Ploidy in Vibrio natriegens: Very Dynamic and Rapidly Changing Copy Numbers of Both Chromosomes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strain, Medium, and Growth Curves
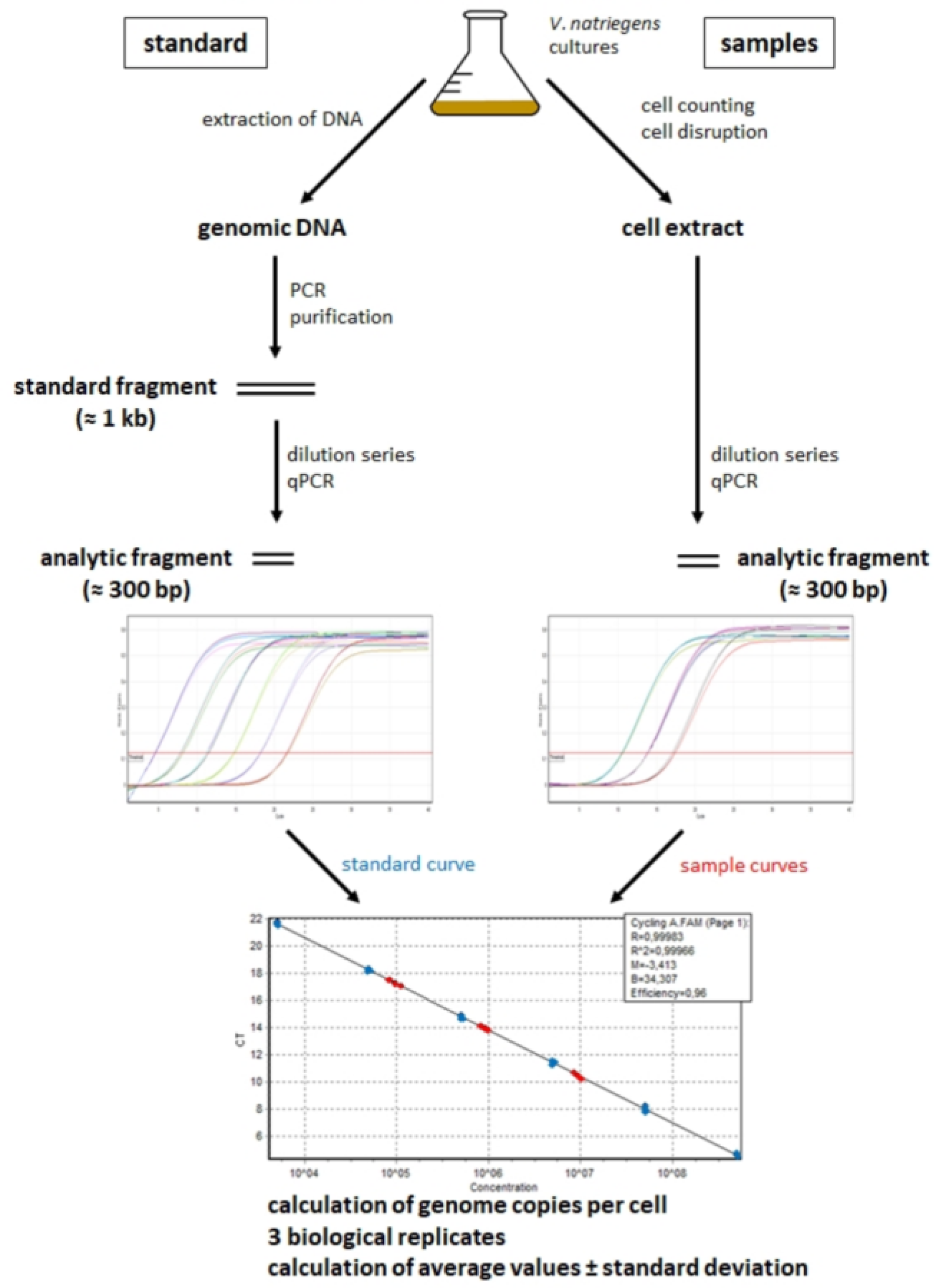
2.2. Quantification of Genome Copy Numbers Using Real-Time PCR
2.2.1. Generation of Standard Curves
2.2.2. Cell Disruption
2.2.3. Quantification of Copy Numbers of Different Chromosomal Sites
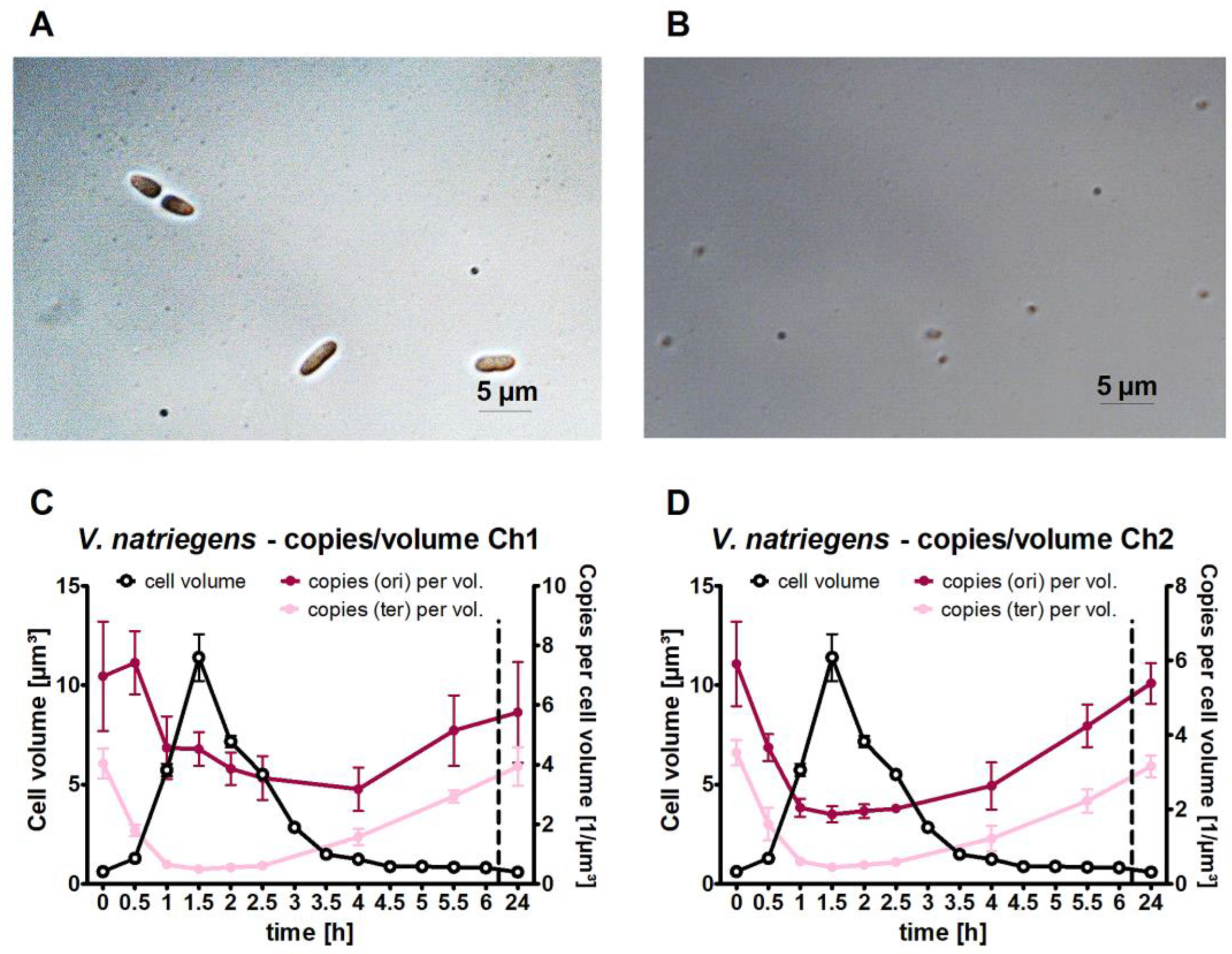
2.3. Quantification of Cell Volumes
2.4. Bioinformatic Analyses
2.5. Marker Frequency Analysis
3. Results
3.1. Optimization of the Real-Time PCR Method for Application to V. natriegens
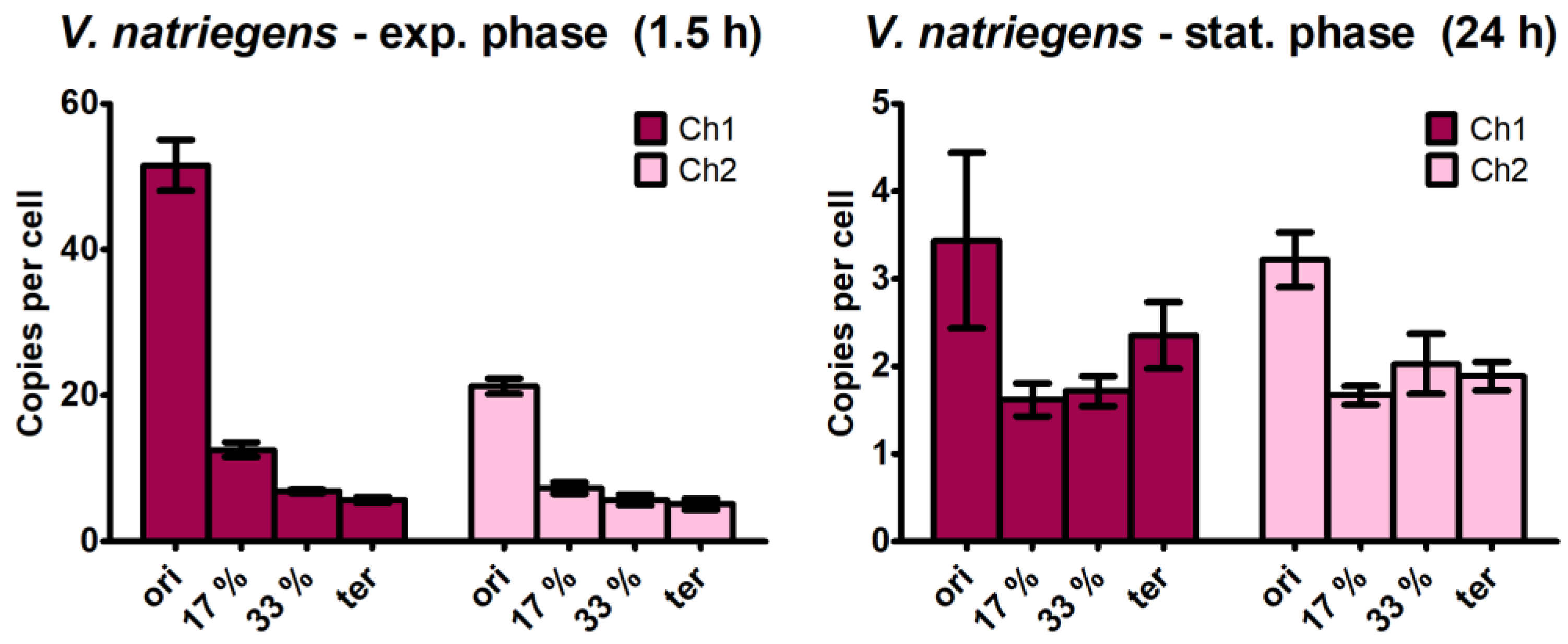
3.2. Quantification of the Copy Numbers of Origins and Termini of Both Chromosomes throughout the Growth Curve
3.3. Quantification of the Copy Numbers of Additional Chromosomal Sites
3.4. Quantification of the Cell Volume and the Copy Numbers per Unit Volume
3.5. Marker Frequency Analysis
4. Discussion
- (1)
- Replication started very early in the lag phase. At thirty minutes after inoculation, ongoing replication was already observed, long before the start of cell division. Additionally, in B. subtilis, the onset of replication during the lag phase was observed after the transfer of stationary-phase cells to a fresh medium [3]. Similarly, when Synechococcus elongatus PCC 7942 was transferred from dark to light conditions, the chromosome number increased from 2–3 to 4–10 before the onset of phototrophic growth [57]. These results obtained with species of three different phylogenetic groups indicate that this strategy is widespread; however, studies with other species are needed.
- (2)
- The replication of chromosome 1 started earlier (0.5 h) than that of chromosome 2 (1 h) (Figure 5). Although this has not yet been described for V. natriegens, it is in line with earlier results obtained for V. cholerae showing that ongoing replication of chromosome 1 licenses the replication initiation of chromosome 2 [51,58,59].
- (3)
- For V. cholerae, it has been shown that only the initiation of replication of the two chromosomes occurs at different times; in contrast, the termination of replication occurs simultaneously and is coordinated with cell division [50]. Our results indicate that this might also be true for V. natriegens because for both chromosomes, there was a slight difference in the read numbers at 4 h, but replication had completely ceased at 5 h.
- (4)
- The ori/ter ratio was much higher for chromosome 1 than for chromosome 2 (Figure 5). This was also observed in a recent study on V. natriegens in the early exponential phase, which reported an ori/ter ratio of 5 for chromosome 1 and 2.5 for chromosome 2 [56]. This was due to the following two reasons: first, the origin copy number increase is much higher for chromosome 1 than for chromosome 2 (Figure 2), and second, the difference depends on the size of the chromosome because larger chromosomes require a longer time between initiation and termination.
- (5)
- The number of origins and the ori/ter ratio had already declined during the exponential growth phase. In contrast, it is thought that the growth rate, number of origins, and ori/ter ratio are constant during fast exponential growth for the mero-oligoploid species E. coli and B. subtilis. However, quantification of the numbers of origins and termini during the whole growth curve, as presented herein, is missing for these two intensively studied model species.
- (1)
- The different results may have been caused by differences in the applied conditions, e.g., temperature, medium, and aeration. For example, marker frequency analyses of exponentially growing Vibrio parahaemolyticus cultures revealed an ori/ter ratio of 4.0 for growth in a complex medium but only 1.4 in a synthetic medium [58]. Similarly, fast-growing E. coli cells contain on average 6.5/6.8 origins, and slow-growing cultures contain only 2.0/2.5 origins [1,13]. As discussed above, in general, fast-growing cultures need to employ multifork replication, whereas slow-growing cultures do not. Notably, these general requirements cannot explain the high copy number of approximately 50 origins at the onset of the exponential growth phase in V. natriegens. Nevertheless, small changes in medium composition can have a very dramatic effect, e.g., a change in medium composition increased toxin production per cell in V. cholerae by more than 1000-fold [64].
- (2)
- For V. natriegens, the results were very sensitive to the exact point of the growth curve; for example, we measured 50 origin copies in the early exponential growth phase, 20 in the mid-exponential growth phase only 1 hour later, and only in the transition phase 2.5 h later (Figure 2). One publication about V. cholerae exists that contradicts all other studies, reporting a highly dynamic change in the origin copy number during the growth curve [52]. Using the qPCR approach, 10–15 origin copies were observed during mid-exponential growth, but 70 origin copies were observed during the transition phase. Therefore, specific conditions and/or strains (see below) exist that also result in highly dynamic origin copy number changes in V. cholerae, albeit in a completely different manner, as observed in this study on V. natriegens.
- (3)
- Another possible reason is species-specific biological differences in the genus Vibrio. For example, one study found an ori/ter ratio of 4.0 for V. parahaemolyticus but a ratio of only 2.0 for V. cholerae and V. vulnificus [58]. Additionally, in Cyanobacteria, species-specific differences within one genus have been reported, e.g., Anabaena variabilis contains 5–8 genome copies, and A. cylindrical contains 25 [2].
- (4)
- Another possible explanation might be strain-specific differences within one species. The species V. cholerae comprises several serotypes as well as strains within these serotypes [65]. A comparison of clinical and environmental isolates revealed that genetic variation exists [66]. However, these strain comparisons did not include quantification of ploidy level. Nevertheless, this might possibly explain the values of 1–2 origins and up to 70 origins for V. cholerae reported in different studies (see above).
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pecoraro, V.; Zerulla, K.; Lange, C.; Soppa, J. Quantification of ploidy in proteobacteria revealed the existence of monoploid, (mero-)oligoploid and polyploid species. PLoS ONE 2011, 6, e16392. [Google Scholar] [CrossRef] [Green Version]
- Griese, M.; Lange, C.; Soppa, J. Ploidy in cyanobacteria. FEMS Microbiol. Lett. 2011, 323, 124–131. [Google Scholar] [CrossRef] [Green Version]
- Böttinger, B.; Semmler, F.; Zerulla, K.; Ludt, K.; Soppa, J. Regulated ploidy of Bacillus subtilis and three new isolates of Bacillus and Paenibacillus. FEMS Microbiol. Lett. 2018, 365, fnx282. [Google Scholar] [CrossRef] [Green Version]
- Breuert, S.; Allers, T.; Spohn, G.; Soppa, J. Regulated polyploidy in halophilic archaea. PLoS ONE 2006, 1, e92. [Google Scholar] [CrossRef]
- Hildenbrand, C.; Stock, T.; Lange, C.; Rother, M.; Soppa, J. Genome copy numbers and gene conversion in methanogenic archaea. J. Bacteriol. 2011, 193, 734–743. [Google Scholar] [CrossRef] [Green Version]
- Angert, E.R. DNA replication and genomic architecture of very large bacteria. Annu. Rev. Microbiol. 2012, 66, 197–212. [Google Scholar] [CrossRef] [PubMed]
- Ionescu, D.; Bizic-Ionescu, M.; de Maio, N.; Cypionka, H.; Grossart, H.-P. Community-like genome in single cells of the sulfur bacterium Achromatium oxaliferum. Nat. Commun. 2017, 8, 455. [Google Scholar] [CrossRef]
- Ludt, K.; Soppa, J. Polyploidy in halophilic archaea: Regulation, evolutionary advantages, and gene conversion. Biochem. Soc. Trans. 2019, 47, 933–944. [Google Scholar] [CrossRef] [PubMed]
- Soppa, J. Polyploidy in archaea and bacteria: About desiccation resistance, giant cell size, long-term survival, enforcement by a eukaryotic host and additional aspects. J. Mol. Microbiol. Biotechnol. 2014, 24, 409–419. [Google Scholar] [CrossRef] [PubMed]
- Comai, L. The advantages and disadvantages of being polyploid. Nat. Rev. Genet. 2005, 6, 836–846. [Google Scholar] [CrossRef]
- Hegarty, M.J.; Hiscock, S.J. Genomic clues to the evolutionary success of polyploid plants. Curr. Biol. 2008, 18, R435–R444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sémon, M.; Wolfe, K.H. Consequences of genome duplication. Curr. Opin. Genet. Dev. 2007, 17, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Bremer, H.; Dennis, P.P. Modulation of Chemical Composition and Other Parameters of the Cell by Growth Rate: Escherichia coli and Salmonella; Neidhardt, F., Ed.; ASM Press: Washington, DC, USA, 1996; pp. 1553–1569. [Google Scholar]
- Fuchino, K.; Wasser, D.; Soppa, J. Genome Copy Number Quantification Revealed That the Ethanologenic α-Proteobacterium Zymomonas mobilis Is Polyploid. Front. Microbiol. 2021, 12, 705895. [Google Scholar] [CrossRef]
- Komaki, K.; Ishikawa, H. Genomic copy number of intracellular bacterial symbionts of aphids varies in response to developmental stage and morph of their host. Insect Biochem. Mol. Biol. 2000, 30, 253–258. [Google Scholar] [CrossRef]
- Payne, W.J.; Eagon, R.G.; Williams, A.K. Some observations on the physiology of Pseudomonas natriegens nov. spec. Antonie Van Leeuwenhoek 1961, 27, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Austin, B.; Zachary, A.; Colwell, R.R. Recognition of Beneckea natriegens (Payne et al.) Baumann et al. as a Member of the Genus Vibrio, as Previously Proposed by Webb and Payne. Int. J. Syst. Bacteriol. 1978, 28, 315–317. [Google Scholar] [CrossRef] [Green Version]
- Eagon, R.G. Pseudomonas natriegens, a marine bacterium with a generation time of less than 10 minutes. J. Bacteriol. 1962, 83, 736–737. [Google Scholar] [CrossRef] [Green Version]
- Dorsch, M.; Lane, D.; Stackebrandt, E. Towards a phylogeny of the genus Vibrio based on 16S rRNA sequences. Int. J. Syst. Bacteriol. 1992, 42, 58–63. [Google Scholar] [CrossRef]
- Maida, I.; Bosi, E.; Perrin, E.; Papaleo, M.C.; Orlandini, V.; Fondi, M.; Fani, R.; Wiegel, J.; Bianconi, G.; Canganella, F. Draft Genome Sequence of the Fast-Growing Bacterium Vibrio natriegens Strain DSMZ 759. Genome Announc. 2013, 1, e00648-13. [Google Scholar] [CrossRef] [Green Version]
- Aiyar, S.E.; Gaal, T.; Gourse, R.L. rRNA promoter activity in the fast-growing bacterium Vibrio natriegens. J. Bacteriol. 2002, 184, 1349–1358. [Google Scholar] [CrossRef] [Green Version]
- Stukenberg, D.; Hoff, J.; Faber, A.; Becker, A. NT-CRISPR, combining natural transformation and CRISPR-Cas9 counterselection for markerless and scarless genome editing in Vibrio natriegens. Commun. Biol. 2022, 5, 265. [Google Scholar] [CrossRef]
- Teufel, M.; Klein, C.A.; Mager, M.; Sobetzko, P. A multifunctional system for genome editing and large-scale interspecies gene transfer. Nat. Commun. 2022, 13, 3430. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Dong, F.; Wu, M.; Tao, R.; Yang, J.; Wu, M.; Jiang, Y.; Yang, S.; Yang, L. Vibrio natriegens as a pET-Compatible Expression Host Complementary to Escherichia coli. Front. Microbiol. 2021, 12, 627181. [Google Scholar] [CrossRef] [PubMed]
- Dalia, T.N.; Hayes, C.A.; Stolyar, S.; Marx, C.J.; McKinlay, J.B.; Dalia, A.B. Multiplex Genome Editing by Natural Transformation (MuGENT) for Synthetic Biology in Vibrio natriegens. ACS Synth. Biol. 2017, 6, 1650–1655. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.H.; Ostrov, N.; Wong, B.G.; Gold, M.A.; Khalil, A.S.; Church, G.M. Functional genomics of the rapidly replicating bacterium Vibrio natriegens by CRISPRi. Nat. Microbiol. 2019, 4, 1105–1113. [Google Scholar] [CrossRef] [Green Version]
- Tietze, L.; Mangold, A.; Hoff, M.W.; Lale, R. Identification and Cross-Characterisation of Artificial Promoters and 5’ Untranslated Regions in Vibrio natriegens. Front. Bioeng. Biotechnol. 2022, 10, 826142. [Google Scholar] [CrossRef] [PubMed]
- Tschirhart, T.; Shukla, V.; Kelly, E.E.; Schultzhaus, Z.; NewRingeisen, E.; Erickson, J.S.; Wang, Z.; Garcia, W.; Curl, E.; Egbert, R.G.; et al. Synthetic Biology Tools for the Fast-Growing Marine Bacterium Vibrio natriegens. ACS Synth. Biol. 2019, 8, 2069–2079. [Google Scholar] [CrossRef]
- Stukenberg, D.; Hensel, T.; Hoff, J.; Daniel, B.; Inckemann, R.; Tedeschi, J.N.; Nousch, F.; Fritz, G. The Marburg Collection: A Golden Gate DNA Assembly Framework for Synthetic Biology Applications in Vibrio natriegens. ACS Synth. Biol. 2021, 10, 1904–1919. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Xu, J.; Zhou, H.; Zhang, H.; Wu, J.; Yang, L. Recombinant Protein Expression Chassis Library of Vibrio natriegens by Fine-Tuning the Expression of T7 RNA Polymerase. ACS Synth. Biol. 2023, 12, 555–564. [Google Scholar] [CrossRef]
- Stadler, K.A.; Becker, W.; Darnhofer, B.; Birner-Gruenberger, R.; Zangger, K. Overexpression of recombinant proteins containing non-canonical amino acids in Vibrio natriegens: P-azido-L-phenylalanine as coupling site for 19F-tags. Amino Acids 2022, 54, 1041–1053. [Google Scholar] [CrossRef]
- González, S.S.; Ad, O.; Shah, B.; Zhang, Z.; Zhang, X.; Chatterjee, A.; Schepartz, A. Genetic Code Expansion in the Engineered Organism Vmax X2: High Yield and Exceptional Fidelity. ACS Cent. Sci. 2021, 7, 1500–1507. [Google Scholar] [CrossRef]
- Zhang, L.; Lin, X.; Wang, T.; Guo, W.; Lu, Y. Development and comparison of cell-free protein synthesis systems derived from typical bacterial chassis. Bioresour. Bioprocess. 2021, 8, 58. [Google Scholar] [CrossRef]
- Zhu, B.; Gan, R.; Cabezas, M.D.; Kojima, T.; Nicol, R.; Jewett, M.C.; Nakano, H. Increasing cell-free gene expression yields from linear templates in Escherichia coli and Vibrio natriegens extracts by using DNA-binding proteins. Biotechnol. Bioeng. 2020, 117, 3849–3857. [Google Scholar] [CrossRef]
- Ozer, E.; Alfonta, L. Genetic Code Expansion of Vibrio natriegens. Front. Bioeng. Biotechnol. 2021, 9, 594429. [Google Scholar] [CrossRef] [PubMed]
- Becker, W.; Wimberger, F.; Zangger, K. Vibrio natriegens: An Alternative Expression System for the High-Yield Production of Isotopically Labeled Proteins. Biochemistry 2019, 58, 2799–2803. [Google Scholar] [CrossRef] [PubMed]
- Wiegand, D.J.; Lee, H.H.; Ostrov, N.; Church, G.M. Cell-free Protein Expression Using the Rapidly Growing Bacterium Vibrio natriegens. J. Vis. Exp. 2019, 145, e59495. [Google Scholar] [CrossRef]
- Wiegand, D.J.; Lee, H.H.; Ostrov, N.; Church, G.M. Establishing a Cell-Free Vibrio natriegens Expression System. ACS Synth. Biol. 2018, 7, 2475–2479. [Google Scholar] [CrossRef]
- Schleicher, L.; Muras, V.; Claussen, B.; Pfannstiel, J.; Blombach, B.; Dibrov, P.; Fritz, G.; Steuber, J. Vibrio natriegens as Host for Expression of Multisubunit Membrane Protein Complexes. Front. Microbiol. 2018, 9, 2537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Failmezger, J.; Scholz, S.; Blombach, B.; Siemann-Herzberg, M. Cell-Free Protein Synthesis From Fast-Growing Vibrio natriegens. Front. Microbiol. 2018, 9, 1146. [Google Scholar] [CrossRef] [Green Version]
- Hoff, J.; Daniel, B.; Stukenberg, D.; Thuronyi, B.W.; Waldminghaus, T.; Fritz, G. Vibrio natriegens: An ultrafast-growing marine bacterium as emerging synthetic biology chassis. Environ. Microbiol. 2020, 22, 4394–4408. [Google Scholar] [CrossRef]
- Xu, J.; Yang, S.; Yang, L. Vibrio natriegens as a host for rapid biotechnology. Trends Biotechnol. 2022, 40, 381–384. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, D.; Chen, Z. Genome-Scale Modeling and Systems Metabolic Engineering of Vibrio natriegens for the Production of 1,3-Propanediol. Methods Mol. Biol. 2023, 2553, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Meng, W.; Zhang, Y.; Ma, L.; Lü, C.; Xu, P.; Ma, C.; Gao, C. Non-Sterilized Fermentation of 2,3-Butanediol with Seawater by Metabolic Engineered Fast-Growing Vibrio natriegens. Front. Bioeng. Biotechnol. 2022, 10, 955097. [Google Scholar] [CrossRef] [PubMed]
- Thoma, F.; Schulze, C.; Gutierrez-Coto, C.; Hädrich, M.; Huber, J.; Gunkel, C.; Thoma, R.; Blombach, B. Metabolic engineering of Vibrio natriegens for anaerobic succinate production. Microb. Biotechnol. 2022, 15, 1671–1684. [Google Scholar] [CrossRef]
- Stella, R.G.; Baumann, P.; Lorke, S.; Münstermann, F.; Wirtz, A.; Wiechert, J.; Marienhagen, J.; Frunzke, J. Biosensor-based isolation of amino acid-producing Vibrio natriegens strains. Metab. Eng. Commun. 2021, 13, e00187. [Google Scholar] [CrossRef]
- Liu, X.; Han, X.; Peng, Y.; Tan, C.; Wang, J.; Xue, H.; Xu, P.; Tao, F. Rapid production of l-DOPA by Vibrio natriegens, an emerging next-generation whole-cell catalysis chassis. Microb. Biotechnol. 2022, 15, 1610–1621. [Google Scholar] [CrossRef]
- Wang, Z.; Tschirhart, T.; Schultzhaus, Z.; Kelly, E.E.; Chen, A.; Oh, E.; Nag, O.; Glaser, E.R.; Kim, E.; Lloyd, P.F.; et al. Melanin Produced by the Fast-Growing Marine Bacterium Vibrio natriegens through Heterologous Biosynthesis: Characterization and Application. Appl. Environ. Microbiol. 2020, 86, e02749-19. [Google Scholar] [CrossRef]
- Fogel, M.A.; Waldor, M.K. Distinct segregation dynamics of the two Vibrio cholerae chromosomes. Mol. Microbiol. 2005, 55, 125–136. [Google Scholar] [CrossRef]
- Val, M.-E.; Marbouty, M.; de Lemos Martins, F.; Kennedy, S.P.; Kemble, H.; Bland, M.J.; Possoz, C.; Koszul, R.; Skovgaard, O.; Mazel, D. A checkpoint control orchestrates the replication of the two chromosomes of Vibrio cholerae. Sci. Adv. 2016, 2, e1501914. [Google Scholar] [CrossRef] [Green Version]
- Ramachandran, R.; Ciaccia, P.N.; Filsuf, T.A.; Jha, J.K.; Chattoraj, D.K. Chromosome 1 licenses chromosome 2 replication in Vibrio cholerae by doubling the crtS gene dosage. PLoS Genet. 2018, 14, e1007426. [Google Scholar] [CrossRef] [PubMed]
- Paranjape, S.S.; Shashidhar, R. The ploidy of Vibrio cholerae is variable and is influenced by growth phase and nutrient levels. FEMS Microbiol. Lett. 2017, 364, fnx190. [Google Scholar] [CrossRef]
- Freese, N.H.; Norris, D.C.; Loraine, A.E. Integrated genome browser: Visual analytics platform for genomics. Bioinformatics 2016, 32, 2089–2095. [Google Scholar] [CrossRef] [Green Version]
- Quinn, W.G.; Sueoka, N. Symmetric replication of the Bacillus subtilis chromosome. Proc. Natl. Acad. Sci. USA 1970, 67, 717–723. [Google Scholar] [CrossRef]
- Cooper, S.; Helmstetter, C.E. Chromosome replication and the division cycle of Escherichia coli B/r. J. Mol. Biol. 1968, 31, 519–540. [Google Scholar] [CrossRef]
- Teufel, M.; Sobetzko, P. Reducing costs for DNA and RNA sequencing by sample pooling using a metagenomic approach. BMC Genom. 2022, 23, 613. [Google Scholar] [CrossRef]
- Watanabe, S.; Ohbayashi, R.; Kanesaki, Y.; Saito, N.; Chibazakura, T.; Soga, T.; Yoshikawa, H. Intensive DNA Replication and Metabolism during the Lag Phase in Cyanobacteria. PLoS ONE 2015, 10, e0136800. [Google Scholar] [CrossRef] [PubMed]
- Dryselius, R.; Izutsu, K.; Honda, T.; Iida, T. Differential replication dynamics for large and small Vibrio chromosomes affect gene dosage, expression and location. BMC Genom. 2008, 9, 559. [Google Scholar] [CrossRef] [Green Version]
- Galli, E.; Poidevin, M.; Le Bars, R.; Desfontaines, J.-M.; Muresan, L.; Paly, E.; Yamaichi, Y.; Barre, F.-X. Cell division licensing in the multi-chromosomal Vibrio cholerae bacterium. Nat. Microbiol. 2016, 1, 16094. [Google Scholar] [CrossRef] [PubMed]
- Yamaichi, Y.; Fogel, M.A.; Waldor, M.K. Par genes and the pathology of chromosome loss in Vibrio cholerae. Proc. Natl. Acad. Sci. USA 2007, 104, 630–635. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, P.; Demarre, G.; Karpova, T.S.; McNally, J.; Chattoraj, D.K. Changes in nucleoid morphology and origin localization upon inhibition or alteration of the actin homolog, MreB, of Vibrio cholerae. J. Bacteriol. 2007, 189, 7450–7463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kadoya, R.; Baek, J.H.; Sarker, A.; Chattoraj, D.K. Participation of chromosome segregation protein ParAI of Vibrio cholerae in chromosome replication. J. Bacteriol. 2011, 193, 1504–1514. [Google Scholar] [CrossRef] [Green Version]
- Demarre, G.; Galli, E.; Muresan, L.; Paly, E.; David, A.; Possoz, C.; Barre, F.-X. Differential management of the replication terminus regions of the two Vibrio cholerae chromosomes during cell division. PLoS Genet. 2014, 10, e1004557. [Google Scholar] [CrossRef] [PubMed]
- Iwanaga, M.; Yamamoto, K. New medium for the production of cholera toxin by Vibrio cholerae O1 biotype El Tor. J. Clin. Microbiol. 1985, 22, 405–408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- COLWELL, R.R. Global climate and infectious disease: The cholera paradigm. Science 1996, 274, 2025–2031. [Google Scholar] [CrossRef] [Green Version]
- Siriphap, A.; Leekitcharoenphon, P.; Kaas, R.S.; Theethakaew, C.; Aarestrup, F.M.; Sutheinkul, O.; Hendriksen, R.S. Characterization and Genetic Variation of Vibrio cholerae Isolated from Clinical and Environmental Sources in Thailand. PLoS ONE 2017, 12, e0169324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
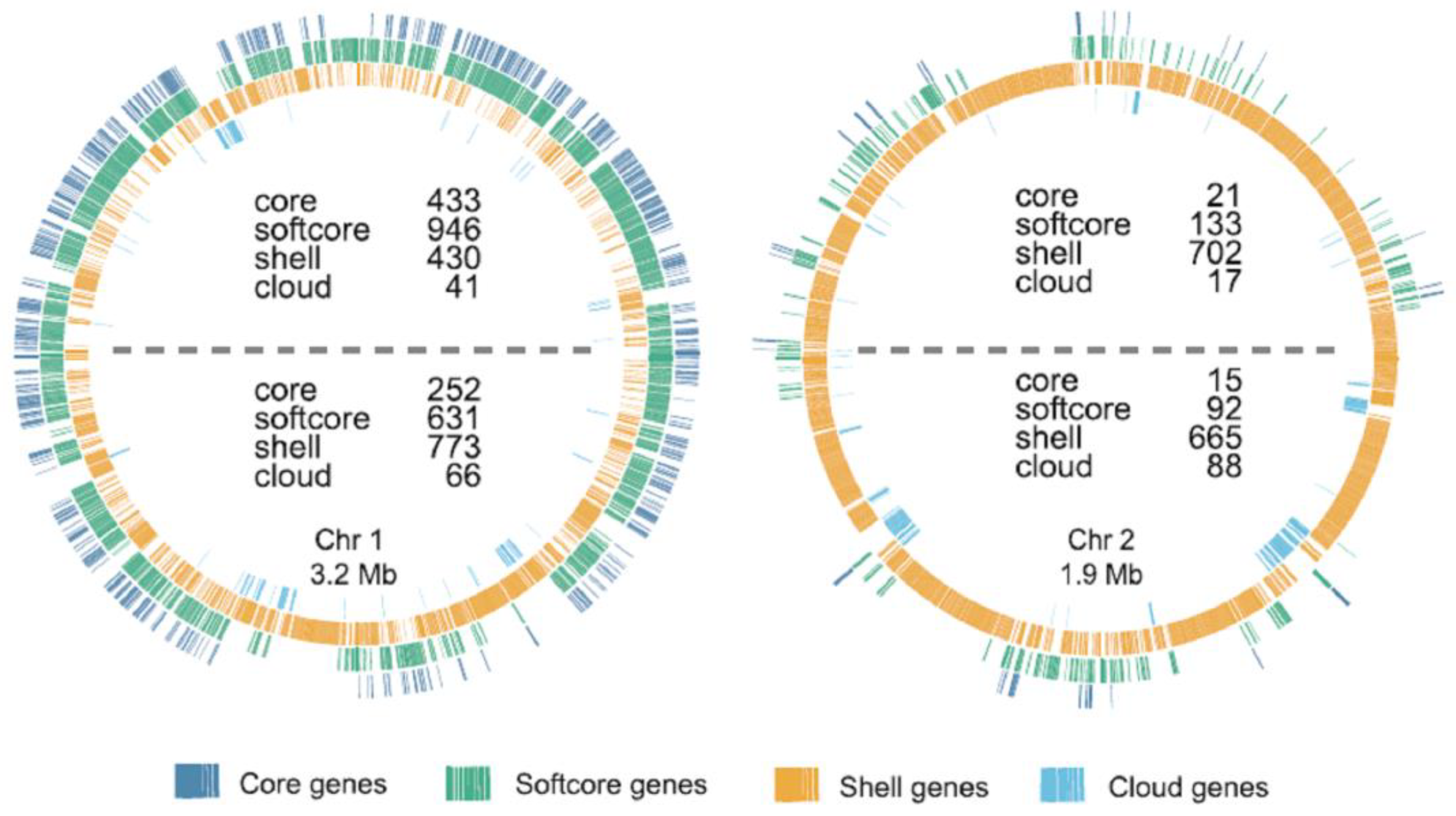
- Sonnenberg, C.B.; Kahlke, T.; Haugen, P. Vibrionaceae core, shell and cloud genes are non-randomly distributed on Chr 1: An hypothesis that links the genomic location of genes with their intracellular placement. BMC Genom. 2020, 21, 695. [Google Scholar] [CrossRef]
- Soler-Bistué, A.; Mondotte, J.A.; Bland, M.J.; Val, M.-E.; Saleh, M.-C.; Mazel, D. Genomic location of the major ribosomal protein gene locus determines Vibrio cholerae global growth and infectivity. PLoS Genet. 2015, 11, e1005156. [Google Scholar] [CrossRef] [Green Version]
- Larotonda, L.; Mornico, D.; Khanna, V.; Bernal-Bayard, J.; Ghigo, J.-M.; Val, M.-E.; Comerci, D.; Mazel, D.; Soler-Bistué, A. Chromosomal Position of Ribosomal Protein Genes Affects Long-Term Evolution of Vibrio cholerae. mBio 2023, 14, e0343222. [Google Scholar] [CrossRef]
- Narula, J.; Kuchina, A.; Lee, D.-Y.D.; Fujita, M.; Süel, G.M.; Igoshin, O.A. Chromosomal Arrangement of Phosphorelay Genes Couples Sporulation and DNA Replication. Cell 2015, 162, 328–337. [Google Scholar] [CrossRef] [Green Version]
- Slager, J.; Kjos, M.; Attaiech, L.; Veening, J.-W. Antibiotic-induced replication stress triggers bacterial competence by increasing gene dosage near the origin. Cell 2014, 157, 395–406. [Google Scholar] [CrossRef] [Green Version]
- Slager, J.; Veening, J.-W. Hard-Wired Control of Bacterial Processes by Chromosomal Gene Location. Trends Microbiol. 2016, 24, 788–800. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Time Point | Average Origin Copy No. | Avg. Terminus Copy No. | Ratio Origin No./Terminus No. |
|---|---|---|---|
| Chromosome 1 | |||
| 1.5 h | 51.6 ± 3.5 | 5.7 ± 0.4 | 9.0 |
| 24 h | 3.4 ± 1.0 | 2.3 ± 0.4 | 1.4 |
| Chromosome 2 | |||
| 1.5 h | 21.3 ± 1.0 | 5.1 ± 0.8 | 4.1 |
| 24 h | 3.2 ± 0.3 | 1.9 ± 0.2 | 1.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brück, P.; Wasser, D.; Soppa, J. Ploidy in Vibrio natriegens: Very Dynamic and Rapidly Changing Copy Numbers of Both Chromosomes. Genes 2023, 14, 1437. https://doi.org/10.3390/genes14071437
Brück P, Wasser D, Soppa J. Ploidy in Vibrio natriegens: Very Dynamic and Rapidly Changing Copy Numbers of Both Chromosomes. Genes. 2023; 14(7):1437. https://doi.org/10.3390/genes14071437
Chicago/Turabian StyleBrück, Patrik, Daniel Wasser, and Jörg Soppa. 2023. "Ploidy in Vibrio natriegens: Very Dynamic and Rapidly Changing Copy Numbers of Both Chromosomes" Genes 14, no. 7: 1437. https://doi.org/10.3390/genes14071437
APA StyleBrück, P., Wasser, D., & Soppa, J. (2023). Ploidy in Vibrio natriegens: Very Dynamic and Rapidly Changing Copy Numbers of Both Chromosomes. Genes, 14(7), 1437. https://doi.org/10.3390/genes14071437







