Molecular Mechanism of Radioresponsiveness in Colorectal Cancer: A Systematic Review
Highlights
- Comprehensive Analysis: The review systematically examines a wide array of molecular mechanisms that influence the radioresponsiveness of colorectal cancer (CRC). It details key epigenetic and genetic expression, providing a thorough overview of the current understanding in this field.
- Signaling Pathways: Using gene set enrichment analysis, the paper delves into critical signaling pathways, such as DNA damage repair and cancer metabolism mechanisms, which play pivotal roles in modulating the response and survival of CRC cells to radiation.
- Therapeutic Insights: We highlight potential biomarkers that could predict radioresponse, suggesting novel therapeutic targets to enhance and predict the efficacy of radiation therapy in CRC. This includes exploring the roles of specific genes and their alterations in influencing treatment outcomes.
- Clinical Implications: The findings emphasize the importance of personalized treatment strategies. By identifying molecular profiles that affect radioresponse, our work aims to guide the development of more targeted and effective radiation therapy protocols, ultimately aiming to improve patient outcomes.
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Study Screening
2.4. Quality Assessment Protocol
2.5. Data Extraction and GSEA
3. Results
3.1. Quality Assessment Results
3.2. Study Characteristics
3.3. Clinical Characteristics
3.4. Outcome Characteristics
3.5. GSEA
4. Discussion
4.1. Potential Molecular Biomarkers for Radioresponse
4.2. Gene Set Analysis Suggests the Potential Molecular Mechanisms of Pathways Affecting Radioresponsiveness
4.3. Limitations and Improvements
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cancer.Net. Colorectal Cancer: Statistics. Available online: https://www.cancer.net/cancer-types/colorectal-cancer/statistics (accessed on 19 August 2024).
- Xi, Y.; Xu, P. Global colorectal cancer burden in 2020 and projections to 2040. Transl. Oncol. 2021, 14, 101174. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Yin, P.; Liu, Y.-N.; Liu, J.-M.; Wang, L.-J.; Qi, J.-L.; You, J.-L.; Lin, L.; Meng, S.-D.; Wang, F.-X. Mortality and years of life lost of colorectal cancer in China, 2005–2020: Findings from the national mortality surveillance system. Chin. Med. J. 2021, 134, 1933–1940. [Google Scholar] [CrossRef]
- John, S.K.P.; George, S.; Primrose, J.N.; Fozard, J.B.J. Symptoms and signs in patients with colorectal cancer. Color. Dis. 2011, 13, 17–25. [Google Scholar] [CrossRef]
- American Cancer Society. Tests to Diagnose and Stage Colorectal Cancer. 2020. Available online: https://www.cancer.org/cancer/colon-rectal-cancer/detection-diagnosis-staging/how-diagnosed.html#written_by (accessed on 19 October 2022).
- Mahmoud, N.N. Colorectal Cancer: Preoperative Evaluation and Staging. Surg. Oncol. Clin. 2022, 31, 127–141. [Google Scholar] [CrossRef]
- Hayman, A.V.; Vasilevsky, C.-A. Colorectal Cancer: Preoperative Evaluation and Staging. In The ASCRS Textbook of Colon and Rectal Surgery; Springer: Cham, Switzerland, 2022; pp. 429–450. [Google Scholar]
- American Cancer Society. Cancer Staging. Available online: https://www.cancer.org/treatment/understanding-your-diagnosis/staging.html (accessed on 19 August 2024).
- Astler, V.B.; Coller, F.A. The prognostic significance of direct extension of carcinoma of the colon and rectum. Ann. Surg. 1954, 139, 846. [Google Scholar] [CrossRef]
- Turnbull, R.; Kyle, K.; Watson, F.R.; Spratt, J. Cancer of the colon: The influence of the no-touch isolation technic on survival rates. CA Cancer J. Clin. 1968, 18, 82–87. [Google Scholar] [CrossRef]
- Patel, V.; Rajak, H. Advances in Chemoradiotherapy for Treatment of Colon Cancer. In Colon Cancer Diagnosis and Therapy Vol. 3; Springer: Cham, Switzerland, 2022; pp. 217–239. [Google Scholar]
- Kim, S.H.; Chang, H.J.; Kim, D.Y.; Park, J.W.; Baek, J.Y.; Kim, S.Y.; Park, S.C.; Oh, J.H.; Yu, A.; Nam, B.-H. What Is the Ideal Tumor Regression Grading System in Rectal Cancer Patients after Preoperative Chemoradiotherapy? Cancer Res. Treat. 2015, 48, 998–1009. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Int. J. Surg. 2021, 88, 105906. [Google Scholar] [CrossRef]
- Jiang, D.; Wang, X.; Wang, Y.; Philips, D.; Meng, W.; Xiong, M.; Zhao, J.; Sun, L.; He, D.; Li, K. Mutation in BRAF and SMAD4 associated with resistance to neoadjuvant chemoradiation therapy in locally advanced rectal cancer. Virchows Arch. 2019, 475, 39–47. [Google Scholar] [CrossRef]
- Zhu, K.; Zhao, Q.; Yue, J.; Shi, P.; Yan, H.; Xu, X.; Wang, R. GOLPH3 overexpression correlates with poor response to neoadjuvant therapy and prognosis in locally advanced rectal cancer. Oncotarget 2016, 7, 68328–68338. [Google Scholar] [CrossRef] [PubMed]
- Molinari, C.; Salvi, S.; Foca, F.; Teodorani, N.; Saragoni, L.; Puccetti, M.; Passardi, A.; Tamberi, S.; Avanzolini, A.; Lucci, E.; et al. miR-17-92a-1 cluster host gene (MIR17HG) evaluation and response to neoadjuvant chemoradiotherapy in rectal cancer. OncoTargets Ther. 2016, 9, 2735–2742. [Google Scholar] [CrossRef][Green Version]
- Senetta, R.; Duregon, E.; Sonetto, C.; Spadi, R.; Mistrangelo, M.; Racca, P.; Chiusa, L.; Munoz, F.H.; Ricardi, U.; Arezzo, A. YKL-40/c-Met expression in rectal cancer biopsies predicts tumor regression following neoadjuvant chemoradiotherapy: A multi-institutional study. PLoS ONE 2015, 10, e0123759. [Google Scholar] [CrossRef]
- Ha, Y.J.; Kim, C.W.; Roh, S.A.; Cho, D.H.; Park, J.L.; Kim, S.Y.; Kim, J.H.; Choi, E.K.; Kim, Y.S.; Kim, J.C. Epigenetic regulation of KLHL34 predictive of pathologic response to preoperative chemoradiation therapy in rectal cancer patients. Int. J. Radiat. Oncol. Biol. Phys. 2015, 91, 650–658. [Google Scholar] [CrossRef] [PubMed]
- Dzhugashvili, M.; Luengo-Gil, G.; García, T.; González-Conejero, R.; Conesa-Zamora, P.; Escolar, P.P.; Calvo, F.; Vicente, V.; Ayala de la Peña, F. Role of genetic polymorphisms in NFKB-mediated inflammatory pathways in response to primary chemoradiation therapy for rectal cancer. Int. J. Radiat. Oncol. Biol. Phys. 2014, 90, 595–602. [Google Scholar] [CrossRef]
- Gantt, G.A.; Chen, Y.; Dejulius, K.; Mace, A.G.; Barnholtz-Sloan, J.; Kalady, M.F. Gene expression profile is associated with chemoradiation resistance in rectal cancer. Color. Dis. 2014, 16, 57–66. [Google Scholar] [CrossRef]
- National Institues of Health. Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies. 2021. Available online: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools (accessed on 19 August 2024).
- Sayers, E.W.; Bolton, E.E.; Brister, J.R.; Canese, K.; Chan, J.; Comeau, D.C.; Connor, R.; Funk, K.; Kelly, C.; Kim, S.; et al. Database resources of the national center for biotechnology information. Nucleic Acids Res. 2022, 50, D20–D26. [Google Scholar] [CrossRef]
- Xie, Z.; Bailey, A.; Kuleshov, M.V.; Clarke, D.J.B.; Evangelista, J.E.; Jenkins, S.L.; Lachmann, A.; Wojciechowicz, M.L.; Kropiwnicki, E.; Jagodnik, K.M.; et al. Gene Set Knowledge Discovery with Enrichr. Curr. Protoc. 2021, 1, e90. [Google Scholar] [CrossRef]
- Liao, Y.; Wang, J.; Jaehnig, E.J.; Shi, Z.; Zhang, B. WebGestalt 2019: Gene set analysis toolkit with revamped UIs and APIs. Nucleic Acids Res. 2019, 47, W199–W205. [Google Scholar] [CrossRef]
- Gallo, G.; Vescio, G.; De Paola, G.; Sammarco, G. Therapeutic targets and tumor microenvironment in colorectal cancer. J. Clin. Med. 2021, 10, 2295. [Google Scholar] [CrossRef]
- Ogunwobi, O.O.; Mahmood, F.; Akingboye, A. Biomarkers in Colorectal Cancer: Current Research and Future Prospects. Int. J. Mol. Sci. 2020, 21, 5311. [Google Scholar] [CrossRef] [PubMed]
- Koncina, E.; Haan, S.; Rauh, S.; Letellier, E. Prognostic and Predictive Molecular Biomarkers for Colorectal Cancer: Updates and Challenges. Cancers 2020, 12, 319. [Google Scholar] [CrossRef] [PubMed]
- Timar, J.; Kashofer, K. Molecular epidemiology and diagnostics of KRAS mutations in human cancer. Cancer Metastasis Rev. 2020, 39, 1029–1038. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Huang, D.; Ni, S.; Peng, Z.; Sheng, W.; Du, X. Plasma microRNAs are promising novel biomarkers for early detection of colorectal cancer. Int. J. Cancer 2010, 127, 118–126. [Google Scholar] [CrossRef]
- Chen, Q.; Xia, H.W.; Ge, X.J.; Zhang, Y.C.; Tang, Q.L.; Bi, F. Serum miR-19a predicts resistance to FOLFOX chemotherapy in advanced colorectal cancer cases. Asian Pac. J. Cancer Prev. 2013, 14, 7421–7426. [Google Scholar] [CrossRef]
- Sun, T.; Yin, Y.F.; Jin, H.G.; Liu, H.R.; Tian, W.C. Exosomal microRNA-19b targets FBXW7 to promote colorectal cancer stem cell stemness and induce resistance to radiotherapy. Kaohsiung J. Med. Sci. 2022, 38, 108–119. [Google Scholar]
- Nazemalhosseini Mojarad, E.; Kuppen, P.J.; Aghdaei, H.A.; Zali, M.R. The CpG island methylator phenotype (CIMP) in colorectal cancer. Gastroenterol. Hepatol. Bed Bench 2013, 6, 120–128. [Google Scholar]
- Cohen, S.A.; Wu, C.; Yu, M.; Gourgioti, G.; Wirtz, R.; Raptou, G.; Gkakou, C.; Kotoula, V.; Pentheroudakis, G.; Papaxoinis, G.; et al. Evaluation of CpG Island Methylator Phenotype as a Biomarker in Colorectal Cancer Treated With Adjuvant Oxaliplatin. Clin. Color. Cancer 2016, 15, 164–169. [Google Scholar] [CrossRef][Green Version]
- Sepulveda, A.R.; Hamilton, S.R.; Allegra, C.J.; Grody, W.; Cushman-Vokoun, A.M.; Funkhouser, W.K.; Kopetz, S.E.; Lieu, C.; Lindor, N.M.; Minsky, B.D.; et al. Molecular Biomarkers for the Evaluation of Colorectal Cancer: Guideline From the American Society for Clinical Pathology, College of American Pathologists, Association for Molecular Pathology, and the American Society of Clinical Oncology. J. Clin. Oncol. 2017, 35, 1453–1486. [Google Scholar] [CrossRef]
- Tang, L.; Wei, F.; Wu, Y.; He, Y.; Shi, L.; Xiong, F.; Gong, Z.; Guo, C.; Li, X.; Deng, H.; et al. Role of metabolism in cancer cell radioresistance and radiosensitization methods. J. Exp. Clin. Cancer Res. 2018, 37, 87. [Google Scholar] [CrossRef]
- Schwab, M.; Thunborg, K.; Azimzadeh, O.; von Toerne, C.; Werner, C.; Shevtsov, M.; Di Genio, T.; Zdralevic, M.; Pouyssegur, J.; Renner, K.; et al. Targeting Cancer Metabolism Breaks Radioresistance by Impairing the Stress Response. Cancers 2021, 13, 3762. [Google Scholar] [CrossRef] [PubMed]
- Sikder, S.; Mondal, A.; Das, C.; Kundu, T.K. Autophagy in Cancer: A Metabolic Perspective. In Metabolism and Epigenetic Regulation: Implications in Cancer; Kundu, T.K., Das, C., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 143–172. [Google Scholar]
- Hu, J.L.; He, G.Y.; Lan, X.L.; Zeng, Z.C.; Guan, J.; Ding, Y.; Qian, X.L.; Liao, W.T.; Ding, Y.Q.; Liang, L. Inhibition of ATG12-mediated autophagy by miR-214 enhances radiosensitivity in colorectal cancer. Oncogenesis 2018, 7, 16. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.-X.; Zhou, P.-K. DNA damage response signaling pathways and targets for radiotherapy sensitization in cancer. Signal Transduct. Target. Ther. 2020, 5, 60. [Google Scholar] [CrossRef] [PubMed]
- Nonnenmacher, L.; Hasslacher, S.; Zimmermann, J.; Karpel-Massler, G.; La Ferla-Brühl, K.; Barry, S.E.; Burster, T.; Siegelin, M.D.; Brühl, O.; Halatsch, M.-E. Cell Death Induction in Cancer Therapy—Past, Present, and Future. Crit. Rev. Oncog. 2016, 21, 253–267. [Google Scholar] [CrossRef]
- He, H.; Lin, K.; Zou, C.; Pan, J.; Fu, W.; Zhou, Y.; Lin, H.; Chen, C.; Su, Y. Knockdown of Annexin A2 Enhances Radiosensitivity by Increasing G2/M-Phase Arrest, Apoptosis and Activating the p38 MAPK-HSP27 Pathway in Nasopharyngeal Carcinoma. Front. Oncol. 2022, 12, 769544. [Google Scholar] [CrossRef]
- Zhou, H.; Tang, L.; Shang, Z.-F.; Zhou, P.-K.; Li, M. PIG3 downregulation enhances the radiosensitivity of NSCLC cells by promoting G2/M cell cycle arrest and apoptosis. Radiat. Med. Prot. 2022, 4, 19–25. [Google Scholar] [CrossRef]
- Coker-Gürkan, A.; Arisan, E.D.; Obakan, P.; Akalın, K.; Özbey, U.; Palavan-Unsal, N. Purvalanol induces endoplasmic reticulum stress-mediated apoptosis and autophagy in a time-dependent manner in HCT116 colon cancer cells. Oncol. Rep. 2015, 33, 2761–2770. [Google Scholar] [CrossRef]
- Qian, H.R.; Shi, Z.Q.; Zhu, H.P.; Gu, L.H.; Wang, X.F.; Yang, Y. Interplay between apoptosis and autophagy in colorectal cancer. Oncotarget 2017, 8, 62759–62768. [Google Scholar] [CrossRef]
- Myung Park, J.; Tougeron, D.; Huang, S.; Okamoto, K.; Sinicrope, F.A. Beclin 1 and UVRAG confer protection from radiation-induced DNA damage and maintain centrosome stability in colorectal cancer cells. PLoS ONE 2014, 9, e100819. [Google Scholar] [CrossRef]
- Kim, W.; Youn, H.; Kang, C.; Youn, B. Inflammation-induced radioresistance is mediated by ROS-dependent inactivation of protein phosphatase 1 in non-small cell lung cancer cells. Apoptosis 2015, 20, 1242–1252. [Google Scholar] [CrossRef] [PubMed]
- Arnold, K.M.; Opdenaker, L.M.; Flynn, N.J.; Appeah, D.K.; Sims-Mourtada, J. Radiation induces an inflammatory response that results in STAT3-dependent changes in cellular plasticity and radioresistance of breast cancer stem-like cells. Int. J. Radiat. Biol. 2020, 96, 434–447. [Google Scholar] [CrossRef] [PubMed]
- Colotta, F.; Allavena, P.; Sica, A.; Garlanda, C.; Mantovani, A. Cancer-related inflammation, the seventh hallmark of cancer: Links to genetic instability. Carcinogenesis 2009, 30, 1073–1081. [Google Scholar] [CrossRef]
- Schmitt, M.; Greten, F.R. The inflammatory pathogenesis of colorectal cancer. Nat. Rev. Immunol. 2021, 21, 653–667. [Google Scholar] [CrossRef]
- Fantini, M.C.; Pallone, F. Cytokines: From gut inflammation to colorectal cancer. Curr. Drug Targets 2008, 9, 375–380. [Google Scholar] [CrossRef]
- Kraus, S.; Arber, N. Inflammation and colorectal cancer. Curr. Opin. Pharmacol. 2009, 9, 405–410. [Google Scholar] [CrossRef]
- Pai, P.; Sukumar, S. HOX genes and the NF-κB pathway: A convergence of developmental biology, inflammation and cancer biology. Biochim. et Biophys. Acta (BBA)-Rev. Cancer 2020, 1874, 188450. [Google Scholar] [CrossRef]
- Zhang, T.; Ma, C.; Zhang, Z.; Zhang, H.; Hu, H. NF-κB signaling in inflammation and cancer. MedComm 2021, 2, 618–653. [Google Scholar] [CrossRef]
- Fujishima, Y.; Nishiumi, S.; Masuda, A.; Inoue, J.; Nguyen, N.M.; Irino, Y.; Komatsu, M.; Tanaka, K.; Kutsumi, H.; Azuma, T.; et al. Autophagy in the intestinal epithelium reduces endotoxin-induced inflammatory responses by inhibiting NF-κB activation. Arch. Biochem. Biophys. 2011, 506, 223–235. [Google Scholar] [CrossRef]
- Lin, X.T.; Zheng, X.B.; Fan, D.J.; Yao, Q.Q.; Hu, J.C.; Lian, L.; Wu, X.J.; Lan, P.; He, X.S. MicroRNA-143 Targets ATG2B to Inhibit Autophagy and Increase Inflammatory Responses in Crohn's Disease. Inflamm. Bowel Dis. 2018, 24, 781–791. [Google Scholar] [CrossRef]
| Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies | ||||||||
|---|---|---|---|---|---|---|---|---|
| Quality Assessed Based on: | Study | |||||||
| Criteria assessed | Question to satisfy | [15] | [16] | [17] | [18] | [19] | [20] | [21] |
| Research Questions | 1. Was the research question or objective in this paper clearly stated? | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Study population | 2. Was the study population clearly specified and defined? | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| 3. Was the participation rate of eligible persons at least 50%? | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Groups recruited from the same population and uniform eligibility criteria | 4. Were all the subjects selected or recruited from the same or similar populations (including the same time period)? Were inclusion and exclusion criteria for being in the study prespecified and applied uniformly to all participants? | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Sample size justifications | 5. Was a sample size justification, power description, or variance and effect estimates provided? | ✗ | ✗ | ✗ | ✓ | ✓ | ✗ | ✗ |
| Exposure assessed prior to outcome measurement | 6. For the analyses in the given paper, were the exposure(s) of interest measured prior to the outcome(s) being measured? | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Sufficient timeframe to see an effect | 7. Was the timeframe sufficient so that one could reasonably expect to see an association between exposure and outcome if it existed? | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Different levels of the exposure interest | 8. For exposures that can vary in amount or level, did the study examine different levels of the exposure as related to the outcome (e.g., categories of exposure, or exposure measured as continuous variable)? | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Exposure measures and assessment | 9. Were the exposure measures (independent variables) clearly defined, valid, reliable, and implemented consistently across all study participants? | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Repeated exposure assessment | 10. Was the exposure(s) assessed more than once over time? | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Outcome measures | 11. Were the outcome measures (dependent variables) clearly defined, valid, reliable, and implemented consistently across all study participants? | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Blinding of outcome assessors | 12. Were the outcome assessors blinded to the exposure status of participants? | ⦸ | ⦸ | ⦸ | ⦸ | ⦸ | ⦸ | ⦸ |
| Follow up rates | 13. Was loss to follow-up after baseline 20% or less? | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Statistical analysis | 14. Were key potential confounding variables measured and adjusted statistically for their impact on the relationship between exposure(s) and outcome(s)? | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Total Points | 12 | 12 | 12 | 13 | 13 | 12 | 12 | |
| Quality Rating | ⬤ | ⬤ | ⬤ | ⬤ | ⬤ | ⬤ | ⬤ | |
| Study Characteristics | ||||||
|---|---|---|---|---|---|---|
| Study | Publication Information | Study Information | ||||
| Year | Country | Recruitment Criteria | Sample Size | Study Age | Gender Size (M/F) | |
| [15] | 2019 | China | Patients with stage II and III rectal cancers treated with pre-operative nCRT. Cases without genetic testing data were excluded due to poor DNA quality. | 74 | >50 (n = 42), ≤50 (n = 32) | 52/22 |
| [16] | 2016 | China | Rectal cancer patients before nCRT therapy. Patients with distant metastasis, declining surgery after nCRT and insufficient tumor tissue samples were excluded. | 148 | <65 (n = 67), ≥65 (n = 81) | 89/59 |
| [17] | 2016 | Italy | LARC patients undergoing homogeneous nCRT. Patients without sufficient quality of pre-therapy DNA were excluded. | 108 | Median = 67 (range: 31–80) | 75/33 |
| [18] | 2015 | Italy | LARC patients eligible for nCRT. Patients with incomplete CRT were excluded. | 81 | Mean = 63 (range: 44–91) | 52/29 |
| [19] | 2014 | Republic of Korea | First step: LARC patients who received preoperative CRT and curative resection were recruited for genome-wide methylation analysis by microarray (Cohort 1). Second step: consisted of a continuous cohort of LARC patients, including 27 patients from first step, with the same therapy regimen were added for validation by pyrosequencing (Cohort 2). | Cohort 1: 45 Cohort 2: 43 | Mean = 59 Cohort 1 SD: ±12 Cohort 2 SD: ±11 | Cohort 1: 34/11 Cohort 2: 29/14 |
| [20] | 2014 | Spain | Patients treated with preoperative CRT for LARC (stages cT3–4 and/or N1–N2). Patients with metastases were excluded from the study | 159 | Mean = 65 (SD: 10.7) Median = 64 (range: 23–84) | 104/55 |
| [21] | 2013 | USA | Middle- or lower-third rectal cancer patients (stage II or III) who met the clinical criteria for nCRT and eventual hepatic mastectomy candidates with ultra-low stage I and stage IV progression. | 33 | Mean = 55.9 (SD: 10.51) | 24/9 |
| Clinical Characteristics | |||||||
|---|---|---|---|---|---|---|---|
| Study | Pathological Information | Treatment Protocol | Remark | ||||
| Pre-Therapeutic Clinical Staging (n) | Tumor Histological Grade (n) | Other Information | CT | RT | Surgical | ||
| [15] | cTNM (AJCC 8th ed): cT2 (2), cT3 (31), cT4a (8), cT4b (33) cAJCC stage: II (25), III (49) | G1 (15), G2 (37), G3 (8) | Carcinoma Type: Adenocarcinoma (60), Mucinous carcinoma (12), Signet ring cell carcinoma (2) | XELOX and mFOLFOX-6 for 1 to 4 cycles before RT. Xeloda or 5-Fu concurrent with RT. | 45 to 50.4 Gy over 28 fractions by IMRT or VMAT. | Curative resection, including TME at 4 to 8 weeks after completion of preoperative CRT. | Staging was evaluated by radiography and endoscopic ultrasonography, followed by surgical resection and IHC. |
| [16] | cTNM (AJCC 7th ed): cT3 (80), cT4 (68) cN0 (54), cN+ (94) M0 (122), M1 (26) | Differentiated (82), Undifferentiated (66) | CEA (ng/mL) <3.4 (70), ≥3.4 (78) Recurrence: Neg (105), Pos (43) Distance from anal verge (cm) <6 (57), ≥6 (91) | 5-Fu or XELOX concurrent with RT. | 50.4 Gy over 28 fractions with 4 fields of irradiation or 3D conformal RT | TME at 4 to 6 weeks after nCRT. | Biopsies were obtained by proctoscopy from patients before therapy to confirm the pathological diagnosis of adenocarcinoma within 15 cm from the anal verge. Physical examination, CEA routine blood test and chest enhancement CT, as well as abdomen and pelvic cavity enhancement CTs were performed before therapy. |
| [17] | uTNM: uT2 (8), uT3 (95), uT4 (3), uT N/A (2) uN0 (53), uN1 (31), uN2 (2), uNx (19), uN N/A (3) | N/A | N/A | Daily dose of 5-Fu during RT or Xeloda twice daily. | 50.4 Gy over 5 weeks with conventional fractionation. | Surgery at 6 to 8 weeks after therapy completion. | Pre-therapeutic TNM staging was performed by ultrasound (uTNM). |
| [18] | N/A | G1 (4), G2 (66), G3 (11) | N/A | 51 patients received standard Xeloda concurrent with RT, whilst 30 patients underwent XELOX | 50.4 Gy in 28 fractions. | Surgery was performed 6 to 8 weeks after end of CRT. | The authors did not provide pre-therapeutic TNM staging. |
| [19] | AJCC 7th ed. stage II/III/IV: Cohort 1 (3/40/2), Cohort 2 (2/40/1) | 4 patients in each cohort have poorly differentiated or mucinous tumor characteristics | CEA (ng/mL) Cohort 1: 4.57 ±6.25, Cohort 2: 13.91 ±52.29 | Xeloda or 5-Fu delivered concurrently with RT | 46 Gy delivered in 23 fractions followed by a 4 Gy boost to the primary tumor. | Radical surgery performed 6 to 8 weeks after CRT. | N/A |
| [20] | cTNM (AJCC 7th ed): cT0–2 (9), cT3–4 (150) cN+ (116) | G1 (16), G2 (134), G3 (9) | Tumor Location: Upper (43), Middle (61), Lower (54), Other (1) | Xeloda concomitant with RT For patients with adverse risk factors, 6 cycles of Xeloda or 6 cycles of XELOX or FOX was given after surgery | 50.4 Gy delivered in 1.8 Gy fractions at 5 times per week by 3D conformal RT with a three-field technique | Radical surgery consisting of APR or LAR was performed 4 to 6 weeks after CRT. | N/A |
| [21] | NCCN stage: I (2), II (11), III (14), IV (4), N/A (2) | Differentiation Well (2), Moderate (29), Poor (1) | Invasion: Vascular (1), Lymphatic (5) | 5-Fu delivered as radiosensitizer | 5040 [c]Gy delivered in 30 fractions | Proctectomy performed approximately 8 to 10 weeks after CRT | Initially, the author reported a 5040 Gy radiation dose, but after confirmation with experts, the dose unit should be cGy (100 cGy = 1 Gy). This could be a minor typo by the literature. |
| Outcome Characteristics | ||||||
|---|---|---|---|---|---|---|
| Study | Tumor Response | Mutation Information | Remark | |||
| TRG Criteria | Grade (n) | Post-Therapeutic Pathological Staging (n) | Gene (Mutation Type) [Frequency (n)] | Detection Method | ||
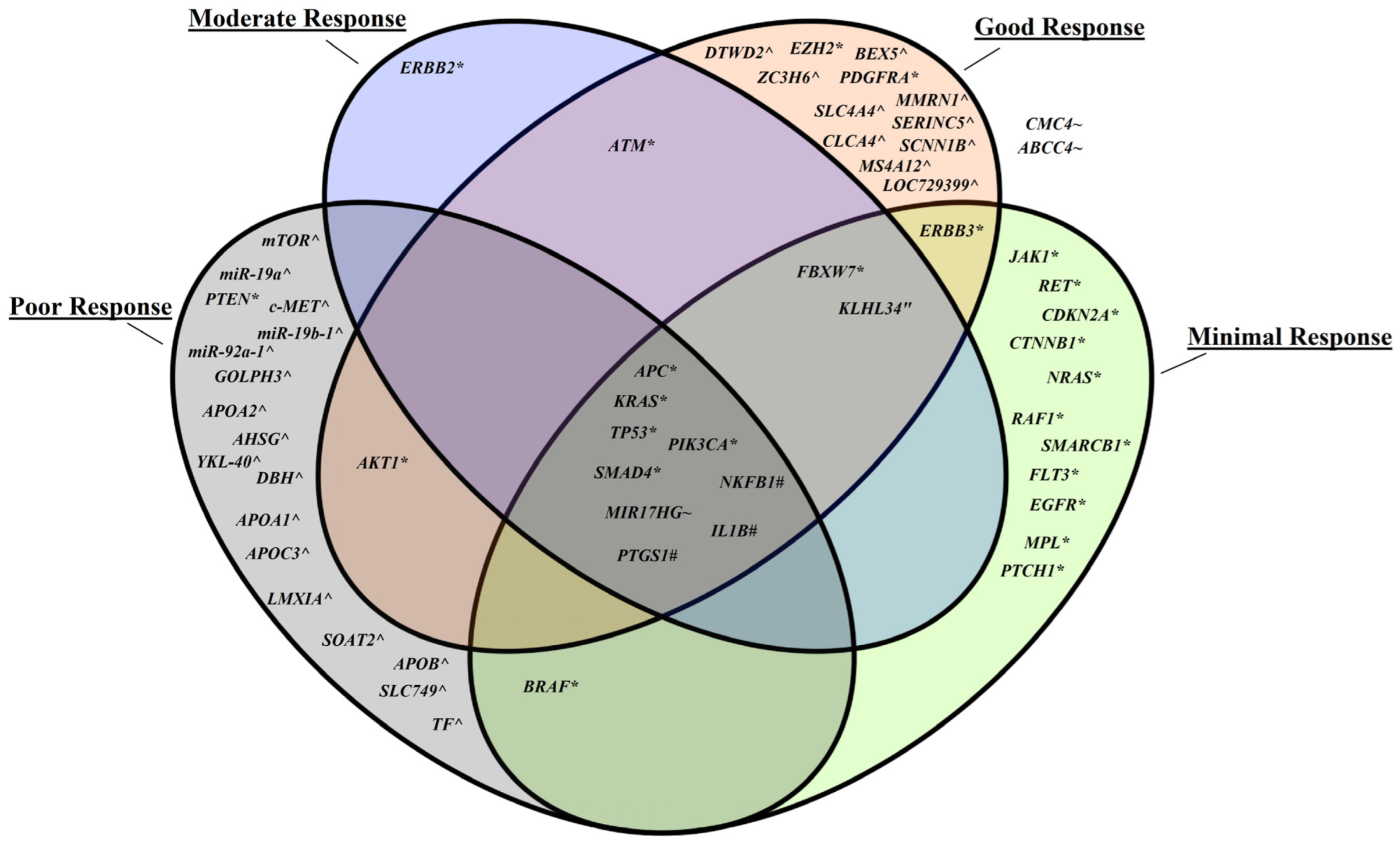
| [15] | NCCN | 0 (13), 1 (19), 2 (30), 3 (12) | ypTNM: ypT0 (13), ypT1 (1), ypT2 (14), ypT3 (38), pT4a (2), ypT4b (6) Downstaging observed (50) ypAJCC_stage: 0 (12), I (11), II (24), III (25), Uncertain (2) | BRAF [TRG2 (1), TRG3 (3)]; RAF1 [TRG2 (1)]; KRAS [TRG0 (4), TRG1 (6), TRG2 (12), TRG3 (4)]; NRAS [TRG2 (1)]; TP53 [TRG0 (8), TRG1 (11), TRG2 (15), TRG3 (6)]; APC [TRG0 (3), TRG1 (4), TRG2 (4), TRG3 (3)]; PIK3CA [TRG2 (1)]; PTEN [TRG3 (1)]; FBXW7 [TRG0 (2), TRG1 (2), TRG2 (3)]; SMAD4 [TRG0 (1), TRG1 (1), TRG2 (2), TRG3 (4)]; ERBB2 [TRG1 (1)]; ERBB3 [TRG0 (1), TRG2 (2)]; ATM [TRG0 (1), TRG1 (2)]; AKT1 [TRG0 (1), TRG3 (1)]; RET [TRG2 (1)]; PDGFRA [TRG0 (1)]; SMARCB1 [TRG2 (2)]; EZH2 [TRG0 (1)]; CDKN2A [TRG2 (1)]; CTNNB1 [TRG2 (1)]; EGFR [TRG2 (1)]; FLT3 [TRG2 (1)]; JAK1 [TRG2 (1)]; MPL [TRG2 (1)]; PTCH1[TRG2 (1)] | Targeted NGS, Sanger sequencing, IHC | Mutation type was not studied in-depth. Downstaging is defined as tumor which has a lower ypT than cT. TRG grading represents the following post therapy tumor response: TRG0—complete response, TRG1—moderate response, TRG2—minimal response, TRG3—poor response. |
| [16] | Dworak | 0–2 (79), 3–4 (69) | ypTNM: ypT0–2 (62), ypT3–4 (86) ypN0 (88), ypN+ (60) Downstaging observed (77) | GOLPH3 (High) [TRG0–2 (49), TRG3–4 (28)], (Low) [TRG0–2 (30), TRG3–4 (41)]; mTOR (High) [in Highly expressed GOLPH3 (53/77)], (Low) [in Low expressed GOLPH3 (43/71)] | IHC | TRG0–2 was defined as poor response, whilst TRG3–4 indicates good response. TRG4 also represents pCR Studied protein expression levels rather than direct gene expression levels. |
| [17] | Dworak | 0 (3), 1 (23), 2 (38), 3 (25), 4 (19) | ypTNM: ypT0 (19), ypT1 (11), ypT2 (31), ypT3 (39), ypT4 (4), ypT information not available (4) ypN0 (77), ypN1 (15), ypN2 (7), ypNx (3), ypN infomation not available (6) | MIR17HG cluster members (High levels of miR-19a, miR-19b-1 and miR-92a-1) [TRG0–1 vs. TRG4 (100%)], (Locus amplified) [Non-responders (41%)], (Deletion) [Responders (41%)]; CMYC; ABCC4 | RT-qPCR | Despite discovering upregulation of a miR levels, no statistical significance with any clinicopathological characteristics was found. No significances in CMYC and ABCC4 expression with nCRT response were found. TRG0–1 and TRG4 denotes absence of response and complete response respectively. |
| [18] | Mandard | 1 (20), 2 (16), 3 (22), 4 (21), 5 (2) | ypTNM: ypT0 (20), ypT1 (4), ypT2 (20), ypT3 (35), ypT4 (2) ypN0 (59), ypN1 (21) Downstaging observed (48) | YKL-40, (Positive) [TRG2–5 (87%)]; c-Met, (Positive) [TRG2–5 (86%)], (co-mutation with YKL-40) [TRG2–5 (94%)] | IHC, FISH | TRG1 describes complete response, whilst TRG2–5 indicates partial or absent response. Complete vs. partial responder distribution was similar for both the RT + capecitabine and XELOXART protocols. Disease status after therapy and surgery: No evidence of disease (n = 59), Alive with disease (n = 18), Died of disease (n = 3). Studied proteomic expression data. |
| [19] | Mandard | For each Cohort 1 against Cohort 2: 1 (7/12), 2 (12/11), 3 (18/12), 4 (8/8), 5 (0/0) | N/A | KLHL34 CpG site: cg14232291 (Hypermethylation) [levels in TRG1–3 = 42.45 ±3.21 vs. non-responders = 27.31 ±4.99] | Pyrosequencing, RT-qPCR, Western Blotting, Microarray | Staging of disease after therapy and radical surgery as well as frequency of mutation in patients was not reported. Positive response is assessed with TRG1–3. |
| [20] | N/A | N/A | ypTNM: ypT0N0 (29), ypT1–2N0 (99), ypT3–4 and/or pN+ (31) | Patients with ypT0N0 or ypT1–2N0 vs. ypT3–4 and/or pN+ NFKB1 polymorphism: rs28362491 (DEL/DEL) [(19) vs. (1)], (INS/INS) [(53) vs. (16)], (INS/DEL) [(56) vs. (14)]; IL1B polymorphism: rs1143627 (A/A) [(61) vs. (9)], (G/A) [(47) vs. (17)], (G/G) [(20) vs. (5)]; IL1B polymorphism: rs16944 (A/A) [(17) vs. (3)], (G/A) [(50) vs. (18)], (G/G) [(61) vs. (10)]; PTGS1 polymorphism: rs1213266, (A/A) [(2) vs. (0)], (G/A) [(26) vs. (4)], (G/G) [(100) vs. (27)]; PTGS1 polymorphism: rs5789 (C/C) [(116) vs. (27)], (C/A) [(11) vs. (3)], (A/A) [(1) vs. (1)]; PTGS2 polymorphism: rs5275 (A/A) [(56) vs. (16)], (G/A) [(58) vs. (13)], (G/G) [(14) vs. (2)] | RT-qPCR | Directly reported mean differences in tumor size (cm) before and after treatment. Pre-treatment (by pelvic MRI): 6.26 (range: 1–12), Post-treatment (by pathological report): 2.26 (range: 0–1.85). Post-therapeutic response assessment was determined as follows: Complete response (ypT0N0), Intermediate or partial response (ypT1–2N0), Poor response (ypT3–4 and/or pN+) Vital status of patients: Deceased with tumor (n = 23), Deceased without tumor (n = 0), Alive with tumor (n = 7), Alive without tumor (n = 129). |
| [21] | AJCC | 0 (6), 1 (7), 2 (13), 3 (7) | N/A | Top 10 (up-regulated) genes: APOA2, AHSG, DBH, APOA1, APOB, APOC3, LMX1A, SOAT2, SLC7A9, TF Top 10 (down-regulated) genes: LOC729399, SERINC5, SCNN1B, ZC3H6, SLC4A4, DTWD2, MS4A12, BEX5, MMRN1, CLCA4 | Microarray | AJCC0–2 were considered responders AJCC3 were considered non-responders. Staging of disease after therapy and radical surgery as well as mutation frequency was not reported. Only reported top 10 up- and down-regulated genes out of 19, 228 target genes for non-responders. |
| Gene Set Enrichment Analysis Summary | ||||
|---|---|---|---|---|
| Apoptosis | DNA Damage Response and Repair | Inflammation | Cancer Metabolism | |
| AKT1 | ✓ | ✓ | ✓ | ✓ |
| APC | ✓ | |||
| ATM | ✓ | |||
| BRAF | ✓ | |||
| CDKN2A | ✓ | ✓ | ||
| CTNNB1 | ✓ | |||
| EGFR | ✓ | ✓ | ||
| ERBB2 | ✓ | ✓ | ||
| FLT3 | ✓ | |||
| KRAS | ✓ | ✓ | ✓ | ✓ |
| MET | ✓ | |||
| mTOR | ✓ | |||
| MYC | ✓ | ✓ | ||
| NFKB1 | ✓ | ✓ | ||
| NRAS | ✓ | ✓ | ✓ | ✓ |
| PDGFRA | ✓ | |||
| PIK3CA | ✓ | ✓ | ✓ | ✓ |
| PTEN | ✓ | ✓ | ✓ | |
| PTGS1 | ✓ | |||
| PTGS2 | ✓ | |||
| RAF1 | ✓ | ✓ | ✓ | |
| RET | ✓ | |||
| SMAD4 | ✓ | |||
| TP53 | ✓ | ✓ | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lau, M.Y.H.; Islam Khan, M.Z.; Law, H.K.W. Molecular Mechanism of Radioresponsiveness in Colorectal Cancer: A Systematic Review. Genes 2024, 15, 1257. https://doi.org/10.3390/genes15101257
Lau MYH, Islam Khan MZ, Law HKW. Molecular Mechanism of Radioresponsiveness in Colorectal Cancer: A Systematic Review. Genes. 2024; 15(10):1257. https://doi.org/10.3390/genes15101257
Chicago/Turabian StyleLau, Matthew Y. H., Md Zahirul Islam Khan, and Helen K. W. Law. 2024. "Molecular Mechanism of Radioresponsiveness in Colorectal Cancer: A Systematic Review" Genes 15, no. 10: 1257. https://doi.org/10.3390/genes15101257
APA StyleLau, M. Y. H., Islam Khan, M. Z., & Law, H. K. W. (2024). Molecular Mechanism of Radioresponsiveness in Colorectal Cancer: A Systematic Review. Genes, 15(10), 1257. https://doi.org/10.3390/genes15101257






