Molecular Characterization of Peroxidase (PRX) Gene Family in Cucumber
Abstract
:1. Introduction
2. Materials and Methods
2.1. Characterization of PRX Gene Family in Cucumber
2.2. Gene Structure and Phylogenetic Relationship of PRX Proteins
2.3. Plant Materials and Treatments
2.4. Gene Expression Analysis
3. Results
3.1. Identification of PRX Genes in Cucumber
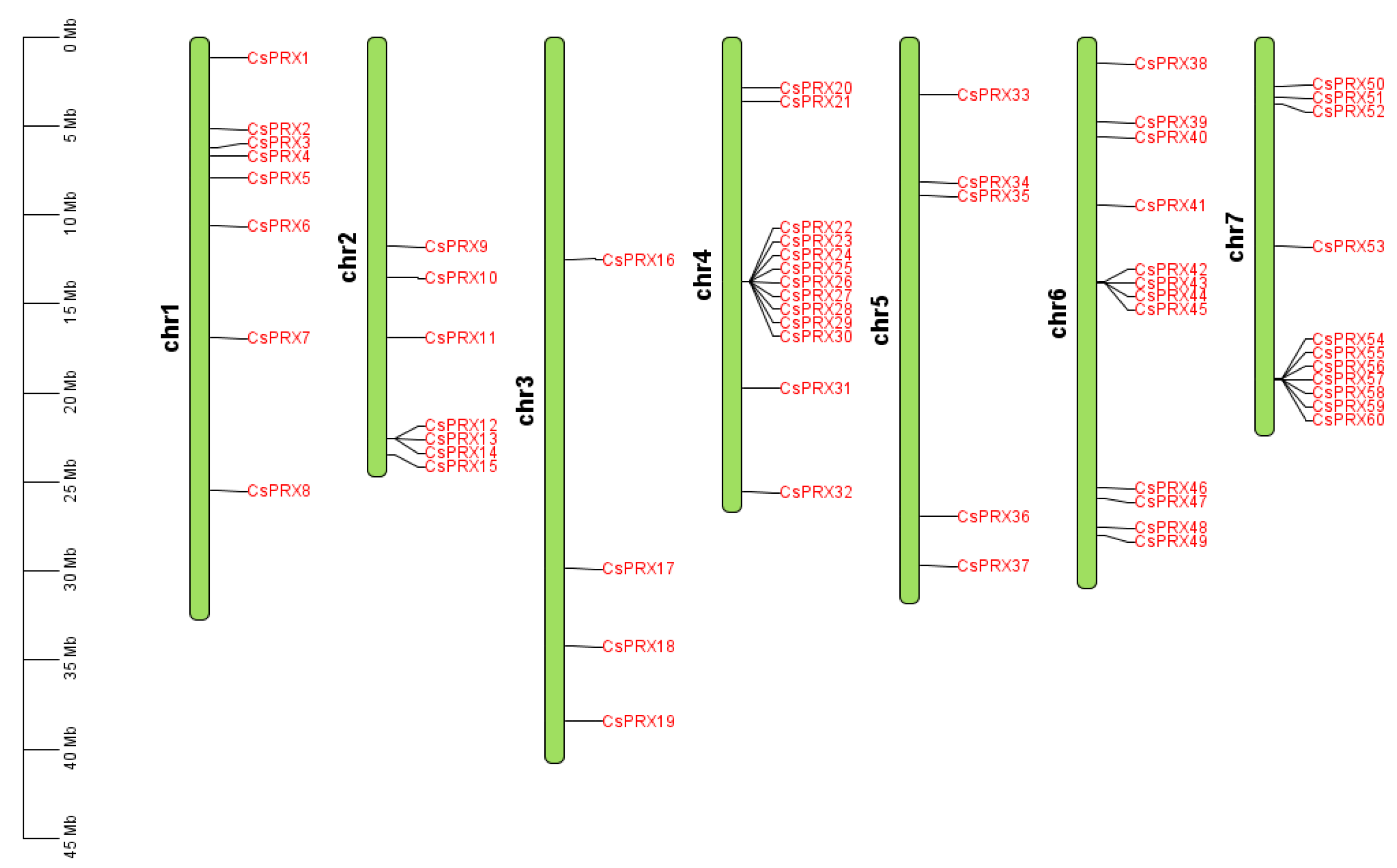
3.2. Chromosomal Location of CsPRX Genes
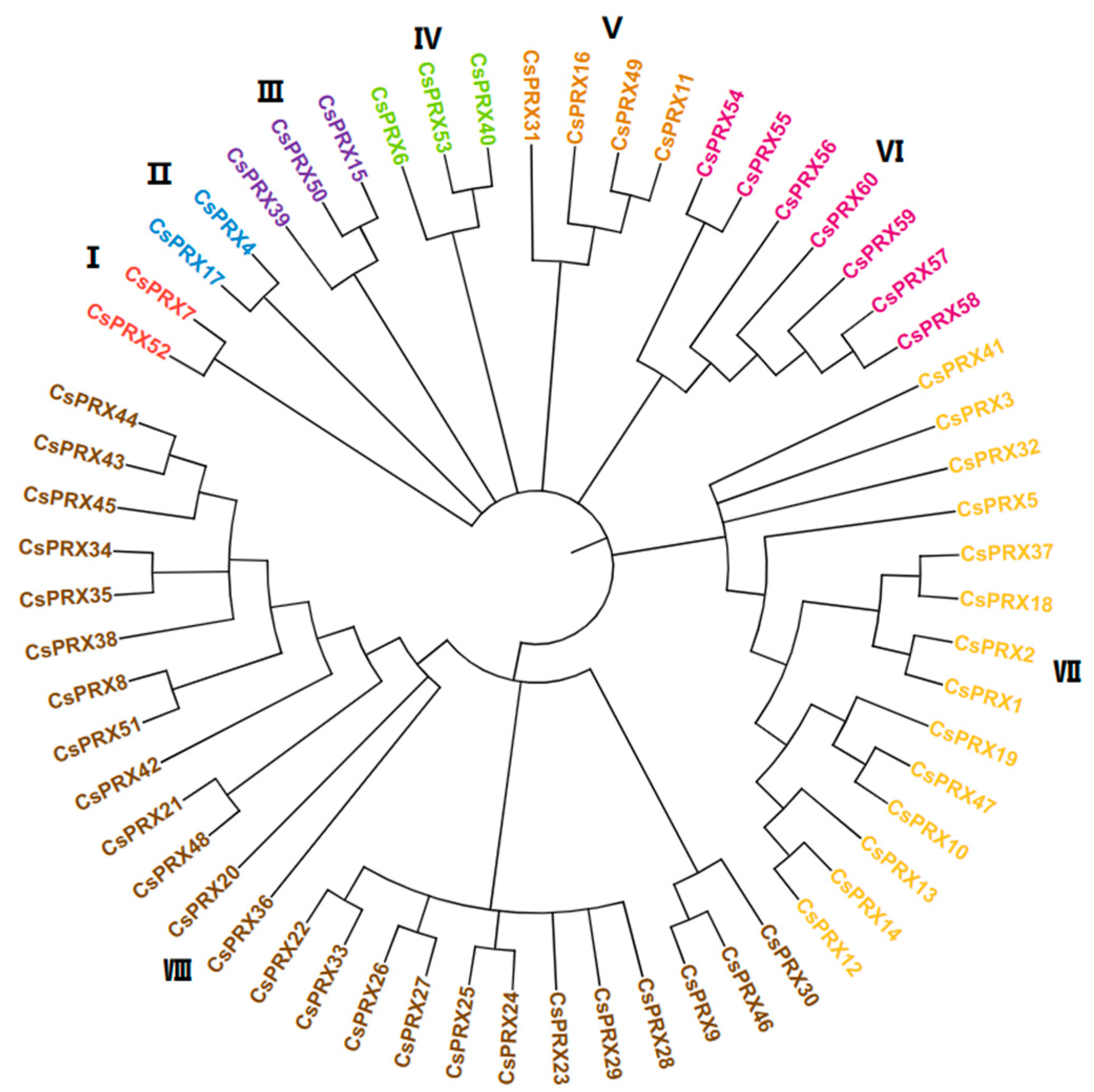
3.3. Phylogenetic Analysis of CsPRX Genes
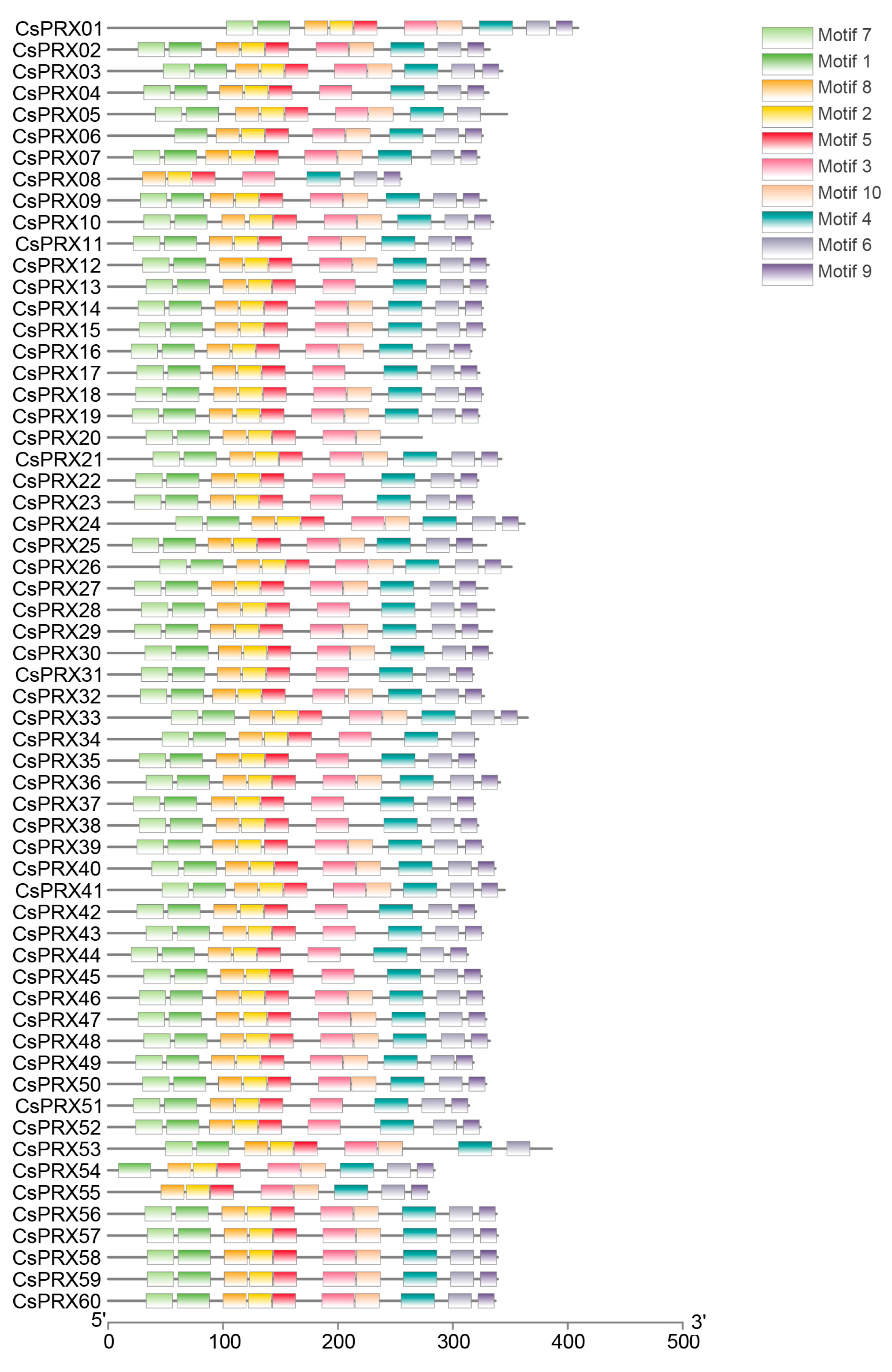
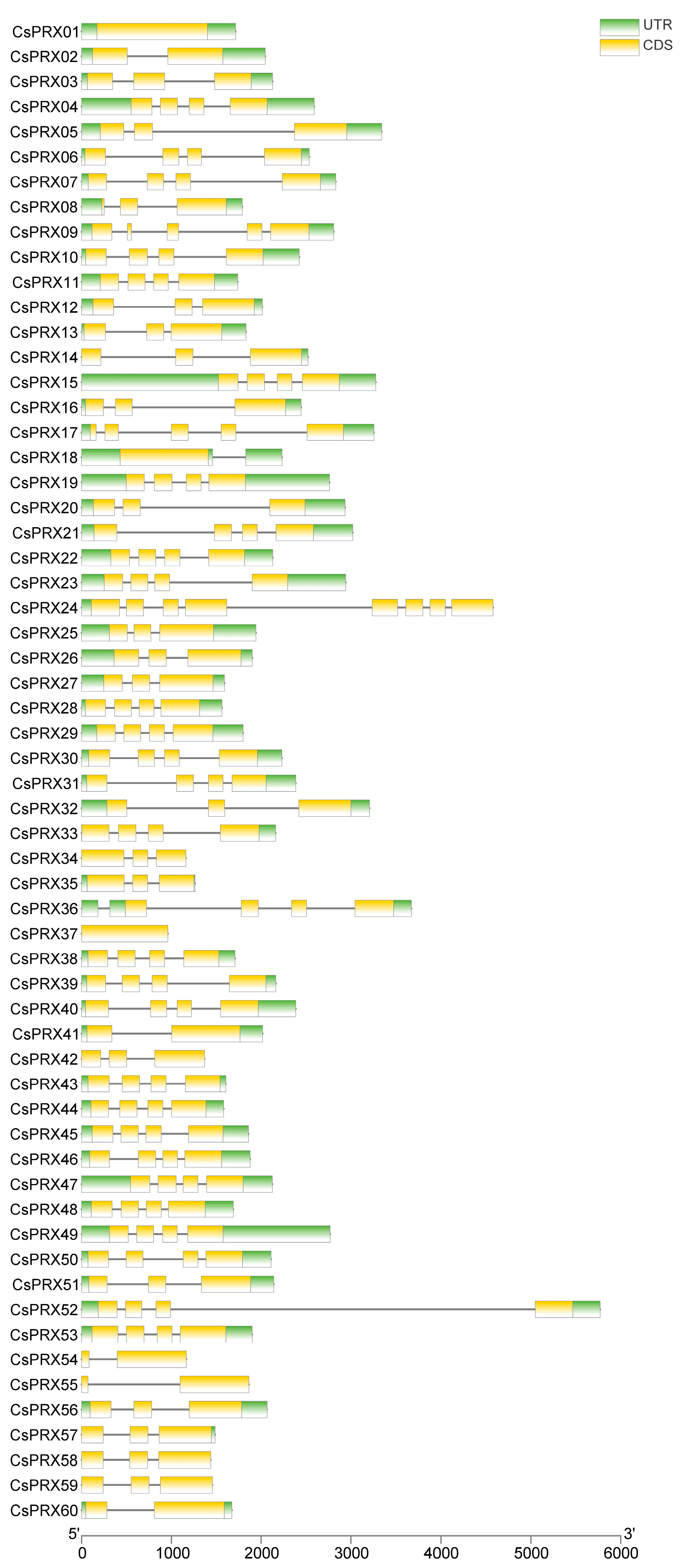
3.4. Conserved Motifs and Gene Structures of CsPRX Genes
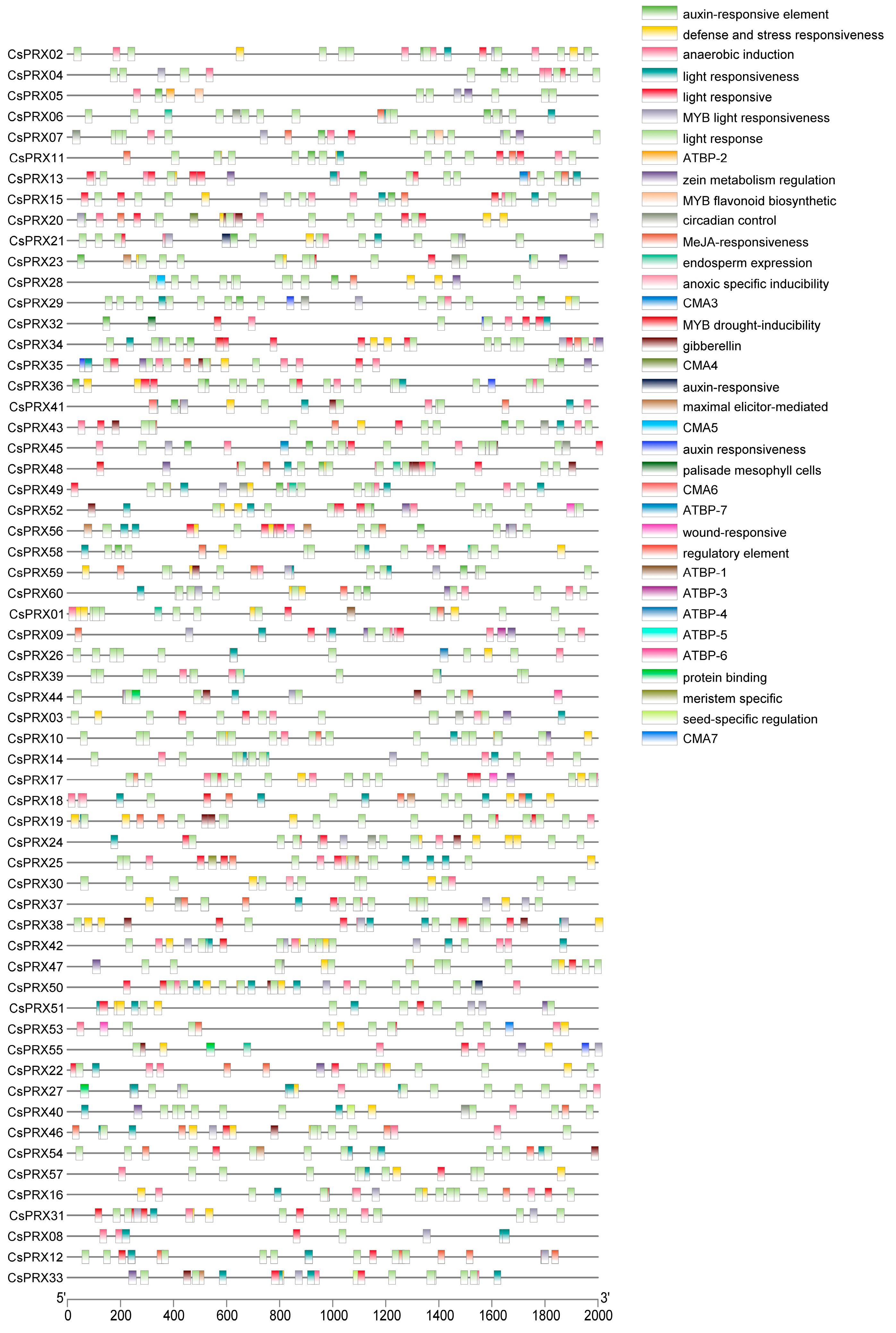
3.5. Cis-Regulatory Elements of CsPRX Genes in Cucumber
3.6. Tissue Specificity of CsPRX Genes in Cucumber
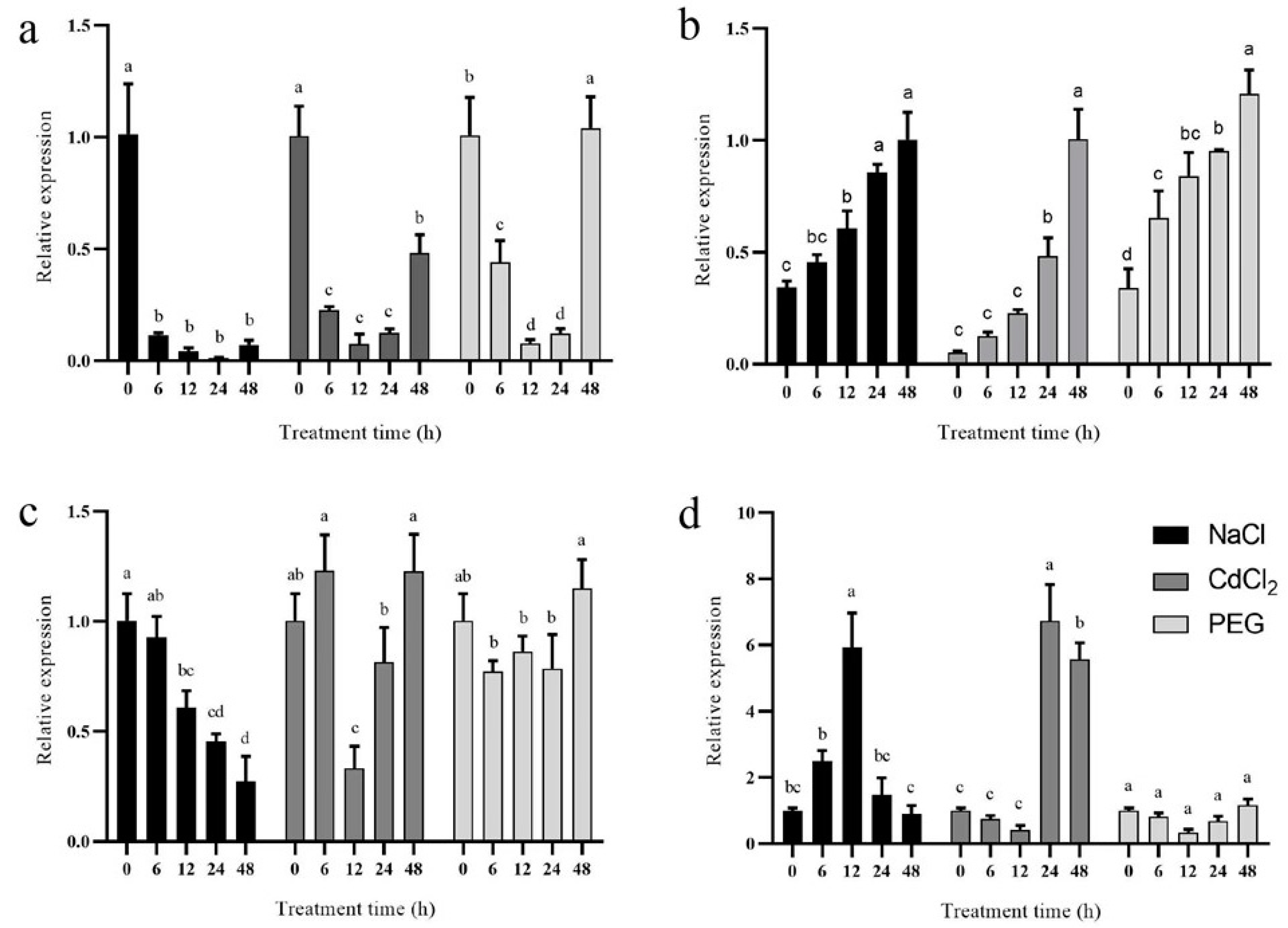
3.7. Expression Profiles of CsPRX Genes under Abiotic Stresses
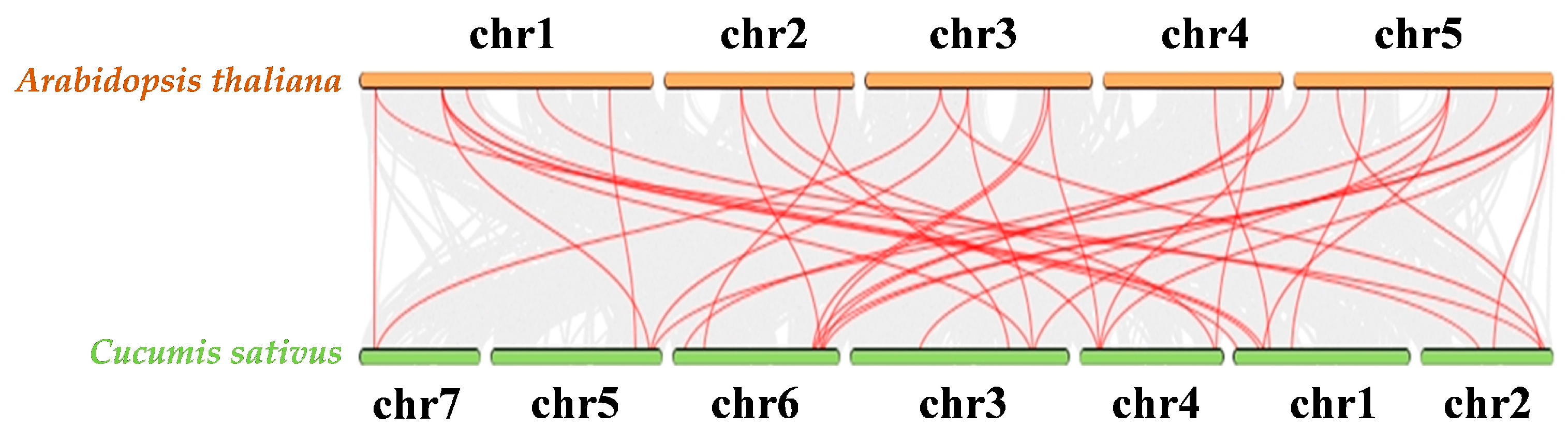
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hiraga, S.; Ichinose, C.; Onogi, T.; Niki, H.; Yamazoe, M. Bidirectional migration of SeqA-bound hemimethylated DNA clusters and pairing of oriC copies in Escherichia coli. Genes Cells 2000, 5, 327–341. [Google Scholar] [CrossRef] [PubMed]
- Hiraga, S.; Sasaki, K.; Ito, H.; Ohashi, Y.; Matsui, H. A large family of class III plant peroxidases. Plant Cell Physiol. 2001, 42, 462–468. [Google Scholar] [CrossRef]
- Passardi, F.; Cosio, C.; Penel, C.; Dunand, C. Peroxidases have more functions than a Swiss army knife. Plant Cell Rep. 2005, 24, 255–265. [Google Scholar] [CrossRef]
- Welinder, K.G. Plant peroxidases: Structure-function relationships. In Plant Peroxidases; Penel, C., Gaspar, T., Greppin, H., Eds.; University of Geneva: Geneva, Switzerland, 1992; pp. 1–24. [Google Scholar]
- Passardi, F.; Longet, D.; Penel, C.; Dunand, C. The class III peroxidase multigenic family in rice and its evolution in land plants. Phytochemistry 2004, 65, 1879–1893. [Google Scholar] [CrossRef]
- Barceló, A.R.; Pomar, F. Oxidation of cinnamyl alcohols and aldehydes by a basic peroxidase from lignifying Zinnia elegans hypocotyls. Phytochemistry 2001, 57, 1105–1113. [Google Scholar] [CrossRef]
- Hiraga, S.; Yamamoto, K.; Ito, H.; Sasaki, K.; Matsui, H.; Honma, M.; Nagamura, Y.; Sasaki, T.; Ohashi, Y. Diverse expression profiles of 21 rice peroxidase genes. FEBS Lett. 2000, 471, 245–250. [Google Scholar] [CrossRef]
- Schopfer, P.; Liszkay, A.; Bechtold, M.K.; Frahry, G.; Wagner, A. Evidence that hydroxyl radicals mediate auxin-induced extension growth. Planta 2002, 214, 821–828. [Google Scholar] [CrossRef]
- Almagro, L.; Gómez Ros, L.V.; Belchi-Navarro, S.; Bru, R.; Ros Barceló, A.; Pedreño, M.A. Class III peroxidases in plant defence reactions. J. Exp. Bot. 2009, 60, 377–390. [Google Scholar] [CrossRef] [PubMed]
- Kidwai, M.; Dhar, Y.V.; Gautam, N.K.; Tiwari, M.; Ahmad, I.Z.; Asif, M.H.; Chakrabarty, D. Oryza sativa class III peroxidase (OsPRX38) overexpression in Arabidopsis thaliana reduces arsenic accumulation due to apoplastic lignification. J. Hazard. Mater. 2019, 362, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Su, P.; Sui, C.; Niu, Y.; Li, J.; Wang, S.; Sun, F.; Yan, J.; Guo, S. Comparative transcriptomic analysis and functional characterization reveals that the class III peroxidase gene TaPRX-2A regulates drought stress tolerance in transgenic wheat. Front. Plant Sci. 2023, 14, 1119162. [Google Scholar] [CrossRef]
- Su, P.; Yan, J.; Li, W.; Wang, L.; Wang, L.; Zhao, J.; Ma, X.; Li, A.; Wang, H.; Kong, L. A member of wheat class III peroxidase gene family, TaPRX-2A, enhanced the tolerance of salt stress. BMC Plant Biol. 2020, 20, 392. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, Z.; Li, X.; Gao, X.; Dai, Z.; Cui, Y.; Zhi, Y.; Liu, Q.; Zhai, H.; Gao, S.; et al. The IbBBX24-IbTOE3-IbPRX17 module enhances abiotic stress tolerance by scavenging reactive oxygen species in sweet potato. New Phytol. 2022, 233, 1133–1152. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Dong, S.; Li, M.; Gu, F.; Yang, G.; Guo, T.; Chen, Z.; Wang, J. The class III peroxidase gene OsPrx30, transcriptionally modulated by the AT-hook protein OsATH1, mediates rice bacterial blight-induced ROS accumulation. J. Integr. Plant Biol. 2021, 63, 393–408. [Google Scholar] [CrossRef] [PubMed]
- Intapruk, C.; Higashimura, N.; Yamamoto, K.; Okada, N.; Shinmyo, A.; Takano, M. Nucleotide sequences of two genomic DNAs encoding peroxidase of Arabidopsis thaliana. Gene 1991, 98, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Tognolli, M.; Penel, C.; Greppin, H.; Simon, P. Analysis and expression of the class III peroxidase large gene family in Arabidopsis thaliana. Gene 2002, 288, 129–138. [Google Scholar] [CrossRef]
- Aleem, M.; Riaz, A.; Raza, Q.; Aleem, M.; Aslam, M.; Kong, K.; Atif, R.M.; Kashif, M.; Bhat, J.A.; Zhao, T. Genome-wide characterization and functional analysis of class III peroxidase gene family in soybean reveal regulatory roles of GsPOD40 in drought tolerance. Genomics 2022, 114, 45–60. [Google Scholar] [CrossRef]
- Yang, X.; Yuan, J.; Luo, W.; Qin, M.; Yang, J.; Wu, W.; Xie, X. Genome-wide identification and expression analysis of the class III peroxidase gene family in potato (Solanum tuberosum L.). Front. Genet. 2020, 11, 593577. [Google Scholar] [CrossRef]
- Xiao, H.; Wang, C.; Khan, N.; Chen, M.; Fu, W.; Guan, L.; Leng, X. Genome-wide identification of the class III POD gene family and their expression profiling in grapevine (Vitis vinifera L.). BMC Genom. 2020, 21, 444. [Google Scholar] [CrossRef]
- Shang, H.; Fang, L.; Qin, L.; Jiang, H.; Duan, Z.; Zhang, H.; Yang, Z.; Cheng, G.; Bao, Y.; Xu, J.; et al. Genome-wide identification of the class III peroxidase gene family of sugarcane and its expression profiles under stresses. Front. Plant Sci. 2023, 14, 1101665. [Google Scholar] [CrossRef]
- Meng, G.; Fan, W.; Rasmussen, S.K.; Ppb, B. Characterisation of the class III peroxidase gene family in carrot taproots and its role in anthocyanin and lignin accumulation. Plant Physiol. Biochem. 2021, 167, 245–256. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations. FAO-STAT Statistical Database; Food and Agriculture Organization of the United Nations: Rome, Italy, 2023. [Google Scholar]
- Wang, Y.; Wang, Q.; Zhao, Y.; Han, G.; Zhu, S. Systematic analysis of maize class III peroxidase gene family reveals a conserved subfamily involved in abiotic stress response. Gene 2015, 566, 95–108. [Google Scholar] [CrossRef] [PubMed]
- Marzol, E.; Borassi, C.; Carignani Sardoy, M.; Ranocha, P.; Aptekmann, A.A.; Bringas, M.; Pennington, J.; Paez-Valencia, J.; Martínez Pacheco, J.; Rodríguez-Garcia, D.R.; et al. Class III peroxidases PRX01, PRX44, and PRX73 control root hair growth in Arabidopsis thaliana. Int. J. Mol. Sci. 2022, 23, 5375. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.J.; Kim, Y.C.; Lee, J.S.; Kim, D.G.; Lee, J.H. Reduced expression of PRX2/ATPRX1, PRX8, PRX35, and PRX73 affects cell elongation, vegetative growth, and vasculature structures in Arabidopsis thaliana. Plants 2022, 11, 3353. [Google Scholar] [CrossRef]
- Xue, Y.J.; Tao, L.; Yang, Z.M. Aluminum-induced cell wall peroxidase activity and lignin synthesis are differentially regulated by jasmonate and nitric oxide. J. Agric. Food Chem. 2008, 56, 9676–9684. [Google Scholar] [CrossRef]
- Chiang, H.C.; Lo, J.C.; Yeh, K.C. Genes associated with heavy metal tolerance and accumulation in Zn/Cd hyperaccumulator Arabidopsis halleri: A genomic survey with cDNA microarray. Environ. Sci. Technol. 2006, 40, 6792–6798. [Google Scholar] [CrossRef]
- Kidwai, M.; Ahmad, I.Z.; Chakrabarty, D. Class III peroxidase: An indispensable enzyme for biotic/abiotic stress tolerance and a potent candidate for crop improvement. Plant Cell Rep. 2020, 39, 1381–1393. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Huang, C.; Deng, X.; Zhou, S.; Chen, L.; Li, Y.; Wang, C.; Ma, Z.; Yuan, Q.; Wang, Y.; et al. TaASR1, a transcription factor gene in wheat, confers drought stress tolerance in transgenic tobacco. Plant Cell Environ. 2013, 36, 1449–1464. [Google Scholar] [CrossRef]
- Choi, H.W.; Hwang, B.K. The pepper extracellular peroxidase CaPO2 is required for salt, drought and oxidative stress tolerance as well as resistance to fungal pathogens. Planta 2012, 235, 1369–1382. [Google Scholar] [CrossRef]
- Kumar, S.; Jaggi, M.; Sinha, A.K. Ectopic overexpression of vacuolar and apoplastic Catharanthus roseus peroxidases confers differential tolerance to salt and dehydration stress in transgenic tobacco. Protoplasma 2012, 249, 423–432. [Google Scholar] [CrossRef]

| Name | Gene ID | AA | MW (Da) | pI | II | GRAVY | Subcellular Localization |
|---|---|---|---|---|---|---|---|
| CsPRX1 | CsaV3_1G001790.1 | 409 | 44,782.37 | 6.57 | 36.19 | −0.106 | endoplasmic reticulum |
| CsPRX2 | CsaV3_1G008260.1 | 332 | 36,902.27 | 6.36 | 30.43 | −0.096 | extracellular |
| CsPRX3 | CsaV3_1G009980.1 | 343 | 38,125.62 | 5.84 | 40.75 | −0.173 | extracellular |
| CsPRX4 | CsaV3_1G010780.1 | 331 | 37,686.25 | 8.47 | 41.32 | −0.352 | vacuole membrane |
| CsPRX5 | CsaV3_1G012650.1 | 347 | 37,950.81 | 9.30 | 39.59 | −0.224 | chloroplasts |
| CsPRX6 | CsaV3_1G014970.1 | 255 | 35,741.98 | 6.53 | 40.29 | −0.029 | chloroplasts |
| CsPRX7 | CsaV3_1G030170.1 | 323 | 35,722.78 | 5.63 | 32.33 | −0..155 | extracellular |
| CsPRX8 | CsaV3_1G040180.1 | 255 | 27,942.32 | 5.15 | 35.64 | −0.314 | cytoplasm |
| CsPRX9 | CsaV3_2G014160.1 | 329 | 36,569.78 | 4.96 | 30.07 | −0.047 | extracellular |
| CsPRX10 | CsaV3_2G016300.1 | 335 | 36,566.83 | 8.86 | 34.73 | −0.092 | vacuole membrane |
| CsPRX11 | CsaV3_2G024340.1 | 317 | 34,683.64 | 9.09 | 49.80 | −0.095 | extracellular |
| CsPRX12 | CsaV3_2G034090.1 | 331 | 35,690.26 | 4.74 | 36.49 | −0.001 | vacuole membrane |
| CsPRX13 | CsaV3_2G034100.1 | 330 | 35,735.71 | 8.74 | 37.72 | −0.137 | cytoplasm |
| CsPRX14 | CsaV3_2G034110.1 | 326 | 34,863.86 | 6.93 | 26.49 | 0.080 | endoplasmic reticulum |
| CsPRX15 | CsaV3_2G035150.1 | 328 | 36,082.20 | 8.10 | 40.23 | −0.139 | vacuole membrane |
| CsPRX16 | CsaV3_3G016670.1 | 316 | 34,565.47 | 7.56 | 38.95 | −0.053 | cell membrane |
| CsPRX17 | CsaV3_3G035690.1 | 323 | 36,388.92 | 8.31 | 34.08 | −0.181 | chloroplasts |
| CsPRX18 | CsaV3_3G042040.1 | 326 | 35,890.94 | 8.63 | 34.12 | −0.196 | chloroplasts |
| CsPRX19 | CsaV3_3G047080.1 | 323 | 36,036.27 | 9.06 | 27.15 | −0.170 | extracellular |
| CsPRX20 | CsaV3_4G004610.1 | 273 | 30,455.90 | 5.40 | 41.04 | −0.041 | chloroplasts |
| CsPRX21 | CsaV3_4G005430.1 | 342 | 37,447.46 | 9.18 | 49.20 | −0.294 | extracellular |
| CsPRX22 | CsaV3_4G023590.1 | 322 | 34,297.21 | 4.94 | 42.45 | −0.153 | vacuole membrane |
| CsPRX23 | CsaV3_4G023610.1 | 318 | 34,844.31 | 6.43 | 52.01 | −0.196 | vacuole membrane |
| CsPRX24 | CsaV3_4G023620.1 | 742 | 80,803.84 | 6.17 | 40.02 | −0.229 | plasma membrane |
| CsPRX25 | CsaV3_4G023630.1 | 329 | 36,469.33 | 6.24 | 46.57 | −0.264 | extracellular |
| CsPRX26 | CsaV3_4G023640.1 | 351 | 38,281.28 | 9.11 | 43.07 | −0.006 | chloroplasts |
| CsPRX27 | CsaV3_4G023650.1 | 330 | 35,502.55 | 5.51 | 37.98 | 0.102 | extracellular |
| CsPRX28 | CsaV3_4G023660.1 | 336 | 36,453.37 | 8.34 | 41.41 | −0.224 | chloroplasts |
| CsPRX29 | CsaV3_4G023670.1 | 334 | 35,554.70 | 4.61 | 43.64 | −0.082 | vacuole membrane |
| CsPRX30 | CsaV3_4G023680.1 | 334 | 36,476.81 | 5.63 | 42.78 | 0.043 | vacuole membrane |
| CsPRX31 | CsaV3_4G029960.1 | 318 | 35,080.10 | 8.10 | 44.63 | −0.092 | chloroplasts |
| CsPRX32 | CsaV3_4G036360.1 | 327 | 34,873.18 | 5.10 | 37.76 | 0.006 | plasma membrane |
| CsPRX33 | CsaV3_5G005000.1 | 365 | 39,538.89 | 5.16 | 42.23 | −0.038 | chloroplasts |
| CsPRX34 | CsaV3_5G012680.1 | 322 | 35,243.14 | 8.16 | 38.21 | −0.012 | endoplasmic reticulum |
| CsPRX35 | CsaV3_5G012840.1 | 320 | 34,594.32 | 7.57 | 43.66 | −0.099 | extracellular |
| CsPRX36 | CsaV3_5G033660.1 | 341 | 37,746.19 | 5.24 | 30.42 | 0.024 | extracellular |
| CsPRX37 | CsaV3_5G037450.1 | 319 | 35,013.07 | 8.36 | 42.21 | −0.002 | chloroplasts |
| CsPRX38 | CsaV3_6G002170.1 | 322 | 34,128.24 | 8.63 | 48.52 | −0.097 | chloroplasts |
| CsPRX39 | CsaV3_6G005630.1 | 326 | 36,159.26 | 9.28 | 49.78 | −0.187 | chloroplasts |
| CsPRX40 | CsaV3_6G006890.1 | 337 | 37,933.75 | 9.14 | 47.17 | −0.461 | vacuole membrane |
| CsPRX41 | CsaV3_6G013360.1 | 345 | 38,494.56 | 9.34 | 33.68 | −0.045 | vacuole membrane |
| CsPRX42 | CsaV3_6G018990.1 | 320 | 34,795.30 | 7.58 | 27.51 | −0.160 | chloroplasts |
| CsPRX43 | CsaV3_6G019010.1 | 326 | 35,456.53 | 9.26 | 40.02 | −0.196 | extracellular |
| CsPRX44 | CsaV3_6G019020.1 | 313 | 34,396.09 | 6.99 | 32.03 | −0.236 | chloroplasts |
| CsPRX45 | CsaV3_6G019040.1 | 325 | 35,404.37 | 9.12 | 42.38 | −0.123 | chloroplasts |
| CsPRX46 | CsaV3_6G043090.1 | 327 | 36,656.70 | 6.43 | 33.14 | −0.165 | extracellular |
| CsPRX47 | CsaV3_6G043930.1 | 329 | 36,083.28 | 9.36 | 36.70 | −0.077 | chloroplasts |
| CsPRX48 | CsaV3_6G046710.1 | 332 | 36,387.16 | 9.04 | 46.80 | −0.319 | chloroplasts |
| CsPRX49 | CsaV3_6G047430.1 | 318 | 34,499.38 | 9.06 | 39.26 | −0.084 | endoplasmic reticulum |
| CsPRX50 | CsaV3_7G003750.1 | 329 | 35,669.05 | 9.20 | 36.63 | −0.030 | chloroplasts |
| CsPRX51 | CsaV3_7G005720.1 | 314 | 34,009.52 | 8.60 | 29.85 | −0.181 | chloroplasts |
| CsPRX52 | CsaV3_7G006200.1 | 324 | 35,012.99 | 6.11 | 41.44 | 0.013 | chloroplasts |
| CsPRX53 | CsaV3_7G022880.1 | 386 | 43,158.60 | 5.79 | 41.10 | −0.294 | chloroplasts |
| CsPRX54 | CsaV3_7G030360.1 | 284 | 30,755.02 | 6.83 | 36.75 | −0.134 | extracellular |
| CsPRX55 | CsaV3_7G030370.1 | 279 | 30,498.63 | 5.20 | 38.27 | −0.200 | endoplasmic reticulum |
| CsPRX56 | CsaV3_7G030380.1 | 338 | 36,567.71 | 9.40 | 42.64 | −0.148 | chloroplasts |
| CsPRX57 | CsaV3_7G030390.1 | 339 | 36,860.73 | 8.04 | 41.24 | −0.180 | mitochondria |
| CsPRX58 | CsaV3_7G030400.1 | 339 | 36,852.71 | 8.32 | 39.80 | −0.183 | mitochondria |
| CsPRX59 | CsaV3_7G030410.1 | 339 | 36,896.89 | 8.03 | 42.37 | −0.158 | chloroplasts |
| CsPRX60 | CsaV3_7G030420.1 | 337 | 36,908.94 | 8.62 | 43.11 | −0.236 | mitochondria |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, W.; Liu, J.; Xu, W.; Zhi, S.; Wang, X.; Sun, Y. Molecular Characterization of Peroxidase (PRX) Gene Family in Cucumber. Genes 2024, 15, 1245. https://doi.org/10.3390/genes15101245
Luo W, Liu J, Xu W, Zhi S, Wang X, Sun Y. Molecular Characterization of Peroxidase (PRX) Gene Family in Cucumber. Genes. 2024; 15(10):1245. https://doi.org/10.3390/genes15101245
Chicago/Turabian StyleLuo, Weirong, Junjun Liu, Wenchen Xu, Shenshen Zhi, Xudong Wang, and Yongdong Sun. 2024. "Molecular Characterization of Peroxidase (PRX) Gene Family in Cucumber" Genes 15, no. 10: 1245. https://doi.org/10.3390/genes15101245





