W., Wu, K., Fang, L., Zeng, S., & Li, L. (2023). The Complete Chloroplast Genomes of Blepharoglossum elegans and B. grossum and Comparative Analysis with Related Species (Orchidaceae, Malaxideae). Genes, 14(5), 1069. https://doi.org/10.3390/genes14051069






