A Circular RNA Derived from the Pumilio 1 Gene Could Regulate PTEN in Human Cumulus Cells
Abstract
1. Introduction
2. Materials and Methods
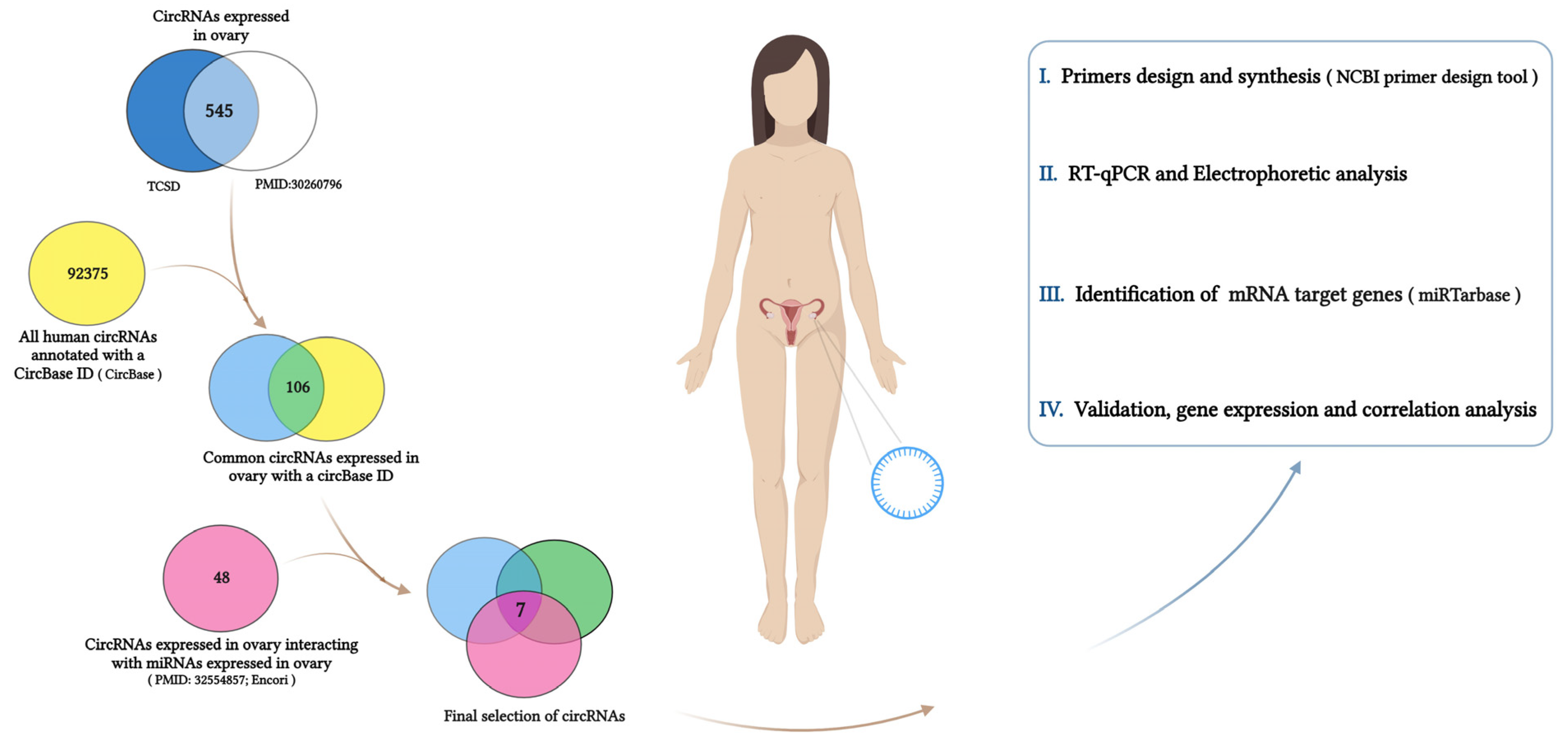
2.1. Selection of circRNAs Expressed in the Ovary
2.2. Patient Enrollment
2.3. Sample Collection
2.4. RNA Isolation and Purification
2.5. Gene Expression Analysis
2.6. Prediction of circRNA–miRNA–mRNA Interactions
2.7. Statistical Analysis
2.8. Genomics of circPUM1
3. Results
3.1. CircRNA Selection
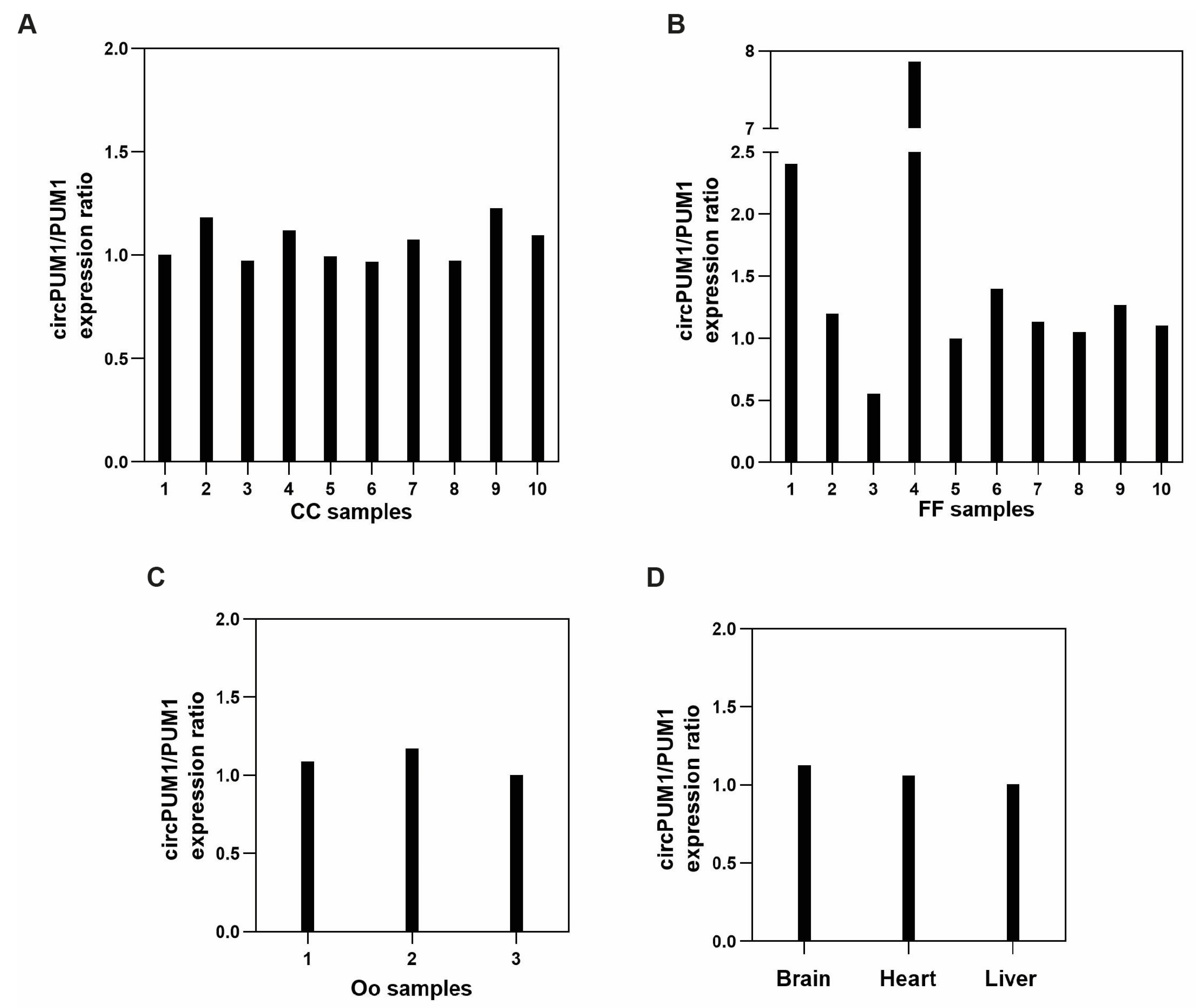
3.2. CircRNA Expression
3.3. Genomics of circPUM1
3.4. CircPUM1 and Its Linear Counterpart Are Expressed in the Follicular Microenvironment
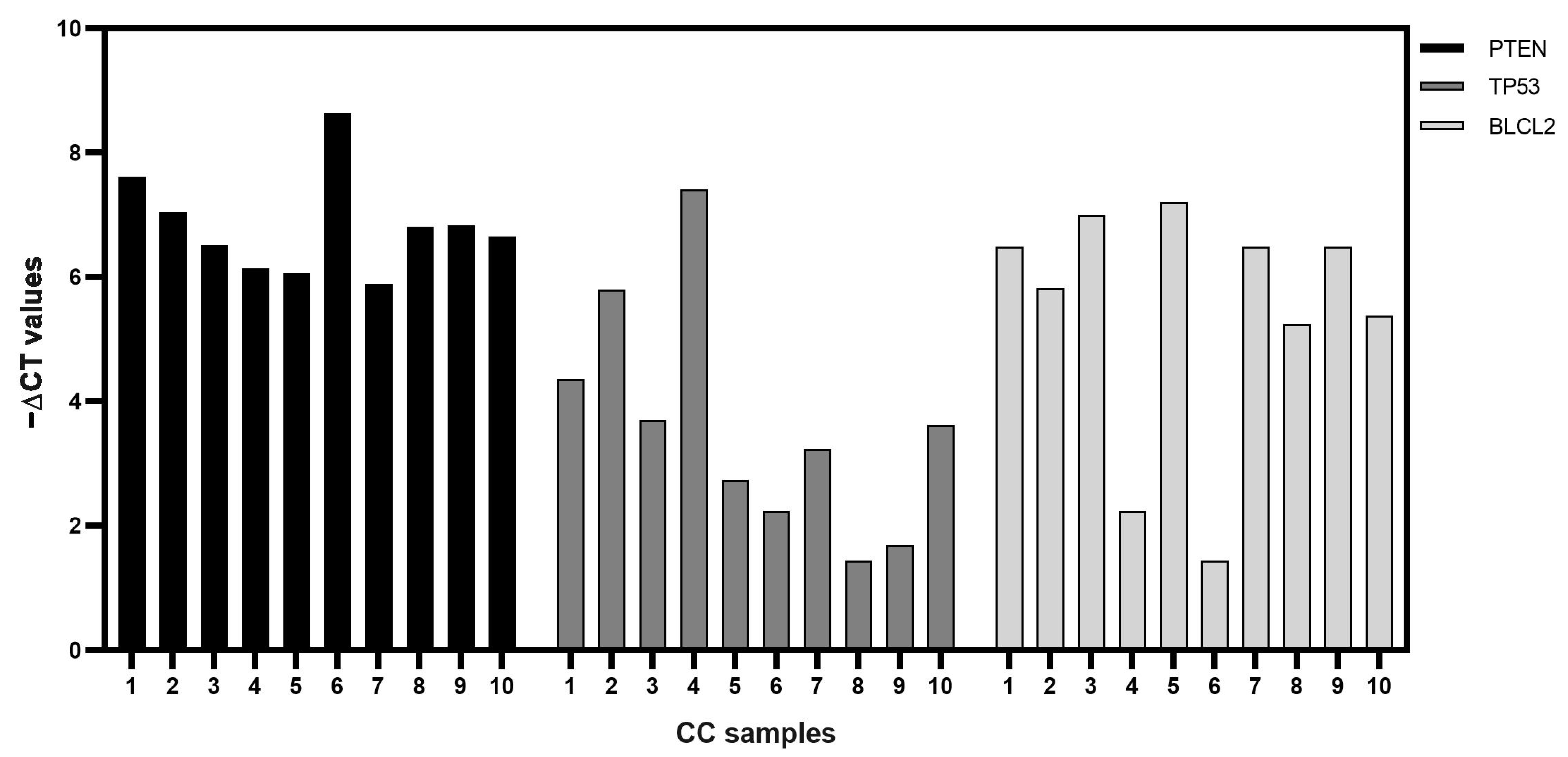
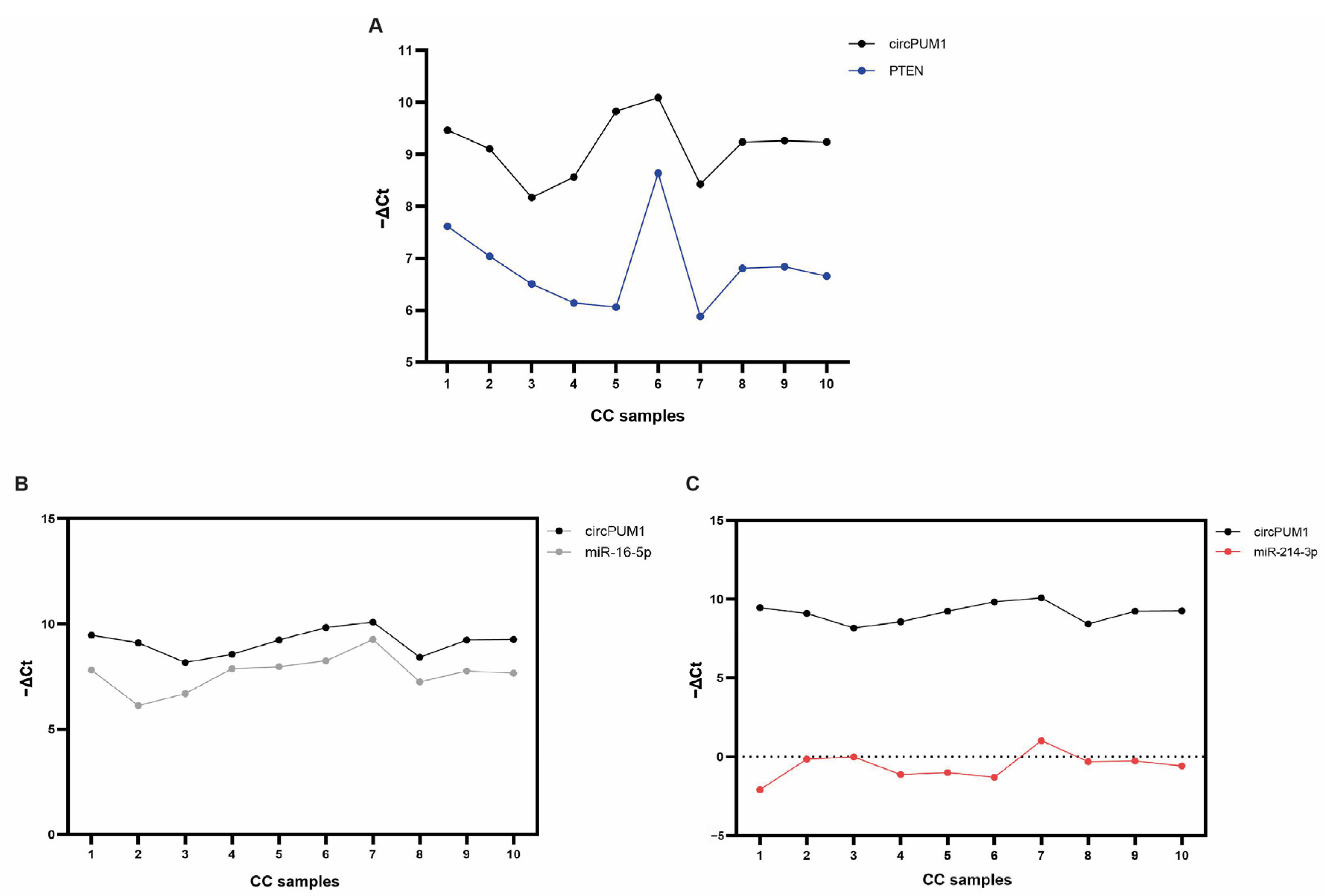
3.5. CircPUM1 and Its RNA Interactors in CC Samples
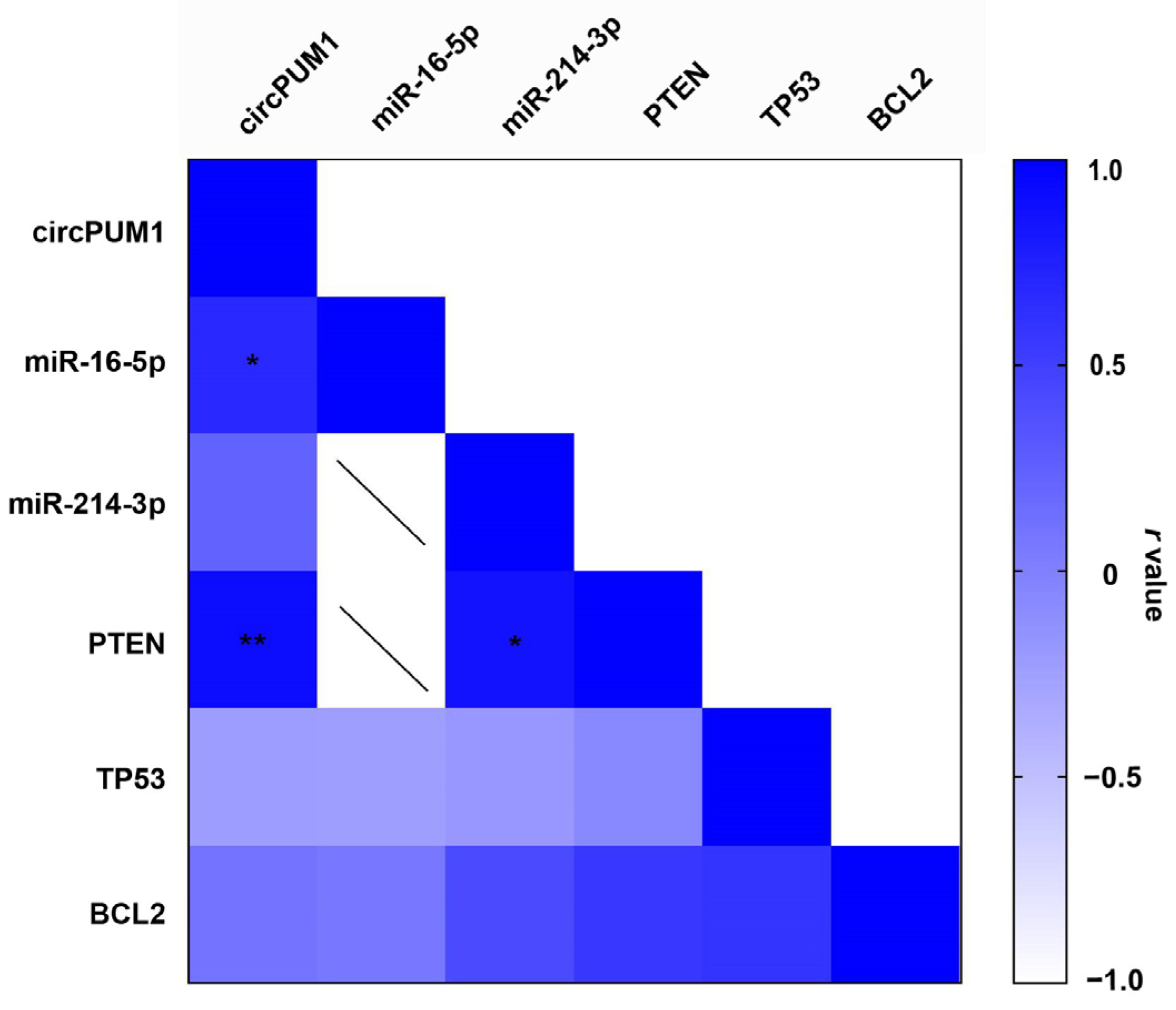
3.6. Correlation between circPUM1 and mRNA Expression Levels in CCs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Palazzo, A.F.; Lee, E.S. Non-coding RNA: What is functional and what is junk? Front. Genet. 2015, 6, 2. [Google Scholar] [CrossRef] [PubMed]
- Elgar, G.; Vavouri, T. Tuning in to the signals: Noncoding sequence conservation in vertebrate genomes. Trends Genet. 2008, 24, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Calore, F.; Lovat, F.; Garofalo, M. Non-coding RNAs and cancer. Int. J. Mol. Sci. 2013, 14, 17085–17110. [Google Scholar] [CrossRef] [PubMed]
- Arraiano, C.M. Regulatory noncoding RNAs: Functions and applications in health and disease. FEBS J. 2021, 288, 6308–6309. [Google Scholar] [CrossRef] [PubMed]
- Caponnetto, A.; Battaglia, R.; Ragusa, M.; Barbagallo, D.; Lunelio, F.; Borzi, P.; Scollo, P.; Purrello, M.; Vento, M.E.; Di Pietro, C. Molecular profiling of follicular fluid microRNAs in young women affected by Hodgkin lymphoma. Reprod. Biomed. Online 2021, 43, 1045–1056. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, R.; Caponnetto, A.; Caringella, A.M.; Cortone, A.; Ferrara, C.; Smirni, S.; Iannitti, R.; Purrello, M.; D’Amato, G.; Fioretti, B.; et al. Resveratrol Treatment Induces Mito-miRNome Modification in Follicular Fluid from Aged Women with a Poor Prognosis for In Vitro Fertilization Cycles. Antioxidants 2022, 11, 1019. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, R.; Alonzo, R.; Pennisi, C.; Caponnetto, A.; Ferrara, C.; Stella, M.; Barbagallo, C.; Barbagallo, D.; Ragusa, M.; Purrello, M.; et al. MicroRNA-Mediated Regulation of the Virus Cycle and Pathogenesis in the SARS-CoV-2 Disease. Int. J. Mol. Sci. 2021, 22, 13192. [Google Scholar] [CrossRef]
- Jeck, W.R.; Sorrentino, J.A.; Wang, K.; Slevin, M.K.; Burd, C.E.; Liu, J.; Marzluff, W.F.; Sharpless, N.E. Circular RNAs are abundant, conserved, and associated with ALU repeats. RNA 2013, 19, 141–157. [Google Scholar] [CrossRef]
- Kristensen, L.S.; Andersen, M.S.; Stagsted, L.V.W.; Ebbesen, K.K.; Hansen, T.B.; Kjems, J. The biogenesis, biology and characterization of circular RNAs. Nat. Rev. Genet. 2019, 20, 675–691. [Google Scholar] [CrossRef]
- Rybak-Wolf, A.; Stottmeister, C.; Glazar, P.; Jens, M.; Pino, N.; Giusti, S.; Hanan, M.; Behm, M.; Bartok, O.; Ashwal-Fluss, R.; et al. Circular RNAs in the Mammalian Brain Are Highly Abundant, Conserved, and Dynamically Expressed. Mol. Cell 2015, 58, 870–885. [Google Scholar] [CrossRef]
- Taulli, R.; Loretelli, C.; Pandolfi, P.P. From pseudo-ceRNAs to circ-ceRNAs: A tale of cross-talk and competition. Nat. Struct. Mol. Biol. 2013, 20, 541–543. [Google Scholar] [CrossRef] [PubMed]
- Thomson, D.W.; Dinger, M.E. Endogenous microRNA sponges: Evidence and controversy. Nat. Rev. Genet. 2016, 17, 272–283. [Google Scholar] [CrossRef] [PubMed]
- Barbagallo, D.; Caponnetto, A.; Brex, D.; Mirabella, F.; Barbagallo, C.; Lauretta, G.; Morrone, A.; Certo, F.; Broggi, G.; Caltabiano, R.; et al. CircSMARCA5 Regulates VEGFA mRNA Splicing and Angiogenesis in Glioblastoma Multiforme Through the Binding of SRSF1. Cancers 2019, 11, 194. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, M.; Yant, L.; Huang, C. Circular RNA in disease: Basic properties and biomedical relevance. Wiley Interdiscip. Rev. RNA 2022, 13, e1723. [Google Scholar] [CrossRef] [PubMed]
- Haque, S.; Harries, L.W. Circular RNAs (circRNAs) in Health and Disease. Genes 2017, 8, 353. [Google Scholar] [CrossRef] [PubMed]
- Merulla, A.E.; Stella, M.; Barbagallo, C.; Battaglia, R.; Caponnetto, A.; Broggi, G.; Altieri, R.; Certo, F.; Caltabiano, R.; Ragusa, M.; et al. CircSMARCA5 Is an Upstream Regulator of the Expression of miR-126-3p, miR-515-5p, and Their mRNA Targets, Insulin-like Growth Factor Binding Protein 2 (IGFBP2) and NRAS Proto-Oncogene, GTPase (NRAS) in Glioblastoma. Int. J. Mol. Sci. 2022, 23, 13676. [Google Scholar] [CrossRef]
- Xu, X.; Jia, S.Z.; Dai, Y.; Zhang, J.J.; Li, X.; Shi, J.; Leng, J.; Lang, J. The Relationship of Circular RNAs With Ovarian Endometriosis. Reprod. Sci. 2018, 25, 1292–1300. [Google Scholar] [CrossRef]
- Liu, L.; Li, L.; Ma, X.; Yue, F.; Wang, Y.; Wang, L.; Jin, P.; Zhang, X. Altered Circular RNA Expression in Patients with Repeated Implantation Failure. Cell Physiol. Biochem. 2017, 44, 303–313. [Google Scholar] [CrossRef]
- Dang, Y.; Yan, L.; Hu, B.; Fan, X.; Ren, Y.; Li, R.; Lian, Y.; Yan, J.; Li, Q.; Zhang, Y.; et al. Tracing the expression of circular RNAs in human pre-implantation embryos. Genome Biol. 2016, 17, 130. [Google Scholar] [CrossRef]
- Che, Q.; Liu, M.; Xu, J.; Liu, Y.; Cao, X.; Dong, X.; Liu, S. Characterization of circular RNA expression profiles in cumulus cells from patients with polycystic ovary syndrome. Fertil. Steril. 2019, 111, 1243–1251.e1241. [Google Scholar] [CrossRef]
- Caponnetto, A.; Battaglia, R.; Ferrara, C.; Vento, M.E.; Borzi, P.; Paradiso, M.; Scollo, P.; Purrello, M.; Longobardi, S.; D’Hooghe, T.; et al. Down-regulation of long non-coding RNAs in reproductive aging and analysis of the lncRNA-miRNA-mRNA networks in human cumulus cells. J. Assist. Reprod. Genet. 2022, 39, 919–931. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Li, J.; Li, J.; Yan, X.; Zhou, W.; Ling, C.; Ye, Y.; Chen, Q.; Li, Y. Circular RNA expression profiling and bioinformatic analysis of cumulus cells in endometriosis infertility patients. Epigenomics 2020, 12, 2093–2108. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Zhao, H.; Zhang, Y.; Liu, X.; Hao, C. Novel circular RNA expression in the cumulus cells of patients with polycystic ovary syndrome. Arch. Gynecol. Obstet. 2019, 299, 1715–1725. [Google Scholar] [CrossRef]
- Wang, L.P.; Peng, X.Y.; Lv, X.Q.; Liu, L.; Li, X.L.; He, X.; Lv, F.; Pan, Y.; Wang, L.; Liu, K.F.; et al. High throughput circRNAs sequencing profile of follicle fluid exosomes of polycystic ovary syndrome patients. J. Cell. Physiol. 2019, 234, 15537–15547. [Google Scholar] [CrossRef]
- Cao, Z.; Gao, D.; Xu, T.; Zhang, L.; Tong, X.; Zhang, D.; Wang, Y.; Ning, W.; Qi, X.; Ma, Y.; et al. Circular RNA profiling in the oocyte and cumulus cells reveals that circARMC4 is essential for porcine oocyte maturation. Aging 2019, 11, 8015–8034. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.Y.; Li, T.C.; Wu, Y.Y.; Yeh, C.H.; Chiang, W.; Chuang, C.Y.; Kuo, H.C. The circular RNA circBIRC6 participates in the molecular circuitry controlling human pluripotency. Nat. Commun. 2017, 8, 1149. [Google Scholar] [CrossRef] [PubMed]
- Nishanth, M.J.; Simon, B. Functions, mechanisms and regulation of Pumilio/Puf family RNA binding proteins: A comprehensive review. Mol. Biol. Rep. 2020, 47, 785–807. [Google Scholar] [CrossRef]
- Cai, H.; Li, Y.; Li, H.; Niringiyumukiza, J.D.; Zhang, M.; Chen, L.; Chen, G.; Xiang, W. Identification and characterization of human ovary-derived circular RNAs and their potential roles in ovarian aging. Aging 2018, 10, 2511–2534. [Google Scholar] [CrossRef]
- Battaglia, R.; Musumeci, P.; Ragusa, M.; Barbagallo, D.; Scalia, M.; Zimbone, M.; Lo Faro, J.M.; Borzi, P.; Scollo, P.; Purrello, M.; et al. Ovarian aging increases small extracellular vesicle CD81(+) release in human follicular fluid and influences miRNA profiles. Aging 2020, 12, 12324–12341. [Google Scholar] [CrossRef]
- Di Pietro, C.; Vento, M.; Ragusa, M.; Barbagallo, D.; Guglielmino, M.R.; Maniscalchi, T.; Duro, L.R.; Tomasello, L.; Majorana, A.; De Palma, A.; et al. Expression analysis of TFIID in single human oocytes: New potential molecular markers of oocyte quality. Reprod. Biomed. Online 2008, 17, 338–349. [Google Scholar] [CrossRef]
- Battaglia, R.; Vento, M.E.; Ragusa, M.; Barbagallo, D.; La Ferlita, A.; Di Emidio, G.; Borzi, P.; Artini, P.G.; Scollo, P.; Tatone, C.; et al. MicroRNAs Are Stored in Human MII Oocyte and Their Expression Profile Changes in Reproductive Aging. Biol. Reprod. 2016, 95, 131. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, M.E.; Rasmussen, I.A.; Kristensen, S.G.; Christensen, S.T.; Mollgard, K.; Wreford Andersen, E.; Byskov, A.G.; Yding Andersen, C. In human granulosa cells from small antral follicles, androgen receptor mRNA and androgen levels in follicular fluid correlate with FSH receptor mRNA. Mol. Hum. Reprod. 2011, 17, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Juusola, J.; Ballantyne, J. mRNA profiling for body fluid identification by multiplex quantitative RT-PCR. J. Forensic. Sci. 2007, 52, 1252–1262. [Google Scholar] [CrossRef] [PubMed]
- Borgbo, T.; Jeppesen, J.V.; Lindgren, I.; Lundberg Giwercman, Y.; Hansen, L.L.; Yding Andersen, C. Effect of the FSH receptor single nucleotide polymorphisms (FSHR 307/680) on the follicular fluid hormone profile and the granulosa cell gene expression in human small antral follicles. Mol. Hum. Reprod. 2015, 21, 255–261. [Google Scholar] [CrossRef]
- Jeppesen, J.V.; Nielsen, M.E.; Kristensen, S.G.; Yding Andersen, C. Concentration of activin A and follistatin in follicular fluid from human small antral follicles associated to gene expression of the corresponding granulosa cells. Mol. Cell Endocrinol. 2012, 356, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.Y.; Lin, Y.C.; Cui, S.; Huang, Y.; Tang, Y.; Xu, J.; Bao, J.; Li, Y.; Wen, J.; Zuo, H.; et al. miRTarBase update 2022: An informative resource for experimentally validated miRNA-target interactions. Nucleic Acids Res. 2022, 50, D222–D230. [Google Scholar] [CrossRef] [PubMed]
- Memczak, S.; Jens, M.; Elefsinioti, A.; Torti, F.; Krueger, J.; Rybak, A.; Maier, L.; Mackowiak, S.D.; Gregersen, L.H.; Munschauer, M.; et al. Circular RNAs are a large class of animal RNAs with regulatory potency. Nature 2013, 495, 333–338. [Google Scholar] [CrossRef]
- Salzman, J.; Chen, R.E.; Olsen, M.N.; Wang, P.L.; Brown, P.O. Cell-type specific features of circular RNA expression. PLoS Genet. 2013, 9, e1003777. [Google Scholar] [CrossRef]
- Maass, P.G.; Glazar, P.; Memczak, S.; Dittmar, G.; Hollfinger, I.; Schreyer, L.; Sauer, A.V.; Toka, O.; Aiuti, A.; Luft, F.C.; et al. A map of human circular RNAs in clinically relevant tissues. J. Mol. Med. 2017, 95, 1179–1189. [Google Scholar] [CrossRef]
- Micheel, J.; Safrastyan, A.; Wollny, D. Advances in Non-Coding RNA Sequencing. Noncoding RNA 2021, 7, 70. [Google Scholar] [CrossRef]
- Ozsolak, F.; Milos, P.M. RNA sequencing: Advances, challenges and opportunities. Nat. Rev. Genet. 2011, 12, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Chen, G.; Zhu, S.; Li, S.; Wen, Z.; Bin, L.; Zheng, Y.; Shi, L. Identification of Tissue-Specific Protein-Coding and Noncoding Transcripts across 14 Human Tissues Using RNA-seq. Sci. Rep. 2016, 6, 28400. [Google Scholar] [CrossRef] [PubMed]
- Guan, X.; Zong, Z.H.; Liu, Y.; Chen, S.; Wang, L.L.; Zhao, Y. circPUM1 Promotes Tumorigenesis and Progression of Ovarian Cancer by Sponging miR-615-5p and miR-6753-5p. Mol. Ther. Nucleic Acids 2019, 18, 882–892. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Shi, L.; Ye, W.; Li, S.; Liu, X.; Zhu, Z. Circular RNA PUM1 (CircPUM1) attenuates trophoblast cell dysfunction and inflammation in recurrent spontaneous abortion via the MicroRNA-30a-5p (miR-30a-5p)/JUNB axis. Bioengineered 2021, 12, 6878–6890. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Xu, S.; Chen, S.; Zong, Z.; Han, X.; Zhao, Y.; Shang, H. CircPUM1 promotes the malignant behavior of lung adenocarcinoma by regulating miR-326. Biochem. Biophys. Res. Commun. 2019, 508, 844–849. [Google Scholar] [CrossRef]
- Gong, W.; Xu, J.; Wang, Y.; Min, Q.; Chen, X.; Zhang, W.; Chen, J.; Zhan, Q. Nuclear genome-derived circular RNA circPUM1 localizes in mitochondria and regulates oxidative phosphorylation in esophageal squamous cell carcinoma. Signal Transduct. Target Ther. 2022, 7, 40. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, D.; Zhu, T.; Yu, J.; Wu, X.; Lin, W.; Zhu, M.; Dai, Y.; Zhu, J. CircPUM1 promotes hepatocellular carcinoma progression through the miR-1208/MAP3K2 axis. J. Cell. Mol. Med. 2021, 25, 600–612. [Google Scholar] [CrossRef]
- Li, M.; Wang, Q.; Zhang, X.; Yan, N.; Li, X. CircPUM1 promotes cell growth and glycolysis in NSCLC via up-regulating METTL3 expression through miR-590-5p. Cell Cycle 2021, 20, 1279–1294. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, Y.; Tao, Q.; Fan, Y.; Ma, C.; Li, D.; Huang, F.; Tang, D. circPUM1 Activates the PI3K/AKT Signaling Pathway by Sponging to Promote the Proliferation, Invasion and Glycolysis of Pancreatic Cancer. Curr. Pharm. Biotechnol. 2022, 23, 1405–1414. [Google Scholar] [CrossRef]
- Spassov, D.S.; Jurecic, R. Cloning and comparative sequence analysis of PUM1 and PUM2 genes, human members of the Pumilio family of RNA-binding proteins. Gene 2002, 299, 195–204. [Google Scholar] [CrossRef]
- Parisi, M.; Lin, H. The Drosophila pumilio gene encodes two functional protein isoforms that play multiple roles in germline development, gonadogenesis, oogenesis and embryogenesis. Genetics 1999, 153, 235–250. [Google Scholar] [CrossRef] [PubMed]
- Miao, J.; Li, J.; Fan, Q.; Li, X.; Li, X.; Cui, L. The Puf-family RNA-binding protein PfPuf2 regulates sexual development and sex differentiation in the malaria parasite Plasmodium falciparum. J. Cell Sci. 2010, 123, 1039–1049. [Google Scholar] [CrossRef] [PubMed]
- Kuo, M.W.; Wang, S.H.; Chang, J.C.; Chang, C.H.; Huang, L.J.; Lin, H.H.; Yu, A.L.; Li, W.H.; Yu, J. A novel puf-A gene predicted from evolutionary analysis is involved in the development of eyes and primordial germ-cells. PLoS ONE 2009, 4, e4980. [Google Scholar] [CrossRef] [PubMed]
- Ota, R.; Kotani, T.; Yamashita, M. Biochemical characterization of Pumilio1 and Pumilio2 in Xenopus oocytes. J. Biol. Chem. 2011, 286, 2853–2863. [Google Scholar] [CrossRef] [PubMed]
- Saitoh, A.; Takada, Y.; Horie, M.; Kotani, T. Pumilio1 phosphorylation precedes translational activation of its target mRNA in zebrafish oocytes. Zygote 2018, 26, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Mak, W.; Xia, J.; Cheng, E.C.; Lowther, K.; Lin, H. A role of Pumilio 1 in mammalian oocyte maturation and maternal phase of embryogenesis. Cell Biosci. 2018, 8, 54. [Google Scholar] [CrossRef] [PubMed]
- Mak, W.; Fang, C.; Holden, T.; Dratver, M.B.; Lin, H. An Important Role of Pumilio 1 in Regulating the Development of the Mammalian Female Germline. Biol. Reprod. 2016, 94, 134. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Li, M.; Xue, C.; Chen, S.; Zheng, L.; Deng, H.; Tang, F.; Li, G.; Xiong, W.; Zeng, Z.; et al. Understanding the roles and regulation patterns of circRNA on its host gene in tumorigenesis and tumor progression. J. Exp. Clin. Cancer Res. 2023, 42, 86. [Google Scholar] [CrossRef]
- Zheng, W.; Nagaraju, G.; Liu, Z.; Liu, K. Functional roles of the phosphatidylinositol 3-kinases (PI3Ks) signaling in the mammalian ovary. Mol. Cell Endocrinol. 2012, 356, 24–30. [Google Scholar] [CrossRef]
- Di Pietro, C. Exosome-mediated communication in the ovarian follicle. J. Assist. Reprod. Genet. 2016, 33, 303–311. [Google Scholar] [CrossRef]
- Yao, J.; Li, M.; Lin, L.; Li, Y.; Zhuang, J.; Huang, Y.; Peng, W.; Lian, J.; Huang, R.; Yang, X. PTEN expression in human cumulus cells is associated with embryo development competence. Zygote 2022, 30, 611–618. [Google Scholar] [CrossRef] [PubMed]
- Reddy, P.; Liu, L.; Adhikari, D.; Jagarlamudi, K.; Rajareddy, S.; Shen, Y.; Du, C.; Tang, W.; Hamalainen, T.; Peng, S.L.; et al. Oocyte-specific deletion of Pten causes premature activation of the primordial follicle pool. Science 2008, 319, 611–613. [Google Scholar] [CrossRef] [PubMed]
- De Felici, M.; Klinger, F.G. PI3K/PTEN/AKT Signaling Pathways in Germ Cell Development and Their Involvement in Germ Cell Tumors and Ovarian Dysfunctions. Int. J. Mol. Sci. 2021, 22, 9838. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Huang, R.; Li, M.; Jiang, Y.; Wu, P.; Li, Y.; Peng, W.; Hua, C.; Huang, Y.; You, H.; et al. PTEN Expression in Human Granulosa Cells Is Associated with Ovarian Responses and Clinical Outcomes in IVF. Reprod. Sci. 2021, 28, 1910–1921. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Sinha, T.; Panda, A.C. Regulation of microRNA by circular RNA. Wiley Interdiscip. Rev. RNA 2023, e1820. [Google Scholar] [CrossRef]
- Vasudevan, S.; Tong, Y.; Steitz, J.A. Switching from repression to activation: MicroRNAs can up-regulate translation. Science 2007, 318, 1931–1934. [Google Scholar] [CrossRef]
- Sadighparvara, S.; Targhazehb, N.; Karimian, A.; Shafiei-Irannejad, V.; Farsad-Akhtar, N.; Rafieian, S.; Salamati, A.; Bastami, M.; Samadi Kafil, H.; Yousefi, M.; et al. Downregulation of microRNA-214 and PTEN in tissue samples of patients with breast cancer. Meta Gene 2020, 24, 100668. [Google Scholar] [CrossRef]
- Xiao, M.; Li, J.; Li, W.; Wang, Y.; Wu, F.; Xi, Y.; Zhang, L.; Ding, C.; Luo, H.; Li, Y.; et al. MicroRNAs activate gene transcription epigenetically as an enhancer trigger. RNA Biol. 2017, 14, 1326–1334. [Google Scholar] [CrossRef]

| # | Gene ID | Host Gene | Forward Primer Sequence | Reverse Primer Sequence | Product Length (bp) |
|---|---|---|---|---|---|
| 1 | hsa_circ_0088943 | NUP188 | TGAAACTGGCATTCTCCGTCA | GGGCACTGAAGTAGCATGTTG | 107 |
| 2 | hsa_circ_0077150 | PHIP | CATCACTACAGGTGAAGTATTACAG | CTCCAGGAACACTTTGAGGAAT | 111 |
| 3 | hsa_circ_0077147 | PHIP | TCTGGGATGCTGGAACCCTT | AGTCATCAGAACCCAGCACTAA | 112 |
| 4 | hsa_circ_0004079 (1) | TRIP12 | CACACGCCAAAAGACCACG | TAGACTGCACTTTGGGTGCC | 125 |
| hsa_circ_0004079 (2) | GCTAGTACCAGGTCACATTTAG | TTAGACTGCACTTTGGGTGC | 98 | ||
| 5 | hsa_circ_0068888 | WHSC1 | AGTGTCGGGTTACCCTTGGT | CACAGAAAAGCAGACAGCTCG | 271 |
| 6 | hsa_circ_0011233 | PUM1 | GAGGCCACGTCCTGTCATTG | TGAGTCCTCCTGCTGGTCTGA | 95 |
| 7 | hsa_circ_0043064 (1) | MYO1D | CCTGAAGGCAAACTGAGCAT | GCACAATCACTCGAGACTTTGA | 121 |
| hsa_circ_0043064 (2) | TACAGAGGTGACCAAGCGAC | CTCCTGGCTGTTGCACAATC | 113 | ||
| 8 | GAPDH | GAPDH | TGCACCACCAACTGCTTAGC | GGCATGGACTGTGGTCATGAG | 87 |
| 9 | linear PUM1 | PUM1 | CCTTCAGACCAGCAGAATGAGA | ATTGCAAAGACTGGGGCTGT | 127 |
| 10 | BCL2 | BCL2 | CATGTGTGTGGAGAGCGTCAA | GCCGGTTCAGGTACTCAGTCA | 83 |
| 11 | PTEN | PTEN | GACAATCATGTTGCAGCAATTCA | CCCATAGAAATCTAGGGCCTCT | 123 |
| 12 | TP53 | TP53 | GAGCACTGCCCAACAACAC | GTCTGGTCCTGAAGGGTGAA | 85 |
| # | circRNA ID | Host Gene | Host Gene ID | miRNAs |
|---|---|---|---|---|
| 1 | hsa_circ_0088943 | Nucleoporin 188 | NUP188 | hsa-miR-16-5p hsa-miR-214-3p |
| 2 | hsa_circ_0077150 | Pleckstrin homology domain Interacting protein | PHIP | hsa-miR-16-5p |
| 3 | hsa_circ_0077147 | hsa-miR-16-5p | ||
| 4 | hsa_circ_0004079 (1) | Thyroid hormone receptor interactor 12 | TRIP12 | hsa-miR-372-3p hsa-miR-18a-5p hsa-miR-214-3p |
| hsa_circ_0004079 (2) | ||||
| 5 | hsa_circ_0068888 | Wolf–Hirschhorn syndrome candidate 1 | WHSC1 | hsa-miR-16-5p hsa-miR-214-3p |
| 6 | hsa_circ_0011233 | Pumilio RNA-binding family member 1 | PUM1 | hsa-miR-16-5p hsa-miR-214-3p |
| 7 | hsa_circ_0043064 (1) | Myosin ID | MYO1D | hsa-miR-16-5p |
| hsa_circ_0043064 (2) |
| circRNA ID | Hsa_circ_0011233 |
| Location | chr1:31422979-31426828 |
| Genomic Length | 3849 bp |
| Spliced Seq Length | 527 bp |
| Best Transcript | NM_014676 |
| Gene Symbol | PUM1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caponnetto, A.; Ferrara, C.; Fazzio, A.; Agosta, N.; Scribano, M.; Vento, M.E.; Borzì, P.; Barbagallo, C.; Stella, M.; Ragusa, M.; et al. A Circular RNA Derived from the Pumilio 1 Gene Could Regulate PTEN in Human Cumulus Cells. Genes 2024, 15, 124. https://doi.org/10.3390/genes15010124
Caponnetto A, Ferrara C, Fazzio A, Agosta N, Scribano M, Vento ME, Borzì P, Barbagallo C, Stella M, Ragusa M, et al. A Circular RNA Derived from the Pumilio 1 Gene Could Regulate PTEN in Human Cumulus Cells. Genes. 2024; 15(1):124. https://doi.org/10.3390/genes15010124
Chicago/Turabian StyleCaponnetto, Angela, Carmen Ferrara, Anna Fazzio, Noemi Agosta, Marianna Scribano, Maria Elena Vento, Placido Borzì, Cristina Barbagallo, Michele Stella, Marco Ragusa, and et al. 2024. "A Circular RNA Derived from the Pumilio 1 Gene Could Regulate PTEN in Human Cumulus Cells" Genes 15, no. 1: 124. https://doi.org/10.3390/genes15010124
APA StyleCaponnetto, A., Ferrara, C., Fazzio, A., Agosta, N., Scribano, M., Vento, M. E., Borzì, P., Barbagallo, C., Stella, M., Ragusa, M., Scollo, P., Barbagallo, D., Purrello, M., Di Pietro, C., & Battaglia, R. (2024). A Circular RNA Derived from the Pumilio 1 Gene Could Regulate PTEN in Human Cumulus Cells. Genes, 15(1), 124. https://doi.org/10.3390/genes15010124









