Single-Cell Analysis in the Omics Era: Technologies and Applications in Cancer
Abstract
1. Introduction
2. Single-Cell Isolation
2.1. Laser Microdissection
2.2. Fluorescence-Activated Cell Sorter (FACS)
2.3. Microfluidics
2.4. Microwell-Based Systems
2.5. Magnetic-Activated Cell Sorting (MACS)
3. Methods for Functional Genomics at Single-Cell Resolution
3.1. Single Cell Genomics
3.2. Single-Cell Epigenomics
3.3. Single-Cell Transcriptomics
4. Single-Cell Data Analysis and Bioinformatic Approaches
4.1. Machine Learning for Functional Single-Cell Omics Data Analysis
4.2. Data Portals Comprising Multi-Omics Data
5. Limitations and Pitfalls of Single-Cell Analysis
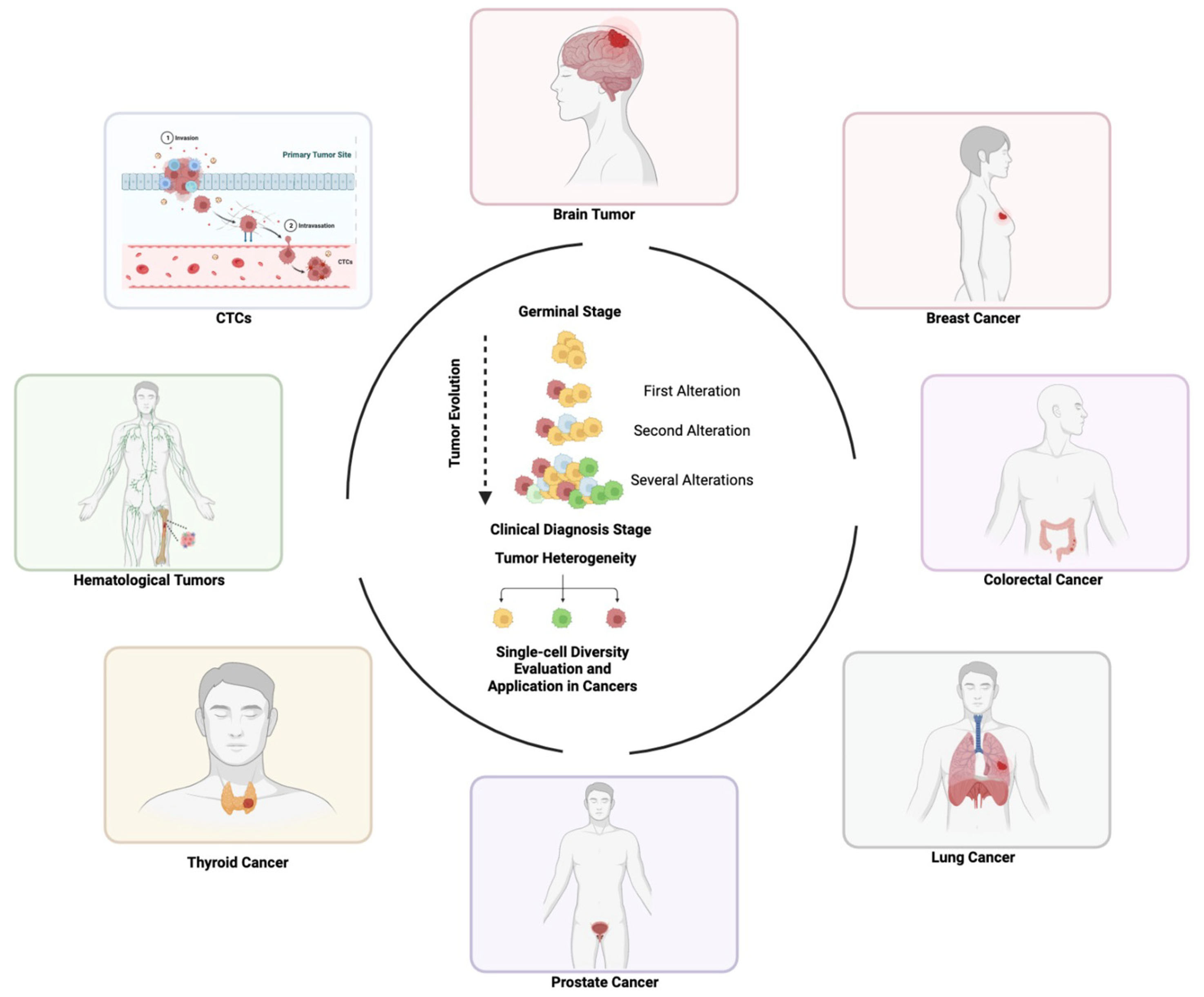
6. Application of Functional Single-Cell Genomics in Cancer
6.1. Brain Tumors
6.2. Breast Cancer
6.3. Colorectal Cancer
6.4. Lung Cancer
6.5. Prostate Cancer
6.6. Thyroid Cancer
6.7. Hematological Malignancies
6.8. Circulating Tumor Cells
7. Summary and Future Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tsimberidou, A.M.; Fountzilas, E.; Nikanjam, M.; Kurzrock, R. Review of precision cancer medicine: Evolution of the treatment paradigm. Cancer Treat. Rev. 2020, 86, 102019. [Google Scholar] [CrossRef]
- Gerlinger, M.; Rowan, A.J.; Horswell, S.; Math, M.; Larkin, J.; Endesfelder, D. Faculty Opinions recommendation of Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N. Engl. J. Med. 2016, 366, 883–892. [Google Scholar] [CrossRef] [PubMed]
- McGranahan, N.; Swanton, C. Clonal Heterogeneity and Tumor Evolution: Past, Present, and the Future. Cell 2017, 168, 613–628. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.; Zhang, W.; Xin, H.; Deng, G. Single Cell Isolation and Analysis. Front. Cell Dev. Biol. 2016, 4, 116. [Google Scholar] [CrossRef] [PubMed]
- Ignatiadis, M.; Sledge, G.W.; Jeffrey, S.S. Liquid biopsy enters the clinic—Implementation issues and future challenges. Nat. Rev. Clin. Oncol. 2021, 18, 297–312. [Google Scholar] [CrossRef]
- Lin, D.; Shen, L.; Luo, M.; Zhang, K.; Li, J.; Yang, Q.; Zhu, F.; Zhou, D.; Zheng, S.; Chen, Y.; et al. Circulating tumor cells: Biology and clinical significance. Signal Transduct. Target. Ther. 2021, 6, 404. [Google Scholar] [CrossRef]
- Visal, T.H.; den Hollander, P.; Cristofanilli, M.; Mani, S.A. Circulating tumour cells in the -omics era: How far are we from achieving the ‘singularity’? Br. J. Cancer 2022, 127, 173–184. [Google Scholar] [CrossRef]
- Radfar, P.; Es, H.A.; Salomon, R.; Kulasinghe, A.; Ramalingam, N.; Sarafraz-Yazdi, E.; Thiery, J.P.; Warkiani, M.E. Single-cell analysis of circulating tumour cells: Enabling technologies and clinical applications. Trends Biotechnol. 2022, 40, 1041–1060. [Google Scholar] [CrossRef]
- Kaur, R.P.; Ludhiadch, A.; Munshi, A. Chapter 9–Single-Cell Genomics: Technology and Applications. In Single-Cell Omics; Barh, D., Azevedo, V., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 179–197. [Google Scholar]
- Zeb, Q.; Wang, C.; Shafiq, S.; Liu, L. Chapter 6–An Overview of Single-Cell Isolation Techniques. In Single-Cell Omics; Barh, D., Azevedo, V., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 101–135. [Google Scholar]
- Lee, J.; Hyeon, D.Y.; Hwang, D. Single-cell multiomics: Technologies and data analysis methods. Exp. Mol. Med. 2020, 52, 1428–1442. [Google Scholar] [CrossRef]
- Ma, Q.; Xu, D. Deep learning shapes single-cell data analysis. Nat. Rev. Mol. Cell Biol. 2022, 23, 303–304. [Google Scholar] [CrossRef]
- Liu, J.; Fan, Z.; Zhao, W.; Zhou, X. Machine Intelligence in Single-Cell Data Analysis: Advances and New Challenges. Front. Genet. 2021, 12, 655536. [Google Scholar] [CrossRef] [PubMed]
- Fend, F.; Raffeld, M. Laser capture microdissection in pathology. J. Clin. Pathol. 2000, 53, 666–672. [Google Scholar] [CrossRef] [PubMed]
- Emmert-Buck, M.R.; Bonner, R.F.; Smith, P.D.; Chuaqui, R.F.; Zhuang, Z.; Goldstein, S.R. Laser capture microdissection. Science 1996, 274, 998–1001. [Google Scholar] [CrossRef]
- Geslewitz, W.E.; Percopo, C.M.; Rosenberg, H.F. FACS isolation of live mouse eosinophils at high purity via a protocol that does not target Siglec F. J. Immunol. Methods 2018, 454, 27–31. [Google Scholar] [CrossRef]
- Hu, Y.; Fan, L.; Zheng, J.; Cui, R.; Liu, W.; He, Y.; Li, X.; Huang, S. Detection of circulating tumor cells in breast cancer patients utilizing multiparameter flow cytometry and assessment of the prognosis of patients in different CTCs levels. Cytom. Part A 2010, 77A, 213–219. [Google Scholar] [CrossRef]
- Al-Mawali, A.; Gillis, D.; Lewis, I. The Role of Multiparameter Flow Cytometry for Detection of Minimal Residual Disease in Acute Myeloid Leukemia. Am. J. Clin. Pathol. 2009, 131, 16–26. [Google Scholar] [CrossRef]
- Schulz, K.R.; Danna, E.A.; Krutzik, P.O.; Nolan, G.P. Single-cell phospho-protein analysis by flow cytometry. Curr. Protoc. Immunol. 2012, 96, 8–17. [Google Scholar] [CrossRef]
- Hwang, B.; Lee, J.H.; Bang, D. Single-cell RNA sequencing technologies and bioinformatics pipelines. Exp. Mol. Med. 2018, 50, 96. [Google Scholar] [CrossRef] [PubMed]
- Shinde, P.; Mohan, L.; Kumar, A.; Dey, K.; Maddi, A.; Patananan, A.N.; Tseng, F.-G.; Chang, H.-Y.; Nagai, M.; Santra, T.S. Current Trends of Microfluidic Single-Cell Technologies. Int. J. Mol. Sci. 2018, 19, 3143. [Google Scholar] [CrossRef]
- Zheng, G.X.; Terry, J.M.; Belgrader, P.; Ryvkin, P.; Bent, Z.W.; Wilson, R.; Ziraldo, S.B.; Wheeler, T.D.; McDermott, G.P.; Zhu, J.; et al. Massively parallel digital transcriptional profiling of single cells. Nat. Commun. 2017, 8, 14049. [Google Scholar] [CrossRef]
- Li, H.; Humphreys, B.D. Single Cell Technologies: Beyond Microfluidics. Kidney360 2021, 2, 1196–1204. [Google Scholar] [CrossRef]
- Abonnenc, M.; Manaresi, N.; Borgatti, M.; Medoro, G.; Fabbri, E.; Romani, A.; Altomare, L.; Tartagni, M.; Rizzo, R.; Baricordi, O.; et al. Programmable Interactions of Functionalized Single Bioparticles in a Dielectrophoresis-Based Microarray Chip. Anal. Chem. 2013, 85, 8219–8224. [Google Scholar] [CrossRef] [PubMed]
- Gossett, D.R.; Weaver, W.M.; Mach, A.J.; Hur, S.C.; Tse, H.T.K.; Lee, W.; Amini, H.; Di Carlo, D. Label-free cell separation and sorting in microfluidic systems. Anal. Bioanal. Chem. 2010, 397, 3249–3267. [Google Scholar] [CrossRef] [PubMed]
- Swennenhuis, J.F.; van Dalum, G.; Zeune, L.L.; Terstappen, L.W. Improving the CellSearch(R) system. Expert Rev. Mol. Diagn. 2016, 16, 1291–1305. [Google Scholar] [CrossRef]
- Sturm, D.; Marselli, L.; Ehehalt, F.; Richter, D.; Distler, M.; Kersting, S. Improved protocol for laser microdissection of human pancreatic islets from surgical specimens. J. Vis. Exp. 2013, 71, 50231. [Google Scholar]
- Esposito, G. Complementary techniques: Laser capture microdissection--increasing specificity of gene expression profiling of cancer specimens. Adv. Exp. Med. Biol. 2007, 593, 54–65. [Google Scholar]
- Allard, W.J.; Matera, J.; Miller, M.C.; Repollet, M.; Connelly, M.C.; Rao, C.; Tibbe, A.G.; Uhr, J.W.; Terstappen, L.W. Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clin. Cancer Res. 2004, 10, 6897–6904. [Google Scholar] [CrossRef] [PubMed]
- Hayes, D.F.; Cristofanilli, M.; Budd, G.T.; Ellis, M.J.; Stopeck, A.; Miller, M.C.; Matera, J.; Allard, W.J.; Doyle, G.V.; Terstappen, L.W. Circulating Tumor Cells at Each Follow-up Time Point during Therapy of Metastatic Breast Cancer Patients Predict Progression-Free and Overall Survival. Clin. Cancer Res. 2006, 12 Pt 1, 4218–4224. [Google Scholar] [CrossRef] [PubMed]
- De Bono, J.S.; Scher, H.I.; Montgomery, R.B.; Parker, C.; Miller, M.C.; Tissing, H.; Doyle, G.V.; Terstappen, L.W.; Pienta, K.J.; Raghavan, D. Faculty Opinions recommendation of Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer. Clin Cancer Res. 2008, 14, 596066. [Google Scholar] [CrossRef]
- Cohen, S.J.A.; Punt, C.J.; Iannotti, N.; Saidman, B.H.; Sabbath, K.D.; Gabrail, N.Y.; Picus, J.; Morse, M.; Mitchell, E.; Miller, M.C.; et al. Relationship of Circulating Tumor Cells to Tumor Response, Progression-Free Survival, and Overall Survival in Patients with Metastatic Colorectal Cancer. J. Clin. Oncol. 2008, 26, 3213–3221. [Google Scholar] [CrossRef]
- Di Trapani, M.; Manaresi, N.; Medoro, G. DEPArray™ system: An automatic image-based sorter for isolation of pure circulating tumor cells. Cytom. Part A 2018, 93, 1260–1266. [Google Scholar] [CrossRef] [PubMed]
- Stevens, M.; Oomens, L.; Broekmaat, J.; Weersink, J.; Abali, F.; Swennenhuis, J.; Tibbe, A. VyCAP’s puncher technology for single cell identification, isolation, and analysis. Cytom. A 2018, 93, 1255–1259. [Google Scholar] [CrossRef] [PubMed]
- Swennenhuis, J.F.; Tibbe, A.G.J.; Stevens, M.; Katika, M.R.; van Dalum, J.; Tong, H.D.; van Rijn, C.J.M.; Terstappen, L.W.M.M. Self-seeding microwell chip for the isolation and characterization of single cells. Lab Chip 2015, 15, 3039–3046. [Google Scholar] [CrossRef] [PubMed]
- Hong, T.H.; Park, W.-Y. Single-cell genomics technology: Perspectives. Exp. Mol. Med. 2020, 52, 1407–1408. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Ma, F.; Chapman, A.; Lu, S.; Xie, X.S. Single-Cell Whole-Genome Amplification and Sequencing: Methodology and Applications. Annu. Rev. Genom. Hum. Genet. 2015, 16, 79–102. [Google Scholar] [CrossRef] [PubMed]
- Himmelbauer, H.; Schalkwyk, L.C.; Lehrach, H. Interspersed repetitive sequence (IRS)-PCR for typing of whole genome radiation hybrid panels. Nucleic Acids Res. 2000, 28, e7. [Google Scholar] [CrossRef]
- Sun, F.; Arnheim, N.; Waterman, M.S. Whole genome amplification of single cells: Mathematical analysis of PEP and tagged PCR. Nucleic Acids Res. 1995, 23, 3034–3040. [Google Scholar] [CrossRef] [PubMed]
- Spits, C.; Le Caignec, C.; De Rycke, M.; Van Haute, L.; Van Steirteghem, A.; Liebaers, I.; Sermon, K. Whole-genome multiple displacement amplification from single cells. Nat. Protoc. 2006, 1, 1965–1970. [Google Scholar] [CrossRef]
- Zong, C. Multiple Annealing and Looping-Based Amplification Cycles (MALBAC) for the Analysis of DNA Copy Number Variation. In Genomic Mosaicism in Neurons and Other Cell Types; Frade, J.M., Gage, F.H., Eds.; Springer: New York, NY, USA, 2017; pp. 133–142. [Google Scholar]
- Zong, C.; Lu, S.; Chapman, A.R.; Xie, X.S. Genome-Wide Detection of Single-Nucleotide and Copy-Number Variations of a Single Human Cell. Science 2012, 338, 1622–1626. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Zhang, F.; Zhang, X.; Yin, J.; Du, M.; Jiang, M.; Liu, L.; Li, J.; Huang, Y.; Wang, J. High-throughput single-cell whole-genome amplification through centrifugal emulsification and eMDA. Commun. Biol. 2019, 2, 147. [Google Scholar] [CrossRef]
- Clark, S.J.; Lee, H.J.; Smallwood, S.A.; Kelsey, G.; Reik, W. Single-cell epigenomics: Powerful new methods for understanding gene regulation and cell identity. Genome Biol. 2016, 17, 72. [Google Scholar] [CrossRef]
- Shema, E.; Bernstein, B.E.; Buenrostro, J.D. Single-cell and single-molecule epigenomics to uncover genome regulation at unprecedented resolution. Nat. Genet. 2019, 51, 19–25. [Google Scholar] [CrossRef]
- Rotem, A.; Ram, O.; Shoresh, N.; Sperling, R.A.; Goren, A.; Weitz, D.A.; Bernstein, B.E. Single-cell ChIP-seq reveals cell subpopulations defined by chromatin state. Nat. Biotechnol. 2015, 33, 1165–1172. [Google Scholar] [CrossRef] [PubMed]
- Sandberg, R. Entering the era of single-cell transcriptomics in biology and medicine. Nat. Methods 2013, 11, 22–24. [Google Scholar] [CrossRef]
- Zucha, D.; Kubista, M.; Valihrach, L. Tutorial: Guidelines for Single-Cell RT-qPCR. Cells 2021, 10, 2607. [Google Scholar] [CrossRef] [PubMed]
- Lebrigand, K.; Magnone, V.; Barbry, P.; Waldmann, R. High throughput error corrected Nanopore single cell transcriptome sequencing. Nat. Commun. 2020, 11, 4025. [Google Scholar] [CrossRef]
- Sheng, K.; Zong, C. Single-Cell RNA-Seq by Multiple Annealing and Tailing-Based Quantitative Single-Cell RNA-Seq (MATQ-Seq). In Single Cell Methods; Proserpio, V., Ed.; Methods in Molecular Biology; Springer: New York, NY, USA, 2019; Volume 1979, pp. 57–71. ISBN 9781493992393. [Google Scholar]
- Picelli, S.; Faridani, O.R.; Björklund, K.; Winberg, G.; Sagasser, S.; Sandberg, R. Full-length RNA-seq from single cells using Smart-seq2. Nat. Protoc. 2014, 9, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Hashimshony, T.; Senderovich, N.; Avital, G.; Klochendler, A.; de Leeuw, Y.; Anavy, L.; Gennert, D.; Li, S.; Livak, K.J.; Rozenblatt-Rosen, O.; et al. CEL-Seq2: Sensitive highly-multiplexed single-cell RNA-Seq. Genome Biol. 2016, 17, 77. [Google Scholar] [CrossRef]
- Jaitin, D.A.; Kenigsberg, E.; Keren-Shaul, H.; Elefant, N.; Paul, F.; Zaretsky, I.; Mildner, A.; Cohen, N.; Jung, S.; Tanay, A.; et al. Massively Parallel Single-Cell RNA-Seq for Marker-Free Decomposition of Tissues into Cell Types. Science 2014, 343, 776–779. [Google Scholar] [CrossRef]
- Kim, J.; Marignani, P.A. Single-Cell RNA Sequencing Analysis Using Fluidigm C1 Platform for Characterization of Heterogeneous Transcriptomes. Methods Mol. Biol. 2022, 2508, 261–278. [Google Scholar] [CrossRef]
- Gong, H.; Do, D.; Ramakrishnan, R. Single-Cell mRNA-Seq Using the Fluidigm C1 System and Integrated Fluidics Circuits. Methods Mol. Biol. 2018, 1783, 193–207. [Google Scholar]
- Marx, V. Method of the Year: Spatially resolved transcriptomics. Nat. Methods 2021, 18, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, X. Spatially resolved single-cell genomics and transcriptomics by imaging. Nat. Methods 2021, 18, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Larsson, L.; Frisén, J.; Lundeberg, J. Spatially resolved transcriptomics adds a new dimension to genomics. Nat. Methods 2021, 18, 15–18. [Google Scholar] [CrossRef] [PubMed]
- Crosetto, N.; Bienko, M.; van Oudenaarden, A. Spatially resolved transcriptomics and beyond. Nat. Rev. Genet. 2014, 16, 57–66. [Google Scholar] [CrossRef]
- Ahmed, R.; Zaman, T.; Chowdhury, F.; Mraiche, F.; Tariq, M.; Ahmad, I.S.; Hasan, A. Single-Cell RNA Sequencing with Spatial Transcriptomics of Cancer Tissues. Int. J. Mol. Sci. 2022, 23, 3042. [Google Scholar] [CrossRef]
- Ståhl, P.L.; Salmén, F.; Vickovic, S.; Lundmark, A.; Navarro, J.F.; Magnusson, J.; Giacomello, S.; Asp, M.; Westholm, J.O.; Huss, M.; et al. Visualization and analysis of gene expression in tissue sections by spatial transcriptomics. Science 2016, 353, 78–82. [Google Scholar] [CrossRef]
- Williams, C.G.; Lee, H.J.; Asatsuma, T.; Vento-Tormo, R.; Haque, A. An introduction to spatial transcriptomics for biomedical research. Genome Med. 2022, 14, 68. [Google Scholar] [CrossRef]
- Wang, N.; Li, X.; Wang, R.; Ding, Z. Spatial transcriptomics and proteomics technologies for deconvoluting the tumor microenvironment. Biotechnol. J. 2021, 16, 2100041. [Google Scholar] [CrossRef]
- Merritt, C.R.; Ong, G.T.; Church, S.E.; Barker, K.; Danaher, P.; Geiss, G.; Hoang, M.; Jung, J.; Liang, Y.; McKay-Fleisch, J.; et al. Multiplex digital spatial profiling of proteins and RNA in fixed tissue. Nat. Biotechnol. 2020, 38, 586–599. [Google Scholar] [CrossRef]
- Hernandez, S.; Lazcano, R.; Serrano, A.; Powell, S.; Kostousov, L.; Mehta, J.; Khan, K.; Lu, W.; Solis, L.M. Challenges and Opportunities for Immunoprofiling Using a Spatial High-Plex Technology: The NanoString GeoMx((R)) Digital Spatial Profiler. Front Oncol. 2022, 12, 890410. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Tran, V.; Vemuri, V.N.P.; Byrne, A.; Borja, M.; Kim, Y.J.; Agarwal, S.; Wang, R.; Awayan, K.; Murti, A.; et al. Concordance of MERFISH spatial transcriptomics with bulk and single-cell RNA sequencing. Life Sci. Alliance 2022, 6, e202201701. [Google Scholar] [CrossRef] [PubMed]
- Xia, C.; Fan, J.; Emanuel, G.; Hao, J.; Zhuang, X. Spatial transcriptome profiling by MERFISH reveals subcellular RNA compartmentalization and cell cycle-dependent gene expression. Proc. Natl. Acad. Sci. USA 2019, 116, 19490–19499. [Google Scholar] [CrossRef]
- La Manno, G.; Soldatov, R.; Zeisel, A.; Braun, E.; Hochgerner, H.; Petukhov, V.; Lidschreiber, K.; Kastriti, M.E.; Lönnerberg, P.; Furlan, A.; et al. RNA velocity of single cells. Nature 2018, 560, 494–498. [Google Scholar] [CrossRef] [PubMed]
- Abdelaal, T.; Lelieveldt, B.P.F.; Reinders, M.J.T.; Mahfouz, A. SIRV: Spatial inference of RNA velocity at the single-cell resolution. bioRxiv 2021. preprint. [Google Scholar] [CrossRef]
- Yalcin, D.; Hakguder, Z.M.; Otu, H.H. Bioinformatics approaches to single-cell analysis in developmental biology. Mol. Hum. Reprod. 2015, 22, 182–192. [Google Scholar] [CrossRef]
- Lawrence, M.S.; Stojanov, P.; Polak, P.; Kryukov, G.V.; Cibulskis, K.; Sivachenko, A.; Carter, S.L.; Stewart, C.; Mermel, C.H.; Roberts, S.A.; et al. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature 2013, 499, 214–218. [Google Scholar] [CrossRef]
- Andersson, R.; Sandelin, A. Determinants of enhancer and promoter activities of regulatory elements. Nat. Rev. Genet. 2019, 21, 71–87. [Google Scholar] [CrossRef]
- Poulos, R.; Olivier, J.; Wong, J.W. The interaction between cytosine methylation and processes of DNA replication and repair shape the mutational landscape of cancer genomes. Nucleic Acids Res. 2017, 45, 7786–7795. [Google Scholar] [CrossRef]
- Daley, T.; Smith, A.D. Modeling genome coverage in single-cell sequencing. Bioinformatics 2014, 30, 3159–3165. [Google Scholar] [CrossRef]
- Deger, T.; Mendelaar, P.A.J.; Kraan, J.; van der Smissen, W.J.P.; Daane, M.V.D.V.; Bindels, E.M.J.; Sieuwerts, A.M.; Sleijfer, S.; Wilting, S.M.; Hollestelle, A.; et al. A pipeline for copy number profiling of single circulating tumour cells to assess intrapatient tumour heterogeneity. Mol. Oncol. 2022, 16, 2981–3000. [Google Scholar] [CrossRef] [PubMed]
- Law, C.W.; Chen, Y.; Shi, W.; Smyth, G.K. Voom: Precision weights unlock linear model analysis tools for RNA-seq read counts. Genome Biol. 2014, 15, R29. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Ritchie, M.E.; Phipson, B.; Wu, D.; Hu, Y.; Law, C.W.; Shi, W.; Smyth, G.K. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015, 43, e47. [Google Scholar] [CrossRef]
- Robinson, M.D.; Oshlack, A. A scaling normalization method for differential expression analysis of RNA-seq data. Genome Biol. 2010, 11, R25. [Google Scholar] [CrossRef]
- Risso, D.; Ngai, J.; Speed, T.P.; Dudoit, S. Normalization of RNA-seq data using factor analysis of control genes or samples. Nat. Biotechnol. 2014, 32, 896–902. [Google Scholar] [CrossRef]
- Sha, Y.; Phan, J.H.; Wang, M.D. Effect of low-expression gene filtering on detection of differentially expressed genes in RNA-seq data. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2015, 2015, 6461–6464. [Google Scholar] [CrossRef]
- Hafemeister, C.; Satija, R. Normalization and variance stabilization of single-cell RNA-seq data using regularized negative binomial regression. Genome Biol. 2019, 20, 296. [Google Scholar] [CrossRef]
- Townes, F.W.; Hicks, S.C.; Aryee, M.J.; Irizarry, R.A. Feature selection and dimension reduction for single-cell RNA-Seq based on a multinomial model. Genome Biol. 2019, 20, 295. [Google Scholar] [CrossRef] [PubMed]
- Vahid, M.R.; Brown, E.L.; Steen, C.B.; Zhang, W.; Jeon, H.S.; Kang, M.; Gentles, A.J.; Newman, A.M. High-resolution alignment of single-cell and spatial transcriptomes with CytoSPACE. Nat. Biotechnol. 2023. online ahead of print. [Google Scholar] [CrossRef]
- Rodriques, S.G.; Stickels, R.R.; Goeva, A.; Martin, C.A.; Murray, E.; Vanderburg, C.R.; Welch, J.; Chen, L.M.; Chen, F.; Macosko, E.Z. Slide-seq: A scalable technology for measuring genome-wide expression at high spatial resolution. Science 2019, 363, 1463–1467. [Google Scholar] [CrossRef] [PubMed]
- Bouras, E.; Karakioulaki, M.; Bougioukas, K.; Aivaliotis, M.; Tzimagiorgis, G.; Chourdakis, M. Gene promoter methylation and cancer: An umbrella review. Gene 2019, 710, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, A.; Nakanishi, M. Navigating the DNA methylation landscape of cancer. Trends Genet. 2021, 37, 1012–1027. [Google Scholar] [CrossRef]
- Hu, Y.; Huang, K.; An, Q.; Du, G.; Hu, G.; Xue, J.; Zhu, X.; Wang, C.-Y.; Xue, Z.; Fan, G. Simultaneous profiling of transcriptome and DNA methylome from a single cell. Genome Biol. 2016, 17, 88. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Rong, H.; Xu, J.; Cao, R.; Li, S.; Gao, Y.; Cheng, B.; Zhou, T. DNA Methylation: An Important Biomarker and Therapeutic Target for Gastric Cancer. Front. Genet. 2022, 13, 823905. [Google Scholar] [CrossRef]
- Farlik, M.; Sheffield, N.C.; Nuzzo, A.; Datlinger, P.; Schönegger, A.; Klughammer, J.; Bock, C. Faculty Opinions recommendation of Single-cell DNA methylome sequencing and bioinformatic inference of epigenomic cell-state dynamics. Cell Rep. 2016, 10, 1386–1397. [Google Scholar] [CrossRef]
- Uzun, Y.; Yu, W.; Chen, C.; Tan, K. SINBAD: A flexible tool for single cell DNA methylation data. bioRxiv 2021. preprint. [Google Scholar] [CrossRef]
- Erfanian, N.; Heydari, A.A.; Ianez, P.; Derakhshani, A.; Ghasemigol, M.; Farahpour, M.; Nasseri, S.; Safarpour, H.; Sahebkar, A. Deep learning applications in single-cell omics data analysis. bioRxiv 2021. preprint. [Google Scholar] [CrossRef]
- Auslander, N.; Wolf, Y.I.; Koonin, E.V. In silico learning of tumor evolution through mutational time series. Proc. Natl. Acad. Sci. USA 2020, 116, 9501–9510. [Google Scholar] [CrossRef]
- Adam, G.; Rampášek, L.; Safikhani, Z.; Smirnov, P.; Haibe-Kains, B.; Goldenberg, A. Machine learning approaches to drug response prediction: Challenges and recent progress. NPJ Precis. Oncol. 2020, 4, 19. [Google Scholar] [CrossRef]
- Welch, J.D.; Kozareva, V.; Ferreira, A.; Vanderburg, C.; Martin, C.; Macosko, E.Z. Single-Cell Multi-omic Integration Compares and Contrasts Features of Brain Cell Identity. Cell 2019, 177, 1873–1887.e17. [Google Scholar] [CrossRef]
- Nativio, R.; Lan, Y.; Donahue, G.; Sidoli, S.; Berson, A.; Srinivasan, A.R.; Shcherbakova, O.; Amlie-Wolf, A.; Nie, J.; Cui, X.; et al. An integrated multi-omics approach identifies epigenetic alterations associated with Alzheimer’s disease. Nat. Genet. 2020, 52, 1024–1035. [Google Scholar] [CrossRef]
- Granja, J.M.; Klemm, S.; McGinnis, L.M.; Kathiria, A.S.; Mezger, A.; Corces, M.R.; Parks, B.; Gars, E.; Liedtke, M.; Zheng, G.X.Y.; et al. Single-cell multiomic analysis identifies regulatory programs in mixed-phenotype acute leukemia. Nat. Biotechnol. 2019, 37, 1458–1465. [Google Scholar] [CrossRef]
- Wold, S.; Esbensen, K.; Geladi, P. Principal component analysis. Chemom. Intell. Lab. Syst. 1987, 2, 37–52. [Google Scholar] [CrossRef]
- Butler, A.; Hoffman, P.; Smibert, P.; Papalexi, E.; Satija, R. Integrating single-cell transcriptomic data across different conditions, technologies, and species. Nat. Biotechnol. 2018, 36, 411–420. [Google Scholar] [CrossRef]
- Rodosthenous, T.; Shahrezaei, V.; Evangelou, M. Integrating multi-OMICS data through sparse canonical correlation analysis for the prediction of complex traits: A comparison study. Bioinformatics 2020, 36, 4616–4625. [Google Scholar] [CrossRef]
- Lin, Y.-Y.; Liu, T.-L.; Fuh, C.-S. Multiple Kernel Learning for Dimensionality Reduction. IEEE Trans. Pattern Anal. Mach. Intell. 2010, 33, 1147–1160. [Google Scholar] [CrossRef] [PubMed]
- Zou, H.; Hastie, T. Regularization and variable selection via the elastic net. J. R. Stat. Soc. Ser. B (Stat. Methodol.) 2005, 67, 301–320. [Google Scholar] [CrossRef]
- Breiman, L. Random Forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Li, H.; Brouwer, C.R.; Luo, W. A universal deep neural network for in-depth cleaning of single-cell RNA-Seq data. Nat. Commun. 2022, 13, 1901. [Google Scholar] [CrossRef]
- Flores, M.; Liu, Z.; Zhang, T.; Hasib, M.M.; Chiu, Y.C.; Ye, Z.; Paniagua, K.; Jo, S.; Zhang, J.; Gao, S.J.; et al. Deep learning tackles single-cell analysis-a survey of deep learning for scRNA-seq analysis. Brief Bioinform. 2022, 23, bbab531. [Google Scholar] [CrossRef]
- Bao, S.; Li, K.; Yan, C.; Zhang, Z.; Qu, J.; Zhou, M. Deep learning-based advances and applications for single-cell RNA-sequencing data analysis. Brief. Bioinform. 2021, 23, bbab473. [Google Scholar] [CrossRef] [PubMed]
- Brendel, M.; Su, C.; Bai, Z.; Zhang, H.; Elemento, O.; Wang, F. Application of Deep Learning on Single-cell RNA Sequencing Data Analysis: A Review. Genom. Proteom. Bioinform. 2022, 20, 814–835. [Google Scholar] [CrossRef] [PubMed]
- Lopez, R.; Regier, J.; Cole, M.B.; Jordan, M.I.; Yosef, N. Deep generative modeling for single-cell transcriptomics. Nat. Methods 2018, 15, 1053–1058. [Google Scholar] [CrossRef]
- Tangherloni, A.; Ricciuti, F.; Besozzi, D.; Liò, P.; Cvejic, A. Analysis of single-cell RNA sequencing data based on autoencoders. bioRxiv 2021. preprint. [Google Scholar] [CrossRef] [PubMed]
- Van Dijk, D.; Sharma, R.; Nainys, J.; Yim, K.; Kathail, P.; Carr, A.J.; Burdziak, C.; Moon, K.R.; Chaffer, C.L.; Pattabiraman, D.; et al. Faculty Opinions recommendation of Recovering Gene Interactions from Single-Cell Data Using Data Diffusion. Cell 2018, 174, 716–729. [Google Scholar] [CrossRef]
- Chen, M.; Zhou, X. VIPER: Variability-preserving imputation for accurate gene expression recovery in single-cell RNA sequencing studies. Genome Biol. 2018, 19, 196. [Google Scholar] [CrossRef]
- Di Ran, D.; Zhang, S.; Lytal, N.; An, L. scDoc: Correcting drop-out events in single-cell RNA-seq data. Bioinformatics 2020, 36, 4233–4239. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, Z.; Bhadani, R.; Cao, S.; Lu, M.; Lytal, N.; Chen, Y.; An, L. NISC: Neural Network-Imputation for Single-Cell RNA Sequencing and Cell Type Clustering. Front. Genet. 2022, 13, 847112. [Google Scholar] [CrossRef]
- Lundberg, S.M.; Lee, S.-I. A unified approach to interpreting model predictions. Adv. Neural Inf. Process. Syst. 2017, 30, 4768–4777. [Google Scholar]
- Ribeiro, M.T.; Singh, S.; Guestrin, C. (Eds.) “Why should i trust you?” Explaining the predictions of any classifier. In Proceedings of the 22nd Acm Sigkdd International Conference on Knowledge Discovery and Data Mining; Association for Computing Machinery: New York, NY, USA, 2016. [Google Scholar]
- Ullah Khan, I.; Ali, T.; Farid, Z.; Scavino, E.; Amiruddin Abd Rahman, M.; Hamdi, M. An improved hybrid indoor positioning system based on surface tessellation artificial neural network. Meas. Control 2020, 53, 1968–1977. [Google Scholar] [CrossRef]
- Consortium ITP-CAoWG. Pan-cancer analysis of whole genomes. Nature 2020, 578, 82–93. [Google Scholar] [CrossRef]
- Hudson, T.J.; Anderson, W.; Artez, A.; Barker, A.D.; Bell, C.; International Cancer Genome. International network of cancer genome projects. Nature 2010, 464, 993–998. [Google Scholar] [PubMed]
- Iorio, F.; Knijnenburg, T.A.; Vis, D.J.; Bignell, G.R.; Menden, M.P.; Schubert, M.; Aben, N.; Gonçalves, E.; Barthorpe, S.; Lightfoot, H.; et al. A Landscape of Pharmacogenomic Interactions in Cancer. Cell 2016, 166, 740–754. [Google Scholar] [CrossRef] [PubMed]
- Tate, J.G.; Bamford, S.; Jubb, H.C.; Sondka, Z.; Beare, D.M.; Bindal, N.; Boutselakis, H.; Cole, C.G.; Creatore, C.; Dawson, E.; et al. COSMIC: The Catalogue of Somatic Mutations in Cancer. Nucleic Acids Res. 2019, 47, D941–D947. [Google Scholar] [CrossRef] [PubMed]
- Van der Wijst, M.G.; de Vries, D.H.; Groot, H.E.; Trynka, G.; Hon, C.C.; Bonder, M.J.; Stegle, O.; Nawijn, M.C.; Idaghdour, Y.; van der Harst, P.; et al. The single-cell eQTLGen consortium. eLife 2020, 9, e52155. [Google Scholar] [CrossRef] [PubMed]
- Osato, N.; Itoh, M.; Konno, H.; Kondo, S.; Shibata, K.; Carninci, P.; Shiraki, T.; Shinagawa, A.; Arakawa, T.; Kikuchi, S.; et al. A Computer-Based Method of Selecting Clones for a Full-Length cDNA Project: Simultaneous Collection of Negligibly Redundant and Variant cDNAs. Genome Res. 2002, 12, 1127–1134. [Google Scholar] [CrossRef] [PubMed]
- Lonsdale, J.; Thomas, J.; Salvatore, M.; Phillips, R.; Lo, E.; Shad, S.; Hasz, R.; Walters, G.; Garcia, F.; Young, N.; et al. The Genotype-Tissue Expression (GTEx) project. Nat Genet. 2013, 45, 580–585. [Google Scholar] [CrossRef]
- Rozenblatt-Rosen, O.; Stubbington, M.J.T.; Regev, A.; Teichmann, S.A. The Human Cell Atlas: From vision to reality. Nature 2017, 550, 451–453. [Google Scholar] [CrossRef]
- Rozenblatt-Rosen, O.; Regev, A.; Oberdoerffer, P.; Nawy, T.; Hupalowska, A.; Rood, J.E.; Ashenberg, O.; Cerami, E.; Coffey, R.J.; Demir, E.; et al. The Human Tumor Atlas Network: Charting Tumor Transitions across Space and Time at Single-Cell Resolution. Cell 2020, 181, 236–249. [Google Scholar] [CrossRef]
- Zong, W.; Kang, H.; Xiong, Z.; Ma, Y.; Jin, T.; Gong, Z.; Yi, L.; Zhang, M.; Wu, S.; Wang, G.; et al. scMethBank: A database for single-cell whole genome DNA methylation maps. Nucleic Acids Res. 2022, 50, D380–D386. [Google Scholar] [CrossRef] [PubMed]
- Megill, C.; Martin, B.; Weaver, C.; Bell, S.; Prins, L.; Badajoz, S.; McCandless, B.; Pisco, A.O.; Kinsella, M.; Griffin, F. cellxgene: A performant, scalable exploration platform for high dimensional sparse matrices. bioRxiv 2021. preprint. [Google Scholar] [CrossRef]
- Tang, X.; Huang, Y.; Lei, J.; Luo, H.; Zhu, X. The single-cell sequencing: New developments and medical applications. Cell Biosci. 2019, 9, 53. [Google Scholar] [CrossRef]
- Guo, X.; Zhang, Y.; Jin, D.; Zhou, M. A Review of Single-Cell Pose Adjustment and Puncture. Adv. Intell. Syst. 2022, 4, 2200096. [Google Scholar] [CrossRef]
- Liu, B.; Li, Y.; Zhang, L. Analysis and Visualization of Spatial Transcriptomic Data. Front. Genet. 2022, 12, 785290. [Google Scholar] [CrossRef]
- Chen, G.; Ning, B.; Shi, T. Single-Cell RNA-Seq Technologies and Related Computational Data Analysis. Front. Genet. 2019, 10, 317. [Google Scholar] [CrossRef]
- Haque, A.; Engel, J.; Teichmann, S.A.; Lönnberg, T. A practical guide to single-cell RNA-sequencing for biomedical research and clinical applications. Genome Med. 2017, 9, 75. [Google Scholar] [CrossRef]
- Kharchenko, P.V. The triumphs and limitations of computational methods for scRNA-seq. Nat. Methods 2021, 18, 723–732. [Google Scholar] [CrossRef] [PubMed]
- Tzur, A.; Kafri, R.; LeBleu, V.S.; Lahav, G.; Kirschner, M.W. Cell Growth and Size Homeostasis in Proliferating Animal Cells. Science 2009, 325, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Tirrò, E.; Massimino, M.; Broggi, G.; Romano, C.; Minasi, S.; Gianno, F.; Antonelli, M.; Motta, G.; Certo, F.; Altieri, R.; et al. A Custom DNA-Based NGS Panel for the Molecular Characterization of Patients with Diffuse Gliomas: Diagnostic and Therapeutic Applications. Front. Oncol. 2022, 12, 861078. [Google Scholar] [CrossRef]
- Venteicher, A.S.; Tirosh, I.; Hebert, C.; Yizhak, K.; Neftel, C.; Filbin, M.G.; Hovestadt, V.; Escalante, L.E.; Shaw, M.L.; Rodman, C.; et al. Decoupling genetics, lineages, and microenvironment in IDH-mutant gliomas by single-cell RNA-seq. Science 2017, 355, eaai8478. [Google Scholar] [CrossRef] [PubMed]
- Tirosh, I.; Venteicher, A.S.; Hebert, C.; Escalante, L.E.; Patel, A.P.; Yizhak, K.; Fisher, J.M.; Rodman, C.; Mount, C.; Filbin, M.G.; et al. Single-cell RNA-seq supports a developmental hierarchy in human oligodendroglioma. Nature 2016, 539, 309–313. [Google Scholar] [CrossRef] [PubMed]
- Abdelfattah, N.; Kumar, P.; Wang, C.; Leu, J.-S.; Flynn, W.F.; Gao, R.; Baskin, D.S.; Pichumani, K.; Ijare, O.B.; Wood, S.L.; et al. Single-cell analysis of human glioma and immune cells identifies S100A4 as an immunotherapy target. Nat. Commun. 2022, 13, 767. [Google Scholar] [CrossRef] [PubMed]
- Stella, S.; Vitale, S.R.; Massimino, M.; Motta, G.; Longhitano, C.; Lanzafame, K.; Martorana, F.; Fazzari, C.; Vecchio, G.M.; Tirro, E.; et al. Molecular Analysis of Luminal Androgen Receptor Reveals Activated Pathways and Potential Therapeutic Targets in Breast Cancer. Cancer Genom. Proteom. 2022, 19, 464–476. [Google Scholar] [CrossRef]
- Lehmann, B.D.; Bauer, J.A.; Chen, X.; Sanders, M.E.; Chakravarthy, A.B.; Shyr, Y.; Pietenpol, J.A. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J. Clin. Investig. 2011, 121, 2750–2767. [Google Scholar] [CrossRef]
- Eliyatkin, N.; Yalcin, E.; Zengel, B.; Aktaş, S.; Vardar, E. Molecular Classification of Breast Carcinoma: From Traditional, Old-Fashioned Way to A New Age, and A New Way. J. Breast Health 2015, 11, 59–66. [Google Scholar] [CrossRef]
- Stella, S.; Vitale, S.R.; Martorana, F.; Massimino, M.; Pavone, G.; Lanzafame, K.; Bianca, S.; Barone, C.; Gorgone, C.; Fichera, M. Mutational Analysis of BRCA1 and BRCA2 Genes in Breast Cancer Patients from Eastern Sicily. Cancer Manag Res. 2022, 14, 1341–1352. [Google Scholar] [CrossRef]
- Gambardella, G.; Viscido, G.; Tumaini, B.; Isacchi, A.; Bosotti, R.; di Bernardo, D. A single-cell analysis of breast cancer cell lines to study tumour heterogeneity and drug response. Nat. Commun. 2022, 13, 1714. [Google Scholar] [CrossRef]
- Ren, L.; Li, J.; Wang, C.; Lou, Z.; Gao, S.; Zhao, L.; Wang, S.; Chaulagain, A.; Zhang, M.; Li, X.; et al. Single cell RNA sequencing for breast cancer: Present and future. Cell Death Discov. 2021, 7, 104. [Google Scholar] [CrossRef]
- Hu, L.; Su, L.; Cheng, H.; Mo, C.; Ouyang, T.; Li, J.; Wang, T.; Fan, Z.; Fan, T.; Lin, B. Single-Cell RNA Sequencing Reveals the Cellular Origin and Evolution of Breast Cancer in BRCA1 Mutation Carriers. Cancer Res. 2021, 81, 2600–2611. [Google Scholar] [CrossRef]
- Casasent, A.K.; Schalck, A.; Gao, R.; Sei, E.; Long, A.; Pangburn, W.; Casasent, T.; Meric-Bernstam, F.; Edgerton, M.E.; Navin, N.E. Multiclonal Invasion in Breast Tumors Identified by Topographic Single Cell Sequencing. Cell 2018, 172, 205–217.e12. [Google Scholar] [CrossRef] [PubMed]
- Mamlouk, S.; Simon, T.; Tomás, L.; Wedge, D.C.; Arnold, A.; Menne, A.; Horst, D.; Capper, D.; Morkel, M.; Posada, D.; et al. Malignant transformation and genetic alterations are uncoupled in early colorectal cancer progression. BMC Biol. 2020, 18, 116. [Google Scholar] [CrossRef] [PubMed]
- Hoorn, S.T.; Trinh, A.; de Jong, J.; Koens, L.; Vermeulen, L. Classification of Colorectal Cancer in Molecular Subtypes by Immunohistochemistry. Methods Mol. Biol. 2018, 1765, 179–191. [Google Scholar] [CrossRef] [PubMed]
- Carlsen, L.; Huntington, K.E.; El-Deiry, W.S. Immunotherapy for Colorectal Cancer: Mechanisms and Predictive Biomarkers. Cancers 2022, 14, 1028. [Google Scholar] [CrossRef]
- Zhang, L.; Yu, X.; Zheng, L.; Zhang, Y.; Li, Y.; Fang, Q.; Gao, R.; Kang, B.; Zhang, Q.; Huang, J.Y.; et al. Lineage tracking reveals dynamic relationships of T cells in colorectal cancer. Nature 2018, 564, 268–272. [Google Scholar] [CrossRef]
- Bian, S.; Hou, Y.; Zhou, X.; Li, X.; Yong, J.; Wang, Y.; Wang, W.; Yan, J.; Hu, B.; Guo, H.; et al. Single-cell multiomics sequencing and analyses of human colorectal cancer. Science 2018, 362, 1060–1063. [Google Scholar] [CrossRef]
- Liu, Y.; Sethi, N.S.; Hinoue, T.; Schneider, B.G.; Cherniack, A.D.; Sanchez-Vega, F.; Seoane, J.A.; Farshidfar, F.; Bowlby, R.; Islam, M.; et al. Faculty Opinions recommendation of Comparative molecular analysis of gastrointestinal adenocarcinomas. Cancer Cell 2018, 33, 721–735.e8. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Wang, P.; Zhou, W.; Luo, M.; Xu, Z.; Cheng, R.; Xu, C.; Jin, X.; Li, Y.; Jiang, Q. Comprehensive analysis of partial methylation domains in colorectal cancer based on single-cell methylation profiles. Briefings Bioinform. 2021, 22, bbab267. [Google Scholar] [CrossRef]
- Maynard, A.; McCoach, C.E.; Rotow, J.K.; Harris, L.; Haderk, F.; Kerr, D.L.; Yu, E.A.; Schenk, E.L.; Tan, W.; Zee, A.; et al. Therapy-Induced Evolution of Human Lung Cancer Revealed by Single-Cell RNA Sequencing. Cell 2020, 182, 1232–1251.e22. [Google Scholar] [CrossRef]
- Wu, F.; Fan, J.; He, Y.; Xiong, A.; Yu, J.; Li, Y.; Zhang, Y.; Zhao, W.; Zhou, F.; Li, W.; et al. Single-cell profiling of tumor heterogeneity and the microenvironment in advanced non-small cell lung cancer. Nat. Commun. 2021, 12, 254. [Google Scholar] [CrossRef]
- Wu, Q.; Guo, J.; Liu, Y.; Zheng, Q.; Li, X.; Wu, C.; Fang, D.; Chen, X.; Ma, L.; Xu, P.; et al. YAP drives fate conversion and chemoresistance of small cell lung cancer. Sci. Adv. 2021, 7, eabg1850. [Google Scholar] [CrossRef]
- DeMarzo, A.M.; Nelson, W.G.; Isaacs, W.B.; Epstein, J. Pathological and molecular aspects of prostate cancer. Lancet 2003, 361, 955–964. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.M.; Abate-Shen, C. Molecular genetics of prostate cancer: New prospects for old challenges. Genes Dev. 2010, 24, 1967–2000. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Weinstein, H.N.; Allegakoen, P.; Wadsworth, M.H., 2nd; Xie, J.; Yang, H.; Castro, E.A.; Lu, K.L.; Stohr, B.A.; Feng, F.Y.; et al. Single-cell analysis of human primary prostate cancer reveals the heterogeneity of tumor-associated epithelial cell states. Nat Commun. 2022, 13, 141. [Google Scholar] [CrossRef]
- Chen, S.; Zhu, G.; Yang, Y.; Wang, F.; Xiao, Y.-T.; Zhang, N.; Bian, X.; Zhu, Y.; Yu, Y.; Liu, F.; et al. Single-cell analysis reveals transcriptomic remodellings in distinct cell types that contribute to human prostate cancer progression. Nature 2021, 23, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Tirrò, E.; Martorana, F.; Romano, C.; Vitale, S.R.; Motta, G.; Di Gregorio, S.; Massimino, M.; Pennisi, M.S.; Stella, S.; Puma, A.; et al. Molecular Alterations in Thyroid Cancer: From Bench to Clinical Practice. Genes 2019, 10, 709. [Google Scholar] [CrossRef]
- Manzella, L.; Massimino, M.; Stella, S.; Tirrò, E.; Pennisi, M.S.; Martorana, F.; Motta, G.; Vitale, S.R.; Puma, A.; Romano, C.; et al. Activation of the IGF Axis in Thyroid Cancer: Implications for Tumorigenesis and Treatment. Int. J. Mol. Sci. 2019, 20, 3258. [Google Scholar] [CrossRef]
- Pu, W.; Shi, X.; Yu, P.; Zhang, M.; Liu, Z.; Tan, L.; Han, P.; Wang, Y.; Ji, D.; Gan, H.; et al. Single-cell transcriptomic analysis of the tumor ecosystems underlying initiation and progression of papillary thyroid carcinoma. Nat. Commun. 2021, 12, 6058. [Google Scholar] [CrossRef]
- Wang, T.; Shi, J.; Li, L.; Zhou, X.; Zhang, H.; Zhang, X.; Wang, Y.; Liu, L.; Sheng, L. Single-Cell Transcriptome Analysis Reveals Inter-Tumor Heterogeneity in Bilateral Papillary Thyroid Carcinoma. Front. Immunol. 2022, 13, 840811. [Google Scholar] [CrossRef]
- Taylor, J.; Xiao, W.; Abdel-Wahab, O. Diagnosis and classification of hematologic malignancies on the basis of genetics. Blood 2017, 130, 410–423. [Google Scholar] [CrossRef]
- Ledergor, G.; Weiner, A.; Zada, M.; Wang, S.-Y.; Cohen, Y.C.; Gatt, M.E.; Snir, N.; Magen, H.; Koren-Michowitz, M.; Herzog-Tzarfati, K.; et al. Single cell dissection of plasma cell heterogeneity in symptomatic and asymptomatic myeloma. Nat. Med. 2018, 24, 1867–1876. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Song, L.; Zhu, P.; Zhang, B.; Tao, Y.; Xu, X.; Li, F.; Wu, K.; Liang, J.; Shao, D.; et al. Single-Cell Exome Sequencing and Monoclonal Evolution of a JAK2-Negative Myeloproliferative Neoplasm. Cell 2012, 148, 873–885. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Yang, B.; Weng, L.; Li, J.; Bai, J.; Wang, T.; Wang, J.; Ye, J.; Jing, H.; Jiao, Y.; et al. Single cell sequencing reveals cell populations that predict primary resistance to imatinib in chronic myeloid leukemia. Aging 2020, 12, 25337–25355. [Google Scholar] [CrossRef] [PubMed]
- Bin Lim, S.; Lim, C.T.; Lim, W.-T. Single-Cell Analysis of Circulating Tumor Cells: Why Heterogeneity Matters. Cancers 2019, 11, 1595. [Google Scholar] [CrossRef]
- Malihi, P.D.; Graf, R.P.; Rodriguez, A.; Ramesh, N.; Lee, J.; Sutton, R.; Jiles, R.; Ruiz Velasco, C.; Sei, E.; Kolatkar, A.; et al. Single-Cell Circulating Tumor Cell Analysis Reveals Genomic Instability as a Distinctive Feature of Aggressive Prostate Cancer. Clin. Cancer Res. 2020, 26, 4143–4153. [Google Scholar] [CrossRef] [PubMed]
- Rothé, F.; Venet, D.; Peeters, D.; Rouas, G.; Rediti, M.; Smeets, D.; Dupont, F.; Campbell, P.; Lambrechts, D.; Dirix, L.; et al. Interrogating breast cancer heterogeneity using single and pooled circulating tumor cell analysis. NPJ Breast Cancer 2022, 8, 79. [Google Scholar] [CrossRef]
- Hofman, V.; Heeke, S.; Marquette, C.H.; Ilie, M.; Hofman, P. Circulating Tumor Cell Detection in Lung Cancer: But to What End? Cancers 2019, 11, 262. [Google Scholar] [CrossRef]
- Weng, X.; Cai, Y. Clinical Significance of Circulating Tumor Cells (CTCs) and Survivin on Predicting Prognosis in Thyroid Cancer Patients. Dis. Markers 2022, 2022, 5188006. [Google Scholar] [CrossRef]
- Bădulescu, I.C.; Bărbuș, E.; Piciu, D. Circulating tumor cells in thyroid carcinoma–The prognostic role of this biomarker. Review of the literature. Med. Pharm. Rep. 2017, 90, 256–261. [Google Scholar] [CrossRef]
- Francescangeli, F.; Magri, V.; De Angelis, M.L.; De Renzi, G.; Gandini, O.; Zeuner, A.; Gazzaniga, P.; Nicolazzo, C. Sequential Isolation and Characterization of Single CTCs and Large CTC Clusters in Metastatic Colorectal Cancer Patients. Cancers 2021, 13, 6362. [Google Scholar] [CrossRef]
- Rossi, E.; Zamarchi, R. Single-Cell Analysis of Circulating Tumor Cells: How Far Have We Come in the -Omics Era? Front Genet. 2019, 10, 958. [Google Scholar] [CrossRef] [PubMed]
- Tirrò, E.; Martorana, F.; Micale, G.; Inzerilli, N.; Carciotto, R.; Romano, C.; Longhitano, C.; Motta, G.; Lanzafame, K.; Stella, S.; et al. Next generation sequencing in a cohort of patients with rare sarcoma histotypes: A single institution experience. Pathol. Res. Pract. 2022, 232, 153820. [Google Scholar] [CrossRef] [PubMed]
- Massimino, M.; Stella, S.; Micale, G.; Motta, L.; Pavone, G.; Broggi, G.; Piombino, E.; Magro, G.; Parra, H.J.S.; Manzella, L.; et al. Mechanistic Translation of Melanoma Genetic Landscape in Enriched Pathways and Oncogenic Protein-Protein Interactions. Cancer Genom. Proteom. 2022, 19, 350–361. [Google Scholar] [CrossRef]
- Malone, E.R.; Oliva, M.; Sabatini, P.J.B.; Stockley, T.; Siu, L.L. Molecular profiling for precision cancer therapies. Genome Med. 2020, 12, 8. [Google Scholar] [CrossRef]
- Lei, Y.; Tang, R.; Xu, J.; Wang, W.; Zhang, B.; Liu, J.; Yu, X.; Shi, S. Applications of single-cell sequencing in cancer research: Progress and perspectives. J. Hematol. Oncol. 2021, 14, 91. [Google Scholar] [CrossRef] [PubMed]

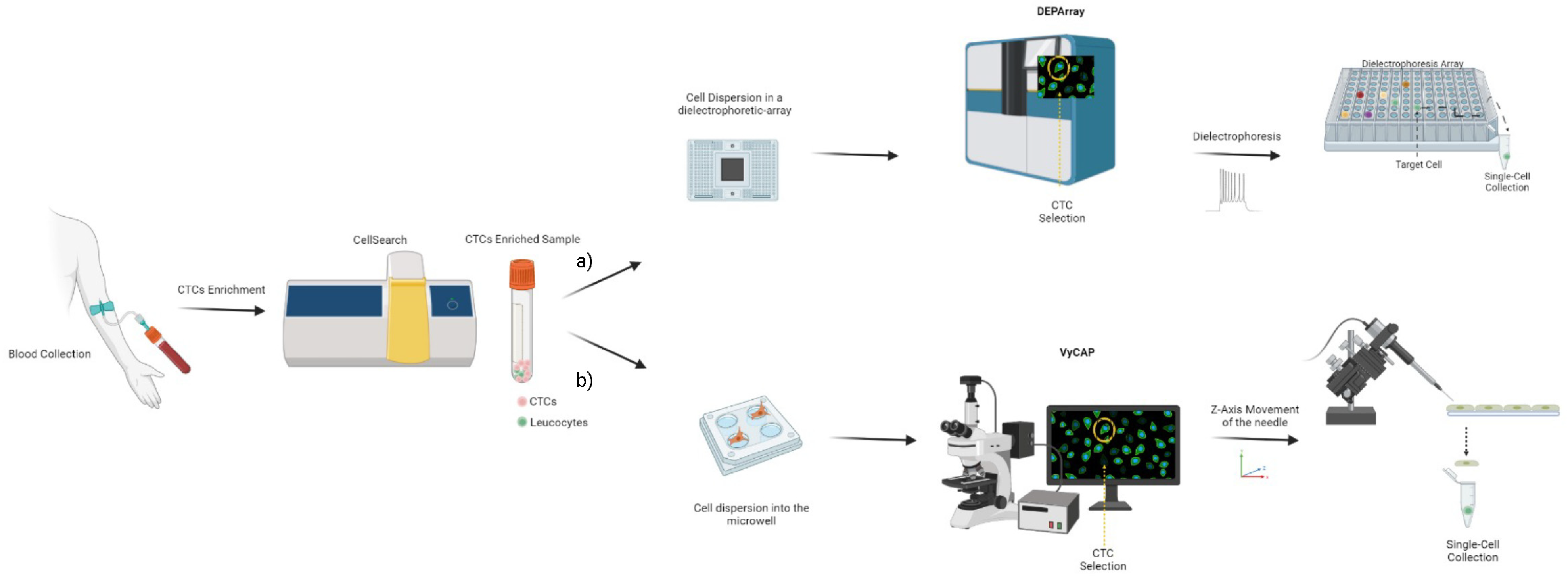
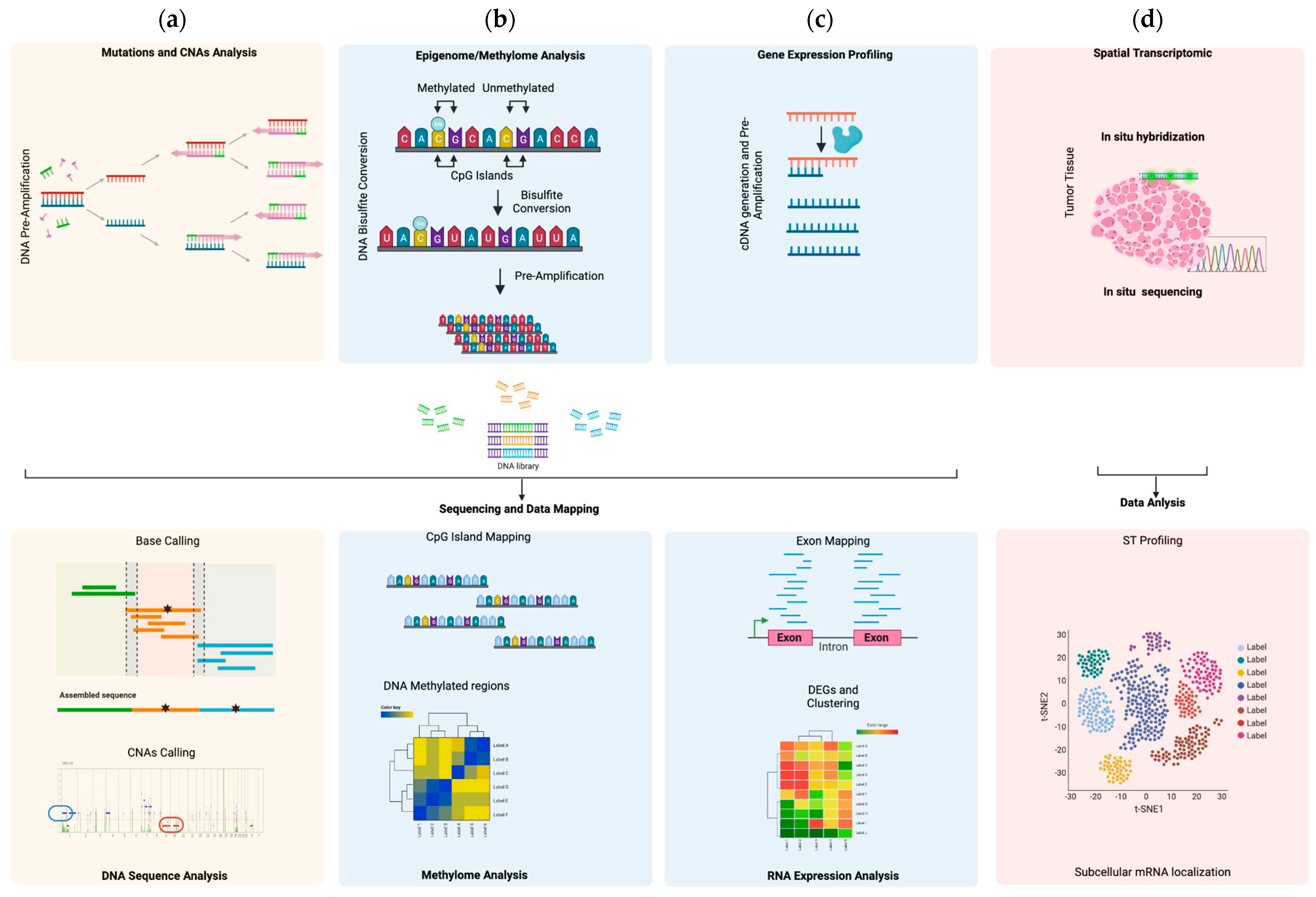

| System | Method | Advantage | Limitations | References |
|---|---|---|---|---|
| Microdissection | Laser dissection of desiderate cell | High-resolution No previous manipulation of the tissue No damage for adjacent tissue | UV laser can damage the nucleic acids structure | [14,15] |
| Facs | Cells are stained and sorted by fluorescence detector | Sensitive including rare cells | Requires tissue dissociation can damage the cell Requires high number of target cells Requires specific cell staining | [9,16,17,18,19,20] |
| Microfluidics | Cell is captured by flow rate according to their physical and chemical features | High-resolution Use of barcodes to start directly with library preparation Can be image-based improving the selection of desiderate cell | Requires tissue dissociation can damage the cell Requires specific cell staining | [9,21,22,23,24] |
| Microwell-based System | Cells are dispersed into the microwell | High-resolution Use of barcodes to start directly with library preparation Can be image-based improving the selection of desiderate cell | Requires tissue dissociation can damage the cell Requires specific cell staining | [23] |
| Macs | Immunogenetic staining | Negative and positive selection | Requires tissue dissociation can damage the cell Only cell enrichment No single-cell isolation The cell suspension needs to be processed further to obtain single separated cell | [9,25,26] |
| Data Portal | cDNA Generation | UMI | References |
|---|---|---|---|
| SCNA UMI-seq | Full length | YES | [50] |
| MATQ-seq | Full length | YES | [51] |
| 10X GENOMICS | 3′-end | YES | [22] |
| CEL-seq2 | 3′-end | YES | [53] |
| SMART-seq2 | Full length | NO | [52] |
| MARS-seq | 3′-end | YES | [54] |
| Fluidigim C1 | 3′-end | YES | [55,56] |
| Data Portal | Source | Links | References |
|---|---|---|---|
| TCGA | Data from 20,000 tumors | URL: https://portal.gdc.cancer.gov/ | [117] |
| ICGC | Incorporates data from TCGA | URL: https://dcc.icgc.org/ | [118] |
| COSMIC | Primary tumors and cell lines | URL: https://cancer.sanger.ac.uk/cosmic | [119,120] |
| SINGLE-CELL eQTLGen | Genetic variations of immune cells and integrate data derived from HCA and HTAN | URL: https://eqtlgen.org/sc/ | [121] |
| FANTOM GTEx | Transcriptomics profiling of human cells | URL: https://fantom.gsc.riken.jp/ https://www.gtexportal.org/home/ | [122,123] |
| HTAN HCA | Genetic signature | URL: https://humantumoratlas.org/ https://www.humancellatlas.org/ | [124,125] |
| scMethBank | A database of single-cell methylation maps for human and mouse | URL: https://ngdc.cncb.ac.cn/methbank/scm/ | [126] |
| CellxGene | Collection of tools useful for downloading and analyzing of single-cells data | URL: https://cellxgene.cziscience.com | [127] |
| Broad Institute | A database of single-cell omics data | URL: https://singlecell.broadinstitute.org/single_cell | / |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Massimino, M.; Martorana, F.; Stella, S.; Vitale, S.R.; Tomarchio, C.; Manzella, L.; Vigneri, P. Single-Cell Analysis in the Omics Era: Technologies and Applications in Cancer. Genes 2023, 14, 1330. https://doi.org/10.3390/genes14071330
Massimino M, Martorana F, Stella S, Vitale SR, Tomarchio C, Manzella L, Vigneri P. Single-Cell Analysis in the Omics Era: Technologies and Applications in Cancer. Genes. 2023; 14(7):1330. https://doi.org/10.3390/genes14071330
Chicago/Turabian StyleMassimino, Michele, Federica Martorana, Stefania Stella, Silvia Rita Vitale, Cristina Tomarchio, Livia Manzella, and Paolo Vigneri. 2023. "Single-Cell Analysis in the Omics Era: Technologies and Applications in Cancer" Genes 14, no. 7: 1330. https://doi.org/10.3390/genes14071330
APA StyleMassimino, M., Martorana, F., Stella, S., Vitale, S. R., Tomarchio, C., Manzella, L., & Vigneri, P. (2023). Single-Cell Analysis in the Omics Era: Technologies and Applications in Cancer. Genes, 14(7), 1330. https://doi.org/10.3390/genes14071330






